Микология и фитопатология, 2019, T. 53, № 5, стр. 301-310
Prevalence of the ability to produce abscisic acid in phytopathogenic fungi
D. S. Syrova 1, 2, *, A. I. Shaposhnikov 1, **, N. M. Makarova 1, ***, T. Yu. Gagkaeva 3, ****, I. A. Khrapalova 4, *****, V. V. Emelyanov 2, ******, Yu. V. Gogolev 5, *******, Ph. B. Gannibal 3, ********, A. A. Belimov 1, 3, *********
1 All-Russia Research Institute for Agricultural Microbiology
196608 St. Petersburg, Russia
2 Saint Petersburg State University
199034 St. Petersburg, Russia
3 All-Russian Institute of Plant Protection
196608 St. Petersburg, Russia
4 N.I. Vavilov Institute of Plant Genetic Resources
190000 St. Petersburg, Russia
5 Kazan Institute of Biochemistry and Biophysics
420111 Kazan, Russia
* E-mail: imperial_phoenix@ro.ru
** E-mail: ai-shaposhnikov@mail.ru
*** E-mail: n.m.46@yandex.ru
**** E-mail: t.gagkaeva@yahoo.com
***** E-mail: i.khrapalova@vir.nw.ru
****** E-mail: bootika@mail.ru
******* E-mail: gogolev.yuri@gmail.com
******** E-mail: phgannibal@yandex.ru
********* E-mail: belimov@rambler.ru
Поступила в редакцию 10.10.2018
После доработки 3.12.2018
Принята к публикации 21.12.2018
Аннотация
Phytohormone abscisic acid (ABA) plays significant role in many physiological processes and response of plants to abiotic and biotic stresses. Phytopathogenic fungi also produce ABA, but the role of this trait in interactions with host plants is poorly understood. In this work 65 collection strains of phytopathogenic fungi (13 genera, 25 species) were screened for ABA production in batch culture using a modified potato dextrose (MPD) and original chemically defined (OCD) media. Analysis of ABA content was carried out by ultra-performance liquid chromatography. Thirty-four strains belonging total of 13 species produced ABA growing on MPD medium, and among them nineteen strains also produced ABA growing on OCD medium. A maximum ABA concentration was detected in MPD culture fluid of strain Apiospora montagnei MF-R13.8 (56.5 ± 0.1 µg L–1), whereas strain MF-S41.5 of the same species was the most active ABA producer (13.4 ± 1.1 µg L–1) growing on OCD medium. For the first time ABA was detected in species Alternaria tenuissima, Apiospora montagnei, Bipolaris sorokiniana, Fusarium avenaceum, F. solani, Pythium ultimum, Sclerotinia sclerotiorum, and Sclerotium varium. No correlation between the ability to produce ABA and host plant, plant organ of isolation or region of strain origin was found. In agar dish culture three tomato cultivars were inoculated with strains of Fusarium solani or F. oxysporum differing in ABA production in vitro to test relationship between the ability of fungi to produce ABA and to appear negative effects on plants. Generally, ABA production didn’t correlate with the effects of fungi of tomato roots, with one exception that ABA production by F. solani strains negatively correlated (r = –0.82, P = 0.046, n = 6) with root length of cultivar Ailsa-Craig. The results suggest possibility for the role of fungal ABA as a positive modulator of pathogenesis, but manifestation of this effect depends on plant genotype and fungus species. The selected ABA-producing strains can be used to study mechanisms underlying involvement of fungal ABA in plant-microbe interactions.
INTRODUCTION
Abscisic acid (ABA) is a phytohormone playing significant role in many physiological processes in plants, including seed and bud dormancy, flowering, root growth, leaf shape and senescence, distribution of assimilates between root and shoot, growth inhibition, seed ripening, as well as in plant responses to abiotic stresses, particularly via regulation of stomata conductance (Davies, Zhang, 1991; Dodd, 2005; Sah, Reddy, 2016). In plants ABA is biosynthesized from carotenoids 9-cis-violaxanthin or 9-cis-neoxanthin via their enzymatic cleavage to xanthoxin followed by subsequent multiplied biochemical steps and finally converting abscisic aldehyde to ABA (Taylor et al., 2000; Oritani, Kiyota, 2003).
At the same time ABA was detected in microorganisms such as bacteria, algae and fungi (reviewed by Hartung, 2010). Many phytopathogenic fungi produce ABA in vitro and information about these fungi including taxonomic position, growth medium and conditions used for batch cultures, ABA concentrations in culture fluids and host plants is summarized in Table 1. Up to now total of eight genera and 14 species of phytopathogenic fungi were described as ABA producers. ABA biosynthesis in fungi was overviewed by Oritani and Kiyota (2003). Briefly, fungal species unable to synthesize 9'-cis-neoxanthin can form ABA directly via farnesyl-diphosphate and different ionylidene derivates, but when 9'-cis-neoxanthin is synthesized its converts to abscisic aldehyde and then to ABA. Another pathway is related to oxidative cleavage of a carotenoid precursor (9Z)-γ-carotene to various forms of γ-ionylideneacetic acid and then to ABA (Oritani, Kiyota, 2003).
Table 1.
Information on phytopathogenic abscisic acid-producing fungi obtained from the literature
| Fungal species | Growth medium | Growth conditions* | Concentration of abscisic acid | Host plant | Reference |
|---|---|---|---|---|---|
| Alternaria alternata | GAMS | 20–40 days, 23°С, CL | 84 mg g–1 DM | NS | Crocol et al. (1991) |
| A. brassicae | PD | 20 days, at 25°C, CL | NS | Canola | Dahiya et al. (1988) |
| Botrytis cinerea | PD | 7 days, 27°C, dark or light | 2 (dark) or 14 (light) mg L–1 | NS | Marumo et al. (1982) |
| B. cinerea | GAMS | 5 days | 2.8 µg L–1 | Lettuce | Dorffling, Petersen (1984) |
| B. cinerea | PD | 7 days, CL | 3.5 mg L–1 | Geranium | Hirai et al. (1986) |
| B. cinerea | PD | 15 days, 28°C | 39.2 mg L–1 | Grape | Wu, Shi (1998) |
| B. cinerea | PD | 20 days, statically, 23°C, dark | 1.4 mg L–1 | Geranium | Inomata et al. (2004) |
| B. cinerea | CD | 7 days, shaking, 20°C | 0.8 mg L–1 | NS | Siewers et al. (2004) |
| Ceratocystis coerulescens | GAMS | 5 days | 1.6 µg L–1 | Pine | Dorffling, Petersen (1984) |
| C. fimbriata | GAMS | 5 days | 2.4 µg L–1 | Aspen | Dorffling, Petersen (1984) |
| Cercospora cruenta | PD | 9 days, shaking, 28°C, CL | 10 mg L–1 | NS | Oritani et al. (1982) |
| C. fici | PD | 30 days, statically, 25°С, 12 h FL/12 h dark | 10 µg L–1 | Pine | Okamoto et al. (1988) |
| C. pinidensiflorae | PD or CzD | 30 days, statically, 25°С, 12 h FL/12 h dark | 110 (PDM) or 380 (CD) µg L–1 | Pine | Okamoto et al. (1988) |
| C. pinidensiflorae | PD | 17 days, 23°C, 12 h FL/12 h dark | NS | Pine | Hirai-et al. (2000) |
| C. rosicola | CD | 7–21 days, shaking, 24–26°C, CL | 1–10 mg g–1 DM | Rose | Norman et al. (1981) |
| C. rosicola | MSGM | 7 days, shaking, 23–24°C, CL | 0.2–13.6 mg L–1 | Rose | Bennet et al. (1981) |
| C. theae | PD | 30 days, statically, 25°С, 12 h light/12 h dark | 10 µg L–1 | Pine | Okamoto et al. (1988) |
| Fusariam culmorum | CzD | 14 days | 0.05 ng g–1 DM | Tomato | Michniewicz (1989) |
| F. oxysporum | GAMS | 5 days | 3.7 µg L–1 | Tomato | Dorffling, Petersen (1984) |
| Fusarium sp. | PD | 15 days, 28°C | 3.1 mg L–1 | Grape | Wu, Shi (1998) |
| Rhizoctonia solani | GAMS | 5 days | 4.6 µg L–1 | Tomato | Dorffling, Petersen (1984) |
| Rhizopus nigricans | GAMS | 20–40 days, 23°С, CL | 202 mg g–1 DM | NS | Crocol et al. (1991) |
| Rhizopus sp. | PD | 15 days, 28°C | 7.2 mg L–1 | Strawberry | Wu, Shi (1998) |
| Verticillum dahliae | PD | 30 days, statically, 25°С, 12 h light/12 h dark | 10 µg L–1 | Pine | Okamoto et al. (1988) |
Note. *Growth conditions mean cultivation period, shaking, temperature and lighting, if available in the corresponding report. Abbreviations: GAMS – glucose-asparagine-mineral salt medium; PD – potato dextrose medium; MSG – mineral salts with glucose medium; CzD – Czapek-Dox medium; CD – various chemically defined media containing mineral salts with glucose or lactose, various amino acids and thiamine (for more details see corresponding references); CL – continuous lighting; NS – not shown; DM – dry mycelium.
The reports describing ability of phytopathogenic fungi to produce ABA aroused interest in the study the role of this trait in interactions between phytopathogen and host plant. It was shown that ABA increased the susceptibility of rice to Magnaporthe oryzae (Matsumoto et al., 1980), soybean to Phytophthora sojae (Mohr and Cahill, 2001), tomato to Botrytis cinerea (Audenaert, et al., 2002) and Arabidopsis thaliana to Peronospora parasitica (Mohr and Cahill, 2003). Low temperature condition increased ABA biosynthesis making rice plants susceptible to Magnaporthe grisea (Koga et al., 2004). ABA suppressed the activity of phenylalanine ammonia-lyase catalyzing the synthesis of polyphenyl compounds involved in defense mechanisms at the transcriptional level (Ward et al., 1989). Suppression of pathogen defense responses related to jasmonate-ethylene (Anderson et al., 2004) and salicylic acid (Audenaert et al. 2002) signaling pathways by ABA was also reported. It was also shown that ABA stimulated spore germination in Botrytis cinerea (Marumo et al., 1982) and mycelium growth of Ceratocystis fimbriata ABA (Stopinska, Michniewicz, 1988). On the other hand, it was shown that ABA increased resistance of Arabidopsis thaliana to Alternaria brassicicola and Plectosphaerella cucumerina via stimulation of callose deposition in a border area with infection zone (Ton, Mauch-Mani, 2004). Stomatal closure caused by high ABA concentrations may prevent invasion of phytopathogens into plant tissues (Asselbergh et al., 2008). Review of these contradictory results leaded to a conclusion that the control of disease resistance by ABA is very complex phenomenon varying from positive to negative depending on fungal and plant species, the timing of infection, growth conditions, presence of abiotic stresses and other unknown factors (Asselbergh et al., 2008; Ton et al., 2009).
This work was aimed to screen the collection strains of different phytopathogenic fungi for ABA production to find new species having this trait and to find relationships between ability to produce ABA and their characteristic features.
MATERIALS AND METHODS
Objects of research. Sixty five strains of phytopathogenic fungi were obtained from the Collection of the All-Russian Institute of Plant Protection and the Russian Collection of Agricultural Microorganisms (RCAM, St. Petersburg, Russia, http://www.arriam.ru/kollekciya-kul-tur1/). Species affiliation and origin of the studied fungal strains are shown in Table 2. The stock cultures of fungi were maintained on Czapek-Dox (CD) agar at 4°C. Tomato (Solanum lycopersicum synonym Lycopersicon esculentum Mill.) cultivar Ailsa-Craig (VIR 1930, England) was obtained from the Moles Seeds (UK, Ltd) and cultivars (cv.) Altai-Ground (VIR 2311, Russia) and Early-Uzbekistan (VIR 4750, Uzbekistan) were obtained from the N.I. Vavilov Institute of Plant Genetic Resources (St. Petersburg, Russia).
Table 2.
Characteristics of the studied fungal strains and their ability to produce abscisic acid in batch culture
| Species | Strain number | Host plant | Region of origin | Abscisic acid production, µg L–1 | ||
|---|---|---|---|---|---|---|
| Species | Organ | MPD medium | OCD medium | |||
| Alternaria radicina | MF-P190-031 | Daucus sativus | leaf | Minsk | ND | ND |
| A. solani | MF-P043-021 | Solanum tuberosum | leaf | Primorsk | ND | ND |
| A. solani | MF-P043-041 | Solanum tuberosum | leaf | Primorsk | 1.2 ± 0.1 | ND |
| A. tenuissima | MF-P480-011 | Triticum aestivum | seed | Primorsk | 0.7 ± 0.1 | 0.1 ± 0.01 |
| Alternariaster helianthi | MF-P16-011 | Helianthus annuus | stem | Krasnodar | ND | ND |
| Apiospora montagnei | MF-S41.4 | Elytrigia repens | leaf | St. Petersburg | 0.2 ± 0.1 | 0.2 ± 0.01 |
| A. montagnei | MF-S41.5 | Heracleum sibiricum | leaf | Novgorod | 6.8 ± 0.6 | 13.4 ± 1.1 |
| Arthrinium arundinis | MF-R13.7 | Brassica napus | seed | St. Petersburg | 0.9 ± 0.07 | 3.9 ± 0.3 |
| A. arundinis | MF-R13.8 | Brassica napus | seed | St. Petersburg | 56.5 ± 0.1 | 19.8 ± 1.7 |
| A. arundinis | MF-R41.5 | Heracleum sibiricum | leaf | Novgorod | 47.8 ± 0.1 | ND |
| Bipolaris sorokiniana | MF-M17.1 | Papaver rhoeas | leaf | Stavropol | ND | ND |
| B. sorokiniana | MF-R16.6 | Brassica napus | seed | St. Petersburg | 0.1 ± 0.1 | ND |
| Botrytis cinerea | MF-R33.7 | Crambe abyssinica | stem | St. Petersburg | 0.7 ± 0.2 | 0.1 ± 0.01 |
| Fusarium avenaceum | MF-W496 | Secale sereale | seed | St. Petersburg | 0.7 ± 0.1 | 0.1 ± 0.01 |
| F. avenaceum | MF-W509 | Helianthus annuus | stem | St. Petersburg | 0.6 ± 0.1 | ND |
| F. culmorum | MF-W993 | Triticum aestivum | seed | Gomel | ND | ND |
| F. culmorum | MF-W30 | Hordeum vulgare | root | St. Petersburg | 0.2 ± 0.1 | 1.5 ± 0.1 |
| F. equiseti | MF-W1081 | Hordeum vulgare | seed | Novgorod | ND | ND |
| F. equiseti | MF-W1090 | Triticum aestivum | seed | Gomel | ND | ND |
| F. graminearum | MF-W218 | Triticum aestivum | seed | North Ossetia | 0.7 ± 0.2 | ND |
| F. oxysporum | MF-W1111 | Ocimum basilicum | seed | St. Petersburg | ND | ND |
| F. oxysporum | MF-W1115 | Cucumis sativus | stem | St. Petersburg | ND | ND |
| F. oxysporum | MF-G58284 | Solanum tuberosum | tuber | St. Petersburg | 4.3 ± 0.4 | 1.9 ± 0.2 |
| F. oxysporum | MF-G58767 | Cucumis sativus | stem | St. Petersburg | ND | ND |
| F. oxysporum | MF-G59014 | Solanum lycopersicum | stem | Krasnodar | 0.5 ± 0.04 | ND |
| F. oxysporum | MF-G59120 | Gossypium sp. | root | Kazahstan | 0.9 ± 0.1 | ND |
| F. oxysporum | MF-G59124 | Beta vulgaris | root | Kazahstan | ND | ND |
| F. oxysporum | MF-G93656 | Capsicum annuum | root | Kiev | ND | ND |
| F. solani | MF-W1109 | Ocimum basilicum | seed | St. Petersburg | 0.5 ± 0.3 | 2.1 ± 1.1 |
| F. solani | MF-W1110 | Cola sp. | stem | St. Petersburg | ND | ND |
| F. solani | MF-W83 | Secale cereale | seed | Riga | 5.4 ± 0.5 | 0.5 ± 0.3 |
| F. solani | MF-W87 | Triticum aestivum | seed | Irkutsk | 1.1 ± 0.2 | ND |
| F. solani | MF-W436 | Avena sativa | seed | Pskov | 10.6 ± 1.0 | ND |
| F. solani | MF-W448 | Zea mays | stem | Krasnodar | 1.2 ± 0.7 | 0.3 ± 0.1 |
| F. solani | MF-W483 | Solanum lycopersicum | stem | Volgograd | 2.3 ± 1.4 | 0.2 ± 0.04 |
| F. solani | MF-W632 | Triticum aestivum | seed | Krasnodar | 1.4 ± 0.2 | 0.6 ± 0.1 |
| F. solani | MF-W723 | Zea mays | stem | Stavropol | 0.9 ± 0.2 | 0.2 ± 0.02 |
| F. solani | MF-W725 | Triticum aestivum | stem | Altay | 8.9 ± 3.3 | 1.7 ± 0.5 |
| F. solani | MF-W728 | Triticum aestivum | seed | Primorsk | 2.3 ± 0.3 | ND |
| F. solani | MF-W841 | Triticum aestivum | stem | Stavropol | ND | ND |
| F. solani | MF-W869 | Cucumis sativus | stem | St. Petersburg | ND | 1.3 ± 0.8 |
| F. solani | MF-W894 | Cannabis sativa | stem | St. Petersburg | 0.6 ± 0.1 | ND |
| F. solani | MF-W898 | Cannabis sativa | stem | St. Petersburg | 0.4 ± 0.1 | ND |
| F. solani | MF-W934 | Papaver rhoeas | stem | St. Petersburg | 3.4 ± 0.4 | ND |
| F. solani | MF-W1014 | Pisum sativum | stem | Voronezh | 7.1 ± 4.2 | ND |
| F. solani | MF-W1099 | Solanum lycopersicum | stem | St. Petersburg | ND | ND |
| F. solani | MF-W1100 | Solanum lycopersicum | stem | St. Petersburg | ND | 0.8 ± 0.4 |
| Macrophomina phaseolina | MF-16-001 | Helianthus annuus | stem | Krasnodar | ND | ND |
| Neocamarosporium betae | MF-Ch16-001 | Beta vulgaris | leaf | Primorsk | ND | ND |
| Phomopsis sojicola | MF-15-002 | Glycine max | seed | Krasnodar | ND | ND |
| Piricularia grisea | MF-S72.1 | Cynodon dactylon | leaf | Krasnodar | ND | ND |
| Plenodomus biglobosus | MF-Br16-001 | Barbarea sp. | stem | Krasnodar | ND | ND |
| P. lindquistii | MF-Ha16-001 | Helianthus annuus | stem | Krasnodar | ND | ND |
| Pythium ultimum | MF-R26.1 | Brassica napus | stem | St. Petersburg | 0.6 ± 0.1 | ND |
| Rhizoctonia solani | MF-R15.2 | Brassica napus | root | St. Petersburg | ND | ND |
| R. solani | MF-R15.6 | Brassica napus | stem | St. Petersburg | ND | ND |
| R. solani | MF-P915-010 | NR | NR | Germany | 0.4 ± 0.1 | ND |
| Sclerotinia sclerotiorum | MF-R22.14 | Brassica campestris | stem | St. Petersburg | 2.2 ± 0.2 | ND |
| S. sclerotiorum | MF-R22.16 | Thlaspi arvense | stem | St. Petersburg | ND | ND |
| S. sclerotiorum | MF-R22.16 | Thlaspi arvense | stem | St. Petersburg | ND | ND |
| Sclerotium rhizodes | MF-S-60.2 | Agrostis tenuis | leaf | St. Petersburg | ND | ND |
| S. rhizodes | MF-S-60.3 | Calamagrostis epigeios | leaf | St. Petersburg | ND | ND |
| S. varium | MF-P992-010 | Raphanus raphanistrum | leaf | St. Petersburg | 3.7 ± 0.3 | 1.4 ± 0.1 |
| Verticillium albo-atrum | MF-M18.5 | Papaver somniferum | stem | Kiev | ND | ND |
Growth media. Two liquid media were used for estimation the ability of fungi to produce ABA. The first medium was a modified potato dextrose (MPD) agar (Okamoto et al, 1988) supplemented after autoclaving with 1 mL L–1 of a juice obtained from fresh potato tubers. For this purpose, fresh potato tubers were homogenized using a household mixer and the juice was collected. Then the juice was centrifuged for 10 min (3000 g, 4°C), sterilized using 0.2 µm filters (Corning, Germany) and stored at –20°C until use. This modification aimed on the enrichment of PD medium with some putative heat sensitive components which can induce ABA production by fungi, but probably were inactivated due to autoclaving of a handmade PD medium. The second medium was an original chemically defined (OCD) medium containing (g L–1): glucose – 10, glutamic acid – 0.2, asparagine – 0.2, aspartic acid – 0.2, serine – 0.2, thiamine – 0.001, MgSO4 – 0.2, KCl – 0.5, CaCl2 – 0.1, KH2PO4 – 0.8, FeCl3 – 0.05 (pH = 6.0). An autoclaved OCD medium was supplemented with sterile solution of micronutrients (µM): H3BO3 – 2, MnSO4 – 1, ZnSO4 – 3, NaCl – 6, Na2MoO4 – 0.06; CoCl2 – 0.06, CuCl2 – 0.06, NiCl2 – 0.06. This medium was composed based on the information available in the literature about chemically defined media used for the study of ABA-producing fungi (see Table 1 for references).
Fungi batch culture. Fungal strains were cultivated for 7 days on CD agar at 28°C. Then water suspensions containing spores and mycelium pieces in the amount of 107 colony forming units (CFU) per mL were prepared via flushing from the agar surface and used as inoculum. One mL of the inoculum was added to the flasks containing 50 mL liquid MPD or OCD media in two replicates for each strain. The uninoculated flasks were used as control treatments and for monitoring the presence of ABA in MPD medium. The flasks were incubated for 20 days at 23°C and lighting of 20 μmol m–2 s–1 with a 12 h photoperiod. The experiments were conducted twice for the strains showing ABA production.
ABA determination. The culture fluids were separated from mycelium using disposable cotton filters, acidified with 1 N HCl to pH = 3.0 and extracted with equal volumes of ethyl acetate. The extracts were evaporated to dryness at 35°C under vacuum on rotary evaporator Heidolph Hei-VAP Advantage (Heidolph, Germany) and the dry residues were dissolved in 0.2 ml of 18% acetonitrile, followed by filtration through 0.2 μm nylon membrane filters (Corning, USA). Ana-lysis of ABA content was carried out by ultra-performance liquid chromatography using a Waters Acquity UPLC H-class system (Waters, USA) on a Waters Acquity UPLC BEH Shield RP18 (Waters, USA) column. Chromatographic separation was carried out for 6 minutes in isocratic mode in 18% aqueous acetonitrile containing 0.1% acetic acid followed by a 3 min flush of the chromatography column with mixture of 80% acetonitrile, 20% water and 0.1% acetic acid. The flow rate of the chromatographic mixture was 0.25 mL min–1. ABA was determined using a Waters PDA diode-matrix UV detector at 265 nm by comparison the retention time and UV spectra (210–400 nm) of ABA peaks in the standard solution of chemically pure ABA (0.1 mg mL–1, and Sigma-Aldrich, USA) with the corresponding peaks in samples. In the end of experiments the uninoculated MPD medium contained some amount of ABA varying from 0.9 to 4.7 µg L–1 depending on the experiment. The source of ABA in MPD medium was potato tubers used for medium preparation. The values of ABA concentration in the uninoculated MPD medium were always subtracted from those detected in the fungal cultures.
Agar dish culture with tomato. Tomato seeds were surface sterilized by 1% Na-hypochlorite for 15 min and germinated on a sterile wet filter paper with tap water for 7 days at 22°C. Germinated seeds were transferred to Petri dishes (3 seedlings per dish) with 25 mL of 1.5% agar nutrient solution (µM): MgSO4 · 7H2O – 500, CaCl2 · 2H2O – 500; K2HPO4 – 500, KH2PO4 – 210, KNO3 – 100, NaFeEDTA – 10, HBO3 – 1; MnSO4 –1; ZnSO4 – 1; Na2MoO4 – 0.03; CuSO4 – 0.8; pH 6.3. Each seedling was inoculated with 30 µL of the above mentioned inoculum. After 10 days incubation in growth chamber at day/night cycle of 16/8 h and temperature of 23°C for 7 days all dishes were inspected and scanned. The obtained images were used to count the number of lateral roots and to measure total root length and using а curvimeter LX-3 (Sprinter, Ukraine).
Statistical analysis of the data was performed using the software Statistica version 10 (StatSoft Inc., USA).
RESULTS AND DISCUSSION
Thirty-four strains of 13 species produced ABA growing on MPD medium (Table 2). The maximum ABA concentrations were found in the culture liquids of both strains MF-R13.8 and MF-R41.5 belonging to Arthrinium arundinis. Nineteen strains produced ABA growing on OCD medium and the maximum ABA concentrations were in culture liquids of strains Apiospora montagnei MF-S-41.5 and A. arundinis MF-R13.8. All the strains producing ABA on MPD medium also did it on OCD medium as well, but among them only 17 strains produced ABA growing on both MPD and OCD media. The results suggest that the ability to produce ABA is a wide spread trait among phytopathogenic fungi of different taxonomic groups isolated from various host plants and environments. Some fungal species were among previously described ABA-producers (Table 1), namely Botrytis cinerea (e.g. Dorffling, Petersen 1984; Inomata-et al., 2004), Fusarium culmorum (Michniewicz, 1989) and Rhizoctonia solani (Dorffling, Petersen, 1984). However, for the first time we detected ABA production in nine species such as Alternaria tenuissima, Apiospora montagnei, Bipolaris sorokiniana, Fusarium avenaceum, F. solani, Pythium ultimum, Sclerotinia sclerotiorum, and Sclerotium varium. Ability to produce ABA highly varied among strains of the same species. For example, only three of eight Fusarium oxysporum strains and 16 of 19 F. solani strains produced ABA growing on the used media. No correlation between the ability to produce ABA and host plant (R = –0.06, P = 0.63, n = 65), plant organ of isolation (R = –0.01, P = 0, 94, n = 65) or region of strain origin (R = 0.06, P = 0.66, n = 65) was found. This suggests that fungal ABA production is not closely associated with these features. Otherwise, it should be mentioned that the presence and concentration of ABA produced by the same fungal strain may significantly vary depending on growth conditions and medium composition (Norman et al., 1981; Okamoto et al., 1988; Oritani, Kiyota, 2003) resulting in the disguise of such correlations. Indeed, the absence of correlations between the in vitro studied traits and the natural properties of biological objects is a well-known fact. The use of knock-out fungal mutants unable to produce ABA or the study of expression of genes related to fungal ABA production in patho-systems may shed light on this problem.
The agar dish culture was applied with three tomato cultivars inoculated with six strains of F. solani and four strains of F. oxysporum differing in ABA production to test relationship between the ability of fungi to produce ABA and to exert negative effects on plants. Similar agar culture we previously applied to investigate effects of ABA-utilizing rhizobacteria on growth and tissue ABA concentrations of tomato cultivar Ailsa-Craig (Belimov et al., 2014). In this study the strains F. solani MF-W1014 and F. oxysporum MF-G58284, MF-G58767 and MF-G59120 significantly inhibited root elongation (Fig. 1a) and root branching (Fig. 1b) of all three tomato cultivars. These treatments also resulted in yellowing of roots and significant reduction of shoot growth (visual observations, data not shown). Root elongation and root branching was also inhibited after inoculation of cultivar Ailsa-Craig by F. solani 725 (Fig. 1). However, the negative effects of strains F. solani MF-W1014 and F. oxysporum MF-G58284 and MF-G59120 on cultivars Altai-Ground and Early-Uzbekistan were more pronounced as compared with Ailsa-Craig (Fig. 1). In general, the root growth inhibiting effects of fungi were similar for all cultivars, since correlations between cultivars for root length (r > 0.75; P < < 0.012; n = 10) and root number (r > 0.76; P < 0.011; n = 10) were significant. This suggests similarity in mechanisms of growth inhibiting effects of the studied strains. It is known that F. solani and F. oxysporum cause disease of various crops, including tomato (Imazaki, Kadota, 2015; Akbar et al., 2018). Most probably the root growth inhibition observed in our study on the inoculated tomato seedlings was due to fungal phytotoxins, particularly fusaric acid which causes negative effect on plants (Bohni et al., 2016; Lopez-Diaz et al., 2018).
Fig. 1.
Effect of Fusarium strains on root length (a) and number of lateral roots (b) of tomato seedlings in agar dish culture. Tomato cultivars: Ailsa-Craig (VIR 1930) (white fill), Altai-Ground (VIR 2311) (black fill) and Early-Uzbekistan (VIR 4750) (speckled fill). Treatments: 1 – uninoculated control, 2 – Fusarium solani MF-W483, 3 – F. solani MF-W725, 4 – F. solani MF-W869, 5 – F. solani MF-W1014, 6 – F. solani MF-W1100, 7 – F. solani MF-W1109, 8 – F. oxysporum MF-G58284, 9 – F. oxysporum MF-G58767, 10 – F. oxysporum MF-G59120, 11 – F. oxysporum MF-G93656. Bars show standard deviations (n = 3).
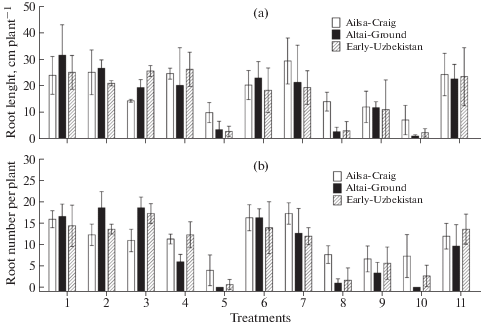
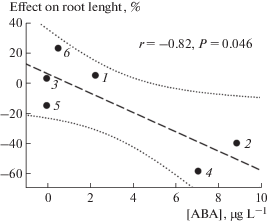
Generally, ABA concentration in culture fluids did not correlate with the effects of fungi on tomato roots, with one exception that ABA production by F. solani strains negatively correlated (r = –0.82, P = 0.046, n = = 6) with root length of cultivar Ailsa-Craig (Fig. 2). The latter observation is in line with previous reports showing the important negative role of fungal ABA in plant disease resistance (Asselbergh et al., 2008; Ton et al., 2009). Whether ABA production is associated with production of some toxins by such fungi needs more detailed study.
Fig. 2.
Linear regression curve (dash line) showing correlation between abscisic acid production in vitro by Fusarium solani strains and their effect on root elongation of tomato cultivar Ailsa-Craig (VIR 1930) in agar dish culture. Strains: 1 – F. solani MF-W483, 2 – F. solani MF-W725, 3 – F. solani MF-W869, 4 – F. solani MF-W1014, 5 – F. solani MF-W1100, 6 – F. solani MF-W1109. Dotted lines show regression confidence area at P = 0.05.
CONCLUSION
In conclusion, we have detected ABA in culture fluids of nine fungal species, for which this property was not previously described, and expanded the species list of ABA-producing phytopathogens. A high variation in the ability to produce ABA was present on both strain and species levels. Production of ABA in a not defined MPD medium, containing extract of potato tubers, was found in more strains and it was generally higher compared with a defined OCD medium. However, the presence of ABA in the uninoculated MPD medium should be taken into account when assessing the ability of fungi to produce ABA. The absence of significant correlations between ABA production and the studied characteristics of strains suggest high complexity of this phenomenon. However, the negative correlation observed here between ABA production by F. solani strains and root length of Ailsa-Craig gave new original information about the role of fungal ABA as a positive modulator of pathogenesis. The selected ABA-producing strains can be used to study mechanisms underlying involvement of fungal ABA in plant-microbe interactions.
The work was supported by the Russian Science Foundation (grant N 17-14-01363).
Список литературы
Akbar A., Hussain S., Ullah K., Fahim M., Ali G.S. Detection, virulence and genetic diversity of Fusarium species infecting tomato in Northern Pakistan. PLoS One. 2018. V. 13 (9): e0203613. https://doi.org/10.1007/s00253-018-9407-5
Anderson J.P., Badruzsaufari E., Schenk P.M., Manners J.M., Desmond O.J., Ehlert C., Maclean D.J., Ebert P.R., Kazan K. Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell. 2004. V. 16 (12). P. 3460–3479. https://doi.org/10.1105/tpc.104.025833
Asselbergh B., Asselbergh B., Vleesschauwer D., Hofte M. Global switches and fine-tuning – ABA modulates plant pathogen defense. Mol. Plant Microbe In. 2008. V. 21 (6). P. 709–719. https://doi.org/10.1094/MPMI-21-6-0709
Audenaert K., Meyer G.B.D., Hofte M. Abscisic acid determines basal susceptibility of tomato to Botrytis cinerea and suppresses salicylic acid-dependent signaling mechanisms. Plant Physiol. 2002. V. 128 (2). P. 491–501. https://doi.org/10.1104/pp.010605
Belimov A.A., Dodd I.C., Safronova V.I., Dumova V.A., Shaposhnikov A.I., Ladatko A.G., Davies W.J. Abscisic acid metabolizing rhizobacteria decrease ABA concentrations in planta and alter plant growth. Plant Physiol Bioch. 2014. V. 74 (1). P. 84–91. https://doi.org/10.1016/j.plaphy.2013.10.032
Bohni N., Hofstetter V., Gindro K., Buyck B., Schumpp O., Bertrand S., Monod M., Wolfender J.L. Production of fusaric acid by Fusarium spp. in pure culture and in solid medium co-cultures. Molecules. 2016. V. 21 (3). P. 370. https://doi.org/10.3390/molecules21030370
Davies W.J., Zhang J. Root signals and the regulation of growth and development of plants in drying soil. Annu. Rev. Plant Phys. 1991. V. 42 (6). P. 55–76. https://doi.org/10.1146/annurev.pp.42.060191.000415
Dodd I.C. Root-to-shoot signalling: assessing the roles of ‘up’ in the up and down world of long-distance signalling in planta. Plant Soil. 2005. V. 274 (1–2). P. 251–270. https://doi.org/10.1007/s11104-004-0966-0
Dorfling K., Petersen W. et al. Abscisic acid in phytopathogenic fungi of the genera Botrytis, Ceratocystis, Fusarium, and Rhizoctonia. Z. Natur-forsch. 1984. V. 39. P. 683–684.
Endo A., Okamoto M., Koshiba T. Abscisic acid: metabolism, transport and signaling. In: D.P. Zhang (ed.). Springer Science, Business Media, Dordrecht, 2014. P. 23–25.
Hartung W. The evolution of abscisic acid (ABA) and ABA function in lower plants, fungi and lichen. Funct. Plant Biol. 2010. V. 37 (9). P. 806–812. https://doi.org/10.1071/FP10058
Hasegawa S., Poling S.M., Mayer V.P., Bennett R.D. Metabolism of abscisic acid: bacterial conversion to dehydrovomifoliol and vomifoliol dehydrogenase activity. Phytochemistry. 1984. V. 23 (12). P. 2769–2771. https://doi.org/10.1016/0031-9422(84)83012-5
Imazaki I., Kadota I. Molecular phylogeny and diversity of Fusarium endophytes isolated from tomato stems. FEMS Microbiol. Ecol. 2015. V. 91 (9). https://doi.org/10.1093/femsec/fiv098
Koga H., Dohi K., Mori M. Abscisic acid and low temperatures suppress the whole plant-specific resistance reaction of rice plants to the infection of Magnaporthe grisea. Physiol. Mol. Plant P. 2004. V. 65 (1). P. 3–9. https://doi.org/10.1016/j.pmpp.2004.11.002
Kriaa M., Hammami I., Sahnoun M., Azebou M.C., Triki M.A., Kammoun R. Biocontrol of tomato plant diseases caused by Fusarium solani using a new isolated Aspergillus tubingensis CTM 507 glucose oxidase. C. R. Biol. 2005. V. 338 (10). P. 666–677. https://doi.org/10.1016/j.crvi.2015.05.007
Kuromori T., Seo M., Shinozaki K. ABA transport and plant water stress responses. Trends Plant Sci. 2018. V. 23 (6). P. 513–522. https://doi.org/10.1016/j.tplants.2018.04.001
Lievens L., Pollier J., Goossens A., Beyaert R., Staal J. Abscisic acid as pathogen effector and immune regulator. Front. Plant Sci. 2017. https://doi.org/10.3389/fpls.2017.00587
López-Díaz C., Rahjoo V., Sulyok M., Ghionna V., Martín-Vicente A., Capilla J., Di Pietro A., López-Berges M.S. Fusaric acid contributes to virulence of Fusarium oxysporum on plant and mammalian hosts. Mol. Plant Pathol. 2018. V. 19 (2). P. 440–453. https://doi.org/10.1111/mpp.12536
Marumo S., Katayama M., Komori E., Ozaki Y., Natsume M., Kondo S. Microbial production of abscisic acid by Botrytis cinerea. Agric. Chem. 1982. V. 46 (7). P. 1967–1968. https://doi.org/10.1080/00021369.1982.10865367
Matsumoto K., Suzuki Y., Mase S., Watanabe T., Sekizawa Y. On the relationship between plant hormones and rice blast disease. Ann. Phytopathol. 1980. V. 46 (3). P. 307–314. https://doi.org/10.1093/mp/sst056
Michniewicz M. Growth regulators formed by Fusaria: Their significance for the fungus growth, sporulation and pathogenicity towards the host plant. In: J. Chelkowski (ed.). Fusarium, mycotoxins, taxonomy and pathogenicity, Elsevier Scientific Publishers, Amsterdam, 1989. P. 227–241.
Mohr P., Cahill D. Abscisic acid influences the susceptibility of Arabidopsis thaliana to Pseudomonas syringae pv. tomato and Peronospora parasitica. Funct. Plant Biol. 2003. V. 30 (4). P. 461–469. https://doi.org/10.1071/FP02231
Mohr P., Cahill D. Relative roles of glyceollin, lignin and the hypersensitive response and the influence of ABA in compatible and incompatible interactions of soybeans with Phytophthora sojae. Physiol. Mol. Plant P. 2001. V. 58 (1). P. 31–41. https://doi.org/10.1006/pmpp.2000.0306
Oritani T., Kiyota H. Biosynthesis and metabolism of abscisic acid and related compounds. Nat. Prod. Rep. 2003. V. 20 (4). P. 414–425. https://doi.org/10.1039/B109859B
Sah S.K., Reddy K.R., Li J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 2016. https://doi.org/10.3389/fpls.2016.00571
Stopinska J., Michniewicz M. Control of growth and development of Ceratocystis fimbriata Ell. et Halst. by plant growth regulators. II. Abscisic acid. B Pol Acad Sci-Biol. 1988. V. 36 (10–12). P. 253–258.
Taylor I.B., Burbidge A., Thompson A.J. Control of abscisic acid biosynthesis. J. Exp. Bot. 2000. V. 51 (350). P. 1563–1574. https://doi.org/10.1093/jexbot/51.350.1563
Ton J., Flors V., Mauch-Mani B. The multifaceted role of ABA in disease resistance. Trends Plant Sci. 2009. V. 14 (6). P. 310–317. https://doi.org/10.1016/j.tplants.2009.03.006
Tudzynski B., Sharon A. Biosynthesis, biological role and application of fungal phytohormones. In: H.D. Osiewacx (ed.). The Mycota X. Industrial applications. Springer-Verlag: Berlin, 2002. P. 183–224.
Vizárová G., Chalanyová M., Janitor A., Dugová O., Bacigálová K., Takác L. Secretion of abscisic acid by hemibiotrophic fungi. Biologia. 1997. V. 52 (6). P. 807–809.
Ward E.W.B., Cahill D.C., Battacharya M.K. Abscisic acid suppression of phenylalanine ammonia-lyase activity and mRNA, and resistance of soybeans to Phytophthora megasperma f. sp. glycinea. Plant Physiol. 1989. V. 91 (1). P. 23–27. https://doi.org/10.1104/pp.91.1.23
Дополнительные материалы отсутствуют.
Инструменты
Микология и фитопатология


