Генетика, 2020, T. 56, № 6, стр. 636-647
Основные сценарии генетически регулируемой клеточной гибели в оогенезе Drosophila melanogaster
Е. У. Болоболова 1, *, Н. В. Дорогова 1, С. А. Федорова 1, 2, **
1 Федеральный исследовательский центр Институт цитологии и генетики Сибирского отделения
Российской академии наук
630090 Новосибирск, Россия
2 Новосибирский национальный исследовательский государственный университет
630090 Новосибирск, Россия
* E-mail: elbol@bionet.nsc.ru
** E-mail: fsveta@bionet.nsc.ru
Поступила в редакцию 01.07.2019
После доработки 25.11.2019
Принята к публикации 17.12.2019
Аннотация
Процесс регулируемой клеточной гибели (РКГ), наряду с пролиферацией и дифференцировкой, является важной и неотъемлемой частью развития любого многоклеточного организма. Существует ряд разнообразных механизмов РКГ, которые могут быть активированы в ответ на сигналы развития и окружающей среды. В данном обзоре суммированы современные представления об основных клеточных событиях и молекулярных механизмах, характеризующих различные процессы гибели клеток в оогенезе Drosophila melanogaster. Во время оогенеза дрозофилы реализуется как минимум пять различных стадиоспецифичных сценариев РКГ: 1) каспаза-зависимая гибель клеток зародышевой линии (КЗЛ) во время раннего оогенеза, которая опосредуется через аутофагию; 2) канонический апоптоз, удаляющий избыток соматических полярных клеток на 4–5-й стадиях оогенеза; 3) РКГ яйцевых камер в среднем оогенезе, начинающаяся с гибели КЗЛ каспаза-зависимым путем при участии аутофагии и завершающаяся их поглощением окружающими фолликулярными клетками (ФК); 4) неапоптозная РКГ питающих клеток в позднем оогенезе, инициируемая и контролируемая окружающими ФК; 5) каспаза-независимая гибель выполнивших свои функции ФК посредством аутофагии в конце 14-й стадии.
Регулируемая (программируемая) клеточная гибель является важным условием выживания организма и необходима на всех этапах его существования – от раннего развития и морфогенеза до поддержания гомеостаза взрослой особи. Избирательной гибели подвергаются как отдельные клетки с невосстанавливаемыми дефектами, так и клеточные популяции, утратившие свои функции в процессе онтогенеза. По морфологическим признакам исторически были выделены три канонических типа регулируемой клеточной гибели (РКГ): апоптоз, смерть клетки посредством аутофагии и некроз [1]. Хотя такая классификация используется до сих пор, комитет по номенклатуре клеточной смерти (Nomenclature Committee on Cell Death), начиная с 2005 г., ведет работу по классификации типов клеточной гибели, учитывающей современные генетические, молекулярные и биохимические данные. Последняя редакция каталога клеточной гибели представляет помимо основных форм (морфотипы апоптоза, аутофагии и некроза) еще 12 подпрограмм, характеризующихся специфичными механизмами [2]. Молекулярно-генетические механизмы и сигнальные пути, осуществляющие клеточную гибель, эволюционно консервативны, имеют высокую степень гомологии у различных эукариот и поэтому эффективно изучаются на модельных организмах, как in vivo, так и in vitro. Одной из таких информативных моделей является яичник дрозофилы. В отличие от системы in vitro, яичник дрозофилы позволяет моделировать и анализировать клеточную гибель на уровне организма, а широкий набор генетических методов и ресурсов дает возможность выявлять межгенные взаимодействия и компоненты сигнальных путей, контролирующих РКГ.
РКГ необходима для нормального функционирования яичника и формирования фертильной яйцеклетки дрозофилы. Генетически запрограммированной гибели подвергаются как клетки зародышевой линии (КЗЛ), так и соматические, эти события инициируются на трех стадиях оогенеза и происходят c использованием разнообразных механизмов.
В данном литературном обзоре мы суммируем современные представления о том, как реализуется программа клеточной гибели в оогенезе дрозофилы, обращая особое внимание на взаимодействие различных молекулярно-генетических механизмов РКГ. Последнее обстоятельство важно для понимания того, что на уровне организма различные типы клеточной гибели не являются взаимоисключающими, они зачастую пересекаются и действуют синергично. В нашем обзоре мы будем называть все типы генетически детерминируемой/контролируемой клеточной гибели общим термином “регулируемая клеточная гибель” (РКГ) согласно рекомендации последнего бюллетеня комитета по номенклатуре клеточной смерти [2].
ЯИЧНИК КАК МОДЕЛЬНАЯ СИСТЕМА
Яичник дрозофилы является ценной информативной модельной системой для изучения путей клеточной гибели. Крупные, легко выделяемые яйцевые камеры хорошо подходят для анализа изображений, и они могут быть культивированы в течение коротких периодов in vitro. Кроме того, для данного модельного объекта существует обширный набор мутантных и трансгенных линий, позволяющий манипулировать экспрессией отдельных генов как на различных стадиях оогенеза, так и в различных клетках [3].
Яичник взрослой самки дрозофилы представляет собой пучок из 15–20 овариол – полых структур, внутри которых последовательно располагаются созревающие яйцевые камеры. Каждая яйцевая камера содержит цисту, состоящую из 16 КЗЛ, окруженных соматическими фолликулярными клетками (ФК). В цисте одна клетка дифференцируется в ооцит, а остальные 15 клеток полиплоидизируются и становятся питающими клетками (ПК), связанными с ооцитом через межклеточные кольцевые каналы и снабжающими ооцит органеллами, белками и РНК. Яйцевые камеры формируются в проксимальных отделах овариол – гермариях, а их созревание происходит в дистальных отделах – вителлариях. В гермарии выделяют четыре функциональных района: 1, 2А, 2В, 3 [4]. В первом районе находятся стволовые клетки, дающие начало КЗЛ, происходит деление стволовых клеток, четыре раунда митотических делений дочерних клеток, сопровождающихся неполным цитокинезом, в результате которых образуются цисты, состоящие из 16 клеток. Во втором районе гермария каждая циста обволакивается ФК. В третьем районе гермария завершается формирование яйцевой камеры, содержащей одну 16-клеточную цисту, окруженную слоем эпителиальных ФК. Покинув гермарий, яйцевая камера продвигается по овариоле, постепенно развиваясь в зрелый ооцит. Процесс развития яйца – оогенез – обычно разделяют на 14 стадий [4, 5]. На восьмой стадии начинается вителлогенез, в процессе которого в ооцит поступают желточные белки из жирового тела и ФК. По мере протекания оогенеза ооцит постепенно растет и в конце процесса заполняет всю камеру. В течение 11–14 стадий ПК сжимаются и исчезают.
В оогенезе Drosophila контроль деления и роста клеток, формирования и развития цист осуществляется экдизоновым и инсулиновым сигнальными путями, которые служат поддержанию жизнеспособности клеток и цист [6–8].
Гибель КЗЛ в яичнике мух дикого типа наблюдается главным образом на трех этапах развития яйцевой камеры: в раннем оогенезе (во втором районе гермария), на стадиях 7–9 (“смерть в среднем оогенезе”) и в конце развития, на стадиях 12–13 (“смерть на поздней стадии”) [3, 9–11] (рис. 1). Гибель клеток в гермарии и на стадиях 7–9, названных “контрольными точками” (англ. – checkpoints) клеточной смерти в оогенезе, при нормальном содержании мух происходит в ответ на аномалии развития и резко возрастает при действии различных стрессорных факторов. Большинство данных о клеточной гибели в гермарии и среднем оогенезе были получены в условиях белкового голодания. В ответ на белковое голодание для экономии ресурсов регулируется производство яиц: в гермарии замедляется скорость пролиферации стволовых клеток, в среднем оогенезе индуцируется гибель некоторых яйцевых камер [12]. Компоненты инсулинового сигнального каскада являются сенсорами наличия достаточного количества аминокислот в клетке [8].
Рис. 1.
Схема оогенеза с указанием стадий (3–14), на которых происходит РКГ (см. текст). Звездочками обозначены контрольные точки. РКГ – регулируемая клеточная гибель, КЗЛ – клетки зародышевой линии, ПК – питающие клетки, ФК – фолликулярные клетки, ПФК – полярные фолликулярные клетки, СФК – стрейч-ФК.
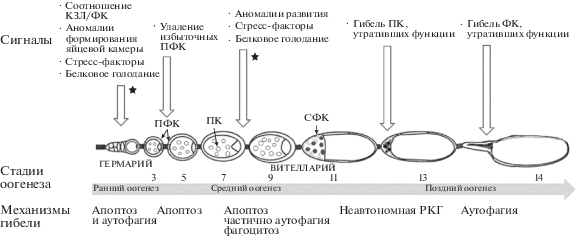
Смерть ПК на поздней стадии происходит в рамках нормального развития каждого яйца. На 11-й стадии ПК переносят свою цитоплазму через кольцевые каналы в ооцит, в процессе, называемом дампингом (англ. – dumping). Остаточные ПК содержат полиплоидные ядра и незначительное количество цитоплазмы, которые исчезают к 14-й стадии, оставляя зрелый ооцит.
Яичник содержит три типа соматических ФК: две пары полярных клеток, находящихся на разных полюсах яйцевой камеры, интерфолликулярные клетки, разделяющие камеры между собой, и эпителиальные ФК, окружающие КЗЛ [13, 14]. В процессе оогенеза большое количество ФК умирает, когда их вспомогательные функции завершены [3, 11].
КРАТКАЯ ХАРАКТЕРИСТИКА ОСНОВНЫХ ТИПОВ КЛЕТОЧНОЙ ГИБЕЛИ, ВСТРЕЧАЮЩИХСЯ В ООГЕНЕЗЕ Drosophila
Апоптоз является универсальным консервативным механизмом, при котором запускается программа саморазрушения клеток с участием протеолитических ферментов, основные из которых относятся к семейству каспаз [15]. У Drosophila семейство каспаз содержит семь белков [16], из которых наиболее важными для апоптоза являются инициаторная каспаза Dronc, функциональный аналог каспазы-9 млекопитающих, эффекторные каспазы Drice и Dcp-1, функциональные аналоги каспазы-3 млекопитающих. В оогенезе дрозофилы важную роль в РКГ играет Dcp-1, имеющая значительную гомологию с Drice [17–21]. Регуляция активности каспаз осуществляется посредством их ингибиторов Diap1, Diap2, dBruce, Deterin, называемых IAP-белками [22–24], и ингибиторов IAP-белков, Reaper, Hid и Grim, вместе часто называемых RHG-белками [25, 26]. В отсутствие проапоптотических сигналов IAP-белки связываются с каспазами и ингибируют их активность. При появлении проапоптотических стимулов RHG-белки присоединяются к IAP-белкам и освобождают каспазы. Не связанная с IAP инициаторная каспаза Dronc взаимодействует с адаптерным белком Ark/Dark (ортолог Apaf-1 млекопитающих) для формирования активационного комплекса, апоптосомы. Апоптосома активирует эффекторные каспазы, которые инициируют серию последующих реакций расщепления [27–29]. Активируемая эффекторной каспазой ДНКаза (dCAD/Rep4) фрагментирует ДНК [30]. Активные эффекторные каспазы расщепляют многие клеточные белки, среди которых компоненты цитоскелета, такие как актин и ламин [31], субъединицы протеасом [32].
У млекопитающих активация внутреннего пути апоптоза происходит с участием митохондрий через регуляцию посредством белков семейства Bcl-2, которые контролируют проницаемость митохондриальной мембраны и таким образом влияют на выход в цитоплазму апоптогенных факторов, таких как цитохром С, апоптоз-индуцирующий фактор AIF, эндонуклеаза G [33, 34]. Однако у Drosophila белки Debcl и Buffy, являющиеся гомологами Bcl-2 белков, не играют центральной роли в регуляции апоптоза, так как индукция и формирование Ark/Dark-апоп-тосомы происходят независимо от цитохрома С [35]. С другой стороны, подобно млекопитающим, белки аппарата слияния/деления (fusion/fission) митохондрий у Drosophila участвуют в регуляции РКГ. Например, было показано, что остановка фрагментации митохондрий путем ингибирования динамин-подобной ГТФазы Drp1 предотвращает гибель клеток дрозофилы, индуцируемую проапоптотическими белками Reaper и Hid [36–40].
Аутофагия – это процесс утилизации клеточных органелл и макромолекул, необходимый для жизнедеятельности любой нормальной клетки. При помощи аутофагии клетка избавляется от неправильно свернутых, частично денатурированных или долгоживущих белков, поврежденных органелл, восполняет недостаток ресурсов, защищается от старения и т.д. Таким образом, умеренная (физиологическая) аутофагия является способом самообновления и выживания клетки. Однако чрезмерная аутофагия может приводить клетку к гибели. Аутофагия сопровождается образованием аутофагосом – специализированных двумембранных структур (вакуолей), которые формируются вокруг органелл и участков цитоплазмы, подлежащих деградации. Биогенез аутофагосом определяют более 30 консервативных генов – atg, большинство из которых были выявлены в генетических скринингах на дрожжах [41–43]. Ортологи atg-генов впоследствии были обнаружены у всех высших эукариот, включая дрозофилу и человека [44, 45]. Клеточная гибель, индуцированная аутофагией, сопровождается избыточным образованием аутофагосом и лизосом, что приводит к перевариванию всех клеточных органелл и закислению цитоплазмы. Этот процесс может происходить независимо от каспаз, параллельно с ними, или один механизм может взаимодействовать с другим. В яичниках дрозофилы белковое голодание индуцирует аутофагию в КЗЛ и ФК. Этот процесс контролируется инсулиновым сигнальным путем через киназу mTOR (Target of rapamicin) [46].
Еще одним типом гибели, который может реализовываться в оогенезе дрозофилы, является программируемый некроз. Морфологически некроз проявляется разжижением цитоплазмы, набуханием органелл, нарушением целостности плазматической мембраны [47]. В последние годы были классифицированы разные формы РКГ, проявляющие признаки некротической морфологии, выявлен ряд генов, требующихся для регулируемого некроза у C. elegans [48], млекопитающих [2, 49, 50], Drosophila [51–53].
РКГ В РАННЕМ ООГЕНЕЗЕ
Стадиоспецифичная гибель цист в гермарии обусловлена необходимостью удаления дефектных вариантов в процессе формирования яйцевых камер, которые, например, появляются при нарушении баланса между скоростями пролиферации КЗЛ и соматических клеток в условиях голодания [10, 12]. Гибель КЗЛ происходит в основном на границе между районами 2a и 2b, где ФК делятся и обволакивают 16-клеточную цисту. Оба этих процесса, гибель клеток и пролиферация ФК, опосредуются инсулиновым сигнальным каскадом [12, 54, 55]. Независимо от инсулинового сигнального пути стероидный гормон экдизон также регулирует пролиферацию и выживание КЗЛ. Действие экдизона опосредуется рецептором экдизона (EcR) и генами-мишенями, включая e74, e75 и broad. Анализ мозаичных клонов показал, что в клетках, гомозиготных по мутации e74, в районах 2 и 3 гермария наблюдаются более интенсивное в сравнении с нормой окрашивание на каспазу и повышенная гибель клеток. Следовательно, отсутствие передачи сигнала экдизона приводит к клеточной смерти [6, 56].
Было установлено, что гибель КЗЛ в гермарии осуществляется совместным действием апоптоза и аутофагии [46, 57, 58]. Погибающие клетки характеризуются конденсацией хроматина, фрагментацией ДНК, присутствием большого количества разнообразных аутофагосом и аутолизосом. В процессе апоптоза участвует эффекторная каспаза Dcp-1. В условиях голодания мутанты, лишенные Dcp-1, характеризуются не только пониженным уровнем фрагментации ДНК, но и редукцией аутофагии по сравнению с диким типом, что предполагает участие каспазы Dcp-1 в регуляции аутофагии [57]. С другой стороны, нарушение экспрессии генов аутофагии atg7 и atg1 приводит к значительному снижению фрагментации ДНК, из чего следует, что аутофагия, вероятно, действует проапоптотически [58]. В настоящее время еще не определен механизм активации эффекторной каспазы Dcp-1. Получены данные, что IAP-белок Bruce в нормальных условиях действует как ингибитор гибели КЗЛ в оогенезе, ограничивая каспазную активность и аутофагию, что свидетельствует о связанной регуляции апоптоза и аутофагии [57]. Таким образом, приведенные данные свидетельствуют о сложном взаимодействии механизмов апоптоза и аутофагии в процессе клеточной гибели в раннем оогенезе.
РКГ В СРЕДНЕМ ООГЕНЕЗЕ
На средних стадиях оогенеза происходят два события, связанных с РКГ: гибель полярных ФК в нормально развивающихся яйцевых камерах и элиминация яйцевых камер в случае аномалий их развития либо в условиях голодания. Гибель полярных ФК является единственным примером РКГ в яичнике, для которой требуются проапоптотические регуляторные белки семейства RHG. Полярные клетки происходят из кластеров 3–6 специализированных ФК, расположенных на переднем и заднем концах яйцевой камеры во время раннего оогенеза. К пятой стадии число полярных клеток в каждом кластере уменьшается до двух, а остальные клетки удаляются каноническим апоптотозным путем: HID ⊣ DIAP1 ⊣ Dronc → Drice [59, 60]. Индукция апоптоза в полярных ФК опосредуется сигнальным путем JAK/STAT [61].
Стадиоспецифичная РКГ яйцевых камер в среднем оогенезе, вероятно, является следствием контроля условий развития фолликула перед началом энергетически затратного вителлогенеза, запускающегося на восьмой стадии. В начале процесса хроматин ПК в погибающей камере конденсируется и фрагментируется. Когда наступают эти события, ФК переключаются на фагоцитарные функции, увеличиваясь в размере и поглощая материал КЗЛ [62–64]. Сигнальные каскады, регулирующие гибель в среднем оогенезе, были частично идентифицированы. На системном уровне (на уровне организма) сенсором достаточности питательных веществ является инсулиновый сигнальный каскад, тогда как на уровне клетки ключевую роль в регуляции метаболизма играет киназа mTOR. Активация mTOR может происходить как финальное событие опосредованного инсулином PI3K/Akt-сигнального каскада, так и независимо от инсулина, в ответ на сигналы от факторов роста, внутриклеточного транспортера аминокислот Slimfast (оценка достаточности аминокислот в клетке), AMPK (сенсора энергии) и других. В условиях белкового голодания в организме (инсулин-продуцирующих клетках мозга и железы corpus allatum) снижается уровень экспрессии инсулино-подобных белков, которые, в том числе, регулируют синтез экдистероидных гормонов [8, 65]. В результате в яичниках происходит повышение концентрации экдизона, которое на восьмой стадии приводит к активации экспрессии определенных транскрипционных факторов, в частности активации в ФК стимулирующего апоптоз фактора E75A (Eip75B), что, по предположению некоторых авторов, может запускать апоптоз по всей яйцевой камере [66, 67]. Активности одного инсулинового каскада недостаточно для индукции РКГ в среднем оогенезе. Большинство яйцевых камер, содержащих КЗЛ, мутантные по генам инсулинового каскада, InR, chico, Akt, S6k, не могут развиться до середины оогенеза, но они также не способны правильно деградировать через фагоцитоз посредством ФК. Эти камеры проявляют специфическую аномальную морфологию с сохранением КЗЛ и потерей ФК как при нормальном питании, так и при белковом голодании. Однако мутации по гену Tor имеют такое же фенотипическое проявление, как и реакция на белковое голодание. На основании этого предположили, что в среднем оогенезе существует независимый путь регуляции РКГ, передающий сигнал через Tor-киназу. Киназа Tor может являться ключевым регулятором РКГ в среднем оогенезе [68].
На начальном этапе гибели яйцевой камеры происходит каспаза-зависимая гибель КЗЛ с участием эффекторной каспазы Dcp-1. При этом в КЗЛ происходят процессы, характерные для апоптоза: конденсация хроматина, разрывы ДНК, фрагментация ядерного материала и цитоплазмы [57, 62, 69, 70]. Однако в отличие от большинства примеров апоптоза у дрозофилы гибель КЗЛ в среднем оогенезе не требует экспрессии проапоптотических генов reaper, hid, grim и гена dark, кодирующего адаптерный белок апоптосомы. Кроме того, мутации в генах инициаторных каспаз слабо влияют на процесс гибели. На основе этих данных предположили, что гибель КЗЛ осуществляется уникальной программой с неканоническим апоптосома-независимым механизмом активации эффекторной каспазы Dcp-1 [71, 72].
В условиях недостатка питательных веществ в яйцевых камерах наблюдается увеличение количества аутофагосом и лизосом. В КЗЛ была выявлена взаимосвязь апоптоза и аутофагии, сходная с гибелью в раннем оогенезе [46, 57]. Кроме того, в экспериментах с индукцией гибели в среднем оогенезе установили, что эффекторная каспаза Dcp-1 взаимодействует с митохондриальной аденин-нуклеотидной транслоказой SesB и в условиях голодания стимулирует аутофагию [73]. Нарушение экспрессии генов atg1, atg7 не приводит к предотвращению гибели камер, но замедляет поглощение и деградацию остатков КЗЛ в ФК. Предположили, что аутофагия принимает участие в деградации дебриса КЗЛ в ФК [74]. Все эти факты свидетельствуют об участии аутофагии в разных событиях клеточной гибели в среднем оогенезе.
Процесс гибели КЗЛ характеризуется специфичной динамикой митохондриального аппарата. Митохондриальные сети фрагментируются (ремоделируются), далее из митохондрий формируются кластеры, которые позже поглощаются окружающими ФК и деградируют там при участии аутофагии. События митохондриальной динамики зависят от каспаз, белков семейства Bc1-2, Debcl и Buffy, белков аппарата слияния/деления Opa1 и Drp1, соответственно. Нарушение экспрессии генов debsy, buffy, opa1, drp1 приводит не только к изменению митохондриальной динамики, но и к значительному ингибированию гибели клеток и яйцевых камер. Эти данные позволили предположить, что гены debsy, buffy, opa1, drp1 действуют проапоптотически и участвуют в регуляции и, возможно, в активации клеточной гибели [74].
Завершающим этапом гибели яйцевой камеры является удаление погибающих КЗЛ с помощью процесса поглощения или фагоцитоза, который включает захват и деградацию остатков (дебриса) клеток. Фагоцитоз осуществляется ФК, выполняющими в яичнике функцию непрофессиональных фагоцитов. ФК синхронно увеличиваются в размере и поглощают погибающие ПК [4, 75]. До сих пор еще относительно мало известно о молекулярных изменениях, происходящих в эпителиальных клетках при инициации программы фагоцитоза in vivo. В результате исследований последних лет были выявлены основные белки и некоторые сигнальные процессы, необходимые для прохождения фагоцитоза в ФК [76]. Было установлено, что два рецептора поглощения, гетеродимер αPS3/βPS интегрина и Draper, а также сигнальный каскад JNK необходимы для фагоцитоза в ФК [64, 77, 78]. Известные по участию в фагоцитозе у различных организмов интегрины и Draper представляют собой трансмембранные белки, локализующиеся на поверхности ФК [79–86]. Интегрины необходимы для процесса захвата материала и предположительно участвуют в прикреплении ФК к КЗЛ. Draper выполняет функцию рецептора узнавания умирающих клеток. Он получает неизвестный сигнал от ПК и передает его в клетку посредством тирозинкиназ Shark и Cdc42. Вероятно, от Draper активируются ГТФаза Rac1, стимулирующая перестройку цитоскелета, и адаптерный белок Сed-12, необходимый для созревания фагосом [87]. После инициации фагоцитоза интегрины и Draper локализуются преимущественно на апикальной поверхности ФК. Было установлено, что для правильного расположения рецепторов необходима экспрессия генов, участвующих в регуляции клеточной полярности, aPKC, baz, par-6, crb и генов Dhc64C, Cdc42 [77, 88].
Деградация остатков ПК в фагосомах происходит посредством канонического пути фагоцитоза с участием малых ГТФаз Rab5 и Rab7, включающего созревание фагосом и их слияние с лизосомами [87, 89–91]. По мере того, как фагоцитоз приближается к завершению, ФК теряют мембранные маркеры, их ядра уплотняются, и в конечном итоге ФК деградируют. Гибель происходит через каспаза-независимый путь, но механизм гибели еще не выяснен [64].
Таким образом, РКГ на средней стадии оогенеза начинается с гибели полярных ФК в нормально развивающихся яйцевых камерах, которая происходит каноническим апоптозом. Гибель КЗЛ и яйцевых камер в целом происходит в ответ на аномалии развития либо при недостатке нутриентов. КЗЛ погибают каспаза-зависимым путем при участии аутофагии. Гибель КЗЛ, в свою очередь, инициирует фагоцитоз, осуществляемый окружающими эпителиальными ФК.
РКГ В ПОЗДНЕМ ООГЕНЕЗЕ
Гибель ПК в позднем оогенезе является частью процесса нормального развития яйцевой камеры. Признаки гибели ПК появляются на стадии 10Б, проявляясь в пермеабилизации ядерной оболочки и высвобождении ядерных белков в цитоплазму ПК [11, 62, 69]. На 11 стадии оогенеза большая часть цитоплазмы ПК перемещается в ооцит. С 11-й по 13-ю стадии оставшиеся компоненты ПК, включая ядро, асинхронно подвергаются РКГ, деградируют и элиминируются. На 14-й стадии оогенеза зрелые яйцевые камеры не содержат ПК.
В процессе гибели в ядрах ПК происходят фрагментация ДНК и деградация ламины. В отличие от гибели на средней стадии, на поздней стадии ядра ПК не фрагментируются, они постепенно конденсируются, ядерный матрикс закисляется [63, 92–94]. Несмотря на то что в ПК наблюдаются активность каспазы Dcp-1 и индукция аутофагии, недавние исследования показали, что апоптоз и аутофагия вносят незначительный вклад в гибель ПК на данной стадии, так как комбинированное ингибирование каспаз и аутофагии слабо блокирует деградацию и элиминацию ПК [94–96]. Предположили, что важную роль в гибели ПК могут играть покрывающие их специфичные для поздних стадий ФК, называемые стрейч-ФК. Гибель стрейч-ФК, индуцированная посредством РНК-интерференции гена Diap1, кодирующего ингибитор каспаз, вызывает значительное предотвращение гибели и элиминации ПК (~73% ядер ПК сохраняются до 14-й стадии). При этом нарушаются основные процессы, связанные с гибелью ПК, такие как пермеабилизация ядерной оболочки, фрагментация ДНК, деградация ламины, закисление ядерного матрикса; кроме того, нарушается перенос цитоплазмы из ПК в ооцит на 11-й стадии. Основываясь на полученных данных, была высказана гипотеза, что гибель ПК на поздних стадиях оогенеза является неавтономной, она вызывается и контролируется стрейч-ФК. Однако поскольку при отсутствии ФК небольшая часть ПК все-таки погибает, предположили, что помимо основного механизма РКГ могут существовать неизвестные клеточно-автономные эффекторы гибели ПК [97, 98].
В гибели и удалении ПК важную роль играют гены фагоцитоза ФК, рецепторов αPS3, βPS интегрина и draper, ген Сed-12. Для элиминации ПК требуются экспрессия связанных с эндоцитозом и лизосомным биогенезом генов shark, Src42A, mbc, Rac1, Gprk2, Rab5, Rab7, Rab35, dor, а также сигнальный каскад JNK. Было показано, что функционирование аппарата фагоцитоза ФК, опосредующего гибель и удаление ПК, различается в среднем и позднем оогенезе. Например, в позднем оогенезе гены фагоцитоза необходимы как для гибели, так и для элиминации ПК, тогда как в среднем оогенезе их роль ограничена элиминацией ПК. Также в позднем оогенезе рецептор Draper не является активатором каскада JNK, для его активации требуется экспрессия Ced-12 (Elmo) [97, 98]. В процессе гибели яйцевых камер в среднем оогенезе ПК поглощаются окружающими ФК путем образования фагосом, содержащих апоптотический дебрис [62, 64]. Однако во время гибели в позднем оогенезе не наблюдается признаков активного везикулярного фагоцитарного поглощения компонентов ПК стрейч-ФК, следовательно, хотя гибель и удаление ПК опосредуются генами фагоцитоза, но происходят преимущественно без деградации дебриса в фагосомах. В то же время целый ряд фактов свидетельствует о важной роли лизосом в гибели и элиминации ПК на поздней стадии. К ним относятся интенсивное окрашивание стрейч-ФК лизотрекером, большая степень сохранения ПК у мутантов по гену dor, кодирующему белок лизосомального трафика Vps18, а также значительное закисление остатков ПК. На основе полученных данных была предложена гипотеза, что несколько стрейч-ФК окружают каждую ПК, а затем используют свой лизосомальный аппарат для закисления и деградации ПК без формирования фагосом [98]. Помимо лизосомного аппарата в гибели ПК могут быть задействованы механизмы программируемого некроза. На это указывают значительное усиление окраски лизотрекером остаточных ПК в процессе гибели, присутствие активных форм кислорода и поглощение пропидия иодида (нарушение целостности мембраны), высвобождение кальция в цитоплазму ПК после пермеабилизации ядерной мембраны [99–101]. Для прояснения данного вопроса необходимы дальнейшие исследования.
В конце 14-й стадии, когда закончен синтез оболочки хориона, происходят гибель и удаление выполнивших свою роль ФК. Исследования у разных представителей Diptera показали, что эти клетки подвергаются гибели по пути клеточной аутофагии, без участия каспаз. В конце оогенеза оставшиеся ФК отсоединяются от оболочки яйца, когда зрелое яйцо выходит из овариолы через яйцевод в матку. Отделившиеся ФК накапливаются у входа в яйцевод, где они могут поглотиться эпителиальными клетками и/или макрофагами [102].
Таким образом, онтогенетически регулируемая неапоптотическая гибель ПК в конце оогенеза происходит с помощью программы, запускаемой ФК. Для создания целостной картины действия путей РКГ в яичнике Drosophila необходимо найти ответы на множество вопросов. Предстоит найти сигналы, запускающие процессы гибели КЗЛ, сигнал, активирующий рецептор Draper и запускающий фагоцитоз в среднем оогенезе. Еще не определено, каким образом ФК активируются для участия в гибели и удалении ПК в конце оогенеза, не выяснено, откуда поступают ранние сигналы, инициирующие гибель, – от ооцита, от ПК или от источника, внешнего по отношению к яичнику. Предстоит продолжить поиск участников процессов, выявление молекулярных механизмов, сигнальных путей и их взаимодействия.
ЗАКЛЮЧЕНИЕ
Регулируемая клеточная гибель является полноправным участником нормального функционирования гонад и созревания яйцеклеток дрозофилы. Хотя оогенез существенно различается у насекомых и позвоночных, любому многоклеточному организму для формирования жизнеспособной, компетентной и качественной яйцеклетки необходимо быстро реагировать на сигналы развития и окружающей среды и эффективно удалять избыточные и дефектные половые клетки. Так, у млекопитающих овулирует менее 1% яйцеклеток, большинство половых клеток (>99%) удаляются из яичника в течение всей репродуктивной жизни [103, 104]. Причины и механизмы такой значительной потери ооцитов остаются недостаточно изученными. Считается, что основным механизмом гибели избыточных и/или дефектных клеток яичников млекопитающих является апоптоз [103], однако накапливаются данные, что другие механизмы РКГ также вовлечены в элиминацию клеток [105–108]. Например, аутофагия также участвует в гибели КЗЛ и гранулезных клеток на разных стадиях оогенеза млекопитающих [105, 106]. В последнее время появились данные, что и некроз может быть задействован в РКГ КЗЛ и ФК позвоночных [107, 108]. Следует отметить, что сведения о РКГ в оогенезе млекопитающих немногочисленны, разрозненны, выполнены на разных видах животных и зачастую противоречивы. Особенности исследования РКГ в оогенезе человека – малое количество доступного материала, большое генетическое разнообразие доноров и невозможность манипулирования генами, что существенно затрудняет выявление механизмов РКГ [103, 104]. Большинство генов, регулирующих клеточную гибель консервативны, поэтому модельные объекты являются важным инструментом для изучения механизмов РКГ в оогенезе [109].
Яичник Drosophila представляет собой ценную модель для изучения разнообразия типов клеточной смерти, включая автономную и неавтономную гибель зародышевой линии с участием неканонического каспаза-зависимого пути и индукцией аутофагии, активацию аппарата фагоцитоза в эпителиальных ФК для удаления клеточного дебриса. Как и у млекопитающих, у дрозофилы центральную роль в выживании клеток яичника играет сигнальный каскад PI3K/Akt, а инициация РКГ зависит от баланса про- и антиапоптотических факторов в каждой конкретной клетке. Участие ФК в смерти зародышевых клеток Drosophila напоминает роль гранулезных клеток в смерти ооцитов у млекопитающих [110]. Исследование индуцируемого фагоцитоза, осуществляемого эпителиальными ФК в яичнике Drosophila, может дать представление о том, как фагоцитарная функция активируется и осуществляется в непрофессиональных фагоцитах [87]. В настоящее время мало известно о молекулярных изменениях, необходимых для инициации в эпителиальных клетках процесса поглощения in vivo, о путях регуляции рецепторов поглощения, таких как интегрины, являющихся критически важными для фагоцитоза как в профессиональных, так и в непрофессиональных фагоцитах у млекопитающих и известных своей ролью в адгезии, миграции и инвазии раковых клеток [77, 111]. Таким образом, изучение РКГ в яичнике дрозофилы может дать понимание многих аспектов регуляции клеточной гибели у млекопитающих.
Работа поддержана Базовым бюджетным проектом 0324-2019-0042-C-01.
Настоящая статья не содержит каких-либо исследований с использованием в качестве объекта животных.
Настоящая статья не содержит каких-либо исследований с участием в качестве объекта людей.
Авторы заявляют, что у них нет конфликта интересов.
Список литературы
Schweichel J.U., Merker H.J. The morphology of various types of cell death in prenatal tissues // Teratology. 1973. V. 7. № 3. P. 253–266. https://doi.org/10.1002/tera.1420070306
Galluzzi L., Vitale I., Aaronson S.A. et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018 // Cell Death Differ. 2018. V. 25. № 3. P. 486–541. https://doi.org/10.1038/s41418-017-0012-4
Jenkins V.K., Timmons A.K., McCall K. Diversity of cell death pathways: insight from the fly ovary // Trends Cell Biol. 2013. V. 23. № 11. P. 567–574. https://doi.org/10.1016/j.tcb.2013.07.005
King R.C. Ovarian Development in Drosophila melanogaster. N.Y.: Acad. Press, 1970.
King R.C., Rubinson A.C., Smith R.F. Oogenesis in adult Drosophila melanogaster // Growth. 1956. V. 20. № 2. P. 121–157.
Ables E.T., Drummond-Barbosa D. The steroid hormone ecdysone functions with intrinsic chromatin remodeling factors to control female germline stem cells in Drosophila // Cell. Stem. Cel. 2010. V. 7. № 5. P. 581–592. https://doi.org/10.1016/j.stem.2010.10.001
Belles X., Piulachs M.D. Ecdysone signalling and ovarian development in insects: from stem cells to ovarian follicle formation // Biochim. Biophys. Acta. 2015. V. 1849. № 2. P. 181–186. https://doi.org/10.1016/j.bbagrm.2014.05.025
Badisco L., Van Wielendaele P., Vanden Broeck J. Eat to reproduce: a key role for the insulin signaling pathway in adult insects // Front. Physiol. 2013. V. 4. art. 202. https://doi.org/10.3389/fphys.2013.00202
Buszczak M., Cooley L. Eggs to die for: cell death during Drosophila oogenesis // Cell Death Differ. 2000. V. 7. № 11. P. 1071–1074. https://doi.org/10.1038/sj.cdd.4400755
McCall K. Eggs over easy: cell death in the Drosophila ovary // Dev. Biol. 2004. V. 274. № 1. P. 3–14. https://doi.org/10.1016/j.ydbio.2004.07.017
Pritchett T.L., Tanner E.A., McCall K. Cracking open cell death in the Drosophila ovary // Apoptosis. 2009. V. 14. № 8. P. 969–979. https://doi.org/10.1007/s10495-009-0369-z
Drummond-Barbosa D., Spradling A.C. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis // Dev. Biol. 2001. V. 231. № 1. P. 265–278. https://doi.org/10.1006/dbio.2000.0135
Огиенко А.А., Федорова С.А., Баричева Э.М. Основные аспекты развития половой системы самок Drosophila melanogaster // Генетика. 2007. Т. 43. № 10. С. 1341–1357.
Wu X., Tanwar P.S., Raftery L.A. Drosophila follicle cells: morphogenesis in an eggshell // Semin. Cell Dev. Biol. 2008. V. 19. № 3. P. 271–282. https://doi.org/10.1016/j.semcdb.2008.01.004
Kumar S. Caspase function in programmed cell death // Cell Death Differ. 2007. V. 14. № 1. P. 32–43. https://doi.org/10.1038/sj.cdd.4402060
Kumar S., Doumanis J. The fly caspases // Cell Death Differ. 2000. V. 7. № 11. P. 1039–1044. https://doi.org/10.1038/sj.cdd.4400756
Dorstyn L., Colussi P.A., Quinn L.M. et al. DRONC, an ecdysone-inducible Drosophila caspase // Proc. Natl Acad. Sci. USA. 1999. V. 96. № 8. P. 4307–4312. https://doi.org/10.1073/pnas.96.8.4307
Fraser A.G., McCarthy N.J., Evan G.I. drICE is an essential caspase required for apoptotic activity in Drosophila cells // EMBO J. 1997. V. 16. № 20. P. 6192–6199. https://doi.org/10.1093/emboj/16.20.6192
Song Z., McCall K., Steller H. DCP-1, a Drosophila cell death protease essential for development // Science. 1997. V. 275. № 5299. P. 536–540. https://doi.org/10.1126/science.275.5299.536
Xu D., Li Y., Arcaro M. et al. The CARD-carrying caspase Dronc is essential for most, but not all, developmental cell death in Drosophila // Development. 2005. V. 132. № 9. P. 2125–2134. https://doi.org/10.1242/dev.01790
Xu D., Wang Y., Willecke R. et al. The effector caspases drICE and dcp-1 have partially overlapping functions in the apoptotic pathway in Drosophila // Cell Death Differ. 2006. V. 13. № 10. P. 1697–1706. https://doi.org/10.1038/sj.cdd.4401920
Deveraux Q.L., Reed J.C. IAP family proteins-suppressors of apoptosis // Genes Dev. 1999. V. 13. № 3. P. 239–252. https://doi.org/10.1101/gad.13.3.239
Orme M., Meier P. Inhibitor of apoptosis proteins in Drosophila: gatekeepers of death // Apoptosis. 2009. V. 14. № 8. P. 950–960. https://doi.org/10.1007/s10495-009-0358-2
Denton D., Aung-Htut M.T., Kumar S. Developmentally programmed cell death in Drosophila // Biochim. Biophys. Acta. 2013. V. 1833. № 12. P. 3499–3506. https://doi.org/10.1016/j.bbamcr.2013.06.014
Goyal L., McCall K., Agapite J. et al. Induction of apoptosis by Drosophila reaper, hid and grim through inhibition of IAP function // EMBO J. 2000. V. 19. № 4. P. 589–597. https://doi.org/10.1093/emboj/19.4.589
Chai J., Yan N., Huh J.R. et al. Molecular mechanism of Reaper-Grim-Hid-mediated suppression of DIAP1‑dependent Dronc ubiquitination // Nat. Struct. Biol. 2003. V. 10. № 11. P. 892–898. https://doi.org/10.1038/nsb989
Rodriguez A., Oliver H., Zou H. et al. Dark is a Drosophila homologue of Apaf-1/CED-4 and functions in an evolutionarily conserved death pathway // Nat. Cell Biol. 1999. V. 1. № 5. P. 272–279. https://doi.org/10.1038/12984
Yu X., Wang L., Acehan D. et al. Three-dimensional structure of a double apoptosome formed by the Drosophila Apaf-1 related killer // J. Mol. Biol. 2006. V. 355. № 3. P. 577–89. https://doi.org/10.1016/j.jmb.2005.10.040
Xu D., Woodfield S.E., Lee T.V. et al. Genetic control of programmed cell death (apoptosis) in Drosophila // Fly. 2009. V. 3. № 1. P. 78–90. https://doi.org/10.4161/fly.3.1.7800
Mukae N., Yokoyama H., Yokokura T. et al. Identification and developmental expression of inhibitor of caspase activated DNase (ICAD) in Drosophila melanogaster // J. Biol. Chem. 2000. V. 275. № 28. P. 21402–21408. https://doi.org/10.1074/jbc.M909611199
Taylor R.C., Cullen S.P., Martin S.J. Apoptosis: controlled demolition at the cellular level // Nat. Rev. Mol. Cell Biol. 2008. V. 9. № 3. P. 231–241. https://doi.org/10.1038/nrm2312
Adrain C., Creagh E.M., Cullen S.P., Martin S.J. Caspase-dependent inactivation of proteasome function during programmed cell death in Drosophila and man // J. Biol. Chem. 2004. V. 279. № 35. P. 36923–36930. https://doi.org/10.1074/jbc.M402638200
Burlacu A. Regulation of apoptosis by Bcl-2 family proteins // J. Cell. Mol. Med. 2003. V. 7. № 3. P. 249–257. https://doi.org/10.1111/j.1582-4934.2003.tb00225.x
Willis S., Day C.L., Hinds M.G., Huang D.C.S. The Bcl-2-regulated apoptotic pathway // J. Cell Sci. 2003. V. 116. № 20. P. 4053–4056. https://doi.org/10.1242/jcs.00754
Pang Y., Bai X.C., Yan C. et al. Structure of the apoptosome: mechanistic insights into activation of an initiator caspase from Drosophila // Genes Dev. 2015. V. 29. № 3. P. 277–287. https://doi.org/10.1101/gad.255877.114
Abdelwahid E., Yokokura T., Krieser R.J. et al. Mitochondrial disruption in Drosophila apoptosis // Dev. Cell. 2007. V. 12. № 5. P. 793–806. https://doi.org/10.1016/j.devcel.2007.04.004
Goyal G., Fell B., Sarin A. et al. Role of mitochondrial remodeling in programmed cell death in Drosophila melanogaster // Dev. Cell. 2007. V. 12. № 5. P. 807–816. https://doi.org/10.1016/j.devcel.2007.02.002
Martinou J.C., Youle R.J. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics // Dev. Cell. 2011. V. 21. № 1. P. 92–101. https://doi.org/10.1016/j.devcel.2011.06.017
Krieser R.J., White K. Inside an enigma: do mitochondria contribute to cell death in Drosophila ? // Apoptosis. 2009. V. 14. № 8. P. 961–968. https://doi.org/10.1007/s10495-009-0362-6
Thomenius M., Freel C.D., Horn S. et al. Mitochondrial fusion is regulated by Reaper to modulate Drosophila programmed cell death // Cell Death Differ. 2011. V. 18. № 10. P. 1640–1650. https://doi.org/10.1038/cdd.2011.26
Das G., Shravage B.V., Baehrecke E.H. Regulation and function of autophagy during cell survival and cell death // Cold Spring Harb. Perspect. Biol. 2012. V. 4. № 6. P. a008813. https://doi.org/10.1101/cshperspect.a008813
Ковалева О.В., Шитова М.С., Зборовская И.Б. Аутофагия: клеточная гибель или способ выживания? // Клиническая онкогематология. 2014. Т. 7. № 2. С. 103–113.
Yu L., Chen Y., Tooze S.A. Autophagy pathway: Cellular and molecular mechanisms // Autophagy. 2018. V. 14. № 2. P. 207–215. https://doi.org/10.1080/15548627.2017.1378838
Zirin J., Perrimon N. Drosophila as a model system to study autophagy // Semin. Immunopathol. 2010. V. 32. № 4. P. 363–372. https://doi.org/10.1007/s00281-010-0223-y
Ryoo H.D., Baehrecke E.H. Distinct death mechanisms in Drosophila development // Curr. Opin. Cell Biol. 2010. V. 22. № 6. P. 889–895. https://doi.org/10.1016/j.ceb.2010.08.022
Barth J.M., Szabad J., Hafen E., Köhler K. Autophagy in Drosophila ovaries is induced by starvation and is required for oogenesis // Cell Death Differ. 2011. V. 18. № 6. P. 915–924. https://doi.org/10.1038/cdd.2010.157
Kroemer G., Galluzzi L., Vandenabeele P. et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009 // Cell Death Differ. 2009. V. 16. № 1. P. 3–11. https://doi.org/0.1038/cdd.2008.150
Vlachos M., Tavernarakis N. Non-apoptotic cell death in Caenorhabditis elegans // Developmental Dynamics. 2010. V. 239. № 5. P. 1337–1351. https://doi.org/10.1002/dvdy.22230
Berghe T., Linkermann A., Jouan-Lanhouet S. et al. Regulated necrosis: the expanding network of non-apoptotic cell death pathways // Nat. Rev. Mol. Cell Biol. 2014. V. 15. № 2. P. 134–146. https://doi.org/10.1038/nrm3737
Dondelinger Y., Hulpiau P., Saeys Y. et al. An evolutionary perspective on the necroptotic pathway // Trends Cell Biol. 2016. V. 26. № 10. P. 721–732. https://doi.org/0.1016/j.tcb.2016.06.004
Kanda H., Igakib T., Okanoa H., Miuradet M. Conserved metabolic energy production pathways govern Eiger/TNF induced nonapoptotic cell death // Proc. Natl Acad. Sci. USA. 2011. V. 108. № 47. P. 18977–18982. https://doi.org/10.1073/pnas.1103242108
Yang Y., Hou L., Li Y., Ni J., Liu L. Neuronal necrosis and spreading death in a Drosophila genetic model // Cell Death Dis. 2013. V. 4. № 7. P. e723. https://doi.org/10.1038/cddis.2013.232
Myllymaki H., Valanne S., Rämet M. The Drosophila imd signaling pathway // J. Immunol. 2014. V. 192. № 8. P. 3455–3462. https://doi.org/10.4049/jimmunol.1303309
LaFever L., Drummond-Barbosa D. Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila // Science. 2005. V. 309. № 5737. P. 1071–1073. https://doi.org/10.1126/science.1111410
Peterson J.S., Timmons A.K., Mondragon A.A., McCall K. The end of the beginning: cell death in the germline // Curr. Top. Dev. Biol. 2015. V. 114. P. 93–119. https://doi.org/10.1016/bs.ctdb.2015.07.025
Carney G.E., Bender M. The Drosophila ecdysone receptor (EcR) gene is required maternally for normal oogenesis // Genetics. 2000. V. 154. № 3. P. 1203–1211.
Hou Y.C., Chittaranjan S., Barbosa S.G. et al. Effector caspase Dcp-1 and IAP protein Bruce regulate starvation-induced autophagy during Drosophila melanogaster oogenesis // J. Cell Biol. 2008. V. 182. № 6. P. 1127–1139.https://doi.org/10.1083/jcb.200712091
Nezis I.P., Lamark T., Velentzas A.D. Cell death during Drosophila melanogaster early oogenesis is mediated through autophagy // Autophagy. 2009. V. 5. № 3. P. 298–302. https://doi.org/10.4161/auto.5.3.7454
Besse F., Pret A.M. Apoptosis-mediated cell death within the ovarian polar cell lineage of Drosophila melanogaster // Development. 2003. V. 130. № 5. P. 1017–1027. https://doi.org/10.1242/dev.00313
Khammari A., Agnès F., Gandille P., Pret A.-M. Physiological apoptosis of polar cells during Drosophila oogenesis is mediated by Hid-dependent regulation of Diap1 // Cell Death Differ. 2011. V. 18. № 5. P. 793–805. https://doi.org/10.1038/cdd.2010.141
Borensztejn A., Boissoneau E., Fernandez G. et al. JAK/STAT autocontrol of ligand-producing cell number through apoptosis // Development. 2013. V. 140. № 1. P. 195–204. https://doi.org/10.1242/dev.079046
Giorgi F., Deri P. Cell death in ovarian chambers of Drosophila melanogaster // J. Emb. Exp. Morph. 1976. V. 35. № 3. P. 521–533.
Nezis I.P., Stravopodis D.J., Papassideri I. et al. Stage-specific apoptotic patterns during Drosophila oogenesis // Eur. J. Cell Biol. 2000. V. 79. № 9. P. 610–620. https://doi.org/10.1078/0171-9335-00088
Etchegaray J.I., Timmons A.K., Klein A.P. et al. Draper acts through the JNK pathway to control synchronous engulfment of dying germline cells by follicular epithelial cells // Development. 2012. V. 139. № 21. P. 4029–4039. https://doi.org/10.1242/dev.082776
Templeman N.M., Murphy C.T. Regulation of reproduction and longevity by nutrient-sensing pathways // J. Cell Biol. 2018. V. 217. № 1. P. 93–106. https://doi.org/10.1083/jcb.201707168
Terashima J., Takaki K., Sakurai S., Bownes M. Nutritional status affects 20-hydroxyecdysone concentration and progression of oogenesis in Drosophila melanogaster // J. Endocrinol. 2005. V. 187. № 1. P. 69–79. https://doi.org/10.1677/joe.1.06220
Terashima J., Bownes M. E75A and E75B have opposite effects on the apoptosis/development choice of the Drosophila egg chamber // Cell Death Differ. 2006. V. 13. № 3. P. 454–464. https://doi.org/10.1038/sj.cdd.4401745
Pritchett T.L., McCall K. Role of the insulin/Tor signaling network in starvation-induced programmed cell death in Drosophila oogenesis // Cell Death Differ. 2012. V. 19. № 6. P. 1069–1079. https://doi.org/10.1038/cdd.2011.200
McCall K., Steller H. Requirement for DCP-1 caspase during Drosophila oogenesis // Science. 1998. V. 279. № 5348. P. 230–234. https://doi.org/0.1126/science.279.5348.230
Laundrie B., Peterson J.S., Baum J.S. et al. Germline cell death is inhibited by P-element insertions disrupting the dcp-1/pita nested gene pair in Drosophila // Genetics. 2003. V. 165. № 4. P. 1881–1888.
Peterson J.S., Bass B.P., Jue D. et al. Noncanonical cell death pathways act during Drosophila oogenesis // Genesis. 2007. V. 45. № 6. P. 396–404. https://doi.org/10.1002/dvg.20306
Baum J.S., Arama E., Steller H., McCall K. The Drosophila caspases Strica and Dronc function redundantly in programmed cell death during oogenesis // Cell Death Differ. 2007. V. 14. № 8. P. 1508–1517. https://doi.org/10.1038/sj.cdd.4402155
DeVorkin L., Go N.E., Hou Y.C. et al. The Drosophila effector caspase Dcp-1 regulates mitochondrial dynamics and autophagic flux via SesB // J. Cell Biol. 2014. V. 205. № 4. P. 477–492. https://doi.org/10.1083/jcb.201303144
Tanner E.A., Blute T.A., Brachmann C.B., McCall K. Bcl-2 proteins and autophagy regulate mitochondrial dynamics during programmed cell death in the Drosophila ovary // Development. 2011. V. 138. № 2. P. 327–338. https://doi.org/10.1242/dev.057943
Lauber K., Blumenthal S.G., Waibel M., Wesselborg S. Clearance of apoptotic cells: getting rid of the corpses // Mol. Cell. 2004. V. 14. № 3. P. 277–287. https://doi.org/10.1016/S1097-2765(04)00237-0
Serizier S.B., McCall K. Scrambled eggs: apoptotic cell clearance by non-professional phagocytes in the Drosophila ovary // Front. Immunol. 2017. V. 8. Article 1642. https://doi.org/10.3389/fimmu.2017.01642
Meehan T.L., Kleinsorge S.E., Timmons A.K. et al. Polarization of the epithelial layer and apical localization of integrins are required for engulfment of apoptotic cells // Dis. Model. Mech. 2015. V. 8. № 12. P. 1603–1614. https://doi.org/10.1242/dmm.021998
Igaki T. Correcting developmental errors by apoptosis: lessons from Drosophila JNK signaling // Apoptosis. 2009. V. 14. № 8. P. 1021–1028. https://doi.org/0.1007/s10495-009-0361-7
Finnemann S.C., Silverstein R.L. Differential roles of CD36 and ανβ5 integrin in photoreceptor phagocytosis by the retinal pigment epithelium // J. Exp. Med. 2001. V. 194. № 9. P. 1289–1298. https://doi.org/10.1084/jem.194.9.1289
Sexton D.W., Blaylock M.G., Walsh G.M. Human alveolar epithelial cells engulf apoptotic eosinophils by means of integrin- and phosphatidylserine receptor-dependent mechanisms: a process upregulated by dexamethasone // J. Allergy Clin. Immunol. 2001. V. 108. № 6. P. 962–969. https://doi.org/10.1067/mai.2001.119414
Flannagan R.S., Canton J., Furuya W. et al. The phosphatidylserine receptor TIM4 utilizes integrins as coreceptors to effect phagocytosis // Mol. Biol. Cell. 2014. V. 25. № 9. P. 1511–1522. https://doi.org/10.1091/mbc.E13-04-0212
Hsieh H.-H., Hsu T.-Y., Jiang H.-S., Wu Y.-C. Integrin α PAT-2/CDC-42 signaling is required for muscle-mediated clearance of apoptotic cells in Caenorhabditis elegans // PLoS Genet. 2012. V. 8. № 5. P. e1002663. https://doi.org/10.1371/journal.pgen.1002663
Manaka J., Kuraishi T., Shiratsuchi A. et al. Draper-mediated and phosphatidylserine-independent phagocytosis of apoptotic cells by Drosophila hemocytes/macrophages // J. Biol. Chem. 2004. V. 279. № 46. P. 48466–48476. https://doi.org/10.1074/jbc.M408597200
Shiratsuchi A., Mori T., Sakurai K. et al. Independent recognition of Staphylococcus aureus by two receptors for phagocytosis in Drosophila // J. Biol. Chem. 2012. V. 287. № 26. P. 21663–21672. https://doi.org/10.1074/jbc.M111.333807
Nonaka S., Nagaosa K., Mori T. et al. Integrin αPS3/βν-mediated phagocytosis of apoptotic cells and bacteria in Drosophila // J. Biol. Chem. 2013. V. 288. № 15. P. 10374–10380. https://doi.org/10.1074/jbc.M113.451427
Evans I.R., Rodrigues F.S., Armitage E.L., Wood W. Draper/CEd-1 mediates an ancient damage response to control inflammatory blood cell migration in vivo // Curr. Biol. 2015. V. 25. № 12. P. 1606–1612. https://doi.org/10.1016/j.cub.2015.04.037
Meehan T.L., Joudi T.F., Timmons A.K. et al. Components of the engulfment machinery have distinct roles in corpse processing // PLoS One. 2016. V. 11. № 6. P. e0158217. https://doi.org/10.1371/journal.pone.0158217
Tepass U. The apical polarity protein network in Drosophila epithelial cells: regulation of polarity, junctions, morphogenesis, cell growth, and survival // Annu. Rev. Cell Dev. Biol. 2012. V. 28. № 1. P. 655–685. https://doi.org/10.1146/annurev-cellbio-092910-154033
Vieira O.V., Bucci C., Harrison R.E. et al. Modulation of Rab5 and Rab7 recruitment to phagosomes by phosphatidylinositol 3-kinase // Mol. Cell. Biol. 2003. V. 23. № 7. P. 2501–2514. https://doi.org/10.1128/MCB.23.7.2501-2514.2003
Kinchen J.M., Doukoumetzidis K., Almendinger J. et al. A pathway for phagosome maturation during engulfment of apoptotic cells // Nat. Cell Biol. 2008. V. 10. № 5. P. 556–566. https://doi.org/10.1038/ncb1718
Gutierrez M.G. Functional role(s) of phagosomal Rab GTPases // Small GTPases. 2013. V. 4. № 3. P. 148–158. https://doi.org/10.4161/sgtp.25604
Cummings M.R., King R.C. Ultrastructural changes in nurse and follicle cells during late stages of oogenesis in Drosophila melanogaster // Z. Zellforsch. 1970. V. 110. P. 1–8
Cavaliere V., Taddei C., Gargiulo G. Apoptosis of nurse cells at the late stages of oogenesis of Drosophila melanogaster // Dev. Genes Evol. 1998. V. 208. № 2. P. 106–112.
Peterson J.S., McCall K. Combined inhibition of autophagy and caspases fails to prevent developmental nurse cell death in the Drosophila melanogaster ovary // PLoS One. 2013. V. 8. № 9. P. e76046. https://doi.org/10.1371/journal.pone.0076046
Peterson J.S., Barkett M., McCall K. Stage-specific regulation of caspase activity in Drosophila oogenesis // Dev. Biol. 2003. V. 260. № 1. P. 113–123. https://doi.org/10.1016/S0012-1606(03)00240-9
Nezis I.P., Shravage B.V., Sagona A.P. et al. Autophagic degradation of dBruce controls DNA fragmentation in nurse cells during late Drosophila melanogaster ooge-nesis // J. Cell Biol. 2010. V. 190. № 4. P. 523–531. https://doi.org/10.1083/jcb.201002035
Timmons A.K., Mondragon A.A., Schenkel C.E. et al. Phagocytosis genes nonautonomously promote deve-lopmental cell death in the Drosophila ovary // Proc. Natl Acad. Sci. USA. 2016. V. 113. № 9. P. 1246–1255. https://doi.org/10.1073/pnas.1522830113
Timmons A.K., Mondragon A.A., Meehan T.L., McCall K. Control of non-apoptotic nurse cell death by engulfment genes in Drosophila // Fly. 2017. V. 11. № 2. P. 104–111. https://doi.org/10.1080/19336934.2016.1238993
Timmons A.K., Meehan T.L., Gartmond T.D., McCall K. Use of necrotic markers in the Drosophila ovary // Methods Mol. Biol. 2013. V. 1004. P. 215–228. https://doi.org/10.1007/978-1-62703-383-1_16
Matova N., Mahajan-Miklos S., Mooseker M.S., Cooley L. Drosophila Quail, a villin-related protein, bundles actin filaments in apoptotic nurse cells // Development. 1999. V. 126. № 24. P. 5645–5657.
Golstein P., Kroemer G. Cell death by necrosis: towards a molecular definition // Trends Biochem. Sci. 2007. V. 32. № 1. P. 37–43. https://doi.org/10.1016/j.tibs.2006.11.001
Nezis I.P., Stravopodis D.J., Margaritis L.H., Papassideri I.S. Autophagy is required for the degeneration of the ovarian follicular epithelium in higher Diptera // Autophagy. 2006. V. 2. № 4. P. 297–298. https://doi.org/10.4161/auto.2858
Marcozzi S., Rossi V., Salustri A. et al. Programmed cell death in the human ovary // Minerva Ginecol. 2018. V. 70. № 5. P. 549–560. https://doi.org/10.23736/S0026-4784.18.04274-0
Зенкина В.Г. Формирование фолликулярного резерва яичников // Бюл. сиб. медицины. 2018. Т. 17. № 3. С. 197–206.
Sun Y.C., Sun X.F., Dyce P.W. et al. The role of germ cell loss during primordial follicle assembly: a review of current advances // Int. J. Biol. Sci. 2017. V. 13. № 4. P. 449–457. https://doi.org/10.7150/ijbs.18836
Yadav P.K., Tiwari M., Gupta A. et al. Germ cell depletion from mammalian ovary: possible involvement of apoptosis and autophagy // J. Biomed. Sci. 2018. V. 25. P. 36. https://doi.org/10.1186/s12929-018-0438-0
Pajokh M., Talaei-Khozani T., Bordbar H., Mesbah F. Apoptosis, autophagy, and necrosis in murine embryonic gonadal ridges and neonatal ovaries: an animal model // I. J. Med. Sci. 2019. V. 44. № 1. P. 36–43.
Chaudhary G.R., Yadav P.K., Yadav A.K. et al. Necroptosis in stressed ovary // J. Biomed. Sci. 2019. V. 26. № 1. P. 1–6. https://doi.org/10.1186/s12929-019-0504-2
Thomson T.C., Fitzpatrick K.E., Johnson J. Intrinsic and extrinsic mechanisms of oocyte loss // Mol. Hum. Reprod. 2010. V. 16. № 12. P. 916–927. https://doi.org/10.1093/molehr/gaq066
Thomson T.C., Johnson J. Inducible somatic oocyte destruction in response to rapamycin requires wild-type regulation of follicle cell epithelial polarity // Cell Death Differ. 2010. V. 17. № 11. P. 1717–1727. https://doi.org/10.1038/cdd.2010.49
Onodera Y., Nam J.M., Sabe H. Intracellular trafficking of integrins in cancer cells // Pharmacol. Ther. 2013. V. 140. № 1. P. 1–9. https://doi.org/10.1016/j.pharmthera.2013.05.007
Дополнительные материалы отсутствуют.


