Микология и фитопатология, 2020, T. 54, № 4, стр. 235-243
Aphyllophoroid fungi of the “Samurskiy” National Park (Dagestan)
1 Komarov Botanical Institute of the Russian Academy of Sciences
197376 St. Petersburg, Russia
* E-mail: sergvolobuev@binran.ru
Поступила в редакцию 10.02.2020
После доработки 10.04.2020
Принята к публикации 11.05.2020
Аннотация
Fifty-six species of wood-inhabiting basidiomycetes including two species with heterobasidia (Aporpium caryae and Auricularia mesenterica) were revealed in lowland deciduous forests of the “Samurskiy” National Park, the Republic of Dagestan, Russia. Twenty-six species (e.g. Botryobasidium curtisii, Dendrothele acerina, Efibula tuberculata, Hydnellum concrescens, Junghuhnia fimbriatella, Mucronella calva, Peniophora laeta, Phanerochaete alnea, Ph. calotricha, Ph. livescens, Phlebiopsis ravenelii, Postia subcaesia, Pseudotomentella tristis, Tomentella lilacinogrisea, Xenasmatella alnicola, etc.) are new to Dagestan and the North-Eastern Caucasus. Most of species registered are presented by saprotrophs on dead wood of main forest-forming trees – Carpinus betulus (25 fungal species), Alnus glutinosa (14 species), Quercus robur subsp. pedunculiflora (9 species). The distribution of the revealed fungi species by size classes (diameter) of wood substrates showed that more species develop on medium-sized and large wood units than on thin dead branches and stems with diameter less than 5 cm. A total of 86% (48 species) of aphyllophoroid fungi found in oak- and hornbeam-dominated forests of the “Samurskyi” National Park are common with deciduous forests of the Central Russian Upland. Ganoderma lucidum and Fistulina hepatica are recommended to be included to the Red Data Book of the Republic of Dagestan.
INTRODUCTION
Despite of a high taxonomical diversity, polyphyletic origin and a wide range of basidiocarp morphological types, aphyllophoroid fungi form a unique ecological group of Agaricomycetes adapted predominantly to the development on woody substrate as saprotrophs, ectomycorrhizal or parasitic species (Bondartseva, 2000; Kotiranta et al., 2009). As wood decomposers, aphyllophoroid fungi play a crucial role in the continuity of the carbon and nutrient cycles and energy transformation in forest ecosystems (Boddy et al., 2008). Some species of aphyllophoroid fungi are used as indicators of biologically valued forests and to assess habitats for nature conservation purposes. It has an exclusive significance since forests with a high number of indicator fungal species normally harbor also other old-forest species from other kingdoms of organisms (vascular plants, invertebrates, etc.) (Andersson et al., 2009).
The Caucasus is known as one of the world biodiversity hotspot, where more than 600 species of aphyllophoroid fungi are listed, including about 500 species mentioned for the Russian part of the Caucasus (Ghobad-Nejhad et al., 2009). At the same time, the territory of the Northern Caucasus has been studied extremely unevenly with a concentration of mycologists’ attention to the Western and the Central parts with more humid climate in opposite to the Eastern Caucasus. Along with many regions of Russia the Republic of Dagestan remains little explored area on the mycologi-cal research map. Data on aphyllophoroid fungi of Dagestan is poorly presented and scattered in the literature, and special systematic studies of this group of fungi have been started here only recently. Until current research altogether 114 species were known for Dagestan (Bagdasarova, 1965; Kõljalg, 1996; Ghobad-Nejhad et al., 2009; Viner, 2017; Ivanushenko et al., 2019; Volobuev et al. 2019a, 2019b), including 61 species have been registered for the territory of “Samurskiy” National Park. The aim of the present work is to obtain new data on species diversity of aphyllophoroid fungi and ecological characteristics on the protected forest area of Dagestan in the Caspian Sea coast.
MATERIALS AND METHODS
The “Samurskiy” National Park was established by the Decree of the Government of Russia N 1839 of the 25th of December 2019 on the basis of the previously existing regional protected areas in Akhtynsky, Derbentsky, Dokuzparinsky and Magaramkentsky districts of the Republic of Dagestan. The National Park has a total area of 482.7 km2 and consists of two sites – “Shalbuzdag” and “Delta Samura”.
The collection of aphyllophoroid fungi specimens was carried out by a route method in the beginning of October 2019. All studied habitats are located within the “Delta Samura” site boundaries of the “Samurskiy” National Park in Magaramkentsky district (Fig. 1).
Fig. 1.
The location of studied area with explored sites numbered according to the list in the text.
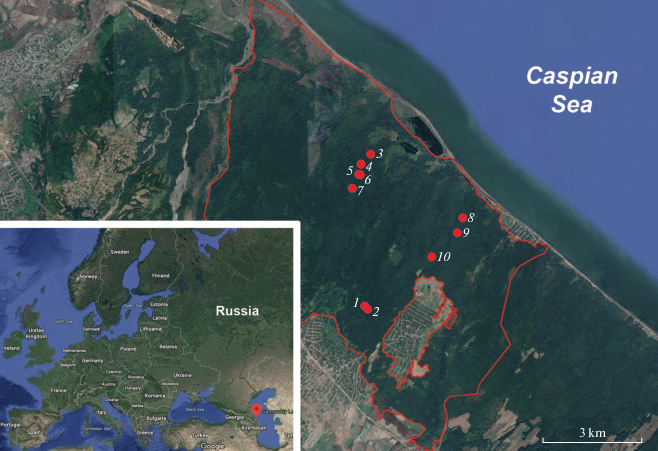
The “Delta Samura” site is located in the south-east of Dagestan, at the border with Azerbaijan. The altitude range of the site is between –25 and 35 m a.s.l. The climate here is warm temperate with an average annual temperature of 12.6°C (the average temperature in January is 1.4°C and 24.5°C in August). Average sum of precipitation is only 400 mm per year, but the relative air humidity is high (78%) due to the close vicinity of the Caspian Sea, the dense river network of the Samur River delta and the high groundwater level (Akaev et al., 1996).
Forest ecosystems of the “Delta Samura” site can be divided into two groups. The first one includes the forests of the central part with dense tree cover and dominated by Acer campestre L., Carpinus betulus L., Quercus robur subsp. pedunculiflora (K. Koch) Menitsky, Ulmus campestris L. Various shrubs [Cornus mas L., C. sanguinea subsp. australis (C.A. Mey.) Jáv., Crataegus spp., Euonymus europaea L., E. verrucosa Scop., Mespilus germanica L., Prunus caspica Kovalev et Ekimov, and Rosa spp.] are common in this forest type, but lianas are absent on most of the trees. Among herbs, Brachypodium sylvaticum (Huds.) P. Beauv., Buglossoides purpurocaerulea (L.) I.M. Johnst., Carex sylvatica Huds., Euphorbia amygdaloides L., and Sanicula europaea L. are prevailed.
The second forest type with sparse tree cover is presented by liana forests (Fig. 2, B) dominated by Alnus glutinosa subsp. barbata (C.A. Mey.) Yalt., Juglans regia L., Populus nigra L., Pyrus caucasica Fed., and Salix alba L. with a rich plant species diversity (70 woody plants and about 300 species of herbs) (Novikova, Polyanskaya, 1994). This warm temperate so-called Liana-tugai forest is a single place in Russia where some Hyrcanian elements occur (e.g. Hedera pastuchovii Woronow, Pyracantha coccinea M. Roem., etc.) (Yarovenko et al., 2004). Tree trunks here are usually strongly covered by lianas (e.g. Clematis orientalis L., C. vitalba L., Hedera pastuchovii, Humulus lupulus L., Lonicera caprifolium L., Periploca graeca L., Rubus spp., Smilax excelsa L. and Vitis silvestris C.C. Gmel.) (Litvinskaya, Murtazaliev, 2015).
Fig. 2.
The habitats of the “Delta Samura” site: a – dead-floor forest dominated by Carpinus betulus; b – liana-tugai forest.

The following numbers pointed in the Fig. 1 are corresponding to the investigated localities and habitats:
1 – N 41°49′36.4″, E 48°31′40.0″, 33 m a.s.l., dead-floor forest dominated by Carpinus betulus (Fig. 2, A);
2 – N 41°49′33.6″, E 48°31′43.7″, 17 m a.s.l., herb-rich mixed forest dominated by C. betulus and Quercus robur subsp. pedunculiflora;
3 – N 41°51′58.7″, E 48°31′44.3″, –11 m a.s.l., forest dominated by Populus nigra with lianas;
4 – N 41°51′49.9″, E 48°31′35.1″, –19 m a.s.l., herb-rich mixed forest dominated by Quercus robur subsp. pedunculiflora and Carpinus betulus;
5 – N 41°51′40.6″, E 48°31′32.4″, –18 m a.s.l., herb-rich mixed forest dominated by Quercus robur subsp. pedunculiflora and Carpinus betulus;
6 – N 41°51′40.2″, E 48°31′33.8″, –15 m a.s.l., herb-rich forest dominated by C. betulus;
7 – N 41°51′27.0″, E 48°31′24.1″, –2 m a.s.l., herb-rich forest dominated by C. betulus;
8 – N 41°50′59.5″, E 48°33′42.9″, –7 m a.s.l., herb-rich mixed forest dominated by C. betulus and Quercus robur subsp. pedunculiflora;
9 – N 41°50′45.4″, E 48°33′36.2″, –9 m a.s.l., herb-rich mixed forest dominated by Carpinus betulus and Quercus robur subsp. pedunculiflora;
10 – N 41°50′23.1″, E 48°33′04.3″, 5 m a.s.l., herb-rich mixed forest dominated by Carpinus betulus and Quercus robur subsp. pedunculiflora.
Microscopic identification of 120 collections was performed using Axio Imager A1 microscope, LOMO Mikmed-6 microscope and a standard set of chemicals (5% KOH, Melzer’s reagent, 0.1% Cotton Blue). Voucher specimens are deposited at the Mycological herbarium of the Komarov Botanical Institute RAS, St. Petersburg (LE).
RESULTS
The list of species revealed is provided with data on occupied substrata and herbarium numbers of specimens examined. The nomenclature of fungal taxa follows the Index Fungorum (2020). The species new to the Republic of Dagestan are marked with “!”, and an asterisk (*) shows the species recorded for the first time for the territory of “Samurskiy” National Park.
Agaricales
!Dendrothele acerina (Pers.) P.A. Lemke – 9: on alive tree of Crataegus sp. (d = 10 cm) (LE F-332367).
!Fistulina hepatica (Schaeff.) With. (Fig. 3, D) – 2, 4, 5: on fallen trunk of Carpinus betulus (d = 60 cm) (LE F-332316) and at base of alive Quercus robur subsp. pedunculiflora (d = 60 cm).
Fig. 3.
Basidiocarps of selected aphyllophoroid fungi species: a – Fuscoporia torulosa; b – Tomentella umbrinospora; c – Botryobasi-dium curtisii [black arrows indicate brownish anamorph (1) and grayish white teleomorph (2)]; d – Fistulina hepatica.

!Mucronella calva (Alb. et Schwein.) Fr. – 4: on fallen trunk of Crataegus sp. (d = 15 cm) (LE F-332338).
Auriculariales
Aporpium caryae (Schwein.) Teixeira et D.P. Ro-gers – 1: on fallen trunk of Carpinus betulus (d = 35 cm) (LE F-332306).
Auricularia mesenterica (Dicks.) Pers. – 2: on fallen branches of Carpinus betulus (d = 5 cm).
Boletales
Coniophora puteana (Schumach.) P. Karst. – 4: on fallen trunk of Alnus sp. (d = 30 cm) (LE F-332342).
Cantharellales
!Botryobasidium curtisii Hallenb. (Fig. 3, C) – 3: on fallen trunk of Alnus sp. (d = 40 cm) (LE F-332325).
B. isabellinum (Fr.) D.P. Rogers – 1: on fallen trunk of Carpinus betulus (d = 20 cm) (LE F-332308).
B. subcoronatum (Höhn. et Litsch.) Donk – 9: on stump of Quercus robur subsp. pedunculiflora (d = = 50 cm) (LE F-332364).
Sistotrema resinicystidium Hallenb. – 8: on fallen trunk of Carpinus betulus (d = 25 cm) (LE F-332354).
Hymenochaetales
Fuscoporia torulosa (Pers.) T. Wagner et M. Fisch. (Fig. 3, A) – 2, 4, 5: on fallen trunk (d = 50 cm) (LE F-332321), on alive tree (d = 30 cm) (LE F-332350) and on stump (d = 70 cm) (LE F-332347) of Quercus robur subsp. pedunculiflora.
Hymenochaete rubiginosa (Dicks.) Lév. – 3, 9: on stump (d = 50 cm) (LE F-332366) and fallen branches (d = 15 cm) (LE F-332327) of Quercus robur subsp. pedunculiflora.
!Lyomyces crustosus (Pers.) P. Karst. – 2, 8: on dead standing stem of Carpinus betulus (d = 40 cm) (LE F-332320) and on fallen branches of Quercus robur subsp. pedunculiflora (d = 10 cm) (LE F-332362).
*Peniophorella pubera (Fr.) P. Karst. – 1, 4, 8, 9: on fallen trunk of Carpinus betulus (d = 20 cm) (LE F-332315), fallen branches of Quercus robur subsp. pedunculiflora (d = 5 cm) (LE F-332359) and fallen trunks of Crataegus sp. (d = 6–15 cm) (LE F-332337).
Sidera vulgaris (Fr.) Miettinen – 4: on fallen trunk of Alnus sp. (d = 40 cm) (LE F-332334).
Trichaptum biforme (Fr.) Ryvarden – 5: on fallen trunk of Quercus robur subsp. pedunculiflora (d = 30 cm).
Xylodon flaviporus (Berk. et M.A. Curtis ex Cooke) Riebesehl et Langer – 1, 8: on fallen trunks (d = 20–35 cm) and fallen branches (d = 5–7 cm) of Carpinus betulus (LE F-332305, LE F-332309, LE F-332358).
!X. raduloides Riebesehl et Langer – 1, 8: on fallen trunks of Carpinus betulus (d = 10 cm) (LE F-332311, LE F-332356).
Polyporales
Byssomerulius corium (Pers.) Parmasto – 3, 4: on fallen branches of Carpinus betulus (d = 5–10 cm) (LE F-332329).
!Daedaleopsis confragosa (Bolton) J. Schröt. – 3: on fallen trunk of Alnus sp. (d = 20 cm) (LE F-332323).
!Efibula tuberculata (P. Karst.) Zmitr. et Spirin – 1: on fallen branches of Carpinus betulus (d = 7 cm) (LE F-332314).
Fomes fomentarius (L.) Fr. – 6: on alive tree of Carpinus betulus (d = 30 cm).
Fomitopsis pinicola (Sw.) P. Karst. – 6: on fallen trunk of Carpinus betulus (d = 10 cm).
Ganoderma applanatum (Pers.) Pat. – 4, 8: at base of alive Quercus robur subsp. pedunculiflora (d = 100 cm) (LE F-332360) and on stump of Alnus sp. (d = 30 cm) (LE F-332341).
G. lucidum (Curtis) P. Karst. – 10: on fallen trunk of Fraxinus excelsior (d = 30 cm) (recorded by R.A. Murtazaliev).
Gloeoporus pannocinctus (Romell) J. Erikss. – 1, 6, 7, 8: on fallen trunks of Carpinus betulus (d = 10–20 cm) (LE F-332351, LE F-332355).
*Hyphoderma setigerum (Fr.) Donk – 8: on fallen trunk (d = 25 cm) (LE F-332353) and fallen branches (d = 5 cm) (LE F-332357) of Carpinus betulus.
!Junghuhnia fimbriatella (Peck) Ryvarden – 1: on fallen trunk of Carpinus betulus (d = 20 cm) (LE F-332312).
Laetiporus sulphureus (Bull.) Murrill – 2: at base of Quercus robur subsp. pedunculiflora (d = 70 cm) (LE F-332322).
!Neofavolus alveolaris (DC.) Sotome et T. Hatt. – 3: on fallen branches of Alnus sp. (d = 5 cm) (LE F-332326).
!Phanerochaete alnea (Fr.) P. Karst. – 4: on fallen trunk of Alnus sp. (d = 30 cm) (LE F-332343).
!Ph. calotricha (P. Karst.) J. Erikss. et Ryvarden – 4: on fallen branches of Carpinus betulus (d = 15 cm) (LE F-332340).
!Ph. laevis (Fr.) J. Erikss. et Ryvarden – 1: on fallen trunk of Carpinus betulus (d = 10 cm) (LE F-332310).
!Ph. livescens (P. Karst.) Volobuev et Spirin – 1, 4, 7: on fallen branches of Carpinus betulus (d = 5 cm) (LE F-332304) and fallen branches of Quercus robur subsp. pedunculiflora (d = 2 cm) (LE F-332346).
!Ph. velutina (DC.) P. Karst. – 1, 8: on fallen trunk (d = 35 cm) (LE F-332307) and branches (d = 5–7 cm) (LE F-332313, LE F-332361) of Carpinus betulus.
!Phlebia rufa (Pers.) M.P. Christ. – 2, 5: on fallen trunk (d = 15 cm) (LE F-332348) and branches (d = = 5 cm) (LE F-332318) of Carpinus betulus.
!Phl. tremellosa (Schrad.) Nakasone et Burds. – 3, 5: on fallen trunk of Carpinus betulus (d = 10 cm) and fallen trunk of Alnus sp. (d = 20 cm) Ph. tremellosa.
!Phlebiopsis ravenelii (Cooke) Hjortstam – 5: on fallen trunk of Quercus robur subsp. pedunculiflora (d = 30 cm) (LE F-332349).
*Postia lactea (Fr.) P. Karst. – 3, 7: on fallen trunk of Alnus sp. (d = 20 cm) (LE F-332324) and fallen trunk of Carpinus betulus (d = 10 cm).
!P. subcaesia (A. David) Jülich – 3: on fallen bran-ches of Carpinus betulus (d = 10 cm) (LE F-332328) and fallen trunk of Alnus sp. (d = 10 cm) (LE F-332332).
*Steccherinum fimbriatum (Pers.) J. Erikss. – 2, 6: on fallen branches of Carpinus betulus (d = 5–7 cm).
Trametes versicolor (L.) Lloyd – 3: on fallen trunk of Alnus sp. (d = 20 cm).
Russulales
!Gloeocystidiellum porosum (Berk. et M.A. Curtis) Donk – 4: on fallen trunk of Alnus sp. (d = 15 cm) (LE F-332345).
!Peniophora laeta (Fr.) Donk – 1: on fallen branches of Carpinus betulus (d = 3 cm) (LE F-332303).
!Stereum hirsutum (Willd.) Pers. – 2, 6: on fallen branches of Carpinus betulus (d = 5–7 cm) and fallen branches of Quercus robur subsp. pedunculiflora (d = = 10 cm).
!Xenasmatella alnicola (Bourdot et Galzin) K.H. Larss. et Ryvarden – 3: on fallen trunk of Alnus sp. (d = 10 cm) (LE F-332333).
Thelephorales
Amaurodon viridis (Alb. et Schwein.) J. Schröt. – 6: on fallen branches of Carpinus betulus (d = 7 cm) (LE F-332352).
!Hydnellum concrescens (Pers.) Banker – 9: on stump of Quercus robur subsp. pedunculiflora (d = 50 cm) (LE F-332363).
*Odontia fibrosa (Berk. et M.A. Curtis) Kõljalg – 9: on fallen trunk of Crataegus sp. (d = 6 cm) (LE F-332368).
!Pseudotomentella tristis (P. Karst.) M.J. Larsen – 4: on fallen trunk of Crataegus sp. (d = 15 cm) (LE F-332339).
*Tomentella bryophila (Pers.) M.J. Larsen – 3: on fallen trunk of Alnus sp. (d = 10 cm) (LE F-332331).
T. ferruginea (Pers.) Pat. – 2: on fallen branches of Carpinus betulus (d = 6 cm) (LE F-332319).
!T. lilacinogrisea Wakef. – 9: on fallen trunk of Crataegus sp. (d = 6 cm) (LE F-332369).
T. pilosa (Burt) Bourdot et Galzin – 9: on stump of Quercus robur subsp. pedunculiflora (d = 50 cm) (LE F-332365).
*T. umbrinospora M.J. Larsen (Fig. 3, B) – 2, 4: on fallen trunk of Carpinus betulus (d = 60 cm) (LE F-332317) and fallen trunks of Alnus sp. (d = 15–40 cm) (LE F-332336, LE F-332344).
DISCUSSION
Fifty-six species of aphyllophoroid fungi from 42 genera and nine orders of Agaricomycetes (Basidiomycota) were revealed in the “Samurskiy” National Park. Among them 33 species are new to the territory of National Park including 26 species are registered for Dagestan for the first time. Most of these species are widely distributed and are known from different regions of the Caucasus (Ghobad-Nejhad et al., 2009), but some of them are rare and should be carefully considered.
One of the most noteworthy findings is presented by Botryobasidium curtisii, which is recorded for the second time in Russia. There was the only occurrence of this species for Russia and the Northern Caucasus from the Chechen Republic territory (Parmasto, Parmasto, 1989). Our record from the “Samurskiy” National Park confirms the distribution of B. curtisii northward from the south-western coast of the Caspian Sea where the species is known and was described from (Ghobad-Nejhad et al., 2009).
The species Junghuhnia fimbriatella has been found for the first time for the Caucasus. This very rare species in Europe, which for known only a few localities in Austria, the Czech Republic, Germany, Poland, and Switzerland (Ryvarden, Melo, 2017), is seems to be a successor of another polypore fungus Ganoderma applanatum (Karasiński, Wołkowycki, 2015) developing perennial basidiocarps also registered in the forests of the “Samurskiy” National Park. In Russia Junguhnia fimbriatella was revealed in the regions of the European part (Arkhangelsk, Leningrad, Pskov, and Tver Regions, Saint Petersburg, the Republic of Karelia and the Republic of Mordovia), and the Russian Far East (Kamchatka Krai) (Bolshakov et al., 2016; Ryvarden, Melo, 2017).
The finding of Peniophora laeta supports its substrate preferences to corticated branches of Carpinus betulus. This fungus has been recorded only four times in Russia – for Kaliningrad (Dedkov et al., 2007), Sverdlovsk Region (Kuznetsova et al., 2015), the Republic of Crimea (Akulov et al., 2003), and Krasnodar Krai (Mukhamedshin, 1992). In the Caucasus outside the Russian part Peniophora laeta is known from Iran and Turkey (Ghobad-Nejhad et al., 2009).
Such species as Sistotrema resinicystidium and Sidera vulgaris were revealed in the territory of the “Samurskiy” National Park earlier (Viner, 2017), but are needed to additional comments on their ecology and known geographical distribution. Sistotrema resinicystidium is a little-known species for Russia and the Caucasus, registered in protected areas of Arkhangelsk, Nizhny Novgorod, Tver, and Sverdlovsk Regions, the Republic of Karelia and the Komi Republic (Bondartseva, Zmitrovich, 2020), the Republic of Dagestan (Viner, 2017) as well as in Siberia from Khanty-Mansi Autonomous Okrug–Yugra (Stavishenko, 2011) and Novosibirsk Region (Zhukoff, 1995). Apparently S. resinicystidium has strictly limited habitats requirements being preferred anthropogenically undisturbed old-growth forests and large-dimension woody substrate and developing its resupinate basidiocarps on both deciduous and coniferous trees (Quercus, Betula, Carpinus, Populus, Picea, and Pinus).
Sidera vulgaris belongs to infrequently collected species distributed predominantly in temperate forests of the European part of Russia (Bolshakov et al., 2016) and included in some regional lists of rare and vulnerable species of lichens and fungi requiring constant monitoring, for instance on the territory of the Republic of Tatarstan (Red Data Book, 2016). The species is also known for the Caucasus from Armenia, Georgia, Iran, and Russia (Ghobad-Nejhad et al., 2009; Viner, 2017), and it is to be expecting that further intensive mycological investigations allow us to reveal new localities of the species in the Caucasus and in particular in Dagestan.
Two genera (Phanerochaete and Tomentella) demonstrated the maximum species richness based on revealed species and each of them presented by five species. The only species from the genus Phanerochaete – Ph. sordida (P. Karst.) J. Erikss. et Ryvarden – was reported from the territory of “Samurskiy” National Park (Viner, 2017) until the present study but was not be found by the author. Simultaneously, among species registered there found the species Ph. alnea showing the similarity with Ph. sordida in macro- and micromorphology for initial stages of fruiting bodies development (Spirin et al., 2017). So far as Ph. sordida inhabits mostly gymnosperm hosts in temperate zone of Eurasia and occurs on deciduous wood only in boreal conifer forests (Volobuev et al., 2015) the record of Ph. sordida for lowland deciduous forests of the “Samurskiy” National Park should be confirmed by morphological re-examination and molecular methods.
Most of aphyllophoroid fungi species revealed are presented by saprotrophs on dead wood of main forest-forming trees – Carpinus betulus (25 fungal species), Alnus glutinosa (14 species), Quercus robur subsp. pedunculiflora (9 species). Five species (Dendrothele acerina, Fistulina hepatica, Fuscoporia torulosa, Fomes fomentarius, Ganoderma applanatum), or 8.9% of all registered fungal species, demonstrated the pathogenic activity by developing their basidiocarps on living trees.
The distribution of registered fungal species by size classes (diameter) of woody substrates are following: ≤ 5 cm – 12 species, 6–10 cm – 22 species, 11–49 cm – 37 species, and ≥ 50 cm – 7 species. Accordingly more than a half of species grow on massive or large-dimension units of wood (fallen trunks, thick branches, dead stumps, etc.) and can be potentially vulnerable in the case of destroying the occupied substrate type. For this reason the species Fistulina hepatica having bright and attractive fruiting bodies (Fig. 3, d) and found here only on big-size wood is recommended to be considered for including to the Red Data Book of the Republic of Dagestan. Moreover, this fungus prefers the wood of Quercus robur, which is locally distributed and poorly regenerated in Dagestan (Novikova, Polyanskaya, 1994). The remarkable finding of Ganoderma lucidum included in the Red Data Book of the Russian Federation (2008) is also recommended for additional protection at the regional level.
Following the analysis of the species composition similarity between the “Samurskiy” National Park and lowland deciduous forests of Central Europe, carried out for lichens (Ismailov et al., 2017), a comparison of the species diversity of fungi between the investigated area and broadleaved forests of the Central Russian Upland was undertaken. In general, 85.7% (48 species) of aphyllophoroid fungi found in oak- and hornbeam-dominated forests of the “Samurskiy” park are common with deciduous forests of Lipetsk and Oryol Regions (Volobuev, 2015; Volobuev et al., 2018). This similarity is very interesting and can be explained by the ability of wood-inhabiting fungi not only to follow a single host tree (e.g. Quercus robur distributed in both compared territories) but also to change a host tree within the same family (e.g. fungal species associated with Carpinus betulus (Betulaceae) in the “Samurskiy” park grow on Betula spp. wood in the Central Russian Upland).
CONCLUSION
The obtained data expand the knowledge on species diversity of aphyllophoroid fungi on the “Samurskiy” National Park, where to date there known 94 species, as well as on species richness of this fungal group in the Republic of Dagestan with a total of 140 species. New localities of cited above species are the contribution to further chorological analysis and modeling of fungal species distribution patterns. The revealed ecological peculiarities of aphyllophoroid fungi are addressed to nature conservation practice and apparently will be taken into account during preparation of the new edition of regional Red Data Book.
The author is grateful to Dr. Aziz B. Ismailov and Dr. Khabagin U. Aliev (Mountain Botanical Garden, the Dagestan Federal Research Center of RAS) for the organization of fieldwork, as well as to Dr. Ramazan A. Murtazaliev (Mountain Botanical Garden, DFRC RAS) kindly provided the data on the Ganoderma lucidum find. This investigation was supported financially by the Russian Science Foundation (RSF project N 19-77-00085). The microscopic identification of fungi was done using equipment of The Core Facilities Center “Cell and Molecular Technologies in Plant Science” at the Komarov Botanical Institute RAS (St. Petersburg, Russia).
Список литературы
Akaev B.A., Ataev Z.V., Gadzhiev B.S. et al. Physical geography of Dagestan. School, Moscow, 1996 (in Russ.).
Akulov A.Yu., Usichenko A.S., Leontyev D.V., Yurchenko E.O., Prydiuk M.P. Annotated checklist of aphyllophoroid fungi of Ukraine. Mycena. 2003. V. 2 (2). P. 1–73.
Andersson L., Alexeeva N., Kuznetsova E. (eds.) Survey of biologically valuable forests in North-Western European Russia. Vol. 2. Identification manual of species to be used during survey at stand level. SPb., 2009 (in Russ.).
Bagdasarova A.F. Fungi of liana forest in the Samur River Delta. In: Botanika, fiziologiya rasteniy i rastenievodtsva. Makhachkala, 1965. P. 64–70 (in Russ.).
Boddy L., Frankland J.C., van West P. Ecology of saprotrophic basidiomycetes. Elsevier Academic Press, London, 2008.
Bolshakov S.Yu., Potapov K.O., Ezhov O.N., Volobuev S.V., Khimich Yu.R., Zmitrovich I.V. New species for regional mycobiotas of Russia. 1. Report 2016. Mikologiya i fitopatologiya. 2016. V. 50 (5). P. 275–286.
Bondartseva M.A. Ecological and biological patterns of functioning of xylotrophic basidiomycetes in forest ecosystems. In: Fungal communities in forest ecosystems: proceedings of coordinating studies. Karelian Scientific Center of RAS, Moscow, Petrozavodsk, 2000. P. 9–25 (in Russ.).
Bondartseva M.A., Zmitrovich I.V. The genus Sistotrema (Cantharellales, Hydnaceae) in Russia. Mikologiya i fitopatologiya. 2020. V. 54 (1). P. 3–15 (in Russ.). https://doi.org/10.31857/S0026364820010043
Dedkov V.P., Volodina A.A., Gubareva I.Yu. Synopsis of fungi of the Kaliningrad Region. In: V.P. Dedkov, I.Yu. Gubareva (eds). Biodiversity of the Kaliningrad Region. Part 1. Fungi, lichens, mosses, clubmosses, horsetails and ferns of the Kaliningrad Region. Kaliningrad, 2007. P. 6–78 (in Russ.).
Ghobad-Nejhad M., Hallenberg N., Parmasto E., Kotiranta H. A first annotated checklist of corticioid and polypore basidiomycetes of the Caucasus region. Mycologia Balcanica. 2009. V. 6 (3). P. 123–168. https://doi.org/10.5281/zenodo.2550071
Index Fungorum. A nomenclature database. http://www.indexfungorum.org/Names/Names.asp. 2020. Accessed 30.03.2020.
Ismailov A., Urbanavichus G., Vondrák J., Pouska V. An old-growth forest at the Caspian Sea coast is similar in epiphytic lichens to lowland deciduous forests in Central Europe. Herzogia. 2017. V. 30. P. 103–125.
Ivanushenko Yu.Yu., Ismailov A.B., Volobuev S.V. First data on polypores of the Gunib Plateau (the Republic of Dagestan). In: Materialy XXI Mezhdunarodnoy konferentsii “Biologicheskoe raznoobrazie Kavkaza i yuga Rossii”. Magas, 2019. P. 163–166 (in Russ.).
Karasiński D., Wołkowycki M. An annotated and illustrated catalogue of polypores (Agaricomycetes) of the Białowieża Forest (NE Poland). Polish Botanical Journal. 2015. V. 60 (2). P. 217–292. https://doi.org/10.1515/pbj-2015-0034
Kõljalg U. Tomentella (Basidiomycota) and related genera in temperate Eurasia. Fungiflora, Oslo, 1996.
Kotiranta H., Saarenoksa R., Kytövuori I. Aphyllophoroid fungi of Finland. A check-list with ecology, distribution, and threat categories. Norrlinia. 2009. V. 19. P. 1–223.
Kuznetsova I.A., Golovatin M.G., Gilev A.V. et al. Specially protected nature territories of the Sverdlovsk Region: environmental monitoring. Izdatelstvo Uralskogo universiteta, Ekaterinburg, 2015 (in Russ.).
Litvinskaya S., Murtazaliev R. Vegetation diversity of the Russian part of the Caucasus in the era of climate change. In: M. Öztürk, K. Hakeem, I. Faridah-Hanum, R. Efe (eds.). Climate change impacts on high-altitude ecosystems. Springer, Cham, 2015. P. 523–544. https://doi.org/10.1007/978-3-319-12859-7_20
Mukhamedshin R.K. Corticioid fungi (Corticiaceae s. lato) of the Northwest Caucasus. Mikologiya i fitopatologiya. 1992. V. 26 (2). P. 104–109 (in Russ.).
Novikova N.M., Polyanskaya A.V. Samursky liana forests: the challenge of biodiversity conservation in a developing water industry. Moscow, 1994 (in Russ.).
Parmasto E., Parmasto I. To the flora of aphyllophoroid fungi of the Chechen-Ingush ASSR. Novosti sistematiki nizshikh rasteniy. V. 26. P. 72–74 (in Russ.).
Red Data Book of the Republic of Tatarstan. Animals, plants, fungi. The third edition. Ed. by A. A. Nazirov. Idel-Press, Kazan, 2016 (in Russ.).
Red Data Book of the Russian Federation (plants and fungi). Tovarishchestvo nauchnykh izdaniy KMK, Moscow, 2008 (in Russ.).
Ryvarden L., Melo I. Poroid fungi of Europe. The second edition. Synopsis Fungorum. 2017. V. 37. P. 1–431.
Spirin V.A., Volobuev S.V., Okun M.V., Miettinen O., Larsson K.-H. What is the type species of Phanerochaete (Polyporales, Basidiomycota)? Mycol. Progress. 2017. V. 16 (2). P. 171–183. https://doi.org/10.1007/s11557-016-1267-8
Stavishenko I.V. Aphyllophoraceous fungi of the Nature Reserve “Malaya Sosva” (Western Siberia). Mikologiya i fitopatologiya. 2011. V. 45 (2). P. 142–157 (in Russ.).
Viner I.A. New records of polypores and corticioid fungi in Dagestan. In: Proceedings of “Dagestanskiy” State Nature Reserve. V. 13. Makhachkala, 2017. P. 13–19 (in Russ.).
Volobuev S., Arzhenenko A., Bolshakov S., Shakhova N., Sarycheva L. New data on aphyllophoroid fungi (Basidiomycota) in forest-steppe communities of the Lipetsk Region, European Russia. Acta Mycologica. 2018. V. 53 (2). P. 1–15. https://doi.org/10.5586/am.1112
Volobuev S., Okun M., Ordynets A., Spirin V. The Phanerochaete sordida group (Polyporales, Basidiomycota) in temperate Eurasia, with a note on Phanerochaete pallida. Mycol. Progress. 2015. V. 14. P. 80. https://doi.org/10.1007/s11557-015-1097-0
Volobuev S.V. Aphyllophoroid fungi of the Oryol Region: Taxonomical composition, distribution, ecology. Lan, Moscow, SPb., 2015 (in Russ.).
Volobuev S.V., Ivanushenko Yu.Yu., Ismailov A.B. Auriporia aurulenta – a proposal to the Red Data Book of the Republic of Dagestan. In: Flora i zapovednoe delo na Kavkaze: istoriya i sovremennoe sostoyanie izuchennosti: materialy Mezhdunarodnoy konferentsii. Pyatigorsk, 2019a. P. 32–33 (in Russ.).
Volobuev S.V., Ivanushenko Yu.Yu., Ismailov A.B. New for Dagestan species of Tomentella (Thelephorales, Basidiomycota). South of Russia: ecology, development. 2019b. V. 14 (2). P. 172–179 (in Russ.). https://doi.org/10.18470/1992-1098-2019-2-172-179
Yarovenko Yu.A., Murtazaliev R.A., Ilyina E.V. Reserved places of Dagestan. Raduga-1, Makhachkala, 2004 (in Russ.).
Zhukoff E.A. Aphyllophorales (Basidiomycetes) from Central Siberia. Mycotaxon. 1995. V. 53. P. 437–445.
Акаев Б.А., Атаев З.В., Гаджиев Б.С., Гаджиева З.X. и др. (Akaev et al.) Физическая география Дагестана. М.: Школа, 1996. 380 с.
Багдасарова А.Ф. (Bagdasarova) Грибы лианового леса дельты реки Самур // Ботаника, физиология растений и растениеводства. Махачкала: Даг. кн. изд., 1965. С. 64–70.
Бондарцева М.А. (Bondartseva) Эколого-биологические закономерности функционирования ксилотрофных базидиомицетов в лесных экосистемах // Грибные сообщества лесных экосистем: материалы координационных исследований. М., Петрозаводск: КарНЦ РАН, 2000. С. 9–25.
Бондарцева М.А., Змитрович И.В. (Bondartseva, Zmitrovich) Род Sistotrema (Cantharellales, Hydnaceae) в России // Микология и фитопатология. 2020. Т. 54. № 1. С. 3–15.
Винер И.А. (Viner) Новые находки трутовых и кортициоидных грибов в Дагестане // Труды государственного природного заповедника “Дагестанский”. Вып. 13. Махачкала: Алеф, 2017. С. 13–19.
Волобуев С.В. (Volobuev) Афиллофороидные грибы Орловской области: Таксономический состав, распространение, экология. СПб.; М.: Лань, 2015. 304 с.
Волобуев С.В., Иванушенко Ю.Ю., Исмаилов А.Б. (Volobuev et al.) Auriporia aurulenta – кандидат в Красную книгу Республики Дагестан // Флора и заповедное дело на Кавказе: история и современное состояние изученности: материалы Международной конференции. Пятигорск, 2019а. С. 32–33.
Волобуев С.В., Иванушенко Ю.Ю., Исмаилов А.Б. (Volobuev et al.) Новые для Дагестана виды рода Tomentella (Thelephorales, Basidiomycota) // Юг России: экология, развитие. 2019b. Т. 14. № 2. С. 172–179.
Выявление и обследование биологически ценных лесов на Северо-Западе Европейской части России. (Andersson et al.) Т. 2. Пособие по определению видов, используемых на уровне выделов / Отв. ред. Л. Андерссон, Н.М. Алексеева, Е.С. Кузнецова. СПб., 2009. 258 с.
Дедков В.П., Володина А.А., Губарева И.Ю. (Dedkov et al.) Конспект грибов Калининградской области // В.П. Дедков, И.Ю. Губарева (ред.). Биоразнообразие Калининградской области. Часть 1. Грибы, лишайники, плауны, хвощи и папоротники Калининградской области. Калининград, 2007. С. 6–78.
Иванушенко Ю.Ю., Исмаилов А.Б., Волобуев С.В. (Ivanushenko et al.) Первые сведения о трутовых грибах плато Гуниб (Республика Дагестан) // Материалы XXI Международной научной конференции “Биологическое разнообразие Кавказа и юга России”. Магас, 2019. С. 163–166.
Красная книга Республики Татарстан. Животные, растения, грибы. Изд. 3 (Red Data Book) / Гл. ред. А.А. Назиров. Казань: Идел-Пресс, 2016. 760 с.
Красная книга Российской Федерации (растения и грибы) (Red Data Book). М.: Товарищество научных изданий КМК, 2008. 855 с.
Кузнецова И.А., Головатин М.Г., Гилев А.В. и др. (Kuznetsova et al.) Особо охраняемые природные территории Свердловской области: мониторинг состояния природной среды. Екатеринбург: Изд-во Урал. ун-та, 2015. 189 с.
Мухамедшин Р.К. (Mukhamedshin) Кортициоидные грибы (Corticiaceae s. lato) Северо-Западного Кавказа // Микология и фитопатология. 1992. Т. 26. № 2. С. 104–109.
Новикова Н.М., Полянская А.В. (Novikova, Polyanskaya) Самурские лиановые леса: проблема сохранения биоразнообразия в условиях развивающегося водного хозяйства. М.: РАСХН, 1994. 106 с.
Пармасто Э., Пармасто И. (Parmasto, Parmasto) К флоре афиллофоровых грибов Чечено-Ингушской АССР // Новости систематики низших растений. 1989. Т. 26. С. 72–74.
Ставишенко И.В. (Stavishenko) Афиллофоровые грибы заповедника “Малая Сосьва” (Западная Сибирь) // Микология и фитопатология. 2011. Т. 45. № 2. С. 142–157.
Яровенко Ю.А., Муртазалиев Р.А., Ильина Е.В. (Yarovenko et al.) Заповедные места Дагестана. Махачкала: Радуга-1, 2004. 96 с.
Дополнительные материалы отсутствуют.
Инструменты
Микология и фитопатология


