ПАРАЗИТОЛОГИЯ, 2020, том 54, № 3, с. 247-258.
Удк 576.895.2 / 576.32/.36
EVOLUTION OF Nf2e1/ dKeap 1 SIGNALING PATHWAY IN ACTIVATING
CYP6M2 GENE REGULATION: POTENTIAL ROLE IN RESISTANCE
TO INSECTICIDES IN ANOPHELES GAMBIAE GILES, 1902
(DIPTERA: CULICIDAE) - A REVIEW
© 2020 B. R. Mohammeda,b,*, M. K. Simona, A. M. Yayoc
a Department of Parasitology and Entomology, Faculty of Veterinary Medicine,
University of Abuja,
P.M.B 117, Abuja, Nigeria
b School of Science, Engineering and Technology, Abertay University, Dundee,
DD1 1HG, United Kingdom (In collaboration with Liverpool School of Tropical Medicine, UK)
c Centre for Infectious Diseases Research, Bayero University,
Kano Department of Medical Microbiology/Parasitology, Faculty of Medicine,
Bayero University, Kano State, Nigeria
*e-mail: balarabemohammed161@yahoo.co.uk
Received 08.04.2020
Received in revised form 25.04.2020
Accepted 23.05.2020
Insecticide resistance is a worldwide menace in the control of vector borne diseases evolving in
substantial vector control program. Cytochrome P450s including CYP6M2 is known to be involved in
the metabolism of insecticides preceding resistance. Information on regulatory mechanisms involved
in the control of P450s in Anopheles gambiae Giles, 1902 is not yet clear. In this review, we analyze the
potential function of Nuclear factor erythroid factor 2 (Nf2e1), which is an ortholog to Nuclear factor
E2-related factor 2 (Nrf2) in vertebrates and Cap ‘n’ collar isoform C (CnCC) in Drosophila melanogaster
in the countenance of the expression of CYP6M2 gene encoding enzymes and conceivably to alienate
insecticide resistance in the control of Anopheles gambiae. Under normal conditions, Nf2e1 aggregates
in the cytoplasm where it synergizes with the actin binding protein, Kelch-like ECH associating protein
1 (Keap1) ortholog AGAP003645, and is instantaneously degenerated by the ubiquitin-proteasome
pathway. This review article depicts contemporary knowledge of the Nf2e1/ AGAP003645 complex,
consolidating chiefly on the molecular mechanism of Nf2e1 regulation and its potential implication in
the control of mosquito borne diseases including malaria.
Keywords: Anopheles gambiae, Drosophila melanogaster, Nf2e1, dKeap 1, insecticide resistance
DOI: 10.31857/S1234567806030050
247
Nuclear factor-erythroid 2 (NF-E2)-related factor 2 (Nrf2) is a redox-sensitive basic
leucine zipper transcription factor, which is implicated in the transcription of antioxidant
defence enzymes including cytochrome P450s (Ramprasath et al., 2014; Swamy et al., 2016).
The body defense system in vertebrates and insects is armed with the amplitude upregulating
expression levels of these target genes including cytochrome P450s (Li et al., 2015). Nrf2
(nuclear factor erythroid 2-related factor 2) is the dominant participant in the empirical
expression insect cellular enzymes (Ben-Yehuda et al., 2016). These Nrf2-regulated enzymes
are differentiated by the existence of a cis-acting element called antioxidant responsive
element (ARE), which lies within the regulatory region (Ramprasath et al., 2012; Jiang et al.,
2015). ARE-mediated response to oxidative stress pathway is conserved in all vertebrates and
invertebrates including mosquitoes (Kumar et al., 2018). In sub-Saharan Africa, Anopheline
mosquito vectors, including Anopheles gambiae s. s. is the target in most insecticide
based malaria control activities, particularly those involving the use of residual pyrethroid
insecticides (Ranson, Lissenden, 2016; Sinka et al., 2016). Resistance to pyrethroids is chiefly
attributed to two principal mechanisms; increased detoxification by enzymes or reduced target
site sensitivity (Horstmann, Sonneck, 2016). The detoxification enzymes typically linked
with insecticide resistance in Anopheles gambiae include; Cytochrome p450s, carboxyl/
choline Esterases and glutathione S-transferase (GSTs) genes (Zhou et al., 2015; Chang et al.,
2017). Additionally, p450s are the largest in comparison to all the other detoxification genes
and are known to have synergistic effects with estrases and GSTs in insecticide resistance
in Anopheles species (Chang et al., 2017). Of the p450s, CYP6 sub-family and especially
CYP6M2 is involved mainly in developmental process and is crucial for the metabolic
detoxification of insecticides in Anopheles gambiae (Guo et al., 2013). CYP6M2 is found
exclusively in insects and repeatedly implicated in resistance to all three distinct classes of
WHO recommended insecticides (Pyrethroids, carbamate, and organophosphates) that are
important in mosquito vector control (Edi et al., 2014; Mohammed et al., 2017; Ibrahim et
al., 2018). Erstwhile microarray investigations on insecticide-resistant mosquitoes, including
Anopheles gambiae has established a comparatively modest number of up-regulated CYP
genes consequent to exposures of the mosquitoes to various concentrations of insecticides
(Liang et al., 2015). Up-regulation of these cytochrome oxidases (P450s) including CYP6M2
has been associated with resistance and enzymatic metabolism of insecticides by CYP6M2
has been demonstrated in vitro (Stevenson et al., 2011; Mitchell et al., 2012; Edi et al., 2014).
The transcriptional up-regulation of CYP6M2 in mosquitoes results in increased levels of
protein production and enzymatic activities, which leads to the development of resistance
(Liu, 2015). Contemporary investigations have established that the evolutionarily conserved
Nrf2 / Keap 1 pathways orthologs perform a significant part in regulation of the correspondent
transcriptional response to xenobiotic compounds in D. melanogaster (Misra et al., 2011;
Jones et al., 2013; Guio et al., 2014; Kuzin et al., 2014).
In Drosophila melanogaster, some genes associated with metabolic activity are
established to be upregulated by the transcription factors Cap ‘n’ collar isoform (CnCC) /
248
Drosophila Kelch-like-ECH-associated protein 1 (dKeap 1). These are orthologs to Nuclear
factor erythroid -2 related factor-2 (Nrf2) / Kelch-like-ECH-associated protein 1 (Keap 1)
signalling pathways in higher mammalians respectively (Cao et al., 2013; Misra et al., 2013;
Das et al., 2014; Siller et al., 2014). No study appears to have been made in understanding
these complex mechanisms regulating CYP6M2 gene expression in insecticide resistance in
Anopheles gambiae.
The recognition of these transcription factor binding sites (TFBSs) will improve
knowledge of how wild populations of Anopheles gambiae become resistant to insecticide
and are activated by different endogenous and exogenous xenobiotic challenges. One of such
pathways is the Nrf2/Keap 1 signaling pathway, which was first shown to regulate P450s.
The orthologs to this gene in Drosophila melanogaster an insect model are also known to be
Cap ‘n’ collar isoform C (CnCC)/Keap 1) (Mohammed et al., 2014; Chatterjee et al., 2016).
Significant explorations with regards to evolutionarily conserved signalling pathways have
been accomplished employing D. melanogaster (Altintas et al., 2016).
Preliminary investigations employing insilico resources revealed the orthologs of CnCC/
dKeap 1 genes in Anopheles gambiae to be Nuclear factor erythroid 2 invertebrate (Nf2e1)/
AGAP003645. In contrast to the detailed studies in higher vertebrates and D. melanogaster,
the complex regulatory mechanism regulating P450 gene including CYP6M2 gene expres-
sion in An. gambiae is yet to be identified. Here we use bioinformatics and molecular biol-
ogy technique to show that Nuclear factor erythroid 2, invertebrate) (Nf2e1) (Nrf2) / dKeap1
pathway play a key role in the regulation of xenobiotic responses in An. gambiae.
As a pre-requisite for understanding the molecular mechanism involved in the regulation
of CYP6M2, searches for potential regulatory elements were made insilico using bioinformatic
resources.
Insilico identification of Nrf2 / are orthologs
Searches were made insilico for the identification of the orthologs of Nrf2 / ARE in
Results? q=%20Nrf2;m%20y=%203;site=ensemblallx=3;;page=1;facetspeciesHuman) was
FBgn 0262975.html) data base was used to search for its orthologs in Drosophila mela-
Gene) was used to search for the orthologs in Anopheles gambiae (Ag).The insilico analysis
results revealed that the orthologs to vertebrate Nrf2 is identified as CnCC in Drosophila
melanogaster and Nf2e1 in Anopheles gambiae gene promoters respectively (Mohammed et
al., 2014) (Table 1).
While the orthologs to higher vertebrate Keap 1 in Drosophila melanogaster is dKeap1
and AGAP003645 in Anopheles gambiae respectively. In this review therefore, we have
described the components mediating Nrf2 signaling in Anopheles gambiae and their
relationship to human and Drosophila melanogaster (Figure 1).
249
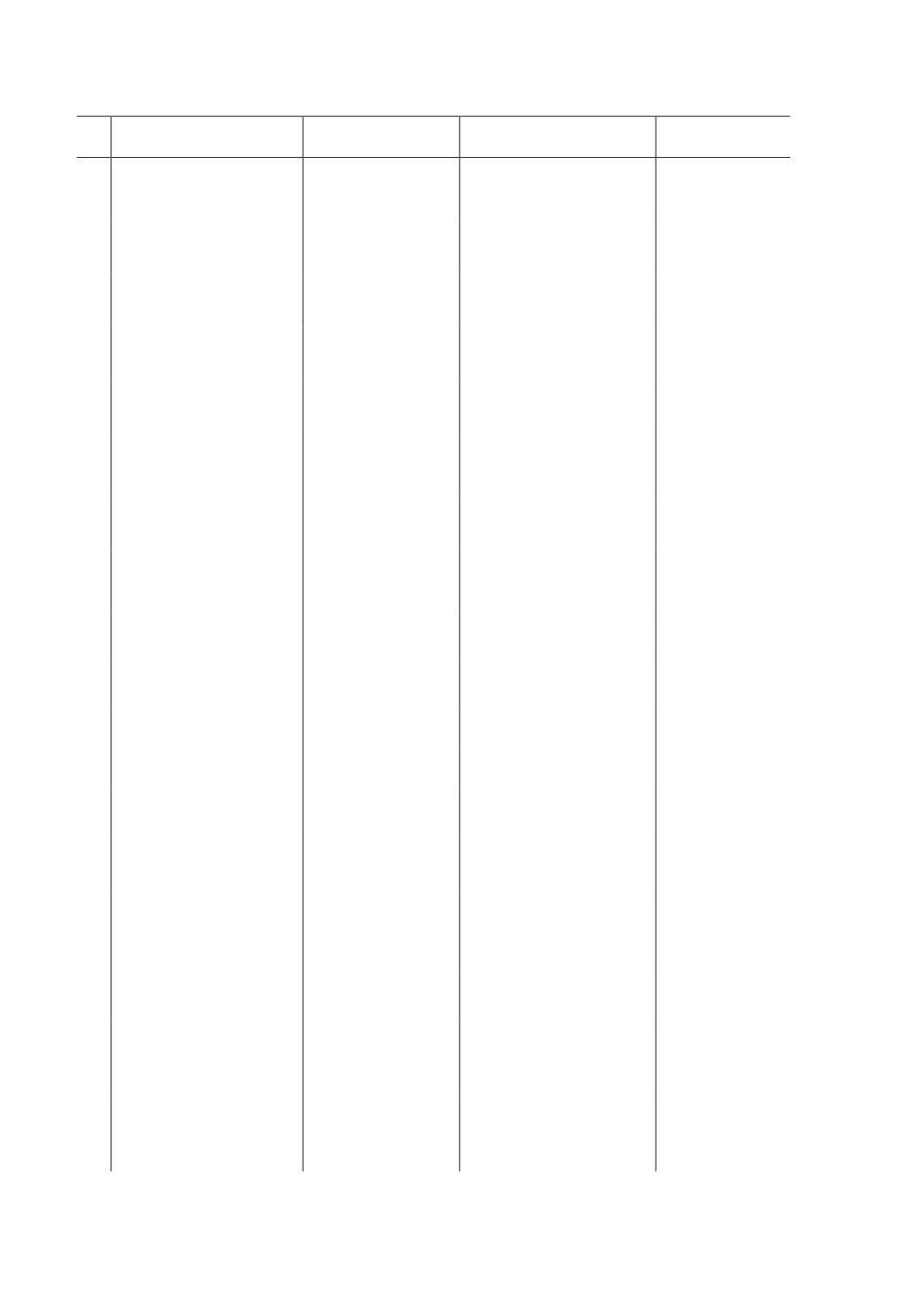
Table 1. The CnCC (ortholog to Nrf2 /Keap 1) of vertebrates
in Drosophila melanogaster and Anopheles gambiae
Vertebrates
D. melanogaster
An. gambiae
Source
Nrf2
CnCC
Nf2e1
Mohammed et al., 2014
Keap 1
dKeap 1
AGAP003645
Mohammed et al., 2014
K e y: Nrf2 - Nuclear factor erythroid-2 related factor-2; CnCC - Cap ‘n’ collar isoform C;
Nf2e1 - Nuclear factor erythroid-2, invertebrate.
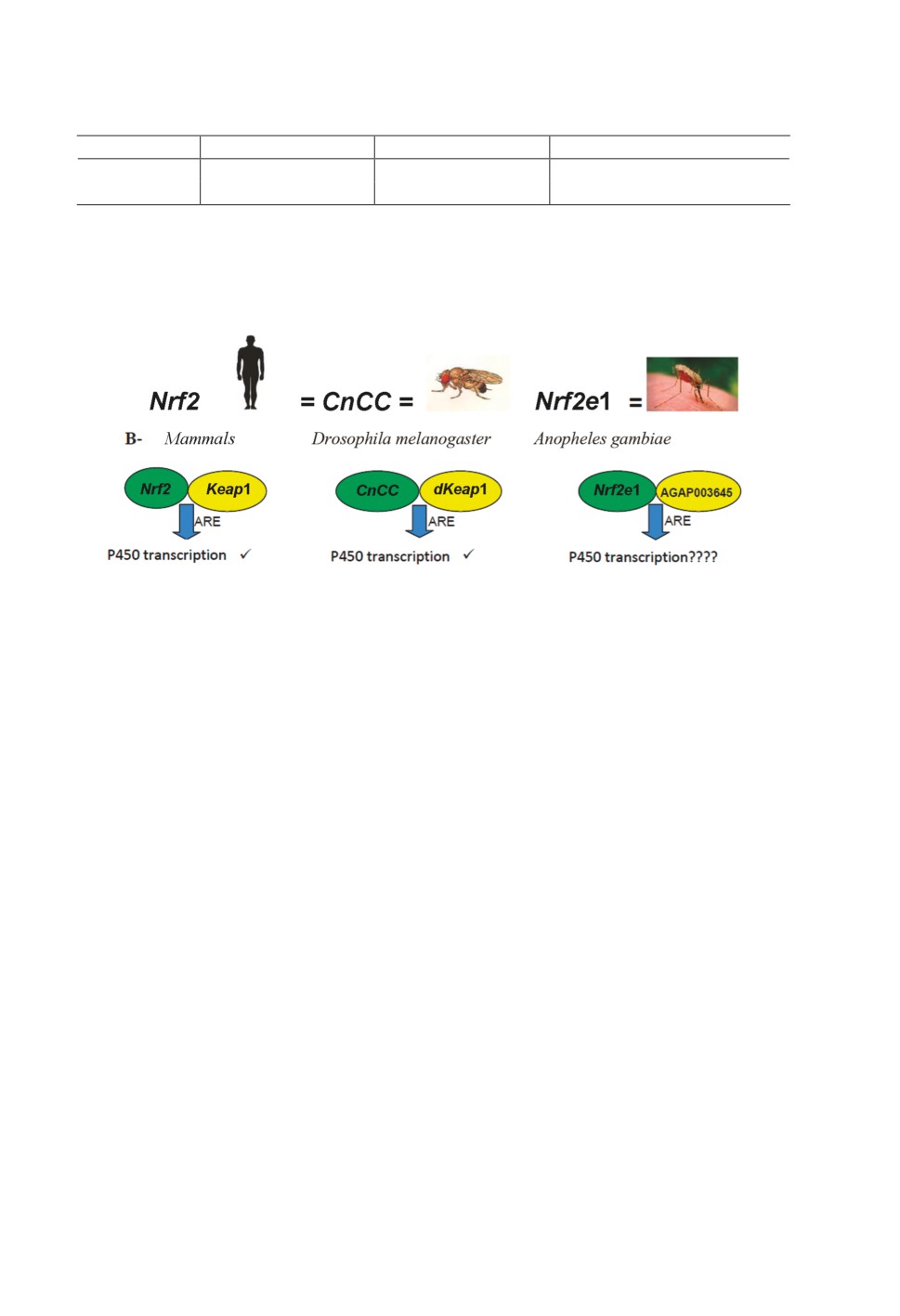
Figure 1. Graphic illustration of the vertebrate Nrf2- Keap1- Kelch-like ECH-Associated Protein
Keap1 and its -Cap’n’ Isoform C CnCC and Nuclear factor 2 invertebrate -Nf2e1-AGAP003645
orthologs of Drosophila melanogaster and Anopheles gambiae respectively and the and ARE-
Antioxidant responsive element (Adopted and modified Mohammed, 2014).
Nrf2 / Keap1 signaling pathway in vertebrates
In unstressed conditions, Nrf2 (Nuclear factor erythroid-2 related factor- 2) in vertebrates,
and CnCC (Cap ‘n’ collar isoform C) in Drosophila are repressed by dKeap1 (Drosophila
Kelch-like ECH-associated protein 1), which also functions as a sensor of oxidants and other
electrophilic compounds (Mohammed et al., 2014; Loboda et al., 2016). In the absence of
stress, Nrf2 is maintained in the cytoplasm by the actin-binding protein Keap1, which also
functions as an E3 ubiquitin ligase to stimulate Nrf2 degradation by the 26S proteasome.
Activation of this pathway through oxidative stress impedes the Nrf2-Keap1 synergy, allow-
ing Nrf2 to translocate to the nucleus, where it can heterodimerize with the small Maf (mus-
cle aponeurosis fibromatosis) proteins and bind to antioxidant response elements (AREs) in
the genome (Atia, Bin Abdullah, 2014). Overexpression of Nrf2 and reduction of Keap1 in
higher vertebrates switches on the transcription of numerous genes including CYP6A2 that
safeguards cells from xenobiotic compounds. Nrf2, Maf and Keap1 are all conserved in D.
melanogaster and exert to maintain cognate regulatory synergy as described in vertebrates
(Si, Liu, 2014; Dhanoa et al., 2013).
250
CnCC / dKeap1 signaling pathway in Drosophila melanogaster
Under oxidative stress situations, the repression of CnCC by dKeap1 are annihilated
permitting these transcription factors to bind, together with other proteins, to ARE sequences
upregulating downstream genes such as P450s. The Drosophila dKeap1 incorporates Kelch
repeats homologous to those that intercedes dKeap1 synergy with Nrf2 as well as a sequence
motif that is mandatory for vertebrate Keap1 export from the nucleus (Deng, Kerppola, 2013).
Overexpression of CnCC and reduction of dKeap1 in Drosophila melanogaster switches on
the transcription of numerous genes including CYP6G1 and CYP6A2 that safeguard cells
from xenobiotic compounds, albeit dKeap1 overexpression supresses their transcription,
demonstrating that the activities of these protein families in the xenobiotic response are con-
served amongst vertebrates and Drosophila (Deng, Kerppola, 2013; Misra et al., 2013).
Activation of this pathway through electrophilic xenobiotics / oxidative stress is neces-
sary and sufficient for xenobiotic-induced transcription of a wide range of detoxification
genes in Drosophila species (Misra et al., 2011; Deng, Kerppola, 2013).
Up-regulation of these cytochrome oxidases (P450s) including CYP6M2 has been
associated with resistance and enzymatic metabolism of insecticides by CYP6M2 has been
shown in vitro (Stevenson et al., 2011; Mitchell et al., 2012; Edi et al., 2014). In Drosophila
melanogaster, some genes involved in metabolic activity are known to be upregulated by the
transcription factors Cap ‘n’ collar isoform (CnCC) / Drosophila Kelch-like-ECH-associated
protein 1 (dKeap 1). These are orthologs to Nuclear factor erythroid -2 related factor-2 (Nrf2)
/ Kelch-like-ECH-associated protein 1 (Keap 1) signalling pathway in higher mammalians
respectively (Cao et al., 2013; Misra et al., 2013; Das et al., 2014; Siller et al., 2014).
The over-expression of Nf2e1 and repression of AGAP003645 (dKeap 1) in Anopheles
gambiae potentially initiates the transcription of many P450 genes including CYP6M2 and
protect cells from xenobiotic compounds, whereas dKeap1 overexpression potentially re-
presses their transcription, suggesting that the functions of these protein families in the xeno-
biotic response are conserved between vertebrates, Drosophila melanogaster and Anopheles
gambiae. This has revealed therefore a connection between Nf2e1 / AGAP003645 signal-
ling pathways and CYP6M2 in insecticide resistance. A proposed scheme for the Nf2e1/
AGAP003645 pathway and that of its inhibition are described in Figures 2 and 3.
The potential nuclear factor erythroid 2 invertebrate (Nf2e1) / AGAP003645
signalling pathway in Anopheles gambiae
Activation of the Nf2e1/ AGAP003645 pathway in Anopheles gambiae
Under oxidative stress situations, the restriction of Nf2e1 by AGAP003645 is abolished
permitting these transcription factors to bind together with other proteins, as maf (muscle
aponeurosis fibromatosis) to ARE sequences up regulating downstream P450 genes such as
CYP6M2 responsible for detoxification of insecticides thereby conferring protection and pos-
sibly resistance to insecticides in Anopheles gambiae as seen in Figure 2.
251
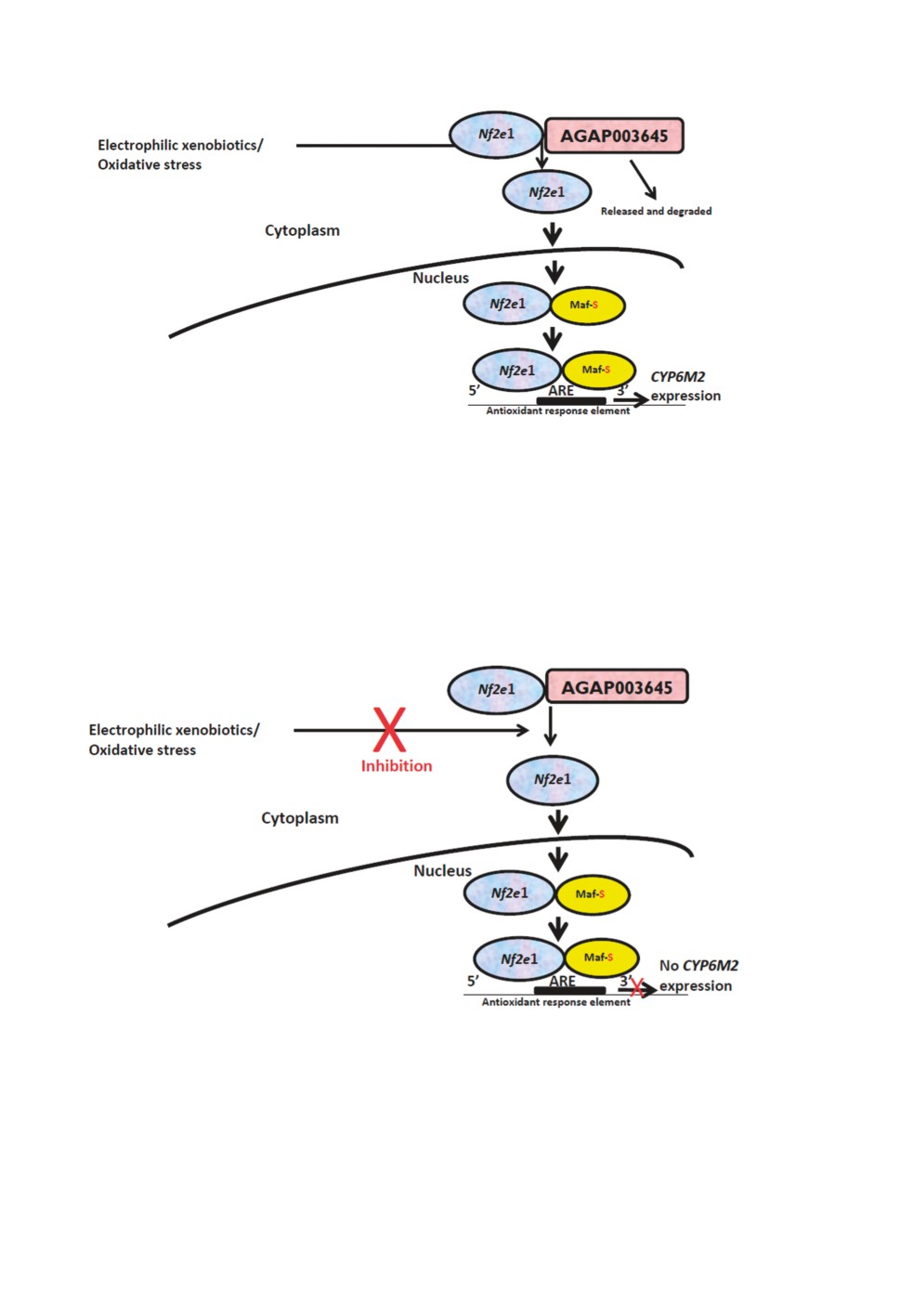
Figure 2. Schematic illustration for the induction of Nf2e1/ AGAP003645-(dKeap1) signalling pathway.
The antioxidant response element (ARE) in the promoter region of select genes allows the coordinated
up-regulation of antioxidant and detoxifying enzymes in response to oxidative/electrophilic stress. This
up-regulation is mediated through Nuclear factor erythroid 2, invertebrate (Nf2e1) that may be activated
by endogenous and exogenous molecules or stressful conditions. These agents disrupt the association
between Nf2e1 and AGAP003645 with subsequent nuclear translocation of Nf2e1. In the cell nucleus,
Nf2e1 interacts with small MAF-S (Muscle apoptosis fibromatosis) protein, forming a heterodimer that
binds to the ARE (Antioxidant response element) sequence in the promoter region and up-regulates
transcription of many genes encoding detoxifying enzymes such as P450s (CYP6M2) (Adopted and
modified from Mohammed, 2014).
Figure 3. Schematic illustration general scheme for the inhibition of NF2e1/ AGAP003645 (dKeap
1) - signalling pathway. This up-regulation is mediated through Nuclear factor erythroid 2 invertebrate
(Nf2e1) that may be activated by endogenous and exogenous molecules including xenobiotics
(ligands) or stressful conditions. When these agents are inhibited, the association between Nf2e1 and
AGAP003645 remains intact in the cytoplasm. This disrupts the up-regulation and transcription of
many genes encoding detoxifying enzymes such as P450s (CYP6M2) (Adopted and modified from
Mohammed, 2014).
252
Inhibition of the Nf2e1/ AGAP003645 pathway in Anopheles gambiae
Figure 3 is an Illustration of the hypothetical inhibition of the Nf2e1/ AGAP003645 sig-
nalling pathway which potentially hinders the up-regulation CYP6M2 responsible for insec-
ticide metabolism in Anopheles gambiae.
However, activation of this pathway is unlikely to be the only factor that contributes to
insecticide resistance in Anopheles gambiae. Nevertheless, inhibition of the Nf2e1 / dKeap
1 (Figure 3) in particular response may potentially improve the efficacy of insecticides and
development of methods to knock down or inhibit these pathways may prove fruitful. This
work has increased our knowledge of the regulatory mechanisms involved in the control of
CYP6M2 in insecticide resistance in Anopheles gambiae. Even if the underlying mechanisms
are still not very clear, the work shows the importance of these regulatory genes in the control
of CYP6M2 in response to insecticide selection.
The functionality of Nf2e1 can only be proven through wet-laboratory experiments with
predetermined parameters, particularly since a potential binding site in a promoter can be
functional in certain cells and non- functional under different conditions (Cartharius et al.,
2005). These findings have implications in the ability to control the spread of malaria due to
the reduction in insecticide resistance in Anopheles gambiae.
Activators and Inhibitors of CnCC signalling pathway
Activators of CnCC signalling pathway
Previous studies have identified Phenobarbital, Paraquat, Caffeine and GAL4UAS system
among others as major activators of CnCC/Keap 1 signaling pathway (Misra et al., 2011;
Deng, Kerppola, 2014) (Table 2).
Inhibitors of Nrf2 signalling pathway
Inhibitors of CnCC, such as Drosophila Keap1 induce beneficial effects on survival
and synaptic function in Drosophila melanogaster (Spiers et al., 2019). Furthermore, over
expression of Drosophila Keap1 is also known to inhibit CnСC activity in vivo (Sykiotis,
Bohmann, 2008). Other inhibitors such as Bromodomain and Extra-Terminal (BET) protein
family in Drosophila, Fs (1) h, as an inhibitor of the stress responsive transcription factor
CnCC, the fly ortholog of Nrf2. The mechanism by which Fs (1) h inhibits CnCC function
is distinct from the canonical mechanism that stimulates Nrf2 function by abrogating
Keap1-dependent proteasomal degradation (Chatterjee et al., 2016). Finally, using an
inducible Drosophila model, it was confirmed that Aβ42 inhibits activity of the fly homolog
of Nrf2 (cap-n-collar isoform C, CnCC (Sykiotis, Bohmann, 2008; Kerr et al., 2017). It is
conceivable that the CnCC inhibition activity plays similar role in Anopheles gambiae.
253
Table 2. Typical activators of CnCC (Nrf2 ortholog) signaling pathway in insects
S/
Insect
Activators
P450
References
No
1
Drosophila melanogaster
Phenobarbital
CYP6D1, CYP6A2,
Misra et al., 2011;
(Fruit fly)
(PB)
CYP6A8 and CYP12D1
Deng, Kerppola,
2014
2
Aphis gossypii
RNA interference
CYP6A2
Peng et al., 2016a
(Glover)
(RNAi)
3
Tribolium castaneum
Double stranded
CYP6BQ
Kalsi, Palli, 2015
(Red flour beetle)
RNA (dsRNA)
4
Aphis gossypii
Paraquat
CYP6DA2
Peng et al., 2016b
(Glover)
5
Drosophila melanogaster
Paraquat
CYP6A2 and CYP6A8
Pitoniak,
(Fruit fly)
Bohmann, 2015
6
Drosophila melanogaster
GAL4/UAS system
CYP6G1, CYP6A2,
Daborn et al.,
(Fruit fly)
CYP6A8 and CYP6A21
2007; Misra et al.,
2011
7
Drosophila melanogaster
chlorpromazine
CYP6A2
Misra et al., 2011
(Fruit fly)
8
Drosophila melanogaster
caffeine
CYP12D1, CYP6A8 and
Misra et al., 2011;
(Fruit fly)
CYP6D5
Coelho et al.,
2015
9
Spodoptera litura
Piperonyl butoxide
CYP6AB12
Lu et el., 2020
(Tobacco cutworm)
10
Bombyx mori
Curcumin
CYP302A1, CYP306A1,
Li et al., 2019
(Silk worm)
High temperature
CYP314A1andCYP315A1
11
Aedes aegypti
Fluoranthene
CYP6M6
Poupardin et al.,
2008
12
Aedes aegypti
Copper
CYP6M11
Poupardin et al.,
2008
13
Tribolium castaneum
Latrophilin
CYP4BN6 and CYP6BQ11
Xiong et al., 2019
(Red flour beetle)
(Lph)
14
Anopheles gambiae
RNA interference
CYP6M2, CYP6Z2,
Ingham et al.,
(RNAi)
CYP6Z3 and CYP6P4
2017
15
Leptinotarsa decemlineata
RRNA interference
CYP6BJ, CYP6BJ1,
Kalsi, Palli, 2017a
(RNAi)
CYP9Z25 and CYP9Z29
Colorado potato beetle
16
Tribolium castaneum
Double stranded
CYP6BQ11
Kalsi, Palli, 2015
RNA (dsRNA)
(Red flour beetle)
17
Leptinotarsa decemlineata
RNA interference
CYP6BJ
Kalsi, Palli,
(RNAi)
2017b
Colorado potato beetle
18
Drosophila melanogaster
Tert-
CYP6BQ
Deng, Kerppola,
butylhydroquinone
2014
(Fruit fly)
(tBHQ)
254
CONCLUSIONS
In this review, we have described findings regarding potential mechanisms by which
Nf2e1 influences insecticide resistance in Anopheles gambiae. The roles of many Nf2e1 and
its orthologs in xenobiotics are remarkably well conserved between higher mammals and
other living organisms. The intervention of the Nf2e1 leads to an extended lifespan all living
organisms. This suggests that the role of Nf2e1 xenobiotics is likely to be conserved across
all living organisms. In addition, genetic variants of Nf2e1 components, including muscle
apoptosis fibromatosis-S (MAF-S) and antioxidant response element (ARE), are associated
with insecticide resistance. Thus, the evidence for the evolutionarily conserved nature of
Nf2e1 and its orthologs-mediated longevity is extremely strong, ranging from invertebrates
to humans. Further wet laboratory experiments such as qPCR (quantitative polymerase
chain reaction) are required to establish the functionality of Nf2e1. We therefore conclude
that inhibition of this Nf2e1 / dKeap 1 may potentially improve the efficacy of insecticides.
Consistent with the previous studies on the Drosophila model pathway, these studies have
established that the Nrf2 / Keap 1(Nf2e1 / dKeap 1) pathway is differentially active as
a key regulator of xenobiotic responses. These studies have implications for understanding
the regulatory mechanisms of acquirement of insecticide resistance and its impact in the
control of mosquito-borne diseases.
ACKNOWLEDGEMENT
This work was supported by Tertiary Education Trust Fund (TETFUND) and University
of Abuja, Nigeria.
CONFLICT OF INTEREST
The authors declare that there are no competing interests regarding the publication of this
paper.
REFERENCES
AltintasO., Park S., Lee S.J.V. 2016. The role of insulin/IGF-1 signaling in the longevity of model invertebrates, C.
elegans and D. melanogaster. BMB reports 49 (2): 81-92.
Atia A.E., Bin Abdallah A. 2014. Modulation of Nrf2 / Keap1 pathway by dietary phytochemicals. International
Journal of Research in Medical Sciences 2 (2): 375-381.
Ben-Yehuda G.M., Ben-Sasson S., Bianco-Peled H., Kohen R. 2016. Skin Redox Balance Maintenance: The Need
for an Nrf2-Activator Delivery System. Cosmetics 3 (1): 1.
Cao C., Wang Z., Niu C., Desneux N., Gao X. 2013. Transcriptome Profiling of Chironomus kiinensis under Phenol
Stress Using Solexa Sequencing Technology. PloS ONE 8 (3): e58914.
Cartharius K., Frech K., Grote K., Klocke B., Haltmeier M., Klingenhoff A., Werner T. 2005. MatInspector and
beyond: promoter analysis based on transcription factor binding sites. Bioinformatics 21 (13): 2933-2942.
Chang K.S., Kim H.C., Klein T.A., Ju Y.R. 2017. Insecticide resistance and cytochrome-P450 activation in unfed
and blood-fed laboratory and field populations of Culex pipiens pallens. Journal of Pest Science 90 (2):
759-771.
Chatterjee N., Tian M., Spirohn K., Boutros M., Bohmann D. 2016. Keap1-Independent Regulation of Nrf2 Activity
by Protein Acetylation and a BET Bromodomain Protein PLoS Genetics 12 (5): e1006072.
Coelho A., Fraichard S., Le Goff G., Faure P., Artur Y., Ferveur J.F., Heydel J.M. 2015. Cytochrome P450-dependent
metabolism of caffeine in Drosophila melanogaster. PLoS One 10 (2): e0117328.
Daborn P.J., Lumb C., Boey A., Wong W., Ffrench-Constant R.H., Batterham P. 2007. Evaluating the insecticide
resistance potential of eight Drosophila melanogaster cytochrome P450 genes by transgenic over-expression.
Insect Biochemistry and Molecular Biology 37 (5): 512-519.
255
Das D.N., Panda P.K., Mukhopadhyay S., Sinha N., Mallick B., Behera B., Maiti T.K., Bhutia S.K. 2014. Prediction
and validation of apoptosis through cytochrome P450 activation by benzo[a]pyrene. Chemico-Biological
Interactions 208: 8-17.
Deng H., Kerppola T.K. 2013. Regulation of Drosophila Metamorphosis by Xenobiotic Response Regulators. PloS
Genetics 9 (2): e1003263.
Dhanoa B.S., Cogliati T., Satish A.G., Friedman J.S. 2013. Update on the Kelch-like (KLHL) gene family. Human
Genomics 7: 13.
Edi C.V., Djogbénou L., Jenkins A.M., Regna K., Muskavitch M.A.T., Poupardin R., Jones C.M., Essandoh J.,
Kétoh G.K., Paine M.J.I, Koudou B.G, Martin J., Donnelly M.J., Ranson H., Weetman D. 2014. CYP6
P450 Enzymes and ACE-1 Duplication Produce Extreme and Multiple Insecticide Resistance in the Malaria
Mosquito Anopheles gambiae. PloS Genetics 10 (3): e1004236.
Guio L., Barron M.G., Gonzalez J. 2014. The transposable element Bari-Jheh mediates oxidative stress response in
Drosophila. Molecular Ecology 23 (8): 2020-2030.
Guo H., Bao Z., Du H., Zhang L., Wang S., Sun L., Hu X. 2013. Identification of Cytochrome P450 (CYP) genes in
Zhikong scallop (Chlamys farreri). Journal of Ocean University of China 12 (1): 97-102.
Horstmann S., Sonneck R., 2016. Contact Bioassays with Phenoxybenzyl and Tetrafluorobenzyl Pyrethroids against
Target-Site and Metabolic Resistant Mosquitoes. PloS One 11 (3): e0149738.
Ibrahim S.S., Amvongo-Adjia N., Wondji M.J., Irving H., Riveron J.M., Wondji C.S. 2018. Pyrethroid resistance in
the major malaria vector Anopheles funestus is exacerbated by overexpression and overactivity of the P450
CYP6AA1 across Africa. Genes 9 (3): 140.
Ingham V.A., Pignatelli P., Moore J.D., Wagstaff S., Ranson H. 2017. The transcription factor Maf-S regulates
metabolic resistance to insecticides in the malaria vector Anopheles gambiae. BMC Genomics 18 (1): 669.
Jiang J., Shi D., Zhou X.Q., Yin L., Feng L., Liu Y., Zhao Y. 2015. Effects of glutamate on growth, antioxidant
capacity, and antioxidant-related signaling molecule expression in primary cultures of fish enterocytes. Fish
Physiology and Biochemistry 41 (5): 1143-1153.
Jones L.M., Rayson S.J., Flemming A.J., Urwin P.E. 2013. Adaptive and Specialised Transcriptional Responses to
Xenobiotic Stress in Caenorhabditis elegans are regulated by Nuclear Hormone Receptors. PloS ONE 8 (7):
e69956.
Kalsi M., Palli S.R. 2015. Transcription factors, CnCC and Maf, regulate expression of CYP6BQ genes responsible
for deltamethrin resistance in Tribolium castaneum. Insect Biochemistry and Molecular Biology 65: 47-56.
Kalsi M., Palli S.R. 2017a. Transcription factor cap n collar C regulates multiple cytochrome P450 genes conferring
adaptation to potato plant allelochemicals and resistance to imidacloprid in Leptinotarsa decemlineata (Say).
Insect Biochemistry and Molecular Biology 83: 1-12.
Kalsi M., Palli S.R. 2017b. Cap n collar transcription factor regulates multiple genes coding for proteins involved
in insecticide detoxification in the red flour beetle, Tribolium castaneum. Insect Biochemistry and Molecular
Biology 90: 43-52.
Kerr F., Sofola-Adesakin O., Ivanov D.K., Gatliff J., Perez-Nievas B.G., Bertrand H.C., Adcott J. 2017. Direct
Keap1-Nrf2 disruption as a potential therapeutic target for Alzheimer’s disease. PLoS Genetics 13 (3):
e1006593.
Kumar A., Srivastava P., Sirisena P.D.N.N., Dubey S., Kumar R., Shrinet J., Sunil S. 2018. Mosquito Innate
Immunity. Insects: 9(3): 95.
Kuzin B.A., Nikitina E.A., Cherezov R.O., Vorontsova J.E., Slezinger M.S., Zatsepina O.G., Simonova O.B.,
Enikolopov G.N., Savvateeva-Popova E.V. 2014. Combination of Hypomorphic Mutations of the Drosophila
Homologues of Aryl Hydrocarbon Receptor and Nucleosome Assembly Protein Family Genes Disrupts
Morphogenesis, Memory and Detoxification. PloS One 9 (4): e94975.
Li J., Mao T., Wang H., Lu Z., Qu J., Fang Y., Gu Z. 2019. The CnCC/keap1 pathway is activated in high
temperature-induced metamorphosis and mediates the expression of Cyp450 genes in silkworm, Bombyx
mori. Biochemical and Biophysical Research Communications 514 (4): 1045-1050.
Li S., Tan H.Y., Wang N., Zhang Z.J., Lao L., Wong C.W., Feng Y. 2015. The role of oxidative stress and antioxidants
in liver diseases. International Journal of Molecular Sciences 16 (11): 26087-26124.
256
Liang X., Xiao D., He Y., Yao J., Zhu G., Zhu K., 2015. Insecticide-mediated up-regulation of cytochrome P450
genes in the red flour beetle (Tribolium castaneum). International Journal of Molecular Sciences 16 (1):
2078-2098.
Liu N. 2015. Insecticide resistance in mosquitoes: impact, mechanisms, and research directions. Annual Review of
Entomology 60: 537-559.
Loboda A., Damulewicz M., Pyza E., Jozkowicz A., Dulak J. 2016. Role of Nrf2/HO-1 system in development,
oxidative stress response and diseases: an evolutionarily conserved mechanism. Cellular and Molecular Life
Sciences 73: 3221-3247.
Lu K., Cheng Y., Li W., Li Y., Zeng R., Song Y. 2020. Activation of CnCC pathway by ROS burst regulates
cytochrome P450 CYP6AB12 responsible for λ-cyhalothrin tolerance in Spodoptera litura. Journal of
Hazardous Materials 387: 121698.
Misra J.R., Horner M.A., Lam G., Thummel C.S., 2011. Transcriptional regulation of xenobiotic detoxification in
Drosophila. Genes and Development 25: 1796-1806.
Misra J.R., Lam G., Thummel C.S. 2013. Constitutive activation of the Nrf2 / Keap1 pathway in insecticide-resistant
strains of Drosophila. Insect Biochemistry and Molecular Biology, 43 (12): 1116-1124.
Mitchell S.N., Stevenson B.J., Müllera P., Wilding C.S., Egyir-Yawsond A., Field S.G., Hemingway J., Mark J. I.P.,
Ranson H., Donnelly M.J. 2012. Identification and validation of a gene causing cross-resistance between
insecticide classes in An. gambiae from Ghana. PNAS 109 (16): 6147-6152.
Mohammed B.R. 2014. Regulatory mechanisms involved in the control of CYP6M2 gene in insecticide resistant
Anopheles gambiae (Diptera: Culicidae). PhD Thesis, Abertay University, Dundee, United Kingdom.
Mohammed B.R., Mailafia S., Kawe M.S., Agbede R.I.S., Finn R.D. 2017. Cytochrome P450s in Anopheles
gambiae (Diptera: Culicidae) and Insecticide Resistance in Africa: A Mini Review. Entomology, Ornithology
and Herpetology 6: 200.
Mohammed B.R., Wilding C.S., Collier P.J., Deeni Y.Y. 2014. Bioinformatic analysis of regulatory elements within
the promoter region of the cytochrome P450 Gene, CYP6M2 in Anopheles gambiae. European Journal of
Biotechnology and Bioscience 2 (1): 24-31.
Peng T., Pan Y., Gao X., Xi J., Zhang L., Yang C., Bi R., Yang S., Xin X., Shang Q. 2016a. Cytochrome P450
CYP6DA2 regulated by cap ‘n’collar isoform C (CnCC) is associated with gossypol tolerance in Aphis
gossypii Glover. Insect Molecular Biology 25 (4): 450-459.
Peng T., Pan Y., Yang C., Gao X., Xi J., Wu Y., Shang Q. 2016b. Over-expression of CYP6A2 is associated with
spirotetramat resistance and cross-resistance in the resistant strain of Aphis gossypii Glover. Pesticide
Biochemistry and Physiology 126: 64-69.
Pitoniak A., Bohmann D. 2015. Mechanisms and functions of Nrf2 signaling in Drosophila. Free Radical Biology
and Medicine 88 (B): 302-313.
Poupardin R., Reynaud S., Strode C., Ranson H., Vontas J., David J.P. 2008. Cross-induction of detoxification genes
by environmental xenobiotics and insecticides in the mosquito Aedes aegypti: impact on larval tolerance to
chemical insecticides. Insect Biochemistry and Molecular Biology 38 (5): 540-551.
Ramprasath T., Kumar P.H., Puhari S.S. M., Murugan P.S., Vasudevan V., Selvam G.S. 2012. L-Arginine ameliorates
cardiac left ventricular oxidative stress by upregulating eNOS and Nrf2 target genes in alloxan-induced
hyperglycemic rats. Biochemical and Biophysical Research Communications 428 (3): 389-394.
Ramprasath T., Senthamizharasi M., Vasudevan V., Sasikumar S., Yuvaraj S., Selvam G.S. 2014. Naringenin confers
protection against oxidative stress through upregulation of Nrf2 target genes in cardiomyoblast cells. Journal
of Physiology and Biochemistry 70(2), 407-415.
Ranson H., Lissenden N. 2016. Insecticide resistance in African anopheles mosquitoes: a worsening situation that
needs urgent action to maintain malaria control. Trends in Parasitology 32 (3): 187-196.
Si H., Liu D. 2014. Dietary antiaging phytochemicals and mechanisms associated with prolonged survival. The
Journal of Nutritional Biochemistry 25 (6): 581-591.
Siller M., Goyal S., Yoshimoto F.K., Xiao Y., Wei S., Guengerich F.P. 2014. Oxidation of Endogenous
N-Arachidonoylserotonin by Human Cytochrome P450 2U1. Journal of Biological and Chemistry 289:
10476-10487.
257
Sinka M.E., Golding N., Massey N.C., Wiebe A., Huang Z., Hay S.I., Moyes C.L. 2016. Modelling the relative
abundance of the primary African vectors of malaria before and after the implementation of indoor,
insecticide-based vector control. Malaria Journal 15 (1): 142.
Spiers J.G., Breda C., Robinson S., Giorgini F., Steinert J.R. 2019. Drosophila Nrf2/Keap1 Mediated Redox
Signaling Supports Synaptic Function and Longevity and Impacts on Circadian Activity. Frontiers in
Molecular Neuroscience 12: 86.
Stevenson B.J., Bibby J., Pignatelli P., Charoen M.S., O’Neill P.M., Lian L.-Y., Müller P., Nikou D., Steven A.,
Hemingway J., Sutcliffe M.J., Paine M.J.I. 2011. Cytochrome P450 6M2 from the malaria vector Anopheles
gambiae metabolizes pyrethroids: sequential metabolism of deltamethrin revealed. Insect Biochemistry and
Molecular Biology 41 (7): 492-502.
Swamy M.K., Akhtar M.S., Sinniah U.R. 2016. Antimicrobial properties of plant essential oils against human
pathogens and their mode of action: an updated review. Evidence-Based Complementary and Alternative
Medicine 2016: 3012462.
Sykiotis G.P., Bohmann D. 2008. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in
Drosophila. Developmental Cell 14 (1): 76-85.
Xiong W., Gao S., Mao J., Wei L., Xie J., Liu J., Li B. 2019. CYP4BN6 and CYP6BQ11 mediate insecticide
susceptibility and their expression is regulated by Latrophilin in Tribolium castaneum. Pest Management
Science 75 (10) 2744-2755.
Zhou D., Liu X., Sun Y., Ma L., Shen B., Zhu C. 2015. Genomic analysis of detoxification supergene families in the
mosquito Anopheles sinensis. PloS One 10 (11): e0143387.
ЭВОЛЮЦИЯ СИГНАЛЬНОГО ПУТИ Nf2e1/ dKeap1
В ПРОЦЕССЕ РЕГУЛЯЦИИ ЭКСПРЕССИИ ГЕНА CYP6M2:
ПОТЕНЦИАЛЬНАЯ РОЛЬ В РЕЗИСТЕНТНОСТИ К ИНСЕКТИЦИДАМ
У ANOPHELES GAMBIAE GILES, 1902 (DIPTERA: CULICIDAE) - ОБЗОР
Б. Р. Мохаммед, М. К. Симон, А. М. Иайо
Ключевые слова: Anopheles gambiae, Drosophila melanogaster, Nf2e1, dKeap 1, резистентность
к инсектицидам
РЕЗЮМЕ
Невосприимчивость (резистентность) к инсектицидам является угрозой мирового значе-
ния, т.к. препятствует борьбе с переносчиками возбудителей инфекций. Известно, что цитох-
ром P450s, включая CYP6M2, вовлечен в метаболизм инсектицидов, и служит одной из причин
возникновения резистентности. Работа регуляторных механизмов, участвующих в контроле
цитохрома P450s у комара Anopheles gambiae, остается неясной. В настоящем обзоре авторы
анализируют потенциальную роль ядерного эритроидного фактора 2 (Nf2e1), который является
ортологом фактора 2 (Nrf2) у позвоночных животных и изоформой С белка Cap-n-Collar (CnCC)
у Drosophila melanogaster, в экспрессии гена CYP6M2, раскодирующего энзимы и возможно
препятствующего возникновению резистентности к инсектицидам у комара Anopheles gambiae.
В нормальных условиях Nf2e1 накапливается в цитоплазме, где он объединяется со связыва-
ющим актин белком Keap 1 (Kelch-like ECH associating protein 1), ортологом AGAP003645, и
мгновенно разрушается в убиквитин-протеасомной системе. В данном обзоре анализируются
современные сведения о комплексе Nf2e1/ AGAP003645, причем особое внимание уделяется
молекулярным механизмам регуляции и потенциальной возможности использования комплекса
для борьбы с инфекционными болезнями, передаваемыми комарами, включая малярию.
258