Микология и фитопатология, 2020, T. 54, № 4, стр. 288-298
Diversity of slime moulds (Myxomycetes = Myxogastrea) in mountain tropical forests of the Phia Oac Reserve (Northern Vietnam) revealed by moist chamber cultures
N. A. Fedorova 1, *, Yu. K. Novozhilov 2, **, V. I. Gmoshinskiy 3, ***
1 Saint Petersburg State University
199034 St. Petersburg, Russia
2 Komarov Botanical Institute of the Russian Academy of Sciences
197376 St. Petersburg, Russia
3 Moscow State University
119992 Moscow, Russia
* E-mail: n.fedorova.a@gmail.com
** E-mail: yurinovozhilov@gmail.com
*** E-mail: rubisco@list.ru
Поступила в редакцию 3.11.2019
После доработки 5.12.2019
Принята к публикации 12.12.2019
Аннотация
The diversity of myxomycetes (plasmodial slime molds) associated with tropical mountain forests of Phia Oắc National Park in Northern Vietnam has been investigated during the field expedition in 2018. Surveys have been carried out in various plant communities at altitudes ranging from 1000 to 1850 m above sea level, where samples for moist chamber cultures were collected. In total, the survey included 306 records obtained from 310 moist chamber cultures prepared with samples taken from the bark surface of living trees and lianas, ground and aerial litter and coarse woody debris. There were 238 records of yielding myxomycete fructifications made. Determinations resulted in 42 taxa (41 morphospecies and 1 variety) from 17 genera and 8 families. One collection of Paradiacheopsis could not be clearly assigned to any described species. Eleven taxa were recorded for Vietnam for the first time, and all taxa were new for the nature reserve. The most significant diversity was observed for corticolous species with small sporocarps inhabiting the bark of living trees and lianas. Substrate cultures work best for the regions with boreal and arid climate. However, they fail to recover a part of the species diversity in tropical areas with a pronounced rainy season, and our results support this assumption.
INTRODUCTION
Vietnam is located on the Southeast Asian Indochinese Peninsula with a total land area of 330 991 square km. The country has an S-shaped outline and stretches over 3200 km from the North to the South. The Northeastern part of Vietnam is crossed by the Song Hong River (the Red River), which delta is surrounded by the highlands. There are several highest peaks in the area, all being a part of isolated granite mountain systems with an altitude of around 2000 meters. However, the rest of the territory to the northeast of the Red River Delta is of only moderate relief (Averyanov et al., 2003).
Myxomycetes (plasmodial slime moulds) are fungus-like organisms that can be easily found on decaying plant materials in various climate zones throughout the world. Although a lot of myxomycetes studies are conducted every year, very few of them cover the associations of tropical forests. Neotropics are well-studied (Farr, 1976; Stephenson et al., 2004; Lado et al., 2007; Rojas et al., 2010), yet there is little information considering Southeast Asia (Ko Ko et al., 2010; Dagamac et al., 2017; Ko et al., 2013), in particular Vietnam (Novozhilov et al., 2017a).
The MCC (moist chamber cultures) technique, first described in H.C. Gilbert and G.W. Martin (1933), can supplement the information obtained from collecting specimens that have fruited in the field under natural conditions and thus represents a major data source for diversity studies in myxomycetes (Eliasson, Lundqvist, 1979; Härkönen, 1977, 1978; Stephenson, 1985; Härkönen, Ukkola, 2000; Wrigley de Basanta et al., 2002). Results from the previous studies have indicated that the MCC technique can be sufficiently effective in revealing myxomycetes in different biomes of the world (Lado et al., 2013; Schnittler et al., 2013; Vlasenko et al., 2013), especially in arid regions (Novozhilov, Schnittler, 2008) and tropics (Stephenson et al., 2004) including tropical forests of Vietnam (Novozhilov et al., 2017a; 2020). First of all, this technique allows a better detection of species with minute fruiting bodies via a dissecting microscope. Moreover, it helps to overcome biases that result from the sporadic and ephemeral nature of myxomycete fruiting.
At the present moment 159 myxomycetes taxa (153 morphospecies and 6 variations) occur in southern Vietnam (Novozhilov et al., 2017a, 2020), including 97 species registered via MCC. Most of these species have been identified from the cultures set with such substrates as ground and air litter and bark of living trees, whereas myxomycetes fruiting bodies rarely appeared on the wood samples. The majority of the most common and widely known lignicolous myxomycetes, including various species of Arcyria, Lycogala, Stemonitis, and Trichia, can be predominantly found in the field.
The primary objectives of this study were to: (1) understand the degree to which substrate cultures are capable of detecting a local myxomycete species richness in various forests and on various substrate types; (2) test the hypothesis about substrate cultures that proved to be more effective in lower montane forests and with the bark of living trees; and (3) reveal any difference between assemblages of the species obtained from MCC in different forests and substrate types in the studied reserve. Moreover, we provide data on rare and new species of myxomycetes revealed via the MCC technique.
MATERIALS AND METHODS
Study area. The Nature Reserve Phia Oắc-Phia Đén (POPD) is situated in Nguyen Binh District, Cao Bắng Province of Vietnam and centered at 22°32′, 22°40′N and 105°49′, 105°57′E. The topography of the study area is dominated by a range of mountains from 900 to 2000 m a.s.l. including the summit Phia Oắc Mt (1935 m a.s.l.) and occupies 10 245.6 ha with mature and undisturbed forest being present on the 77% of the territory. This is a natural habitat for many endangered species, such as, in case of flora, Paphiopedilum emersonii and Xanthocyparis vietnamensis (Trần et al., 2014b).
The climate in the reserve shares the features of the Northern climatic zone of Vietnam. All four seasons are quite distinct; summers are hot and damp with abundant torrential rains of 1423 mm on the average (Le, 2005). The dry season, that extends from November to April, is particularly known for its freezing temperatures and occasional snowfalls. The mean annual temperature ranges from 20 to 22°C and the mean precipitation number equals to 1718 mm.
Such conditions are favorable for various forest types, especially for low and high-altitude montane forests. The highland area associated with Hoang Lien Son Range is outlined in modern biogeography as the southeastern part of Sikang-Yunnan floristic province of Holarctic floristic kingdom (Averyanov et al., 2003). Generally, mountain systems here are composed of magmatic silicate rocks, granite and quartzite in particular, and of solid, highly pressed and deeply metamorphosed sandstone with high amount of silicates consolidated with more or less large numerous quartzite dikes (Dovzikov et al., 1965a, b; Trần et al., 2014a).
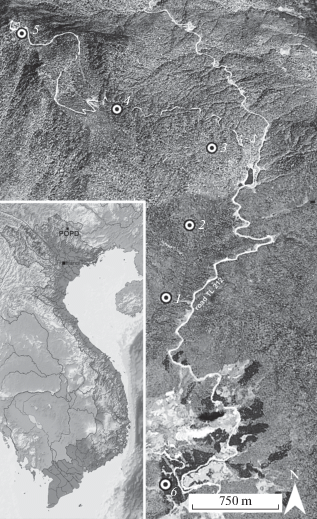
Study plots and habitat types. Substrate samples for MCC were obtained from six principal collecting plots situated along studied landscape profiles at different elevations in most typical and representative plant communities. The plot numbers refer to Fig. 1; nomenclature used for tree species follows (Averyanov et al., 2003). Geographical coordinates, elevation above sea level and slope exposure were measured for each site, using the GPS receiver of the iPhone 8 plus and the program Gaia v2020.3 for iOS. Moreover, brief characteristics of ground plant litter, coarse woody debris and bark of living trees (pH, texture, and coverage of epiphytic plants) were given.
Fig. 1.
Schematic map of the study area. Sampled localities are indicated by numbers and circles; the black square shows a geographical position of Phia Oắc-Phia Đen National Park (POPD). Source: Google Earth (modified).
A. Closed canopy evergreen tropical monsoon (seasonal) submontane broad-leaf forest. This vegetation type can be found at elevations between 1000 and 1400 m a.s.l., and three strata are distinguished here. Such broad-leaf trees as Castanopsis sp., Cinnamomum sp., Lithocarpus sp., Chisocheton paniculatus and Quercus sp. proved to be dominant in the first forest stratum, whilst taking in consideration only gentle slopes. The shrub stratum consists of bamboo Dendrocalamus sp., Musa sp., a woody fern Cyathea sp. and various giant grasses, that are especially common in open wet areas. The slope inclination differs from 3° to 40°. Well-drained montane dark-brown, gray-brown or brown, distinctly friable, rich in humus soils prevail. Ground leaf litter is 3–8 cm thick; last year litter remains are often found under fresh litter and appear to be 3–12 cm thick (commonly 4–8 cm), overlaid by a layer of fragments of unidentifiable leaves and twigs 1.5–3 cm thick. Plot 1: 22°35′25.16″N, 10°52′27.40″E, 950 m a.s.l.; Plot 2: 22°35′46.46″N, 105°52′38.89″E, 1200 m a.s.l.; Plot 3: 22°36′9.40″N, 105°52′50.20″E, 1350 m a.s.l.
B. Closed canopy evergreen tropical monsoon (seasonal) lower montane broad-leaf forest. This vegetation type can be found at elevations between 1600 and 1900 m a.s.l. The main dominant trees of the first forest stratum are broad-leaf trees like species of Acer, Archidendron, Cryptocarya, Eberhardtia, Eleocarpus, Exbucklandia, Ficus, Lithocarpus, Quercus, Litsea, Magnolia, Manglietia, Michelia, Rehderodendron, Rhoiptelea, Schima, Sideroxylon, Symplocos, etc. These trees are abundant on gentle slopes and can reach 20–30 m high. Their trunks are bent and twisted, and the bark is usually covered with dense mats of mosses and numerous epiphytic plants (Orchidaceae, Ericaceae, Pteridophyta). The understory layer contains dense bamboo thicket, which is of a middle height (1–2 m). The slope inclination is from 15° to 40°. Well-drained montane dark-brown, gray-brown or brown, distinctly friable, rich in humus soils prevail. Soils are rich in humus, principally well-drained montane brown to dark brown and are located on granite. Ground leaf litter is 2–5 cm thick; underneath there are often found the last year litter remains, which are from 4 to 8 cm thick and overlaid by fragments of unidentifiable leaves and twigs 2–7 cm thick. Plot 4: 22°36′24.03″N, 105°52′18.53″E, 1650 m a.s.l.; Plot 5: 22°36′50″N, 105°51′49.72″E, 1850 m a.s.l.
C. Artificial plantation of Pinus massoniana. The reserve is surrounded by a number of azonal artificial vegetation types that tend to have unusual plant communities with specific taxonomic composition. In this study the material was collected from the artificial plantation of P. massoniana. with separate trees of Lithocarpus sp. The lowest part of the transect is represented by trees 20–40 cm in diameter and 10–15 m in height. The slope inclination is 3–10°. As for soils, well-drained montane dark-brown and gray-brown, that are rich in humus, prevail. The majority of leaf litter is 3–5 cm thick; the last year litter remains are found to be 3–12 cm (usually 4–8 cm) thick, overlaid by fragments of needles, pine cones, leaves, and many pine twigs of 1.5–3 cm in diameter. Plot 6: 22°34′28.96″N, 105°52′18.22″E, 1030 m a.s.l.
Specimen collection and substrate sampling. All samples of plant organic material were collected during the field work in the middle of November, 2018. The bark samples were obtained from 10 different trees at each study site. The examined trees were located at least 10 meters apart, and a small, sharp-edged chisel was used to remove pieces of bark from living trees. Samples from a single tree had all been placed in a shared small paper bag. They were allowed to air-dry for a few days and then stored with silica gel. In the laboratory the moist chamber cultures were set up in the beginning of January, 2019 according to the classical protocol (Stephenson, Stempen, 1994; Novozhilov et al.; 2017b).
All myxomycete substrates were classified as following: “b” (98 samples) for bark of living trees, “l” for ground litter (53 samples) including leafy forest ground litter, decaying twigs, woody fruits of trees and lianas, litter of fleshy herbaceous plant parts such as shoots and leaves of Musa sp., located directly on the ground, thus taking up moisture from the soil; “alit” (9) for plant aerial litter attached to or trapped in the branches of living trees, lianas or giant grasses; “w” (51) for decaying coarse wood debris.
On the whole 310 Petri dishes have been prepared within 2–3 months after returning from the field survey. The first and the second authors took part in the moist chamber cultures preparation. Filter paper was placed in every dish, then covered with sufficient amount of substrate and fully moistened with distilled water adjusted to pH 7.0. Twenty-four hours later the pH values of waterlogged substrates were measured using a pH meter Hanna HI 98128. The experiment was divided into three stages separated in time. Cultures that took part in the first stage underwent examination on 8 occasions in a span of 3 months. Dishes set up for the following two parts of the method were examined 5 times. The examination was carried out using a dissecting microscope and all the data was recorded properly for the further usage in the ecological analysis. Sporocarps and particularly spores were preserved as semipermanent slides in polyvinyl lactophenol or prepared as temporary slides in watered-down potassium hydroxide in order to distinguish microscopic features. Air-dried sporocarps were studied with a Zeiss motorized stereo microscope (DM) Discovery V20. The material fixed on semipermanent and temporary slides was observed and measured with differential interface contrast (DIC) lenses mounted on a Zeiss Axio Imager A1 compound microscope via the program Axio Vision 4.8.0.0 (Carl Zeiss Imaging Solutions) or without DIC using Micromed 3 var. 3 LED M microscope via ToupView software.
Specimens were identified to the lowest possible taxonomic level according to (Martin, Alexopoulos, 1969) and various original descriptions from the literature (Farr 1976, Poulain et al., 2011) applying a morphospecies concept. Nomenclature followed Lado (2005–2020).
Fruiting bodies were mounted in herbarium boxes and are currently deposited in the herbarium of the Komarov Botanical Institute of Russian Academy of Sciences, Laboratory of Systematics and Geography of Fungi (LE).
Data analysis. The species identification was based on the morphological species concept. The myxomycetes which were still in the plasmodial stage were not considered.
In order to estimate the degree to which the survey was exhaustive, individual-based species accumulation curves (SAC) were plotted using the EstimateS version 9.0 software (Colwell, 2014). The number of recorded species to the number of expected species according to the Chao1 estimator (S × 100/Chao1) ratio was used to evaluate the local species inventory completeness. Graphs were created via SigmaPlot version 12.5 software.
The species abundance was rated on the basis of the adapted ACOR scale (Stephenson et al., 1993). It is based on the number of certain species records to the total number of records ratio: R – rare (<0.5%, 1 record for this survey), O – occasional (0.5–1.5%, 2–3 records), C – common (1.5–3%, 4–8 records), A – abundant (>3%, more than 8 records). Species diversity (alpha-diversity) was calculated using Shannon’s diversity index H' = –ΣPi – ln Pi, where Pi is the particular species relative abundance (the total number of individuals or records represented by the i-th species ratio), and inverse Simpson’s dominance index $D = {\text{1/}}\Sigma {\text{P}}_{i}^{2}$ (Magurran, 2004). The mean number of species per genus (S/G) was used as an indicator of the overall taxonomic diversity.
RESULTS AND DISCUSSION
The following checklist consists of all myxomycete species recorded during the present study that have been arranged alphabetically by genus and then species. Every taxon name is supported by the following data: 1) abundance according to the ACOR scale and total number of this species records from moist chamber cultures (given in brackets); 2) occurrence of the species in three different vegetation types and on four substrate types using the indication given above; 3) all localities where a species was found; 4) all or some (indicated by the string “...”) specimen numbers as given in the herbarium (LE).
An exclamation mark in superscript (!) indicates a species recorded as a new one for Vietnam. For the taxa already reported for Vietnam the references are given as numbers in superscript preceding the taxon name: 1 – Van Hooff (2009), 2 – Trần et al. (2014a), 3 – Novozhilov et al. (2017); 4 – Novozhilov et al. (2020).
1,2,3,4Arcyria cinerea (Bull.) Pers. [A, 15] A: 7, B: 1, C: 2; b: 3, l: 2, alit: 6; Loc. 1, 2, 3, 5, 6; LE324515, LE324519, LE324532. This is one of the most common and widely distributed of all myxomycetes and to date has been recorded in moist chamber cultures on different substrates in every survey carried out in Vietnam.
1,2,3,4A. marginoundulata Nann.-Bremek. et Y. Yamam. [A, 9] A: 9; b: 1, l: 8; Loc. 1, 2, 3; LE324482, LE324484, LE324538. The specimens are morphologically similar to the collections found in the lowland forests of the following national parks: Cát Tiên, Bidoup-Núi Bà and Chư Yang Sin. We have previously given a detailed description of this species morphology and its difference from Arcyria cinerea and A. globosa Schwein (Novozhilov et al. 2017).
2,3,4Clastoderma debaryanum A. Blytt [A, 9] A: 3, B: 5, C: 1; b: 3, w: 6; Loc. 2, 4, 5, 6; LE324485, LE324495, LE324527.
2,3,4Collaria arcyrionema (Rostaf.) Nann.-Bremek. ex Lado [R, 1] A: 1; w: 1; Loc. 1. LE324548
!C. rubens (Lister) Nann.-Bremek. [R, 1] A: 1; l: 1; Loc. 2 LE324548 (Fig. 2, a, b). The sporocarps were observed in a small group, 1.5–2.3 mm in height, long-stalked. The sporotheca is ovate, pinkish-brown, 0.3–0.5 mm wide. The stalk is 1.0–1.5 mm in length, continuously tapering upwards, black, fibrous under the microscope, opaque. The peridium is fugacious, except for the conspicuous collar at the sporotheca base, smooth and very pale brown being examined via transmitted light microscopy. The columella is reaching to 2/3 of the sporangial height. The capillitium is formed by 4–6 main branches throughout the entire columella, with relatively sparse, curved and slender threads, ending freely at the periphery without any surface net, pale brown under the microscope. The spores are in mass pinkish-brown and very pale brown in the light, globose, minutely spinulose, (7)7.5–7.8(8) μm in diameter. The specimen fits the description given by Nannenga-Bremekamp (1991) except for the collar which should have been more prominent in our case.
Fig. 2.
Collaria rubens (LE324548): a sporocarp, as visible with the light microscope (LM); b – the same sporocarp (LM); Colloderma oculatum [(LE324529 (c, d, f, g); LE324480 (e)]: с – spores (LM); d – capillitium and spores (LM); e – sporocarp on the early stage of development under the dissecting microscope (DM); f – fully developed sporocarp (DM); g – scanning electron micrograph (SEM) of capillitium and spores; Comatricha laxa (LE324512): h – spherical sporocarp (DM); i – ovoid sporocarp (DM); j – capillitium and spores (LM); k – capillitium and spores; Hemitrichia leiotricha (LE324643): l – sporocarp (DM); m – end of the capillitial thread (LM); n – capillitium and spores (LM). Scale bars: h, l – 200 μm; b, e, f, i – 100 μm; a, k – 50 μm; c, d, g, j, m, n – 10 μm.
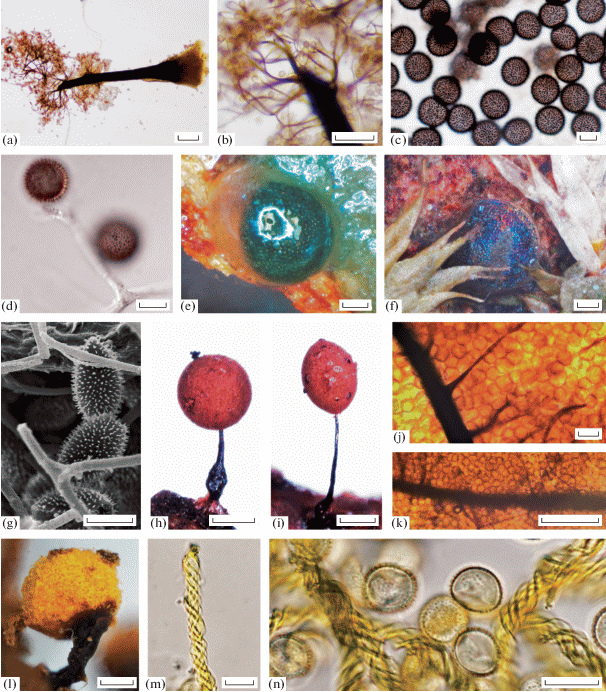
!Colloderma oculatum (C. Lippert) G. Lister [O, 2] A: 1, B: 1; b: 1, w: 1; Loc. 2, 4; LE324480, LE324529 (Fig. 2, c–g). The sporocarps are sessile and widely scattered, globose, 0.2–0.4 μm in diameter, covered by a gelatinous peridial outer layer when fresh and iridescent with brilliant blue colours after drying. The peridium is very thin, colourless and smooth under the microscope. A columella is absent. The capillitium is represented by a branched network arising from the sporocarp base, with colourless to pale violet-brown, mostly free-ending threads, which are very lax, 0.5 to 1.5 μm wide, with branching ends and almost colourless finest tips, sometimes with darker zones up to 3 μm thick (Fig. 2, g). The structure is also surrounded by a fine, inconspicuous, colourless sheath (Fig. 2d). The spores are dull brown to black in mass, dusty violet-brown under the microscope (Fig. 2, c), globose, ornamented with regularly distributed spines, (10.5)11–12.5(13) μm in diameter.
Apparently rare in tropics. Currently in Vietnam this species was found only in POPD and cultivated in moist chamber cultures on a substrate consisting of mosses from living tree bark and coarse wood debris. In the northern hemisphere it is often associated with Physarum viride, Lepidoderma tigrinum, Lamproderma columbinum, and Barbeyella minutissima (Schnittler et al., 2000). All these species form a distinct ecological guild on large wet coarse coniferous wood debris covered by mosses and liverworts in the boreal forests (Schnittler, Novozhilov, 1998). The interesting thing is, in spite of our intensive studies in the montane forests of the Đà Lạt Plateau we could not find this species in suitable habitats. Large mossy wet pine logs, where we were able to register other species from the guild mentioned above, did not yield any Colloderma oculatum specimens (Novozhilov et al., 2020).
3,4Comatricha elegans (Racib.) G. Lister [R, 1] A: 1; w: 1; Loc. 2. LE324559.
!C. laxa Rostaf. [R, 1] A: 1; l: 1; Loc. 3. LE324512 (Fig. 2h–k). Our single collection represents a typical form with the ovoid or short cylindrical reddish-brown sporotheca. The capillitium is composed of main parallel branches that begin throughout the entire columella at right angles without forming a surface net and have many pointed free ends.
3,4C. pulchella (C. Bab.) Rostaf. [O, 2] A: 2; l: 2; Loc. 2; LE324545, LE324647.
3,4C. spinispora Novozh. et D. W. Mitch. [C, 4] A: 4; b: 1, l: 3; Loc. 2, 3. LE324500, LE3245002, LE324509. The sporocarps are long-stalked with small (0.15–0.4 mm in diam.), pinkish-grey, subglobose or ovoid sporotheca. The spores are ornamented by scattered long spines 0.5–0.8 μm long with coral-like tips consisting of 6–8 very small additional spines which are the key characters of this species. Comatricha spinispora is mostly found on leafy litter from Lithocarpus sp. and Quercus sp. accumulations in high montane cloud forests (Novozhilov et al., 2014; Novozhilov et al., 2020).
3,4C. confusa Nann.-Bremek. et Y. Yamam. [O, 3] A: 1, C: 2; b: 3; Loc. 2, 6; LE324518, LE324602, LE32462.
2,3,4C. microcarpa (Schrad.) Pers. [A, 28] A: 16, B: 8, C: 4; b: 5, l: 2, w: 21; Loc. 1, 2, 3, 4, 5, 6; LE324488, LE324513, LE324521…
1,2,3C. tecta Hooff [R, 1] A: 1; b: 1; Loc. 1. LE324581. This species distinguishing characteristic is the sporotheca morphology with a convex lid, which forms a “parachute-like” structure attached by threads to the calyculus.
1,2,3,4C. violacea Rex [O, 3] A: 3; b: 1, alit: 1, w: 1; Loc. 1, 3. LE324514, LE324576, LE327815.
1,2,3Diachea leucopodia (Bull.) Rostaf. [O, 3] A: 2, C: 1; b: 1, l: 2; Loc. 3, 6. LE324505, LE324506, LE324668.
!Diderma chondrioderma cf (de Bary et Rostaf. ) G. Lister [R, 1] A: 1; b: 1; Loc. 2. LE324526. Our collection is represented by the typical effused plasmodiocarps 1–2 mm in diam., with the scanty columellae and the dark-brown capillitial filaments with several expansions. The spores are brown in mass, pale brown in transmitted light, (11)12–13(14) μm in diam., spinulose.
3,4D. deplanatum Fr. [A, 22] A: 11, B: 2, C: 9; b: 18, l: 4; Loc. 1, 2, 3, 5, 6. LE324517, LE324603, LE324669…
4Didymium difforme (Pers.) Gray [C, 5] A: 3, C: 2; b: 5; Loc. 1, 2, 6. LE324528, LE324600, LE324659…
2,3,4D. floccoides Nann.-Bremek. et Y. Yamam. [R, 1] A: 1; b:1; Loc. 1. LE324580.
3,4D. iridis (Ditmar) Fr. [C, 4] A: 2, B: 2; b: 3, alit: 1; Loc. 1, 5. LE324578, LE324589, LE324599.
2,3,4D. nigripes (Link) Fr. [R, 1] A: 1; b: 1; Loc. 1. LE324575.
1,2,3,4Echinostelium minutum de Bary [A, 17] A: 4, B: 2, C: 11; b: 9, l: 3, w: 5; Loc. 1, 2, 6; LE324481, LE324492, LE324537.
!Hemitrichia leiotricha (Lister) G. Lister [R, 1] A: 1; b: 1; Loc. 2; LE324643 (Fig. 2, l–n). There was only one specimen found. Some features bring him closer to H. intorta (Lister) Lister including the short-stalked ochraceum-yellow sporotheca, which is brown towards the base, and the dark brown stalk which length equals to the ½ of sporocarp. The spores are yellow in transmitted light, 8–10 μm in diam., verrucose. However, what distinguishes the sample from typical H. intorta is the almost complete absence of spines on the capillitium threads surface. They are orange-yellow, long, 3–4 μm in diam. and ornamented only with spirals, which are more typical for H. leiotricha.
3,4H. pardina (Minakata) Ing [R, 1] A: 1; b: 1; Loc. 2. LE324535.
2,3,4H. serpula (Scop.) Rostaf. ex Lister [R, 1] A: 1; b: 1; Loc. 1. LE327819.
!Licea bulbosa Nann.-Bremek. et Y. Yamam. [A, 17] A: 5, B: 12; b: 1, l: 16; Loc. 2; LE324472, LE324473, LE324474 (Fig. 3, a-g). One of the most common litter inhabiting species in the study area. It is easily recognizable by the transparent base of the sporotheca and the darkened brownish band below the area of dehiscence, the metallic, glossy lid of peridium bearing knobs on its inner surface (Fig. 3, c). In our specimens, the equatorial thinner band was observed as a light line in developing sporocarps as soon as the sporotheca formed as a transparent bulb at the top of the stalk (Fig. 3, a), before the spore formation and the darkening of the upper half. The inner peridial layer is membranous and smooth except for the area of dehiscence, where some warts can be seen (Fig. 3, d). The spores are free, pale yellow or greyish green to almost hyaline, globose, 10–11 μm in diam., smooth; spore wall is evenly thick (Fig. 3, g).
Fig. 3.
Licea bulbosa [LE 324473 (a, c); LE324472 (b, d, e, f, g)]: a – sporocarps (DM); b – outer side of peridium and spores (SEM); c – sporocarp (DM); d – spore ornamentation (SEM); e – sporocarp with scattered spores (LM); f – sporocarp with scattered spores (SEM); g – spores (LM); Paradiacheopsis cf. sp (LE324507): h – sporocarp (SEM); i – sporocarps (DM); j – stalk base and spores (LM); k – peridium and spores (LM); l – spore surface (SEM); m – peridium and spores (SEM); Trichia ambigua [LE324652 (n,p); LE324613 (o); LE324630 (q, r)]: n – sporocarps (DM); o – sporocarps (DM); p – capillitium and spores (LM); q – spore ornamentation (SEM); r – capillitium thread (SEM); Trichia decipiens var. hemitrichioides (LE324634): s – sporocarp (DM); t – capillitium thread (SEM); u – spore ornamentation (SEM); v – capillitium thread (LM). Scale bars: o – 500 μm; i, n, s – 200 μm; a, b, c, e – 100 μm; h, f – 50 μm; j, k, p, v – 10 μm; b, m – 5 μm; t, r – 2 μm; d, q, u – 1 μm; l – 0.5 μm.
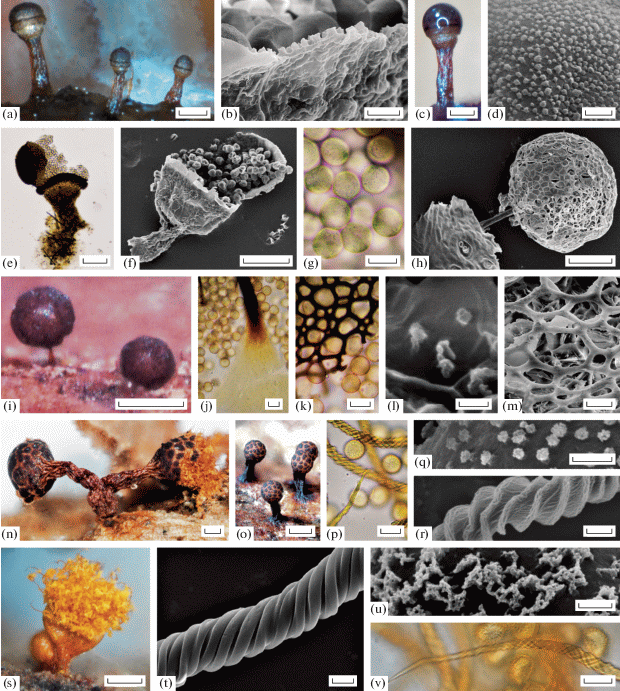
2,3,4L. operculata (Wingate) G. W. Martin [A, 19] A: 11, B: 6, C: 2; b: 17, l: 1, w: 1; Loc. 1, 2, 6; LE324469, LE324470, LE324471.
3,4L. pygmaea (Meyl.) Ing [O, 2] A: 1, B: 1; w: 2; Loc. 2; LE324556, LE324639.
!Macbrideola cornea cf (G. Lister et Cran) Alexop. [R, 1] A: 1; b: 1; Loc. 2; LE324524. Our specimen consists of only several sporocarps with typical brown-reddish, tubulate, horny, translucent stalks, a well-developed capillitium and the peridium remaining as a small basal collar. However, due to scanty material there should be a more detailed phylogenetic revision of the specimen undertaken to confirm such a tentative determination.
!Paradiacheopsis cf. sp. A: 1; l: 1; Loc. 3. LE324507 (Fig. 3, h–m). This morphospecies was found only once on the leaf litter and does not fit into any known species of myxomycetes, hence it may represent a new taxon. Our specimen includes a group of well-developed stipitate, reddish-browgn, slightly shiny, globose sporocarps 250–300 μm high and 150–250 μm in diam (Fig. 3, i). The stalk is reddish-yellow at the base, dark brown above, fibrous (Fig. 3, j). The peridium is covered by the thickenings of the dark-brown flatted ribs that merge into a reticulum with small round meshes 5–15 μm in diam. The net might be filled with the remnants of the peridial membranous inner part (Fig. 3, k, m). The spores are dark brown in mass, brown-lilac in the transmitted light, globose, free, 8–10 µm in diam. The spore wall is covered with rough scattered warts (Fig. 3, l).
This taxon has the unique peridium and capillitium morphology. A detailed molecular-phylogenetic study is required to find out the correct taxonomical position of this species.
4Paradiacheopsis longipes Hoof et Nann.-Bremek. [A, 15] A: 14, B: 1; b: 3, l: 5, alit: 1, w: 6; Loc. 1, 2; LE324498, LE324499, LE324501. The key characters of this species are a very long slender stalk which is about 7/8–9/10 of the sporocarp total height and the loose capillitium threads spreading out from the top of the columella and branched without any anastomoses. The spores are pale grey in the transmitted light, 7–9 μm in diam. and ornamented by scattered warts.
4Paradiacheopsis solitaria (Nann.-Bremek.) Nann.-Bremek. [C, 4] A: 1, C: 3; b: 4; Loc. 2, 6; LE32453, LE324608, LE324610.
!Perichaena calongei Lado, D. Wrigley et Estrada [O, 2] A: 2; alit: 2; Loc. 1; LE324586, LE324661. Our specimens have some very distinctive characters of this morphospecies including the dark-edged polygonal peridial plates and the peridium petaloid dehiscence as well as the typical capillitium ornamentation represented by short spines (0.5–2 μm long) and a reticulum.
3,4P. dictyonema Rammeloo [R, 1] A: 1, B: 0, C: 0; alit: 1; Loc. 1; LE324660.
3P. pedata (Lister et G. Lister) Lister ex E. Jahn [O, 2] A: 2; l: 1, alit: 1; Loc. 1; LE324590, LE327805.
2,3,4Physarum album (Bull.) Chevall. [R, 1] A: 1; l: 1; Loc. 2; LE324550.
1,2,3,4Ph. melleum (Berk. et Broome) Massee [R, 1] A: 1; l: 1; Loc. 1; LE 324568.
3,4Ph. oblatum T. Macbr. [R, 1] A: 1; w: 1; Loc. 2; LE 324648.
1,2,3,4Ph. pusillum (Berk. et M.A. Curtis) G. Lister [R, 1] A: 1; w: 1; Loc. 1; LE324563.
2,3Ph. roseum Berk. et Broome [R, 1] B: 1; b: 1; Loc. 4; LE324632.
!Trichia ambigua Schirmer, L.G. Krieglst. et Flatau [A, 8] A: 1, C: 7; b: 7, w: 1; Loc. 2, 6; LE324613, LE324624, LE324628 (Fig. 3, n–r). Our collection consists of numerous sporocarps with some typical features for this species. The sporangia are gregarious to fascicled, oval, ovoid to obovoid, 0.6–1.2 in diam., up to 2 mm high, clay-coloured to brown or even reddish brown (Fig. 3, o). Stipe is rugose, opaque, brown to dark reddish (Fig. 3, n). The peridium is double, with the inner peridial layer firmly attached to the outer layer, light yellowish, transparent. The peridial outer layer possesses more or less distinct dark granular thickenings in the sporocarp upper part, forming an areolate pattern (5–8 thickenings) throughout the whole sporotheca (Fig. 3, o). The polygonal, partly roundish meshes break open along the yellow dehiscent lines. After dehiscence the peridium remains as a deep cup. The capillitial elaters are inelastic, 4–5 μm in diam., decorated with 4–5 distinct spiral bands (Fig. 3, r), often with weak thickenings before the ends. The elaters ends are medium long (30–50 μm), pointed, yellow or olive yellow, brownish-yellow to light red-brown in TL. The spores are light olive-yellow in mass (Fig. 3, n), globose, 11–12.5 μm in diam. and verrucose (Fig. 3, p). The warts are grouped in small clusters, that are evenly distributed over the spore surface (Fig. 3, q). Macroscopically this species resembles T. botrytis (J.F. Gmel.) Pers., T. flavicoma (Lister) Ing, T. munda (Lister) Meyl., and T. subfusca Rex. However, T. botrytis differs in the peridial pattern of large areolations and in highly elongated elaters. T. flavicoma sporocarps have a small-areolated pattern on the peridium, but are much smaller and do not form clusters. The sporocarps of T. munda are very tiny and have never been observed in clusters too. T. subfusca differs from T. ambigua by the lack of the areolate pattern and very short, slightly elongated elater ends (Schirmer et al., 2015).
!T. decipiens var. hemitrichioides Brandza [R, 1] B: 1; b:1; Loc. 4. LE324634 (Fig. 3, t-v).
Our specimen consists of several small sporocarps 0.8–1.0 mm high (Fig. 3, s) with typical characters of this rare variety of T. decipiens (Pers.) T. Macbr. The spores are bright yellow in mass (Fig. 3, s), pale yellow in the transmitted light; 11–12 μm in diam., marked with a delicate reticulum of warts (Fig. 3, u).
4T. scabra Rostaf. [R, 1] B: 1; b:1; Loc. 4. LE324633.
On the whole there were 238 records made, including undetermined species, and 210 specimens were clearly assigned to the already known ones. However, one specimen was not recognized as a described morphospecies, but assumed to be Paradiacheopsis cf. sp. So, there was a putatively new species among 42 taxa. There was the possibility of determining specimens only to generic level as material was either scanty or damaged by fungi material in 26 cases. Almost half of all taxa (21) were classified as rare for the whole study area (accounting for less than 0.5% of all records) and all of them were represented by singletons. This study reports 11 species (including one variation) found in Vietnam for the first time and 42 taxa registered as new for Phia Oắc-Phia Đen National Park.
The moist chamber cultures productivity including non-fruiting plasmodia (calculated as a moist chamber cultures yielding myxomycete fructifications and/or plasmodia to the total number of cultures ratio) varied to some extent. Myxomycete fructifications were observed in ca. 40–50% of the prepared moist chambers, with the lowest number for the artificial plantation (C, 37.9%). Much fewer chambers were positive for plasmodia and the numbers fluctuated from 5.8% for lower montane forest (B) to 25.8% for submontane ones (A).
The completeness of the fulfilled study was evaluated with the Chao1 estimator (Fig. 4), which showed the exhaustive revelation (98%) of the artificial plantation myxomycete diversity (vegetation type C). However, this trend may be explained by the small number of prepared moist chambers (66). Considering the data as a whole, the sampling effort did not prove to be sufficient: the Chao 1 estimator suggested that only half of the predicted species inventory was revealed (43 species, 78 estimated). Some figures for the partial data represented the same trend: estimators both for wood (w, 14 species, 51 records) and submontane broad-leaf forests (A, 40 species, 123 records) displayed respectively 50.5% and 48.9% of survey efficiency. The lowest number was obtained for the bark: only 17.7% of completeness was reached (b, 29 species, 97 records), however the standard deviation was also high (67.4). These observations might be explained by the multiple myxomycete assemblages in heterogenic microhabitats on this substrate type (H' = 2.80). Predictably, submontane forests appeared to be the most diverse according to the Shannon index (H' = 3.16).
Fig. 4.
The graphs of the three vegetation types, including all records from the whole studied area (4a), and three of four studied substrate types (4b). The thick lines indicate individual-based species accumulation curves and the thin jagged lines show the Chao1 (mean) estimator value of expected morphospecies richness.
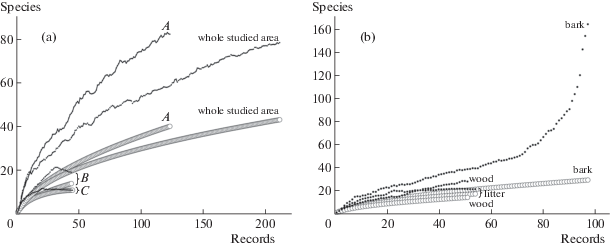
Regarding the substrate types, diversity declined from bark (H' = 2.80) over litter (H' = 2.38) and wood (H' = 2.01) to aerial litter (H' = 1.89). The similar trend held true for the number of species per MC, starting with bark (0.76), decreasing over litter (0.68) and aerial litter (0.60), although the wooden substrate displayed the smallest value (0.57). The distribution remained the same considering the species to genus ratio (Table 1).
Table 1.
Statistical data for myxomycetes obtained from moist chamber cultures on four substrate types (bark, litter, aerial litter and wood) and from three different vegetation types
| Parameters | A | B | C | b | l | alit | w |
|---|---|---|---|---|---|---|---|
| Rec | 123 | 44 | 44 | 97 | 54 | 9 | 51 |
| Sp | 40 | 14 | 11 | 29 | 17 | 7 | 14 |
| G | 17 | 11 | 11 | 15 | 11 | 5 | 11 |
| Sp/G | 2.35 | 1.27 | 1.00 | 1.93 | 1.55 | 1.40 | 1.27 |
| Chao1 | 81.82 | 19.13 | 11.20 | 163.60 | 21.12 | 9.96 | 27.73 |
| SD | 24.56 | 5.26 | 0.61 | 67.4 | 4.27 | 3.72 | 13.00 |
| Comp (%) | 48.89 | 73.18 | 98.21 | 17.73 | 80.49 | 70.28 | 50.49 |
| H' | 3.16 | 2.21 | 2.10 | 2.80 | 2.38 | 1.89 | 2.01 |
| D | 15.91 | 6.72 | 6.59 | 10.80 | 7.25 | 6.23 | 4.65 |
| MC | 155 | 89 | 66 | 127 | 79 | 15 | 89 |
| % MC (spc) | 51.60 | 41.60 | 37.90 | 50.40 | 40.50 | 53.30 | 42.70 |
| % MC (pls) | 25.80 | 5.80 | 9.10 | 13.40 | 36.70 | 20.00 | 16.90 |
| Sp per MC | 0.79 | 0.49 | 0.67 | 0.76 | 0.68 | 0.60 | 0.57 |
| SD | 1.10 | 0.71 | 1.01 | 1.08 | 1.12 | 0.74 | 0.75 |
Note. Rec – number of myxomycete specimens identified to species level; Sp – number of morphospecies; G – number of genera; Sp/G – species/genus ratio; Chao1 – individual-based richness estimator; SD – standard deviation of Chao1; Comp (%) – degree of completeness that was achieved for the data set (recorded species/Chao 1 ratio); H' – Shannon index of diversity; D – Simpson (inverse) diversity index; MC – number of the prepared moist chamber cultures; % MC (spc) – number of positive moist chamber cultures in where sporocarps have developed; % MC (pls) – number of positive moist chamber cultures where plasmodia were registered; Sp per MC – the mean value of the species number per one moist chamber culture; SD – the mean value standard deviation of the species number per one moist chamber culture; abbreviations of the vegetation and substrate types as given in the text.
The species showing the highest rate of occurrence was Cribraria microcarpa with the total of 28 records and 21 of them being found on the wood. At the same time, the most abundant species on the bark was Diderma deplanatum (18 records out of 22). Licea bulbosa was unambiguously associated with ground litter (16 out of 17) and L. operculata was predominantly corticulous (17 out of 19). At the same time, Paradiacheopsis longipes did not reveal any substrate-based trend, since it was recorded on the all represented types, but common only in submontane forests (14 out of 15). Such a trend held true for Arcyria marginoundulata too (9 out of 9), although without any records from either aerial litter or bark. Echinostelium minutum was quite abundant in all described categories, including 4 substrate and 3 habitat types.
The MCC technique demonstrated its effectiveness in revealing corticolous myxomycetes in different biomes (Novozhilov et al., 2000). The number of revealed species and moist chambers that proved to be positive rise from tropical forests, over boreal and deciduous broad-leaf forests, over Mediterranean forests and ultimately to grasslands and deserts (Novozhilov et al., 2017b). A high epiphyte cover seems to be one, but not the only reason for the low abundance and diversity of corticolous myxomycetes in the tropics (Schnittler, Stephenson, 2000). The most possible explanation of this phenomenon is excess rainfall in connection with the fact that the bark surface of tree trunks in closed-canopy cloud forests rarely dries out and is often extensively covered with liverworts and mosses. Furthermore, heavy tropical rains probably remove myxomycete plasmodia and spores from the bark surface. In addition to this mechanical factor, a leaching effect caused by the sheer amount of rainfall cannot be ruled out, with soluble nutrients and microorganisms being removed from the bark. Overall, our results obtained for lower montane forest of Phia Oắc Nature Reserve appeared to support this hypothesis (in case of disregarding the excessive and overestimated study completeness of the artificial plantation). Further investigations including the field collection review are required to reveal the more accurate myxomycetes species inventory in Phia Oắc Nature Reserve.
We gratefully acknowledge the technical support (SEM) provided by Ludmila A. Kartzeva (The Core Facility Center “Cell and Molecular Technologies in Plant Science” at the Komarov Botanical Institute RAS, St. Petersburg, Russia). The authors are grateful to the administration of the Joint Russian-Vietnamese Tropical Research and Technological Centre. Expeditions and laboratory work was supported by the program Ecolan-1.2 of the Russian-Vietnamese Tropical Research and Technological Centre, the program “Taxonomic diversity, ecology and physiological and biochemical features of fungi and fungus-like protists of Vietnam” AAAA-A19-119080990059-1 (Komarov Botanical Institute RAS), and by Russian Foundation for Basic Research (project 18-04-01232 А). The work of the third author was supported by the Moscow State University Grant for Leading Scientific Schools “Depository of the Living Systems”.
Список литературы
Averyanov L.V., Loc P.K., Hiep N.T., Harder D.K. Phytogeographic review of Vietnam and adjacent areas of Eastern Indochina. Komarovia. 2003. V. 3. P. 1–83.
Chao A., Chazdon R.L., Colwell R.K., Shen T.J. A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecology Letters. 2005. V. 8 (2). P. 148–159. https://doi.org/10.1111/j.1461-0248.2004.00707.x
Chao A., Chazdon R.L., Colwell R.K., Shen T.J. Abundance-based similarity indices and their estimation when there are unseen species in samples. Biometrics. 2006. V. 62 (2). P. 361–371. https://doi.org/10.1111/j.1541-0420.2005.00489.x
Colwell R.K. EstimateS 9.10 User’s Guide. 2014. http://viceroy.eeb.uconn.edu/estimates
Dagamac N.H.A., Stephenson S.L., Dela Cruz T.E.E. Occurrence, distribution and diversity of myxomycetes (plasmodial slime moulds) along two transects in Mt. Arayat National Park, Pampanga, Philippines. Mycology. 2012. V. 3 (2). P. 119–126.
Dagamac N., Dela Cruz T.E., Rea-Maminta M.A.D., Cruz A.D., Jeane V., Schnittler M. Rapid assessment of myxomycete diversity in the Bicol Peninsula, Philippines. Nova Hedwigia. 2017. V. 104 (1–2). P. 31–46. https://doi.org/10.1127/nova_hedwigia/2015/0252
Dagamac N., Heherson A., Novozhilov Y.K., Stephenson S., Lado C., Rojas C., Dela Cruz, T.E., Unterseher M., Schnittler M. Biogeographical assessment of myxomycete assemblages from Neotropical and Asian Palaeotropical forests. Journal of Biogeography. 2017. V. 44 (7). P. 1524–1536. https://doi.org/10.1111/jbi.12985
Dovzikov A.E. et al. Geological map of Vietnam 1:500 000. Main Geological Department of DRV, Hanoi, 1965a.
Dovzikov A.E. et al. Geology of the North Vietnam. Description for geological map of the North Vietnam 1:500 000. Main Geological Department of DRV, Hanoi, 1965b (in Russ.).
Eliasson U., Lundqvist N. Fimicolous myxomycetes. Bot. Not. 1979. V. 132. P. 551–568.
Farr M.L. Flora Neotropica Monograph No. 16 Myxomycetes. New York Botanical Garden, New York, 1976.
Gilber H.C., Martin G.W. Myxomycetes found on the bark of living trees. Univ. Iowa Stud. University Iowa Stud. Nat. Hist. 1933. V. 15 (3). P. 3–8.
Härkönen M. Corticolous myxomycetes in three different habitats in southern Finland. Karstenia. 1977. V. 17 (1). P. 19–32.
Härkönen M. On corticolous myxomycetes in Northern Finland and Norway. Annales Botanici Fennici, Finnish Botanical Publishing Board. 1978. V. 15. P. 32–37.
Härkönen M., Ukkola, T. Conclusions on myxomycetes compiled over twenty-five years from 4793 moist chamber cultures. 2000.
Ko Ko T.W., Tran H.T.M., Clayton M.E., Stephenson S.L. First records of myxomycetes from Laos. Nova Hedwigia. 2013. V. 96 (1–2), P. 73–81.
Ko Ko T.W., Tran H.T.M., Stephenson S.L., Mitchell D.W., Rojas C., Hyde K.D., Lumyoung S. Myxomycetes of Thailand. Sydowia. 2010. V. 62. P. 243–260. https://doi.org/10.1127/0029-5035/2012/0047
Lado C., Estrada-Torres A., Stephenson S.L. Myxomycetes collected in the first phase of a north-south transect of Chile. Fungal Diversity. 2007. V. 25. P. 81–101. https://doi.org/10.13039/100000001
Lado C., Wrigley de Basanta D., Estrada-Torres A., Stephenson S.L. The biodiversity of myxomycetes in central Chile. Fungal Diversity. V. 59. P. 3–32. 2013. https://doi.org/10.1007/s13225-012-0159-8
Lap V.T. Natural geography of Vietnam. Education Publishing House, Ha Noi, 1999.
Le V.C. Contribution to Mammal Study in Phia Oac, Cao Bang Province. Ph.D. Thesis, Hanoi, Vietnam, 2005 (in Vietn.).
Magurran A.E. Measuring biological diversity. Blackwell, Oxford, 2004.
Martin G.W., Alexopoulos C.J. The myxomycetes. University of Iowa Press, Iowa City, 1969.
Novozhilov Yu.K., Mitchell D.W. A new species of Comatricha (Myxomycetes) from southern Vietnam. Novosti Sist. Nizsh. Rast. 2014. V. 48. P. 188–195.
Novozhilov Yu.K., Erastova D A., Shchepin O.N., Schnittler M., Alexandrova A.V., Popov E.S., Kuznetov A.N. Myxomycetes associated with monsoon lowland tropical forests in southern Vietnam. Nova Hedwigia. 2017a. V. 104(1), P. 143–182. https://doi.org/10.1127/nova_hedwigia/2016/0395
Novozhilov Yu.K., Rollins A., Schnittler M. Ecology and distribution of Myxomycetes. In: S.L. Stephenson (ed.). Myxomycetes. Academic Press, London, 2017b. P. 253–297.
Novozhilov Yu.K., Schnittler M. Myxomycete diversity and ecology in arid regions of the Great Lake Basin of western Mongolia. Fungal Divers. 2008. V. 30 (1). P. 97–119.
Novozhilov Yu.K., Shchepin O.N., Schnittler M., Dagamac N.H., Alexandrova A.V., Popov E.S., Kuznetsov A.N. Myxomycetes associated with mountain tropical forests of Bidoup Nui Ba and Chu Yang Sin national parks (Dalat Plateau, southern Vietnam). Nova Hedwigia. 2020. V. 110 (1–2). P. 185–224. https://doi.org/10.1127/nova_hedwigia/2019/0560
Novozhilov Yu.K., Schnittler M., Zemlianskaia I.V., Fefelov K.A. Biodiversity of plasmodial slime moulds (Myxogastrea): measurement and interpretation. Protistology. 2000. V. 1 (4). P. 161–178.
Pouilain M., Meyer M., Bozonnet J. Les Myxomycètes. Federation mycologique et botanique Dauphine-Savoie. Sevrier. 2011.
Rojas C., Valverde R., Stephenson S.L., Vargas M.J. Ecological patterns of Costa Rican myxomycetes. Fungal Ecology. 2010. V. 3 (3). P. 139–147. https://doi.org/10.1016/j.funeco.2009.08.002
Schnittler M., Novozhilov Yu.K. Late-autumn myxomycetes of the Northern Ammergauer Alps. Nova Hedwigia. 1998. V. 66 (1). P. 205–222.
Schnittler M., Novozhilov Yu.K., Carvajal E., Spiegel F.W. Myxomycete diversity in the Tarim basin and eastern Tian-Shan, Xinjiang Prov., China. Fungal Diversity. 2013. V. 59. P. 91–108. https://doi.org/10.1007/s13225-012-0186-5
Schnittler M., Stephenson S.L., Novozhilov Yu.K. Ecology and world distribution of Barbeyella minutissima (Myxomycetes). Mycological Research. 2000. V. 104. P. 1518–1523. https://doi.org/10.1017/S0953756200002975
Schnittler M., Stephenson S.L. Myxomycete biodiversity in four different forest types in Costa Rica. Mycologia. 2000. V. 92. P. 626–637.
Stephenson S.L. Slime moulds in the laboratory II: Moist chamber cultures. American Biology Teacher. 1985. V. 47 (8). P. 487.
Stephenson S.L., Kalyanasundaram I., Lakhanpal T.N. A comparative biogeographical study of myxomycetes in the mid-Appalachians of eastern North America and two regions of India. Journal of Biogeography. 1993. P. 645–657.
Stephenson S.L., Novozhilov Yu.K., Almadrones-Reyes K.J., Dagamac N.H.A., Schnittler M. New records of Barbeyella minutissima (Myxomycetes, Echinosteliales) with an updated distribution map. Nova Hedwigia. 2019. V. 109(1–2). P. 177–186. https://doi.org/10.1127/nova_hedwigia/2019/0530
Stephenson S.L., Schnittler M., Lado, C. Ecological characterization of a tropical myxomycete assemblage – Maquipucuna Cloud Forest Reserve, Ecuador. Mycologia. 2004. V. 96 (3). P. 488–497. https://doi.org/10.1080/15572536.2005.11832948
Stephenson S.L., Stempen H. Myxomycetes: A Handbook of Slime Molds. Timber Press, Portland. 1994.
Trần D.Q., Nguyen H.T.N., Tran H.T.M., Stephenson S.L. Myxomycetes recorded from three lowland tropical forests in Vietnam. Mycosphere. 2014a. V. 5 (5). P. 662–672. https://doi.org/10.5943/mycosphere/5/5/7
Trần T.T.T., La Q.D., Hoang V.H. Floristic of Phia Oắc-Phia Đén Natural Protected Area at Nguyen Binh district, Cao Bang province: Biodiversity and impacting factors. Tập chí khoa học và công nghệ. 2014b. V. 119. P. 107–112 (in Vietn.).
Van Hooff, J.P.M. Cribraria tecta, a new myxomycete from Vietnam. Boletín de la Sociedad Micológica de Madrid. 2009. V. 33. P. 129–136.
Vlasenko A.V., Novozhilov Yu.K., Vlasenko V.A. Myxomycetes of the steppe communities plains of the Altai territory. Vestnik Novosibirskogo Gosudarstvennogo Universiteta. 2013. V. 11 (4). P. 5–12 (in Russ.).
Wrigley de Basanta D., Lado C., Stephenson S.L., Estrada-Torres A. Myxomycetes from moist chamber cultures of Neotropical substrates. Scripta Botanica Belgica. 2002. V. 22. P. 100.
Дополнительные материалы отсутствуют.
Инструменты
Микология и фитопатология


