ПАРАЗИТОЛОГИЯ, 2020, том 54, № 2, с. 145-151.
УДК 616.936
MOLECULAR DETECTION OF PLASMODIUM VIVAX ON DRY BLOOD
SPOT IN EASTERN SUDAN
© 2020 S. G. Abdallaa, H. A. Musab,*, I. Adamc, S. E. Elzaki d,
A. H. Malika, M. A. Elsheike, M. I. Saeeda
a Faculty of Medical laboratory, The National RibatUniversity, Sudan
b Faculty of Medicine, The National Ribat University, Sudan
c Faculty of medicine, University of Khartoum, Sudan
d National Center for research, Coordinate of malaria research projects, Sudan
e Faculty of Medicine, The National Ribat Hospital, Sudan
⁕e-mail: moibsaeed@yahoo.com
Received 09.01.2020
Received after revision 06.02.2020
Accepted 07.02.2020
Plasmodium vivax nowadays is emerging as one of the common causative species of malaria
mainly in Sudan. Laboratory studies based on genomic approaches provide an alternative to identify
the increased frequency of recurrent relapses of malaria infections and cases of low parasitemia such
as P. vivax. The main objective of this study was to compare the performance of PCR and RDT to the
gold standard diagnostics microscopy as a mean of detecting Plasmodium vivax parasites during active
malaria. A total of 572 febrile patients were enrolled in the present study from Kassala, Halfa, and
Eastern Nile area of Sudan. The sample was diagnosed by quality, insured microscopy, ICT (Immune-
Chromatography Test) and PCR methods. The results indicated that the incidence of P. vivax infections
among suspected malaria cases was relatively high. The total positive samples number of P. vivax by
three methods was 164; while the three methods detected 71 (28.7%), 70 (28.3%), and 123 (38.8%),
respectively.
The study findings indicated the changing Plasmodium vivax distribution pattern which seemly
attributed to the recent demographic movement and high rate of immigration from neighboring
countries to the east region in the recent years; ending with such rising trend of P.vivax malaria
in eastern Sudan due to which management of the dormant hypnozoite stage when treating the cases
of relapsing malaria. In conclusion, detection of Plasmodium vivax gene showed superior capability
to identify cases of low parasitemia compared to the gold standard diagnostic microscope methods
and reliable mean for adequate detection and primarily tool for eliminating Plasmodium vivax malaria.
Keywords: Malaria, Plasmodium vivax, Molecular ICT, Eastern Sudan
DOI: 10.31857/S1234567806020054
145
Malaria is an infectious febrile disease of humans and other animal species; it’s caused by
Plasmodium parasites (Centers for Disease Control, 2015). According to the WHO estimates,
there were about 228 million cases of malaria in 2018 in the world (WHO, 2019). Reduction
of malaria mortality rates varied from 533 000 in 2010 to 380 000 in 2018 in the WHO
African Region (WHO, 2019). Although malaria case incidence has fallen globally since
2010, the rate of decline has stalled and even reversed in some regions since 2014. Globally,
the total malaria deaths reached 445 000 deaths, about the same number was reported in
2015. In WHO report 2016, 91 countries reported a total of 216 million cases of malaria,
with an increase of 5 million cases over the previous year. The African Region continues to
account for about 90% of malaria cases and deaths worldwidewhile fifteen countries - all but
one in sub-Saharan Africa - carry 80% of the global malaria burden (Mbacham etal., 2019).
Plasmodium vivax is the most frequent and widely distributed cause of recurring (benign
tertian) malaria. P. vivax is one of the five species of malaria parasites that commonly infect
humans. It is less virulent than Plasmodium falciparum, the deadliest of the five, but vivax
malaria can also lead to severe disease and death (Anstey et al., 2012).
Plasmodium vivax infection is becominga major health problem in Sudan. This parasite
species has the broadest geographic distribution of the five malaria species known to infect
humans (Guerra et al., 2009). There are about 2.5 billion people at risk of malaria and an
estimated 80 to 300 million clinical cases of P. vivax annually. Although P. vivax is mainly
endemic in Southeast Asia and Latin America, it has recently been observed in Ethiopia and
Sudan (Mahgoub et al., 2012). However, in recent years many clinicians observed recurrent
relapses of malaria infections in different areas in Sudan suggesting perhaps a higher than
expected transmission of non-falciparum malaria parasites most likely P. vivax since it is
the second most important malaria parasite species in Sudan. The objective of this study
was to compare the reliability of the diagnostics methods for the detection of P. vivaxand to
recommend the best diagnostic option for detection this spices and co-infection.
MATERIALS AND METHODS
Ethical Considerations
The study was approved by the ethical research committee of the Faculty of Medicine, the University
of Ribat, Khartoum, Sudan.
Study Area and Sample Collection
This was a cross-sectional study carried out in eastern area of Sudan. It is the region of Sudan lying
to the west and south of Gedaref state to the Eritrean border. The area is considered mesoendemic for
malaria; transmission follows mainly the vector breeding in the rainy season (July to OctoberWhole-
blood samples were collected from patients with malaria - like symptoms, including fever and/or
146
chills, sweats, headaches, muscle pains, nausea and vomiting. About 3ml of venous blood samples were
collected into an EDTA anticoagulant tube. Additionally, the venous peripheral blood was prepared
as dried blood spots: two 50 μl aliquots of blood from the same patient were applied to filter paper
Whatman Grade No. 3 (Whatman plc, Maidstone, UK), air-dried immediately, placed individually in
sealed plastic bags and the specimens were transported for molecular detection by PCR in the National
center for tropical medicine research,Department of molecular epidemiology.
Lab Diagnosis of Malaria
Thick and thin blood smears were made in the same slide and the rapid diagnostic test (ICT) were
performed immediately.
The collected fresh blood samples were diagnosed for malaria using blood film microscopy and ICT
and confirmed with PCR. Microscopic examination was performed on both thick and thin blood films,
microscopic fields were read at least twice, and the procedure was followed according to quality control
guidelines of WHO. PCR was performed for P. vivaxwith positive and negative control included. Genomic
DNA was extracted from whole blood samples using Chelex method. A fragment of the plasmodial
18S rRNA gene with 121 bp size was amplified by PCR and species identification was
performed with species-specific oligoprobes using the following P. vivaxprimers; rVIV1
(CGCTTCTAGCTTAATCCACAT AACTGATAC), and rVIV2 (ACTTCCAAGCCGAAGCAAAGA
AAGTCCTTA), using the following PCR cycling steps: 95°C for 5 min. Initial denaturation, 94°C for
1 min. Denaturation, 64°C for 2 min. Annealing, 72°C for 2 min. Extension, according to the protocol
adopted from Snounou, Singh (2002).
Statistical Analysis
Data were analyzed using SPSS (statistical package for the social sciences) version twentieth
software.
RESULTS
The participant gender distribution in the study was as follows: more males were affected
by malaria; however, the percentage of females was 53.1% while the percentage of males
was 46.9%.
Comparison of the rapid diagnostic test (RDT) and polymerase chain reaction (PCR)
with the microscopic gold-standard method demonstrated the following. Out of 572 samples,
the total positive malaria patients revealed by microscopy in the three areas of the study
resulted in 71 positive samples (12.4%) due to Plasmodium vivax, in different areas (Table 1).
When the RDT was used, among total number of positive samples, 70(12.3 %) were
positive for Plasmodium vivax (Table 1). On the other hand, among the total number of the
positive samples revealed by polymerase chain reaction (PCR), 123(23.3%) were positive
for Plasmodium vivax (Table 1). According to the method of gold standard microscopy, the
fraction of P. vivax in Halfa, Kassala, and Eastern Nile constituted 12.4,14.1, and 10.9%,
respectively (Table 2).
147
DISCUSSION
In the present study, males were found to be the gender more affected with malaria
(53.1%).
Throughout the entire studied area, the fraction of P. vivax infections among suspected
malaria cases was relatively high (about 38.8 % by PCR). This result is similar to that
previously done in Aljabalain area located in the White Nile state in central Sudan. The most
remarkable result in this study was the unexpected high proportion (about 40% by PCR)
of P. vivax infections among suspected malaria cases, eight times more than that previously
reported in Sudan (Makarim et al., 2016). These results suggest that the change in the infection
pattern is most likely explained by the recent changed composition of the community
resulting from several migrations of people from several Asian and African countries to work
at petroleum and new sugar companies in White Nile area. This can be true especially for
migrants from Ethiopia, where high prevalence of P. vivax infection (31%) among malaria
cases was found (Lo et al., 2015).
The prevalence of P. vivax had been estimated in this work for three areas of study. The
results showed that no statistically significant differences between the three areas of the study
(P-value >0.05) were revealed. This comes with an agreement with a study performed in
relation to the epidemiology and distribution of Plasmodium vivax malaria in Sudan, where
the overall fraction of P. vivax among the malaria cases constituted 26.6% (Amandaet al.,
2017). The prevalence showed significant variations between the states (p<0.001), which
could be explained by differences in population movement, the presence of refugees, and
proximity to Plasmodium vivax endemic neighboring countries. It also varied significantly
with residence status (p<0.001), reflecting the stability of transmission (Amanda et al., 2017).
Accurate diagnosis of Plasmodium species is important not only for establishing the
correct treatment regimen, but also for applying effective malaria control strategies in
endemic regions as in Sudan. The present study compared microscopy and ICT with PCR.
It was found that results obtained by PCR method were superior to those obtained by
microscopy. Sensitivity of microscopy, ICT and PCR were evaluated in this study in order to
determine the most sensitive method that detects more positive cases. The result proved that
PCR was the most sensitive technique (detected 47.0% of the total positive samples) (P-value
<0.001). These results are in agreement with many studies done worldwide for different sero-
prevalence studies comparing the sensitivity of different techniques.
As diagnostic resources are limited in the study area, without a reference laboratory, the
gold standard microscopy remains the reliable, affordable and applicable laboratory method
for diagnosing malaria. PCR diagnosis for malaria is accurate especially for differentiating
between plasmodia species, but it is more expensive and needs well-trained personnel.
148
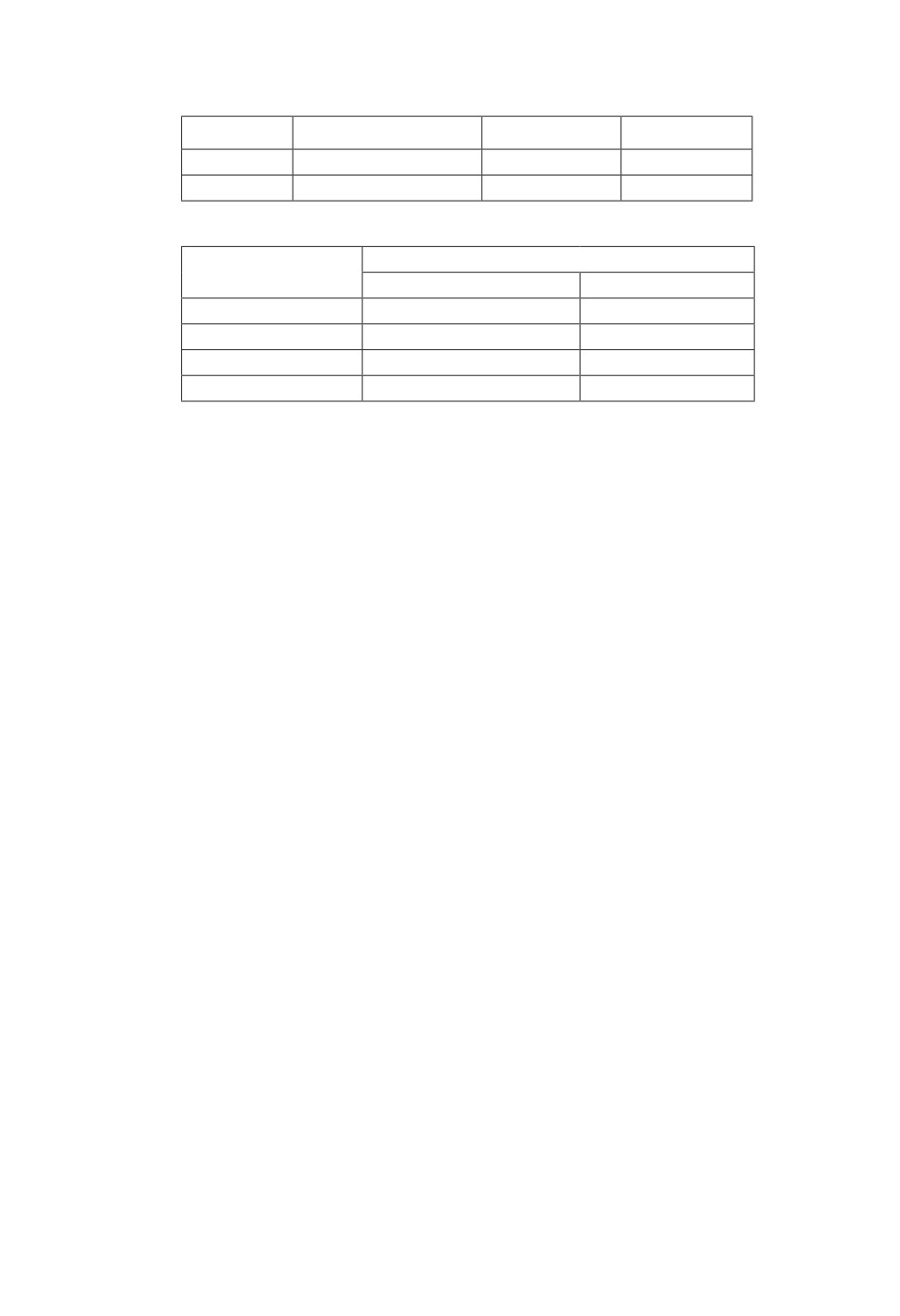
Table 1. Prevalence of P. vivax infections detected in malaria cases
Detection
Microscopy, %
RDT, %
PCR, %
P. vivax
71(12.4)
70(12.2)
123(21.5)
Negative
501(87.6)
502 (87.8)
449 (78.5)
Table 2. Prevalence of P. vivax infections detected by microscopy from different sites
Sites
P. vivax
Positive n, %
Negative n, %
Halfa
24(12.4)
170(87.6)
Kassala
28(14.1)
170(85.9)
Eastern Nile
19(10.9)
161(89.4)
Total
71(12.4)
501(87.6)
In all the cases, P-value<0.05 statistical significantly different.
Currently, the Sudanese National Malaria Control Program recommends the use of
RDT in those settings where no expert microscopy is available, and maintains microscopic
examination in those places where microscopy is of an adequate level ( Elmardi et al., 2009).
This RDT strategy was investigated earlier in Sudan for the home management of malaria
using artemisinin-based combination therapy ( Elmardi et al., 2009). This is in agreement
with a study performed at Gadarif teaching hospital in eastern Sudan. Based on the findings
of his study, it appears likely that implementation of malaria RDT in Sudan in settings where
microscopic expertise is available should not be recommended (Awadalla et al., 2013).
CONCLUSION
We can conclude that Plasmodium vivax malaria remains a major public health problem
in eastern Sudan. The possibility of low parisitemia infections is increasing and seems to be
more prevalent in future. PCR detects more cases than has been revealed by microscopy,
while RDT reveals similar cases of malaria parasitic infections. The results indicate the
superior capacity of PCR in detection of more cases and raise queries about the possibility of
asymptomatic carrier, recurrent infections, or presence of drug resistance of local or newly
imported resistant strains.
ACKNOWLEDGEMENT
We would like to acknowledge the National Ribat University for the funding of this
research project.
Conflict of interests: all authors declare no conflict of interest.
149
REFERENCES
Amanda G.G., Salah A., Rania T., Elsir A., Badria B.A. 2017. Epidemiology and distribution of Plasmodium vivax
malaria in Sudan. Transactions of The Royal Society of Tropical Medicine and Hygiene, trz044. Available at
Anstey N.M., Douglas N.M., Poespoprodjo J.R., Price R.N. 2012. Plasmodium vivax: clinical spectrum, risk factors
and pathogenesis. Advances in Parasitology 80: 151-201.
Awadalla H.K., Gamal K.A., Ahmed A.M., Salah E.E., Ahmed M.A., Ishag A. 2013. Reliability of rapid diagnostic
test for diagnosing peripheral and placental malaria in an area of unstable malaria transmission in Eastern
Sudan. Diagnost Pathology 8: 59.
malaria_worldwide/index.html
Elmardi K.A., Malik E.M., Abdelgadir T., Ali S.H., Elsyed A.H., Mudather M.A., Elhassan A.H., Adam I. 2009.
Feasibility and acceptability of home-based management of malaria strategy adapted to Sudan’s conditions
using artemisinin-based combination therapy and rapid diagnostic test. Malaria Journal 8: 39.
Guerra C.A., Howes R.E., Patil A.P., Gething P.W., Van Boeckel T.P., Temperley W.H., Kabaria C.W., Tatem A.J.,
Manh B.H., Elyazar I.R., Baird J.K., Snow R.W., Hay S.I. 2009. The international limits and population at
risk of Plasmodium vivax transmission in 2009. PLoSNeglected Tropical Diseases. 4: e774. doi: 10.1371/
journal.pntd.000077
Lo E., Yewhalaw D., Zhong D., Zemene E., Degefa T., Tushune K., Ha M., Lee M.-C.,James A.A., Yan G. et al. 2015.
Molecular epidemiology of Plasmodium vivax and Plasmodium falciparum malaria among duffy-positive
and duffy-negative populations in Ethiopia. Malaria Journal 14, article 84. doi: 10.1186/s12936-015-0596-4
Mahgoub H., Gasim G.I., Musa I.R., Adam I. 2012. Severe Plasmodium vivax malaria among Sudanese children at
New Halfa Hospital Eastern Sudan. Parasites and Vectors 5: 154. doi: 10.1186/1756-3305-5-154
Mbacham W.F., Ayong L., Guewo-Fokeng M., Makoge V. 2019. Current situation of malaria in Africa. In: Malaria
Control and Elimination. New York, Humana, 29-44.
Snounou G., Singh B. 2002. Nested PCR analysis of Plasmodium parasites. In: Malaria methods and protocols. New
York, Humana Press, 189-203.
World Health Organization (WHO), 2019. World malaria report 2019 ISBN 978-92-4-156572-1
150
ВЫЯВЛЕНИЕ PLASMODIUM VIVAX В ПЯТНАХ СУХОЙ КРОВИ
МОЛЕКУЛЯРНЫМИ МЕТОДАМИ
С. Г. Абдалла1, Х. А. Муза2,*, И. Адам3, С. Е. Г. Эльзаки4, А. Х. Малик1,
М. А. Эльшейк5,С. М. Ибрахим1
1 Факультет медицинской лаборатории, Национальный университет Рибата,
Хартум, Судан
2 Отдел бактериологии, Медицинский факультет, Национальный университет Рибата,
Хартум, Судан
3 Медицинский факультет, Хартумский университет, Судан
4 Национальный исследовательский центр,
Центр исследовательских проектов по малярии, Хартум, Судан
5 Медицинский факультет, Национальный госпиталь Рибата, Хартум, Судан
⁕e-mail: moibsaeed@yahoo.com
Ключевые слова: малярия, Plasmodium vivax, молекулярные методы, Восточный
Судан
РЕЗЮМЕ
В настоящее время Plasmodium vivax становится одним из самых распространенных воз-
будителей малярии в Судане. Лабораторные исследования, основанные на геномных подходах,
служат альтернативой при изучении возросшей частоты повторных рецидивов малярийных ин-
фекций и случаев пониженной паразитемии, наблюдаемых у P. vivax. Целью настоящей работы
было сравнение методов ПЦР и RDT (rapid diagnostic test) со стандартными методами светооп-
тической диагностики Plasmodium vivax. Были исследованы 572 пациента с явно выраженными
признаками лихорадки из Кассалы, Халфы и территории Восточного Нила (Судан). Было про-
ведено сравнение стандартных методов с методами иммунной хроматографии и методами ПЦР.
Результаты показали, что заражение P. vivax среди всех обнаруженных случаев малярии было
относительно высоким. Общее количество положительных реакций на P. vivax всеми методами
составило 164, при этом различные методики определили 71(28.7%), 70 (28.3%) и 23 (38.8%).
В нашем исследовании были обнаружены изменения в характере распространения
Plasmodium vivax в Судане, что, возможно, объясняется последними демографическими изме-
нениями. Эти изменения связаны с эмиграцией в Судан жителей соседних африканских стран,
усилившейся в последние годы. Определение гипнозоитов, покоящейся стадии малярийного
плазмодия, является насущной задачей в выявлении рецидивов малярии. Наше исследование
показало, что выявление генов Plasmodium viva продемонстрировало свое преимущество при
определении плазмодия при низких уровнях паразитемии в сравнении со стандартными свето-
оптическими методами и является адекватным методом для выявления и последующей ликви-
дации малярии.
151