РАСПЛАВЫ
2 · 2019
УДК 549.5+544.31
A NEW MULTIPURPOSE THERMODYNAMIC DATABASE FOR OXIDE SYSTEMS
© 2019 г. E. Yazhenskikha, *, T. Jantzenb, K. Hackb, M. Müllera
aForschungszentrum Jülich GmbH, Institute of Energy and Climate Research (IEK 2), Jülich, Germany
bGTT Technologies, Herzogenrath, Germany
*e mail: e.yazhenskikh@fz juelich.de
Received 02.07.2018
After completion 09.07.2018
Accepted for publication 15.07.2018
A new thermodynamic database for oxides has been developed in order to perform calcu
lations and predictions of equilibria for various scientific and industrial applications (slags,
ashes, glasses, minerals, ceramics, refractories, etc.). The database containing all known
phases (gas, stoichiometric compounds, solid and liquid solutions) for combinations of
27 different components provides proper descriptions for many different processes under
varying conditions (temperature, pressure, overall composition) including interactions be
tween materials and environments, and thus supports the development of new materials.
Keywords: thermodynamic assessment, CALPHAD modelling, database development,
phase equilibria, oxide systems, associate model, calculations of thermodynamic properties.
DOI: 10.1134/S0235010619010237
INTRODUCTION
Thermodynamic properties of complex oxide systems containing high amounts of silica,
alumina, iron oxides, alkali earth and alkali oxides, for which the measurements are experi
mentally difficult due to high temperatures, high volatility, high viscosity, etc., can be calculat
ed and predicted by thermodynamic modelling on the basis of reliable experimental data and
appropriate Gibbs energy models for the various phases. The available commercial databases
are not always sufficient to describe the complete system containing slag, ashes, biomass, gas,
coal etc., so a new database is necessary. In the past 15 years an oxide database has been devel
oped in the framework of a series of national projects (concerning coal gasification and combus
tion processes) supported by funding from the German government and industrial partners [1].
THERMODYNAMIC MODELS
Detailed reports on several of the assessments of subsystems can be found in respective pub
lications e.g. [2-4]. The thermodynamic database has to contain reliable descriptions of all
relevant phases (gas, stoichiometric compounds, solid and liquid solutions) in order to per
form proper calculations and predictions of thermodynamic properties and phase constella
tions. Stoichiometric compounds, i.e. solids with a fixed chemical formula such as Al2O3 or
CaSiO3 are modelled with a temperature dependent G°(T) function:
G° = A + B · T + C · T · ln(T) + D · T2 + E · T3 + F/T.
(1)
In case of non stoichiometric phase, i.e. solid and liquid solutions, the molar Gibbs energy of
ref
the solution is a three term expression with contributions of the reference part(G
),
the ideal
id
ex
(G
)
and the excess
(G
)
part as:
ref
id
G
m
=
G
+
G
+
G
ex.
(2)
Depending on the solution model chosen the three parts take on different mathematical form.
A new multipurpose thermodynamic database for oxide systems
117
T, °C
2500
Slag
[6]
2300
2100
1900
1700
C2S_C3P
1500
1300
C2S_C3P + C3P(s2)
1100
C2S_C3P + C3P(s)
C2S_Prime
900
Ca5P2SiO12 + Ca3(PO4)2(s)
700
Ca5P2SiO12
500
Ca7P2Si2O16
C2S_Prime + Ca2SiO4
300
0
20
40
60
80
100
wt % Ca3P2O8
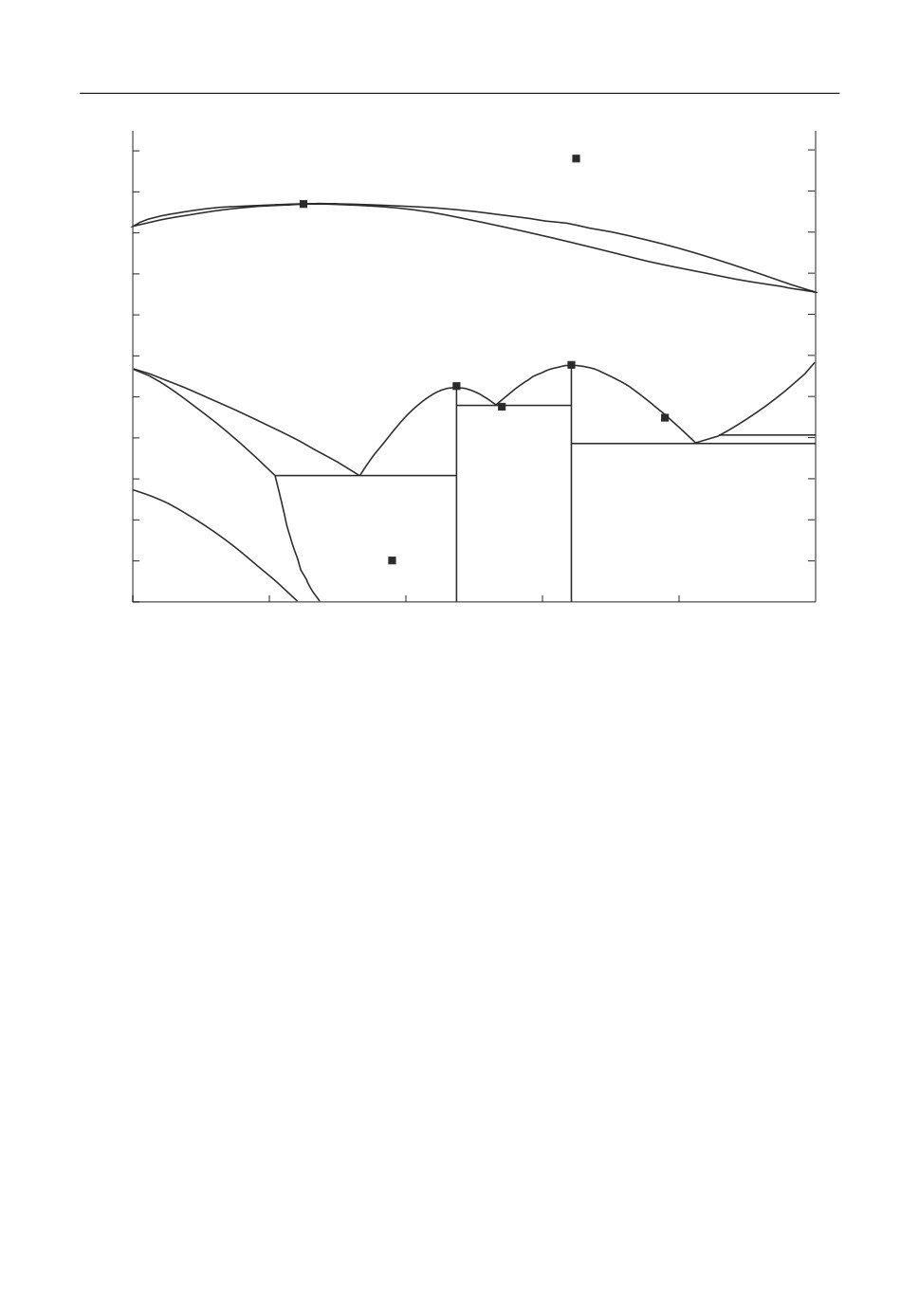
Fig. 1. Isopleth Ca2SiO4-Ca3P2O8.
Solid solutions are usually treated using the multi sublattice approach. For example, the
high temperature modifications of dicalcium silicate Ca2SiO4 and tricalcium phosphate
Ca3P2O8 have the same structure and Pearson symbol [5]. According to the high temperature
phase equilibria investigations [6] in the quasi binary system Ca2SiO4-Ca3P2O8 a continuous
series of solid solutions is formed at steelmaking temperatures. This is termed the C2S C3P
phase with the phase formula (Ca2+)3(Ca2+,Va)(P5+,Si4+)2(O2-)8. The first and fourth sublattic
es have fixed occupancy, while the third sublattice can be occupied by phosphor and silicon
cations and the second sublattice represents an interstitial position. This formula allows de
scribing the unsymmetrical end members Ca2SiO4 (C2S) and Ca3P2O8 (C3P) and the contin
uous transition between them. As shown in Figure 1, the solid solution between the two end
members C2S and C3P is complete above 1500°C.
Chromium, manganese and magnesium cations are introduced on the second sublattice to
describe their experimentally determined solubilities in C2S-C3P. Eke and Brett [7] reported
considerable solubility also of Vanadium oxide in the α Ca2SiO4. The positioning of V as a pen
tavalent cation on the third sublattice leads to a good reproduction of the experimental solubility
as shown in Figure 2. The full thermodynamic description of the phase C2S-C3P is presented
using the following formula (Ca+2)3(Ca2+,Mg2+,Cr3+,Mn2+,Va)(P5+,Si4+,V5+)2(O2-)8.
The liquid phase (slag), which exhibits short range ordering as well as ranges of immiscibil
ity, is modelled using the non ideal modified associate model according to Spear and Bes
mann [8]. The model was successfully applied for many types of systems with strong interac
tions [2-4]. Moreover, this model can give a reliable phase internal distribution of associate
species, which is consistent with experimentally determined Qn species distributions [9]. Fur
118
E. Yazhenskikh, T. Jantzen, K. Hack, M. Müller
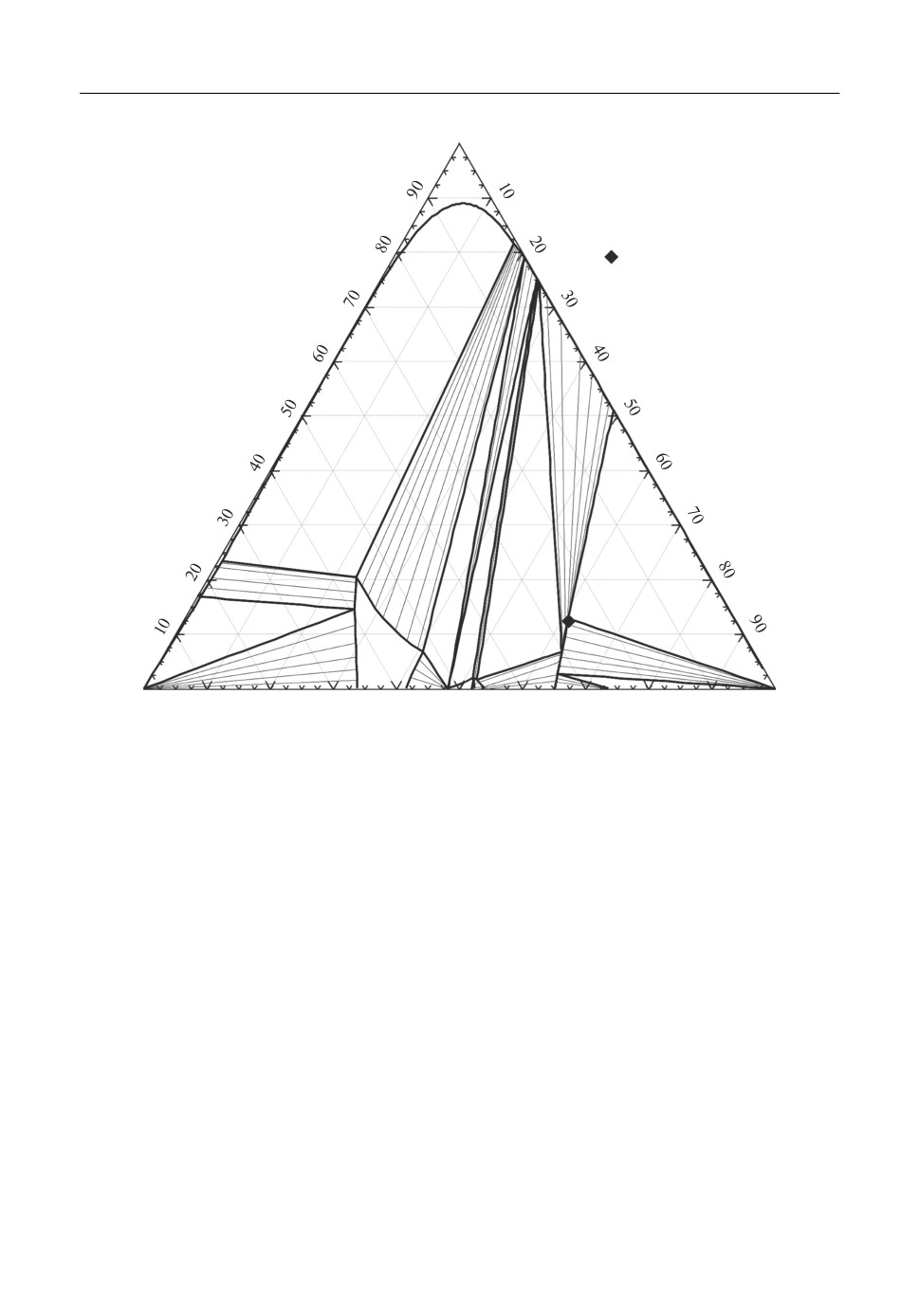
V2O5
Slag
[7]
Slag
C2S_C3P
Slag
SiO2
90
80
70
60
50
40
30
20
10
CaO
CaSiO3(s2)
Ca3SiO5
percent by weight
Fig. 2. Isothermal section at 1500°C in CaO-V2O5-SiO2 system.
thermore, the associate model representing the internal structure of the molten slag provides
an excellent basis for the modelling of melt viscosity [9]. The liquid component oxides as well
as binary and ternary associate species are considered as solution components. In addition, in
teractions between species were introduced in order to fine tune the thermodynamic descrip
tion especially in regions of immiscibility. The excess term of G (Eq. (2)) is expressed by
Redlich-Kister polynomials. The associate species were given the same stoichiometries in
comparable systems in order to provide a handle for their use in multi component systems. As
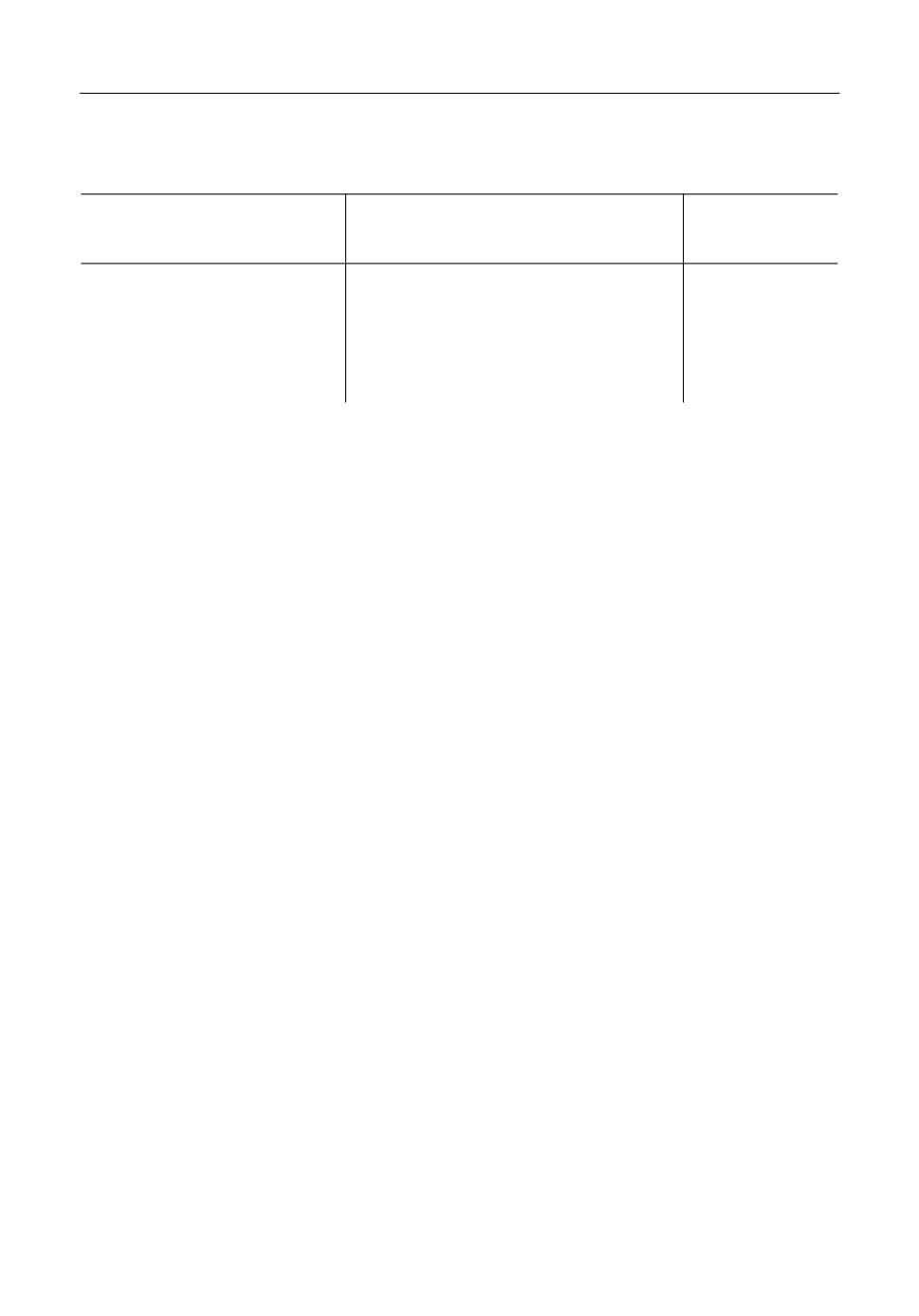
an example of consistent description, all P2O5 containing liquid species are listed in Table 1.
ASSESSMENT OF THERMODYNAMIC PARAMETERS
According to CALPHAD type modelling all available experimental data (phase equilibria,
mixing properties, activity, etc.) are critically analysed in terms of their consistency. Each
phase in the system is treated by an appropriate Gibbs energy model with adjustable parame
ters (Gibbs energy of constituents, interaction parameters, etc.). The Gibbs energy parameters
are optimised in accordance with the experimental information in order to generate a self
consistent dataset of Gibbs energies of all phases in a system.
A new multipurpose thermodynamic database for oxide systems
119
Table 1
Liquid components (associate species) in the P containing systems
Description
System
Associate species with P2O5
MeO
x : P2O5
MeO2-P2O5 with Me = Si, Ti
SiP2O7 · 2/3, Si3P4O16 · 2/7, TiP2O7 · 2/3,
1 : 1, 3 : 2
Me2O3-P2O5 with Me = Al, Cr, Fe
AlPO4, CrPO4, FePO4
1 : 1
MeO-P2O5 with Mn = Mg, Ca, Fe, Zn
Me3P2O8 · 2/5, Me2P2O7 · 1/2, MeP2O6 · 2/3
3 : 1, 2 : 1, 1 : 1
Alk2O-P2O5 with Alk = Na, K
Alk3PO4 · 1/2, Alk4P2O7 · 1/3, AlkPO3
3 : 1, 2 : 1, 1 : 1
The database, called GTOX, is under constant development and at present covers the fol
lowing oxide and sulphide components: Al2O3-Al2S3-CaO-CaF2-CaS-CrOx-FeOx-FeS-
MgO-MgS-MnOx-MnS-K2O-K2S-Na2O-Na2S-NiO-P2O5-SiO2-SO3-TiOx-VOx.
DATABASE APPLICATION
In the following examples of application calculations using the GTOX database will be pre
sented.
Slagging and fouling. An effective use of the world’s fossil fuels is obligatory in terms of their
limited supply and the worldwide increase in demand for energy. Hence, a fundamental
knowledge of the thermophysical and thermochemical processes during the conversion of the
fuel is necessary. Combustion and gasification request an essential understanding of the prop
erties of the inorganic residue (ash). The high temperature causes the ash to melt and, there
fore, the slag characteristically influences the conditions and limitations of the processes. The
behaviour of the liquid phase (melting temperature, crystallisation on cooling) is strongly de
pendent on the overall composition of the fuel (coal, biomass) and, particularly, of the residual
mineral part (ash). Thermodynamic calculations with reliable thermodynamic data can be
helpful in modelling the processes depending on various parameters (temperature, partial
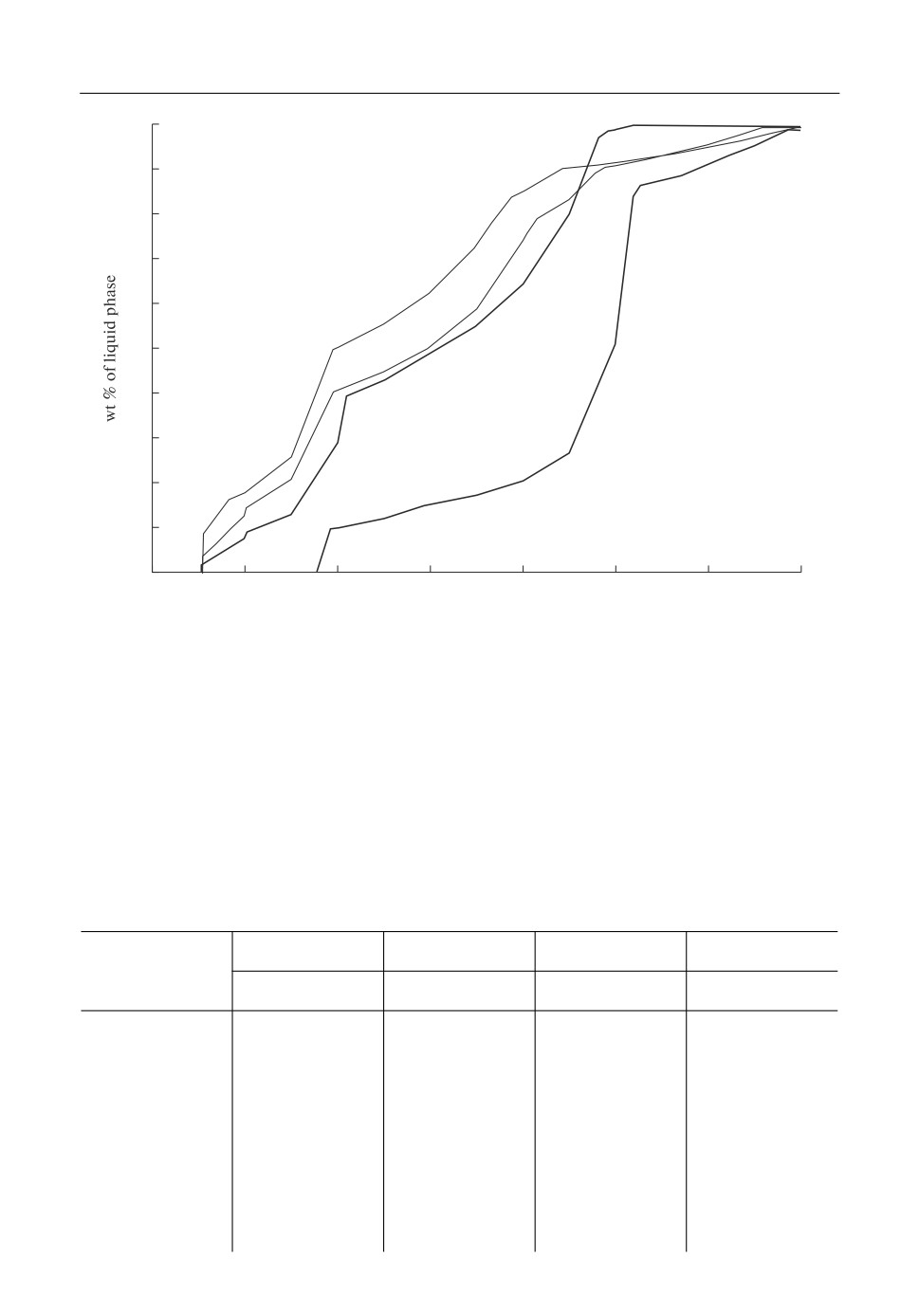
pressure of oxygen, etc.). Table 2 shows the compositions of several ashes from different hard
coals. Taking into account all possible transitions occurring in the system (phase formation
and decomposition, melting, structure transitions, etc.) it is possible to represent the fraction
(in wt %) of the liquid phase as a function of temperature (Fig. 3). The ash SKK containing
the highest amount of alumina and CaO and the smallest one of SiO2 is predicted to begin
melting at higher temperature (about 870°C) compared to the other compositions. The liquid
us temperature (mass fraction of liquid = 100%) is higher as well (1400°C) due to the high
melting temperature of alumosilicates. The SKC composition on the other hand, with mini
mal content of CaO and Al2O3 and maximal SiO2 has a low melting range (onset at 750 and
end at 1150°C). The phase transitions can be calculated in order to describe the processes un
der cooling or heating at equilibrium conditions. Slagging and fouling in a coal fired power
plant (formation of liquid and/or solid deposits) are determined by the composition of the con
densed phases formed. Furthermore, the composition and formation temperature of solid phas
es influence the thermochemical and thermophysical properties of the slag (e.g. viscosity).
Dephosphorization. Dephosphorisation of steels by a metal slag reaction is important for
the steelmaking chemistry. The GTOX database can be used in this application field for the
calculation of dephosphorisation equilibria between slag and iron melts determining the equi
librium distribution of phosphorus between slag and metal for basic slags of various composi
120
E. Yazhenskikh, T. Jantzen, K. Hack, M. Müller
100
90
SKK
80
SKR
70
60
SKU
SKC
50
40
30
20
10
0
700
800
900
1000
1100
1200
1300
1400
T, °C
Fig. 3. Melting of different ashes (hard coals).
tions. A critical thermodynamic aspect of these processes is the equilibrium phosphorus parti
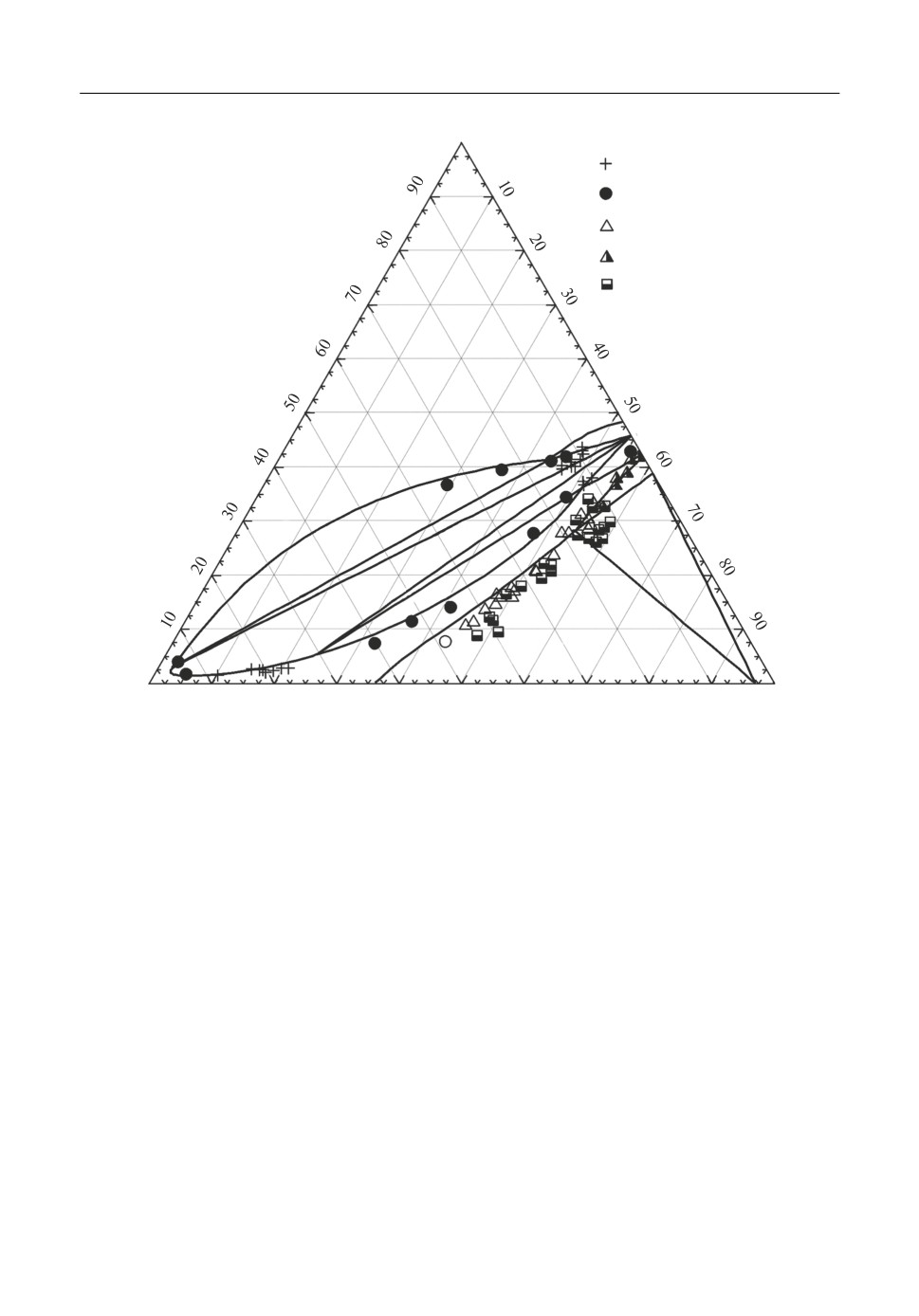
tion ratios Lp(%P)Slag/[%P]Fe) between slag and metal. The ternary CaO-FeO-P2O5 system is
a most important system for the Basic Oxygen Furnace (BOF) process. This system is charac
terized by a wide field of immiscibility extending in the liquid phase towards the FeO rich cor
ner reported by many investigators [10, 11]. The calculated isothermal section at 1600°C pre
Table 2
Compositions (in wt %) of the ashes
Columbia
South Africa
Russia
USA
Oxides
SKC
SKK
SKR
SKU
Al2O3
14.6
25.9
22.1
20.6
CaO
2.1
7.1
4.9
3.7
Fe2O3
15.5
15.4
6.8
14.6
K2O
1.4
0.7
2.9
2.4
MgO
1.1
0.1
0.2
0.9
Na2O
1.8
0.2
1.3
0.7
P2O5
0.1
1.5
0.5
0.2
SiO2
60.7
45.4
57.1
52.6
SO3
1.9
2.5
3.2
3.0
TiO2
0.8
1.4
0.9
1.1
A new multipurpose thermodynamic database for oxide systems
121
P2O5
[10]
[11]
CaO, [12]
CaO + Ca4P2O9, [12]
[13]
Slag + Liquid
C3P(s2)
C4P
Slag + Slag#2 + Liquid
Slag + Liquid + C3P(s3)
MeO + Slag + Liquid
FeO
90
80
70
60
50
40
30
20
10
CaO
percent by weight
Fig. 4. Isothermal section at 1500°C in CaO-FeO-P2O5 system in equilibrium with Fe.
sented in Figure 4 is in good agreement with known experimental data [10-13]. The phospho
rus distribution between molten slag and liquid Fe in the CaO FeO P2O5 system along the
CaO saturation was studied by several researchers in the temperature range 1550-1650°C
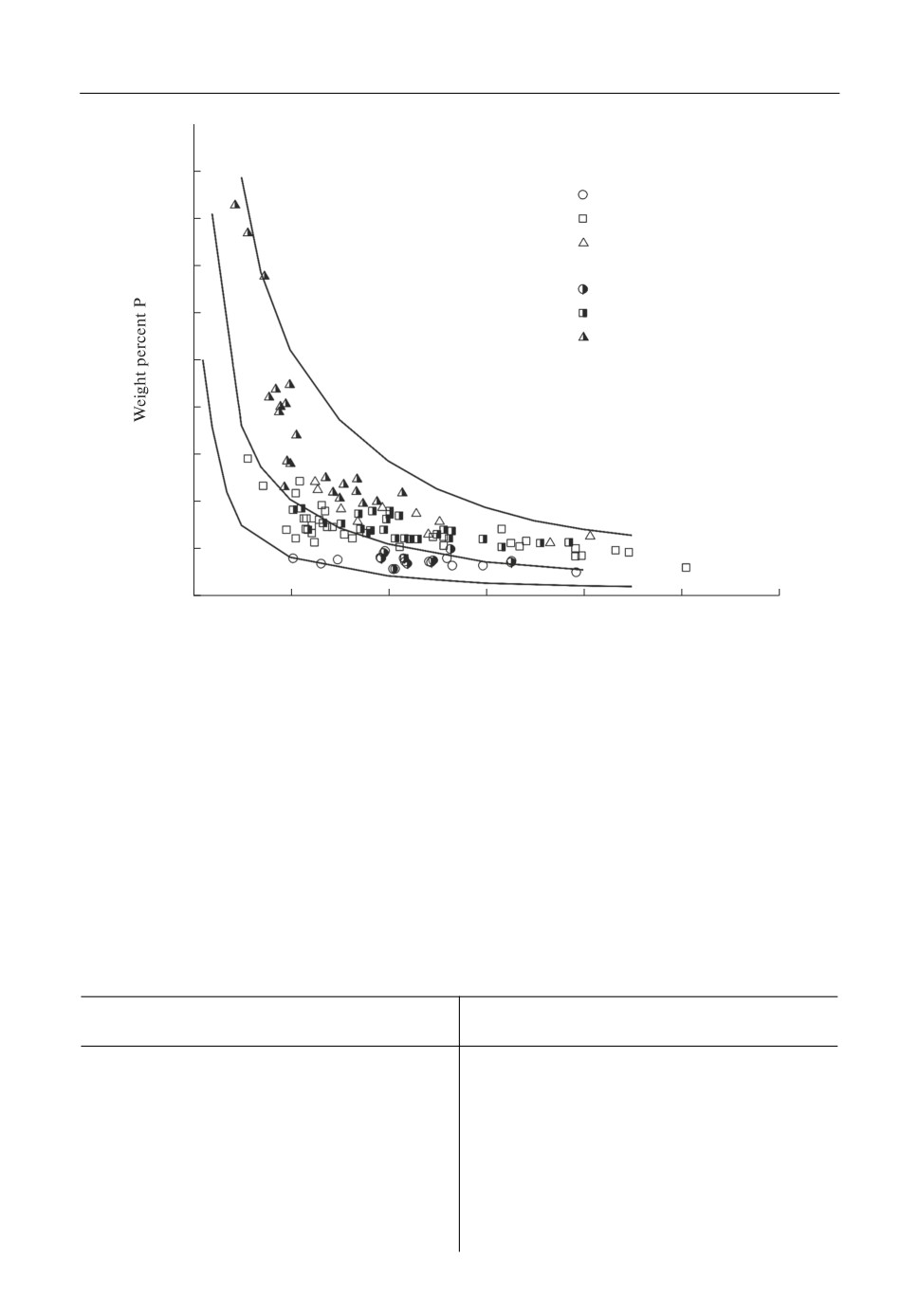
[13, 14]. Figure 5 shows the relationship between the amount of phosphorus in liquid iron and
the content of FeO in CaO saturated molten slags at different temperatures. The phosphorous
concentration in liquid Fe depends on the temperature and the amount of FeO in the molten
slag. It increases with increasing temperature and decreasing amount of FeO. The experimen
tal behaviour could be well reproduced by the calculations (Fig. 5).
Inclusion of CaF2. An other important non oxide addition in the GTOX oxide database is
Fluorspar (CaF2) because of its wide application in ladle treatment of steel and especially elec
trometallurgy. The addition of CaF2 decreases the melting temperature and furthermore the
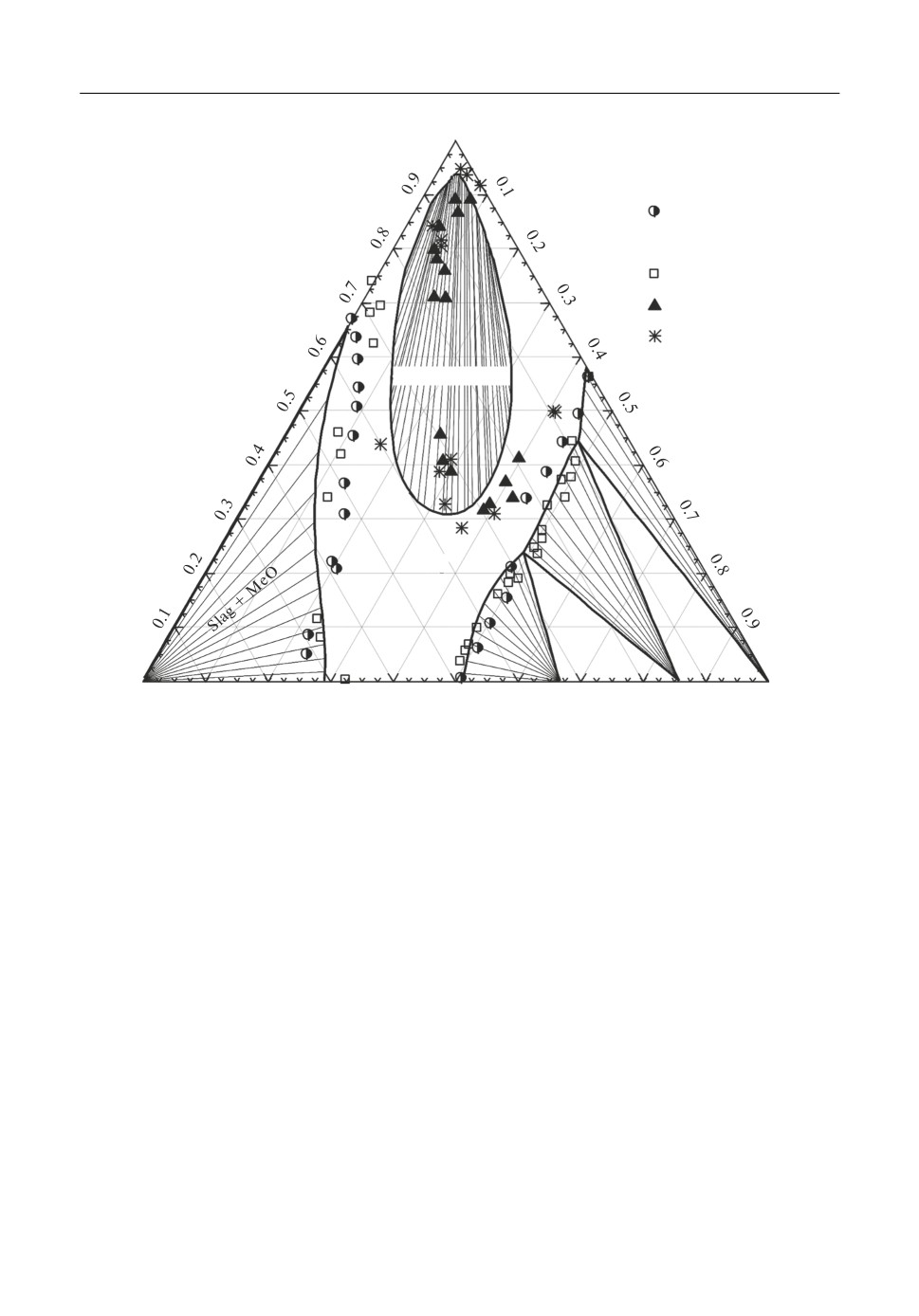
viscosity of the slags. Figure 6 shows the isothermal section at 1600°C through the Al2O3-
CaO-CaF2 system compared with experimental data [15, 16]. The calculated lines of satura
tion with CaO, Al2O3, CA6 and CA2 are in good agreement with experimental data, also the
computed wide zone of liquid immiscibility in the high fluoride region.
Addition of sulphur. Sulfur is commonly contained as an essential component in many natu
ral and manufactured materials. Sulfide glasses can be used for high refractory index materials.
122
E. Yazhenskikh, T. Jantzen, K. Hack, M. Müller
0.10
[13]
0.09
1550°C
0.08
1600°C
1650°C
0.07
[14]
1550°C
0.06
1600°C
1650°C
1650°C
0.05
1600°C
0.04
1550°C
0.03
0.02
0.01
0
10
20
30
40
50
60
Weight percent FeO
Fig. 5. Relationship between [%P] in liquid Fe and (FeO) in slag.
Sulfur species are present in radioactive and toxic wastes. Sulfides are in focus of attention due
to their importance for ferrous and copper metallurgical processes. The thermal properties
and stability ranges of sulfide containing systems is necessary for understanding and control of
the processes of desulfurization of molten alloys and precipitation of the sulfides during the
solidification process. The Ca-Cr-Fe-Mg-Mn-S sulfide sub system in combination with
corresponding oxides is part of the GTOX database (Table 3) and can be used and can be used
for thermodynamic calculations [4].
Table 3
GTOX database
Contents
Year 2018
Binary systems
149
Ternary systems
131
Quaternary systems
7
Solid solution phases
117
Compounds
702
Slag Atlas pages
1001
A new multipurpose thermodynamic database for oxide systems
123
CaF2
[15]
[16]
Phase boundry
Two liquids
One liquid
Slag + Slag#2
Slag
CaO
0.9
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
Al2O3
Mole fraction
CaAl4O7
CaAl
12O19
Fig. 6. Isothermal section at 1600°C in Al2O3-CaO-CaF2 system.
CONCLUSIONS
A self consistent thermodynamic database for the system Al2O3-Al2S3-CaO-CaF2-CaS-
CrOx-FeOx-FeS-MgO-MgS-MnOx-MnS-K2O-K2S-Na2O-Na2S-NiO-P2O5-SiO2-
SO3-TiOx-VOx which permits the calculation of phase diagrams and thermodynamic proper
ties for any composition and temperature has been established. Phase diagrams calculated us
ing this database show generally good agreement with the experimental phase boundaries. All
phase diagrams assessed are presented in a Slag Atlas provided by GTT Technologies in the
framework of the HotVeGas project [17]. The database obtained is successfully used in a very
wide range of applications (coal combustion and gasification metallurgy, dephosphorization,
fluxing, viscosity modelling).
ACKNOWLEDGEMENTS
Both GTT technologies and IEK 2, Forschungszentrum Jülich, gratefully acknowledge fi
nancial support of the HotVeGas project by the Ministry of Economic Affairs of the Federal
Republic of Germany under (FKZ 0327773L) and (FKZ 0327773K).
REFERENCES
124
E. Yazhenskikh, T. Jantzen, K. Hack, M. Müller
2. Hack K., Jantzen T., Müller M., Yazhenskikh E., Wu G. A novel thermodynamic database for
slag systems and refractory materials // Proceedings of the 5th Int. Congress on the Science and Tech
nology of Steelmaking. ICS 2012. Dresden. Germany. 2012.
3. Yazhenskikh E., Jantzen T., Hack K., Müller M. Critical thermodynamic evaluation of oxide
system relevant to fuel ashes and slags: Potassium oxide magnesium oxide silica // Calphad. 2014. 47.
P. 35-49.
4. Jantzen T., Hack K., Yazhenskikh E., Müller M. Evaluation of thermodynamic data and phase
equilibria in the system Ca-Cr-Cu-Fe-Mg-Mn-S: Part I: Binary and quasi binary subsystems //
Calphad. 2016. 56. P. 270-285; Part II: Ternary and quasi ternary subsystems // Calphad. 2017. 56.
P. 286-302.
5. Pearson W.B. A Handbook of Lattice Spacings and Structures of Metals and Alloys. Pergamon
Press, Oxford. 1967. V. 2.
6. Fix W., Heymann H., Heinke R. Subsolidus relations in the system 2CaO · SiO2-3CaO · P2O5 //
J. Am. Ceram. Soc. 1969. 52. № 6. P. 346-347.
7. Eke M., Brett N.H. Phase equilibriums in the system CaO-MgO-SiO2-V2O5 // Trans. J. Br.
Ceram. Soc. 1973. 72. № 5. P. 195-201.
8. Besmann T.M., Spear K.E. Thermodynamic modelling of oxide glasses. // J. Am. Ceram. Soc.
2002. 85. № 12. P. 2887-2894.
9. Wu G., Seebold S., Yazhenskikh E., Hack K., Müller M. Viscosity model for oxide melts rele
vant to fuel slags. Part 3: The iron oxide containing low order systems in the system SiO2-Al2O3-
CaO-MgO-Na2O-K2O-FeO-Fe2O3 // Fuel Processing Technology. 2018. 171. P. 339-349.
10. Trömmel G., Fritze H.W. Equilibriums between iron and lime containing phosphate slags. //
Arch. Eisenhüttenwes. 1959. 30. P. 461-472.
11. Turkdogan E.T., Pearson J. Activities of constituents of iron and steelmaking slags. III Phos
phorus pentoxide. // J. Iron Steel Inst. London 1953. 175. P. 398-401.
12. Trömmel G., Fix W. The equilibrium between an iron melt and lime containing phosphate
slags in the presence of silica and manganese oxide // Arch. Eisenhüttenwes. 1962. 33. P. 745-755.
13. Nagabayashi R., Hino M., Banya S. Distribution pf phosphorus between liquid iron and FetO-
(CaO+MgO)-(SiO2+P2O5) phosphate slags // Tetsu to Nagane. 1988. 74. P. 1770-1777.
14. Trömmel G., Fix W. The system lime phosphorus pentoxide // Arch. Eisenhüttenwes. 1961.
32. P. 209-272.
15. Zhmoidin G.I., Chatterjee A.K. Slags for Metal Refining. Properties Variations of System
CaO-Al2O3-CaF2 // Metallurgiya, Moscow. 1986. P. 286-296.
16. Ries R., Schwerdtfeger K. Contribution to the phase diagram calcium fluoride calcium oxide
alumina // Arch. Eisenhuettenwes. 1980. 51. № 4. P. 123-129.
17. Slag Atlas on request of GTT Technologies, info@gtt technologies.de.