ПАРАЗИТОЛОГИЯ, 2020, том 54, № 4, с. 312-321.
УДК 595.122.2; 59.087
MICRO-CT AS A METHOD
TO VISUALIZE INTRAMOLLUSCAN STAGES OF DIGENEA
© 2020 G. A. Kremneva, *, L. Y. Kryuchkovab, A. A. Miroliubovc,
V. A. Kalashnikovaa, D. Y. Krupenkoa
a Department of Invertebrate Zoology, Saint Petersburg University,
Universitetskaya emb. 7-9, Saint Petersburg, 199034 Russia
b Research Centre for X-ray Diffraction Studies, Saint Petersburg University
c Zoological Institute, Russian Academy of Sciences,
Universitetskaya emb. 1, Saint Petersburg, 199034 Russia
* e-mail: glistoved69@gmail.com, st022767@student.spbu.ru
Received 26.03.2020
Received in revised form 19.05.2020
Accepted 22.05.2020
X-ray micro-computed tomography (micro-CT) is a non-destructive method widely used for vi-
sualization of three-dimensional structures. Application of micro-CT for comparative morphology is
limited due to low x-ray contrast of soft animal tissues, but staining can increase image quality for such
specimens. We suggest that micro-CT may be used for rough visualization of branched sporocysts of
Digenea within intact hosts, and tested this approach on sporocyst of Leucochloridium paradoxum.
Two infected mollusks were treated following two different protocols. One specimen was scanned in
ethanol; the other was dried before scanning. Anatomical features of the host were better visible on
microtomographic sections of the dried specimen. Regardless of the sample preparation, full-grown
and underdeveloped broodsacs of the sporocyst were visible, but we could not trace its central part. We
suggest how the micro-CT protocol can be modified for better results on branched digenean sporocysts.
Keywords: micro-CT, phosphotungstic acid, critical point drying, Digenea, Leucochloridium par-
adoxum, sporocyst, metacercariae, Gastropoda, Succinea putris
DOI: 10.31857/S1234567806040045
312
X-ray micro-computed tomography (micro-CT) is a non-destructive method applicable
for visualization of three-dimensional structures, widely used in the studies on various free-
living (e.g. Parapar et al., 2018; Marcondes Machado et al., 2019) and parasitic (e.g. Noever
et al., 2016; Martín-Vega et al., 2018; O'Sullivan et al., 2018) invertebrates. However, the
application of this method for comparative morphology is limited due to low x-ray contrast
of soft animal tissues (Metscher, 2009). Staining with phosphotungstic acid (PTA) and IKI
(1 % iodine metal + 2 % potassium iodide in water) with subsequent scanning in ethanol
is the main tool to increase contrast and quality of micro-CT images for such specimens
(Metscher, 2009). Staining could be combined with incubation in dimethyl sulfoxide (Mar-
condes Machado et al., 2019) or freeze-drying after staining (Noever et al., 2016) for better
results.
In micro-CT studies of digeneans authors used basic protocols (Lee et al., 2007; Mar-
tín-Vega et al., 2018) and critical point drying after staining (Bulantová et al., 2016). This
method was successfully adopted to determine the localization of metacercariae and juvenile
adults within the second or definitive host organs (Lee et al., 2007; Bulantová et al., 2016;
Martín-Vega et al., 2018). Nevertheless, all these works focused on the hermaphroditic gen-
eration of digeneans while parthenogenetic generations (sporocysts and rediae) have so far
been neglected. In a few digenean taxa sporocysts have branched bodies with modular orga-
nization, where each module is different in terms of function and morphology (Galaktionov
et al., 2014). This makes them quite hard to study with traditional approaches like dissecting
from host tissues or histological sections with subsequent 3D reconstruction. Though histo-
logical techniques provide essential information on the internal structure and tissue organiza-
tion, this method is time consuming and gives sections in one plane only. Micro-CT does not
reveal the histological structure but it provides sections of three planes, it is relatively fast
and easy, and may be used for rough visualization of branched digenean sporocysts within
the host body and for estimation of branch numbers. Micro-CT also could be applied to de-
termine the host/parasite volume ratio, as was demonstrated for Rhizocephala (Nagler et al.,
2017). We tested this approach on sporocysts of Leucochloridium paradoxum Carus, 1835
(Brachylaimoidea: Leucochloridiidae). The sporocyst of this species is well-known for its
colored broodsacs and extremely branched body composed of functionally and morphologi-
cally different regions: a central part, broodsacs with infective metacercariae, narrowed stalks
connecting the broodsacs with the central part and underdeveloped broodsacs with develop-
ing metacercariae (Pojmanska, Machaj, 1991; Ataev et al., 2013; Ataev, Tokmakova, 2015).
Our study is a first attempt to apply micro-CT for intramolluscan stages of Digenea.
313
MATERIAL AND METHODS
Two specimens of amber snails, Succinea putris L., 1758, infected with L. paradoxum (referred
here and onwards as S1 and S2) were collected at the Yuzno-Primorskij park of Saint-Petersburg in
September 2017 and 2018. Sporocysts were visible through thin transparent shell of the hosts. Shells of
mollusks were crushed and a small puncture was made in the mantle to provide a better infiltration by
fixative and contrasting solutions. Samples remained intact without any dissection of the host. Infected
mollusks were fixed in Zenker’s solution with 100 % acetic acid (10:1). After 2 h fixation and 2 h rins-
ing in water, specimens were incubated in 70 % ethanol with iodine for 1 h and stored in 70 % ethanol
for one week.
Contrasting protocol was generally adopted from studies of Metscher (2009) and Marcondes Mach-
ado et al. (2019). S1 was stained in 0.3 % ethanol solution of phosphotungstic acid (PTA) for 12 h,
rinsed in 70 % ethanol for one hour to remove PTA, transferred to 96 % ethanol and stored in it for
three days inside a 1.5 ml plastic tube. S2 was stained in mixture of 0.3 % PTA with 3 % dimethyl
sulfoxide (DMSO) (10:1) for one week (Table 1). Next, S2 was gradually dehydrated through 70 %,
96 % ethanol, 96 % ethanol-acetone (3:1, 1:1, 1:3) and pure acetone (1 h in each liquid), and dried using
a critical point dryer (CPD) (Hitachi HCP-2). Both samples were scanned using a microtomography
scanner (Bruker SkyScan 1172). S1 was scanned twice in a 1.5 ml plastic tube filled with 96 % ethanol
under slightly different scanning parameters (referred here and onwards as S1-1 and S1-2, Table 1).
S2 was scanned once in a 1.5 ml plastic tube under a single set of scanning parameters (Table 1). Ob-
tained data was processed using CTVox® and DataViewer® software packages (Bruker Micro-CT).
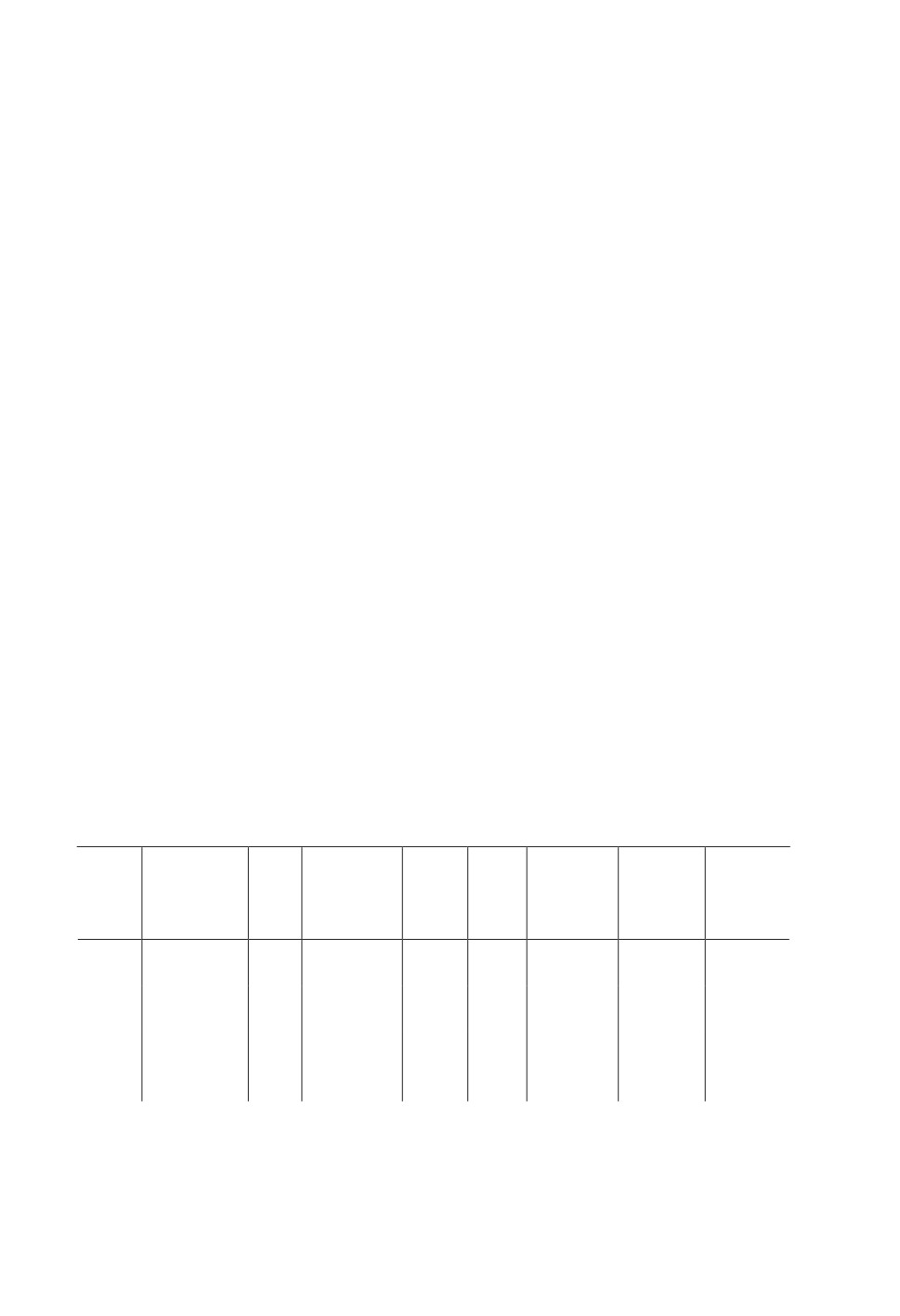
Table 1. Sample preparation and key parameters used for scannin
Frame
Dried
Source
Acceleration
Resolution,
averaging
Exposure,
Sample
Contrasting
in
current,
Filter
voltage, kV
μm
for one
ms
CPD
uA
scanning
12 h in 0.3 %
S1-1
-
74
100
No
4.3
3
460
PTA
12 h in 0.3 %
S1-2
-
74
108
No
2.5
5
470
PTA
1 w in 0.3 %
Al 0.5
S2
PTA with
+
58
161
3.03
3
1050
mm
3 % DMSO
Sample rotation angle for all samples was 0.2 grade.
314
RESULTS AND DISCUSSION
In total we received three datasets, two for the first sample (S1-1, Fig. 1A; 2; S1-2,
Fig. 1B; 3) and one for the second (S2, Fig. 4). S2 was overexposed, possibly due to extensive
staining of the albumin gland by PTA (Fig. 4). Therefore, a microtomography scanner filter
was applied to S2 during scanning to increase the contrast of other tissues (Table 1). We visu-
alized all datasets with DataViewer® to generate three planes of microtomographic sections
(Figs 2-4). Bright spots were observed in all datasets (Fig. 1-4), which are likely a scanning
artifact. 3D reconstructions in CTVox® were performed only for S1-1 and S1-2 (Fig. 1).
A 3D reconstruction for the S2 dataset is not shown here since the scanned wall of a plastic
tube (Fig. 4) made it impossible to receive a high resolution image in CTVox®. This artifact
possibly appeared due to the scanning parameters of S2, including filter.
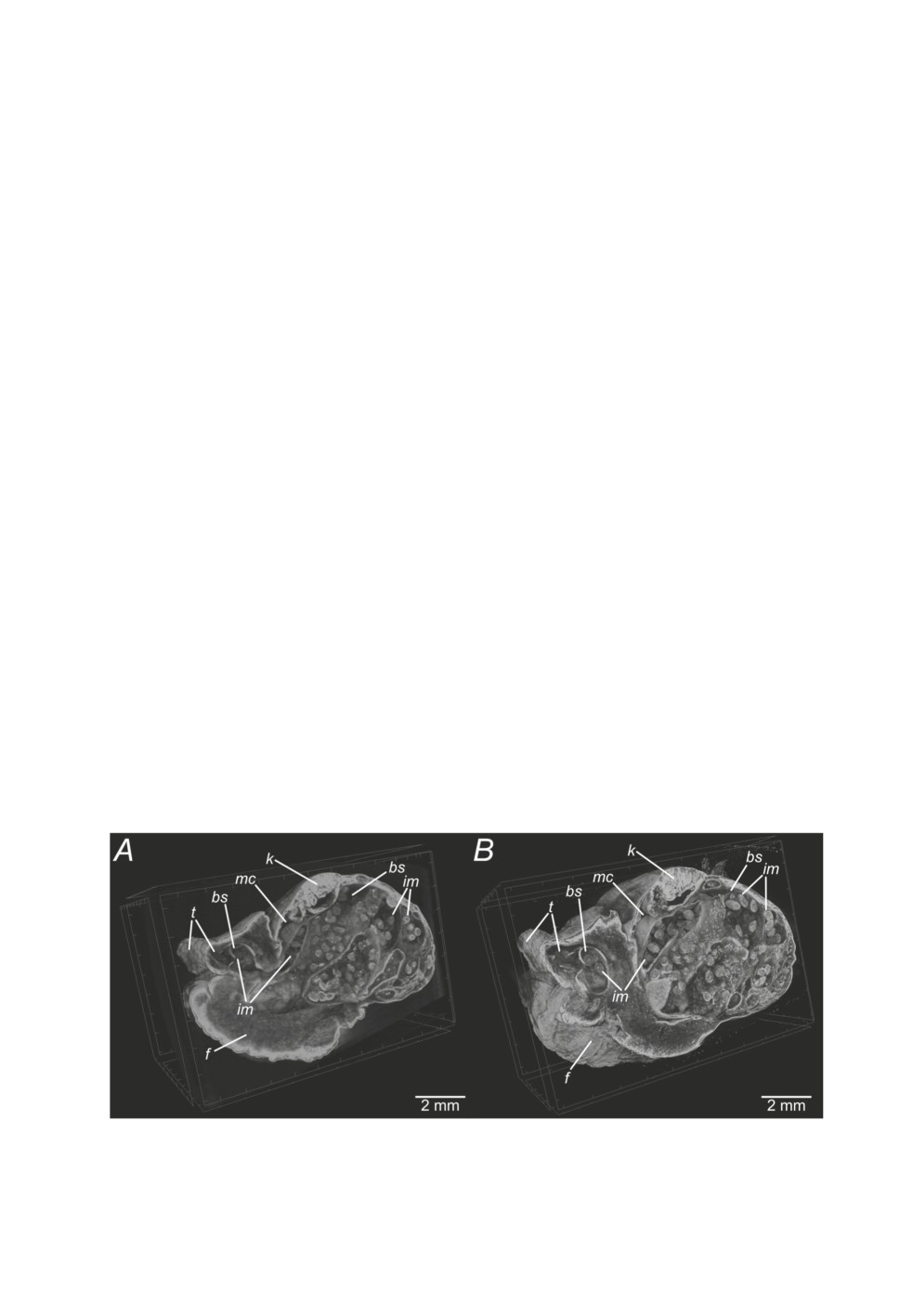
3D reconstructions of both S1-1 (Fig. 1A) and S1-2 (Fig. 1B) give a general picture of
the hosts morphology: head, foot, tentacles, lung and kidney are visible. Of the sporocyst,
only broodsacs containing infective metacercariae could be clearly identified (Fig. 1). The
resolution of S1-2 was higher than in S1-1, resulting in a better 3D reconstruction from S1-2
(Fig. 1B).
Microtomographic sections were more informative than 3D reconstructions (Figs 2-4).
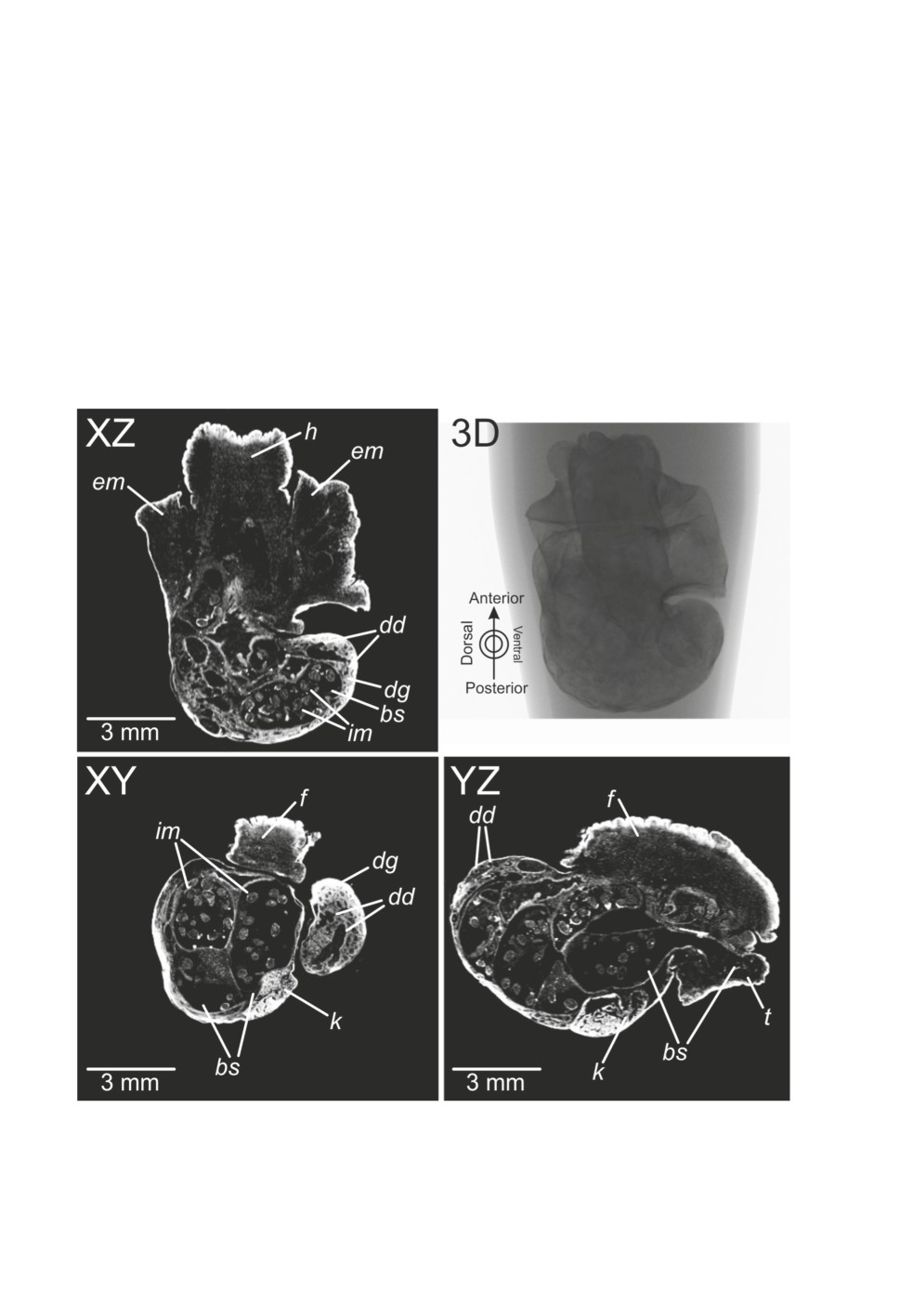
Anatomy of the snail was recognizable to different extents between the samples. On S1-1
the lung, kidney, digestive gland and its ducts were visible, but other organs were not
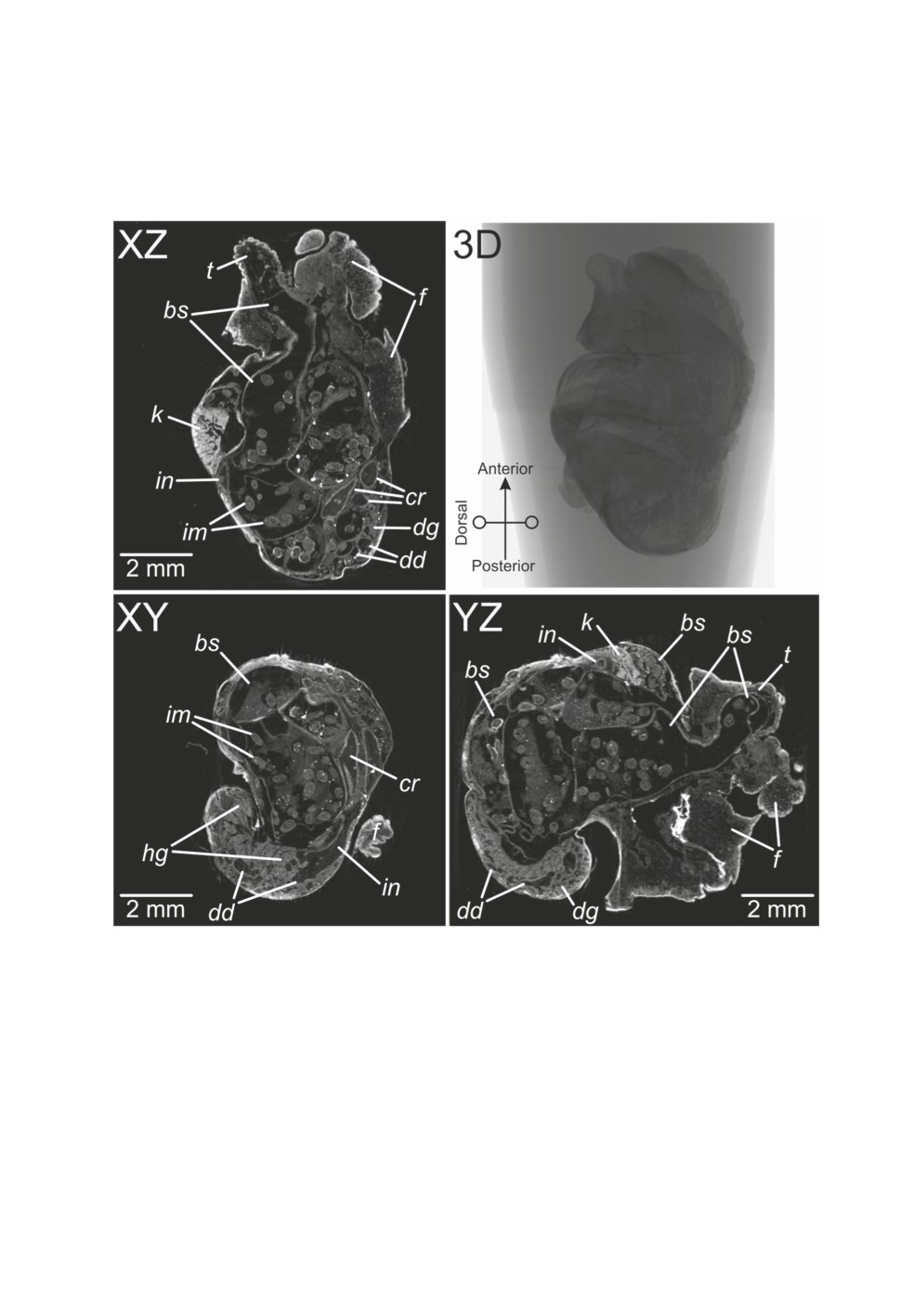
(Fig. 2). On S1-2 sections, other parts of the digestive system including crop and parts of
the intestine were visible, as well as the lung, kidney and urinary duct (Fig. 3). The only part
of reproductive system that could be distinguished on S1-2 was the hermaphrodite gland
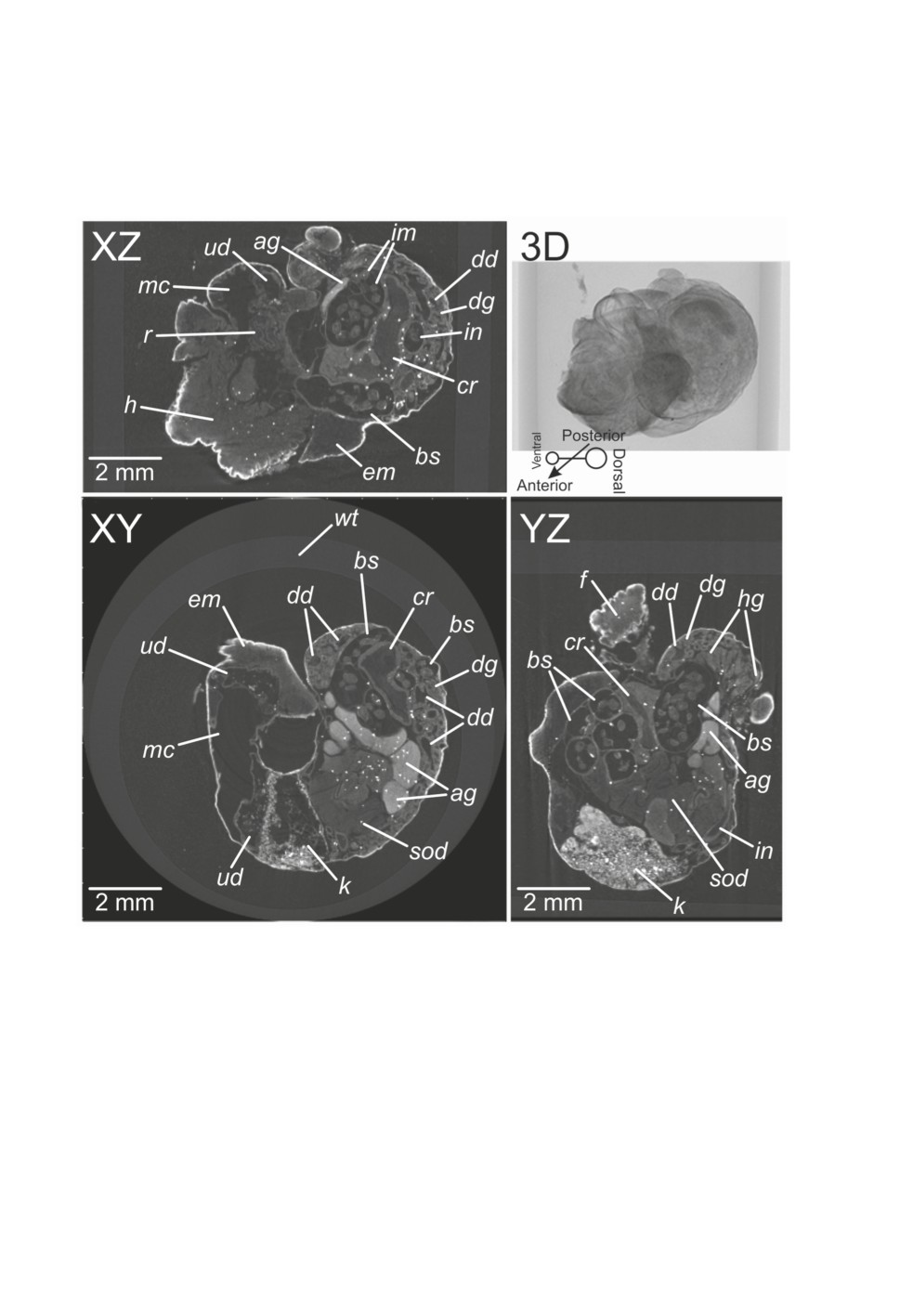
(Fig. 3). Sections of the S2 dataset provided the most detailed overview of snail anatomy
Figure 1. 3D reconstructions of datasets S1-1 (A) and S1-2 (B) made in CTVox®. Abbreviations:
bs - broodsac, f - foot, im - infective metacercariae, k - kidney, mc - mantle cavity (lung), t - tentacle.
315
(Fig. 4), with kidney, urinary duct and lung clearly distinguishable. All parts of the diges-
tive system were visible including the buccal cavity, crop, stomach, digestive gland with
its ducts, whole intestine, and rectum. Besides the hermaphroditic gland many other organs
of the reproductive system could be seen: the albumen gland, spermoviduct and distal parts
of the reproductive ducts. However, the difference in reproductive systems of S1-2 and S2
might also be caused due to different degrees of reproductive system degradation caused
by the sporocyst. The presence of a hermaphrodite gland in both samples is quite interest-
ing since digeneans usually cause castration of their host via the disruption of the gonad
(e.g. Lauckner, 1987; Tétreault et al., 2000).
Figure 2. Microtomographic sections (dataset S1-1) visualized with DataViewer®. Abbreviations:
bs - broodsac, dd - ducts of digestive gland, dg - digestive gland, em - edge of mantle, f - foot,
h - head, im - infective metacercariae, k - kidney, t - tentacle.
316
Figure 3. Microtomographic sections (dataset S1-2) visualized with DataViewer®. Abbreviations:
bs - broodsac, cr - crop, dd - ducts of digestive gland, dg - digestive gland, f - foot,
hg - hermaphrodite gland, im - infective metacercariae, in - intestine, k - kidney, t - tentacle.
317
Figure 4. Microtomographic sections (dataset S2) visualized with DataViewer®. Abbreviations:
ag - albumen gland, bs - broodsac, cr - crop, dd - ducts of digestive gland, dg - digestive gland,
em - edge of mantle, f - foot, h - head, hg - hermaphrodite gland, im - infective metacercariae,
in - intestine, k - kidney, mc - mantle cavity (lung), r - rectum, sod - spermoviduct,
ud - urinary duct, wt - wall of plastic tube.
318
Broodsacs with infective metacercariae of L. paradoxum could be easily distinguished
on sections from all three datasets (Figs 2-4) and some underdeveloped broodsacs were vis-
ible as well. Suckers and ceca of metacercariae could clearly be seen in S1-2 (Fig. 3) and
S2 (Fig. 4) datasets but were hardly discernible on S1-1 (Fig. 2). Unfortunately, regardless
of sample preparation, we could not trace the stalks of the broodsacs and the central part of
the sporocyst.
The following advantages of the obtained micro-CT datasets can be outlined. Firstly,
basic anatomical features of snails, including parts of digestive and reproductive systems,
were easy to trace and locate. Secondly, localization of sporocyst broodsacs within the intact
mollusk body can be readily studied. It is important to note that the protocol including drying
of the sample provided the most contrast dataset with the highest resolution. However, the
applied sample preparation and scanning parameters did not allow us to visualize the entire
sporocyst. The central part of the sporocyst remained obscure possibly due to its low contrast
with surrounding tissues of the host. Nevertheless, micro-CT seems to be applicable for stud-
ies on L. paradoxum sporocyst but changes in the protocol should be tested in future studies.
The contrast of the sporocyst might be further increased by injection of the PTA or IKI into
the sporocyst broodsac. Such technique was successfully applied by Nagler et al. (2017) for
visualization of root system in two rhizocephalan species. A similar approach can be tested
on other branched sporocysts of Digenea.
To conclude, micro-CT can be applied to study the localization of digenean sporocysts
or rediae among the host organs, and in perspective - to make spatial reconstructions of
branched sporocysts. However, this method provides no information on the tissue organiza-
tion of the parasite comparing to the histological serial sections.
ACKNOWLEDGEMENTS
This study was a part of the initiative project of Saint Petersburg University 1.52.1489.2017.
During this work we utilized equipment of the resource center “Center for X-ray Diffraction
Studies” of the Research park of Saint Petersburg University and “Taxon” Research Resource
of Sciences. This study was partly supported by the state laboratory theme of Zoological
Institute AAAA-A19-119020690109-2. Authors thanks Vladimir Lebedenkov, a student of
Saint Petersburg University for his help with sampling.
319
REFERENCES
Ataev G.L., Dobrovolskij A.A., Tokmakova A.S. 2013. Reproduction of trematode Leucochloridium paradoxum
sporocysts (Trematoda: Leucochloridiidae). Parazitologiya 47 (2): 178-182 [In Russian].
Ataev G.L., Tokmakova A.S. 2015. Seasonal changes in the biology of Leucochloridium paradoxum (Trematoda,
Leucochloridiomorphidae). Parazitologiya 49 (3): 200-207 [In Russian].
Bulantová J., Macháček T., Panská L., Krejčí F., Karch J., Jährling N., Saghaf S., Dodt H.U. Horák, P. 2016. Tricho-
bilharzia regenti (Schistosomatidae): 3D imaging techniques in characterization of larval migration through
the CNS of vertebrates. Micron 83: 62-71.
Galaktionov K.V., Dobrovolsky A.A., Podvyaznaya I.M. 2014. Evolution of the morpho-functional organization of
parthenogenetic generations of trematodes. Zoological journal 93 (3): 426-442 [In Russian].
Lauckner G. 1987. Ecological effects of larval trematode infestation on littoral marine invertebrate populations.
International Journal for Parasitology 17 (2): 391-398.
Lee C.H., Im J. G., Goo J.M., Lee H.J., Hong S.T., Shen C.H., Chung D.H., Son K.R., Chang J.M., Eo H. 2007. Se-
rial CT findings of Paragonimus infested dogs and the Micro-CT findings of the worm cysts. Korean Journal
of Radiology 8 (5): 372-381.
Marcondes Machado F., Passos F.D., Giribet, G. 2019. The use of micro-computed tomography as a minimally in-
vasive tool for anatomical study of bivalves (Mollusca: Bivalvia). Zoological Journal of the Linnean Society
186 (1): 46-75.
Martín-Vega D., Garbout A., Ahmed F., Wicklein M., Goater C.P., Colwell D.D., Hall M.J. 2018. 3D virtual histol-
ogy at the host/parasite interface: visualisation of the master manipulator, Dicrocoelium dendriticum, in the
brain of its ant host. Scientific reports 8 (1): 1-10.
Metscher B.D. 2009. MicroCT for comparative morphology: simple staining methods allow high-contrast 3D imag-
ing of diverse non-mineralized animal tissues. BMC physiology 9 (1): 11.
Nagler C., Hörnig M.K., Haug J.T., Noever C., Høeg J.T., Glenner H. 2017. The bigger, the better? Volume measure-
ments of parasites and hosts: Parasitic barnacles (Cirripedia, Rhizocephala) and their decapod hosts. PloS
one 12 (7).
Noever C., Keiler J., Glenner H. 2016. First 3D reconstruction of the rhizocephalan root system using MicroCT.
Journal of Sea Research 113: 58-64.
O'Sullivan J.D., Behnsen J., Starborg T., MacDonald A.S., Phythian-Adams A.T., Else K.J., Cruickshank S.M.,
Withers P.J. 2018. X-ray micro-computed tomography (μCT): an emerging opportunity in parasite imaging.
Parasitology 145 (7): 848-854.
Parapar J., Zhadan A., Tzetlin A., Vortsepneva E., Moreira J. 2018. Exploring the anatomy of Cossura pygodactylata
Jones, 1956 (Annelida, Cossuridae) using micro-computed tomography, with special emphasis on gut archi-
tecture. Marine Biodiversity 48 (2): 751 -761.
Pojmanska T., Machaj K. 1991. Differentiation of the ultrastructure of the body wall of the sporocyst of Leucochlo-
ridwm paradoxum. International journal for parasitology 21 (6): 651-659.
Tétreault F., Himmelman J.H., Measures L. 2000. Impact of a castrating trematode, Neophasis sp., on the common
whelk, Buccinum undatum, in the Northern Gulf of St. Lawrence. The Biological Bulletin 198 (2): 261-271.
320
КОМПЬЮТЕРНАЯ МИКРОТОМОГРАФИЯ КАК МЕТОД ВИЗУАЛИЗАЦИИ
ПАРТЕНИТ ТРЕМАТОД ВНУТРИ МОЛЛЮСКА-ХОЗЯИНА
Г. А. Кремнев, Л. Ю. Крючкова, А. А. Миролюбов,
В. А. Калашникова, Д. Ю. Крупенко
Ключевые слова: компьютерная микротомография, фосфорновольфрамовая кислота, суш-
ка через критическую точку, трематоды, Leucochloridium paradoxum, спороциста, метацеркарии,
Gastropoda, Succinea putris
Рентгеновская компьютерная микротомография позволяет визуализировать трехмерную
структуру объектов без нарушения их целостности. Использование этого метода в сравнитель-
ной морфологии ограничено, поскольку мягкие ткани животных имеют низкую плотность и
практически не ослабляют поток рентгеновских лучей. Для увеличения контрастности изобра-
жения такие образцы обрабатывают специальными красителями. Мы предположили, что ком-
пьютерная микротомография может быть использована для приблизительной визуализации раз-
ветвленных спороцист трематод в теле моллюска-хозяина, и попробовали протестировать этот
метод на спороцисте Leucochloridium paradoxum. Два зараженных моллюска были обработаны
в соответствии с двумя различными протоколами. Один образец был отсканирован в этиловом
спирте, второй был предварительно высушен. Анатомические признаки хозяина лучше читались
на микротомографических сечениях высушенного образца. Вне зависимости от примененной
пробоподготовки, нам удалось различить только полностью сформированные и созревающие
отростки спороцисты, содержащие зрелых и развивающихся метацеркарий, тогда как централь-
ную часть спороцисты проследить не удалось. В данной работе мы предлагаем возможные из-
менения протокола для более точной визуализации спороцисты L. paradoxum с помощью ком-
пьютерной микротомографии.
321