ПАРАЗИТОЛОГИЯ, 2023, том 57, № 1, с. 64-76.
УДК 579.64:632.654
ACARICIDAL EFFECT OF SECONDARY METABOLITES
FROM SYMBIOTIC BACTERIA XENORHABDUS BOVIENII
AND X. NEMATOPHILA OF ENTOMOPATHOGENIC NEMATODES
ON SPIDER MITE TETRANYCHUS URTICAE
(TROMBIDIFORMES, TETRANYCHIDAE)
© 2023 L. G. Danilov1, G. P. Ivanova1,
V. G. Kaplin1,*, E. A. Varfolomeeva2
1All-Russia Institute of Plant Protection, Pushkin, Saint Petersburg, 196608 Russia
2Komarov Botanical Institute of the Russian Academy of Sciences,
Professora Popova str., 2, Saint Petersburg, 197376 Russia
*e-mail: ctenolepisma@mail.ru
Received June 18, 2022
Received in revised form January 17, 2023
Accepted January 22, 2023
In laboratory, the highest mortality rates of Tetranychus urticae after the use of metabolic products
of symbiotic bacteria with a titer of 1 × 107 were observed in Xenorhabdus bovienii at 6-8 days
post application (dpa) in the experiment with live and at 8 dpa of autoclaved culture (about 95%).
In experiments with live and autoclaved culture with a titer of 1 × 107, the mortality mites at 8 dpa
in X. bovienii was almost the same, but in X. nematophila it was slightly higher in autoclaved culture.
At 8 dpa, the efficacy of the live and autoclaved metabolic products of Xenorhabdus bovienii and
X. nematophila against the spider mite with a titer of 1 × 105 was about 1.4 times lower compared
to the culture with a titer of 1 × 107. The relationship between the mortality of spider mites (%) and
the exposure time (days) to bacterial metabolism products most reliably reflects by the polynomial
dependence with the accuracy of approximation 0.93-1.0. In the greenhouse, the effectiveness of
the bacterial metabolic products of X. bovienii against spider mite was highest in experiments with
live culture with a titer of 1 × 108. In experiments with live culture of X. bovienii with a titer of
1 x 107 (in vivo) the mortality rate of spider mites on leaves of shrub Dracaena sanderiana
at 8 dpa increased from 84% on the ground floor to 90% on the second floor. The overall efficacy
of the bacterial metabolic products of X. bovienii (in vivo, titer 1 × 107) against adults, larvae and
nymphs of T. urticae on the leaves of perennial marsh grasses (Potenderia cordata, Thalia geniculata
and T. dealbata) was about 98-99%.
Keywords: Steinernema, live bacterial culture, autoclaved culture, laboratory conditions, green-
houses, toxic secondary metabolites, efficiency
DOI: 10.31857/S0031184723010064; EDN: FJTRQQ
64
Introduction
Bacteria of the genus Xenorhabdus are symbionts of entomopathogenic nematodes
(EPNs) in the genus Steinernema. The bacterium colonizes a specialized intestinal pocket
within the infective stage of the third age nematode, which transports the bacteria between
insects that are killed and consumed by the pair for reproduction. The infectious stage
of the third-instar nematode, which lives in the soil without feeding, penetrates into the
body of soil insects and regurgitates symbiotic bacteria that contribute to the death and
digestion of the host’s internal organs. Nematodes begin to feed on digested foods, turn-
ing into larvae of the fourth instar, then into males and females. Bacteria of the genus
Xenorhabdus produce a large number of secondary metabolites that weaken the immune
system of insects, causing their death, suppressing the development of other microorgan-
isms. These include toxic peptides, amino acids, polypeptides, antimicrobial and antifungal
substances with antibiotic properties (fabclavines, xenocoumacins and others). They have
found wide application in plant protection against nematodes - parasites of stems and
leaves, the root system of plants, their diseases and pests (Hazir et al., 2016; Dreyer et al.,
2018; Eroglu et al., 2019; Abebew et al., 2022; Yüksel, 2022; Yüksel et al., 2022; Zhang
et al., 2022). To protect plants from diseases and pests, three main forms of biological
products containing toxic secondary metabolites of Xenorhabdus bacteria are used: culture
fluids containing metabolites and of live (in vivo) or dead (in vitro) bacteria; cell-free cul-
ture supernatants. The cultivation of bacteria from stock cultures is initially carried out in
Petri dishes on agar plates at 28°C for 24 h. One of the bacterial colonies is transferred to
flasks containing sterile Tryptic Soy Broth and the flasks are incubated at 30°C and 150 rpm
(revolution per minute) for 24 h. The density of bacterial culture cells is measured by
a spectrophotometer. To extract the cell-free supernatants, the bacterial culture in the
broth suspension is centrifuged at 20.000 revolutions per minute (rpm) for 15 min at 4°C
in 50 ml Falcon tubes. The centrifuged supernatant solution is separated from the bacterial
cells by passing through a 0.22 μm millipore filter. The filtrated solution is checked for
the presence of bacterial cells by streaking onto NBTA agar (Hazir et al., 2016). Autocla-
ving at 121°C for 10 min do not influence the antibiotic activities of the cell-free cultures
of Xenorhabdus (Fodor et al., 2010). Cell-free bacterial cultures and supernatants can be
stored at 4°C for 2 weeks before use in experiments (Hazir et al., 2016). Xenorhabdus
nematophila, X. bovienii and X. szentirmaii supernatants could be used as potential con-
trol agents against T. urticae (Incedayi et al., 2021). The effect of secondary metabolites
of Xenorhabdus bacteria on plant diseases and pests has been studied mainly in laboratory
conditions in Petri dishes and in pots.
Tetranychus urticae C.L. Koch, 1836 (two-spotted spider mite) is a widespread polypha-
gous, cosmopolitan species. It has been recorded from most countries in North, Central
and South America, Europe, Asia, Africa and Australia. T. urticae infests about 1167 spe-
cies host plants from 127 families, annuals, perennial grasses, shrubs and trees, wild and
65
cultivated both in field conditions and in greenhouses. The lower temperature threshold for
it development is about 12°C and the upper limit for development is about 40°C, optimal
temperature 26-30°C, air humidity 60-80%. The life cycle ranges from 8 days to 40 days.
T. urticae is one of the most serious agricultural pests in the world. About 88 cultural host
plants are infested by this pest, such as bean, soybean, cotton, cucumber, tomato, melon,
peanut, vine, banana, papaya, corn, ornamental crops and others (Riahi et al., 2013; Mi-
geon, Dorkeld, 2019). In most agricultural crops, the use of synthetic pesticides is the main
method to control T. urticae. However, because environmental adverse effects of these
pesticides, the development of pesticide resistance in the target pest, and potential impacts
on biodiversity and Human health (Dermauw et al., 2013), alternative methods should be
developed. T. urticae is very difficult to control with acaricides because most populations
developed resistance to chemical groups after a few years of use (Cranham, Helle, 1985).
In Turkey, Eroglu et al. (2019) were investigated the effects of secondary metabolites
produced by 6 species symbiotic bacteria of the genera Xenorhabdus and Photorhabdus
on different stages of Tetranychus urticae using cell-free bacterial supernatants in Petri
dishes and in pot experiments. The number of living and dead individuals was recorded
at 2, 5 and 7 day post application (dpa) of the cell-free bacterial supernants. Depending
on the bacterial supernatant, mortality in the was less than 4% for eggs, 46-97% for lar-
vae, 30-96% for protonymphs, 41-92% for deutonymphs, 92-100% for adult males and
46-93% for adult females.
The purpose of our research was to evaluate the effectiveness of exposure to secondary
metabolites of live and autoclaved bacterial cultures of the genus Xenorhabdus in labora-
tory conditions and in greenhouses against Tetranychus urticae by spraying these cultures
of experimental mite-infested plants.
MATERIALS AND METHODS
The research was carried out in the Laboratory of Microbiology of the All-Russian Institute of
Plant Protection and in the greenhouses of the Botanical Garden of the Institute of Botany of the
Russian Academy of Sciences in March, April and May 2020 and 2021.
Cultures of symbiotic bacteria Xenorhabdus bovienii and Xenorhabdus nematophila were obtained
indirectly from the nematodes by sampling the haemocoel of Galleria mellonella (L.) (Lepidop-
tera, Pyralidae) Iarvae of older ages infected by nematodes Steinernema feltiae strain RP18-91 and
Steinernema carpocapsae strain “agriotes” and that stored in distilled water at 5-7°C within two
weeks. The pathogenicity of the two forms of symbiotic bacteria the X. bovienii and X. nematophila
was compared by estimation of LD50 following intrahaemocoelic injection of Galleria larvae. The
concentration of cells in shaken, 24 h broth cultures was estimated by use of a counting slide. Each
culture was then serially diluted with sterile Ringer’s solution (Akhurst, 1980).
The initial titer of 1 × 108 was taken as the maximum in the experiments. The concentration of
bacterial cells with a titer of 1 × 107 and 1 × 105 was obtained by diluting the culture liquid with
a titer of 1 × 108 with sterile water. Part of the resulting culture liquids were autoclaved at a tem-
66
perature of 121°C, pressure of 1 atmosphere for 30 min. As known, symbiotic bacteria of the genus
Xenorhabdus produce both heat-labile and heat-stabile toxins, enzymes and antimicrobials. Their
heat-stabile components are active after heat sterilization and can be used against different species of
bacteria and pests (Inman, Holmes, 2012). In experiments against spider mites, live and autoclaved
bacterial cultures were used in laboratory conditions and in greenhouses.
In the laboratory, the study of the effect of the products of the metabolism of symbiotic bacteria
of entomopathogenic nematodes on the spider mite was carried out according to toxicological methods
(Sukhoruchenko, Ivanova, 2013). The laboratory population of the spider mate was maintained in
cages on bean plants (Phaseolus vulgaris) at a temperature of 22-24°C, relative humidity of 65-70%,
photoperiod of L18: D6. The beans for the experiments were grown on water in glass jars with
a volume of 0.5 l, closed with plastic lids with holes where the bean sprouts were inserted. Then
the plants of bean with a height of 9-10 cm with roots and one leaf were placed in conical cones
with a volume of 100 ml. Before the experiments, 20 or 25 female mites were placed on the leaf
with a soft brush (depending on the size of the leaf) 2 hours before they are treated with bacterial
preparations, so that the mites on the leaf began to feed. The number of mites on the leaf of bean
in each experiment was the same. Then the plants with the mites were removed from the cone,
carefully dipped in solutions of live or autoclaved bacterial culture with titers 1 × 105 or 1 × 107 for
3 seconds, allowed to drain excess moisture and placed back in the cones. Cones with mite-infested
plants were placed on pallets with water to avoid their migration from one plant to another and kept
under the above conditions of temperature, humidity and photoperiod. Control plants were dipped
in water. The number of live and dead individuals of mite were recorded at 1, 4, 6 and 8 dpa (day
post application) after reatment with bacterial preparations (Table 1). The replication of each experi-
ment was 4-fold. Before the appearance of the larvae, the number of live females was counted on
the leaf, and after the hatching of the larvae, the total number of individuals was calculated. In the
control, female spider mites laid eggs in Petri dishes and the larvae hatched on the 5th-6th day. Mite
mortality was determined taking into account changes in their number in the control according to the
formula of Henderson and Tilton (1955): E = 100 × (1 - Oe × Cc / On × Cn), where: E - efficiency
expressed as the percentage of pest population reduction adjusted for control;
Oe, Cc - the number of live individuals before processing in the experiment and in the control;
On, Cn - the number of live individuals after processing and in the control, respectively, by the
accounting.
The effect of the live and autoclaved products of the metabolism of symbiotic bacteria
Xenorhabdus bovienii of entomopathogenic nematode Steinernema feltiae with titers 1 × 107 and 1 ×
108 was also tested against the spider mite in the greenhouses of the Botanical Garden of the Botani-
cal Institute of the Russian Academy of Sciences (St Petersburg) 3.05-10.05.2020, 30.03-7.04 and
12.05-20.05 2021 (Tables 2, 3). In the Leningrad region, the spider mite develops in 8-10 genera-
tions per year. With a decrease in the duration of the daylight less than 16 hours fertilized female
spider mites enter winter diapause, which is observed in St Petersburg since the beginning of August.
Overwintered females appear on plants in early May when the air temperature rises above 12-14°C,
feed and lay eggs among the cobwebs on the underside of the leaves. In other words, during the first
period of experiments on plant protection from spider mite at the end of March - the first decade
67
of April, when an air temperature in greenhouses was of 19-21°C, overwintered females during the
egg laying period were dominated. Females lay eggs for 15-20 days. The development from an egg
to an adult takes about from 7 to 20 days, depending on the air temperature. During the second pe-
riod of experiments (12-20.05) at an air temperature in greenhouses of 28-32°C, all stages of spider
mite development (eggs, larvae, protonymphs, deutonymphs and adults) were presented. Spraying
of experimental mite-infested plants was carried out with manual sprayers Marolex Profession, 5 l.
The experimental plants were represented by trees (Bolusanthus speciosus (Fabaceae), Ziziphus mau-
ritiana (Rhamnaceae)), shrub (Dracaena sanderiana (Asparagaceae)) and perennial marsh grasses
(Pontederia cordata (Pontederiaceae), Thalia geniculata and T. dealbata (Marantaceae)). Experimental
plants were sprayed in two experience options: with metabolic products of live and autoclaved culture
of X. bovienii with titers 1 × 108 and 1 × 107 in 3-fold replication in each option. The consumption
of bacterial cultural liquid for spraying of experimental plants in greenhouses was about 5 l at a titer
of 1 × 108 and 10-15 l at a titer 1 × 107.
The numbers of spider mites were taken into account visually on the leaves of each experimen-
tal plant in ind./leaf in 10-fold replication before spraying and at 4, 6 and 8 dpa of spraying with
bacterial preparations. The mortality of spider mites and the effectiveness of bacterial preparations
against them were determined taking into account changes in their numbers in the control as well
as in the laboratory conditions.
Statistical processing was performed in Microsoft Exel and Sigma Plot 12.0 programs. Biological
efficiency was calculated using the Abbott’s formula (Abbot, 1925), adjusted for control (Fleming,
Retnakan, 1985).
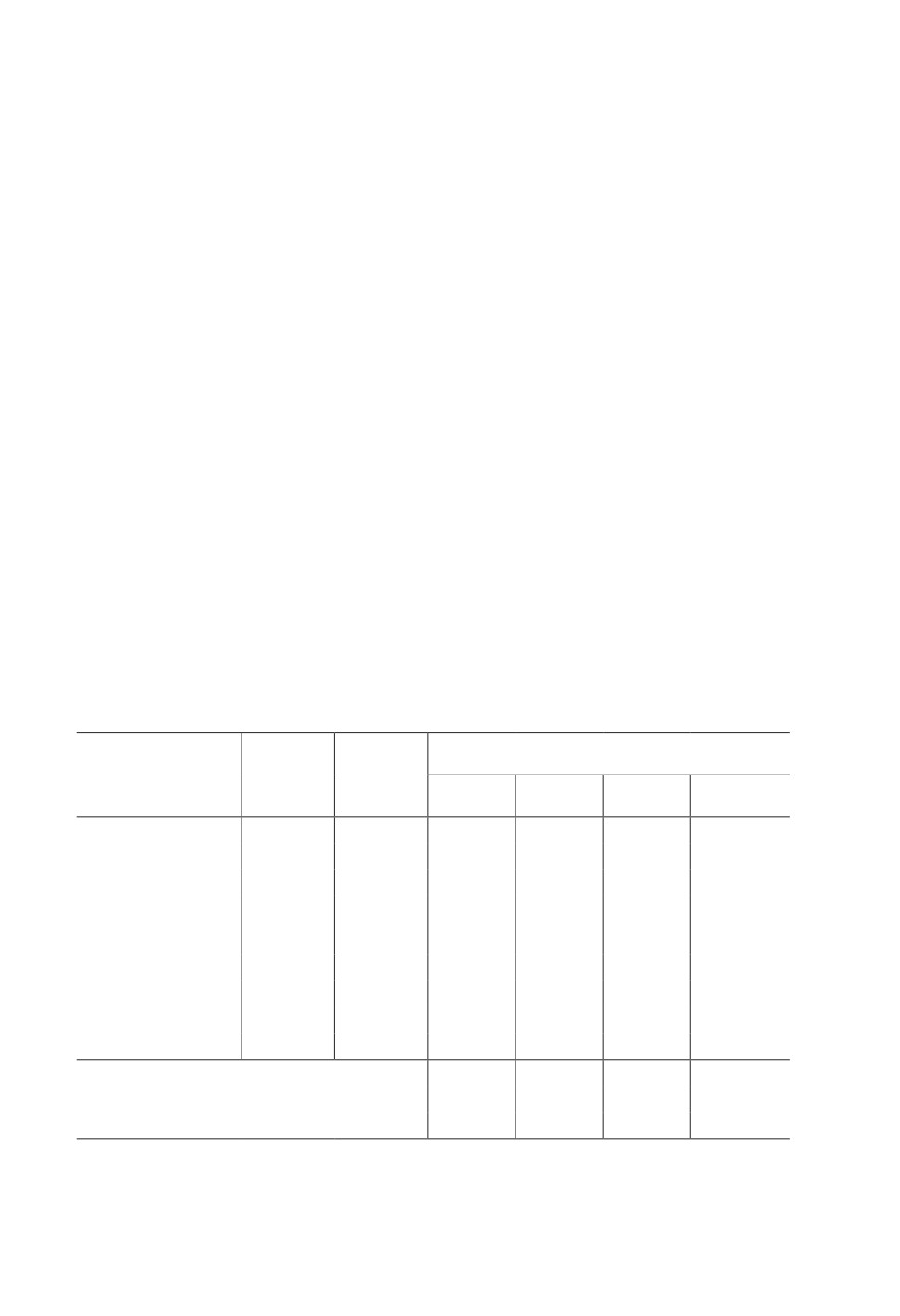
Table 1. Effect of secondary metabolic products of Xenorhabdus bovienii on the mortality of
Tetranychus urticae in laboratory conditions (25 females in 4-fold replication)
The titer
Mortality rate of mites after application
Symbiotic bacterium
of bacterial
Bacterial
of bacterial culture, %
(entomopathogenic
cells,
culture
nematode)
1
4
6
8
n × ml-1
Xenorhabdus bovienii
1 × 107
Live
48.0 ± 2.8
82.0 ± 2.0
94.5 ± 1.1
95.4 ± 0.7
(Steinernema feltiae)
(in vivo)
1 × 105
2.0 ± 2.0
33.0 ± 1.9
38.6 ± 2.8
61.4 ± 3.6
1 × 107
Autoclaved
57.0 ± 1.9
89.6 ± 1.2
90.0 ± 1.3
95.1 ± 0.2
at 121°C
1 × 105
26.0 ± 2.6
45.0 ± 1.9
60.7 ± 5.2
73.2 ± 2.9
for
10 min
Xenorhabdus
1 × 107
In vivo
59.0 ± 1.9
80.0 ± 2.8
87.6 ± 1.8
85.6 ± 2.3
nematophila
1 × 105
13.0 ± 2.5
40.0 ± 1.6
58.6 ± 1.2
62.4 ± 2.3
(Steinernema
1 × 107
Autoclaved
25.8 ± 2.3
81.0 ± 4.1
87.6 ± 2.6
91.3 ± 2.7
carpocapsae)
1 × 105
1.0 ± 1.0
36.0 ± 2.8
55.6 ± 2.0
72.6 ± 3.6
LSD0.05 (titer)
7.8
10.2
11.3
12.8
LSD0.05 (species of Xenorhabdus, titer 1 × 107)
6.5
4.2
1.6
2.8
T. urticae, control, ind./leaf (dish)
25
25
36.2 ± 0.4*
133.8 ± 4.9*
Notes. Air temperature 22-24°C, relative humidity 65-70%, photoperiod L18: D6.
1, 4, 6, 8 - day post application. *females and larvae.
68
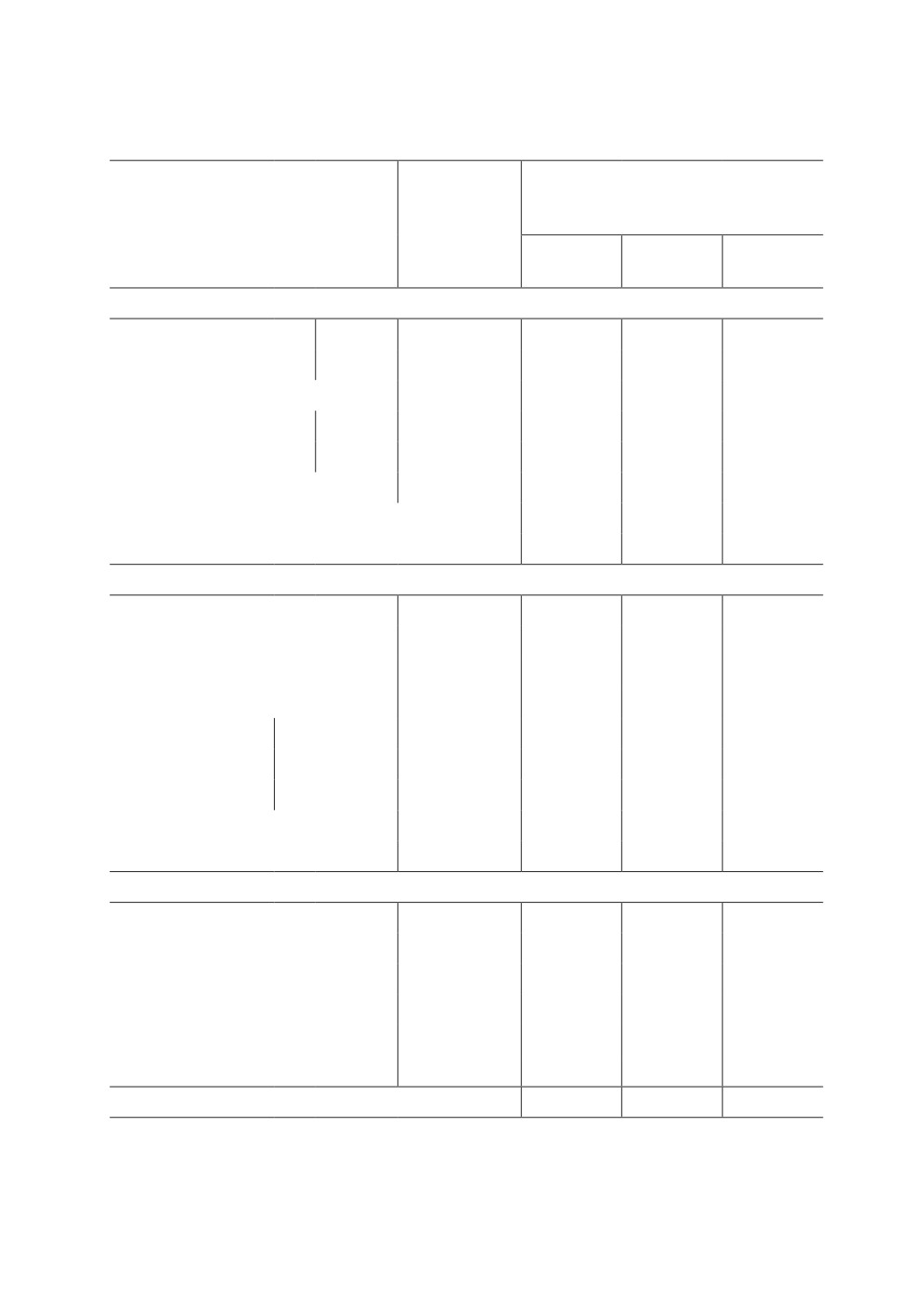
Table 2. Effect of metabolic products of Xenorhabdus bovienii (titer 1 x 107) of entomopathogenic
nematode (Steinernema feltiae) on the mortality of females Tetranychus urticae in greenhouse
conditions (leaves in 10-fold replication on plants in 3-fold replication)
Number
Mortality rate of mites after application
of adult
of bacterial culture, %
mites before
Dates, plants, bacterial culture, titer
treatment,
individuals/
4
6
8
leaf
3-10.05.2020
Bolusanthus speciosus,
1 × 108
20.0 ± 2.2
56.6 ± 2.5
84.4 ± 3.2
98.8 ± 0.8
in vivo
1 × 107
20.1 ± 2.1
41.7 ± 3.4
59.7 ± 2.2
88.6 ± 0.5
T. urticae, control, ind./leaf
19.2 ± 1.4
20.0 ± 1.8
18.5 ± 1.6
19.4 ± 1.2
Ziziphus mauritiana,
1 × 108
19.4 ± 1.5
39.1 ± 3.2
55.6 ± 5.0
90.8 ± 2.9
autoclaved culture
1 × 107
21.3 ± 3.7
24.1 ± 3.5
46.5 ± 2.7
87.5 ± 2.8
T. urticae, control, ind./leaf
18.7 ± 1.3
19.0 ± 1.6
21.5 ± 1.5
23.4 ± 1.2
LSD0.05 (in vivo and autoclaved culture)
8.5
4.6
3.8
LSD0.05 (titer)
6.8
7.5
4.2
30.03-8.04.2021 (females), 1 × 107, in vivo
Bolusanthus speciosus (Fabaceae)
19.5 ± 1.6
55.1 ± 3.5
86.2 ± 4.3
99.0 ± 2.2
T. urticae, control, ind./leaf
18.5 ± 1.2
23.2 ± 2.1
25.4 ± 2.3
28.6 ± 3.2
Ziziphus mauritiana (Rhamnaceae)
22.3 ± 2.0
54.3 ± 3.0
85.6 ± 2.2
98.9 ± 0.6
T. urticae, control, ind./leaf
19.0 ± 2.0
20.0 ± 1.8
21.5 ± 1.9
24.6 ± 1.8
Ground floor
20.1 ± 2.0
42.8 ± 3.1
59.7 ± 2.8
84.1 ± 1.5
Dracaena sanderiana
First floor
23.3 ± 2.1
51.9 ± 4.2
65.2 ± 3.1
88.0 ± 0.8
(Asparagaceae)
Second floor
26.4 ± 2.3
54.5 ± 4.2
69.7 ± 3.8
90.2 ± 1.3
T. urticae, control, ind./leaf
23.2
20.1 ± 1.4
16.6 ± 1.2
17.3 ± 1.5
LSD0.05 (Dracaena sanderiana) (floors)
2.5
6.3
4.5
2.8
30.03-8.04.2021 (females), 1 × 107, autoclaved culture
Pontederia cordata (Potenderiaceae)
21.4 ± 2.1
43.6 ± 3.3
47.0 ± 2.4
87.8 ± 2.8
T. urticae, control, ind./leaf
15.3 ± 1.5
16.8 ± 1.5
18.5 ± 1.2
18.9 ± 1.0
Thalia geniculata (Marantaceae)
15.1 ± 0.7
42.7 ± 4.2
47.0 ± 4.4
81.4 ± 3.5
T. urticae, control, ind./leaf
15.0 ± 0.9
16.5 ± 1.2
17.6 ± 0.8
18.9 ± 1.3
Thalia dealbata
12.4 ± 0.8
40.3 ± 2.6
56.4 ± 3.5
90.1 ± 4.2
T. urticae, control, ind./leaf
15.2 ± 1.1
16.3 ± 1.3
17.8 ± 1.0
19.7 ± 1.4
LSD0.05 (plants sprayed with bacterial culture)
1.5
4.2
3.9
Notes. Peter the Great Botanical Garden, St. Petersburg, air temperature 19-21°C,
relative humidity 85-90%. 4, 6, 8 - day post application.
69
Statistical analyses. Analysis of variance (ANOVA) was used to assess effect of trial to deter-
mine whether there were significant differences between the experiment repeats (P ≤ 0.05). Data are
presented as means ± standard error and least significant difference (LSD0.05). Any difference between
means larger than the LSD is considered a significant result.
RESULTS
The effect of the live and autoclaved metabolic products of symbiotic bacteria
Xenorhabdus bovienii and X. nematophilus entomopathogenic nematodes, respectively,
Steinernema feltiae, and S. carpocapsae on the death (%) of the common spider mite
(Tetranychus urticae Koch) depending on the titer of bacterial cells (1 × 108, 1 × 107 and
1 × 105) and the exposure time (days). 1, 4, 6 and 8 dpa in laboratory and greenhouses
have been investigated (Tables 1-3). In general, the mortality rate of mites with an increase
in the titer of bacterial cells in the corresponding culture fluids, as well as with an increase
in the exposure time (days). At the same time, the mortality rate of females and mite larvae
significantly increased in two bacterial species (X. bovienii and X. nematophilus) with an
increase in the exposure time (days).
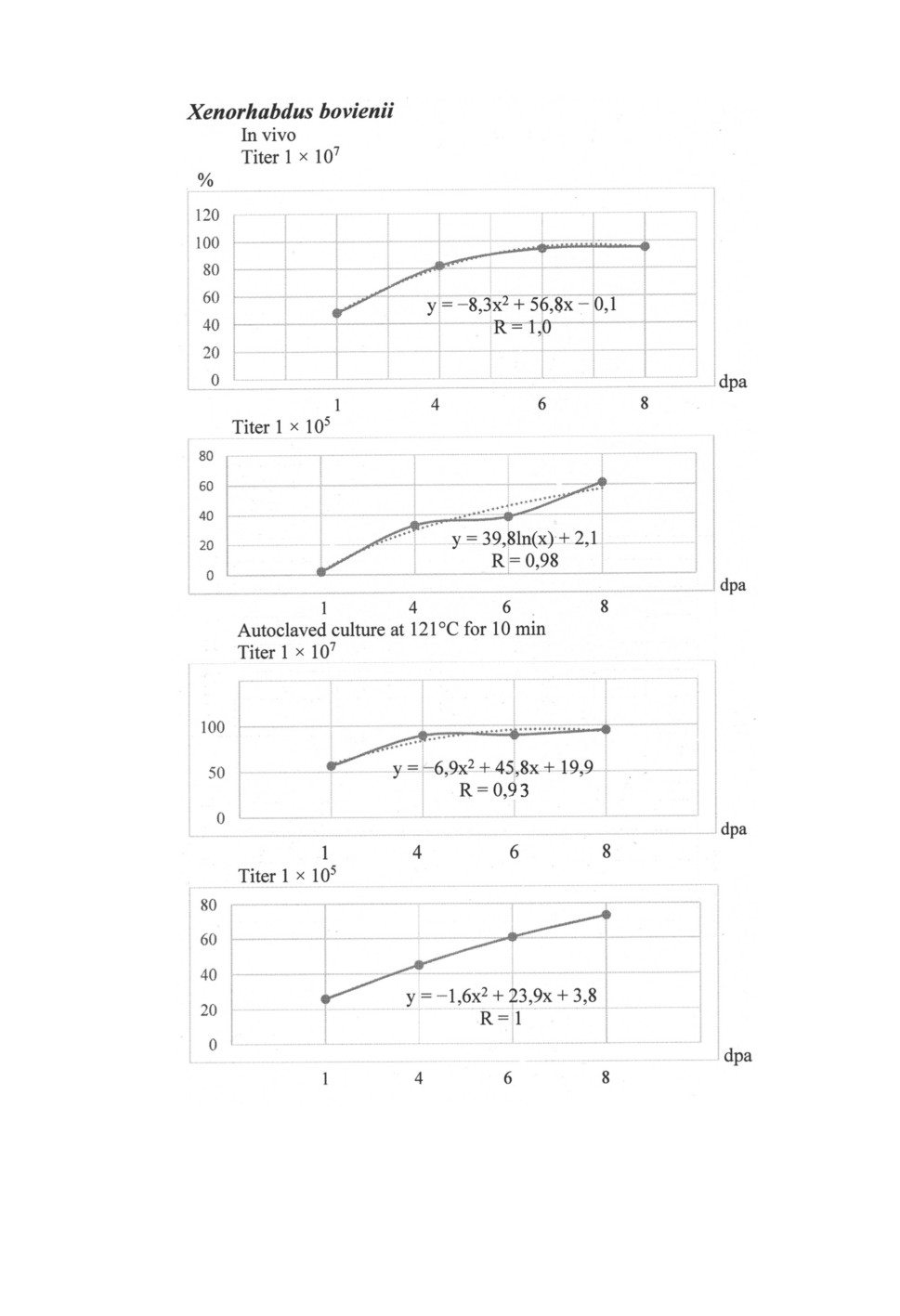
In laboratory conditions, the highest mortality rates were observed in X. bovienii bac-
teria at 6 and 8 dpa with a titer of 1 × 107 culture fluid (about 95%). The mortality
mites at 8 dpa in options with live and autoclaved culture fluid with a titer of 1 × 107 in
X. bovienii was almost the same, but in X. nematophilus it was 5.7% higher in autoclaved
culture fluid. At 8 dpa, the efficacy of the live and autoclaved metabolic products of the
bacteria Xenorhabdites bovienii and X. nematophilus against the spider mite with a titer of
1 × 105 was lower compared to the culture with a titer of 1 × 107, respectively, by 1.3-1.6
and 1.3-1.4 times. Means of mortality rate of mites after application of bacterial culture (%)
of Xenorhabdus bovienii and X. nematophila at titer 1 × 107 larger than the least significant
difference is considered a significant result (Table 1). The relationship between the mortality
of spider mites (%) and the exposure time (days) to bacterial metabolism products most
reliably reflects mainly by the polynomial dependence with the accuracy of approximation
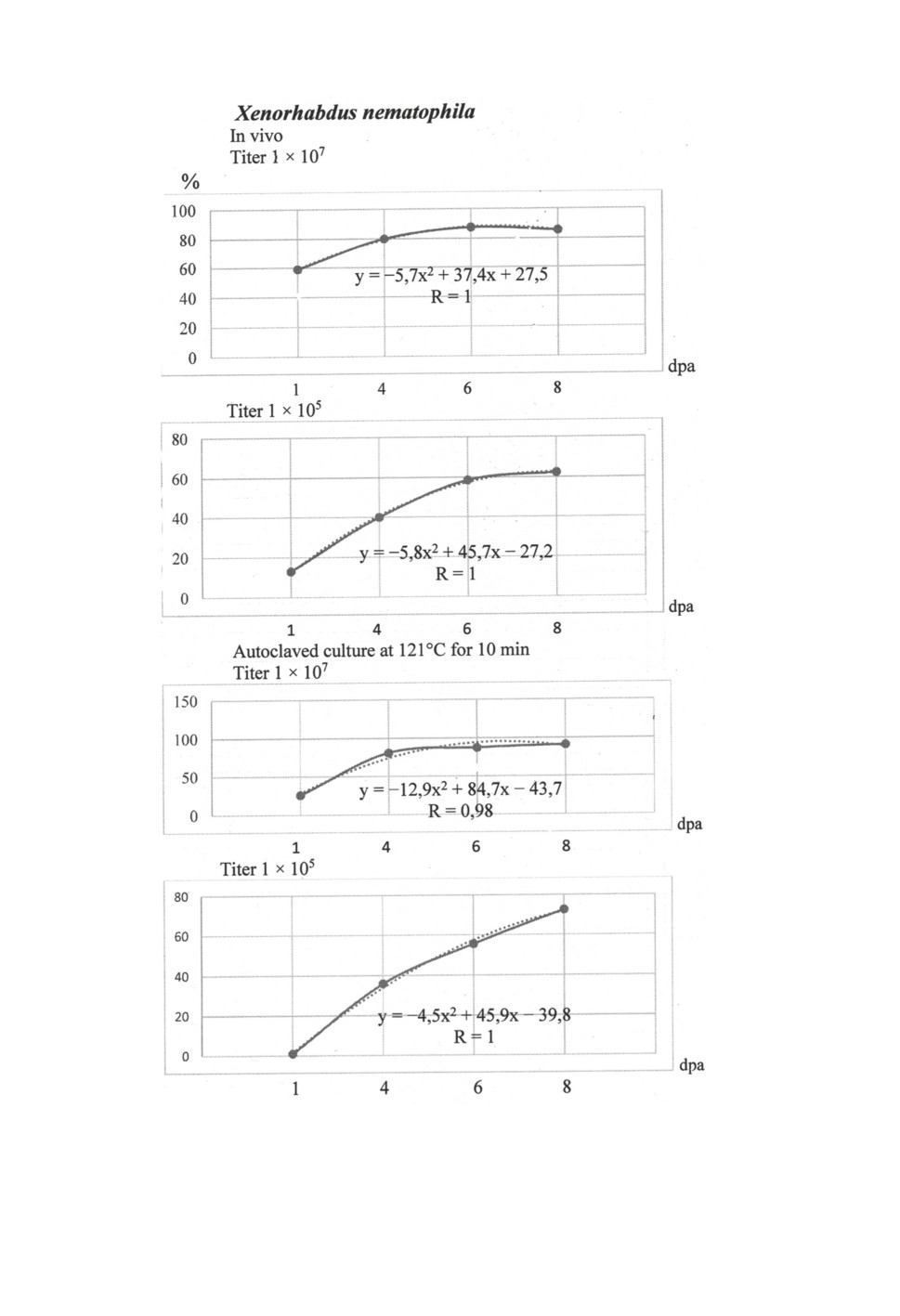
0.95-1.0 (Figs 1, 2). The maximum mortality of spider mites occurs the faster the higher
the concentration of metabolic products of live cultures X. bovienii and X. nematophila in
the preparation, in at a titer of 1 × 107 respectively on 8 and 6 dpa and autoclaved culture
on 8 dpa in both species of bacteria, and at a titer of 1 × 105 more than 8 dpa later.
In the greenhouse in the first decade of May 2020, the effectiveness of the bacterial
metabolic products of X. bovienii against spider mite was highest in experiments with live
culture with a titer of 1 × 108 (Table 2). In experiments with an autoclaved culture with
a titer of 1 × 108 at 8 dpa, it decreased by 8%, and with a titer of 1 × 107 by 1.1% com-
pared with a live culture of X. bovienii. The average mortality rates of spider mites (%)
after using live and autoclaved bacterial cultures of X. bovienii, with their titers of 1 × 107
and 1 × 108 exceed the least significant difference (LSD), which are considered a significant
70
Figure 1. Efficiency of secondary metabolic products of Xenorhabdus bovienii
on the mortality of Tetranychus urticae in laboratory conditions, %: dpa - day post application,
R - the accuracy coefficient of the approximation.
71
Figure 2. Efficiency of secondary metabolic products of Xenorhabdus nematophila
on the mortality of Tetranychus urticae in laboratory conditions, %: dpa - day post application,
R - the accuracy coefficient of the approximation.
72
result (Table 2). Similar experimental results were also obtained against overwintered fe-
males by the influence of the plant species on mite mortality at the end of March early
April 2021. The floor arrangement of plants in the greenhouse also influenced the effec-
tiveness of bacterial metabolism products against spider mites. In experiments with live
culture fluid of X. bovienii with a titer of 1 x 107, the mortality rate of spider mites on
leaves of shrub Dracaena sanderiana at 8 dpa was about 84% on the ground floor, 88%
on the first and 90% on the second floor (Table 2). In the second decade of May 2021 the
overall efficacy of the bacterial metabolic products of X. bovienii (in vivo, titer 1 × 107)
against adults, larvae and nymphs of T. urticae on the leaves of perennial marsh grasses
(Potenderia cordata, Thalia geniculata and T. dealbata) was about 98-99% (Table 3).
Table 3. Effect of metabolic products of Xenorhabdus bovienii (in vivo, titer 1 x 107)
of entomopathogenic nematode (Steinernema feltiae) on the mortality of Tetranychus urticae
in greenhouse conditions (leaves in 10-fold replication on plants in 3-fold replication)
Composition of spider
Number
Mortality rate of mites
mite populations, %
of mites before
after application
Plant
Larvae
treatment,
of bacterial culture, %
Adults
and nymphs
ind./leaf
4
6
8
Pontederia cordata
50
50
6.7 ± 0.8
62.4 ± 4.9
87.8 ± 3.4
99.1 ± 0.9
Thalia dealbata
100
0
9.2 ± 0.9
58.3 ± 5.4
80.2 ± 3.5
98.3 ± 1.1
Thalia geniculata
60
40
9.2 ± 1.2
53.2 ± 5.0
73.8 ± 6.0
98.0 ± 1.4
Average
70
30
8.4 ± 1.0
58.0 ± 5.1
80.6 ± 4.3
98.5 ± 1.1
Notes. Peter the Great Botanical Garden, St. Petersburg, date of the experiment
12.05-20.05.2021, air temperature 28-32°C, relative humidity 85-90%. 4, 6, 8 - day post application.
DISCUSSION
In the world practice, symbiotic bacterial-parasitic complexes of invasive larvae of the
genus Steinernema and bacteria of the genus Xenorhabdus are widely used in the biologi-
cal protection of agricultural crops mainly from soil insect pests and root-knot nematodes
of the genus Meloidogyne in greenhouses and in the field (Lee, 2009; Lewis et al., 2001;
Perez, Lewis, 2002; Lacey, Georgis, 2012 and others). Two biological preparations (Ento-
nem and Nemabact) based on entomopathogenic nematodes and their symbiotic bacteria
against insect pests were obtained and found practical application in Russia. They were
included in the State Catalog of Pesticides and Agrochemicals Approved for Use on the
territory of the Russian Federation (Kaplin, 2012).
Bacteria of the genus Xenorhabdus, when cultivated, form a large number of second-
ary mainly protein toxic metabolites against other microorganisms, soil nematodes, insects
and spider mite Tetranychus urticae. Currently, live cultures are obtained in laboratories,
as well as autoclaving culture at 121°C for 10 min and cell-free supernatants obtained by
centrifuging cultures at 4°C for 15 min. They are widely tested in many countries in labo-
ratories in Petri dishes and pots against pests and plant diseases. We have investigated the
73
effectiveness of exposure to secondary metabolites of live and autoclaved bacterial cultures
of the genus Xenorhabdus in laboratory conditions and for the first time in greenhouses
against T. urticae by spraying these cultures of experimental mite-infested plants.
CONCLUSION
In our laboratory investigations, the highest mortality rates of spider mites were ob-
served in in experiments with X. bovienii at 8 dpa with a titer of 1 × 107 culture fluid
(about 95%). The mortality mites at 8 dpa in experiments with live and autoclaved culture
with a titer of 1 × 107 in X. bovienii was almost the same, but in X. nematophila it was
slightly higher in vitro. After 8 dpa, the efficacy of the live and autoclaved metabolic
products of Xenorhabdites bovienii and X. nematophilus against the spider mite with a
titer of 1 × 105 was about 1.4 times lower compared to the culture with a titer of 1 × 107.
In the greenhouses, the effectiveness of the bacterial metabolic products of X. bovienii
against spider mite was highest in experiments with live culture with a titer of 1 × 108.
The efficiency of an autoclaved culture with a titer of 108 was 8%, and with a titer of
107 about 1% lower than in experiments with live culture. This was probably due to the
negative effect of culture autoclaving at 121°C on the stability of some peptide secondary
metabolites of the bacterium Xenorhabdus bovienii. In experiments with live culture of
X. bovienii with a titer of 1 x 107 (in vivo) the mortality rate of spider mites on leaves of
shrub Dracaena sanderiana at 8 dpa increased from 84% on the ground floor to 88% on
the first floor and to 90% on the second floor. The overall efficacy of the bacterial meta-
bolic products of X. bovienii (in vivo, titer 1 × 107) against adults, larvae and nymphs of
T. urticae on the leaves of perennial marsh grasses (Potenderia cordata, Thalia geniculata
and T. dealbata) was about 98-99%.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
ACKNOWLEDGEMENTS
The authors are much obliged to reviewer for valuable comments and corrections of
article.
REFERENCES
Abbott W.S. 1925. A method of computing the effectiveness of an insecticide. Journal of Economic Entomology
18: 265-267.
Abebew D., Sayedain F.S., Edna Bode E., Bode H.B. 2022. Uncovering Nematicidal Natural Products from
Xenorhabdus Bacteria. Journal of Agricultural and Food Chemistry 70: 498-506.
Akhurst R.J. 1980. Morphological and functional dimorphism in Xenorhabdus spp. bacteria symbiotically as-
sociated with the insect pathogenic nematodes Neoaplectana and Heterorhabditis. Journal of General
Cranham J.E., Helle W. 1985. Pesticide resistance in Tetranychidae. In: Helle W., Sabelis M.W., (eds). Spider
Mites: Their Biology, Natural Enemies and Control 1 (B), Elsevier, Amsterdam-New York, 405-421.
74
Dermauw W., Wybouw N., Rombauts S., Menten B., Vontas J., Grbić M., Clark R.M., René Feyereisen R.,
Leeuwen T.V. 2013. A link between host plant adaptation and pesticide resistance in the polyphagous
spider mite Tetranychus urticae. Proceedings of the National Academy of Sciences 110 (2): 113-122.
https://doi.org/10.1073/pnas.1213214110
Dreyer J., Malan A.P., Dicks L.M.T. 2018. Bacteria of the Genus Xenorhabdus, a Novel Source of Bioactive
Eroglu C., Cimen H., Ulug D., Karagoz M., Hazir S., Cakmak I. 2019. Acaricidal effect of cell-free supernatants
from Xenorhabdus and Photorhabdus bacteria against Tetranychus urticae (Acari: Tetranychidae). Journal
Fleming R., Retnakaran A. 1985. Evaluating Single Treatment Data Using Abbot’s Formula With Ref-
erence to Insecticides. Journal of Economic Entomology 78: 1179-1181. Available online at:
Fodor A., Fodor A.M., Forst S., Hogan J.S., Klein M.G., Lengyel K., Sáringer G., Stackebrandt E., Taylor
R.A.J., Lehoczky É. 2010. Comparative analysis of antibacterial activities of Xenorhabdus species
on related and non-related bacteria in vivo. Journal of Microbiology and Antimicrobials 2 (4): 36-46.
Hazir S., Shapiro-Ilan D.I., Bock C.H., Hazir C., Leite L.G., Hotchkiss M.W. 2016. Relative potency of culture
supernatants of Xenorhabdus and Photorhabdus spp. on growth of some fungal phytopathogens. European
Henderson C.F., Tilton E.W. 1955. Tests with acaricides against the brow wheat mite. Journal of Economic
Entomology 48: 157-161.
Incedayi G., Cimen H., Ulug D., Touray M., Bode E., Bode H.B., Yaylagül E.Ö., Hazir S., Cakmak
I. 2021. Relative potency of a novel acaricidal compound from Xenorhabdus, a bacterial ge-
nus mutualistically associated with entomopathogenic nematodes. Scientific Reports 11, 11253.
Inman F.L., Holmes L. 2012. Effect of heat sterilization on the bioactivity of antibacterial metabolites se-
creted by Xenorhabdus nematophila. Pakistan Journal of Biological Sciences 15 (20): 997-1000.
Kaplin V.G. 2012. Applied Nematology. Samara, 384 pp. [In Russian].
Lacey L.A., Georgis R. 2012. Entomopathogenic Nematodes for Control of Insect Pests Above and Below Ground
with Comments on Commercial Production. Journal of Nematology 44 (2): 218-225.
Lee Ming-Min 2009. A phylogenetic hypothesis on the evolution and interactions on Xenorhabdus spp.
(γ-Proteobacteria) and their Steinernema hosts (Nematoda: Steinermatidae). Master’s Theses. USA: The
Lewis E.E., Grewal P.S., Sardanelli S. 2001. Interactions between the Steinernema feltiae-Xenorhabdus bovienii
Insect Pathogen Complex and the Root-Knot Nematode Meloidogyne incognita. Biological Control 21:
Migeon A., Dorkeld F. 2019. Spider Mites Web: a comprehensive database for the Tetranychidae. Tetrahychus
Perez E.E., Lewis E.E. 2002. Use of entomopathogenic nematodes to suppress Meloidogyne incognita on green-
house tomatoes. Journal of Nematology 34: 171-174.
Riahi Е., Shishehbor P., Nemati A.R., Saeidi Z. 2013. Temperature Effects on Development and Life Table Pa-
rameters of Tetranychus urticae (Acari: Tetranychidae). Journal of Agricultural Science and Technology
15: 661-672.
Sukhoruchenko G.I., Ivanova G.P. 2013. Common spider mite. In: Monitoring of pesticide resistance in popula-
tions of harmful arthropods. Methodological recommendations. St. Petersburg, VISR: 14-16 [In Russian].
Yüksel E. 2022. Biocontrol potential of endosymbiotic bacteria of entomopathogenic nematodes against the to-
mato leaf miner, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Egyptian Journal of Biological Pest
75
Yüksel E., Imren M., Ozdemir E., Bozbuğa R., Ramazan Canhilal R. 2022. Insecticidal effect of entomopatho-
genic nematodes and the cell-free supernatants from their symbiotic bacteria against different larval instars
of Agrotis segetum (Denis & Schiffermüller) (Lepidoptera: Noctuidae). Egyptian Journal of Biological Pest
Zhang Y., Fang Wang F., Zhao Z. 2022. Metabonomics reveals that entomopathogenic nematodes mediate
fmicb.2022.1042145
АКАРИЦИДНОЕ ВЛИЯНИЕ ВТОРИЧНЫХ МЕТАБОЛИТОВ
СИМБИОТИЧЕСКИХ БАКТЕРИЙ XENORHABDUS BOVIENII И X. NEMATOPHILA
ЭНТОМОПАТОГЕННЫХ НЕМАТОД НА ПАУТИННОГО КЛЕЩА
TETRANYCHUS URTICAE (TROMBIDIFORMES, TETRANYCHIDAE)
Л. Г. Данилов, Г. П. Иванова, В. Г. Каплин, Е. А. Варфоломеева
Ключевые слова: Steinernema, живая бактериальная культура, автоклавирован-
ная культура, лабораторные условия, теплицы, токсичные вторичные метаболиты,
эффективность
РЕЗЮМЕ
В лабораторных условиях самые высокие показатели смертности Tetranychus urticae после
применения продуктов метаболизма симбиотических бактерий с титром 1 × 107 наблюдались
у Xenorhabdus bovienii на 6-8-й день в опыте с живой и на 8-й день с автоклавированной
культуральной жидкостью (около 95%). В экспериментах с живой и автоклавированной куль-
турой с титром 1 × 107 смертность клещей на 8-й день после применения у X. bovienii была
почти одинаковой, но у X. nematophila она была немного выше в опыте с автоклавированной
культурой. Через 8 дней после применения продукты метаболизма живых и автоклавированных
бактерий X. bovienii и X. nematophila против паутинного клеща с титром 1 × 105 были примерно
в 1.4 раза менее эффективны, чем аналогичные продукты с титром 1 × 107. Взаимосвязь между
смертностью паутинных клещей (%) и временем воздействия (дни) продуктов бактериального
метаболизма наиболее достоверно отражает полиномиальная зависимость с точностью прибли-
жения 0.93-1.0. В теплицах эффективность продуктов бактериального метаболизма X. bovienii
против паутинного клеща была самой высокой в экспериментах с живой культурой с титром
1 × 108. В экспериментах с живой культурой X. bovienii с титром 1 × 107 (in vivo) уровень
смертности паутинных клещей на листьях кустарника Dracaena sanderiana на 8-й день после
применения увеличился с 84% на первом этаже до 90% на третьем этаже. Общая эффектив-
ность продуктов бактериального метаболизма X. bovienii (in vivo, титр 1 × 107) против взрослых
особей, личинок и нимф T. urticae на листьях многолетних болотных трав (Potenderia cordata,
Thalia geniculata и T. dealbata) составила около 98%, X. nematophila - 99%.
76