Журнал эволюционной биохимии и физиологии, 2020, T. 56, № 7, стр. 738-738
Effects of Omecamtiv Mecarbil on the Interaction of Atrial and Ventricular Myosin with Actin
S. Yu. Bershitskiy 1, *, D. V. Shchepkin 1, S. R. Nabiev 1, L. V. Nikitina 1, A. M. Kochurova 1, V. Y. Berg 1, G. V. Kopylova 1
1 Institute of Immunology and Physiology UB RAS
Yekaterinburg, Russia
* E-mail: serg.bersh@gmail.com
Introduction. In heart failure, the contractile function of the ventricles is reduced. To compensate for the reduction in the ejection fraction, the ventricular myosin activator omecamtiv mecarbil (OM) was created (Teerlink, 2009). OM prolongs the state of strong binding to F-actin by binding the catalytic domain of cardiac myosin, thus increasing the number of myosin force-generating heads, reduces the step size of myosin molecule and the sliding velocity of F-actin over cardiac myosin in an in vitro motility assay. The mechanism of the OM effect was studied on cardiomyocytes and myosin of the left ventricle. But it is known that atrial and ventricular isoforms of myosin differ in the composition of heavy (MHC) and light (LC) chains. Ventricular myosin consists of β-TCM and ventricular LC and atrial of α-MHC and atrial LC. Isoforms of myosin atria and ventricles differ in kinetic and mechanical properties.
Methods. We compared the effect of OM on the characteristics of the single interactions of the native atrial myosin and pig’s ventricle with skeletal actin in an optical trap. The impact of OM on the kinetics of the interaction of atrial and ventricular myosin with F-actin was evaluated by the sliding velocity of actin filaments in the in vitro motility assay.
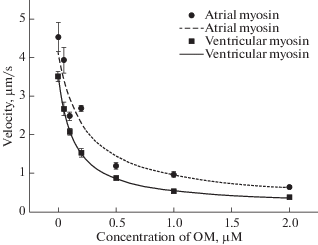
Results. OM increased the lifetime of the myosin head on actin; 0.7 μM OM halved the step. OM reduced the sliding velocity of F-actin in ventricular myosin by seven times, in the atrial by four times (Fig. 1).
Discussion. The difference in the sensitivity of myosin isoforms to OM is explained by the difference in their affinity for actin in the nucleotide-bound state. OM is closely associated with myosin before the power stroke (ADP · Pi state), and the lifetime in the nucleotide-bound state in the cycle of myosin isoforms with α- and β-TCM is different. We suggest that the difference in the kinetics of hydrolysis determines the kinetic and mechanical properties of atrial and ventricular myosin. Results at the molecular level explain why OM more significantly affects the characteristics of the atria than the ventricle.
Supported by RFBR 18-015-000252, the State Program (AAAA-A18-118020590135-3).
Список литературы отсутствует.
Дополнительные материалы отсутствуют.
Инструменты
Журнал эволюционной биохимии и физиологии