Микология и фитопатология, 2019, T. 53, № 6, стр. 354-362
GANODERMA APPLANATUM (POLYPORALES, BASIDIOMYCOTA) AT THE SAINT PETERSBURG AREA
I.V. Zmitrovich 1, *, S.V. Volobuev 1, **, V.A. Dudka 1, ***, E.A. Zhukova 2, ****, M.V. Sidelnikova 3, *****, M.A. Bondartseva 1, ******
1 Komarov Botanical Institute of the Russian Academy of Sciences
197376 St. Petersburg, Russia
2 The State Russian Museum
191186 St. Petersburg, Russia
3 St. Petersburg State Agrarian University
196601 St. Petersburg, Russia
* E-mail: iv_zmitrovich@mail.ru
** E-mail: sergvolobuev@mail.ru
*** E-mail: dudkavasiliy.a@gmail.com
**** E-mail: ealukmazova@mail.ru
***** E-mail: kapa0505@mail.ru
****** E-mail: bondartseva@mail.ru
Поступила в редакцию 2.04.2019
После доработки 10.04.2019
Принята к публикации 16.05.2019
Аннотация
Ganoderma applanatum is one of the most common polypores in the world. However, due to the variability of its macro- and even micro-characters, it is rather difficult to compile an exact picture of the distribution of this fungus both in the world as a whole and in its different regions. At the same time, in cities all the Ganoderma representatives are subject of control, because the pathogenesis of parkland is often associated with these species. Therefore, an exact identification of the pathogen is not only a subject of basic scientific, but also of practical importance. The aim of this paper is molecular testing of G. applanatum-coll. representatives to understand one or more phylospecies are distributed over area in question. In the present study, we analyzed 20 model samples collected in various St. Petersburg localities. The analysis of ITS sequences has showed that only one phylospecies (G. applanatum) characterized by significant biomorphological plasticity is distributed over St. Petersburg area. It has shown that, according to the ratio of pileus diameter to basidiome thickness, two basic morphotypes can be distinguished: subfomitoid one (the ratio of the pileus radius to its thickness ≤ 2) and applanate one (the ratio of the cap radius to its thickness ≥ 2). A severity of this character depends on basidiome age, and on the content of easily immobilized metabolites in host tree. According to their substrate location, basidiomes can be grouped onto laterally attached on vertical surfaces and complex rosette-shaped mantle formations on buried wood. Variability of basidiomes associated with interaction with insects or vertebrates was separately noted. It was found that the age structure of model St. Petersburg population of G. applanatum is characterized by predominance of 1–2-years-old basidiomes, whereas the largest age of basidiomes doesn’t exceed 7 years. It has been suggested that relatively young age of perennial basidiomes is rather constitutional feature of the species in boreal zone, characterized by mycelium freezing in marginal zones of basidiome. Variability of G. applanatum basidiospores was considered, too. It was shown that specimens with longest (and partly widest) basidiospores are confined to living trees. G. applanatum combines two trophic strategies, a pathogenic saprotroph growing on living trees and causing chronic rot, and a pure saprotroph, which continues to develop on dead trunks as well as stumps, dead and buried wood. As a pathogen, the species manifests itself by colonizing the frost holes of the butt area, characteristic of broad-leaved species under conditions of degradation of artificial drainage of broadleaf stands. As a pathogen control measure, furrowing of the bark of spring shoots of broad-leaved seedlings was proposed to prevent increased formation of frost cracks as well as the prevention the degradation of drainage systems of broad-leaved stands. A modern diagnosis of the species was given taking into account the data obtained on its boundaries and variability at St. Petersburg areas.
INTRODUCTION
Ganoderma applanatum (Polyporales, Basidiomycota) is one of polypores the most common in the world. It grows on deciduous and coniferous species, causing a lump, roots, or stems white rot, and is able to begin its development on living trees, but continue also a dead wood colonization (Bondartsev, 1953; Ryvarden, Gilbertson, 1993; Bondartseva, 1998; Ryvarden, Melo, 2017). However, due to its huge variability of macro- and even micro-characters, which was a reason for repeated re-descriptions of this species, an exact picture of distribution of this polypore is rather difficult to establishing both for the global space and for its different regions.
The species has been described as Boletus applanatus by Persoon (1800). An authentic type specimen of this species is kept in the Leiden herbarium (Steyaert, 1967). Authentic material was not preserved for B. lipsiensis, which was described a little earlier (Batsch, 1786), while contemporaries doubted the identity of B. applanatus with B. lipsiensis (Persoon, 1801), therefore, this second name should be considered today rather as avoidable (Moncalvo, Ryvarden, 1997).
Later, the species Polyporus leucophaeus (Montagne, 1856) was described in North America. The lectotype of this species was tested and recognized as pale variety of Ganoderma applanatum (Ryvarden, 1982). The situation with G. australe (type Polyporus australis) (Fries, 1828) is more complicated, because for this species in Kew kept an associated European specimen, apparently unrelated to the original collection (Steyaert, 1975). In Europe, this species is understood either widely (Ryvarden, Gilbertson, 1993; Bondartseva, 1998), or narrowly and then referred to Ganoderma europaeum (Steyaert, 1961) or G. adspersum (Donk, 1969). This latter, due to the lack of molecular testing of type material till now, is very often mixed with G. applanatum in European guides.
In Saint Petersburg, located in the softwood zone, but covered with a broad-leaved plantations on artificially drained grounds, in principle, there are conditions for meeting both a ubiquitous G. applanatum and more southern Central European taxa of the genus, such as G. adspersum or G. pfeifferi. G. applanatum was reported for Saint Petersburg areas in a range of papers (Bondartseva et al., 2014; Zmitrovich et al., 2018; etc.). G. adspersum was also preliminary indicated for this territory (Zhukova et al., 2017) in accordance with widespread morphological concept of the species. However, the morphological concept of Ganoderma spp. is very unreliable issue due to huge variability of macro- and micro-morphological characters over wide range even for one species (Steyaert, 1980; Wasser et al., 2006). At the same time, Ganoderma representatives in cities are subject to control, since the pathogenesis of parkland is associated with these species, therefore, an exact identification of pathogen has not only basic scientific, but also applied importance.
Taking into account aforementioned, the aim of the present paper is a molecular testing of Saint Petersburg G. applanatum-coll. representatives in order to understand one or more phylospecies we are dealing with in area in question. In case of successful solution of this problem it would help reassess the morphological boundaries of the units previously described in the literature as well as streamline the understanding of measurements of morphological intraspecific variability of G. applanatum and clarify the diagnosis of this species.
MATERIALS AND METHODS
The present study has involved an isolation of DNA from field material coupled with morphological testing of the same samples. A total of 20 samples were selected, covering the range of morphological variability of basidiomes as much as possible, main areas of St. Petersburg, and substrates on which the species was recorded most often.
Table 1 shows a geographic coordinates of each find, the herbarium documentation (voucher specimens) and GenBank number under which the obtained nucleotide sequence is defended.
Table 1.
Geographical coordinates and other documentation of Ganoderma applanatum specimens studied
| OSU* | Field number** | Coordinates | Locality | GenBank accession number | Voucher -specimen number |
|---|---|---|---|---|---|
| 1 | IZ 1 | 59°58'12''N, 30°19'31"E | Peter the Great Botanical Garden | MN435132 | LE 287658 |
| 2 | IZ 2 | 59°58'12''N, 30°19'31"E | »» | MN435133 | LE 287659 |
| 3 | IZ 3 | 59°58'12''N, 30°19'31"E | »» | – | LE 287660 |
| 4 | IZ 4 | 59°58'12''N, 30°19'31"E | »» | – | LE 287661 |
| 5 | IZ 5 | 59°58'12''N, 30°19'31"E | »» | MN435134 | LE 287662 |
| 6 | IZ 6 | 60°00'57''N, 30°24'27''E | Grazhdanka green space | MN435135 | LE 287663 |
| 7 | IZ 7 | 60°00'57''N, 30°24'27''E | »» | MN435136 | LE 287664 |
| 8 | IZ 8 | 59°59'59''N, 30°24'26''E | Piskarevskiy forest park | – | LE 287665 |
| 9 | IZ 9 | 59°59'21''N, 30°23'43''E | Bogoslovskoye Cemetery | – | LE 287667 |
| 10 | IZ 10 | 59°59'21''N, 30°23'43''E | »» | MN435137 | LE 287668 |
| 11 | EZ 13-35 | 59°94'63''N, 30°33'26''E | Summer Garden | MN435138 | LE 287669 |
| 12 | EZ 12-24 | 59°94'55''N, 30°33'66''E | »» | MN435139 | LE 287670 |
| 13 | EZ 98 | 59°94'37''N, 30°33'51''E | Engineering Square | MN435140 | LE 287671 |
| 14 | EZ 698 | 59°94'85''N, 30°33'58''E | Mikhailovskiy Garden | MN435141 | LE 313851*** |
| 15 | EZ 893 | 59°94'53''N, 30°33'71''E | »» | MN435142 | LE 287672 |
| 16 | MS 3 | 59°41'54''N, 30°28'16''E | Pavlovskiy Park | MN435143 | LE 287673 |
| 17 | MS 11 | 59°55'56''N, 30°16'56''E | St. Petersburg University Botanical Garden | – | LE 287674 |
| 18 | MS 7 | 59°41'54''N, 30°28'15''E | Pavlovsky Park | MN435144 | LE 287675 |
| 19 | MS 6 | 59°41'54''N, 30°28'17''E | »» | MN435145 | LE 287676 |
| 20 | MS 10 | 59°55'56''N, 30°16'56''E | St. Petersburg University Botanical Garden | MN435146 | LE 287677 |
The material collection involved both remnants of the zonal vegetation of Saint Petersburg Region (such as Piskarevskiy Forest Park) as well as old broadleaf stands, namely the Summer Garden, Mikhailovskiy Garden, Engineering Square, Peter the Great Botanical Garden, St. Petersburg University Botanical Garden.
Material collection and herbarization were carried out according to standard methods (Bondartsev, Singer, 1950).
DNA was extracted from small pieces of dried basidiocarps using NucleoSpin® Plant II (Macherey-Nagel, Germany). The ribosomal ITS1–5.8S–ITS2 region was amplified with the fungal specific primers ITS1F and ITS4B (Gardes, Bruns, 1993). PCR products were visualized using agarose gel electrophoresis and Gel Red staining, and subsequently purified with the Fermentas Genomic DNA Purification Kit (Thermo Fisher Scientific, MA, USA). Purified PCR products were sequenced on an ABI model 3130 Genetic Analyzer (Applied Biosystems, CA, USA). Raw data were edited and assembled in MEGA 6 (Tamura et al., 2013). Newly generated sequences have been deposited in the GenBank.
For this study, 15 ITS sequences were generated and additional 12 ITS sequences of other species including Amauroderma sprucei JX982568 chosen as outgroup were retrieved from GenBank (www.ncbi.nlm.nih. gov/genbank/). Sequences were aligned with the MAFFT version 7 web tool (Katoh, Toh, 2008; http://mafft.cbrc.jp/alignment/server/) using the G-INS-1 option. The final ITS alignment contained 624 characters (including gaps). ML analysis was performed in the RAxML BlackBox, v0.9.0 (Kozlov et al., 2019), under a GTR + G model with 100 rapid bootstrap replicates. Bootstrap (BS) values ≥ 70% are considered significant.
The macroscopic descriptions were based on study of fresh and dried specimens. The materials of the herbarium of Komarov Botanical Institute (St. Petersburg, Russia, LE) were studied. Microscopic preparations were mounted from dried material in Melzer’s solution, 10% ammoniacal Congo Red and 5% aqueous solution of KOH, using a LOMO Micmed-6 light microscope. The hyphal system was revealed and described according to updated technique (Zmitrovich et al., 2009). The size of mature spores was measured on 30 spores in distilled water and Melzer’s solution. The variability of basidiospores was studied according to the methods proposed by Parmasto et al. (1987).
Visualization and graphing were performed using the programming language R 3.3.3 (R Core Team, 2012) in the software environment RStudio 1.0.136 (R Studio Team, 2017). The graphs were performed using the packages Ggplot2 (Wickham, 2009), Scales (Scales, 2019), Ggpubr (RPackages 2019), as well as the online program Sankey Diagram Generator v1.2 (Sankey, 2019).
RESULTS AND DISCUSSION
Phylogenetic analysis. Since approximately 2/3 of tested fungal organisms have an intraspecific variability of ITS1–ITS2 sequences lies in the range 0–1% and approximately 3/4 of fungi have their intraspecific variability ca 1–2%. Therefore, in fungi this region can be used as a marker of species divergence (Nilsson et al., 2008). This region we used in this study. 15 of the 20 samples involved in the study succeeded in amplifying this region. Five samples (IZ3, IZ4, IZ8, IZ9, MS11) have produced dubious products and we excluded them from further analysis, all the more so because all of them had a typical morphology of “neutral applanatum type”.
The analysis of ITS region of all 20 operational sample units (OSUs) showed that all differences between the lines are absent, or (MS-10) lie within a few hundredths of the expected changes per site (noise is possible due to intragenomic rDNA variability) which is typical for a single phylospecies. We did not set the task of populations marking by microsatellite polymorphisms, but our task was to identify whether several deeply diverging species were hiding behind the morphologically motley conglomerates of the G. applanatum collective. In the Fig. 1, all OSUs are marked with a rough morphobiological characteristic and it turns out that the distribution of morphobiotypes through phylogenetic multifurcation is quite random. It is obviously that here we can talk about the age and modification variability of basidiomes, that will be described in more detail in the next section of the paper.
Fig. 1.
Phylogenetic tree derived from Maximum Likelihood analysis, based on nrITS1–5.8S–ITS2 data. Bootstrap values (BS > 70%) are shown to the left of a node (field numbers abbreviations – see Table 1). Scale bar – a number of expected changes per site.
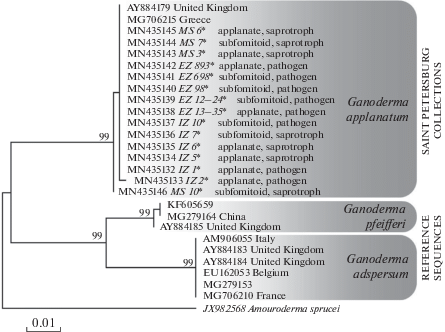
It should also be noted that the more divergent sequences introduced into the topology, such as G. adspersum or G. pfeifferi, although represent separate lineages of the species level, are in no way correlated with nomenclatural types of these taxa. This represents the main problem of the Ganoderma taxonomy. With the exception of a few common ubiquitous species, the authenticity of which does not cause doubts, the identification of most other ganodermas without resorting to type material and a few standard revisions is impossible – for this reason such names as “G. lucidum”, “G. resinaceum”, “G. tsugae”, “G. australe” can be found in different clusters of the same molecular cladogram, and the question of their relation to the corresponding types remains to be open (Zmitrovich, 2018). We guess that in the future it will be necessary to provide a critical type study of G. adspersum with the corresponding epitypification procedures (in case it is impossible to obtain a holotype DNA) in order to understand in which part of the Ganoderma-tree this taxon is really nested.
The fact of belonging of samples identified by keybooks as G. australe – G. adspersum, allows us to outline new approaches to differentiating the boundaries of these taxa, but this issue should also be addressed in connection with the molecular critical type studies.
Overview of morphological variability. As can be seen from Table 2 and Fig. 2, the variability spectra of the individual features of model samples intersect, and, as a result, there is the impression of a fine species diffe-rentiation. Particularly noteworthy are fungi infesting living trees and having wide white margin that sometimes really want to associate with some southern Ganoderma taxa. In fact, we are dealing with an incubation of the age and modification variability of basidiomes, in which the following directions can be distinguished.
Table 2.
Substrates and morphological features of studied Ganoderma applanatum operational sample units (OSU)
| OSU | Basidiome age (year layers) | Pileus radius (cm) | Pileus radius/ base thickness | Basidiospores (l × w limits, μm) | Qm | Host | State* |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 4.5 | 3.0 | 7.8–10.4 × 5.2–6.2 | 1.65 | Tilia platyphyllos | 1 |
| 2 | 2 | 3.8 | 2.5 | 7.8–10.6 × 5.2–6.2 | 1.57 | Ulmus glabra | 1 |
| 3 | 5 | 12.5 | 3.2 | 7.8–10.4 × 5.2 –6.5 | 1.59 | Sorbus aucuparia | 1 |
| 4 | 1 | 3.5 | 1.8 | 7.0–8.5 × 4.4–5.7 | 1.50 | Salix alba | 1 |
| 5 | 2 | 5.5 | 2.5 | 7.8–10.4 × 4.4–6.5 | 1.52 | »» | 2 |
| 6 | 3 | 11.2 | 2.1 | 7.8–9.1 × 5.2–6.2 | 1.48 | Betula pendula | 5 |
| 7 | 2 | 9.0 | 1.9 | 7.8–10.4 × 4.9–6.2 | 1.52 | Populus nigra | 3 |
| 8 | 2 | 4.0 | 2.8 | 7.8–8.5 × 5.2–5.9 | 1.52 | Betula pubescens | 3 |
| 9 | 7 | 35.0 | 2.3 | 7.5–10.4 × 4.9–6.2 | 1.55 | Populus nigra | 2 |
| 10 | 1 | 4.0 | 1.7 | 7.0–9.1 × 4.4–5.7 | 1.44 | Sorbus aucuparia | 1 |
| 11 | 1 | 9 | 2.3 | 7.8–10.9 × 5.2–5.9 | 1.73 | Tilia cordata | 1 |
| 12 | 1 | 4 | 2.0 | 7.8–10.1 × 5.2–5.9 | 1.56 | »» | 1 |
| 13 | 2 | 5.5 | 1.7 | 5.7–8.5 × 4.6–5.7 | 1.43 | Fraxinus excelsior | 1 |
| 14 | 1 | 3 | 1.2 | 7.8–11.1 × 5.2–7.8 | 1.44 | Tilia cordata | 1 |
| 15 | 1 | 4.5 | 3.0 | 7.8–9.1 × 5.2–5.9 | 1.47 | Fraxinus excelsior | 1 |
| 16 | 5 | 12 | 3.1 | 7.5–8.3 × 4.9–5.7 | 1.49 | Tilia cordata | 3 |
| 17 | 4 | 14 | 2.0 | 7.8–10.4 × 4.9–6.2 | 1.53 | ?Ulmus glabra | 3 |
| 18 | 1 | 6 | 1.1 | 7.2–10.4 × 5.2–5.9 | 1.55 | Tilia cordata | 3 |
| 19 | 4 | 8 | 3.2 | 7.8–10.4 × 5.2–5.9 | 1.51 | »» | 3 |
| 20 | 7 | 15 | 2.0 | 5.9–8.3 × 4.9–5.7 | 1.51 | Salix alba | 3 |
Fig. 2.
The age and modification variability of Ganoderma applanatum. The first range – basic morphotypes: subfomitoid (a), applanate (b); the second range – substrate location: with an orthotropic tendency on submerged wood (c), lateral on standing trees (d); the third range – basic ecades: eutrophic ecade of the first year with hypertrophic margin (e), mesotrophic ecade of the second year with thinning margin (f); the fourth range – morphological changes due to interaction with environmental factors: channel penetration from insect activity (g), collapse of anamixoderm with formation of polished surface under impact of vertebrates (h).

According to the ratio of the cap diameter to the thickness of the basidiome, two basic morphotypes can be distinguished: subfomitoid one (the ratio of radius of the pileus to its thickness ≤ 2), and applanate one (the ratio of radius of the pileus to its thickness ≥ 2). A severity of this trait depends on basidiome age (perennial pilei have an applanate tendency) and the content of easily immobilized metabolites in host tree (on living trees, the sterile layer is thicker, which marks a subfomitoid tendency) (Zmitrovich, Spirin, 2005).
Another variability dimension is connected with a substrate location of basidiomes. In addition to classic case of laterally attached pilei, this species is characterized by the formation of rosette-shaped mantle structures, rooted in roots buried wood. Sometimes individual marginal pilei rise on stalk-like base resembling representatives of the G. lucidum-complex.
It should also be noted the variability of basidiomes associated with interaction with insects or vertebrates. Insects form galls and capsules, channels and external deposits of processed fungal tissue. Vertebrates (including humans) can affect the growing edge and surface of the pileus, causing the growing felt part of anamixoderm to collapse and an obliterated surface to remain polished spots, with a hint of the G. lucidum-complex and G. pfeifferi, but this is impession only, because true pileocystidia are absent into G. applanatum crust.
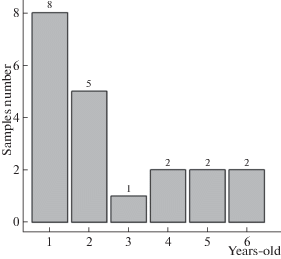
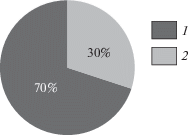
It is interesting, but the age population structure of G. applanatum is characterized by predominance of basidiomes of the first year or biennial ones, while the maximum age of basidiomes in Saint Petersburg model population does not exceed 7 years (Fig. 3). At the same time, species of the genera Phellinus and Fomes in the surrounding boreal forests often reach 25–30 years. Dr. M. Szczepka (personal communication) has reported the presence of 20-year-old G. pfeifferi basidiome found in Southern Poland. The cause of the short-lived state of G. applanatum basidiomes may be the same insects and vertebrates, leading to their destruction. Parasitic mycophilous fungi, e.g. Sporophagomyces chrysostomus (Donk, 1974), may play some role, too. However, the general basidiome design of the fungus seems to be not in perfect harmony with boreal environments and, possibly, like Fomitopsis pinicola, short-lived state of Ganoderma applanatum basidiomes is incorporated structurally (the impossibility of preserving mycelial nests in marginal zones after overwintering). As a result, the basidiomes of small age with wide zone of hypertrophy (subfomitoid) in the Saint Petersburg areas prevail over mature applanate ones, characterized by maximum ages (Fig. 4).
Fig. 4.
Distribution of St. Petersburg Ganoderma applanatum model samples on basic morphotypes: subfomitoid (1), applanate (2).
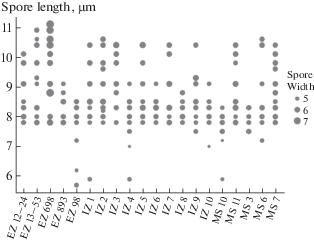
The visualization of spore variability of G. applanatum model population is presented on Fig. 5. The samples with the longest (and partly widest) basidiospores are mainly associated with living trees. This can also be partly explained by better supply of mycelium with easily immobilized metabolites, which leads to hypertrophy and hyperplasia of elements of aerial mycelium.
Fig. 5.
Variability in the sizes of basidiospores of St. Petersburg Ganoderma applanatum model samples (field numbers abbreviations – see Table 1).
The modern diagnosis of G. applanatum. In view of our new data on the boundaries and morphological variability of G. applanatum based on Saint Petersburg material, below we provide an updated diagnosis of this taxon. Outside the scope of this paper, the molecular type revision of southern applanatum-like polypores remains. Only after such revision it would be possible to compose any species keys in boreal guides.
Ganoderma applanatum (Pers.) Pat., Hyménomyc. Eur.: 143, 1887. – Boletus applanatus Pers., Observ. Mycol. 2: 2, 1800; Polyporus merismoides Corda in Sturm, Deutschl. Fl. 4: 139, 1837; P. stevenii Lév., Ann. Sci. Nat. Bot. 2: 91, 1844; P. megaloma Lév., Annls Sci. Nat., Bot., 1846; P. leucophaeus Mont. (ut ‘leucophaeum’), Syll. Gen. Sp. Crypt.: 157, 1856; P. incrassatus Berk., J. Linn. Soc. Bot. 16: 41, 1878; P. concentricus Cooke, Grevillea 9: 13, 1880; Fomes gelsicola Berl., Malpighia 3: 373, 1889; F. longoporus Lloyd, Mycol. Writ. 6: 940, 1920.
Basidiocarps perennial (up to 7 years in boreal forests), subfomitoid to applanate, sessile of arising from buried wood, woody to corky, in section triquetrous or ungulate; up to 70 cm in diam. and 10 cm thick near the base. The pilei often clustered, radiating, with orthotropic tendency on rosette-like formations. Upperside crustose, cinnamon, grayish or brown, some cracking and protruded insect pores, usually covered with rusty-brown spore deposits, appearing dusty, irregular to scrupose, radially zonate. Margin round, white, bolster-like, firstly thick, later more slender. Context corky, purplish-brown, usually mottled with whitish streaks and patches, with weak fruit odour. Hymenophore stratified: tube layers concolorous with context, separated by a layer of context tissue, each layer up to 1 cm thick. Pore surface white on fresh specimens, quickly bruising brown when handled, becoming dull buff with age, pores circular, 4–7 per mm.
Hyphal system dimitic with sympodially branched sclerohyphae (according to Zmitrovich, 2018) or tri-mitic (Corner, 1983). Generative hyphae 2–5 μm in diam., thin-walled, with clamps. Skeletal hyphae 3–6.5 μm in diam., thick-walled, brown, non-septate, occasionally branched, the extremities tapering to acute apices. Cystidia none. Basidia mostly 18–25 × × 8–10 μm, but occasionally with elongated bases, broadly clavate, 4-spored, some tapering abruptly to a narrow base, arising from intercalary positions on the subhymenial hyphae, with a basal clamp. Basidiospores 5.9–10.4 × 4.4–6.2 μm, ovoid, truncate at the distal end, with ornamented exosporium enveloping with transparent perisporium, inamyloid, faintly cyanophilous. Specimens associated with living trees usually have, on average, larger basidiospores.
Pathogenicity and control. G. applanatum combines two trophic strategies. It can grow as pathogenic saprotroph (Zmitrovich et al., 2015) which infests living trees and causes a chronic rot and as pure saprotroph which continues its development on dead trunks as well as stumps, dead and buried wood. As pathogen, G. applanatum is subject for control (Ţura et al., 2016).
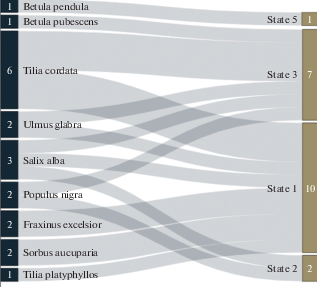
The Fig. 6 shows the Sankey-visualization of relationship of species belonging to tree hosts inhabited by model G. applanatum samples and state of the wood substrate (living trees, dead trees, stumps, fallen logs, buried wood). From this diagram, in particular, it can be seen that only as a saprotroph does the species manifest itself on zonal stand-formers (Betula spp.), while it exhibits pathogenic properties in broadleaf stands reproduced in Saint Petersburg under conditions of anthropogenic ground drainage. Prior to testing the (subsequently rejected) two-species hypothesis, there was a great temptation to associate the pathogenic populations with some southern Ganoderma species, whereas the saprotrophic ones with autochthonous boreal one. However, since it was turned out that only one species is widespread in Saint Petersburg areas, some other explanations are needed. One of them boils down to the fact that broadleaf species on water-rich soils are prone to ripening of wood and the formation of frost cracks at the base, where G. applanatum rushes. We studied frost-cracks in the model objects of Peter the Great Botanical Garden (Firsov et al., 2018). When a frost-crack is infested by ganodermas in the buttress area, the pathogen quickly captures the sapwood and core wood, and such a tree becomes threatening due to its tendency to easy windfall. As control measures, we proposed the furrowing the bark of spring shoots of broadleaf seedlings to prevent increased formation of frost-cracks as well as the preventing a degradation of drainage systems of broadleaf stands (Zmitrovich, Volobuev, 2019).
Fig. 6.
Sankey-visualization of relationship between species attribution of host trees inhabited by model specimens and the state of wood substrate. Substrate states: 1 – living trees, 2 – dead trees, 3 – stumps, 4 – fallen logs, 5 – buried wood.
The work was supported by the Program of basic research of the Russian Academy of Sciences (I.2.41), Project “Resource potential of plants and fungi of Russia”. The work of S.V. Volobuev was partially supported by the Grant of the President of the Russian Federation, project MK-3216.2019.11. The research was done using equipment of The Core Facilities Center “Cell and Molecular Technologies in Plant Science” at the Komarov Botanical Institute RAS (St.-Petersburg, Russia).
Список литературы
Bondartsev A.S. Polyporaceae of European part of USSR and Caucasus. Moscow, Leningrad, Academy of Science USSR, 1953 (in Russ.).
Bondartsev A.S., Singer R.A. A guide to the collection of higher basidiomycetes for their scientific study. Moscow, Leningrad, 1950 (in Russ.).
Bondartseva M.A. Definitorium fungorum Rossicum. Aphyllophorales 2. Albatrellaceae, Aporpiaceae, Boletopsidaceae, Bondarzewiaceae, Ganodermataceae, Corticiaceae (species porioideis), Lachnocladiaceae (species tubuli-ferae), Polyporaceae (genera poroidea), Poriaceae, Rigidoporaceae, Phaeolaceae, Fistulinaceae. Saint Petersburg, Nauka, 1998 (in Russ.).
Bondartseva M.A., Kotkova V.M., Zmitrovich I.V., Volobuev S.V. Aphyllophoroid fungi and heterobasidiomycetes of the Peter the Great Botanical Garden of the Komarov Botanical Institute (St. Petersburg). In: Botany: history, theory, practice (on the 300th anniversary of the founding of the V.L. Komarov Botanical Institute of the Russian Academy of Sciences). SPb., pp. 23–30 (in Russ.).
Corner E.J.H. Ad Polyporaceas I. Amauroderma and Ganoderma. Beih. Nova Hedwigia. 1983. V. 75. P. 1–182.
Donk M.A. Notes on European polypores – IV. On some species of Ganoderma. Proc. K. Ned. Akad. Wet. C. 1969. V. 72. P. 273–282.
Donk M.A. Check list of European polypores. Verh. Koninkl. Ned. Akad. Wet. Nat. Reeks. 1974. V. 62. P. 1–469.
Firsov G.A., Zmitrovich I.V., Bondartseva M.A., Bolshakov S.Yu., Volobuev S.V. Frost cracks of trees and basidiomycetes are the causative agents of chronic rot in the Peter the Great Botanical Garden. Hortus botanicus. 2018. V. 13. P. 160–194. https://doi.org/ (in Russ.).https://doi.org/10.15393/j4.art.2018.5042
Fries E.M. Elenchus fungorum, sistens commentarium in Systema mycologicum, I. Gryphiswald, 1828.
Gardes M., Bruns T.D. ITS primers with enhanced specificity for basidiomycetes application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993. V. 2. P. 132–118. https://doi.org/10.1111/j.1365-294x.1993.tb00005.x
Katoh K., Toh H. Improved accuracy of multiple ncRNA alignment by incorporating 16 structural 11 information into a MAFFT-based framework. BMC Bioinformatics. 2008. V. 9. № 13. https://doi.org/10.1186/1471-2105-9-212
Kozlov A.M., Darriba D., Flouri T., Morel B., Stamatakis A. RAxML-NG: A fast, scalable, and user-friendly tool for maximum likelihood phylogenetic inference // Bioinformatics. 2019. btz305 https://doi.org/10.1093/bioinformatics/btz305
Montagne J.F.C. Sylloge generum specierumque plantarum cryptogamarum. Paris, 1856.
Nilsson R.H., Kristiansson E., Ryberg M., Hallenberg N., Larsson K.-H. Intraspecific ITS variability in the kingdom Fungi as expressed in the international sequence databases and its implications for molecular species identification. Evolutionary Bioinformatics. 2008. V. 4. P. 193–201.
Parmasto E., Parmasto I., Möls T. Variation of basidiospores in the Hymenomycetes and its significance to their taxonomy. Bibltheca Mycol. 1987. V. 115. P. 1–168.
Persoon C.H. Observationes mycologicae. V. 2. Leipzig, Wolf, 1800.
Persoon C.H. Synopsis methodica fungorum. Gotting, 1801.
R Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, 2012. http://www.R-project.org.
R Packages. 2019. https://rpkgs.datanovia.com.
R Studio Team. RStudio: Integrated Development for R. RStudio, Inc., Boston, 2017. http://www.rstudio.com/Saleh-Rasti.
Ryvarden L. Type studies in the Polyporaceae. 11. Species des-cribed by J.F.C. Montagne, either alone or with other authors. Nord. J. Bot. 1982. V. 2. P. 75–84.
Ryvarden L., Gilbertson R.L. European polypores. Part 1. Abortiporus–Lindtneria. Synopsis Fung. 1993. V. 6. P. 1–387.
Ryvarden L., Melo I. Poroid fungi of Europe / with photos by T. Niemelä and drawings by I. Melo and T. Niemelä. Synopsis Fung. 2017. V. 37. P. 1–430.
Sankey Diagram Generator. 2019. http://sankey-diagram-generator.acquireprocure.com.
Scales 1.0.0. 2019. https://scales.r-lib.org.
Steyaert R.L. Genus Ganoderma (Polyporaceae). Taxa nova. I. Bull. Jard. Bot. l’État à Bruxelles. 1961. V. 31. P. 69–83.
Steyaert R.L. Considération générale sur le genre Ganoderma et plus spécialement sur les espèses européenes. Bull. Soc. Roy. Bot. Belg. 1967. V. 100. P. 189–211.
Steyaert R.L. The concept and circumscription of G. tornatum. Trans. Brit. Mycol. Soc. 1975. V. 65. P. 451–467.
Steyaert R.L. Study of some Ganoderma species. Bull. Gard. Bot. Nat. Belg. 1980. V. 50. P. 135–186.
Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013. V. 30. P. 2725–2729. https://doi.org/10.1093/molbev/mst197
Ţura D., Zmitrovich I.V., Wasser S.P. Wood-inhabiting fungi: Applied aspects. In: Fungi: Applications and management strategies. London etc., CRC Press, 2016, pp. 245–292.
Wasser S.P., Zmitrovich I.V., Didukh M.Ya., Spirin W.A., Malysheva V.F. Morphological traits of Ganoderma lucidum complex highlighting G. tsugae var. jannieae: The current generalization. Ruggell, A.R.A. Gantner Verlag K.-G., 2006.
Wickham H. Ggplot2: Elegant graphics for data analysis. Springer-Verlag, N.Y., 2009.
Zhukova E.A., Morozova O.V., Volobuev S.V., Bryantseva Yu.S. Basidiomycetous macromycetes and their influence on the state of green spaces in the gardens of the Russian Museum (St. Petersburg). Mikologiya i fitopatologiya. 2017. V. 51. № 6. P. 328–339 (in Russ.).
Zmitrovich I.V. Conspectus systematis Polyporacearum v. 1.0. Folia Cryptogamica Petropolitana. 2018. № 6. P. 3–145.
Zmitrovich I.V., Spirin W.A. Ecological aspects of speciation in higher fungi. Vestnik ekologii, lesovedeniya i landshaftovedeniya. 2005. № 6. 46–68 (in Russ.).
Zmitrovich I.V., Malysheva V.F., Malysheva E.F. The hyphal types of polyporoid and pleurotoid fungi: a terminology revision. Ukrainian botanical journal. 2009. V. 66. № 1. P. 71–87 (in Russ.).
Zmitrovich I.V., Wasser S.P., Ţura D. Wood-inhabiting fungi. In: Fungi from different substrates N. Y., Taylor and Francis group, 2015, pp. 17–74.
Zmitrovich I.V., Firsov G.A., Bondartseva M.A., Volobuev S.V., Bolshakov S.Yu. Basidiomycetes as causal agents of chronic rot of trees of the Peter the Great Botanical Garden of the Komarov Botanical Institute: diagnostics, biology, and distribution over the territory // Hortus botanicus. 2018. V. 13. P. 137–159. (in Russ.).https://doi.org/10.15393/j4.art.2018.5082
Zmitrovich I.V., Volobuev S.V. Xylobiont aphyllophoroid fungi in old growth parks of St. Petersburg. In: The life of palace gardens and parks: the problems of preserving historical plantings and the safety of visitors. SPb., 2019 (in Russ., in press).
Бондарцев А.С. (Bondartsev) Трутовые грибы Европейской части СССР и Кавказа. М.; Л.: АН СССР, 1953. 1106 с.
Бондарцев А.С., Зингер Р.А. (Bondartsev, Singer) Руководство по сбору высших базидиальных грибов для научного их изучения. М.; Л.: АН СССР, 1950. 73 с.
Бондарцева М.А. (Bondartseva) Определитель грибов России. Порядок афиллофоровые; Вып. 2. Семейства альбатрелловые, апорпиевые, болетопсиевые, бондарцевиевые, ганодермовые, кортициевые (виды с порообразным гименофором), лахнокладиевые (виды с трубчатым гименофором), полипоровые (роды с трубчатым гименофором), пориевые, ригидопоровые, феоловые, фистулиновые. СПб.: Наука, 1998. 391 с.
Бондарцева М.А., Коткова В.М., Змитрович И.В., Волобуев С.В. (Bondartseva et al.) Афиллофороидные и гетеробазидиальные грибы Ботанического сада Петра Великого Ботанического института им. В.Л. Комарова РАН (Санкт-Петербург) // Ботаника: история, теория, практика (к 300-летию основания Ботанического института им. В.Л. Комарова Российской академии наук): Труды международной научной конференции / Отв. ред. Д.В. Гельтман. СПб.: Изд-во СПбГЭТУ, 2014. С. 23–30.
Жукова Е.А., Морозова О.В., Волобуев С.В., Брянцева Ю.С. (Zhukova et al.) Базидиальные макромицеты и их влияние на состояние зеленых насаждений садов Русского музея (Санкт-Петерубрг) // Микология и фитопатология. 2017. Т. 51. № 6. С. 328–339.
Змитрович И.В., Спирин В.А. (Zmitrovich, Spirin) Экологические аспекты видообразования у высших грибов // Вестник экологии, лесоведения и ландшафтоведения. 2005. Вып. 6. С. 46–68.
Змитрович И.В., Малышева В.Ф., Малышева Е.Ф. (Zmitrovich et al.) Типы гиф полипороидных и плевротоидных грибов: терминологическая ревизия // Укр. бот. журн. 2009. Т. 66. № 1. С. 71–87.
Змитрович И.В., Фирсов Г.А., Бондарцева М.А., Волобуев С.В., Большаков С.Ю. (Zmitrovich et al.) Базидиомицеты – возбудители хронических гнилей деревьев Ботанического сада Петра Великого Ботанического института имени В.Л. Комарова РАН: диагностика, биология, распределение по территории // Hortus botanicus. 2018. Т. 13. С. 137–159.
Змитрович И.В., Волобуев С.В. (Zmitrovich, Volobuev) Ксилобионтные афиллофороидные грибы в старовозрастных парковых насаждениях Санкт-Петербурга // Жизнь дворцовых садов и парков: проблемы сохранения исторических насаждений и безопасности посетителей. СПб., 2019 (в печати).
Фирсов Г.А., Змитрович И.В., Бондарцева М.А., Большаков С.Ю., Волобуев С.В. (Firsov et al.) Морозобоины деревьев и базидиомицеты – возбудители хронических гнилей в Ботаническом саду Петра Великого // Hortus botanicus. 2018. Т. 13, 2018–5042. С. 160–194.
Дополнительные материалы отсутствуют.
Инструменты
Микология и фитопатология