Журнал высшей нервной деятельности им. И.П. Павлова, 2023, T. 73, № 3, стр. 369-383
Нейровизуализационные (фМРТ покоя) и нейропсихологические особенности “неманифестировавших” пациентов из группы клинически высокого риска шизофрении
И. С. Лебедева 1, *, Я. Р. Паникратова 1, Е. Г. Абдуллина 1, В. В. Мигалина 1, Д. В. Тихонов 1, М. А. Омельченко 1, В. Г. Каледа 1
1 ФГБНУ Научный центр психического здоровья
Москва, Россия
* E-mail: lebedeva-i@yandex.ru
Поступила в редакцию 17.10.2022
После доработки 16.02.2023
Принята к публикации 27.02.2023
- EDN: TTGUVK
- DOI: 10.31857/S0044467723030097
Аннотация
В рамках концепции “клинически высокого риска манифестации психоза” была поставлена цель выявления функциональных особенностей головного мозга (по данным фМРТ покоя) и нейропсихологических характеристик у 27 больных с непсихотическими психическими заболеваниями с аттенуированными симптомами шизофрении, психоз у которых не манифестировал в течение длительного периода наблюдения, по сравнению с 24 пациентами с первым эпизодом шизофрении и 27 психически здоровыми испытуемыми. Основная группа характеризовалась более высокой согласованностью BOLD-сигнала в зрительной коре правого полушария и большей функциональной связанностью между затылочным компонентом зрительной сети и компонентом сети определения значимости в правой префронтальной коре (по сравнению с больными шизофренией). В обеих группах пациентов было обнаружено снижение показателей продуктивности вербальных ассоциаций. Выявленные нейровизуализационные и нейропсихологические особенности пациентов основной группы можно рассматривать как отражение действия “защитных” и “патологических” механизмов при высоком риске манифестации шизофрении.
ВВЕДЕНИЕ
Согласно современным представлениям, шизофрения обусловлена кумулятивным эффектом длительных нелинейных многоуровневых процессов и связана с множеством различных факторов как внутренней, так и внешней среды, при этом развитие патологических процессов длится десятилетиями. Однако, несмотря на накопленный колоссальный объем научной информации, точные механизмы этиопатогенеза так до конца и не выявлены.
Одним из наиболее перспективных подходов к изучению механизмов заболевания стали исследования в рамках парадигмы “клинически высокого риска манифестации психоза” (КВР). Эта концепция опирается на тот факт, что на инициальных этапах развития, задолго до появления очерченной клинической картины болезни, могут выявляться ослабленные (аттенуированные) симптомы шизофрении, представленные психопатологическими феноменами из спектра позитивных, негативных симптомов и симптомов дезорганизации (Newton et al., 2018; Patel et al., 2014) со степенью выраженности, достаточной для клинического распознавания, но не удовлетворяющей критериям, позволяющим верифицировать текущий шизофренический процесс (Омельченко, 2020). При этом фокусной группой риска являются молодые люди в возрасте 20–29 лет (Hafner, 2019; van der Werf et al., 2014), впервые обратившиеся к психиатру, чаще всего по поводу депрессии (Addington et al., 2017; Bosanac, Castle, 2013).
При проспективной клинической оценке группу КВР, как правило, делят на подгруппы, у одной из которых за время наблюдения произошла манифестация психоза (КВР(+)), а у другой не произошла (КВР(−)), помимо этого, значимыми являются и показатели функционального исхода.
Следует отметить, что если ранее при изучении нейрофизиологических и нейропсихологических факторов манифестации психоза основное внимание уделялось группе КВР(+), то особой тенденцией последнего времени стало прицельное изучение и выборок пациентов, у которых не развился психоз в течение длительного периода наблюдения (Smieskova et al., 2012). Подобный интерес вызван тем, что, помимо патологических изменений, одновременно происходит и разворачивание защитных механизмов, которые наиболее выраженно могут проявляться именно в данной группе пациентов. Понимание не только про-, но и контрпатологических процессов является ключевым для целей профилактики и раннего вмешательства.
Определение нейровизуализационных “факторов предрасположенности” и “факторов устойчивости/защиты” может осуществляться во всех МРТ-модальностях, однако содержанием настоящего исследования стали данные, полученные методом фМРТ покоя (resting-state fMRI).
За прошедшие годы были выявлены многочисленные аномалии функционирования головного мозга по данным фМРТ у пациентов с КВР, в том числе и по данным фМРТ покоя (обзор Andreou, Borgwardt, 2020), хотя число работ, где отдельно выделялись группы КВР(+) и КВР(−), существенно меньше.
Так, в мультицентровом исследовании в группе КВР были обнаружены снижение функциональной связанности (ФС) таламуса с префронтальной корой и мозжечком и повышение ФС таламуса с сенсомоторными зонами, наиболее выраженные в группе КВР(+). При этом группа КВР(−) демонстрировала промежуточные с психически здоровыми испытуемыми результаты (Anticevic et al., 2015).
Сходная закономерность, т.е. максимальная выраженность у КВР(+) и промежуточный результат у КВР(−), наблюдалась и при использовании фМРТ как в покое, так и при выполнении различных задач – в своем исследовании авторы фокусируют внимание на повышенной связанности в мозжечково-таламо-кортикальной сети (Cao et al., 2018).
Анализ модулярной структуры коннектома показал сходство психически здоровых испытуемых и пациентов, у которых не манифестировал психоз. При этом в группе КВР(+) обнаружили исключение орбитофронтальной коры из лимбического модуля и включение в него (вместо сенсомоторного модуля) верхней височной извилины билатерально, а также наличие цингулярно-оперкулярного модуля, отсутствующего в других двух группах. В целом, испытуемые с нарушением модулярной структуры коннектома заболевали в три раза чаще (Collin et al., 2020).
Wang et al. (2018) не обнаружили у пациентов КВР(−) отличий от здоровых испытуемых по функциональной архитектуре мозга, в то время как в группе КВР(+) регистрировали выраженную реорганизацию сетевой структуры, затрагивающую сеть определения значимости, дорзальную сеть внимания, дефолтную сеть, сенсомоторную и лимбическую сети.
Очевидно, что подобные публикации все еще немногочисленны, что видно и по обзорным статьям (Ellis et al., 2020), охватывают лишь отдельные аспекты функциональных показателей и достаточно гетерогенны по результатам.
Отдельное направление исследований в группе КВР связано с анализом состояния когнитивных функций. Уровень когнитивного функционирования этих пациентов обычно является промежуточным между показателями здоровых испытуемых и больных с первым приступом психоза (Catalan et al., 2021). При этом состояние вербальной памяти, регуляторных функций и скорости переработки информации различается между подгруппами КВР(+) и КВР(−) (Addington et al., 2019; Catalan et al., 2021), и, в совокупности с продуктивностью вербальных ассоциаций (вербальная беглость; verbal fluency), описаны как когнитивные предикторы “конверсии” (conversion to psychosis; Bolt et al., 2019; Riecher-Rossler et al., 2009). Скорость переработки информации и вербальные ассоциации также являются предикторами ролевого (работа, учеба, ведение домашнего хозяйства) и социального функционирования (Bolt et al., 2019).
Следует отметить, однако, что для выделения маркеров предрасположенности и “устойчивости” наиболее информативным является мультидисциплинарный подход, предполагающий, в частности, совместный анализ данных нейровизуализации, нейропсихологических и клинических данных (Addington et al., 2019).
В свете вышесказанного целью нашего исследования стало определение функциональных особенностей головного мозга (по данным фМРТ покоя) и нейропсихологических особенностей пациентов с КВР(−), у которых психоз не манифестировал в течение длительного периода катамнестического наблюдения, по сравнению с пациентами с первым эпизодом шизофренического психоза и психически здоровыми испытуемыми. Ожидалось, что сопоставление данных в этих группах позволит выделить характеристики, являющиеся потенциальными маркерами “защитных” процессов (сходство/отличие от психически здоровых испытуемых + отличие от пациентов с шизофренией) или “патологических” процессов (сходство с пациентами с шизофренией + отличие от психически здоровых испытуемых).
МЕТОДИКА
Исследование проводилось согласно принципам Хельсинкской декларации. Все испытуемые подписывали добровольное информированное согласие.
Общими критериями исключения при отборе участников исследования являлось наличие неврологических заболеваний, соматических заболеваний в состоянии обострения или тяжелых хронических соматических заболеваний, наркотической или алкогольной зависимости, черепно-мозговых травм с сопутствующей потерей сознания более 5 мин в анамнезе, задержки психического развития, а также леворукость, нарушения слуха и наличие противопоказаний к МРТ.
Основная группа: пациенты с КВР(−)
Основная группа включала 27 пациентов (ср. возраст 27.5 ± 3.1 лет), у которых психоз не манифестировал в течение длительного периода катамнестического наблюдения (5–11 лет, в среднем 7.3 ± 1.7 лет).
Исходная группа больных мужского пола с клинически высоким риском манифестации психоза формировалась с 2010 по 2016 год и включала 70 пациентов, госпитализированных в клинику ФГБНУ НЦПЗ.
Критериями включения являлись: юношеский возраст (17–25 лет), наличие аттенуированных позитивных симптомов11, оцениваемых по соответствующей субшкале Шкалы оценки продромальных симптомов (Scale of Prodromal Symptoms, SOPS; Miller et al., 1999), наличие депрессивных расстройств. Критерии исключения включали наличие другой психической клинически значимой патологии (психотические приступы, органическое психическое расстройство, умственная отсталость), иные критерии исключения совпадали с указанными выше.
При катамнестическом обследовании в 2020–2022 гг. удалось собрать сведения у 43 больных. На повторное обследование согласились прийти 3722 человек, в том числе 30 неманифестировавших больных. Из последних полное мультимодальное обследование (см. ниже) прошли 27 человек, которые и сформировали основную группу.
На момент обследования шестнадцать больных не принимали психотропную терапию, у оставшихся одиннадцати средние суточные дозы нейролептиков составили 171.1 ± ± 378 мг/сут в хлорпромазиновом эквиваленте.
Психометрическая оценка проводилась по шкалам SOPS и HDRS (Hamilton Depression Rating Scale; Hamilton, 1960), суммарные оценки представлены в табл. 1.
Таблица 1.
Результаты психометрического шкалирования в группе КВР(−) Table 1. Clinical data for patients with clinical high risk for psychosis
| SOPS: субшкала позитивных симптомов (M ± SD) | 4.4 ± 4.6 |
| SOPS: субшкала негативных симптомов (M ± SD) | 12.9 ± 6.3 |
| SOPS: субшкала симптомов дезорганизации (M ± SD) | 4.9 ± 3.6 |
| SOPS: субшкала симптомов общей психопатологии (M ± SD) | 7.4 ± 4.1 |
| SOPS: сумма баллов по всей шкале (M ± SD) | 29.4 ± 17.1 |
| HDRS: сумма баллов по всей шкале (M ± SD) | 14.7 ± 8.7 |
Оценка по шкале PSP (Personal and Social Performance Scale, Шкала персонального и социального функционирования; Morosini et al., 2000) проводилась с помощью присвоения каждому пациенту одного из десяти равнозначных рангов внутри диапазона 1–100 баллов (чем ниже ранг, тем больше выраженность нарушений). На этой основе пациенты были разделены на две группы (шестой ранг и выше, n = 19; пятый ранг и ниже, n = 8). Исход заболевания оценивался качественно. Четыре пациента характеризовались благоприятным исходом заболевания, одиннадцать – относительно благоприятным (подгруппа 1, 15 человек), пять – неблагоприятным и семь – относительно неблагоприятным (подгруппа 2, 12 человек). Указанное выше деление на подгруппы было использовано для межгруппового анализа.
Контроль 1: больные с первым эпизодом шизофрении
Выборка включала 24 пациента мужского пола с первым эпизодом эндогенного психоза, средний возраст 20.6 ± 3.6 лет33, средняя длительность заболевания 11.1 ± 12.3 мес. Пациенты находились на стационарном лечении в клинике ФГБНУ НЦПЗ и получали индивидуально подобранную антипсихотическую терапию. Величины дневной дозы нейролептиков оценивались в виде хлорпромазинового эквивалента, в среднем по группе – 554 ± 259 мг/сут.
Мультидисциплинарное обследование больные проходили при отсутствии психомоторного возбуждения и дезорганизации поведения, что являлось необходимым условием. Суммарная оценка по шкале PANSS (Positive and Negative Syndrome Scale; Kay et al., 1987) для группы составляла 89.5 ± 25.0, оценка по субшкале позитивных симптомов – 21.7 ± ± 8.0, негативных симптомов – 21.7 ± 5.9, симптомов общей психопатологии – 46.1 ± ± 13.8 балла.
Контроль 2: психически здоровые испытуемые
Группа психически здоровых испытуемых была отобрана из имеющейся базы данных и включала 27 испытуемых мужского пола, средний возраст которых составлял 27.3 ± 4 лет.
Магнитно-резонансная томография. Обследование проводили на магнитно-резонансном томографе 3Т Philips Ingenia (Голландия). Функциональные T2*-изображения получали с помощью последовательности EPI: TR = 3 с, TE = 35 мс, FA = 90°, 100 объемов, 35 срезов, матрица 128 × 128, размер воксела 1.8 × 1.8 × 4 мм, межсрезовое расстояние 0 мм, примерное время сканирования 5 мин. Участникам исследования давали инструкцию – в течение функционального сканирования избегать любой систематической мыслительной деятельности и лежать неподвижно с открытыми глазами, фиксируя взгляд на белом кресте в центре серого поля монитора. Для каждого участника исследования функциональные изображения были дополнены T1-взвешенными изображениями, полученными с использованием последовательности TFE: TR = 7.9 мс, TE = 3.5 мс, FA = 8°, размер воксела 1 × 1 × 1 мм, 170 срезов, межсрезовое расстояние 0 мм.
Предобработку изображений проводили с использованием пакета SPM12 (https:// www.fil.ion.ucl.ac.uk). Изображения ориентировали параллельно плоскости, проходящей через переднюю и заднюю комиссуры. Производили корректировку смещения во времени измерений в рамках одного объема головного мозга. Затем корректировали артефакты движения в функциональных изображениях. Далее осуществляли совмещение функциональных изображений с анатомическими, сегментацию анатомических изображений на объемы серого, белого вещества и спинномозговой жидкости, приведение всех изображений к координатам пространства MNI, пространственное сглаживание функциональных изображений с использованием фильтра Гаусса (8 мм).
Дальнейшую обработку данных фМРТ покоя проводили с помощью CONN-fMRI toolbox 19.с (www.nitrc.org/projects/conn). Сначала корректировали артефакты, связанные с движением головы испытуемых, и физиологические артефакты (ART-based identification of outlier scans for scrubbing, aCompCor, частотный фильтр 0.008–0.09 Гц; включение индивидуальных параметров движения в модель в качестве ковариат первого уровня). Регрессия глобального сигнала (global signal regression) не выполнялась, поскольку этот метод может приводить к искажениям (Murphy et al., 2009) и нивелировать потенциально значимые компоненты анализа (Chai et al., 2012).
Далее было проведено три анализа данных фМРТ покоя. В каждом из них с применением общей линейной модели со случайными эффектами анализировались различия между тремя группами участников исследования (one-way ANCOVA) по различным показателям. Возраст, количество поврежденных из-за движения изображений и хлорпромазиновые эквиваленты были включены во все анализы в качестве ковариат второго уровня.
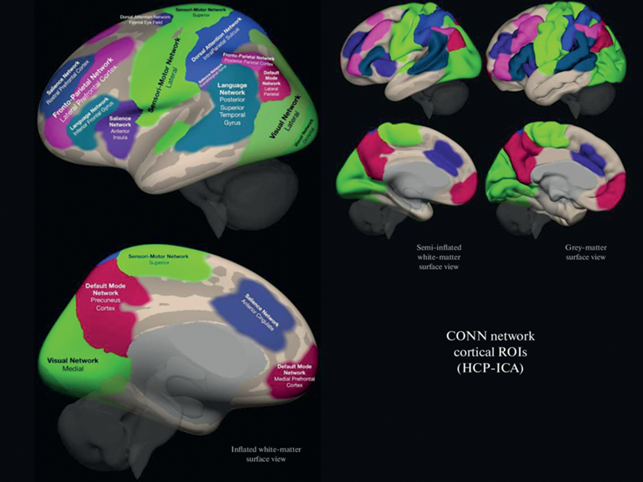
1. ФС между зонами интереса (ROI-to-ROI analysis). Для анализа использовали атлас функциональных сетей CONN, разработанный на основе анализа данных Human Connectome Project (497 человек) методом независимых компонент (www.nitrc.org/projects/conn, рис. 1). Атлас включает следующие сети: дефолтную (default mode network, 4 компонента), сенсомоторную (sensorimotor network, 3 компонента), зрительную (visual network, 4 компонента), сеть определения значимости (salience network, 7 компонентов), дорсальную сеть внимания (dorsal attention network, 4 компонента), фронтопариетальную (frontoparietal network, 4 компонента), речевую (language network, 4 компонента), мозжечковую (cerebellar network, 2 компонента), суммарно 32 зоны интереса. Каждый из 32 компонентов выступал как отдельная зона интереса. ФС между зонами интереса оценивали с помощью коэффициентов корреляции с трансформацией Фишера (Z Фишера). Анализировались различия между тремя группами участников исследования по ФС между всеми парами из 32 компонентов функциональных сетей (т.е. одновременно анализировалась ФС как между компонентами внутри каждой сети, так и между компонентами различных сетей). Уровень значимости p корректировали на общее количество связей между всеми парами зон интереса, pFDR < 0.05.
Рис. 1.
Атлас функциональных сетей CONN (www.nitrc.org/projects/conn). Fig. 1. CONN functional network atlas (www.nitrc.org/projects/conn).
2. Локальные корреляции (Local Correlation, LCOR) – анализ локальной согласованности гемодинамического сигнала в каждом вокселе с областью соседних вокселов (в нашем анализе FWHM = 25 мм). Локальная согласованность определяется как среднее значение коэффициентов корреляции между данным вокселом и областью соседних вокселов. Анализировались различия между тремя группами участников исследования по показателям локальной согласованности сигнала во всем головном мозге (p < 0.001 повоксельно, pFDR < 0.05 на уровне кластера).
3. Амплитуда низкочастотных флуктуаций (Amplitude of Low-Frequency Fluctuations, ALFF) – измерение мощности гемодинамического сигнала в определенном частотном диапазоне (в нашем случае 0.008–0.09 Гц). ALFF определяется как среднее квадратичное значение гемодинамического сигнала в каждом вокселе после применения частотного фильтра. Анализировались различия между тремя группами участников исследования по показателям ALFF во всем головном мозге (p < 0.001 повоксельно, pFDR < 0.05 на уровне кластера).
В случае получения различий между тремя группами по какому-либо показателю проводился post hoc анализ. Для этого извлекались индивидуальные показатели ФС/LCOR/ALFF, затем с помощью общей линейной модели (с учетом всех вышеупомянутых ковариат) три группы попарно сравнивались по указанным показателям. Статистические пороги корректировались с помощью поправки Бонферрони (p < 0.05/3, т.е. p < 0.017).
Нейропсихологическое обследование
Тест “Повторение цифровых рядов” из теста интеллекта Векслера (Филимоненко, Тимофеев, 1995) состоял из двух субтестов: повторение цифровых рядов в прямом и в обратном порядке. Оценивались продуктивность в каждом субтесте, а также продуктивность повторения цифр в обратном порядке, по сравнению с продуктивностью повторения цифр в прямом порядке (с целью контроля эффекта объема кратковременной слухоречевой памяти).
Тест словесно-цветовой интерференции (Color-Word Interference Test) из Батареи оценки регуляторных функций Делиса-Каплан (Delis-Kaplan Executive Function System, D-KEFS; Delis et al., 2001). В субтесте простого называния цветов необходимо было как можно быстрее называть цвета квадратов, в субтесте с интерференцией – называть цвета чернил, которыми написаны названия цветов (например, для слова “красный”, написанного синим цветом, верным ответом является “синий”). Субтест с интерференцией и переключением предполагал переключение между называнием цвета чернил слов, которые напечатаны без рамки, и чтением слов, заключенных в рамку. Для каждого субтеста оценивались время выполнения и сумма самостоятельно скорректированных и нескорректированных ошибок. Кроме того, подсчитывалась разница показателей (время выполнения и ошибки) субтеста с интерференцией и субтеста простого называния цветов, а также субтеста с интерференцией и переключением и субтеста с интерференцией. В первом случае контролировался эффект скорости переработки информации, а во втором – эффект оттормаживания.
Проба вербальных ассоциаций (Verbal Fluency). В каждом из субтестов вербальных ассоциаций участнику исследования необходимо было назвать как можно больше слов в течение 1 мин в соответствии с инструкцией: слова, начинающиеся на букву К, кроме имен собственных, числительных и однокоренных слов (фонологический субтест, D-KEFS; Delis et al., 2001); слова, относящиеся к категории “растения” (категориальный субтест; Ахутина, 2016); поочередно называть то фрукт, то мебель (субтест с переключением между категориями, D-KEFS; Delis et al., 2001). Оценивались количество верных ответов (продуктивность), количество повторов, а также количество ошибок по типу потери инструкции.
Три группы участников исследования сравнивались между собой по каждому из нейропсихологических показателей (всего 22 показателя) с помощью непараметрического критерия H Краскела–Уоллиса. Для тех показателей, по которым были обнаружены статистически значимые различия между тремя группами, проводился post hoc анализ с применением непараметрического критерия U Манна–Уитни и поправки Холма–Бонферрони.
Дополнительные анализы проводились только для тех нейровизуализационных и нейропсихологических показателей, по которым были обнаружены статистически значимые различия между тремя группами и различия по которым в post hoc анализах для группы КВР(−) проходили коррекцию на множественные сравнения. Они включали:
1. Корреляции показателей фМРТ/нейропсихологических показателей с клиническими данными (субшкалы позитивных, негативных симптомов, симптомов дезорганизации и симптомов общей психопатологии SOPS, общая сумма баллов по шкале SOPS, общая сумма баллов по шкале HDRS) в группе КВР(−).
2. Корреляции показателей фМРТ с нейропсихологическими показателями в группе КВР(−).
3. Сравнения подгрупп пациентов КВР(−), выделенных на основе оценок PSP и качественной оценки исхода, по показателям фМРТ/нейропсихологическим показателям.
Все дополнительные анализы проводились с использованием общей линейной модели.
РЕЗУЛЬТАТЫ ИССЛЕДОВАНИЙ
фМРТ покоя
ФС между зонами интереса (ROI-to-ROI analysis). Три группы участников исследования различались по ФС между затылочным компонентом зрительной сети и компонентом сети определения значимости в правой ростральной префронтальной коре (F (2, 72) = = 11.28; puncorrected < 0.001; pFDR = 0.027; рис. 2).
Рис. 2.
(а): Статистически значимые межгрупповые различия были выявлены для ФС между следующими зонами интереса (ROI-to-ROI analysis): затылочным компонентом зрительной сети и компонентом сети определения значимости в правой префронтальной коре (результаты наложены на шаблон серого вещества в пространстве MNI). (б): Значения ФС в основной группе (CHR(−)), в группах психически здоровых испытуемых (HC) и пациентов с шизофренией (Sch). Большие светлые точки – реальные значения (observed values), маленькие темные точки – предсказания модели (fitted values). Звездочками обозначены межгрупповые различия, прошедшие поправку на множественные сравнения для post hoc анализов. Fig. 2. (а): Statistically significant between-group differences in functional connectivity were revealed between the following regions of interest (ROI-to-ROI analysis): the occipital component of the visual network and the component of salience network in the right prefrontal cortex (the results are overlaid on the gray matter MNI template). (б): The functional connectivity values for clinical high risk subjects (CHR(−)), healthy controls (HC), and schizophrnia patients (Sch). The large bright dots are the observed values and the small dark dots are the fitted values. Asterisks mark the between-group differences that survived the correction for multiple analyses for post hoc tests.
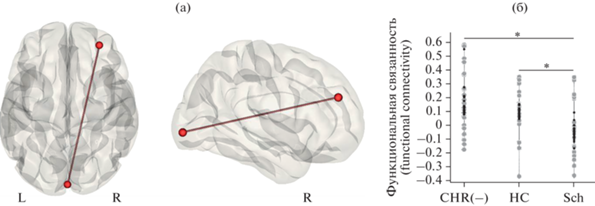
По результатам post hoc анализа ФС между указанными компонентами была выше в группе КВР(−) (обозначение на рисунке CHR(−)), чем в группе пациентов с шизофренией (Sch) (T (46) = 3.74; p < 0.001; Cohen’s d = = 1.06) и выше в группе здоровых испытуемых (HC), чем у пациентов с шизофренией (T (46) = 4.01; p < 0.001; Cohen’s d = 1.14). Группа КВР(−) и группа нормы между собой не различались.
Дополнительный анализ (в программном обеспечении IBM SPSS Statistics 26) показал, что полученный эффект не связан с возрастом (F (1, 72) = 1.5; p = 0.23) или взаимодействием возраста и группы (F (2, 72) = 0.003; p = 0.99).
Локальные корреляции (LCOR). Межгрупповые различия были выявлены по согласованности сигнала во внутри- и надшпорной коре, клине правого полушария (объем кластера 2328 мм3, {10; –86; 22}; F (2,72) = 13.65; puncorrected < 0.001; pFDR = 0.004 на уровне кластера; рис. 3).
Рис. 3.
(а): Кластер в затылочной коре правого полушария, локальная согласованность сигнала в котором статистически значимо различалась между тремя группами (результаты наложены на шаблон серого вещества в пространстве MNI). (б): Диаграмма значений локальной согласованности сигнала в основной группе (CHR(−)), группах психически здоровых испытуемых (HC) и пациентов с шизофренией (Sch). Большие светлые точки – реальные значения (observed values), маленькие темные точки – предсказания модели (fitted values). Звездочкой обозначены межгрупповые различия, прошедшие поправку на множественные сравнения для post hoc анализов. Fig. 3. (а): Local coherence of hemodynamic signal in the right occipital cortex was different between three groups (the results are overlaid on the gray matter MNI template). (б): Values of signal local coherence for clinical high risk subjects (CHR(−)), healthy controls (HC), and schizophrenia patients (Sch). The large bright dots are the observed values and the small dark dots are the fitted values. Asterisks mark the between-group differences that survived the correction for multiple analyses for post hoc tests.
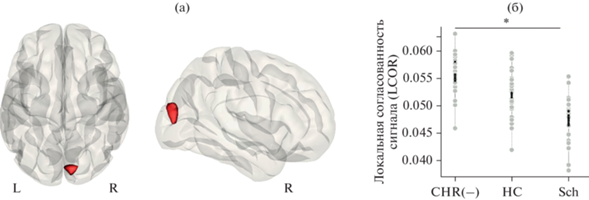
По результатам post hoc анализа согласованность сигнала в указанном кластере была выше в группе КВР(−) (CHR(−)), по сравнению с больными шизофренией (Sch) (T (46) = = 4.27; p < 0.001; Cohen’s d = 1.21). Различия между группой КВР(−) и группой психически здоровых испытуемых (HC) (T (49) = = 2.22; p = 0.031; Cohen’s d = 0.61) и между последней группой и больными шизофренией (T (46) = 2.10; p = 0.042; Cohen’s d = 0.60) не прошли поправку на множественные сравнения (порог p < 0.017).
Дополнительный анализ (в программном обеспечении IBM SPSS Statistics 26) показал, что полученный эффект не связан с возрастом (F (1, 72) = 0.01; p = 0.92) или взаимодействием возраста и группы (F (2, 72) = 0.65; p = = 0.53).
Амплитуда низкочастотных флуктуаций (ALFF). Значимых различий между тремя группами участников исследования не обнаружено.
Нейропсихологические показатели
Статистически значимые различия между тремя группами испытуемых были обнаружены для продуктивности повторения цифр в прямом и обратном порядке, ряда показателей теста словесно-цветовой интерференции (время выполнения и ошибки в субтесте простого называния цветов (I), время выполнения субтеста с интерференцией (III)), продуктивности в трех субтестах вербальных ассоциаций: фонологическом, семантическом, с переключением между категориями.
Далее анализировались только те показатели, по которым в post hoc анализе группа КВР(−) отличалась от других групп. Поправку Холма-Бонферрони прошли только три последних показателя (табл. 2, рис. 4).
Таблица 2.
Результаты межгруппового сравнения по данным нейропсихологического обследования: показатели, в post hoc анализах для которых получены статистически значимые и прошедшие поправку Холма-Бонферрони различия для группы КВР(−) Table 2. Between-group differences in neuropsychological data: indices that differed between the clinical high risk group and other groups and that survived the Holm-Bonferroni correction for post hoc analyses
| Показатель | H | Post hoc (U) |
|---|---|---|
| Продуктивность в фонологическом субтесте вербальных ассоциаций (называние слов на букву “К”) | H (2) = 14.19; p = 0.001 | HC > CHR(−) U = 232; p = 0.021 Размер эффекта: r = 0.31 HC > Sch U = 139.5; p < 0.001 Размер эффекта: r = 0.49 CHR(−) > Sch U = 219; p = 0.047 Размер эффекта: r = 0.28 |
| Продуктивность в семантическом субтесте вербальных ассоциаций (называние растений) | H (2) = 20.8; p < 0.0001 | HC > CHR(−) U = 154; p < 0.001 Размер эффекта: r = 0.5 HC > Sch U = 105.5; p < 0.001 Размер эффекта: r = 0.58 |
| Продуктивность в субтесте вербальных ассоциаций с переключением между категориями (фрукты/мебель) | H (2) = 25.12; p < 0.0001 | HC > CHR(−) U = 112; p < 0.001 Размер эффекта: r = 0.6 HC > Sch U = 107; p < 0.001 Размер эффекта: r = 0.58 |
Примечание: CHR(−) (clinical high risk) – основная группа, Sch (schizophrenia) – пациенты с шизофренией, HC (healthy controls) – группа психически здоровых испытуемых. Все представленные в таблице результаты post hoc анализа прошли поправку Холма–Бонферрони. Размер эффекта оценивается как большой при r ≥ 0.5, средний при 0.3 ≤ r < 0.5, маленький при r < 0.3. Note: CHR(−) are non-converters with clinical high risk for psychosis, Sch are schizophrenia patients, and HC are healthy controls. All results of the post hoc analysis, presented in the table, survived Holm-Bonferroni correction. Effect size is high if r ≥ 0.5, medium if 0.3 ≤ r < 0.5, and small if r < 0.3.
Рис. 4.
Продуктивность вербальных ассоциаций (медианы) в основной группе (CHR(−)), группах здоровых испытуемых (HC) и пациентов с шизофренией (Sch). Столбцы ошибок 95%-го доверительного интервала. Звездочками обозначены межгрупповые различия, прошедшие поправку на множественные сравнения для post hoc анализов. Fig. 4. Verbal fluency productivity (medians) for clinical high risk subjects (CHR(−)), healthy controls (HC), and schizophrenia patients (Sch). Error bars: 95% confidence intervals. Asterisks mark the between-group differences that survived the correction for multiple analyses for post hoc tests.
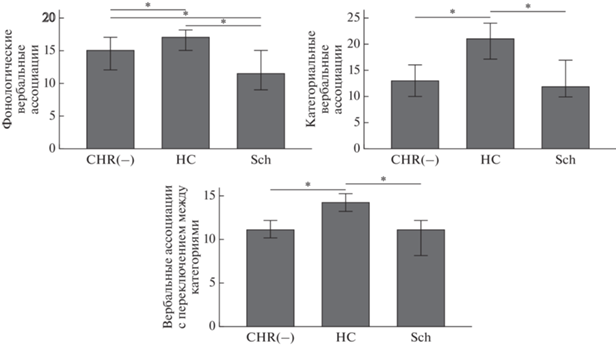
Продуктивность повторения цифр в прямом и обратном порядке была ниже в группе КВР(−) (CHR(−)) по сравнению с группой психически здоровых испытуемых (HC) (U = = 251.5, p = 0.042; U = 249, p = 0.041, соответственно), сходно с тем, что наблюдали у больных шизофренией (Sch) (U = 153, p < 0.001; U = 197.5, p = 0.015 соответственно), но поправку прошли только различия пациентов с шизофренией и группы нормы. Время выполнения субтеста с интерференцией (III) было выше у пациентов с шизофренией по сравнению с группой нормы (U = 180.5; p = = 0.011), а у группы КВР(−) ниже, чем у пациентов с шизофренией (U = 197; p = 0.041), однако результаты для КВР(−) не прошли поправку.
Дополнительный анализ (в программном обеспечении IBM SPSS Statistics 26, корреляции Спирмена), проведенный в каждой из групп по отдельности, показал, что продуктивность в трех субтестах вербальных ассоциаций не коррелирует с возрастом.
Статистически значимых корреляций между показателями фМРТ, нейропсихологическими и клиническими показателями обнаружено не было.
Сравнение подгрупп КВР(−), различающихся по социальному функционированию и оценке функционального исхода, не выявило статистически значимых различий.
ОБСУЖДЕНИЕ РЕЗУЛЬТАТОВ
Результаты межгруппового сравнения по данным фМРТ покоя указывают на снижение ФС между затылочным компонентом зрительной сети и компонентом сети определения значимости в ростральной префронтальной коре правого полушария у пациентов с шизофренией, по сравнению с группой КВР(−) и психически здоровыми испытуемыми (ROI-to-ROI анализ).
Сходные результаты были показаны в работе (Chechko et al., 2018), где выявлялось снижение ФС между правой префронтальной корой и (в том числе) нижней и средней затылочными извилинами в обоих полушариях при шизофрении (хотя топография кластеров лишь частично перекрывалась с нашими данными).
Описанный выше компонент зрительной сети располагался преимущественно в затылочном полюсе, распространяясь на нижнюю часть латеральной затылочной коры и височную часть веретеновидной извилины – эти области мозга связаны с обеспечением как низкоуровневых, так и высокоуровневых процессов зрительного восприятия (Grill-Spector, Malach, 2004). Ростральная префронтальная кора правого полушария в нашем анализе являлась компонентом сети определения значимости (salience network). Функции данной сети заключаются в выявлении важных (как с точки зрения биологического функционирования организма, так и с точки зрения когнитивной оценки) внешних и внутренних событий, что необходимо для обновления ожиданий и регуляции поведения (Kronke et al., 2020). Согласно ряду гипотез, нарушение данных процессов и связанная с ним неправильная оценка ошибки предсказания, то есть степени расхождения между ожиданиями и реально поступающей информацией, являются ключевыми для возникновения психотических симптомов (salience theory: Kapur, 2003; теории, основанные на теореме Байеса: Williams, 2018).
Интересно, что второй результат, полученный на основе межгруппового сравнения по данным фМРТ покоя, был также связан со зрительной системой. В группе КВР(−), по сравнению с пациентами с шизофренией (и даже с психически здоровыми испытуемыми, хотя эти различия не прошли поправку Холма–Бонферрони), наблюдалась более высокая согласованность гемодинамического сигнала в кластере, включающем внутри- и надшпорную кору и клин правого полушария. Указанные области мозга включают преимущественно первичную и вторичную зрительную кору (Grill-Spector, Malach, 2004; результаты также сопоставлялись с вероятностным атласом Wang et al., 2015).
Существование аномалий в зрительной системе головного мозга, в том числе во время ранних этапов обработки информации и касающихся как восходящих, так и нисходящих процессов, отмечено многими исследователями и у пациентов с шизофренией, и в группах КВР (например, Adamek et al., 2022; Keri, Benedek, 2007). Хотя однозначная нейро- или психофизиологическая интерпретация полученных нами результатов касательно кластера в первичной и вторичной зрительной коре затруднена (в том числе, так как не изучались периферические элементы зрительной системы), они, скорее, могут рассматриваться как отражение более синхронизированных (и, возможно, как следствие, более эффективных) процессов, имеющих потенциально компенсаторный характер на данном уровне обработки зрительной информации.
Полученные паттерны межгрупповых различий по локальной согласованности флуктуаций гемодинамического сигнала (в группе КВР(−) выше, чем в контрольных группах) и по ФС между затылочным компонентом зрительной сети и префронтальным компонентом сети определения значимости (в группе КВР(−) выше, чем у больных шизофренией, без статистически значимых различий с группой психически здоровых испытуемых) соответствует описанным выше критериям маркеров “защитных” процессов, хотя лежащие в их основе механизмы еще предстоит исследовать.
Продуктивность выполнения фонологического субтеста вербальных ассоциаций значимо различалась не только между группой КВР(−) и здоровыми испытуемыми, но и между группой КВР(−) и пациентами с первым эпизодом психоза. Таким образом, группа КВР(−) занимала промежуточное положение между пациентами с шизофренией и неклинической группой контроля, что согласуется с данными предшествующих работ (Addington et al., 2019).
В то же время по продуктивности в семантическом субтесте и в субтесте с переключением между категориями был выявлен следующий паттерн различий: группа КВР(−) и пациенты с первым эпизодом психоза не различались, но обе клинические группы выполняли эти субтесты хуже здоровых испытуемых, что согласуется с данными литературы о снижении продуктивности вербальных ассоциаций в группах КВР (Hedges et al., 2022).
Это можно трактовать как проявление в группе КВР(−) “патологических процессов”, сходных с наблюдаемыми у пациентов с расстройствами шизофренического спектра.
Методика вербальных ассоциаций чувствительна к состоянию речевых и регуляторных функций (Aita et al., 2019), и выполнение этой пробы моделирует произвольную регуляцию порождения речи (хотя и не касается синтаксических аспектов речи). Методика требует целенаправленного извлечения подходящих слов из семантической памяти, оттормаживания ситуативных ассоциаций и нерелевантных слов из близких семантических категорий (Shao et al., 2014); сохранной рабочей памяти для удержания инструкции и слов, которые уже были названы; эффективного переключения с элемента на элемент (Gustavson et al., 2019). Продуктивность вербальных ассоциаций нарушена при шизофрении и – что является ключевым в контексте наших результатов – часто рассматривается как эндофенотип44 данного заболевания (Kim et al., 2015; Liang et al., 2016).
Вместе с тем нами не было получено корреляций между показателями фМРТ и продуктивностью вербальных ассоциаций в группе КВР(−).
В отдельных работах у психически здоровых испытуемых обнаруживались корреляции ФС между лобными и затылочными областями головного мозга в покое и продуктивностью вербальных ассоциаций (например, Panikratova et al., 2020). Однако следует отметить, что в нашей работе не ставилась цель исследовать мозговые механизмы вербальных ассоциаций в группе КВР(−); корреляционный анализ был направлен на проверку наличия связей между конкретными обнаруженными нами нейропсихологическими и нейровизуализационными особенностями пациентов с КВР(−). По всей видимости, выявленные нами паттерны можно с осторожностью рассматривать как не сопряженные.
Особо следует подчеркнуть отсутствие в группе КВР(−) корреляций между выявленными нейровизуализационными и нейропсихологическими особенностями, с одной стороны, и клиническими показателями, а также отсутствие межгрупповых различий в подгруппах КВР(−) с разным функциональным исходом. Возможной причиной может быть отсутствие линейной взаимосвязи между анализируемыми показателями. Тестируемые нами функциональные особенности головного мозга и нейропсихологические параметры могут являться лишь отдельными элементами сложной иерархии, определяющей клинические симптомы при психических заболеваниях и включающей показатели, не анализируемые в настоящей работе (например, структурные характеристики головного мозга или социальное окружение). Также отмеченные феномены могут быть эндофенотипами, которые по определению слабо ассоциированы с текущим состоянием пациента (Gottesman, Gould, 2003).
Можно выделить ряд ограничений данного исследования. Во-первых, возраст больных шизофренией был статистически значимо ниже, чем в двух других группах. Тем не менее дополнительные анализы (см. раздел Результаты) показали, что полученные нами эффекты не связаны с возрастом или взаимодействием возраста и группы. Во-вторых, все участники исследования являлись мужчинами, что затрудняет обобщение выводов на женскую часть популяции. В-третьих, общей проблемой исследований, подобных нашему, является определенная клиническая гетерогенность групп КВР, что уже отмечалось рядом исследователей (van Os, Guloksuz, 2017). В-четвертых, в нашей работе не корректировались метрические искажения изображений, возникающие вследствие неоднородности магнитного поля (ввиду длительности сбора материала в контрольной группе, у части испытуемых не были получены соответствующие данные) – следует, правда, отметить, что ряд авторов считает возможным не проводить подобную коррекцию (например, Lombardo et al., 2018; Montchal et al., 2019). И, наконец, в нашем исследовании отсутствовала такая группа сравнения, как больные непсихотическими психическими заболеваниями без симптомов клинического риска, что не позволяет однозначно оценить специфичность найденных феноменов именно для КВР(−).
ЗАКЛЮЧЕНИЕ
Проведенное нейровизуализационное и нейропсихологическое исследование “неманифестировавших” пациентов с клинически высоким риском психоза позволило выявить характеристики, потенциально отражающие “защитные” и “патологические” процессы в патогенезе шизофрении. К первым может быть отнесена высокая согласованность гемодинамического сигнала в кластере, расположенном в зрительной коре правого полушария, – показатель, максимальный в группе КВР(−) по сравнению с больными шизофренией и психически здоровыми испытуемыми, – а также сходная с психически здоровыми испытуемыми функциональная связанность между затылочным элементом зрительной сети и элементом сети определения значимости в правой префронтальной коре. К маркерам “патологических” процессов можно отнести показатели продуктивности вербальных ассоциаций – в семантическом субтесте и в субтесте с переключением между категориями, – отличающие и группу КВР(−), и пациентов с шизофренией от психически здоровых испытуемых.
Отсутствие статистически значимых корреляций между выявленными паттернами и клиническими данными, а также отсутствие межгрупповых различий в подгруппах с разным функциональным исходом, вероятно, отражает сложную нелинейную иерархию мозговых механизмов, определяющую клинические симптомы; также отмеченные феномены могут быть эндофенотипами, которые по определению слабо ассоциированы с текущим состоянием пациента.
Список литературы
Ахутина Т.В. (ред.) Методы нейропсихологического обследования детей 6–9 лет. М.: В. Секачев, 2016. С. 280.
Омельченко М.А. Клинические предпосылки высокого риска развития эндогенного психоза: вопросы диагностики и терапии. Психиатрия. 2020. 18 (2): 82–91. https://doi.org/10.30629/2618-6667-2020-18-2-82-91
Филимоненко Ю.И., Тимофеев В.И. Руководство к методике исследования интеллекта для взрослых Д. Векслера (WAIS). СПб.: ИМАТОН, 1995. С. 57.
Adamek P., Langova V., Horacek J. Early-stage visual perception impairment in schizophrenia, bottom-up and back again. Schizophrenia (Heidelb). 2022. 8 (1): 27. https://doi.org/10.1038/s41537-022-00237-9
Addington J., Farris M., Stowkowy J., Santesteban-Echarri O., Metzak P., Kalathil M.S. Predictors of Transition to Psychosis in Individuals at Clinical High Risk. Curr. Psychiatry Rep. 2019. 21 (6): 39. https://doi.org/10.1007/s11920-019-1027-y
Addington J., Piskulic D., Liu L., Lockwood J., Cadenhead K.S., Cannon T.D., Cornblatt B.A., McGlashan T.H., Perkins D.O., Seidman L.J., Tsuang M.T., Walker E.F., Bearden C.E., Mathalon D.H., Woods S.W. Comorbid diagnoses for youth at clinical high risk of psychosis. Schizophr. Res. 2017. 190: 90–95. https://doi.org/10.1016/j.schres.2017.03.043
Aita S.L., Beach J.D., Taylor S.E., Borgogna N.C., Harrell M.N., Hill B.D. Executive, language, or both? An examination of the construct validity of verbal fluency measures. Appl. Neuropsychol. Adult. 2019. 26 (5): 441–451. https://doi.org/10.1080/23279095.2018.1439830
Andreou C., Borgwardt S. Structural and functional imaging markers for susceptibility to psychosis. Mol. Psychiatry. 2020. 25 (11): 2773–2785. https://doi.org/10.1038/s41380-020-0679-7
Anticevic A., Haut K., Murray J.D., Repovs G., Yang G.J., Diehl C., McEwen S.C., Bearden C.E., Addington J., Goodyear B., Cadenhead K.S., Mirzakhanian H., Cornblatt B.A., Olvet D., Mathalon D.H., McGlashan T.H., Perkins D.O., Belger A., Seidman L.J., Tsuang M.T., van Erp T.G., Walker E.F., Hamann S., Woods S.W., Qiu M., Cannon T.D. Association of Thalamic Dysconnectivity and Conversion to Psychosis in Youth and Young Adults at Elevated Clinical Risk. JAMA Psychiatry. 2015. 72 (9): 882–891. https://doi.org/10.1001/jamapsychiatry.2015.0566
Bolt L.K., Amminger G.P., Farhall J., McGorry P.D., Nelson B., Markulev C., Yuen H.P., Schafer M.R., Mossaheb N., Schlogelhofer M., Smesny S., Hickie I.B., Berger G.E., Chen E.Y.H., de Haan L., Nieman D.H., Nordentoft M., Riecher-Rossler A., Verma S., Thompson A., Yung A.R., Allott K.A. Neurocognition as a predictor of transition to psychotic disorder and functional outcomes in ultra-high risk participants: Findings from the NEURAPRO randomized clinical trial. Schizophr. Res. 2019. 206: 67–74. https://doi.org/10.1016/j.schres.2018.12.013
Bosanac P., Castle D.J. Schizophrenia and depression. Med J Aust. 2013. 199 (S6): S36–39. https://doi.org/10.5694/mja12.10516
Cao H., Chen O.Y., Chung Y., Forsyth J.K., McEwen S.C., Gee D.G., Bearden C.E., Addington J., Goodyear B., Cadenhead K.S., Mirzakhanian H., Cornblatt B.A., Carrion R.E., Mathalon D.H., McGlashan T.H., Perkins D.O., Belger A., Seidman L.J., Thermenos H., Tsuang M.T., van Erp T.G.M., Walker E.F., Hamann S., Anticevic A., Woods S.W., Cannon T.D. Cerebello-thalamo-cortical hyperconnectivity as a state-independent functional neural signature for psychosis prediction and characterization. Nat Commun. 2018. 9 (1): 3836. https://doi.org/10.1038/s41467-018-06350-7
Catalan A., Salazar de Pablo G., Aymerich C., Damiani S., Sordi V., Radua J., Oliver D., McGuire P., Giuliano A.J., Stone W.S., Fusar-Poli P. Neurocognitive Functioning in Individuals at Clinical High Risk for Psychosis: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2021. https://doi.org/10.1001/jamapsychiatry.2021.1290
Chai X.J., Castanon A.N., Ongur D., Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012. 59 (2): 1420–1428. https://doi.org/10.1016/j.neuroimage.2011.08.048
Chechko N., Cieslik E.C., Muller V.I., Nickl-Jockschat T., Derntl B., Kogler L., Aleman A., Jardri R., Sommer I.E., Gruber O., Eickhoff S.B. Differential Resting-State Connectivity Patterns of the Right Anterior and Posterior Dorsolateral Prefrontal Cortices (DLPFC) in Schizophrenia. Front. Psychiatry. 2018. 9: 211. https://doi.org/10.3389/fpsyt.2018.00211
Collin G., Seidman L.J., Keshavan M.S., Stone W.S., Qi Z., Zhang T., Tang Y., Li H., Anteraper S.A., Niznikiewicz M.A., McCarley R.W., Shenton M.E., Wang J., Whitfield-Gabrieli S. Functional connectome organization predicts conversion to psychosis in clinical high-risk youth from the SHARP program. Mol. Psychiatry. 2020. 25 (10): 2431–2440. https://doi.org/10.1038/s41380-018-0288-x
Delis D.C., Kaplan E., Kramer J.H. Delis-Kaplan Executive Function System: Examiner’s Manual. San Antonio, TX: The Psychological Corporation, 2001. P. 388.
Ellis J.K., Walker E.F., Goldsmith D.R. Selective Review of Neuroimaging Findings in Youth at Clinical High Risk for Psychosis: On the Path to Biomarkers for Conversion. Front. Psychiatry. 2020. 11: 567534. https://doi.org/10.3389/fpsyt.2020.567534
Gottesman I.I., Gould T.D. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003. 160 (4): 636–645. https://doi.org/10.1176/appi.ajp.160.4.636
Grill-Spector K., Malach R. The human visual cortex. Annu Rev Neurosci. 2004. 27: 649–677. https://doi.org/10.1146/annurev.neuro.27.0702-03.144220
Gustavson D.E., Panizzon M.S., Franz C.E., Reynolds C.A., Corley R.P., Hewitt J.K., Lyons M.J., Kremen W.S., Friedman N.P. Integrating verbal fluency with executive functions: Evidence from twin studies in adolescence and middle age. J. Exp. Psychol Gen. 2019. 148 (12): 2104–2119. https://doi.org/10.1037/xge0000589
Hafner H. From Onset and Prodromal Stage to a Life-Long Course of Schizophrenia and Its Symptom Dimensions: How Sex, Age, and Other Risk Factors Influence Incidence and Course of Illness. Psychiatry J. 2019. 2019: 9804836. https://doi.org/10.1155/2019/9804836
Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. 1960. 23: 56–62. https://doi.org/10.1136/jnnp.23.1.56
Hedges E.P., Dickson H., Tognin S., Modinos G., Antoniades M., van der Gaag M., de Haan L., McGorry P., Pantelis C., Riecher-Rossler A., Bressan R., Barrantes-Vidal N., Krebs M.O., Nordentoft M., Ruhrmann S., Sachs G., Rutten B.P., van Os J., Study E.-G.H.R., Valmaggia L.R., McGuire P., Kempton M.J. Verbal memory performance predicts remission and functional outcome in people at clinical high-risk for psychosis. Schizophr. Res. Cogn. 2022. 28: 100222. https://doi.org/10.1016/j.scog.2021.100222
Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am. J. Psychiatry. 2003. 160 (1): 13–23. https://doi.org/10.1176/appi.ajp.160.1.13
Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987. 13 (2): 261–276. https://doi.org/10.1093/schbul/13.2.261
Keri S., Benedek G. Visual contrast sensitivity alterations in inferred magnocellular pathways and anomalous perceptual experiences in people at high-risk for psychosis. Vis. Neurosci. 2007. 24 (2): 183–189. https://doi.org/10.1017/S0952523807070253
Kim D., Kim J.W., Koo T.H., Yun H.R., Won S.H. Shared and distinct neurocognitive endophenotypes of schizophrenia and psychotic bipolar disorder. Clin. Psychopharmacol. Neurosci. 2015. 13 (1): 94–102. https://doi.org/10.9758/cpn.2015.13.1.94
Kronke K.M., Wolff M., Shi Y., Kraplin A., Smolka M.N., Buhringer G., Goschke T. Functional connectivity in a triple-network saliency model is associated with real-life self-control. Neuropsychologia. 2020. 149: 107667. https://doi.org/10.1016/j.neuropsychologia.2020.-107667
Liang S., Deng W., Wang Q., Ma X., Li M., Brown M.R., Hu X., Li X., Greenshaw A.J., Li T. Performance of Verbal Fluency as an Endophenotype in Patients with Familial versus Sporadic Schizophrenia and Their Parents. Sci. Rep. 2016. 6: 32597. https://doi.org/10.1038/srep32597
Lombardo M.V., Pramparo T., Gazestani V., Warrier V., Bethlehem R.A.I., Carter Barnes C., Lopez L., Lewis N.E., Eyler L., Pierce K., Courchesne E. Large-scale associations between the leukocyte transcriptome and BOLD responses to speech differ in autism early language outcome subtypes. Nat. Neurosci. 2018. 21 (12): 1680–1688. https://doi.org/10.1038/s41593-018-0281-3
Miller T.J., McGlashan T.H., Woods S.W., Stein K., Driesen N., Corcoran C.M., Hoffman R., Davidson L. Symptom assessment in schizophrenic prodromal states. Psychiatr Q. 1999. 70 (4): 273–287. https://doi.org/10.1023/a:1022034115078
Montchal M.E., Reagh Z.M., Yassa M.A. Precise temporal memories are supported by the lateral entorhinal cortex in humans. Nat. Neurosci. 2019. 22 (2): 284–288. https://doi.org/10.1038/s41593-018-0303-1
Morosini P.L., Magliano L., Brambilla L., Ugolini S., Pioli R. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social funtioning. Acta Psychiatrica Scandinavica. 2000. 101 (4): 323–329. https://doi.org/10.1034/j.1600-0447.2000.10100-4323.x
Murphy K., Birn R.M., Handwerker D.A., Jones T.B., Bandettini P.A. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009. 44 (3): 893–905. https://doi.org/10.1016/j.neuroimage.2008.09.036
Newton R., Rouleau A., Nylander A.G., Loze J.Y., Resemann H.K., Steeves S., Crespo-Facorro B. Diverse definitions of the early course of schizophrenia-a targeted literature review. NPJ Schizophr. 2018. 4 (1): 21. https://doi.org/10.1038/s41537-018-0063-7
Panikratova Y.R., Vlasova R.M., Akhutina T.V., Korneev A.A., Sinitsyn V.E., Pechenkova E.V. Functional connectivity of the dorsolateral prefrontal cortex contributes to different components of executive functions. Int. J. Psychophysiol. 2020. 151: 70–79. https://doi.org/10.1016/j.ijpsycho.2020.02.013
Patel K.R., Cherian J., Gohil K., Atkinson D. Schizophrenia: Overview and Treatment Options. Pharmacy & Therapeutics. 2014. 39 (9): 638–645.
Riecher-Rossler A., Pflueger M.O., Aston J., Borgwardt S.J., Brewer W.J., Gschwandtner U., Stieglitz R.D. Efficacy of using cognitive status in predicting psychosis: a 7-year follow-up. Biol Psychiatry. 2009. 66 (11): 1023–1030. https://doi.org/10.1016/j.biopsych.2009.07.020
Shao Z., Janse E., Visser K., Meyer A.S. What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Front. Psychol. 2014. 5: 772. https://doi.org/10.3389/fpsyg.2014.00772
Smieskova R., Fusar-Poli P., Riecher-Rossler A., Borgwardt S. Neuroimaging and resilience factors–staging of the at-risk mental state? Curr. Pharm. Des. 2012. 18 (4): 416–421. https://doi.org/10.2174/138161212799316046
van der Werf M., Hanssen M., Kohler S., Verkaaik M., Verhey F.R., Investigators R., van Winkel R., van Os J., Allardyce J. Systematic review and collaborative recalculation of 133,693 incident cases of schizophrenia. Psychol. Med. 2014. 44 (1): 9–16. https://doi.org/10.1017/S0033291712002796
van Os J., Guloksuz S. A critique of the “ultra-high risk” and “transition” paradigm. World Psychiatry. 2017. 16 (2): 200–206. https://doi.org/10.1002/wps.20423
Wang C., Lee J., Ho N.F., Lim J.K.W., Poh J.S., Rekhi G., Krishnan R., Keefe R.S.E., Adcock R.A., Wood S.J., Fornito A., Chee M.W.L., Zhou J. Large-Scale Network Topology Reveals Heterogeneity in Individuals With at Risk Mental State for Psychosis: Findings From the Longitudinal Youth-at-Risk Study. Cereb. Cortex. 2018. 28 (12): 4234–4243. https://doi.org/10.1093/cercor/bhx278
Wang L., Mruczek R.E., Arcaro M.J., Kastner S. Probabilistic Maps of Visual Topography in Human Cortex. Cereb. Cortex. 2015. 25 (10): 3911–3931. https://doi.org/10.1093/cercor/bhu277
Williams D. Hierarchical Bayesian models of delusion. Conscious Cogn. 2018. 61: 129–147. https://doi.org/10.1016/j.concog.2018.03.003
Дополнительные материалы отсутствуют.
Инструменты
Журнал высшей нервной деятельности им. И.П. Павлова


