ПАРАЗИТОЛОГИЯ, 2023, том 57, № 6, с. 498-503.
УДК [595.122-155.7:574.523](262.5)
HELICOMETRA FASCIATA (RUDOLPHI, 1819) COMPLEX
FROM NEW FISH HOST IN THE BLACK SEA,
THE BROADNOSED PIPEFISH
SYNGNATHUS TYPHLE LINNAEUS, 1758,
WITH NOTES ON BIOLOGY OF THIS TREMATODE SPECIES
© 2023 Yu. M. Kornyychuk*
The A.O. Kovalevsky Institute of Biology of the Southern Seas,
Russian Academy of Sciences, 2 Nakhimov ave., Sevastopol, 299011 Russia
*e-mail: miju2811@mail.ru
Received September 09, 2023
Revised November 04,2023
Accepted November 09,2023
This paper reports on the first record of Helicometra fasciata (Rudolphi, 1819) complex (Opecoe-
lidae) maritae from black-striped pipefish, Syngnathus typhle Linnaeus, 1758, in the Black Sea. Ten
fish specimens caught near the Kerch Strait (north-eastern part of the Black Sea) in July 2007 were
examined and one of them was found to be parasitized by a single H. fasciata complex ovigerous
marita. Description and drawing of the trematode found are given. S. typhle and fish of the Syn-
gnathidae family as a whole are believed to be accidental definitive hosts of H. fasciata complex.
Cases of Helicometra spp. ovigerous maritae records in fresh waters are discussed. The expansion
of H. fasciata complex definitive host range in the Black Sea (S. typhle is its 33d known fish host
from here) reflects a complexity of food webs in the shelf zone of this sea.
Keywords: first record, Trematode, Opecoelidae, Syngnathidae, Black Sea, food webs
DOI: 10.31857/S0031184723060042; EDN: RWJCIU
The Black Sea Syngnathidae are known as hosts of many helminth species with Trema-
toda as the most abundant class among them (Kornyychuk et al., 2022). Nevertheless,
studying the slides deposited in the Collection of Marine Parasites of the A.O. Kova-
we identified one more digenean which have not been previously mentioned from these fish
hosts, namely Helicometra fasciata (Rudolphi, 1819) complex. According to Katokhin and
Kornyychuk (2020) and Sokolov et al. (2022), H. fasciata is actually a species complex
consisting of at least two species in the Black Sea with a clear genetic differentiation. The
present paper is devoted to morphological description of this find.
MATERIAL AND METHODS
The slide No 1404.Tr.39.v43 deposited in IBSS Collection of Marine Parasites was studied;
according to our field journal entry, host specimens (broadnosed pipefish Syngnathus typhle Lin-
naeus, 1758) were sampled in the Black Sea (Kerch Strait near Naberezhnoe village, 45°08′20″N,
498
36°25′00″E) in July 2007 by hand nets at depths 0-1 m; identification of fishes to species was made
using conventional keys (Svetovidov, 1964; Vasil’eva, 2007).
Fish hosts were dissection immediately after catching. For light microscopy, the digenean was
stored in 70% ethanol and the whole mount was prepared as follows: stained with borax carmine,
dehydrated in a graded ethanol series (70-100) and mounted in Canada balm on a glass slide
(Bykhovskaya-Pavlovskaya, 1985).
Trematoda species identification was taken using an Olympus CX-41 microscope with digital
camera CAM-SC50 and СellSens Standard v. 1.18 software; all the measurements in the text are in
micrometers.
RESULTS
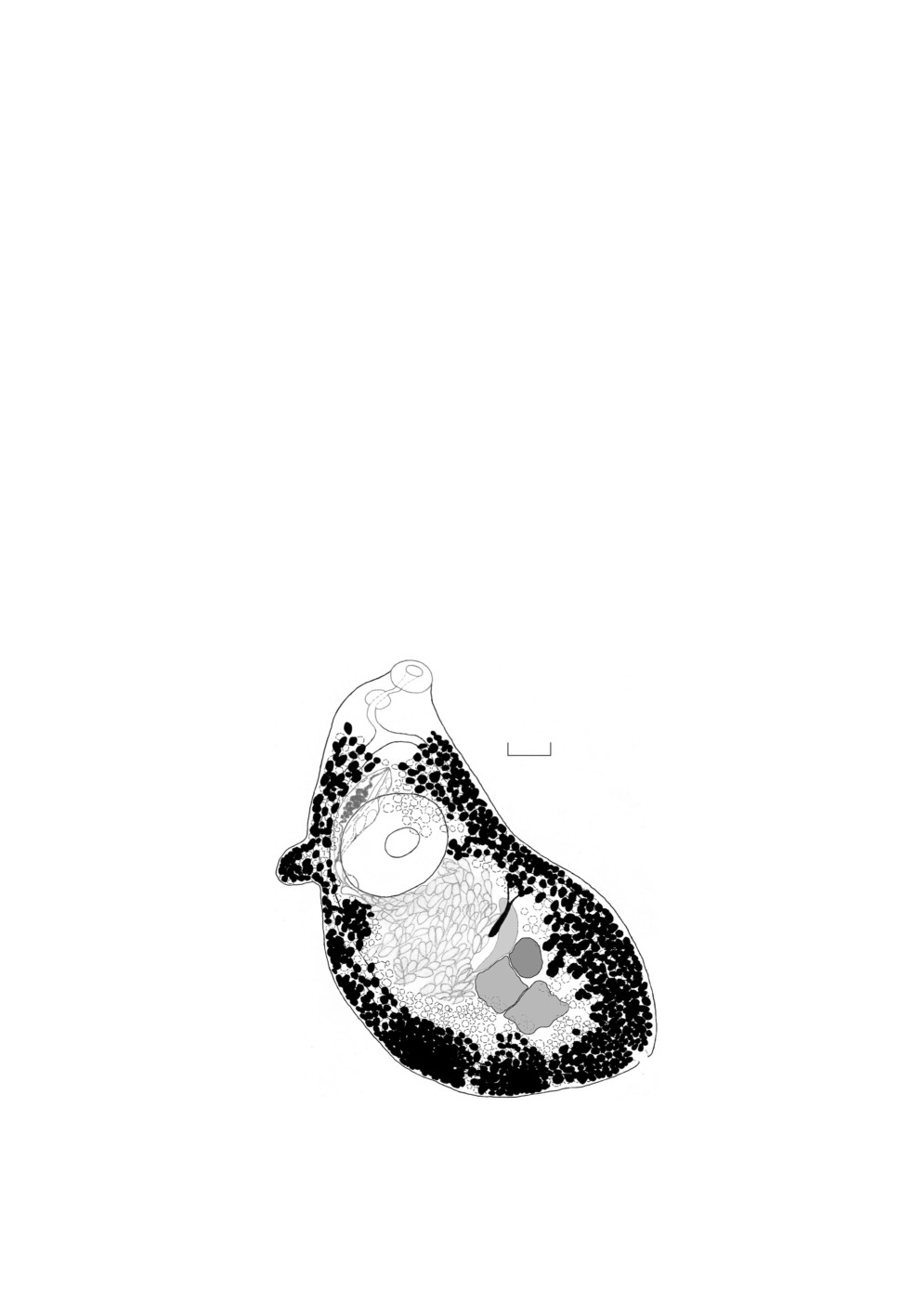
The only gravid marita of H. fasciata species complex (Fig. 1) is fixed on the slide in
a slightly curved position.
Body oval, 1234.4 х 598.7; body W:L ratio 2.06.
Tegument unarmed.
Oral sucker subterminal, 103.8 х 123.5. Ventral sucker 210.1 х 239.9. Sucker length
ratio 1:2.02 and sucker width ratio 1:1.94.
Pharynx 53.3 х 68.2, prepharynx present, esophagus short.
Caeca blind, extend to posterior end of the body.
Forebody: 306.9 (25% of body length).
Testes tandem, contiguous, slightly indented. Anterior testis 108.9 x 132, posterior testis
132 x 158.4.
Posttesticular space: 238.1 (19% of body length).
Cirrus sac 188.3 x 51.4, its posteriormost extent slightly posterior to midlevel of ventral
sucker, well developed, enclosing a seminal vesicle.
Figure 1. Helicometra fasciata (Rud., 1819) complex (Trematoda, Opecoelidae)
marita from the Black Sea broadnosed pipefish Syngnathus typhle Linnaeus, 1758,
ventral view. Scale bar - 100 µ.
499
Ovary 95.7 x 66, sinistro-submedian, at level of anterior testis, oval.
Genital pore median, between caeca bifurcation and anterior edge of ventral sucker.
Anterior extremity to genital pore distance 267.3 (21.7% of body length).
Vitellaria follicular. Follicles of varying sizes, in lateral fields, dorsal, ventral and lat-
eral to caeca, extending from level of caeca bifurcation to caeca ends, confluent dorsally
in forebody and ventrally and dorsally in posttesticular space. The specimen studied has
unusual vitellaria follicles grouped randomly into different-size globules.
Common vitelline reservoir slightly submedian, anterior to ovary.
Uterus coiled, preovarian. Metraterm along left edge of cirrus sac.
Eggs (n = 15) 53.45-59.67 (56.15) х 22.9-31.06 (25.82), with long unipolar filament.
The marita studied was found in the gut of one of eleven broadnosed pipefish specimens
cought off Kerch Strait region (prevalence 10%, abundance 0.1). Taking into account total
number of previously studied S. typhle from another parts of the Crimean Black Sea shelf
(n = 107), the prevalence is 0.9%, abundance 0.009.
DISCUSSION
The trematode specimen we found matches morphologically with H. fasciata complex
description, namely: a ventral sucker in the anterior part of the body, two tandem slightly
indented testes, filamented eggs, a median genital pore near the caeca bifurcation, vitelline
follicles in the lateral fields reaching far in the forebody (Blend, Dronen, 2014; Sokolov
et al., 2022).
The body length of H. fasciata complex marita from broadnosed pipefish is in the
previously established (Korniychuk, 2009a) limits of mature Black Sea representatives of
this species complex (414-3795). Eggs sizes are in the frames (52-73 x 24-41) known
for the Black Sea H. fasciata complex (Korniychuk, 2009a), too, and in the middle of ap-
propriate limits (38-100 × 16-42) mentioned for H. fasciata complex by Blend, Dronen
(2014). Strongly submedian position of ovary (“triangle” arrangement of gonads) sinistrally
to anterior testis has also been previously identified in H. fasciata complex from Black Sea
fishes, both in live and fixed worms (Korniychuk, 2009a).
There are only two cases of Helicometra spp. maritae records from Syngnathidae fish
hosts have been known before (Blend, Dronen, 2014).
The first of them referrers to Nora Sproston’s (1938) finding of “some” Helicometra
sinuata (Rudolphi, 1819) (=H. fasciata s. lato) specimens from the only male of a short
snouted seahorse, Hippocampus hippocampus (Linnaeus, 1758) (=Hippocampus antiquorum
Leach, 1814) she studied, the fish was caught near French coast of the English Channel.
To our knowledge, it was also a first report of opecoelid marita maritae from Syngnathidae
fish hosts. One more finding of Helicometra Odhner, 1902 maritae from Syngnathidae was
made by Alexandra Chaplina and Ljudmila Antsishkina (1961) who studied fish parasite
fauna in small rivers (Berda, Obitochnaja, Lozovatka, Korsak, Big and Small Utljuk, Tash-
enak) flowing to the north part of the Sea of Azov and registered Helicometra pulchella
(Rudolphi, 1819) (=H. fasciata s. lato) from the black-striped pipefish, Syngnathus abaster,
without noting the exact locality of this find. The digeneans found were not described by
the authors: there were no figures, measurements of the parasites as well as a remark on
their maturity. The limits of infection cannot be obtained from the table in the text - very
many”, as the authors stated; the prevalence at 50% seems exceptionally high comparing
with data on another fish hosts in the region (Kornjychuk, 2017) but the authors didn’t
specify total number of the pipefish studied.
500
So, we believe Syngnathidae syngnathid fishes to be accidental definitive hosts of
H. fasciata complex regardless of the true species affiliation of representatives of this
digenean species complex.
The digeneans belonging to Helicometra are known mostly from fish hosts inhabiting
marine and brackish waters (Blend, Dronen, 2014); there are also two records of Helicom-
etra spp. in fresh waters, they are the above mentioned find of Chaplina and Antsishkina
(1961) and a find of two ovigerous maritae in Kahovsky water reservoir on Dnieper river,
from two fish hosts: the monkey goby, Neogobius fluviatilis (Gobiidae), and the pike perch,
Sander lucioperca (Linnaeus, 1758) (=Lucioperca lucioperca (Linnaeus, 1758) (Koval’
et al., 1975) and worth noting: judging by the drawing, the last find referrers to H. fasciata
s. lato. These two cases raise a question of possible ways of fish host infection.
The trochid gastropods Steromphala adriatica (R. A. Philippi, 1844) (=Gibbula adriatica
(Philippi, 1844) acting as H. fasciata complex first intermediate hosts in the Black Sea
(Machkevsky et al., 1997) are numerous along coasts of this sea at a depth of up to 50 m,
on rocks and algae (Chukhchin, 1984). Concerning these mollusks in the Sea of Azov,
Steromphala (=Gibbula) spp. are known there from the only place - near the Biryuchiy
Island spit (north-western part of the sea) but are able to spread to the east along the
northern coast of the Sea of Azov beyond the Belosaraiskaya Spit (bounding the Taganrog
Bay from the north) (Anistratenko et al., 2011). The rivers studied by Chaplina and Ant-
sishkina (1961) flows into the Sea of Azov just in this region. Nevertheless, Helicometra
parthenogenetic generations have not been found yet as from S. adriatica inhabiting the
Sea of Azov as from any other mollusks in the rivers flowing into the northern part of the
Sea of Azov (Kudlay, 2011).
Metacercariae of H. fasciata complex were recorded from prawns Palaemon elegans
Rathke, 1836 and Palaemon adspersus Rathke, 1836 inhabiting Crimean part of the Black
Sea shelf zone (Mordvinova, 1979; Machkevsky et al., 1997; Korniychuk, 2008, 2009b;
Tkachuk, Mordvinova, 1999) and from the same hosts in the Sea of Azov (Mordvinova,
1979).
Palaemon spp. prawn are the most common second intermediate hosts of Helicometra
in different regions of the World Ocean; other then Palaemonidae hosts known to harbor
Helicometra spp. metacercariae are prawns belonging to genuses Hippolyte Leach, 1814
(Hippolytidae), Alpheus Fabricius, 1798 (Alpheidae), Crangon Fabricius, 1798 (Crangoni-
dae) and Gammarus Fabricius, 1775 (Gammaridae) (Blend, Dronen, 2014). Of them Cran-
gon spp. and Gammarus spp. are able to live in fresh waters but crustaceans from these
genera are not recorded as hosts of H. fasciata complex metacercariae (Mordvinova, 1979).
The black-striped pipefish, S. abaster, inhabit relatively shallow waters around seaweed
and sea grass in the Black Seas and are also known from brackish waters and can enter the
rivers; they feed on small crustaceans, fish fry, and sometimes small adult fish (Svetovidov,
1964). So, in terms of routes of infection, these pipefish are able to ingest Palaemon spp.
infected with H. fasciata complex metacercariae. Nevertheless, taking into account the
above-mentioned data on S. adriatica areal in the Sea of Azov, we believe registration of
H. fasciata complex trematode in fresh waters of North Azov region (Chaplina, Antsishkina,
1961) to be accidental and assuming infection of fish hosts, S. abaster, somewhere in the
Sea of Azov and subsequent pipefish’s migrations into the rivers. The same way we believe
possible to explain the appropriate find by Koval’ et al. (1975).
CONCLUSIONS
The previously summarized data on H. fasciata complex definitive host range in the
Black Sea (Kornjychuk, 2017) emphasized extremely wide host specificity of this trema-
501
todes at marita stage in the Black Sea (32 fish host species but excepting Syngnathidae)
and on different importance of fish hosts. We also dissected more than 100 S. typhle
specimens caught in the Crimean part of the Black Sea shelf (Kornyychuk et al., 2022)
but did not find H. fasciata complex - as well as other researchers. So, the role of these
fish in maintaining Helicometra maritae hemipopulations we believe to be insignificant.
Nevertheless, the expansion of H. fasciata complex definitive hosts range reflects grows
of our understanding of food chains complexity in the Black Sea shelf zone.
FUNDING
This study was funded and conducted in the frames of the A.O. Kovalevsky Insti-
tute of Biology of the Southern Seas of Russian Academy of Sciences state assignment
(№ 121030100028-0). No additional grants to carry out or direct this particular research
were obtained.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This work does not contain any studies involving human and animal subjects.
CONFLICT OF INTEREST
The author of this work declares that he has no conflicts of interest.
REFERENCES
Anistratenko V.V., Khaliman I.A., Anistratenko O.Yu. 2011. Mollusks of the Sea of Azov. Kiev, Naukova Dumka,
707 pp. [in Russian].
Blend C.K., Dronen N.O. 2014. A review of the genus Helicometra Odhner, 1902 (Digenea: Opecoelidae: Pla-
gioporinae) with a key to species including Helicometra overstreeti n. sp. from the cusk-eel Luciobrotula
corethromycter Cohn, 1964 (Ophidiiformes: Ophidiidae) from the Gulf of Mexico. Marine Biodiversity
45(2): 183-270. DOI: 10.1007/s12526-014-0250-3
Bykhovskaya-Pavlovskaya I.E. 1985. Parasitological investigation of fish. Leningrad, Nauka, 121 pp. [in Russian].
DOI: 10.21072/bykhovskaya-pavlovskaya-1985
Chaplina O.M., Antsishkina L.M. 1961. Data on the fish parasites in small rivers of the northern Azov area.
Proceedings of Ukrainian Academy of Sciences 2: 247-250 [in Ukrainian].
Chukhchin V.D. 1984. Ecology of gastropods in the Black Sea. Kiev, Naukova dumka, 176 pp. [in Russian].
Katokhin A.V., Kornyychuk Yu.M. 2020. Genotyping of Black Sea trematodes of the family Opecoelidae by
mitochondrial markers. Marine Biological Journal 5 (4): 15-27 [in Russian, with English Summary]. ht-
tps://doi.org/10.21072/mbj.2020.05.4.02
Korniychuk Y.M. 2008. Seasonal dynamics of abundance and qualitative composition of trematode, Helicometra
fasciata, metacercaria hemipopulation in coastal biocenosys of Southwestern Crimea. Ecology of the sea
75: 9-15 [In Russian, with English Summary].
Korniychuk Yu.M. 2009а. Additional description of hermaphroditic generation of trematodes Black Sea Fishes,
Helicometra fasciata (Trematoda, Opecoelidae). Vestnik zoologii, spec. iss. 23: 63-68 [in Russian, with
English Summary].
Korniychuk Yu.M. 2009b. Fauna of shrimp parasites in the Black and Azov seas. Ecology of the sea 77: 44-48
[in Russian, with English Summary].
Kornjychuk Yu.M. 2017. Polyhostal helminthes: how to make a quantitative estimation of their final host signifi-
cance. Journal of General Biology 78 (6): 3-15 [in Russian, with English Summary].
Kornyychuk Y., Polyakova T., Pronkina N. 2022. New data on pipefishes’ and seahorse’s endohelminths off
Koval’ V.P., Vagushenko A.M., Seregina L.J., Pashkevichute A.C. 1975. Parasite fauna of fishes of the Kahovsky
Reservoir (in its upper part) on the fourteenth year of its existence. Bulleting of Kiev State University,
Biological Series 17: 105-108 [in Russian].
Kudlay O.S. 2011. Trematode fauna of Gastropoda from water bodies in region adjacent to the northern Azov
Sea. PhD thesis. Schmalhausen Institute of Zoology of National Academy of Sciences of Ukraine, Kyiv.
Machkevsky V.K., Pronkina N.V., Gaevskaia A.V., Korniychuk J.M. 1997. Life cycle of Helicometra fasciata
(Rud., 1819) (Trematoda: Opecoelidae) in the rock biocenosis from the Black Sea. Ecology of the sea
(Kiev) 46: 58-62 [in Russian, with English Summary].
502
Mordvinova T.N. 1979. Higher Crustacea as intermediate hosts of fish helminths. VII All-Union meeting on
parasites and diseases of fish, Leningrad, Nauka, 74-75 [in Russian].
Sokolov S.G., Shchenkov S.V., Khasanov F.K., Kornyychuk Y.M., Gordeev I.I. 2022. Redescription and phy-
logenetic assessment of Helicometra antarcticae Holloway & Bier, 1968 (Trematoda, Opecoelidae), with
evidence of nonmonophyletic status of the genus Helicometra Odhner, 1902. Zoosystema 44 (15): 423-433.
Sproston N.G. 1938. Notes sur la faune parasitaire des poissons à Roscoff. Proceedings of Roscoff Biological
Station 16: 33-58 [in French].
Svetovidov A.N. 1964. Fish of the Black Sea. Moscow-Leningrad, Nauka, 552 pp. [in Russian].
10.21072/Black_Sea_Fish
Tkachuk L.P., Mordvinova T.N. 1999. On parasitic infection of the shrimp, Palaemon elegans from two coastal
regions of the Black Sea. Ecology of the sea (Kiev) 49: 21-23 [in Russian, with English Summary].
Vasil’eva E.D. 2007. Fish of the Black Sea. Key to marine, brackish-water, euryhaline, and anadromous species
with color illustrations collected by S.V. Bogorodsky. VNIRO Publishing, 222 pp. [in Russian].
HELICOMETRA FASCIATA (RUDOLPHI, 1819) COMPLEX ИЗ НОВОГО ХОЗЯИНА
В ЧЁРНОМ МОРЕ, ДЛИННОРЫЛОЙ ИГЛЫ-РЫБЫ SYNGNATHUS TYPHLE
LINNAEUS, 1758, С ЗАМЕТКАМИ О БИОЛОГИИ ЭТОЙ ТРЕМАТОДЫ
Ю. М. Корнийчук
Ключевые слова: новый дефинитивный хозяин, Trematode, Opecoelidae, Syngnathi-
dae, Чёрное море, пищевые сети
РЕЗЮМЕ
Сообщается о первой находке мариты комплекса Helicometra fasciata (Rudolphi, 1819)
(Opecoelidae) у обитающей в Чёрном море длиннорылой иглы-рыбы (иглы-трубкорота)
Syngnathus typhle Linnaeus, 1758: единственный зрелый (с яйцами) экземпляр этой трематоды
был найден у одного из 10 экземпляров иглы-трубкорота, выловленных в северо-восточной
части Чёрного моря (район Керченского пролива) в июле 2007 г. Приведены описание и рисунок
этого паразита. Syngnathus typhle и рыб семейства Syngnathidae в целом мы относим к числу
случайных дефинитивных хозяев комплекса H. fasciata. Обсуждаются находки зрелых марит
H. fasciata complex у пресноводных рыб-хозяев в пресных водоемах. Полученные данные
о расширении круга известных дефинитивных хозяев H. fasciata complex в Чёрном море
(S. typhle - 33-й известный здесь её окончательный хозяин) отражают сложность пищевых
сетей в его шельфовой зоне.
503