Вопросы ихтиологии, 2022, T. 62, № 2, стр. 211-238
Рыбы как источники кайромонов – химических сигналов для водных животных
А. О. Касумян *
Московский государственный университет
Москва, Россия
* E-mail: alex_kasumyan@mail.ru
Поступила в редакцию 11.03.2021
После доработки 15.03.2021
Принята к публикации 16.03.2021
- EDN: TNRTGY
- DOI: 10.31857/S0042875222020114
Аннотация
Систематизированы сведения об эффектах, оказываемых кайромонами (межвидовыми химическими сигналами) рыб, на водных животных – ракообразных, моллюсков, личинок и имаго насекомых, амфибий и других организмов, а также на некоторых животных вне воды. Рассмотрено разнообразие релизерных (поведенческих) и праймерных (фенотипических) реакций животных на запахи рыб и взаимоотношения, регулируемые этими сигналами. Оценено влияние биотических и абиотических факторов и условий существования (биотопическое сходство взаимодействующих видов, их ареал, образ жизни, тип питания и пищевое поведение, возраст и жизненная стадия, заражённость паразитами, накормленность, суточная и сезонная динамика и др.) на выделение кайромонов рыбами-донорами и восприимчивость к ним животных-реципиентов. Обобщены данные о химической природе кайромонов рыб. Обсуждается роль кайромонов рыб в регуляции связей между организмами в водных сообществах.
В исследованиях хемокоммуникаций рыб традиционно основное внимание уделяют выяснению разнообразия химических сигналов, воспринимаемых рыбами и регулирующих их внутривидовые и межвидовые взаимоотношения и различные формы поведения (Kasumyan, 2004). Источниками таких запахов чаще всего являются экзометаболиты – сложные смеси веществ разной природы, постоянно выделяемые рыбами в окружающую среду в результате жизнедеятельности (Wisenden, 2015). Некоторые из этих веществ эволюционно приобретают информационную функцию и становятся феромонами – химическими маркерами вида, популяции, социального статуса особи, индивидуальной и половой принадлежности, готовности к нересту и т.п. (Brönmark, Hansson, 2012; Sorensen, Wisenden, 2015). Другие вещества обеспечивают химическую коммуникацию между рыбами разных видов, а также рыб с другими представителями водных сообществ. Из межвидовых химических сигналов наиболее распространены кайромоны – запахи, продуцируемые особями одного вида, но содержащие полезную информацию для реципиентов, принадлежащих к другим видам.
Генерация запахов и создание рыбами химических информационных полей экспериментально показана уже почти около века назад (Wrede, 1932; Göz, 1941). Последующие исследования на многочисленных примерах подтвердили, что вода, в которой какое-то время пребывали рыбы, неизбежно приобретает специфический запах, улавливаемый другими рыбами и несущий им различную информацию (Sorensen, Wisenden, 2015). Формирование рыбами химического следа в воде подтверждает также детектирование в природных водоёмах экологической ДНК рыб − эДНК (environmental DNA − eDNA), что позволяет использовать этот метод для обнаружения рыб, мониторинга их популяций, определения сроков миграций, уточнения ареалов и т.п. (Никифоров и др., 2018; Coulter et al., 2019). Водные животные, сосуществующие с рыбами в одних и тех же водоёмах и биотопах, обладают хорошо развитой хеморецепцией (Kamio, Derby, 2017; Derby, 2020). Поэтому вещества, распространяемые рыбами, доступны для них в той же мере, что и самим рыбам. Однако исследования сигнальной функции и значения запахов рыб для других животных пока ещё не получили такого же внимания, как изучение хемокоммуникаций рыб. Но, несмотря на малочисленность и спорадичность экспериментальных работ, сведения, накопленные к настоящему времени в этой области химической экологии, свидетельствуют о том, что запахи рыб служат для многих водных животных важнейшими химическими регуляторами их поведения и метаболизма (Mitchell et al., 2017).
Большинство рыб питаются животной пищей и занимают верхние уровни в трофической иерархии водных экосистем (Wootton, 1998). Являясь хищниками по отношению к остальным гидробионтам, рыбы представляют для них опасность при прямом столкновении. Для успешного сосуществования с рыбами потенциальные жертвы должны обладать разнообразными защитными адаптациями, такими как шипы, колючки и других выросты, прочные внешние покровы, быстрое созревание и повышенная плодовитость, хорошие локомоторные возможности, образование скоплений и стай, способность к мимикрии, наличие химической защиты (токсичность, ядовитые железы, репеллентность, вкусовая детеррентность и т.п.) и многими другими (Lima, Dill, 1990; Paul et al., 2007; Ferrari et al., 2010). В число таких адаптаций входит обладание большими сенсорными возможностями и широким диапазоном воспринимаемых сигналов. Это позволяет жертвам заблаговременно обнаруживать опасность и вовремя реализовать двигательные реакции и иные защитные механизмы. Из-за особенностей водной среды дистантная хеморецепция имеет ряд несомненных преимуществ по сравнению с другими сенсорными системами водных животных, например, зрительной и акустической, сигналы которых формируют менее устойчивые и менее протяжённые информационные поля, подверженные нарушениям из-за интерференции и других ограничений (Atema, 2012).
Цель настоящего обзора − систематизация и анализ имеющихся сведений о роли и значении кайромонов в регуляции взаимоотношений рыб с другими представителями водных сообществ. В основные задачи обзора входит оценка разнообразия эффектов, оказываемых кайромонами рыб на водных животных различных групп, рассмотрение зависимости этих эффектов от образа жизни доноров и реципиентов кайромонов, от биотических и абиотических факторов среды, обобщение данных о соотношении врождённых и приобретённых компонентов в проявлении ответов животных на кайромоны рыб, анализ сведений о химической природе этих сигналов.
Исследования, рассматривающие влияние кайромонов рыб на водных животных, выполняются по двум основным направлениям. Первое касается релизерных реакций, т.е. быстро проявляющихся поведенческих ответов животных. В работах второго направления рассматриваются праймерные эффекты кайромонов рыб – медленно развивающиеся латентные реакции, затрагивающие физиологические процессы в организме реципиента и приводящие к отставленным по времени фенотипическим и иным изменениям при хроническом воздействии. В обоих направлениях исследований наибольший объём данных получен на примере ракообразных, брюхоногих моллюсков, водных личинок насекомых и личинок амфибий. Объекты исследований, за редкими исключениями, являются обитателями пресных вод. Другие группы водных животных изучены значительно слабее. Условия получения кайромонов рыб в некоторых экспериментах представлены в табл. 1.
Таблица 1.
Условия получения кайромонов рыб
| Рыба (донор кайромонов) | Длина рыб, см (масса, г) | Экспозиция | Источник информации | ||
|---|---|---|---|---|---|
| объём воды, л | время, ч |
температура, °С | |||
| Атлантическая сельдь Clupea harengus | – | 2500 | § | 10 | Hamrén, Hansson, 1999 |
| Радужная форель Oncorhynchus mykiss | – | § | 12 | 16 | Mathis, Hoback, 1997 |
| Ручьевой голец Salvelinus fontinalis | 20–25 | 200 | § | − | McIntosh, Peckarsky, 1996 |
| То же | 13 | 25 | 3* | 17 | Huryn, Chivers, 1999 |
| Щука Esox lucius | 18.2 | 12 | 72 | 22 | Wudkevich et al., 1997 |
| То же | 16 | 1.2 | 72 | − | Chivers et al., 1996 |
| Верховка Leuciscus delineatus | 5 | 5 | 24 | 20 | Pijanowska et al., 2006а |
| Язь Leuciscus idus | – | 4 | 24 | − | Tams et al., 2018 |
| Золотой карась Carassius carassius | – | 0.5 | 24 | − | Pijanowska et al., 2020 |
| То же | 6 | 10 | 24 | – | Dawidowicz et al., 2010 |
| Гольян Phoxinus phoxinus | 2 | 1 | 24 | – | Tollrian, Heibl, 2004 |
| Трёхиглая колюшка Gasterosteus aculeatus | – | 1.5 | 2 | – | Jensen et al., 1998 |
| Окунь Perca fluviatilis | 20.6 | 10 | 1 | – | Laurila, 2000 |
| То же | 4 | 2 | – | – | Reede, 1995 |
| Perca fluviatilis, Gobiomorphus cotidianus |
– | 2.5 | 24 | – | Lagrue, Poulin, 2007 |
| Perca flavescens, Ambloplites rupestris, Etheostoma nigrum, E.exile | – | – | § | – | Keller, Moore, 1999 |
| Большеротый окунь Micropterus salmoides | 20.5 | 22.7 | 40 | – | Kenison et al., 2018 |
| Зелёный солнечник Lepomis cyanellus | 9 | 100 | 24 | 21 | Holomuzki, Short, 1988 |
| То же | 7–9 | 10 | 0.3–0.4 | 21 | Holomuzki, Short, 1988 |
| » | 5 | 38 | 24 | 20 | Lauridsen, Lodge, 1996 |
| Солнечник Lepomis megalottis | 9.7 (25.6) | 20 | 72 | 18 | Holomuzki, Hatchett, 1994 |
| Обыкновенный солнечник Lepomis gibbosus | 5–7 | 4 | 1.5 | 21 | Macchiusi, Baker, 1992 |
| То же | 6 | 8 | – | 21 | Gyssels, Stoks, 2006 |
| Полосатая зубатка Anarhichas lupus | 61 (1830) | 20 | 1 | 7 | Hagen et al., 2002 |
| Атлантическая менидия Menidia menidia | 6–8 | 4 | 72 | 23–25 | Cieri, Stearns, 1999 |
| Цихлида Nimbochromis venustus | – | 1.5 | 24 | 19–20 | Stabell et al., 2003 |
РЕЛИЗЕРНОЕ ДЕЙСТВИЕ КАЙРОМОНОВ РЫБ
Механизмы релизерных ответов водных животных на запахи рыб основаны на хемосенсорных системах, обеспечивающих рецепцию этих сигналов. У беспозвоночных животных таких систем много, уровень их развития позволяет улавливать следовые концентрации различных запахов (Valentinčič, 1991; Moore, Bergman, 2005; Mobley et al., 2008; Motti et al., 2018). Поскольку рыбы являются потенциальными хищниками для многих гидробионтов, поведенческие ответы на кайромоны рыб представляют собой защитные реакции.
Кайромоны рыб – сигналы для жертв
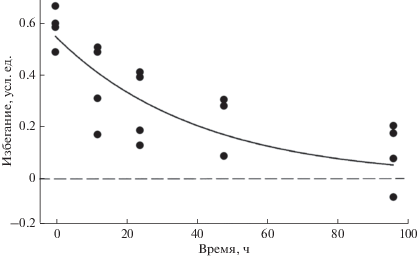
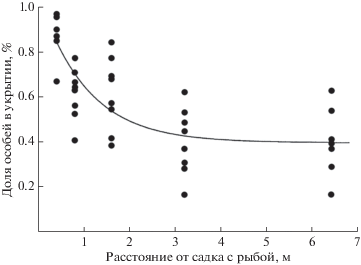
Моллюски (Mollusca). Запах линя Tinca tinca стимулирует брюхоногих моллюсков (Gastropoda) – большого прудовика Lymnaea stagnalis и пупырчатую физу Physa fontinalis, имеющих относительно тонкую раковину, подниматься к поверхности воды и выползать на надводные предметы. Моллюскам с более прочной раковиной – Lymnaea peregra, Planorbis planorbis и Anisus vortex − присущ уход на дно в укрытия (Rundle, Brönmark, 2001; Dalesman et al., 2006). Физа Physella gyrina при наличии в воде запаха обыкновенного солнечника Lepomis gibbosus предпочитает больше времени находиться в укрытии, тогда как запах рака Faxonius (=Orconectes) rusticus (Decapoda) вынуждает моллюска избегать перемещений по дну и подниматься к поверхности воды (Turner et al., 1999; Bernot, Turner, 2001). Чем ближе моллюск находится к источнику запаха – сетчатому садку с живой рыбой внутри, тем сильнее реакция (рис. 1) (Turner, Montgomery, 2003). Запах (раствор кожной слизи) обыкновенного фундулюса Fundulus heteroclitus вызывает испуг и уход у живущих на литорали брюхоногих моллюсков Littoraria irorata и Ilyanassa obsoleta (Rahman et al., 2000).
Рис. 1.
Зависимость доли особей физы Physella acute (Gastropoda), пребывающих в укрытии, от расстояния до источника кайромонов рыб – сетчатого садка с обыкновенным солнечником Lepomis gibbosus (по: Turner, Montgomery, 2003, с изменениями).
У дрейссены Dreissena polymorpha (Bivalvia) запах плотвы Rutilus rutilus подавляет фильтрационную активность: уменьшает скорость осветления воды и снижает продукцию пеллет (Naddafi et al., 2007). Сходным образом другой вид дрейссены (D. rostriformis) реагирует на запахи черноротого бычка Neogobius melanostomus и солнечника L. gibbosus, что указывает на отсутствие заметной специфичности в ответах моллюсков на кайромоны симпатрических и аллопатрических рыб (Naddafi, Rudstam, 2013).
Иглокожие (Echinodermata). Молодь морского ежа Strongylocentrotus droebachiensis избегает запах губана Tautogolabrus adspersus, питающегося в природе молодью ежа (Scheibling, Hamm, 1991).
Ракообразные (Crustacea). Значительное число публикаций посвящено изучению влияния кайромонов рыб на поведение мелких планктонных ракообразных рода Daphnia – представителей ветвистоусых (Cladocera). Наиболее хорошо изучен феномен усиления их суточных вертикальных миграций в присутствии запаха рыб. Уход на глубину при повышении освещённости является защитной адаптацией, позволяющей планктонным организмам выйти из-под пресса планктоноядных рыб (Ringelberg, 1995). Наблюдается взаимосвязь между численностью рыб в водоёмах и вертикальными перемещениями зоопланктона (Dini, Carpenter, 1991; Ringelberg et al., 1991).
В воде с запахом верховки Leucaspius delineatus дафнии D. magna совершают регулярные вертикальные миграции в соответствие с суточным ритмом освещённости, тогда как в воде без запаха таких перемещений не происходит: дафнии постоянно распределяются в верхних слоях воды (Dawidowicz, Loose, 1992). Кайромоны хищников, преследующих и схватывающих своих жертв поштучно, вызывают у дафний горизонтальные перемещения. В частности, таким образом дафнии реагируют на запах личинок коретры – комаров рода Chaoborus (Loose et al., 1993).
Запах синежаберного солнечника Lepomis macrochirus не только усиливает вертикальные миграции разных видов дафний, но и достаточен для их инициирования (Dodson, 1988). Действие кайромонов L. macrochirus усиливает чувствительность дафний к небольшим гидродинамическим возмущениям, а также снижает их уязвимость при прямом столкновении с мелкими хищниками – гуппи Poecilia reticulata (Brewer et al., 1999). Опускание дафний в более глубокие слои воды становится заметным уже через 5 мин после начала стимуляции, интенсивность реакции зависит от концентрации экзометаболитов рыб (рис. 2) (Van Gool, Ringelberg, 1995; von Elert, Pohnert, 2000). Запах плотвы стимулирует уход на глубину у дафнии D. magna всех исследованных клонов (16) независимо от их происхождения (водоёма). При этом зрительная доступность рыб для реагирующих дафний на силу ответа не влияет. При возвращении в чистую воду поведение дафний нормализуется через несколько дней (De Meester, 1993). При дефиците пищи эффект кайромонов проявляется слабо (Van Gool, Ringelberg, 1995, 1998).
Рис. 2.
Глубина (M ± m) погружения дафнии Daphnia magna в зависимости от концентрации кайромонов (литров воды на одну рыбу, экспозиция 24 ч) золотого карася Carassius carassius (по: von Elert, Pohnert, 2000).

В присутствии запаха плотвы дафния D. magna образует более компактные скопления и становится менее подвижной, хотя частота прыжков увеличивается (Pijanowska, Kowalczewski, 1997). При стимуляции запахом трёхиглой колюшки Gasterosteus aculeatus перемещения дафний D. pulex в скоплениях более согласованы, чем в чистой воде (Jensen et al., 1998). Повышение агрегированности и синхронности плавания может обеспечивать лучшую защищённость, как это наблюдается в стаях рыб (Касумян, Павлов, 2018).
На мелководье запах рыб может побуждать дафний уходить в укрытия. Так, запах зелёного солнечника L. cyanellus вынуждает дафний D. magna покидать свободную центральную часть аквариума и скрываться среди водных растений, куда в норме дафнии заходить избегают (табл. 2) (Lauridsen, Lodge, 1996). Солёность воды может модифицировать ответы дафний на запах уклеи Alburnus escherichii (Bezirci et al., 2012).
Таблица 2.
Эффекты, оказываемые кайромонами некоторых видов рыб на водных и других животных
| Рыба-донор кайромонов | Животные-реципиенты кайромонов | Краткая характеристика ответа | Источник информации |
|---|---|---|---|
| Релизерные ответы | |||
| Радужная форель Oncorhynchus mykiss | Gammarus pseudolimnaeus (Amphipoda) | Подавление пассивного ската в темноте и перемещения навстречу потоку днём | Williams, Moore, 1982 |
| Gammarus pseudolimnaeus (Amphipoda) | Быстрое образование пары и вступление в амплексус | Mathis, Hoback, 1997 | |
| Личинки подёнки Baetis bicaudatus (Ephemeroptera) | Снижение длительности нахождения в потоке ночью | Alvarez et al., 2014 | |
| Миксоспоридия Myxobolus cerebralis (Myxozoa, Cnidaria) | Выброс филамента и закрепление споры на хозяине | Kallert et al., 2005, 2011 | |
| Щука Esox lucius | Гаммарус Gammarus lacustris (Amphipoda) | Сокращение продолжительности плавания, увеличение времени неподвижного пребывания на дне | Wudkevich et al., 1997 |
| Молодь Astacus astacus и Pacifastacus leniuscus (Decapoda) | Снижение двигательной активности, увеличение времени нахождения в укрытии | Appelberg et al., 1993; Blake, Hart, 1993 |
|
| Личинки стрекоз-стрелок Enallagma spp. (Odonata, Zygoptera) | Снижение охотничьей активности | Chivers et al., 1996 | |
| Головастики лягушек прудовой Pelophylax lessonae и съедобной P. esculenta (Amphibia) | Снижение подвижности, стремление находиться на максимальном удалении от источника запаха | Staufer, Semlitsch, 1993 | |
| Плотва Rutilus rutilus | Дрейссена Dreissena polymorpha (Bivalvia) | Подавление фильтрационной активности | Naddafi et al., 2007 |
| Daphnia magna (Cladocera) | Вертикальные миграции | De Meester, 1993; von Elert, Loose, 1996 |
|
| То же | Повышение агрегированности, снижение подвижности, усиление прыжков | Pijanowska, Kowalczewski, 1997 | |
| Личинки комаров Chironomus riparius (Diptera) | Уход в более глубокие слои грунта | Hölker, Stief, 2005 | |
| Головастики остромордой Rana arvalis, травяной R. temporaria и прудовой Pelophylax lessonae лягушек и серой жабы Bufo bufo (Amphibia) | Избегание запаха | Мантейфель, Жушев, 1998 | |
| Верховка Leucaspius delineatus | Daphnia magna (Cladocera) | Вертикальные миграции | Dawidowicz, Loose, 1992; Pijanowska et al., 2006b |
| Линь Tinca tinca | Большой прудовик Lymnaea stagnalis, физа пупырчатая Physa fontinalis (Gastropoda) | Подъём к поверхности воды, выползание на надводные предметы | Rundle, Brönmark, 2001; Dalesman et al., 2006 |
| Золотой карась Carassius carassius | Gammarus roeseli, G. pulex (Amphipoda) | Избегание запаха; снижение двигательной активности | Baumgärtner et al., 2002; Åbjörnsson et al., 2004 |
| Daphnia spp. (Cladocera) | Инициирование и усиление вертикальных миграций | Van Gool, Ringelberg, 1995; von Elert, Loose, 1996; von Elert, Pohnert, 2000 |
|
| Гамбузия Gambusia affinis | Имаго комаров Culex spp. (Diptera) | Избегание водоёмов с кайромонами при поиске мест для откладки яиц | Angelon, Petranka, 2002; van Dam, Walton, 2008; Eveland et al., 2016 |
| Взрослые особи саламандры Eurycea sosorum (Amphibia) | Подавление двигательной активности | DeSantis et al., 2013 | |
| Налим Lota lota | Gammarus roeseli (Amphipoda) | Избегание запаха | Baumgärtner et al., 2002, 2003 |
| Молодь Astacus astacus и Pacifastacus leniuscus (Decapoda) | Снижение двигательной активности, увеличение времени нахождения в укрытии | Appelberg et al., 1993; Blake, Hart, 1993 |
|
| Трёхиглая колюшка Gasterosteus aculeatus | Daphnia pulex (Cladocera) | Повышение согласованности перемещений в скоплениях | Jensen et al., 1998 |
| Личинки коретры Chaoborus flavicans (Diptera) | Уход в придонные и донные слои, поиск укрытий | Dawidowicz et al., 1990 | |
| Окунь Perca fluviatilis | Молодь раков Astacus astacus и Pacifastacus leniuscus (Decapoda) | Снижение двигательной активности, увеличение времени нахождения в укрытии | Appelberg et al., 1993; Blake, Hart, 1993 |
| Жук-плавунец Acilius sulcatus (Coleoptera) | Снижение плавательной активности в воде | Åbjörnsson et al., 1997 | |
| Водолюбы Hydroporus incognitus и H. nigrita (Coleoptera) | Избегание ёмкостей с запахом для откладки яиц | Brodin et al., 2006 | |
| Головастики остромордой Rana arvalis, травяной R. temporaria и прудовой Pelophylax lessonae лягушек и серой жабы Bufo bufo (Amphibia) | Избегание запаха | Мантейфель, Жушев, 1998 | |
| Головастики травяной лягушки Rana temporaria | Снижение подвижности | Laurila, 2000 | |
| Gammarus pulex (Amphipoda) | Избегание запаха здоровыми особями и предпочтение после инфицирования скребнем Pomphorhynchus laevis (Acanthocephala) | Baldauf et al., 2007 | |
| Молодь рака Astacus astacus (Decapoda) | Снижение двигательной активности, увеличение времени нахождения в укрытии | Appelberg et al., 1993 | |
| Жук-плавунец Acilius sulcatus (Coleoptera) | Снижение плавательной активности в воде | Åbjörnsson et al., 1997 | |
| Большеротый окунь Micropterus salmoides | Faxonius (=Orconectes) rusticus (Decapoda) | Снижение подвижности, замирание, движение клешнями и антеннами, переход в укрытие | Kenison et al., 2018 |
| Личинки коретры Chaoborus punctipennis (Diptera) | Отпугивающее действие, уход в противоположную от источника запаха в сторону | O’Bryan, Forrester, 1997 | |
| Взрослые особи саламандры Eurycea sosorum (Amphibia) | Подавление двигательной активности | DeSantis et al., 2013 | |
| Головастики американской жабы Bufo americanus, лягушки-быка Rana catesbeiana и крикликовой лягушки R. clamitans (Amphibia) | Снижение активности, пребывание в водных зарослях и др. | Smith et al., 2008 | |
| Зелёный солнечник Lepomis cyanellus | Daphnia magna (Cladocera), Lirceus fontinalis (Isopoda) | Снижение двигательной активности, уход из предпочитаемой открытой части аквариума в заросли растений | Holomuzki, Short, 1988; Lauridsen, Lodge, 1996 |
| Gammarus minus (Amphipoda) | Сокращение времени плавания | Holomuzki, Hoyle, 1990 | |
| Lirceus fontinalis (Isopoda) | Подавление пищевого поведения | Short, Holomuzki, 1992 | |
| Личинки саламандры Ambystoma texanum и Eurycea bislineata; головастика квакши Hyla chrysoscelis (Amphibia) | Сокращение времени, проводимого на открытых участках | Petranka et al., 1987; Kats, 1988; Kats et al., 1988 |
|
| Синежаберный солнечник L. macrochirus | Головастики американской жабы Bufo americanus, лягушки-быка Rana catesbeiana и крикликовой лягушки R. clamitans (Amphibia) | Снижение активности, пребывание в водных зарослях и др. | Smith et al., 2008 |
| Daphnia spp. (Cladocera) | Инициирование и усиление вертикальных миграций | Dodson, 1988 | |
| Имаго двукрылых насекомых (Diptera) | Избегание водоёмов с кайромонами при поиске мест для откладки яиц | Petranka, Fakhoury, 1991 | |
| Праймерные ответы | |||
| Верховка Leucaspius delineatus | Daphnia magna (Cladocera) | Синхронизация репродуктивных циклов, повышение доли самок с покоящимися яйцами | Pijanowska, Stolpe, 1996; Pijanowska et al., 2006a |
| То же | Усиление экспрессии гена, контролирующего фолдинг (скручивание полипептидной цепи и приобретение белками пространственной структуры) | Schwarzenberger et al., 2009 | |
| Линь Tinca tinca | Прудовик Radix balthica (Gastropoda) | Изменение формы и увеличение толщины стенок раковины | Lakowitz et al., 2008; Brönmark et al., 2011 |
| Трёхиглая колюшка Gasterosteus aculeatus | Daphnia lumholtzi (Cladocera) | Индуцирование роста хвостовой иглы | Tollrian, 1994 |
| Науплии Temora longicornis (Copepoda) | Повышение темпа роста | Bjærke et al., 2014 | |
| Головастики Amphibia | Изменений пропорций туловища, длины хвоста и размера хвостовых мышц | Relyea, 2001; Teplitsky et al., 2005 |
|
| Личинки стрекозы Lestes viridis (Odonata) | Замедление роста и накопления жиров, отставание в развитии иммунной системы | Stoks et al., 2006 | |
| Окунь Perca fluviatilis | Трематода Coitocaecum parvum (Digenea) в промежуточном хозяине (амфипода Paracalliope fluviatilis) | Снижение прогенеза (созревание половых клеток до достижения организмом взрослой стадии) | Lagrue, Poulin, 2007 |
| Daphnia galeata × D. hyaline (Cladocera) | Сокращение времени созревания и снижение размеров впервые созревающих дафний | Reede, 1995 | |
| Зелёный солнечник Lepomis cyanellus | Личинки саламандры Ambystoma barbouri (Amphibia) | Увеличение длительности инкубационного периода | Moore et al., 1996 |
| Синежаберный солнечник L. macrochirus | Daphnia lumholtzi (Cladocera) | Индуцирование роста длинного шипа на голове и хвостовой иглы | Dzialowski et al., 2003; Engel, Tollrian, 2009 |
Запахи разных видов рыб (верховка, плотва, золотой карась Carassius carassius) не имеют выраженной видовой специфичности в своём влиянии на поведение дафний, поскольку опасность, исходящая от них мелким планктонным жертвам, сходная (von Elert, Loose, 1996). Не находят существенных различий между кайромонами разных рыб и по химическим свойствам (см. ниже).
Данные о влиянии запаха рыб на проявление дафниями вертикальных миграций, полученные в лаборатории, подтверждаются экспериментами и наблюдениями, выполненными в природе. Так, изъятие из озера (~1 га) 90% всех рыб (Phoxinus eos, P. neogaeus, Umbra limi) и вселение большеротого окуня Micropterus salmoides привело к повышению численности дафний и к отсутствию или слабо выраженному проявлению ими суточных вертикальных перемещений. Но после возвращения карповых рыб в водоём миграции дафний вновь становятся хорошо заметными (Dini, Carpenter, 1991).
Жаброногие (Branchiopoda). У науплий артемии Artemia franciscana уход на глубину при повышении освещённости выражен сильнее, если в воде присутствует запах обыкновенного фундулюса, атлантического менхэдена Brevoortia tyrannus или колючей чопы Lagadon rhomboides; действие кайромонов заметно уже в первые минуты после подачи запаха и зависит от его концентрации (McKelvey, Forward, 1995).
Бокоплавы (Amphipoda). Запах щуки Esox lucius приводит к сокращению времени, затрачиваемому гаммарусом Gammarus lacustris на плавание в аквариуме в толще воды, но увеличивает длительность неподвижного нахождения на дне, что делает его менее заметным для щуки (табл. 2). Реагируя на запах хищника, охотящегося иным образом – личинок стрекозы Aeshna eremita, гаммарус старается не опускаться ко дну или находиться на грунте, т.е. избегает зоны водоёма, занимаемые личинками стрекоз (Wudkevich et al., 1997).
Содержание радужной форели Oncorhynchus mykiss внутри сетчатого садка, из которого запах беспрепятственно попадает в гидродинамическую установку, подавляет (в течение нескольких минут) пассивный скат амфиподы Gammarus pseudolimnaeus в темноте. При дневной освещённости запах радужной форели блокирует перемещение гаммаруса навстречу потоку (Williams, Moore, 1982). Такие же ответы вызывают запахи других симпатрических рыб – озёрного гольца Salvelinus namaycush, арктического хариуса Thymallus arcticus, ринихтов Rhinichthys spp., дартеров Etheostoma spp., нотрописов Notropis spp., а также аллопатрической африканской цихлиды Pelmatochromis kribensi (Williams, Moore, 1985). Запах зелёного солнечника L. cyanellus сокращает время плавания амфиподы Gammarus minus (Holomuzki, Hoyle, 1990). Запах хищных личинок насекомых, ведущих донный образ жизни, в отличие от запаха рыб не подавляет, а усиливает ночной дрифт мелких ракообразных (Wooster, Sih, 1995).
Ночной дрифт гаммарид в реках и ручьях, населённых рыбами, менее интенсивный по сравнению с тем, что наблюдается там, где рыбы отсутствуют (Friberg et al., 1994). Внесение садка с кумжей Salmo trutta в небольшой безрыбный ручей сразу снижает ночной скат обитающей здесь амфиподы Gammarus pulex (Andersen et al., 1993). Сходным образом эта амфипода реагирует и на запах европейского обыкновенного подкаменщика Cottus gobio. После помещения нескольких сотен подкамещиков в отгороженный участок небольшой реки интенсивность ночного дрифта амфипод в ручье ниже по течению от этого участка уменьшается по сравнению с дрифтом выше по течению от участка с рыбой. Реакция на запах подкамещика наблюдается и в лабораторных опытах. Но если подкамещика поместить в стеклянную ёмкость, блокирующую распространение запаха, но сохраняющую возможность жертвам для зрительного контроля за хищником, изменений в поведении амфипод не происходит, что подтверждает важность хемосенсорного контакта между жертвой и хищником (Andersson et al., 1986).
Амфиподы, реагирующие на запах рыб (кумжа), способны благодаря изогнутой форме тела и движениям плеопод создавать локальный противоток, благодаря которому амфиподы получают запаховый сигнал от источника, располагающегося ниже по течению. У амфиподы G. pulex это расстояние не превышает 1 см. За счёт неоднородности донного рельефа могут создаваться локальные противотоки ещё большей протяженности. Эти особенности гидродинамики важны для таких животных дрифта, как амфиподы, поскольку позволяют им хемосенсорно детектировать рыб и других хищников, располагающихся в большинстве случаев ниже по течению (Dahl et al., 1998).
Кайромоны рыб не только изменяют двигательную активность, но и приводят к перераспределению или перемещению животных благодаря реакциям предпочтения/избегания. При возможности выбора одного из двух отсеков проточного лабиринта, лишённого каких-либо укрытий, амфипода Gammarus roeseli избегает отсек с запахом налима Lota lota или золотого карася (табл. 2). Реакция отсутствует, если концентрация запаха недостаточно высокая (снижена на один порядок). Запах окуня Perca fluviatilis, в меньшей степени склонного к поиску пищи в грунте, где предпочитают находиться скрывающиеся от хищников амфиподы, избегания не вызывает (Baumgärtner et al., 2002). Запах налима не только вынуждает G. roeseli избегать потенциально опасные зоны, но и смещает биотопические предпочтения: амфипода начинает избирать донный субстрат из мелкого галечника, в котором хищнику её сложнее обнаружить (Baumgärtner et al., 2002, 2003).
В присутствии запаха радужной форели самцы амфиподы Gammarus pseudolimnaeus быстрее образуют пару и вступают в амплексус с самками меньшего размера, чем в чистой воде (Mathis, Hoback, 1997).
Равноногие (Isopoda). При появлении запаха зелёного солнечника L. cyanellus изопода Lirceus fontinalis снижает двигательную активность на открытых участках и реже покидает укрытия − скопления нитчатых водорослей Cladophora (табл. 2) (Holomuzki, Short, 1988). Двигательная активность изоподы L. fontinalis снижается и при действии запаха длинноухого солнечника Lepomis megalotis (Holomuzki, Hatchett, 1994). Запах рыб подавляет пищевое поведение L. fontinalis, причём запах хищных рыб (зелёный солнечник) действует сильнее, чем рыб, питающихся преимущественно водорослями или бентосом (необычная кампостома Campostoma anomalum) (Short, Holomuzki, 1992).
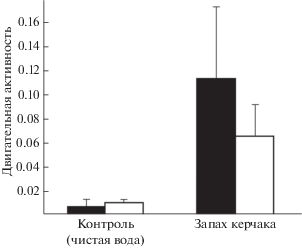
Запах европейского керчака Myoxocephalus scorpius вынуждает морскую изоподу Saduria entomon в десять раз больше времени находиться в грунте и не выходить из него (рис. 3), реже и на меньшую высоту выставлять над грунтом антенны, на которых располагаются хеморецепторы (Ejdung, 1998).
Рис. 3.
Влияние запаха европейского керчака Myoxocephalus scorpius на двигательную активность (длительность пребывания вне грунта и плавание, M ± m, усл. ед.) одиночной изоподы Saduria entomon в темноте (◼) и на свету (◻) в аквариуме с объектами её питания – амфиподами Monoporeia affinis; различия между двигательной активностью изоподы в темноте и на свету в обоих вариантах опыта недостоверны (p > > 0.05) (по: Ejdung, 1998, с изменениями).
Мизиды (Mysida). В присутствии запаха атлантической сельди Clupea harengus мизиды Mysis mixta в несколько раз снижают интенсивность питания науплиями артемии. Предполагают, что запах сельди и других планктоноядных рыб может также стимулировать защитные вертикальные миграции мизид в более глубокие и холодные и менее кормные слои воды, но более безопасные для них в дневное время (Hamrén, Hansson, 1999).
Веслоногие (Copepoda). В воде, содержащей кайромоны атлантической менидии Menidia menida, веслоногие рачки Acartia tonsa и A. hudsonica менее интенсивно питаются планктонными диатомовыми водорослями. Эффект проявляется только при освещённости, достаточной для зрительного питания менидии (Cieri, Stearns, 1999).
Ракушковые (Ostracoda). Добавление небольшого количества воды (3 мл), взятой из аквариума с молодью леща Abramis brama, в установку, где находятся ракушковые рачки Cypridopsis vidua, приводит к их перераспределению и избиранию безопасных условий – отсека с густыми зарослями хары Chara fragilis (Roca et al., 1993).
Десятиногие раки (Decapoda). Запах окуня, щуки, налима и европейского угря Anguilla anguilla подавляет двигательную активность и вынуждает молодь широкопалого речного рака Astacus astacus и североамериканского рака Pacifastacus leniuscus больше времени проводить в укрытии, когда она находится в темноте и максимально активна (Appelberg et al., 1993; Blake, Hart, 1993). Но запах плотвы, не представляющей опасности для A. astacus, индифферентен для этого рака (Appelberg et al., 1993). Результаты подтверждают способность жертв дифференцированно реагировать на запах разных хищников, симпатрических по отношению к жертвам. Запах европейского угря вызывает у рака P. leniusculus, интродуцированного в водоёмы Финляндии, поисковое поведение – нахождение вне укрытий и повышенную двигательную активность, т.е. поведение, противоположное ответу на запах угря у рака A. astacus (Hirvonen et al., 2007).
Однако запахи красноглазого каменного окуня Ambloplites rupestris, жёлтого окуня Perca flavescens, чёрного дартера Etheostoma nigrum и изящного дартера E. exile, являющихся опасными хищниками для молоди рака Faxonius (=Orconectes) virilis, влияния на её подвижность не оказывают (Keller, Moore, 1999). В то же время запах этих рыб стимулирует у молоди рака демонстрацию позы угрозы (Hazlett, Schoolmaster, 1998), что, как полагают, отражает настороженность и готовность проявить защитную реакцию (Keller, Moore, 1999). Возможно, сохранение двигательной активности у F. virilis связано с недостаточной концентрацией запаха, поскольку в другом исследовании, выполненном на близком виде – F. rusticus, запах большеротого окуня M. salmoides, питающегося в природе этими раками, снижает их подвижность, вызывает замирание, движение клешнями и антеннами, переход в укрытие (Kenison et al., 2018). Запах малоротого окуня Micropterus dolomieu приводит к снижению двигательной активности у F. virilis (Ramberg-Pihl, Yurewicz, 2020).
Запах бронзового керчака Myoxocephalus aenaeus вынуждает молодь американского омара Homarus americanus в несколько раз больше времени проводить в укрытии, чем это наблюдается в чистой воде (Wahle, 1992). Планктонные личинки краба Rhithropanopeus harrisii (зоеа) становятся более чувствительными к затенению как к сигналу начала вертикальной миграции, если испытывают действие запаха обыкновенного фундулюса (Cohen, Forward, 2003).
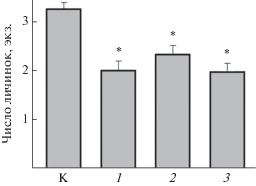
Насекомые (Insecta). Водные личинки. Личинки североамериканской подёнки Baetis bicaudatus (Ephemeroptera), взятые из речек, где много питающихся ими рыб, ночью в проточных бассейнах, куда постоянно поступает запах ручьевого гольца S. fontinalis, снижают длительность нахождения в потоке воды (McIntosh, Peckarsky, 1996). Такой же эффект на личинок оказывают кайромоны других симпатрических, но разных по экологии питания и систематике рыб – радужной форели, лосося Кларка Oncorhynchus clarkii, обыкновенного чукучана Catostomus catostomus, интродуцированной в местные реки кумжи. Под действием запаха этих рыб личинки подёнки реже выходят на открытую донную поверхность для питания (рис. 4) (Alvarez et al., 2014). Запах золотой рыбки Carassius auratus, аллопатрической по отношению к подёнкам B. bicaudatus и не представляющей для них потенциальной угрозы в качестве хищника, на дрифт личинок влияния не оказывает (McIntosh, Peckarsky, 2004). Личинки обитающей на европейском континенте подёнки Baetis rhodani реагируют только на запах кумжи и не чувствительны к запаху карпа Cyprinis carpio и тюрбо Psetta maxima (Alvarez et al., 2014).
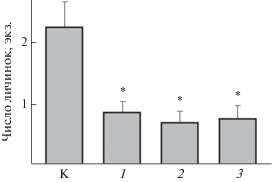
Рис. 4.
Число (M ± m) питающихся личинок североамериканской подёнки Baetis bicaudatus на открытой поверхности дна в чистой воде (К) и в присутствии кайромонов рыб: 1 – ручьевой голец Salvelinus fontinalis, 2 – лосось Кларка Oncorhynchus clarkii, 3 – радужная форель O. mykiss; * отличия от контроля достоверны при p < 0.05 (по: Alvarez et al., 2014, с изменениями).
Реакция водных животных на кайромоны рыб зависит от биологии рыб. Так, личинки подёнки Ephemerella aurivill в ответ на запах бентосоядного длинноносого ринихта Rhinichthys cataractae не снижают, а усиливают ночью скат по течению воды. Личинки подёнок Paraleptophlebiahe teronea и Baetis tricaudatus в ответ на запах этой рыбы поведение в потоке не изменяют (Scrimgeour et al., 1994). Адаптивное значение усиления ската личинок E. aurivill при получении химического сигнала о присутствии питающегося бентосом хищника очевидно. Но какими особенностями биологии обусловлено отсутствие реакции на этот сигнал у личинок других подёнок, не выяснено.
Предположение, что кайромоны рыб регулируют интенсивность суточных ритмов ската личинок подёнок в водоёмах (Douglas et al., 1994), подтверждают эксперименты в природе. Локальное внесение запаха ручьевого гольца S. fontinalis в небольшую речку, населённую этими рыбами, вызывает снижение числа крупных личинок подёнок, попавших в ловушки в ночные часы. Эффект заметен уже через 5 мин (McIntosh et al., 1999).
Под действием веществ, выделяемых в воду щукой, хищные личинки стрекоз-стрелок Enallagma spp. (Odonata, Zygoptera) снижают свою охотничью активность: реже изгибают характерным образом тело и реже совершают направленные перемещения и броски (табл. 2) (Chivers et al., 1996).
Запах американского карликового сома Ameiurus nebulosus и большеротого окуня оказывает репеллентное действие на личинок коретры Chaoborus punctipennis (Diptera). В отличие от дафний и других планктонных Cladocera личинки коретры не уходят на глубину, а перемещаются по горизонтали в сторону, противоположную источнику запаха (O’Bryan, Forrester, 1997). Присутствие в установке в отгороженном сетчатом отсеке трёхиглой колюшки вызывает у личинок коретры C. flavicans уход в придонные и донные слои и попытки найти там укрытие. Реакция проявляется на протяжении всех 15 сут наблюдений (Dawidowicz et al., 1990). Личинки комаров Chironomus riparius (Diptera) при наличии в воде запаха плотвы быстрее зарываются в грунт и уходят в его более глубокие слои; эта реакция усиливается с повышением концентрации запаха и длительности воздействия (рис. 5, 6) (Hölker, Stief, 2005). В присутствии запаха обыкновенного солнечника выращенные в лаборатории личинки комаров C. tentans менее активны и реже покидают свои домики-трубки (Macchiusi, Baker, 1992).
Рис. 5.
Динамика ухода личинок комаров Chironomus riparius с поверхности грунта в глубину в зависимости от концентрации кайромонов плотвы Rutilus rutilus: (◻) – контроль (чистая вода), ( ) – запах одной рыбы, (◼) – запах десяти рыб (по: Hölker, Stief, 2005, с изменениями).
) – запах одной рыбы, (◼) – запах десяти рыб (по: Hölker, Stief, 2005, с изменениями).
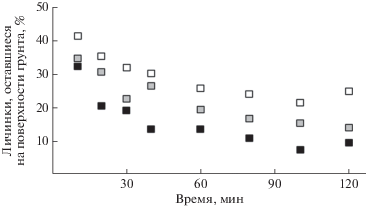
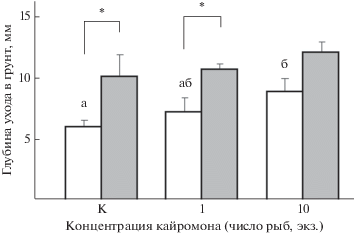
Рис. 6.
Глубина ухода (M ± m) личинок комаров Chironomus riparius в грунт при экспозиция 3 (◻) и 5 ( ) сут в чистой воде (К) и при разной концентрации кайромонов плотвы Rutilus rutilus; разные буквы обозначают наличие достоверных различий при экспозиции 3 сут, p < 0.05; * достоверные различия между глубиной ухода в грунт при экспозиции 3 и 5
сут, p < 0.05 (по: Hölker, Stief, 2005, с изменениями).
) сут в чистой воде (К) и при разной концентрации кайромонов плотвы Rutilus rutilus; разные буквы обозначают наличие достоверных различий при экспозиции 3 сут, p < 0.05; * достоверные различия между глубиной ухода в грунт при экспозиции 3 и 5
сут, p < 0.05 (по: Hölker, Stief, 2005, с изменениями).
Имаго. Вещества, выделяемые водными организмами, не только хорошо растворимы в воде, но и могут обладать летучестью, подниматься в воздух и нести полезную информацию для наземных или воздушных обитателей (Fink, 2007). Хорошо известен пример с веществами диметилсульфидной природы, выделяемыми морским фитопланктоном в местах скопления. Переходя в воздух и поднимаясь вверх, они служат важными запаховыми ориентирами для рыбоядных птиц, легко обнаруживающих таким образом питающихся планктоном рыб (Nevitt et al., 1995; Nevitt, 2008).
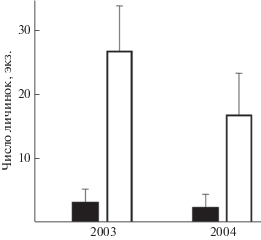
Жук-плавунец Acilius sulcatus (Coleoptera), постоянно живущий в водоёмах и лишь изредка покидающий их для расселения, при появлении запаха окуня снижает свою плавательную активность. Реакция наблюдается в темноте и в условиях слабой видимости, но отсутствует при дневном освещении (Åbjörnsson et al., 1997). При расселении, когда жуки-плавунцы и некоторые другие жесткокрылые (Coleoptera) перелетают в новые водоёмы, запахи рыб, поднимающиеся в воздух, влияют на выбор мигрирующими жуками нового места обитания. В эксперименте жуки-плавунцы Dytiscidae и водолюбы Hydrophilidae избегают колонизировать искусственные водоёмы-ёмкости, вода в которых содержит кайромоны Lepomis, Gambusia, Pimephales, Enneacanthus и Umbra. Исключением является запах насекомоядного пиратоокуня Aphredoderus sayanus (Aphredoderidae, Percosiformes), который, как полагают, обладает уникальным для рыб свойством – запаховым камуфляжем (Resetarits, Binckley, 2013). Присутствие запаха окуня P. fluviatilis в ёмкостях с водой препятствует откладке яиц водолюбами Hydroporus incognitus и H. nigrita (рис. 7), однако на двигательную активность личинок жуков этот запах не влияет (Brodin et al., 2006).
Рис. 7.
Число личинок (M ± m) жуков-водолюбов Hydroporus incognitus и H. nigrita в мезокосмах с сетчатыми садками с окунем Perca fluviatilis (◼) и без него (◻) после пяти недель экспонирования вблизи природных водоёмов в 2003 и 2004 гг. (по: Brodin et al., 2006, с изменениями).
Двукрылые насекомые (Diptera), жизненный цикл которых связан с водой, при откладке яиц отдают предпочтение тем водоёмам, в которых рыбы отсутствуют или плотность их популяции невысокая (Vonesh, Blaustein, 2010). Число яиц, откладываемых самками Aedes taeniorchyncus в прудах, населённых рыбами, ниже, чем в прудах без рыб, пропорционально численности рыбного населения (Ritchie, Laidlaw-Bell, 1994). В бассейнах, в которых в отгороженных сетчатых отсеках содержится синежаберный солнечник L. macrochirus, личинок комаров меньше, чем в бассейнах без рыб (Petranka, Fakhoury, 1991). Количество личинок комаров Culex spp., обнаруживаемых в имитирующих при-родные водоёмы ёмкостях, закономерно уменьшается по мере увеличения концентрации экзометаболитов гамбузии Gambusia affinis (Angelon, Petranka, 2002). Наличие в воде запаха G. affinis, но не зелёного солнечника L. cyanellus, препятствует откладке яиц комарами Culex restuans (Eveland et al., 2016).
Запах рыб по-разному влияет на самок комаров, различающихся экологией размножения. Запах гамбузии G. affinis отпугивает самок комаров Culex tarsalis, обычно использующих для размножения водоёмы, населённые рыбами. У самок Culex quinquefasciatus, редко использующих для размножения водоёмы, где может быть рыба, избегание запаха гамбузии выражено слабо. Добавление в воду кайромонов гамбузии не оказывает никакого влияния на выбор ёмкости у самок Aedes aegypti, которые в природе используют для размножения дупла деревьев и другие небольшие углубления с водой (van Dam, Walton, 2008). Экзометаболиты краснопёрки Scardinius erythrophthalmus и девятииглой колюшки Pungitius pungitius препятствуют откладке яиц самками тех видов коретры (Chaoborus crystallinus и C. obscuripes), которые в природе размножаются в водоёмах, где нет рыб. Для самок коретры C. flavicans, размножающихся в водоёмах, обычно населённых рыбами, запах краснопёрки и колюшки не оказывает какого-либо влияния на выбор контейнеров с водой для откладки яиц (Berendonk, 1999).
Амфибии (Amphibia). Многие амфибии, главным образом их личинки, являются объектами питания рыб. Кайромоны рыб служат для личинок химическими сигналами, предупреждающими об опасности и вызывающими у них защитное поведение. Запах окуня, плотвы, ротана Perccottus glenii и особенно ерша Gymnocephalus cernuus избегают головастики разных бесхвостых амфибий – остромордой Rana arvalis, травяной R. temporaria и прудовой Pelophylax lessonae лягушек и серой жабы Bufo bufo (Мантейфель, Жушев, 1998). Вода, поступающая из ёмкости с зелёным солнечником L. cyanellus, снижает время, проводимое личинками саламандр Ambystoma texanum и Eurycea bislineata и квакши Hyla chrysoscelis на открытых участках, где они более доступны для хищников, и увеличивает длительность пребывания в убежище (рис. 8) (Petranka et al., 1987; Kats, 1988; Kats et al., 1988). Если запах рыб застаёт личинок Ambystoma barbourin на открытом дне, то они следуют к укрытию по прямой траектории (Sih, Kats, 1991). Экзометаболиты американской евдошки Umbra limi уменьшают подвижность и продолжительность питания и вызывают стремление покинуть запаховую зону у головастиков шести видов североамериканских бесхвостых амфибий (Relyea, 2001). Запах гамбузии, солнечника L. auratus и особенно большеротого окуня подавляет двигательную активность у взрослой саламандры Eurycea sosorum (рис. 9) (DeSantis et al., 2013). После блокирования органа обоняния реакция у личинок саламандр не проявляется (Kats, 1988; Kats et al., 1988). Запах солнечника L. cyanellus эффективен в основном для личинок саламандр и бесхвостых амфибий Северной Америки (Hylidae, Ranidae, Bufonidae, Plethodontidae, Ambystomatidae, Salamandridae), обитающих в водоёмах, населённых рыбами (Kats et al., 1988). Если реакция на запах хищных рыб (L. cyanellus, O. mykiss) у личинок амфибий отсутствует, то у них обнаруживают вкусовую детеррентность – другой способ химической защиты от рыб (Kats et al., 1988; Kiesecker et al., 1996; Nyström, Åbjörnsson, 2000).
Рис. 8.
Время (M ± m), проводимое в убежище личинками саламандры Ambystoma texanum, в зависимости от присутствия (⚪) или отсутствия (⚫) в воде кайромонов зелёного солнечника Lepomis cyanellus; (↑) − момент внесения в воду кайромонов (по: Kats et al., 1988).
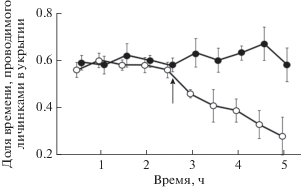
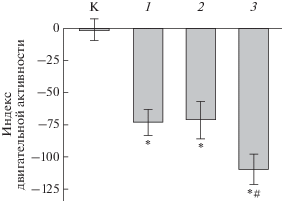
Рис. 9.
Влияние кайромонов рыб на двигательную активность (M ± m) взрослых особей саламандры Eurycea sosorum. Индекс двигательной активности – разность между временем плавания или перемещения по грунту одиночной саламандры до и после подачи в экспериментальный аквариум (4.5 л) 50 мл чистой воды (К − контроль) или воды с запахом рыб: 1 − гамбузия Gambusia affinis, 2 – солнечник Lepomis auratus, 3 – большеротый окунь Micropterus salmoides; отличия достоверны при p < 0.05: * от контроля, # от воздействия кайромонов гамбузии и солнечника (по: DeSantis et al., 2013).
В присутствии запаха окуня головастики травяной лягушки R. temporaria снижают свою подвижность (табл. 2) (Laurila, 2000), головастики прудовой Pelophylax lessonae и съедобной P. esculenta лягушек снижают подвижность и стремятся находиться на максимальном удалении от источника запаха щуки (Staufer, Semlitsch, 1993).
Экометаболиты синежаберного солнечника, большеротого окуня и золотой рыбки оказывают влияние на поведение головастиков американской жабы Bufo americanus, лягушки-быка Rana catesbeiana и крикливой лягушки R. clamitans, однако изменения, вызываемые у каждой из этих жертв (снижение активности, пребывание в водных зарослях и др.), имеют свои особенности и зависят от размера головастиков (Smith et al., 2008).
В присутствии запаха золотой рыбки повышается смертность головастиков дальневосточной квакши Hyla japonica, находящихся в аквариуме совместно с хищными личинками стрекозы Anax parthenope (Odonata) или флоридским раком Procambarus clarkii (Decapoda). Эффект связывают с увеличением доступности жертв, снижающих двигательную активность в ответ на восприятие запаха рыб (Takahara et al., 2003).
Искусственные водоёмы, в которых некоторое время содержали разных североамериканских пресноводных рыб (Esocidae, Salmonidae, Amiuridae, Centrarchidae, Umbridae, Poeciliidae), менее предпочитаемы для откладки икры квакшами Hyla chrysoscelis, H. femoralis и H. squirella (Hylidae). Исключение – запах пиратоокуня A. sayanus, обладающего химическим (запаховым) камуфляжем (Resetarits, Wilbur, 1989; Binckley, Resetarits, 2003; Resetarits, Binckley, 2013).
Кайромоны рыб – сигналы для хищников
Экзометаболиты, продуцируемые рыбами, в некоторых случаях служат сигналами, информирующими не только жертв об опасности, но и хищников о наличии добычи. Стрекательные клетки хищной сифонофоры, португальского кораблика Physalia physalis (Hydrozoa), охотящегося в том числе и на рыб, в электрофизиологических опытах отвечают на стимуляцию запахом паралихта Paralichthys lethostigma (Purcell, Anderson, 1995).
Кайромоны рыб – сигналы для паразитов
Вещества, выделяемые рыбами в окружающую среду, служат химическими ориентирами для свободных форм многих паразитических организмов и облегчают им поиск и обнаружение рыб-хозяев. Экзометаболиты и отдельные компоненты кожных выделений (гликопротеины, аминокислоты) обыкновенной пецилии Xiphophorus maculatus привлекают расселяющихся теронтов (бродяжки) ихтиофтириуса Ichthyophthirius multifiliis – паразитической реснитчатой инфузории (Hymenostomatida, Ciliophora). Экзометаболиты, по-видимому гликоконъюгаты, вызывают у теронтов реакцию избегания. Предполагают, что реакция на запах рыб-хозяев возможна, когда теронты в результате поиска оказываются на близком к ним расстоянии (Haas et al., 1999). Компоненты кожной слизи радужной форели, карпа, леща и последующая механическая стимуляция спор миксоспоридий Myxobolus cerebralis (Myxozoa, Cnidaria) вызывают у них выброс филамента и закрепление на хозяине (Kallert et al., 2005, 2011). Запахи атлантического лосося Salmo salar и некоторых других рыб повышают подвижность лососёвой вши Lepeophtheirus salmonis (Copepoda) и инициируют её перемещение к источнику запаха (Devine et al., 2000).
Расселяющиеся церкарии трематоды Cryptocotyle lingua (Digenea) под влиянием запаха атлантической сельди сокращают продолжительность периодов покоя и увеличивают время активного плавания, чаще меняют направление движения, что, как полагают, повышает вероятность столкновения с будущим хозяином (Chapman, 1974; Haas, 1994). Церкарии трематоды Acanthostomum brauni быстро прикрепляются к агаровому субстрату, содержащему компоненты слизи карпа (Ostrowski de Nuñez, Haas, 1991). Под действием запаха окуня и гобиоморфа Gobiomorphus cotidianus, являющихся окончательными хозяевами для трематоды Coitocaecum parvum, паразит, находящийся в промежуточном хозяине (амфипода Paracalliope fluviatilis), изменяет жизненную стратегию: снижает проявление прогенеза (созревание половых клеток до достижения организмом взрослой стадии) (Lagrue, Poulin, 2007). Инфицированная скребнем Pomphorhynchus laevis (Acanthocephala) амфипода G. pulex предпочитает запах окуня, тогда как незаражённые амфиподы запах хищника избегают. Реакция на зрительный образ окуня у амфиподы не изменяется (Baldauf et al., 2007). Аналогичным образом изменяется поведение циклопа Macrocyclops albidus (Copepoda) после инфицирования процеркоидами цестоды Schistocephalus solidus (Cestoda) (Jakobsen, Wedekind, 1998). Имеются другие примеры влияния кайромонов рыб на состояние паразитов и их манипуляцию хозяевами (Baldauf et al., 2007; Гопко, Михеев, 2017).
ПРАЙМЕРНОЕ ДЕЙСТВИЕ КАЙРОМОНОВ РЫБ
Столкновение водных животных с запахами рыб происходит не только случайно или неожиданно. В населённых рыбами водоёмах (причём не только в относительно небольших и замкнутых) эти запахи постоянно присутствуют в воде. Косвенно об этом свидетельствуют результаты экологической генетики, выявляющие молекулярные следы рыб в водоёмах (Coulter et al., 2019). Химическая природа и скорость распада веществ, формирующих запах рыб, неизвестны, а реальная продолжительность сохранения кайромонами активности в природной воде исследована крайне слабо. Однако, судя по косвенным данным, этот срок всё же достаточен для накопления и достижения этими веществами концентраций, способных при хроническом действии вызывать у животных ответы праймерного типа. В целом структурные и функциональные изменения, возникающие у жертв в результате длительного влияния на них кайромонов, следует рассматривать как эпигенетические. В литературе они получили название индуцированной защиты. Наиболее полно такие ответы изучены у пресноводных ветвистоусых и личинок амфибий.
Морфология
Изменения в морфологии являются пластическими (фенотипическими) адаптациями и требуют перераспределения или дополнительных затрат энергии, которые могут быть оправданными только при перманентном присутствии опасности (Auld et al., 2010). Как и все праймерные ответы, эти изменения требуют определённого времени и условий для их реализации. Являясь по своей сути защитными морфологическими адаптациями, они направлены на снижение риска жертв быть атакованными, схваченными и истреблёнными хищниками.
Моллюски (Mollusca). Под влиянием запаха линя у прудовика Radix balthica меняется форма раковины: она становится более округлой и с менее заострённой вершиной, что делает её более устойчивой к механическим воздействиям. Кроме того, увеличивается толщина стенок раковины, что также повышает защищённость моллюска. Такие же особенности приобретает раковина у этих моллюсков, живущих в водоёмах, населённых рыбами (Lakowitz et al., 2008; Brönmark et al., 2011).
Ветвистоусые (Cladocera). Постоянное присутствие в воде кайромонов язя Leuciscus idus вызывает у дафний разных видов рост хвостовой иглы, но только при высокой обеспеченности дафний пищей, что подчёркивает энергозатратность таких адаптаций (Spaak, Boersma, 1997). Запах синежаберного солнечника L. macrochirus индуцирует рост длинного шипа на голове и хвостовой иглы у молоди дафнии D. lumholtzi. Для этого достаточно пребывания самок в среде с запахом, т.е. непосредственного воздействии запаха на молодь не требуется (Dzialowski et al., 2003; Engel, Tollrian, 2009). Повышенная выживаемость дафний с удлинёнными выростами находит экспериментальное подтверждение (Engel et al., 2014).
Веслоногие (Copepoda). В присутствии запаха трёхиглой колюшки науплии морской копеподы Temora longicornis быстрее растут, особенно на поздних возрастных стадиях (Bjærke et al., 2014).
Амфибии (Amphibia). Инкубация икры саламандры Ambystoma barbouri при постоянном присутствии запаха зелёного солнечника L. cyanellus, охотно питающегося молодью саламандры в природе, задерживает вылупление личинок не менее чем в два раза. Выходящие из икры личинки значительно крупнее и более развиты, чем инкубировавшиеся в воде без запаха. Полагают, что удлинение инкубационного периода и увеличение размеров снижают вероятность гибели молоди в условиях, когда существует потенциальная опасность (Moore et al., 1996).
Выращивание до стадии метаморфоза личинок леопардовой лягушки Lithobates pipiens в воде с запахом американского карликового сома Ameiurus nebulosus снижает темпы развития и роста, приводит к формированию у головастиков более высокого хвостового плавника, что позволяет совершать резкие повороты при плавании (Balaa, Blouin-Demers, 2013).
В присутствии запаха американской евдошки Umbra limi у головастиков шести видов североамериканских бесхвостых амфибий через пять недель изменяются пропорции тела, хвоста и хвостового плавника (длина, высота), причём в разной мере у головастиков разных видов. Аналогичные изменения индуцируют запахи и других водных хищников (трёхиглой колюшки, личинки стрекоз Anax spp., жуков-плавунцов Colymbetes sp. и Dytiscus sp., водяных клопов Belostoma sp. и саламандры Ambystoma tigrinum, взрослых тритонов Notophthalmus viridescens), однако запах евдошки в большинстве случаев наиболее эффективен. Такие морфологические изменения обеспечивают бóльшую скорость и манёвренность плавания (Relyea, 2001; Teplitsky et al., 2005).
Влияние кайромонов на репродукцию жертв
Кроме влияния на поведение животных или индуцирования медленно проявляющихся фенотипических изменений кайромоны рыб могут изменять скорость полового созревания и репродуктивный потенциал жертв (Lass, Spaak, 2003; Ferrari et al., 2010).
Ветвистоусые (Cladocera). Экспозиция в среде с запахом верховки синхронизирует репродуктивные циклы у дафний D. magna и повышает долю самок с покоящимися яйцами (табл. 2) (Pijanowska, Stolpe, 1996; Pijanowska et al., 2006a). Запах язя ускоряет созревание и снижает размеры впервые созревающих дафний D. hyaline, увеличивает плодовитость при первой репродукции, но уменьшает размеры производимой молоди (Stibor, 1992). К таким же эффектам приводит выращивание гибридных особей D. galeata × D. hyalina в воде с запахом окуня, сила эффекта зависит от концентрации кайромонов (рис. 10) (Reede, 1995). Не найдено существенных отличий между кайромонами гамбузии G. holbrooki и солнечника L. gibbosus: они сходным образом влияют на скорость роста, созревания, число яиц первой порции и размеры молоди D. longispina (Castro et al., 2007). При сочетании кайромонов с высокой обеспеченностью пищей скорость роста и плодовитость дафний в последовательных кладках снижается, но созревание происходит быстрее, что компенсирует снижение плодовитости (Hülsmann et al., 2004; Pijanowska et al., 2006a). Влияние запахов рыб на рост и репродукцию дафний лучше выражено в летние месяцы и слабо проявляется зимой, когда интенсивность питания рыб ниже (Stibor, Lampert, 2000).
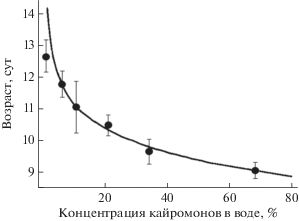
Рис. 10.
Зависимость возраста полового созревания (M ± m) гибридных особей дафнии Daphnia galeata × × D. hyalina от концентрации запаха окуня Perca fluviatilis (по: Reede, 1995, с изменениями).
Таким образом, длительная экспозиция в воде с запахом рыб перераспределяет энерготраты у дафний и смещает метаболизм с соматического на генеративный. Это приводит к сдвигу воспроизводства дафний на более ранний возраст. Они достигают половозрелости при меньших размерах, продуцируют мелкие и более многочисленные яйца, из которых выходит мелкая молодь. В итоге жертвы становятся менее уязвимыми для рыб-планктонофагов, предпочитающих питаться более крупными объектами (Lass, Spaak, 2003). Запах личинок коретры Chaoborus, неспособных питаться крупными жертвами, стимулирует у дафний не генеративный, а соматический рост (Tollrian, 1994). При совместном воздействии кайромонов окуня и коретры химические сигналы нейтрализуют друг друга и эффект не проявляется (Weber, Declerck, 1997).
Восприимчивость к запаху рыб у дафний разного происхождения может не совпадать. Так, длительное пребывание D. galeata в воде с запахом язя снижает скорость роста у 17 клонов и приводит к более раннему созреванию у 13 клонов из 24 исследованных (Tams et al., 2018). Влияние имеет и эколого-географическая совместимость хищника и жертвы. Так, для моины Moina macrocopa, сосуществующей с глазчатым горчаком Rhodeus ocellatus в одних и тех же водоёмах, запах горчака влияет на сроки созревания, плодовитость и подвижность значительно сильнее, чем запахи выращенных в искусственных условиях золотой рыбки и данио Danio rerio (Gu et al., 2017).
Хроническое действие кайромонов золотого карася C. auratus сокращает в среднем на 20% продолжительность жизни дафний D. hyalina и Diaphanosoma brachyurum (Dawidowicz et al., 2010). Развитие в среде с запахом гольяна Phoxinus phoxinus снижает пигментированность дафний D. pulex, что делает их менее заметными для рыб, полагающихся на зрение при поиске мелких планктонных жертв (Tollrian, Heibl, 2004). Сила действия запаха плотвы на жизненный цикл дафнии D.galeata зависит от их возрастной стадии, длительности экспозиции (Macháček, 1995) и концентрации кайромонов в воде (Reede, 1995; Castro et al., 2007).
Запах верховки усиливает у дафнии D. magna экспрессию гена, контролирующего фолдинг – скручивание полипептидной цепи и приобретение белками уникальной пространственной структуры. Полагают, это может иметь отношение к механизмам структурных (фенотипических) преобразований, вызываемых кайромонами (Schwarzenberger et al., 2009).
Насекомые (Insecta). В воде, в которой постоянно присутствует запах ручьевого гольца S. fontinalis, замедляется рост личинок подёнок B. bicaudatus: при переходе к стадии имаго они уступают по размерам личинкам, выросшим в воде без кайромонов (Peckarsky, McIntosh, 1998). В воде, содержащей последовательно кайромоны трёхиглой колюшки и солнечника L. gibbosus, личинки стрекозы зелёной лютки Lestes viridis (Odonata) растут медленнее. Они отстают по массе тела и накопленным энергоёмким веществам (жиры), их иммунная система хуже развита, чем у личинок в чистой воде (Stoks et al., 2006).
Влияние кайромонов на сенсорные системы жертв
Кайромоны хищных рыб не только служат жертвам сигналом о потенциальной угрозе, вызывают поведенческие реакции и индуцируют пластические изменения, но и обостряют чувствительность к стимулам различных модальностей, что способствует раннему обнаружению опасности. Показано, что даже непродолжительное (1–3 ч) содержание в воде с запахом обыкновенного фундулюса (экстракт кожной слизи) повышает восприимчивость к свету молоди (зоеа) морских крабов Rhithropanopeus harrisii и Hemigrapsus sanguineus. Снижение пороговой освещённости происходит за счёт морфологических преобразований зрительных рецепторных клеток личинок, причём эти изменения носят концентрационно-зависимый характер (Charpentier, Cohen, 2015). Столь же быстрые и такие же морфофункциональные преобразования сетчатки и зрительных возможностей запах рыб вызывает у взрослых особей артемии A. franciscana (Charpentier, Cohen, 2018). Присутствие в воде кайромонов L. macrochirus повышает восприимчивость дафнии D. pulicaria не только к изменению освещённости (Dawidowicz, Loose, 1992; Ringelberg, 1995; Forward, Rittschof, 1999; Charpentier et al., 2019), но и к небольшим гидродинамическим возмущениям, имитирующим плавание мелких планктоноядных рыб при питании (Brewer et al., 1999).
ВРОЖДЁННЫЕ РЕАКЦИИ И ПРИОБРЕТЁННЫЙ ОПЫТ
Результаты многих исследований указывают на врождённый характер защитного поведения, вызываемого кайромонами рыб (Dalesman et al., 2006; Epp, Gabor, 2008; DeSantis et al., 2013). Так, наивная молодь широкопалого речного рака, выращенная в искусственных условиях и не имевшая опыта встреч с хищниками, становится менее активной и стремится больше времени проводить в укрытиях, если в аквариум попадает запах окуня, щуки, налима и европейского угря (табл. 2) (Appelberg et al., 1993). На запах гамбузии G. affinis, солнечника L. auratus и большеротого окуня реагируют выращенные в искусственных условиях взрослые особи саламандры Eurycea sosorum (DeSantis et al., 2013). Запах окуня подавляет в равной мере подвижность как головастиков травяной лягушки Rana temporaria, отловленных из водоёмов, населённых питающимися ими рыбами, так и головастиков из безрыбных водоёмов (Laurila, 2000). На запах ручьевого гольца S. fontinalis реагируют личинки подёнок (Ephemeroptera) и веснянок (Plecoptera), выращенных в лаборатории (Peckarsky, McIntosh, 1998).
Однако индивидуальный опыт столкновений с хищниками может влиять на проявление защитной реакции. Так, личинки подёнки B. bicaudatus, взятые из речек, населённых рыбами, реагируют сильнее отловленных в водоёмах без рыб (McIntosh, Peckarsky, 1996). Личинки коретры C. flavicans, ранее сталкивавшиеся с запахом трёхиглой колюшки, реагируют на него более интенсивно (Dawidowicz et al., 1990). Предварительное пребывание в воде с кайромоном линя усиливает ответ у большого прудовика (Dalesman et al., 2006). Преэкспозиция в среде с кайромонами верховки Leuciscus delineatus и плотвы усиливает уход дафнии на глубину (De Meester, 1993; Pijanowska et al., 2006b). Реакция ухода на глубину в ответ на запах обыкновенного фундулюса, атлантического менхэдена и колючей чопы у науплий артемии A. franciscana проявляется лишь в том случае, если они предварительно содержались в воде с кайромонами в течение суток (Forward, Rittschof, 1993; McKelvey, Forward, 1995). Механизм активации чувствительности к кайромонам не изучен. Личинки подёнки Ischnura elegans реагируют снижением двигательной активности на запах солнечника L. gibbosus только в тех случаях, когда несут на себе следы прошлых атак хищников – регенерирующие сегменты хвостовой части тела, потерянные ранее. Такое поведение характерно для личинок как из водоёма, населённого рыбами, так и из водоёма без рыб, что может указывать на врождённый характер ответов на кайромоны (Gyssels, Stoks, 2006). Насколько устойчивы приобретённые личинками индивидуальные навыки и закреплены ли генетически популяционные различия в поведении, остаётся невыясненным.
При сочетании с сильными врождёнными стимулами, информирующими об опасности, такими как феромон тревоги, котрый попадает в воду при физическом нарушении целостности жертв, запахи рыб могут усиливать своё действие или становиться эффективными. Запах кумжи, ручьевого гольца, тигровой (S. trutta × S. fontinalis) и радужной форели не влияет на активность головастиков древесной лягушки Lithobates sylvaticus (Ranidae, Anura). Однако однократное сочетание запаха этих рыб с феромоном тревоги головастика приводит последнего к обучению распознавать кайромоны и реагировать на них резким снижением плавания (Chivers et al., 2015). Наивные личинки стрекоз-стрелок Enallagma spp. начинают реагировать на запах щуки после однократного совместного предъявления этого запаха вместе с феромоном тревоги (Wisenden et al., 1997). При сочетании с феромоном тревоги головастики лягушки Pelophylax perezi начинают реагировать на ранее инертный для них запах данио и сохраняют эту способность более недели (Gonzalo et al., 2009).
Уменьшение размеров тела и увеличение плодовитости в ответ на запах язя демонстрируют те клоны дафний, которые взяты из водоёмов, населённых рыбами (Boersma et al., 1999). Амфиподы G. pulex из водоёма, населённого рыбами, в ответ на запах золотого карася снижают двигательную активность, тогда как амфиподы из водоёма, где рыбы отсутствуют, её усиливают. Эти различия сохраняются у следующего поколения амфипод (F1), что указывает на наследуемость этих качеств, т.е. на существование популяционного своеобразия защитного поведения, вызываемого кайромонами (Åbjörnsson et al., 2004). У выращенных в искусственных условиях особей поколения F1 большого прудовика и пупырчатой физы защитное поведение в ответ на запах линя также сохраняется (Dalesman et al., 2006).
Существуют данные, указывающие на возможность привыкания жертв к запаху хищника. После длительного (3, 9, 15 сут) нахождения в среде с запахом длинноухого солнечника изопода Lirceus fontinalis, помещённая для опытов в чистую воду, реагирует на него слабее, чем содержащаяся в среде без запаха (Holomuzki, Hatchett, 1994).
ИСТОЧНИКИ И ПРОИСХОЖДЕНИЕ КАЙРОМОНОВ
У рыб крайне редко находят специальные железы, секретирующие запаховые вещества; как правило, кайромоны представляют собой продукты внешней экскреции (Kasumyan, 2004). Это могут быть вещества, выделяющиеся из кожи и кожной слизи, попадающие в воду вместе с мочой и фекалиями, с жаберными экскретами. Так, запах (экзометаболиты) радужной форели, а также экстракт кожной слизи и фекалий, содержащих кишечную слизь, блокируют пассивный скат вниз по течению в темноте амфиподы G. pseudolimnaeus (Williams, Moore, 1985). Вещества кожной слизи разных видов Salmonidae ответственны за сокращение времени пребывания в потоке воды при ночном скате личинок подёнки B. bicaudatus (Alvarez et al., 2014). Как полагают, запах рыб может образовываться за счёт микробных преобразований более сложных веществ-предшественников кожной слизи. На такую возможность указывает ослабление эффекта кайромонов после предварительной обработки рыб-доноров запаха антибиотиком (Ringelberg, van Gool, 1998; Beklioglu et al., 2006).
Являются ли кайромоны рыб самостоятельными сигналами или представляют собой некие дериваты сигнальных веществ, находящихся в потреблённых жертвах, – вопрос дискуссионный. Внимание этой проблеме уделено во многих исследованиях. Накоплено большое число данных, показывающих, что эффективность кайромонов зависит от питания хищников, точнее – от того, входили ли в их рацион жертвы, реакция которых исследуется. Если таких организмов в питании хищника не было, то ответы на его запах слабые или отсутствуют. Если же рыбы получают эти организмы в качестве пищи, то действие запаха проявляется или усиливается (Chivers et al., 1996; Mathis, Hoback, 1997; Chivers, Mirza, 2001; Stabell et al., 2003; Laforsch et al., 2006; Ferrari et al., 2010; Kenison et al., 2018). Например, личинки подёнок Siphlonurus и Siphlonisca снижают свою двигательную активность при стимуляции запахом ручьевого гольца только в том случае, если гольцам предварительно скармливали личинок любых из этих подёнок. Эффект отсутствовал, если рыбы питались артемией. Длительность питания гольцов подёнками и величина рациона, достаточные или необходимые для проявления эффекта, не исследовались. Максимальное снижение двигательной активности вызывал водный экстракт самих личинок (Huryn, Chivers, 1999).
Личинки стрекоз-стрелок Enallagma spp., взятые из водоёма, где отсутствует щука, на запах этого хищника проявляют своё типичное охотничье поведение (изгиб тела, направленные перемещения, броски), если щуку предварительно (12 сут) кормили мучными червями Tenebrio molitor. Но запах щуки, получавшей в качестве пищи личинок стрекоз-стрелок, подавляет пищевое поведение личинок (Chivers et al., 1996). Сравнительное тестирование личинок стрекоз-стрелок Enallagma spp., полученных из водоёмов с щукой и без неё, показало, что наивные по отношению к этому хищнику личинки реагируют лишь на собственный феромон тревоги, тогда как личинки из водоёмов с щукой реагируют на оба запаха – на феромон тревоги и на запах щуки (Wisenden et al., 1997). Запах полосатой зубатки Anarhichas lupus, питавшейся несколько недель мидией Mytilus edulis, слабо эффективен для морского ежа Strongylocentrotus droebachiensis. Однако после двух недель кормления зубатки ежами, её запах вызывает у ежей сильный ответ – смену направления движения на противоположное или длительное замирание. Водный экстракт ежей для ежей также эффективен, но по силе воздействия уступает запаху зубатки, питавшейся ежами (Hagen et al., 2002).
Предполагается, что эффект, который приобретает запах хищников после того как они некоторое время питаются исследуемыми жертвами, обеспечивается не компонентами собственного запаха хищников, а феромоном тревоги, содержащимся в жертвах. Проходя через пищеварительный тракт хищника, феромон тревоги выходит наружу неизменённым либо модифицированным. Запах хищника приобретает таким путём сигнальную активность, которая маркирует его, что послужило основанием назвать приобретённую активность пищевой меткой (Wisenden et al., 1997; Ferrari et al., 2007). Другие исследователи называют этот сигнал покоящимся феромоном или латентным сигналом опасности (Stabell et al., 2003; Stabell, 2005).
Однако имеются исследования, согласно которым рыбам совершенно необязательно питаться какое-то время жертвами, чтобы быть для них источниками сигналов опасности и вызывать релизерные или праймерные ответы (von Elert, Loose, 1996; von Elert, Pohnert, 2000; von Elert, Stibor, 2006; Paterson et al., 2013). Сообщается, что для эффективности кайромонов рыб не имеет значения, питались ли рыбы-доноры конкретными жертвами или нет (Loose et al., 1993). Для подёнки B. bicaudatus кайромонами, вызывающими защитное поведение, являются компоненты кожной слизи рыб, но не вещества, происхождение которых имеет отношение к самим жертвам – феромон тревоги или продукты его преобразования в пищеварительном тракте хищника (рис. 11) (Alvarez et al., 2014). Запах американского карликового сома Ameiurus nebulosus, питавшегося искусственным кормом или головастиками леопардовой лягушки Lithobates pipiens, имеет сходное влияние на темпы развития, роста и пропорции тела этих головастиков (Balaa, Blouin-Demers, 2013).
Рис. 11.
Число (M ± m) личинок североамериканской подёнки Baetis bicaudatus, находящихся в потоке воды в присутствии кайромонов ручьевого гольца Salvelinus fontinalis, получавшего разный рацион: 1 – голодные рыбы, 2 – рыбы, питавшиеся личинками B. bicaudatus, 3 – рыбы, питавшиеся растительным белком; * отличия от контроля (К) достоверны при p < 0.05 (по: Alvarez et al., 2014, с изменениями).
Во многих экспериментальных работах для контроля фактора питания используют в качестве доноров запаха голодающих рыб либо рыб, питавшихся организмами, не имеющими отношения к исследуемым жертвам с точки зрения экологии, ареала и систематики. Но накормленность хищника, а не только состав его рациона могут быть самостоятельным фактором, влияющим на сигнальность его запаха – экзометаболиты сытого окуня, в отличие от голодного, не эффективны для жука-плавунца Acilius sulcatus (Åbjörnsson et al., 1997). Запахи сытого и голодающего окуня различаются по действию на широкопалого речного рака (Appelberg et al., 1993).
ХИМИЧЕСКАЯ ПРИРОДА КАЙРОМОНОВ РЫБ
Состав экзометаболитов рыб крайне сложен. Попытки установить химическую природу запаховых веществ рыб, вызывающих у водных животных поведенческие и иные ответы, предпринимались неоднократно. Неспецифичность реакции некоторых животных (амфиподы, дафнии, моллюски) на запахи разных видов рыб, в том числе аллопатрических (Williams, Moore, 1985; von Elert, Loose, 1996; Naddafi, Rudstam, 2013), дало основание предположить, что основными компонентами запаха могут быть некие общие для всех рыб вещества (Williams, Moore, 1985). Молекулярная масса этих веществ, по разным данным, варьирует в широком диапазоне. Эффективными компонентами запаха рыб для португальского кораблика P. physalis являются вещества с молекулярной массой ≤ 3000 Да, более высокомолекулярные вещества оказались полностью инертными (Purcell, Anderson, 1995). Вещества из кожной слизи карпа, вызывающие реакцию прикрепления к хозяину у церкарий паразитической трематоды Acanthostomum brauni, имеют молекулярную массу > 10 000 Да и представляют собой гликопротеины, но их углеводные компоненты активностью не обладают (Haas, Ostrowski de Nuñez, 1988). Молекулярная масса веществ обыкновенного фундулюса, усиливающих уход науплий A. franciscana от света на глубину, не превышает 10 000 Да. Эти вещества могут быть продуктами распада более сложных молекул кожной слизи (глюкозаминогликаны, гликопротеины), скорее всего, дисахаридами гиалуроновой кислоты, особенно с сульфамино- или ацетиламиногруппой у второго углеродного атома. Эффект таких гексозаминов прослеживается вплоть до концентрации 10–9 М (Forward, Rittschof, 1999; Rittschof, Cohen, 2004).
Активным компонентом запаха лососёвых рыб, стимулирующего личинок подёнок уходить в убежища, являются гексозамины, входящие в более сложные соединения – глюкозаминогликаны, при бактериальном разрушении которых высвобождается действующий компонент. Пептидная составляющая глюкозаминогликанов активностью не обладает (Landeira-Dabarca et al., 2019). Химическими компонентами кожной слизи рыб, активирующими выброс филамента у спор миксоспоридий, являются относительно низкомолекулярные вещества, скорее всего, нуклеозиды (Kallert et al., 2011). Попытка выделить действующие вещества из экзометаболитов налима методом твёрдофазной экстракции с помощью С18-SPE показало, что кайромон либо не обладает липофильными свойствами, либо эти вещества не могут быть элюированы метанолом (Baumgärtner et al., 2002).
Большую историю имеют эксперименты по идентификации химической природы кайромонов рыб, стимулирующих вертикальные перемещения у планктонных ракообразных. Установлено, что вещества, обладающие такой активностью, относительно небольшие по размеру молекулы (<500 Да), хорошо растворимы в воде и нелетучи, устойчивы к температуре и рН, имеют невысокую липофильность, подвергаются быстрой деградации микроорганизмами. Наличие в молекуле амино, карбокси, сульфатных и фосфатных групп, по-видимому, не обязательно или роль этих групп в обеспечении активности невысока. Глюкуроновая кислота – одна из основных составляющих слизи рыб − также не связана с активностью запаха. Эффективность не теряется после воздействия различными пептидазами, что указывает на их непептидную природу. Существенные различия между кайромонами разных представителей карповых и других рыб не обнаружены (верховка, золотой карась, плотва, трёхиглая колюшка, щука) (Loose et al., 1993; von Elert, Loose, 1996; von Elert, Pohnert, 2000). Интересно, что активность экзометаболитов коретры Chaoborus, обладающих сходным действием на дафний, связывают с наличием карбоксильных и гидроксильных групп (Tollrian, von Elert, 1994). Были получены данные о том, что активным компонентом запаха рыб, влияющим на вертикальные перемещения дафний, может быть триметиламин (ТМА), предшественник триметиламин-N-оксида, играющего у рыб важную роль в процессах осморегуляции (Boriss et al., 1999). Однако эти выводы впоследствии были отвергнуты, поскольку экзометаболиты рыб после удаление ТМА из их состава не снижали своего эффекта на дафний (Pohnert, von Elert, 2000). На основании сходства эффектов запаха рыб и выделяемых ими в воду желчных солей, а также самой желчи предположено, что кайромонами могут быть желчные вещества (Pijanowska et al., 2020).
В обычной воде кайромоны рыб (запах обыкновенного солнечника) довольно устойчивы и могут сохраняться в течение нескольких суток хранения, но закономерно теряют свою активность (рис. 12) (Turner, Montgomery, 2003).
ЗАКЛЮЧЕНИЕ
Несмотря на различия в экспериментальных подходах и методах, в целом имеющиеся данные свидетельствуют о важной роли, которую запахи рыб играют в регуляции отношений между хищниками (рыбы) и их жертвами в водных сообществах (Paterson et al., 2013). Эти связи затрагивают разных животных, прежде всего тех, кто обычно является объектами питания рыб, – моллюсков, различных ракообразных, насекомых, амфибий и других (рис. 13, табл. 2). Учитывая высокий уровень развития хеморецепции у водных животных (Kamio, Derby, 2017), вряд ли можно ожидать каких-либо исключений из этого правила при расширении круга исследованных видов и систематических групп, который пока остаётся ограниченным. Кайромоны рыб могут влиять не только на водных животных, но и на тех, у которых с водной средой связана лишь часть жизненного цикла. Такие данные получены пока лишь для некоторых имаго насекомых и взрослых амфибий, но, возможно, таких животных больше.
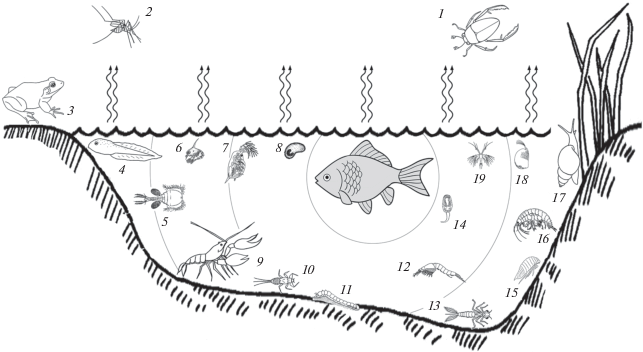
Рис. 13.
Некоторые животные, проявляющие релизерные и праймерные реакции на кайромоны рыб: 1 – жуки-плавунцы и водолюбы (Coleoptera), 2 – двукрылые насекомые (Diptera); 3, 4 – взрослые и личинки бесхвостых (Anura) и хвостатых (Cuadata) земноводных (Amphibia), 5 – веслоногие (Copepoda), 6 – личинки крабов (Decapoda), 7 – ветвистоусые (Cladocera), 8 – теронт (бродяжка) паразитической реснитчатой инфузории Ichthyophthirius multifiliis (Ciliophora), 9 – раки и омары (Decapoda), 10 – личинки подёнок (Ephemeroptera), 11 – личинки двукрылых насекомых (Diptera), 12 – мизиды (Mysida), 13 – личинки стрекоз (Odonata), 14 – церкарий трематоды (Digenea), 15 – равноногие (Isopoda), 16 – бокоплавы (Amphipoda), 17 – брюхоногие (Gastropoda), 18 – ракушечные рачки (Ostracoda), 19 – науплии жаброногих (Branchiopoda). Пропорции размеров тела животных не соблюдены.
Кайромоны вызывают у жертв широкий спектр быстрых поведенческих реакций и индуцируют медленное развитие многообразных эпигенетических изменений (табл. 2). Один и тот же кайромон может быть для жертвы одновременно сигналом-релизером и сигналом-праймером, но такие примеры получены для запахов не рыб, а других водных хищников (Bourdeau et al., 2015). Информация, которую несут водным животным запахи рыб, касается главным образом взаимоотношений хищник−жертва и паразит−хозяин. Защитное поведение, морфологические адаптации и изменения репродуктивной стратегии у жертв определяются образом их жизни и особенностями охоты рыб (Smith et al., 2008). Это оптимизирует защитное поведение жертв и повышает его эффективность, но усложняет питание хищникам и неизбежно вызывает развитие контрадаптаций (Lima, Dill, 1990; Kats, Dill, 1998). Запахи хищника являются сильными стрессорами для жертв. Получение таких сигналов запускает каскад физиологических преобразований, активирующих эндокринную систему и метаболизм. Это повышает готовность жертв к проявлению быстрого поведенческого ответа, но представление об этих процессах пока недостаточно ясное (Mitchell et al., 2017).
У большинства водных животных способность реагировать на запахи, распространяемые рыбами в воде, закреплена генетически, но может усиливаться или даже возникать в результате приобретаемого жертвами индивидуального опыта. Каким образом соотносится врождённость и приобретённый индивидуальный опыт в проявлении этой способности, насколько разным это соотношение может быть у разных взаимодействующих видов − эти вопросы лишь начинают разрабатываться. Слабо изучены и другие направления, например, физиологические механизмы, лежащие в основе развития у жертв фенотипических изменений в ответ на хроническое воздействие кайромонов. Явно недостаточно знаний о химической природе кайромонов рыб, что объясняется сложностью работ по идентификации сложных высокомолекулярных веществ, к которым, судя по имеющимся данным, принадлежат многие кайромоны рыб.
Нельзя исключать, что многие несоответствия полученных данных о реагировании гидробионтов на запахи рыб обусловлены несовершенством используемых методов, прежде всего отсутствием стандартизации условий и способов получения запаховых стимулов. Известно, что сила проявляемых водными животными поведенческих реакций на кайромоны рыб носит дозозависимый характер (Reede, 1995; Kats, Dill, 1998; Castro et al., 2007; Charpentier, Cohen, 2015). Однако в большинстве исследований мало внимания уделяют концентрации действующего стимула или этот важный параметр не учитывают вовсе. Отсутствие знаний о химической природе кайромонов рыб не даёт возможности выражать их концентрацию общепринятыми единицами. Но в таких случаях можно использовать условные единицы, учитывающие размеры (массу тела) доноров запаха, длительность экспозиции и объём воды или скорость водообмена, например г ⋅ ч/л (Малюкина и др., 1983; Касумян, Пономарев, 1986; Mathis, Hoback, 1997; Мантейфель, Жушев, 1998). Во многих статьях, к сожалению, не упоминаются размеры рыб-доноров, температура и объём воды, в которых их содержали, продолжительность экспозиции и другие условия (табл. 1). Внимание и более строгий подход к количественному выражению концентрации кайромонов позволит решить важные вопросы, например, сравнить эффективность кайромонов рыб, использующих разные стратегии пищевого поведения (угонщики/засадчики) или оценить концентрации кайромонов, которые требуются для стимуляции у жертв ответов разных типов (релизерные и/или праймерные).
К слабо разработанным относится вопрос о существовании в онтогенезе водных животных чувствительных периодов, когда восприимчивость к кайромонам обострена или морфологические последствия у жертв, попадающих под их влияние, наиболее выражены. Отсутствуют чёткие представления о продолжительности хронического действия кайромонов, достаточной для индуцирования у жертв пластических изменений, и относительного вклада в эти изменения длительности и силы (концентрации) этого воздействия. Ещё меньше знаний об обратимости индуцированных изменений (Relyea, 2003).
Почти все рыбы, эффект запахов которых исследовали, принадлежат к пресноводным видам. Среди них слабо представлены виды, которые по типу охоты относятся к подстерегающим хищникам (засадчикам) и представляют для жертв наибольшую опасность. Крайне ограничены наши знания о влиянии на способность водных животных обнаруживать и реагировать на кайромоны рыб разных внешних факторов, таких как температура и солёность воды, содержание загрязняющих веществ и др. (Lürling, Scheffer, 2007; Araújo et al., 2020; Cothran et al., 2020). Требуются более детальные исследования связи между питанием рыб (состав и размер рациона, длительность его получения) и эффективностью воздействия их кайромонов на жертв. Учитывая несомненную важность этих и других новых сведений в этой области водной химической экологии, многие из ещё недостаточно разработанных направлений, несомненно, получат дальнейшее развитие уже в ближайшее время.
Список литературы
Гопко М.В., Михеев В.Н. 2017. Паразитические манипуляции фенотипом хозяина: эффекты во внутренней и внешней среде // Журн. общ. биологии. Т. 78. № 6. С. 16–48.
Касумян А.О., Павлов Д.С. 2018. Стайное поведение рыб. М.: Т-во науч. изд. КМК, 273 с.
Касумян А.О., Пономарев В.Ю. 1986. Исследование поведения данио-рерио Brachidanio rerio Hamilton-Buchanan (Cypriniformes, Cyprinidae) при действии естественных химических пищевых сигналов // Вопр. ихтиологии. Т. 26. Вып. 4. С. 665–673.
Малюкина Г.А., Марусов Е.А., Карпов А.К. 1983. Некоторые особенности химической сигнализации у беломорской трески Gadus morhua marisalbi Derjugin (Gadidae) // Там же. Т. 23. Вып. 5. С. 839–844.
Мантейфель Ю.Б., Жушев А.В. 1998. Поведенческие реакции личинок четырех видов бесхвостых амфибий на химические стимулы от хищников // Журн. общ. биологии. Т. 59. № 2. С. 192–208.
Никифоров А.И., Гаврилов Б.А., Круглова Д.К. и др. 2018. Исследования с использованием выделенной из водной среды ДНК: состояния и перспективы // Успехи соврем. биологии. Т. 138. № 1. С. 18–30. https://doi.org/10.7868/S0040364418010039
Åbjörnsson K., Wagner B.M.A., Axelsson A. et al. 1997. Responses of Acilius sulcatus (Coleoptera: Dytiscidae) to chemical cues from perch (Perca fluviatilis) // Oecologia. V. 111. P. 166–171. https://doi.org/10.1007/s004420050221
Åbjörnsson K., Hansson L.-A., Brönmark C. 2004. Responses of prey from habitats with different predator regimes: local adaptation and heritability // Ecology. V. 85. № 7. P. 1859–1866. https://doi.org/10.1890/03-0074
Alvarez M., Landeira-Dabarca A., Peckarsky B. 2014. Origin and specificity of predatory fish cues detected by Baetis larvae (Ephemeroptera; Insecta) // Anim. Behav. V. 96. P. 141–149. https://doi.org/10.1016/j.anbehav.2014.07.017
Andersen T.H., Friberg N., Hansen H.O. et al. 1993. The effects of introduction of brown trout (Salmo trutta L.) on Gammarus pulex L. drift and density in two fishless Danish streams // Arch. Hydrobiol. V. 126. P. 361–371.
Andersson K.G., Bronmark C., Herrmann J. et al. 1986. Presence of sculpins (Cottus gobio) reduces drift and activity of Gammarus pulex (Amphipoda) // Hydrobiologia. V. 133. P. 209–215. https://doi.org/10.1007/BF00005592
Angelon K.A., Petranka J.W. 2002. Chemicals of predatory mosquitofish (Gambusia affinis) influence selection of oviposition site by Culex mosquitoes // J. Chem. Ecol. V. 28. № 4. P. 797–806. https://doi.org/10.1023/A:1015292827514
Appelberg M., Söderbäck B., Odelström T. 1993. Predator detection of predation risk in the crayfish Astacus astacus L. // Nord. J. Freshwat. Res. V. 68. P. 55–62.
Araújo C.V.M., Pereira K.C., Sparaventi E. et al. 2020. Contamination may induce behavioural plasticity in the habitat selection by shrimps: a cost-benefits balance involving contamination, shelter and predation // Environ. Pollut. V. 263B. Article 114545. https://doi.org/10.1016/j.envpol.2020.114545
Atema J. 2012. Aquatic odour dispersal fields: opportunities and limits of detection, communication, and navigation // Chemical ecology in aquatic systems / Eds. Brönmark C., Hansson L.-A. Oxford: Oxford Univ. Press. P. 1–18.
Auld J.R., Agrawal A.A., Relyea R.A. 2010. Re-evaluating the costs and limits of adaptive phenotypic plasticity // Proc. Roy. Soc. Biol. Sci. V. 277B. P. 503–511. https://doi.org/10.1098/rspb.2009.1355
Balaa R.E., Blouin-Demers G. 2013. Does exposure to cues of fish predators fed different diets affect morphology and performance of Northern Leopard Frog (Lithobates pipiens) larvae? // Can. J. Zool. V. 91. P. 203–211. https://doi.org/10.1139/cjz-2012-0232
Baldauf S.A., Thüken T., Frommen J.G. et al. 2007. Infection with an acanthocephalan manipulates an amphipod’s reaction to a fish predator’s odours // Int. J. Parasitol. V. 37. P. 61–65. https://doi.org/10.1016/j.ijpara.2006.09.003
Baumgärtner D., Jungbluth A.-D., Koch U., von Elert E. 2002. Effects of infochemicals on microhabitat choice by the freshwater amphipod Gammarus roeseli // Arch. Hydrobiol. V. 155. P. 353–367. https://doi.org/10.1127/archiv-hydrobiol/155/2002/353
Baumgärtner D., Koch U., Rothhaupt K.-O. 2003. Alteration of kairomone-induced antipredator response of the freshwater amphipod Gammarus roeseli by sediment type // J. Chem. Ecol. V. 29. № 6. P. 1391–1401. https://doi.org/10.1023/A:1024213403537
Beklioglu M., Telli M., Gozen A.G. 2006. Fish and mucus-dwelling bacteria interact to produce a kairomone that induces diel vertical migration in Daphnia // Freshwat. Biol. V. 51. P. 2200–2206. https://doi.org/10.1111/j.1365-2427.2006.01642.x
Berendonk T.U. 1999. Influence of fish kairomones on the ovipositing behavior of Chaoborus imagines // Limnol. Oceanogr. V. 44. № 2. P. 454–458. https://doi.org/10.4319/lo.1999.44.2.0454
Bernot R.J., Turner A.M. 2001. Predator identity and trait-mediated indirect effects in a littoral food web // Oecologia. V. 129. P. 139–146. https://doi.org/10.1007/s004420100705
Bezirci G., Akkas S.B., Rinke K. et al. 2012. Impacts of salinity and fish-exuded kairomone on the survival and macromolecular profile of Daphnia pulex // Ecotoxicology. V. 21. P. 601–614. https://doi.org/10.1007/s10646-011-0820-0
Binckley C.A., Resetarits W.J., Jr. 2003. Functional equivalence of non-lethal effects: generalized fish avoidance determines distribution of gray treefrog, Hyla chrysoscelis, larvae // Oikos. V. 102. P. 623–629. https://doi.org/10.1034/j.1600-0706.2003.12483.x
Bjærke O., Andersen T., Titelman J. 2014. Predator chemical cues increase growth and alter development in nauplii of a marine copepod // Mar. Ecol. Prog. Ser. V. 510. P. 15–24. https://doi.org/10.3354/meps10918
Blake M.A., Hart P.J.B. 1993. The behavioural responses of juvenile signal crayfish Pacifastacus leniusculus to stimuli from perch and eels // Freshwat. Biol. V. 29. № 1. P. 89–97. https://doi.org/10.1111/j.1365-2427.1993.tb00747.x
Boersma M., De Meester L., Spaak P. 1999. Environmental stress and local adaptation in Daphnia magna // Limnol. Oceanogr. V. 44. № 2. P. 393–402. https://doi.org/10.4319/lo.1999.44.2.0393
Boriss H., Boersma M., Wiltshire K.H. 1999. Trimethylamine induces migration of waterfleas // Nature. V. 398. P. 382–382. https://doi.org/10.1038/18796
Bourdeau P.E., Butlin R.K., Brönmark C. et al. 2015. What can aquatic gastropods tell us about phenotypic plasticity: a review and meta-analysis // Heredity. V. 115. P. 312–321. https://doi.org/10.1038/hdy.2015.58
Brewer M.C., Dawidiwicz P., Dodson S.I. 1999. Interactive effects of fish kairomone and light on Daphnia escape behavior // J. Plankton Res. V. 21. № 7. P. 1317–1335. https://doi.org/10.1093/plankt/21.7.1317
Brodin T., Johansson F., Bergsten J. 2006. Predator related oviposition site selection of aquatic beetles (Hydroporus spp.) and effects on offspring life-history // Freshwat. Biol. V. 51. P. 1277–1285. https://doi.org/10.1111/j.1365-2427.2006.01563.x
Brönmark C., Hansson L.-A. (eds.). 2012. Chemical ecology in aquatic systems. Oxford: Oxford Univ. Press, 291 p.
Brönmark C., Lakowitz T., Hollander J. 2011. Predator-induced morphological plasticity across local populations of a freshwater snail // PLoS ONE. V. 6. № 7. Article e21773. https://doi.org/10.1371/journal.pone.0021773
Castro B.B., Consciência S., Gonçalves F. 2007. Life history responses of Daphnia longispina to mosquitofish (Gambusia holbrooki) and pumpkinseed (Lepomis gibbosus) kairomones // Hydrobiologia. V. 594. P. 165–174. https://doi.org/10.1007/s10750-007-9074-5
Chapman H.D. 1974. The behaviour of the cercaria of Cryptocotyle lingua // Z. Parasitenkunde. V. 44. P. 211–226.
Charpentier C.L., Cohen J.H. 2015. Chemical cues from fish heighten visual sensitivity in larval crabs through changes in photoreceptor structure and function // J. Exp. Biol. V. 218. P. 3381–3390. https://doi.org/10.1242/jeb.125229
Charpentier C.L., Cohen J.H. 2018. Kairomones from an estuarine fish increase visual sensitivity in brine shrimp (Artemia franciscana) from Great Salt Lake, Utah, USA // J. Comp. Physiol. V. 204 A. P. 197–208. https://doi.org/10.1007/s00359-017-1230-4
Charpentier C.L., Angell C.S., Duffy P.I., Cohen J.H. 2019. Natural variations in estuarine fish, fish odor, and zooplankton photobehavior // Mar. Freshwat. Behav. Physiol. V. 52. № 3. P. 265−282. https://doi.org/10.1080/10236244.2020.1713701
Chivers D.P., Mirza R.S. 2001. Predator diet cues and the assessment of predation risk by aquatic vertebrates a review and prospectus // Chemical signals in vertebrates / Eds. Marchlewska-Koj A. et al. N.Y.: Kluwer Acad. Plenum Publ. P. 277–284.
Chivers D.P., Wisenden B.D., Smith R.J.F. 1996. Damselfly larvae learn to recognize predators from chemical cues in the predator’s diet // Anim. Behav. V. 52. P. 315–320. https://doi.org/10.1006/anbe.1996.0177
Chivers D.P., Mathiron A., Sloychuk J.R., Ferrari M.C.O. 2015. Responses of tadpoles to hybrid predator odours: strong maternal signatures and the potential risk/response mismatch // Proc. Roy. Soc. Biol. Sci. V. 282 B. Article 1809. https://doi.org/10.1098/rspb.2015.0365
Cieri M.D., Stearns D.E. 1999. Reduction of grazing activity of two estuarine copepods in response to the exudate of a visual predator // Mar. Ecol. Prog. Ser. V. 177. P. 157–163. https://doi.org/10.3354/meps177157
Cohen J.H., Forward R.B., Jr. 2003. Ctenophore kairomones and modified aminosugar disaccharides alter the shadow response in a larval crab // J. Plankton Res. V. 25. № 2. P. 203–213. https://doi.org/10.1093/plankt/25.2.203
Cothran R.D., Monahan P.J., Relyea R.A. 2020. Antipredator behaviour affected by prey condition, food availability and pH-mediated info-disruption // Anim. Behav. V. 171. P. 111–118. https://doi.org/10.1016/j.anbehav.2020.11.007
Coulter D.P., Wang P., Coulter A.A. et al. 2019. Nonlinear relationship between silver carp density and their eDNA concentration in a large river // PLoS ONE. V. 14. № 6. Article e0218823. https://doi.org/10.1371/journal.pone.0218823
Dahl J., Nilsson P.A., Pettersson L.B. 1998. Against the flow: chemical detection of downstream predators in running waters // Proc. Roy. Soc. Biol. Sci. V. 265. № 1403. P. 1339–1344. https://doi.org/10.1098/rspb.1998.0439
Dalesman S., Rundle S.D., Coleman R.A., Cotton P.A. 2006. Cue association and antipredator behavior in a pulmonate snail, Lymnaea stagnalis // Anim. Behav. V. 71. P. 789–797. https://doi.org/10.1016/j.anbehav.2005.05.028
Dawidowicz P., Loose C.J. 1992. Metabolic costs during predator-induced diel vertical migration of Daphnia // Limnol. Oceanogr. V. 37. № 8. P. 1589–1595. https://doi.org/10.4319/lo.1992.37.8.1589
Dawidowicz P., Pijanowska J., Ciechomski K. 1990. Vertical migration of Chaoborus larvae is induced by the presence of fish // Ibid. V. 35. № 7. P. 1631–1637. https://doi.org/10.4319/l0.1990.35.7.1631
Dawidowicz P., Prędki P., Pietrzak B. 2010. Shortened lifespan: another cost of fish-predator avoidance in cladocerans? // Hydrobiologia. V. 643. P. 27–32. https://doi.org/10.1007/s10750-010-0132-z
De Meester L. 1993. Genotype, fish-mediated chemicals, and phototactic behavior in Daphnia magna // Ecology. V. 74. № 5. P. 1467–1474. https://doi.org/10.2307/1940075
Derby C.D. 2020. Chemoreception in aquatic invertebrates // The senses: a comprehensive reference. V. 3 / Ed. Meyerhof W. N.Y.: Acad. Press. P. 65–84. https://doi.org/10.1016/B978-0-12-809324-5.23775-9
DeSantis D.L., Davis D.R., Gabor C.R. 2013. Chemically mediated predator avoidance in the Barton Springs salamander (Eurycea sosorum) // Herpetologica. V. 69. № 3. P. 291–297. https://doi.org/10.1655/HERPETOLOGICA-D-13-00017
Devine G.J., Ingvarsdóttir A., Mordue W. et al. 2000. Salmon lice, Lepeophtheirus salmonis, exhibit specific chemotactic responses to semiochemicals originating from the salmonid, Salmo salar // J. Chem. Ecol. V. 26. № 8. P. 1833–1847. https://doi.org/10.1023/A:1005592606682
Dini M.L., Carpenter S.R. 1991. The effect of whole-lake fish community manipulations on Daphnia migratory behavior // Limnol. Oceanogr. V. 36. № 2. P. 370–377. https://doi.org/10.4319/lo.1991.36.2.0370
Dodson S.I. 1988. The ecological role of chemical stimuli for the zooplankton: predator-avoidance behavior in Daphnia // Ibid. V. 33. № 6. P. 1431–1439. https://doi.org/10.4319/lo.1988.33.6part2.1431
Douglas P.L., Forrester G.T., Cooper S.D. 1994. Effects of trout on the diel periodicity of drifting in baetid mayflies // Oecologia. V. 98. P. 48–56. https://doi.org/10.1007/BF00326089
Dzialowski A.R., Lennon J.T., O’Brien W.J., Smith V.H. 2003. Predator-induced phenotypic plasticity in the exotic cladoceran Daphnia lumholtzi // Freshwat. Biol. V. 48. P. 1593–1602. https://doi.org/10.1046/j.1365-2427.2003.01111.x
Ejdung G. 1998. Behavioural responses to chemical cues of predation risk in a three-trophic-level Baltic Sea food chain // Mar. Ecol. Prog. Ser. V. 165. P. 137–144. https://doi.org/10.3354/meps165137
Engel K., Tollrian R. 2009. Inducible defences as key adaptations for the successful invasion of Daphnia lumholtzi in North America? // Proc. R. Soc. B. V. 276. № 1663. P. 1865–1873. https://doi.org/10.1098/rspb.2008.1861.
Engel K., Schreder T., Tollrian R. 2014. Morphological defences of invasive Daphnia lumholtzi protect against vertebrate and invertebrate predators // J. Plankton Res. V. 36. № 4. P. 1140–1145. https://doi.org/10.1093/plankt/fbu023
Epp K.J., Gabor C.R. 2008. Innate and learned predator recognition mediated by chemical signals in Eurycea nana // Ethology. V. 114. P. 607–615. https://doi.org/10.1111/j.1439-0310.2008.01494.x
Eveland L.L., Bohenek J.R., Silberbush A., Resetarits W.J., Jr. 2016. Detection of fish and newt kairomones by ovipositing mosquitoes // Chemical signals in vertebrates. V. 13 / Eds. Schulte B.A. et al. N.Y.: Springer. P. 247–259. https://doi.org/10.1007/978-3-319-22026-0_18.
Ferrari M.C.O., Brown M.R., Pollock M.S., Chivers D.P. 2007. The paradox of risk assessment: comparing responses of fathead minnows to capturereleased and diet-released alarm cues from two different predators // Chemoecology. V. 17. P. 157–161. https://doi.org/10.1007/s00049-007-0373-0
Ferrari M.C.O., Chivers D.P., Wisenden B.D. 2010. Chemical ecology of predator-prey interactions in aquatic ecosystems: a review and prospectus // Can. J. Zool. V. 88. P. 698–724. https://doi.org/10.1139/Z10-029
Fink P. 2007. Ecological functions of volatile organic compounds in aquatic systems // Mar. Freshwat. Behav. Physiol. V. 40. P. 155–168. https://doi.org/10.1080/10236240701602218
Forward R.B., Rittschof D. 1993. Activation of photoresponses of brine shrimp nauplii involved in diel vertical migration by chemical cues from fish // J. Plankton Res. V. 15. P. 693–701. https://doi.org/10.1093/plankt/15.6.693
Forward R.B., Rittschof D. 1999. Brine shrimp larval photoresponses involved in diel vertical migration: activation by fish mucus and modified amino sugars // Limnol. Oceanogr. V. 44. № 8. P. 1904–1916. https://doi.org/10.4319/lo.1999.44.8.1904
Friberg N., Andersen T.H., Hansen H.O. et al. 1994. The effect of brown trout (Salmo trutta L.) on stream invertebrate drift, with special reference to Gammarus pulex L. // Hydrobiologia. V. 294. P. 105–110. https://doi.org/10.1007/BF00016850
Gonzalo A., López P., Martín J. 2009. Learning, memorizing and apparent forgetting of chemical cues from new predators by Iberian green frog tadpoles // Anim. Cogn. V. 12. P. 745–750. https://doi.org/10.1007/s10071-009-0232-1
Göz H. 1941. Űber den Art- und Individualgeruch bei Fischen // Z. Vergl. Physiol. V. 29. P. 1–45.
Gu L., Lyu K., Dai Z. et al. 2017. Predator-specific responses of Moina macrocopa to kaironmones from different fishes // Int. Rev. Hydrobiol. V. 102. P. 83−89. https://doi.org/10.1002/iroh.201601872
Gyssels F., Stoks R. 2006. Behavioral responses to fish kairomones and autotomy in a damselfly // J. Ethol. V. 24. P. 79–83. https://doi.org/10.1007/s10164-005-0165-3
Haas W. 1994. Physiological analyses of host-finding behavior in trematode cercariae: adaptations for transmission success // Parasitology. V. 109. P. SI5–S29. https://doi.org/10.1017/S003118200008505X
Haas W., Ostrowski de Nuñez M. 1988. Chemical signals of fish skin for the attachment response of Acanthostomum brauni cercariae // Parasitol. Res. V. 74. P. 552–557. https://doi.org/10.1007/BF00531633
Haas W., Haberl B., Hofmann M. et al. 1999. Ichthyophthirius multifiliis invasive stages find their fish hosts with complex behavior patterns and in response to different chemical signals // Europ. J. Protistol. V. 35. P. 129–135. https://doi.org/10.1016/S0932-4739(99)80030-3
Hagen N.T., Andersen Å., Stabell O.B. 2002. Alarm responses of the green sea urchin, Strongylocentrotus droebachiensis, induced by chemically labelled durophagous predators and simulated acts of predation // Mar. Biol. V. 140. P. 365–374. https://doi.org/10.1007/s002270100694
Hamrén U., Hansson S. 1999. A mysid shrimp (Mysis mixta) is able to detect the odour of its predator (Clupea harengus) // Ophelia. V. 51. № 3. P. 187–191.
Hazlett B.A., Schoolmaster D.R. 1998. Responses of cambarid crayfish to predator odor // J. Chem. Ecol. V. 24. P. 1757–1770. https://doi.org/10.1023/A:1022347214559
Hirvonen H., Holopainen S., Lempiäinen N. et al. 2007. Sniffing the trade-off: Effects of eel odours on nocturnal foraging activity of native and introduced crayfish juveniles // Mar. Freshwat. Behav. Physiol. V. 40. № 3. P. 213–218. https://doi.org/10.1080/10236240701556919
Hölker F., Stief P. 2005. Adaptive behaviour of chironomid larvae (Chironomus riparius) in response to chemical stimuli from predators and resource density // Behav. Ecol. Sociobiol. V. 58. № 3. P. 256–263. https://doi.org/10.1007/s00265-005-0932-8
Holomuzki J.R., Hatchett L.A. 1994. Predator avoidance costs and habituation to fish chemicals by a stream isopod // Freshwat. Biol. V. 32. № 3. P. 585–592. https://doi.org/10.1111/j.1365-2427.1994.tb01149.x
Holomuzki J.R., Hoyle J.D. 1990. Effect of predatory fish presence on habitat use and diel movement of the stream amphipod, Gammarus minus // Ibid. V. 24. № 3. P. 509–517. https://doi.org/10.1111/j.1365-2427.1990.tb00728.x
Holomuzki J.R., Short T.M. 1988. Habitat use and fish avoidance behaviors by the stream-dwelling isopod Lirceus fontinalis // Oikos. V. 52. № 1. P. 79–86.
Hülsmann S., Vijverberg J., Boersma M., Mooij W.M. 2004. Effects of infochemicals released by gape-limited fish on life history traits of Daphnia: a maladaptive response? // J. Plankton Res. V. 26. P. 535–543. https://doi.org/10.1093/plankt/fbh054
Huryn A.D., Chivers D.P. 1999. Contrasting behavioural responses by detrivorous and predatory mayflies to chemicals released by injured conspecifics and their predators // J. Chem. Ecol. V. 25. P. 2729–2740. https://doi.org/10.1023/A:1020851524335
Jakobsen P.J., Wedekind C. 1998. Copepod reaction to odor stimuli influenced by cestode infection // Behav. Ecol. V. 9. № 4. P. 414–418. https://doi.org/10.1093/beheco/9.4.414
Jensen K.H., Jakobsen P.J., Kleiven O.T. 1998. Fish kairomone regulation of internal swarm structure in Daphnia pulex (Cladocera: Crustacea) // Hydrobiologia. V. 368. P. 123–127. https://doi.org/10.1023/A:1003233728870
Kallert D.M., El-Matbouli M., Haas W. 2005. Polar filament discharge of Myxobolus cerebralis actinospores is triggered by combined non-specific mechanical and chemical cues // Parasitology. V. 131. P. 609–616. https://doi.org/10.1017/S0031182005008383
Kallert D.M., Bauer W., Haas W., El-Matbouli M. 2011. No shot in the dark: myxozoans chemically detect fresh fish // Int. J. Parasitol. V. 41. P. 271–276. https://doi.org/10.1016/j.ijpara.2010.09.012
Kamio M., Derby C.D. 2017. Finding food: how marine invertebrates use chemical cues to track and select food // Nat. Prod. Rept. V. 34. P. 514–528. https://doi.org/10.1039/C6NP00121A
Kasumyan A.O. 2004. The olfactory system in fish: structure, function, and role in behavior // J. Ichthyol. V. 44. Suppl. 2. P. S180–S223.
Kats L.B. 1988. The detection of certain predators via olfaction by small-mouthed salamander larvae (Ambystoma texanum) // Behav. Neural. Biol. V. 50. P. 126–131.
Kats L.B., Dill L.M. 1998. The scent of death: chemosensory assessment of predation risk by prey animals // Écoscience. V. 5. № 3. P. 361–394.
Kats L.B., Petranka J.W., Sih A. 1988. Antipredator defenses and the persistence of amphibian larvae with fishes // Ecology. V. 69. № 6. P. 1865–1870. https://doi.org/10.2307/1941163
Keller T.A., Moore P.A. 1999. Effects of ontogeny and odors on behavior: the influence of crayfish size and fish odors on crayfish movement // Mar. Freshwat. Behav. Physiol. V. 33. P. 35–50. https://doi.org/10.1080/10236249909387080
Kenison E.K., Weldy P.Y., Williams R.N. 2018. There must be something in the water: assessing the behavioral responses of rusty crayfish (Orconectes rusticus) to fish and amphibian predator kairomones // J. Ethol. V. 36. P. 77–84. https://doi.org/10.1007/s10164-017-0529-5
Kiesecker J.M., Chivers D.P., Blaustein A.R. 1996. The use of chemical cues in predator recognition by western toad tadpoles // Anim. Behav. V. 52. P. 1237–1245. https://doi.org/10.1006/anbe.1996.0271
Laforsch C., Beccara L., Tollrian R. 2006. Inducible defenses: the relevance of chemical alarm cues in Daphnia // Limnol. Oceanogr. V. 51. № 3. P. 1466–1472. https://doi.org/10.4319/lo.2006.51.3.1466
Lagrue C., Poulin R. 2007. Life cycle abbreviation in the trematode Coitocaecum parvum: can parasites adjust to variable conditions? // J. Evol. Biol. V. 20. P. 1189–1195. https://doi.org/10.1111/j.1420-9101.2006.01277.x
Lakowitz T., Bronmark C., Nystrom P. 2008. Tuning in to multiple predators: conflicting demands for shell morphology in a freshwater snail // Freshwat. Biol. V. 53. P. 2184–2191. https://doi.org/10.1111/j.1365-2427.2008.02045.x
Landeira-Dabarca A., Álvarez M., Peckarsky B. 2019. Mayflies avoid sweets: fish skin mucus amino sugars stimulate predator avoidance behaviour of Baetis larvae // Anim. Behav. V. 158. P. 35–45. https://doi.org/10.1016/j.anbehav.2019.10.003
Lass S., Spaak P. 2003. Chemically induced anti-predator defences in plankton: a review // Hydrobiologia. V. 491. P. 221–239. https://doi.org/10.1023/A:1024487804497
Lauridsen T.L., Lodge D.M. 1996. Avoidance by Daphnia magna of fish and macrophytes: chemical cues and predator-mediated use of macrophyte habitat // Limniol. Oceanol. V. 41. P. 794–798. https://doi.org/10.4319/lo.1996.41.4.0794
Laurila A. 2000. Behavioural responses to predator chemical cues and local variation in antipredator performance in Rana temporaria tadpoles // Oikos. V. 88. P. 159–168. https://doi.org/10.1034/j.1600-0706.2000.880118.x
Lima S.L., Dill L.M. 1990. Behavioral decisions made under the risk of predation: a review and prospectus // Can. J. Zool. V. 68. P. 619–640. https://doi.org/10.1139/z90-092
Loose C.J., von Elert E., Dawidowicz P. 1993. Chemically induced diel vertical migration in Daphnia: a new bioassay for kairomones exuded by fish // Arch. Hydrobiol. V. 126. P. 329–337.
Lürling M., Scheffer M. 2007. Info-disruption: pollution and the transfer of chemical information between organisms // Trends Ecol. Evol. V. 22. P. 374–379. https://doi.org/10.1016/j.tree.2007.04.002
Macchiusi F., Baker R.L. 1992. Effects of predators and food availability on activity and growth of Chironomus tentans (Chironomidae: Diptera) // Freshwat. Biol. V. 28. P. 207–216. https://doi.org/10.1111/j.1365-2427.1992.tb00577.x
Macháček J. 1995. Inducibility of life history chenges by fish kairomone in various developmental stages of Daphnia // J. Plankton Res. V. 17. P. 1513–1520. https://doi.org/10.1093/plankt/17.7.1513
Mathis A., Hoback W.W. 1997. The influence of chemical stimuli from predators on precopulatory pairing by the amphipod, Gammarus pseudolimnaeus // Ethology. V. 103. P. 33–40. https://doi.org/10.1111/j.1439-0310.1997.tb00004.x
McIntosh A.R., Peckarsky B.L. 1996. Differential behavioural responses of mayflies from streams with and without fish to trout odour // Freshwat. Biol. V. 35. P. 141–148. https://doi.org/10.1046/j.1365-2427.1996.00489.x
McIntosh A.R., Peckarsky B.L. 2004. Are mayfly anti-predator responses to fish odour proportional to risk? // Arch. Hydrobiol. V. 160. № 2. P. 145–151. https://doi.org/10.1127/0003-9136/2004/0160-0145
McIntosh A.R., Peckarsky B.L., Taylor B.W. 1999. Rapid size-specific changes in the drift of Baetis bicaudatus (Ephemeroptera) caused by alterations in fish odour concentration // Oecologia. V. 118. P. 256–264. https://doi.org/10.1007/s004420050726
McKelvey L.M., Forward R.B., Jr. 1995. Activation of brine shrimp nauplii photoresponses involved in diel vertical migration by chemical cues from visual and non-visual predators // J. Plankton Res. V. 17. № 12. P. 2191–2206. https://doi.org/10.1093/plankt/17.12.2191
Mitchell M.D., Bairos-Novak K.R., Ferrari M.C.O. 2017. Mechanisms underlying the control of responses to predator odours in aquatic prey // J. Exp. Biol. V. 220. P. 1937–1946. https://doi.org/10.1242/jeb.135137
Mobley A.S., Michel W.C., Lucero M.T. 2008. Odorant responsiveness of squid olfactory receptor neurons // Anat. Rec. V. 291. P. 763–774. https://doi.org/10.1002/ar.20704
Moore P.A., Bergman D.A. 2005. The smell of success and failure: the role of intrinsic and extrinsic chemical signals on the social behavior of crayfish // Integr. Comp. Biol. V. 45. P. 650–657. https://doi.org/10.1093/icb/45.4.650
Moore R.D., Newton B., Sih A. 1996. Delayed hatching as a response of streamside salamander eggs to chemical cues from predatory sunfish // Oikos. V. 77. P. 331–335.
Motti C.A., Bose U., Roberts R.E. et al. 2018. Chemical ecology of chemosensation in Asteroidea: insights towards management strategies of pest species // J. Chem. Ecol. V. 44. P. 147–177. https://doi.org/10.1007/s10886-018-0926-4
Naddafi R., Rudstam L.G. 2013. Predator-induced behavioural defences in two competitive invasive species: the zebra mussel and the quagga mussel // Anim. Behav. V. 86. P. 1275–1284. https://doi.org/10.1016/j.anbehav.2013.09.032
Naddafi R., Eklöv P., Pettersson K. 2007. Non-lethal predator effects on the feeding rate and prey selection of the exotic zebra mussel Dreissena polymorpha // Oikos. V. 116. P. 1289–1298. https://doi.org/10.1111/j.2007.0030-1299.15695.x
Nevitt G.A. 2008. Sensory ecology on the high seas: the odor world of the procellariiform seabirds // J. Exp. Biol. V. 211. P. 1706–1713. https://doi.org/10.1242/jeb.015412
Nevitt G.A., Veit R.R., Kareiva P. 1995. Dimethyl sulphide as a foraging cue for Antarctic procellariiform seabirds // Nature. V. 376. P. 681–682. https://doi.org/10.1038/376680ao
Nyström P., Åbjörnsson K. 2000. Effects of fish chemical cues on the interactions between tadpoles and crayfish // Oikos. V. 88. № 1. P. 181–190. https://doi.org/10.1034/j.1600-0706.2000.880120.x
O’Bryan L.M., Forrester G.E. 1997. Effects of fish presence and simulated moonlight gradients on nighttime horizontal movements of a predatory zooplankter, Chaoborus punctipennis // J. Plankton Res. V. 19. № 10. P. 1441–1453. https://doi.org/10.1093/plankt/19.10.1441
Ostrowski de Nuñez M., Haas W. 1991. Penetration stimuli of fish skin for Acanthostomum brauni cercariae // Parasitology. V. 102. P. 101–104. https://doi.org/10.1017/S003118200006039X
Paterson R.A., Pritchard D.W., Dick J.T.A. et al. 2013. Predator cue studies reveal strong trait-mediated effects in communities despite variation in experimental designs // Anim. Behav. V. 86. P. 1301–1313. https://doi.org/10.1016/j.anbehav.2013.09.036
Paul V.J., Arthur K.E., Ritson-Williams R. et al. 2007. Chemical defenses: from compounds to communities // Biol. Bull. V. 213. P. 226–251. https://doi.org/10.2307/25066642
Peckarsky B.L., McIntosh A.R. 1998. Fitness and community consequences of avoiding multiple predators // Oecologia. V. 113. P. 565–576. https://doi.org/0.1007/s004420050410
Petranka J.W., Fakhoury K. 1991. Evidence of a chemically mediated avoidance response of ovipositing insects to bluegills and green frog tadpoles // Copeia. № 1. P. 234–239. https://doi.org/10.2307/1446271
Petranka J.W., Kats L.B., Sih A. 1987. Predator-prey interactions among fish and larval amphibians: use of chemical cues to detect predatory fish // Anim. Behav. V. 35. P. 420–425. https://doi.org/10.1016/S0003-3472(87)80266-X
Pijanowska J., Kowalczewski A. 1997. Predators can induce swarming behaviour and locomotory responses in Daphnia // Freshwat. Biol. V. 37. P. 649–656. https://doi.org/10.1046/j.1365-2427.1997.00192.x
Pijanowska J., Stolpe G. 1996. Summer diapause in Daphnia as a reaction to the presence of fish // J. Plankton Res. V. 18. № 8. P. 1407–1412. https://doi.org/10.1093/plankt/18.8.1407
Pijanowska J., Dawidowicz P., Howe A., Weider L.J. 2006a. Predator-induced shifts in Daphnia life-histories under different food regimes // Arch. Hydrobiol. V. 167. № 1–4. P. 37–54. https://doi.org/10.1127/0003-9136/2006/0167-0037
Pijanowska J., Dawidowicz P., Weider L.J. 2006b. Predator-induced escape response in Daphnia // Ibid. V. 167. № 1–4. P. 77–87. https://doi.org/10.1127/0003-9136/2006/0167-0077
Pijanowska J., Markowska M., Ruszczyńska A. et al. 2020. Kairomone-like activity of bile and bile components: a step towards revealing the chemical nature of fish kairomone // Sci. Rept. V. 10. Article 7037. https://doi.org/10.1038/s41598-020-63456-z
Pohnert G., von Elert E. 2000. No ecological relevance of trimethylamine in fish – Daphnia interactions // Limnol. Oceanogr. V. 45. P. 1153–1156. https://doi.org/10.4319/lo.2000.45.5.1153
Purcell J.E., Anderson P.A.V. 1995. Electrical responses to water soluble components of fish mucus recorded from the cnidocytes of a fish predator, Physalia physalis // Mar. Freshwat. Behav. Physiol. V. 26. P. 149–162. https://doi.org/10.1080/10236249509378936
Rahman Y.J., Forward R.B., Jr., Rittschof D. 2000. Responses of mud snails and periwinkles to environmental odors and disaccacharide mimics of fish odor // J. Chem. Ecol. V. 26. № 3. P. 679–696. https://doi.org/10.1023/A:1005428221952
Ramberg-Pihl N.C., Yurewicz K.L. 2020. Behavioral responses of northern crayfish (Faxonius virilis) to conspecific alarm cues and predator cues from smallmouth bass (Micropterus dolomieu) // Mar. Freshwat. Behav. Physiol. V. 53. № 1. P. 1−16. https://doi.org/10.1080/10236244.2020.1717338
Reede T. 1995. Life history shifts in response to different levels of fish kairomones in Daphnia // J. Plankton Res. V. 17. № 8. P. 1661–1633. https://doi.org/10.1093/plankt/17.8.1661
Relyea R.A. 2001. Morphological and behavioral plasticity of larval anurans in response to different predators // Ecology. V. 82. № 2. P. 523–540. https://doi.org/10.1890/0012-9658(2001)082[0523:MABPOL]2.0.CO;2
Relyea R.A. 2003. Predators come and predators go: the reversibility of predator-induced traits // Ibid. V. 84. № 7. P. 1840–1848. https://doi.org/10.1890/0012-9658(2003)084[1840:PCAPGT]2.0.CO;2
Resetarits W.J., Jr., Binckley C.A. 2013. Is the pirate really a ghost? Evidence for generalized chemical camouflage in an aquatic predator, pirate perch Aphredoderus sayanus // Amer. Naturalist. V. 181. № 5. P. 690–699. https://doi.org/10.1086/670016
Resetarits W.J., Jr., Wilbur H.M. 1989. Choice of oviposition site by Hyla chrysoscelis: role of predators and competitors // Ecology. V. 70. № 1. P. 220–228. https://doi.org/10.2307/1938428
Ringelberg J. 1995. Changes in light intensity and diel vertical migration: a comparison of marine and freshwater environments // J. Mar. Biol. Assoc. UK. V. 75. № 1. P. 15–25. https://doi.org/10.1017/S0025315400015162
Ringelberg J., van Gool E. 1998. Do bacteria, not fish, produce “fish kairomone”? // J. Plankton Res. V. 20. № 9. P. 1847–1852. https://doi.org/10.1093/plankt/20.9.1847
Ringelberg J., Flik B.J.G., Ljndenaar D., Royackers K. 1991. Diel vertical migration of Daphnia hyalina (sensu latiori) in Lake Maarsseveen: Part I. Aspects of seasonal and daily timing // Arch. Hydrobiol. V. 121. P. 129–145.
Ritchie S.A., Laidlaw-Bell C. 1994. Do fish repel oviposition by Aedes taeniorhychus? // J. Amer. Mosquito Control Assoc. V. 10. № 3. P. 380–384.
Rittschof D., Cohen J.H. 2004. Crustacean peptide and peptide-like pheromones and odor // Peptides. V. 25. P. 1503–1516. https://doi.org/10.1016/j.peptides.2003.10.024
Roca J.R., Baltanas A., Uiblein F. 1993. Adaptive responses in Cypridopsis vidua (Crustacea: Ostracoda) to food and shelter offered by a macrophyte (Chara fragilis) // Hydrobiologia. V. 262. P. 127–131. https://doi.org/10.1007/BF00007513
Rundle S.D., Brönmark C. 2001. Inter- and intraspecific trait compensation of defense mechanisms in freshwater snails // Proc. Roy. Soc. Biol. Sci. V. 268. P. 1463–1468. https://doi.org/10.1098/rspb.2001.1682
Scheibling R.E., Hamm J. 1991. Interactions between sea urchins (Strongylocentrotus droebachiensis) and their predators in field and laboratory experiments // Mar. Biol. V. 110. P. 105–116. https://doi.org/10.1007/BF01313097
Schwarzenberger A., Courts C., von Elert E. 2009. Target gene approaches: gene expression in Daphnia magna exposed to predator-borne kairomones or to microcystin-producing and microcystin-free Microcystis aeruginosa // BMC Genomics. V. 10. Article 527. https://doi.org/10.1186/1471-2164-10-527
Scrimgeour G.J., Culp J.M., Kevin J. 1994. Anti-predator responses of mayfly larvae to conspecific and predator stimuli // J. N. Amer. Bentholog. Soc. V. 13. № 2. P. 299–309.
Short T.M., Holomuzki J.R. 1992. Indirect effects of fish on foraging behaviour and leaf processing by the isopod Lirceus fontinalis // Freshwat. Biol. V. 27. P. 91–97. https://doi.org/10.1111/j.1365-2427.1992.tb00526.x
Sih A., Kats L.B. 1991. Effects of refuge availability on the responses of salamander larvae to chemical cues from predatory green sunfish // Anim. Behav. V. 42. P. 330–332. https://doi.org/10.1016/S0003-3472(05)80569-X
Smith G.R., Burgett A.A., Temple K.G. et al. 2008. The ability of three species of tadpoles to differentiate among potential fish predators // Ethology. V. 114. P. 701–710. https://doi.org/10.1111/j.1439-0310.2008.01505.x
Sorensen P.W., Wisenden B.D. (eds.). 2015. Fish pheromones and related cues. Iowa: Wiley Blackwell, 296 p.
Spaak P., Boersma M. 1997. Tail spine length in the Daphnia galeata complex: costs and benefits of induction by fish // Aquat. Ecol. V. 31. P. 89–98. https://doi.org/10.1023/A:1009935100804
Stabell O.B. 2005. Latent alarm signals: are they present in vertebrates // Chemical signals in vertebrates. V. 10 / Eds. Mason R.T. et al. N.Y.: Springer. P. 381–388.
Stabell O.B., Ogbebo F., Primicerio R. 2003. Inducible defences in Daphnia depend on latent alarm signals from conspecific prey activated in predators // Chem. Senses. V. 28. P. 141–153. https://doi.org/10.1093/chemse/28.2.141
Staufer H.-P., Semlitsch R.D. 1993. Effects of visual, chemical and tactile cues of fish on the behavioural response of tadpoles // Anim. Behav. V. 46. P. 355–364. https://doi.org/10.1006/anbe.1993.1197
Stibor H. 1992. Predator induced life-history shifts in a freshwater cladoceran // Oecologia. V. 92. P. 162–165. https://doi.org/10.1007/BF00317358
Stibor H., Lampert W. 2000. Components of additive variance in life-history traits of Daphnia hayalina: seasonal differences in the response to predator signals // Oikos. V. 88. № 1. P. 129–138. https://doi.org/10.1034/j.1600-0706.2000.880115.x
Stoks E., De Block M., Slos S. et al. 2006. Time constrains mediate predator-induced plasticity in immune function, condition, and life history // Ecology. V. 87. P. 809–815. https://doi.org/10.1890/0012-9658(2006)87[809:TCMPPI]2.0.CO;2
Takahara T., Kohmatsu Y., Maruyama A., Yamaoka R. 2003. Effects of fish chemical cues on tadpole survival // Ecol. Res. V. 18. P. 793–796. https://doi.org/10.1111/j.1440-1703.2003.00598.x
Tams V., Lüneburg J., Seddar L. et al. 2018. Intraspecific phenotypic variation in life history traits of Daphnia galeata populations in response to fish kairomones // PeerJ. V. 6. Article e5746. https://doi.org/10.7717/peerj.5746
Teplitsky C., Plenet S., Lena J.-P. et al. 2005. Escape behaviour and ultimate causes of specific induced defences in an anuran tadpole // J. Evol. Biol. V. 18. P. 180–190. https://doi.org/10.1111/j.1420-9101.2004.00790.x
Tollrian R. 1994. Fish-kairomone induced morphological changes in Daphnia lumholtzi (Sars) // Arch. Hydrobiol. V. 130. P. 69–75.
Tollrian R., Heibl C. 2004. Phenotypic plasticity in pigmentation in Daphnia induced by UV radiation and fish kairomones // Function. Ecol. V. 18. P. 497–502. https://doi.org/10.1111/j.0269-8463.2004.00870.x
Tollrian R., von Elert E. 1994. Enrichment and purification of Chaoborus kairomone from water: further steps toward its chemical characterization // Limnol. Oceanogr. V. 39. № 4. P. 788–796. https://doi.org/10.4319/lo.1994.39.4.0788
Turner A.M., Montgomery S.L. 2003. Spatial and temporal scales of predator avoidance: experiments with fish and snails // Ecology. V. 84. № 3. P. 616–622. https://doi.org/10.1890/0012-9658(2003)084[0616:SATSOP]2.0.CO;2
Turner A.M., Fetterolf S.A., Bernot R.J. 1999. Predator identity and consumer behavior: differential effects of fish and crayfish on the habitat use of a freshwater snail // Oecologia. V. 118. P. 242–247. https://doi.org/10.1007/s004420050724
Valentinčič T. 1991. Behavioral responses of the brittle star Ophiura ophiura to chemical stimuli during adaptation of amino acid chemoreceptors // Chem. Senses. V. 16. P. 267–275. https://doi.org/10.1093/chemse/16.3.267
Van Dam A.R., Walton W.E. 2008. The effect of predatory fish exudates on the ovipostional behaviour of three mosquito species: Culex quinquefasciatus, Aedes aegypti and Culex tarsalis // Med. Vet. Entomol. V. 22. P. 399–404. https://doi.org/10.1111/j.1365-2915.2008.00764.x
Van Gool E., Ringelberg J. 1995. Swimming of Daphnia galeata × hyalina in response to changing light intensity: influence of food availability and predator kairomone // Mar. Freshwat. Behav. Physiol. V. 26. P. 259–265. https://doi.org/10.1080/10236249509378944
Van Gool E., Ringelberg J. 1998. Light-induced migration behavior of Daphnia modified by food and predator kairomone // Anim. Behav. V. 56. P. 741–747. https://doi.org/10.1006/anbe.1998.0821
Von Elert E., Loose C.J. 1996. Predator-induced diel vertical migration in Daphnia: enrichment and preliminary chemical characterization of a kairomone exuded by fish // J. Chem. Ecol. V. 22. № 5. P. 885–895. https://doi.org/10.1007/BF02029942
Von Elert E., Pohnert G. 2000. Predator specificity of kairomones in diel vertical migration of Daphnia: a chemical approach // Oikos. V. 88. № 1. P. 119–128. https://doi.org/10.1034/j.1600-0706.2000.880114.x
Von Elert E., Stibor H. 2006. Predator-mediated life history shifts in Daphnia: enrichment and preliminary chemical characterisation of a kairomone exuded by fish // Arch. Hydrobiol. V. 167. P. 21–35. https://doi.org/10.1127/0003-9136/2006/0167-0021
Vonesh J.R., Blaustein L. 2010. Predator-induced shifts in mosquito oviposition site selection: a meta-analysis and implications for vector control // Isr. J. Ecol. Evol. V. 56. P. 123–139. https://doi.org/10.1560/IJEE.56.2-3.123
Wahle R.A. 1992. Body-size dependent anti-predator mechanisms of the American lobster // Oikos. V. 65. № 1. P. 52–60.
Weber A., Declerck S. 1997. Phenotypic plasticity of Daphnia life history traits in response to predator kairomones: genetic variability and evolutionary potential // Hydrobiologia. V. 360. P. 89–99. https://doi.org/10.1023/A:1003188331933
Williams D.D., Moore K.A. 1982. The effect of environmental factors on the activity of Gammarus pseudolimnaeus (Amphipoda) // Ibid. V. 96. P. 137–147. https://doi.org/10.1007/BF02185429
Williams D.D., Moore K.A. 1985. The role of semiochemicals in benthic community relationships of the lotic amphipod Gammarus pseudolimnaeus: a laboratory analysis // Oikos. V. 44. № 2. P. 280–286.
Wisenden B.D. 2015. The cue–signal continuum: a hypothesized evolutionary trajectory for chemical communication in fishes // Fish pheromones and related cues / Eds. Sorensen P.W., Wisenden B.D. Iowa: Wiley Blackwell, 296 p.
Wisenden B.D., Chivers D.P., Smith R.J.F. 1997. Learned recognition of predation risk by Ennalagma damselfly larvae (Odonata, Zygoptera) on the basis of chemical cues // J. Chem. Ecol. V. 23. № 1. P. 137–121. https://doi.org/10.1023/B:JOEC.0000006350.66424.3d
Wooster D., Sih A. 1995. A review of the drift and activity responses of stream prey to predator presence // Oikos. V. 73. P. 3–8.
Wootton R.J. 1998. Ecology of teleost fishes. Dordrecht: Kluwer Acad. Publ., 386 p.
Wrede W.L. 1932. Versiche űber den Artduft der Elritzen // Z. Vergl. Physiol. V. 17. № 3. P. 510–519.
Wudkevich K.N., Wisenden B.D., Chivers D.P., Smith R.J.F. 1997. Reactions of Gammarus lacustris to chemical stimuli from natural predators and injured conspecifics // J. Chem. Ecol. V. 23. № 4. P. 1163–1173. https://doi.org/10.1023/B:JOEC.0000006393.92013.36
Дополнительные материалы отсутствуют.
Инструменты
Вопросы ихтиологии