Pis’ma v ZhETF, vol. 109, iss. 10, pp. 677 - 678
© 2019
May 25
Comparative study on interatomic force constants and elastic
properties of zinc-blende AlN, AlP and AlAs
H. Wang1), Q. Tan, X. Zeng
Department of Physics and Electronic Information Engineering, Xiangnan University,
423000 Chenzhou, The People’s Republic of China
Submitted 25 February 2019
Resubmitted 25 February 2019
Accepted 21
March 2019
DOI: 10.1134/S0370274X19100060
Almost all epitaxial AlN, AlP and AlAs films are
Oppenheimer (BO) total energy surface of the system
expected to contain some residual strain. A knowl-
(electrons plus clamped ions) where Cion and Celec are
edge of their elastic constants and strain deforma-
the ionic contribution and electronic contribution to the
tion potentials is indispensable. Although there are
force constants, respectively for other variables, see [8].
many theoretical [1-3] and experimental [4, 5] results,
For this reason, IFC offer a convenient way of storing
there is a lack of systematic research on their elastic
the information contained in the dynamical matrix at
constants and behaviors under hydrostatic and uniax-
any q into a few independent parameters. Therefore, it
ial stress. The purpose of this study is to study the
is necessary to fully describe the ion motion in DFPT.
above properties of these compounds by pseudopotential
Fourier analysis is used to calculate and tabulate a set of
method, and the linear response by density functional
force constant matrices on uniform meshes in reciprocal
theory (DFT) and density functional perturbation the-
space. Real space force constants can be easily obtained:
ory (DFPT). The norm-conserving non-local Troulliers-
1∑
Martins pseudopotentials [6] is employed. The Kohn-
Ckα,k′β(R) =
eiqR
Ckα,k′β(q),
(2)
N
Sham orbits are expanded in plane waves basis set. The
q
Troullier and Martins programs generate soft core pseu-
Here N is the number of unit cells in the crystal. Af-
ter calculating the real space constants by this method,
dopotential. The theoretical calculation is performed by
using the local density approximation of the exchange-
the reciprocal-space dynamic matrix can be obtained
by inverse Fourier transform at any q point of Brillouin
correlation Hamiltonian as implemented in abinit pack-
age [7].
region.
In this calculation, Al
(3s23p1), N
(2s22p3),
The dynamical matrices have been calculated on a
P (3s23p3), and As (4s24p3) shells are used as valence
(8 × 8 × 8) reciprocal space face centered cubic (FCC)
band elections. The ground state equilibrium volumes
grid. Fourier deconvolution on its mesh yields real-space
of zinc-blende AlN, AlP and AlAs are determined
interatomic force constants up to the ninth neighbor
by calculating the total energy of each primitive unit
shell. This process is equivalent to calculate real space
force constant using the FCC supercell, which linear size
cell as a function of V. The calculated energy volume
data are fitted with Monahan’s equation of state. The
is four times that of the primitive zinc-blende cell, so it
contains 128 atoms.
obtained structural parameters are
4.352Å,
5.442Å
and 5.613Å respectively.
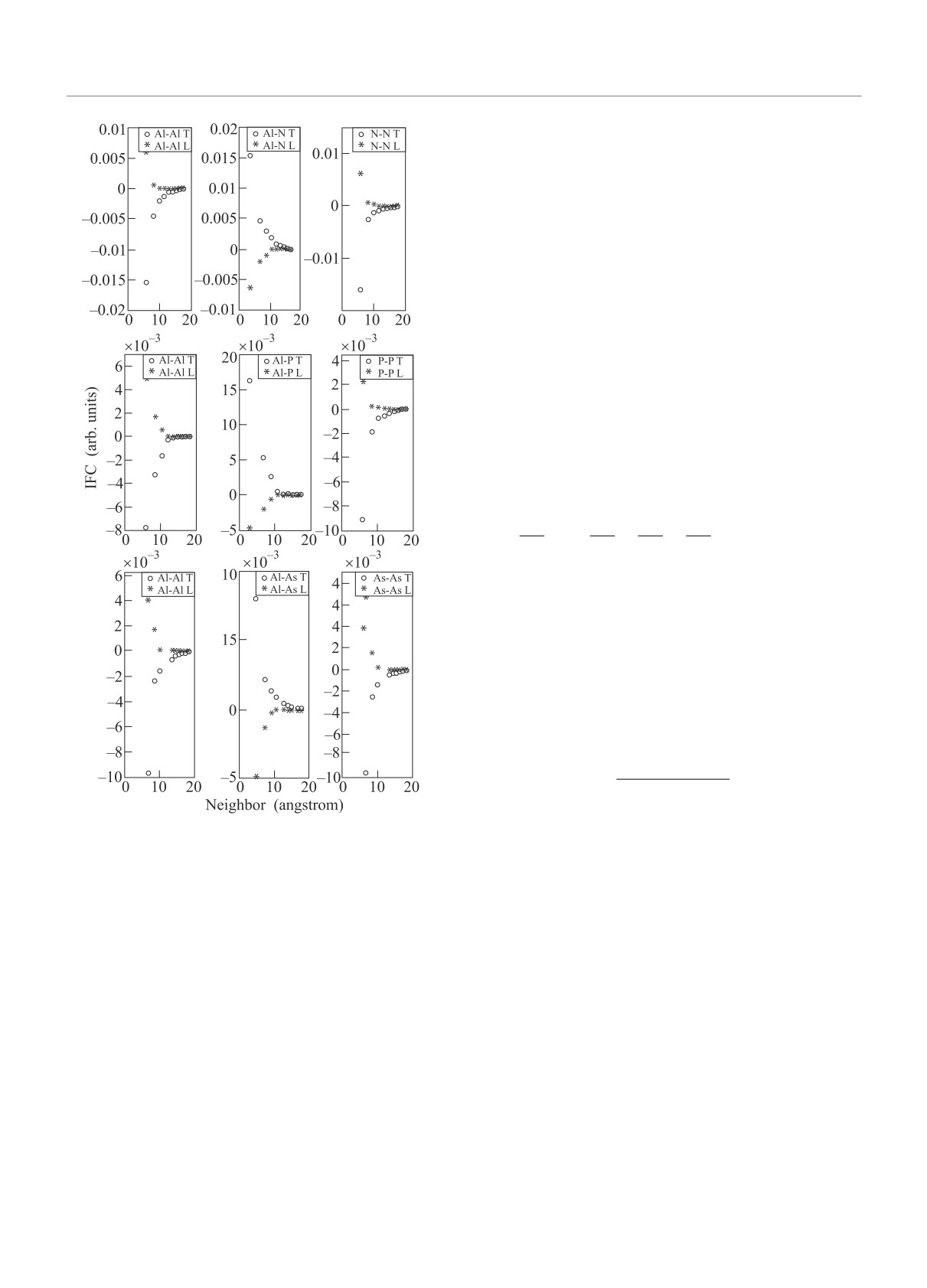
As seen from Fig. 1, in general, the decay of local
The interactomic force constants (IFC) describing
interaction for cation-cation, cation-anion, and anion-
the atomic interactions in a crystalline solid are defined
anion is faster than the total interaction decay. In the
in real space as [8]:
fourth neighbor, the local part of each species pair tends
to zero, while the total part of cation-cation, cation-
∂2E
Ckα,k′β(a, b) =
=
anion and anion-anion interaction of AlAs tends to zero
∂τakα∂τb
k′β
only in the eighth neighbor, and the rest in the ninth
= Cionkα,k′β(a, b) + Celeckα,k′β(a, b).
(1)
neighbor has not yet tended to zero.
The elastic constants of solids provide interesting in-
Here, τakα is the displacement vector of k-th atom in
formation about their mechanical and dynamic proper-
the a-th primitive cell along α axis. E is the Born-
ties and provide a link between the dynamics and me-
1)e-mail: whycs@163.com
chanical behavior of crystals. The calculated elastic con-
Письма в ЖЭТФ том 109 вып. 9 - 10
2019
677