Микология и фитопатология, 2020, T. 54, № 5, стр. 340-346
Influence of Verticillium Wilt Infection on The Functional Activity of the Cotton Photosynthetic Apparatus
M. M. Khotamov 1, *, V. S. Agishev 2, **, I. G. Akhmedzhanov 2, ***
1 Institute of Genetics and Plant Experimental Biology, Uzbek Academy of Sciences
111208 Yukoriyuz, Tashkent Region, Uzbekistan
b Institute of Biophysics and Biochemistry, the National University of Uzbekistan
100047 Tashkent, Uzbekistan
* E-mail: mansurhatamov@mail.ru
** E-mail: _vlad@mail.ru
*** E-mail: iskakhm@mail.ru
Поступила в редакцию 16.04.2020
После доработки 28.04.2020
Принята к публикации 11.05.2020
Аннотация
The effect of infection with the Verticillium dahliae pathogen on the functional state of the photosynthetic apparatus of leaves of the susceptible cotton variety S-4727 was studied by the method of chlorophyll fluorescence induction. It was found that the leaves of infected plants reduced the content of chlorophylls a and b and their ratio, which is accompanied by changes in the fluorescence spectra and its kinetics, i.e. a significant increase in the ratio of fluorescence intensities at 690 and 730 nm and a shift of the spectra by 10 nm to the short-wave region, as well as a significant decrease in the parameter value (FM – FT)/FM. Such drastic changes in the parameters of induced chlorophyll fluorescence indicate that when cotton is infected with wilt, the native structure of chlorophyll – protein complexes is disrupted, which leads to a change in the energy migration between the forms of chlorophyll itself and to a violation of the interaction of two pigment photosystems (FSI and FSII). The possibility of using the revealed changes in the characteristics of the spectral-kinetic curves of fluorescence of leaves of plants infected with the fungus V. dahliae as diagnostic indicators of the wilt resistance of cotton is discussed.
INTRODUCTION
Among the numerous works carried out in the field of phytoimmunity, a special place is occupied by research on cotton wilt. Physiological and biochemical studies of diseased plants showed that the infection of the plant with Verticillium wilt pathogen fungus V. dah-liae Kleb. has a significant effect on the processes of energy metabolism in the plant cell, directly related to photosynthesis, in the early stages of the disease (Ibragimov, 1978).
In this case, changes in the amount and ratio of pigments of the photosynthetic apparatus (PSA) occur (Rubin et al., 1974; Akinshina et al., 2016). The revealed changes in the content of photosynthetic pigments can be associated with a destruction of their native complexes, which can lead to a change in the energy migration between the forms of chlorophyll itself and to a disruption in the interaction of two pigment photosystems (PS I and PS II). Considering that disturbances of this kind should affect the characteristics of the induced fluorescence of chlorophyll in the leaves of diseased plants, conducting fluorescence studies can provide valuable information on the state of PSA pigment-protein complexes (Korneev, 2002). In addition, a comparative study of the fluorescence of leaves of sick and healthy plants, due to their high sensitivity, makes it possible to identify signs of the disease and related damage to the pigment apparatus in the early stages of the development of the disease (Kshirsagar et al., 2001; Mandal et al., 2009; Caldero’n et al., 2014; Fang, Ramaraja, 2015; Babar et al., 2018; Aleynikov, Mineev, 2019).
At present, when studying the activity of the photosynthetic apparatus of plants, the method of inducing chlorophyll fluorescence is widely used (Zlatev, Yordanov, 2004; Pikulenko, Bulichev, 2007; Pascual et al., 2010; Posudin et al., 2010; Caldero’n et al., 2014; Ptuchenko et al., 2014; Martinez-Ferri et al., 2016; Babar et al., 2018; Aleynikov, Mineev, 2019; Cristhian et al., 2019), since chlorophyll located in photosynthetic membranes serves as a kind of natural sensor of the state of algae and higher plants cells in changing environmental conditions (Veselovsky, Veselova, 1990). In this case, fluorimeters of various designs are used (Agishev et al., 2002; Raimondi et al., 2009; Romanov et al., 2010; Akhmedzhanov et al., 2013), which provide quick testing of plant resistance to adverse effects.
The aim of this work was to study the effect of artificial infection of plants with a phytopathogenic fungus – the causative agent of Verticillium wilt on the state of the photosynthetic apparatus of cotton, estimated by spectral-luminescent analysis and the content of green pigments – chlorophylls a and b.
MATERIALS AND METHODS
In the experiments, cotton plant of variety S-4727 (Gossypium hirsutum) was used, which was grown in vegetation vessels on sterilized vermiculite enriched with Belousov’s nutritious mixture (Zhurbitsky, 1968). In a phase of 6–7 true leaves, cotton plants were infected with a dosed inoculum of race-2 of the fungus V. dahliae from the collection of plant pathogens of the Institute of Genetics and Experimental Plant Biology of the Academy of Sciences of Uzbekistan at a rate of 2.5 million spores/ml. Plants were used as control, into the stems of which distilled water was introduced using a capillary (Avazkhodjaev et al., 1995).
A monosporous culture of V. dahliae was grown for 8 days in test tubes on a solid Chapek medium of the following composition: NaNO3 – 3 g, KH2PO4 – 1 g, MgSO4 – 0.5 g, KCl – 0.5 g, FeSO4 – 0.01 g, sucrose – 30 g, agar-agar – 20 g per 1 liter of distilled water. Sowing was carried out by the “injection” method on the surface of an agar medium. As seed material, conidia or microsclerosis of micromycetes were used separately, as well as a mixture of these fungal structures. Then, using a microbiological loop under sterile conditions, part of the conidia was transferred to Petri dishes with Chapek’s medium, where they were germinated in an incubator at a temperature of 27°C in complete darkness. A fungal spore suspension was prepared by shaking 2 ml of sterile distilled water in a test tube with a fungal culture of 10–15 days of age (Avazkhodjaev, Zeltser, 1980). The density of fungal spores in suspension was calculated according to the method described in (Israel et al., 1968). The suspension of conidia of the fungus obtained in vitro, after calculating its density, was diluted in the required concentration.
Plants were infected by injection of inoculum using a triple injection with a syringe into the stem. The inoculum was released from the syringe as a drop of suspension at the end of the needle. The needle was inserted into the stem at an angle of 45°. A drop was absorbed into the stem, and this gave visible confirmation of inoculation. The appearance of chlorosis on the lower leaves of cotton, yellowing of tissues, and necrotization of leaf blade sections indicated damage to infected plants by the wilt (Avazkhodjaev, Zeltzer, 1980).
Content of chlorophyll a and b was determined spectrophotometrically (SQ-2800 UV-Vis, Cole Parmer Co, USA) using method (Vorobyov et al., 2013), after a quick homogenization of a leaf cut, dried at room temperature in a porcelain mortar and extraction in 85% acetone solution in cold.
Induced fluorescence of chlorophyll of the control (uninfected) leaves and infected with the pathogen of Verticillium wilt plants was measured on 3, 5, 8 and 11 days after infection. The functional activity of the photosynthetic apparatus (PSA) of assimilating cotton tissues was evaluated by indicators of chlorophyll fluorescence induction (CFI) using a portable fluorimeter (Akhmedzhanov et al., 2013): light source – LED, 450–470 nm; receiver – P-I-N photodiode; fluorescence kinetics recording time up to 10 min with a resolution of 0.01 s. In this case the following ratio of parameters of the leaf fluorescence induction curve was used: (FM – FT)/FM – degree of decrease in chlorophyll fluorescence intensity characterizing the integral activity of the photosynthetic apparatus, where FM – maximum value of fluorescence induction, FT – stationary value of fluorescence after light adaptation of a leaf (Posudin et al., 2010; Romanov et al., 2010; Akhmedzhanov et al., 2013).
The fluorescence spectra of the leaves were measured on a Lidar installation (Agishev et al., 2002), the main elemental base of which is: emitter – helium-neon laser, wavelength of exciting light 632 nm, radiation power 100 mW, light beam diameter 1 cm. Receiver – Newton system telescope with 110 mm working mirror diameter. Spectral selection of the signal was carried out using the diffraction grating of the MUM monochromator. The laser mode, monochromator spectrum sweep, and the display of the results are programmed. The intensity ratio I690/I730 was used as a parameter characterizing changes in the fluorescence spectra. CFI was measured on the leaves of the middle tier in plants 6–10.
RESULTS AND DISCUSSION
In the process of verticillosis of the leaves of the wilt susceptible cotton variety S-4727 (Avazkhodjaev et al., 1995) under the influence of toxic pathogen metabolites, visible signs of a wilt disease on the leaves appear on the 18–20 days after infection, resulting in the formation of characteristic chlorosis, which has a pale green coloring.
The appearance of chlorosis indicates that, as a result of exposure to the parasite, disturbances in the photosynthetic apparatus occur, accompanied by changes in the pigment systems of the infected plant (Pavlovskaya et al., 1973). Regarding this, we conducted a comparative study of the content of chlorophyll a and b in the leaves of infected plants, and in similar areas cut from the leaves of healthy plants.
Table 1 shows the results of study of chlorophyll a and b in leaf tissues of healthy and infected with Verticillium wilt pathogen. As expected, the content of chlorophyll pigments in plants not affected by Verticillium wilt was higher. In the tissues of infected plants, the amount of chlorophyll a and b decreased by 17% compared with the control in the absence of infection. In this case, more significant changes were observed for chlorophyll a that mainly determined sharp reduction ratio of chlorophylls a and b (21%).
Table 1.
The effect of infection with Verticillium wilt on the content of photosynthetic pigments in the leaves of cotton variety S-4727
| Pigments | Uninfected plants | Infected plants | % of healthy plants |
|---|---|---|---|
| Chlorophyll a, mg/g dry weight | 2.69 ± 0.228 | 2.05±0.246 | 76.2 |
| Chlorophyll b, mg/g dry weight | 1.17 ± 0.142 | 1.14 ± 0.161 | 97.4 |
| The sum of chlorophylls a and b, mg/g dry weight | 3.86 ± 0.372 | 3.19 ± 0.296 | 82.6 |
| The ratio of chlorophyll a to chlorophyll b | 2.29 | 1.81 | 79.0 |
Thus, the data in table indicate that the destruction of the pigment apparatus of diseased leaves is expressed in a decrease in the number of green pigments and in a decrease in the ratio of chlorophyll a to chlorophyll b.
The revealed changes in the content of green pigments can be associated with a destruction of their native complexes, which can lead to a change in energy migration between the forms of chlorophyll itself and to a disruption in the interaction of two pigment photosystems (PS I and PS II ). Destructions of this kind should be reflected in the characteristics of the induced fluorescence of chlorophyll in the leaves of diseased plants.
As expected, changes in the induction curves and fluorescence spectra of cotton leaves of variety S-4727 occur upon infection with the pathogen of Verticillium wilt. Thus, the parameter (FM – FT)/FM of the kinetic fluorescence curves characterizing the processes occurring in the electron-transport chain of PSA (Nesterenko, Sidko, 1993) undergoes significant changes after infection of plants with a wilt. The greatest change in fluorescence kinetics parameter occurs at 8–11 days after infection and by day 11, a decrease in the ratio (FM–FT)/FM, calculated for induction curves measured at two wavelengths (690 and 730 nm) reaches almost 3 and 7 times, respectively (Fig. 1, A, B).
Fig. 1.
Changes in the characteristics of the induction curves of chlorophyll fluorescence (FM – FT)/FM depending on the time of infection of the cotton plant of variety S-4727. Measurement at a wavelength of 690 nm (a) and 730 nm (b): 1 – healthy plants; 2 – plants infected with a Verticillium wilt. The confidence interval for the average values was at least 95% (P ≤ 0.05).
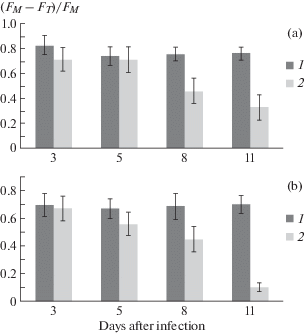
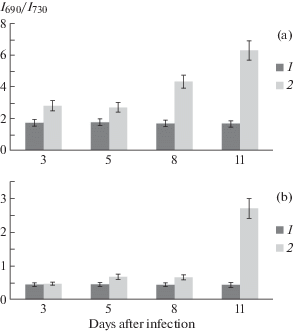
Figure 2, A shows the fluorescence spectra of leaves of healthy and infected cotton plants of variety S-4727, measured at room temperature. Results showed that at day 3 after infection occurs a slight increase in the ratio of I690/I730 compared with the control, on day 5 there is a slight decrease in its value, then, from day 8, it rises sharply and on day 11, the value I690/I730 becomes almost 2.5 times larger than on day 3. When this value of the ratio I690/I730 in healthy plants remained virtually unchanged, slightly varying within measurement error and throughout the period of measurement of fluorescence spectra (Fig. 3, А).
Fig. 2.
Fluorescence spectra of cotton leaves of variety S-4727 at room temperature (A) and 77 K (B). A1, В1 – control (days after the infecting lines): 3 days – (A2, В2), 5 days – (A3, В3), 8 days – (A4, В4), 11 days – (A5, В5). On the x-axis – wavelength, nm; on the y-axis – fluorescence intensity, rel. units.
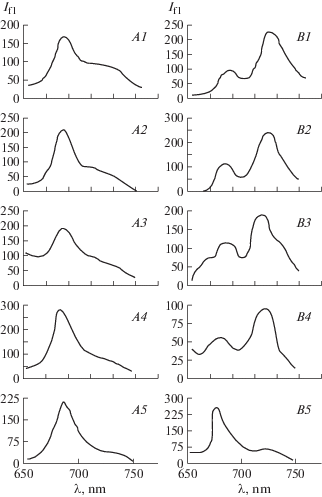
Fig. 3.
Changes in spectral characteristics of the laser-induced fluorescence of chlorophyll (LIFC) depending on the time of infection of S-4727 cotton variety. а – room temperature, в – liquid nitrogen temperature (77 К). 1 – control, 2 – infected plants. The confidence interval for the average values was at least 95% (P ≤ 0.05).
Changes in ratio I690/I730 are defined, on the one hand, by a significant increase in fluorescence intensity at 690 nm, which is apparently due to impairment of the native structure of the electron-transport chain (ETC) connecting photosystems I and II. The introduction of pathogen metabolites into the membranes of the host plant leads to the reduction of PS II, which contributes to the waste of the energy of excited molecules on luminescence. On the other hand, significant contribution to the increase of the ratio I690/I730 makes a sharp decrease in the intensity of fluorescence in the region of 730 nm, due likely to destruction of PS I (maximum luminescence spectrum – 730 nm).
Figure 2, B shows the fluorescence spectra of S-4727 cotton variety leaves, measured at liquid nitrogen temperature (77 K). It is known that at such temperatures, photosynthetic processes are completely stopped, while the excitation energy is almost completely spent on fluorescence (Voronkov et al., 1976; Veselovsky, Veselova, 1990). Another advantage of measuring low-temperature fluorescence spectra is that PS I practically does not fluoresce at room temperature, and the quantum yield is very small (Voronkov et al., 1976). Thus, freezing leaves to liquid nitrogen temperature has a dramatic effect on the ratio of the intensities of the maxima of the low-temperature fluorescence spectra of the control sample I690/I730, whose value changes to the opposite (Fig. 3, В). On day 5 after infection of plants, the fluorescence intensity at 730 nm decreases due to a shift of the new wavelength maximum of the spectrum to 725 nm, which leads to an increase in the ratio I690/I730 by almost 1.5 times compared to the control. Even more significant shift observed for the short-range maximum, which at day 11 after infection becomes fixed at about 680 nm, shifting approximately 10 nm to the shorter wavelengths. In this case, the low-temperature spectrum becomes similar to the spectra measured at room temperature, in which the intensity of the short-wave maximum is greater than the intensity of the long-wave, which mainly determines a significant, more than 4-fold increase in the value of I690/I730 compared to its value on day 5 (Fig. 3, В).
According to the data of Voronkov et al. (1976), in both I and II photosystems, the long-wavelength forms of chlorophyll are most sensitive to Verticillium wilt, although the pigments that determine fluorescence in the short-wave region also change, but to a much lesser extent. In this regard, the revealed changes in the low-temperature fluorescence spectra of the leaves of infected plants associated with a sharp decrease in the fluorescence intensity in the long-wavelength region of the spectrum are most likely due to the destruction of long-wavelength forms of chlorophyll-protein complexes (Korneev, 2002).
Many researchers (Pavlovskaya et al., 1973; Voronkov et al., 1976; Kshirsagar et al., 2001; Mandal et al., 2009; Pascual et al., 2010; Akinshina et al., 2016; Aleynikov, Mineev, 2019; Cristhian et al., 2019) note a significant decrease in photosynthetic activity of plants when they are affected by phytopathogenic organisms, which may be associated with a decrease in the content of photosynthetic pigments and a interruption of the outflow of photosynthesis products due to PSA damage. In this case, disturbances in the activity of PSA are effectively recorded by the CFI method, the parameters of which vary depending on the degree of damage to the plant (Kshirsagar et al., 2001; Martinez-Ferri et al., 2016; Yi Fang, Ramaraja, 2016, Babar et al., 2018).
Our results revealed that the infection of susceptible cotton S-4727 variety with Verticillium wilt pathogen reduced total chlorophyll content and the ratio of chlorophyll a to chlorophyll b accompanied by changes in fluorescence spectra and its kinetics, i.e. a significant increase in the ratio of fluorescence intensities at 690 and 730 nm and 10 nm shift of low-temperature spectra toward shorter wavelengths, as well as a significant decrease in the parameter value (FM – FT)/FM. Such changes in parameters of spectral and induction fluorescence curves of plant leaves can occur with significant damages to the native structure of chlorophyll-protein complexes and the interaction of two pigment photosystems (PS I and PS II).
The obtained data generally correspond to the conclusions of a number of scientific articles on the disruptions in the photosynthetic activity of various plants infected with phytopathogenic organisms, recorded by the IFC method (Kshirsagar et al., 2001; Mandal et al., 2009; Caldero’n et al., 2014; Yi Fang, Ramaraja, 2015; Babar et al., 2018; Aleynikov, Mineev, 2019). In this regard, the revealed changes in the characteristics of the spectral-kinetic fluorescence curves of leaves of plants infected with the fungus V. dahliae suggest the possibility of their use as a diagnostic indicator of cotton’s wilt resistance. However, to confirm this hypothesis, it is necessary to carry out similar studies of fluorescence of leaves of cotton genotypes contrasting in wilt resistance.
Список литературы
Agishev V.S., Khusainov I.A., Zinoviev A.V., Usmanov T.B., Akhmedzhanov I.G. Investigation of the spectral and temporal characteristics of the luminescence of higher plants upon excitation by laser radiation with various energy and time parameters. Uzbekskiy biologicheskiy zhurnal. 2002. № 5–6. P. 80–83 (in Russ.).
Akhmedzhanov I.G., Agishev V.S., Dzholdasova K.B. et al. The use of a portable fluorimeter to study the effect of water deficit on the characteristics of delayed fluorescence of cotton leaves. Doklady Akademii nauk Uzbekistana. 2013. № 3. P. 58–60 (in Russ.).
Akinshina N.G., Rashidova D.K., Azizov A.A. Seed encapsulation in chitosan and its derivatives restores levels of chlorophyll and photosynthesis in wilt-affected cotton (Gossypium L., 1753) plants. Selskokhozyaistvennaya biologiya. 2016. V. 51 (5). P. 696–704.
Aleynikov A.F., Mineev V.V. Effect of the fungus of Ramularia tulasnei Sacc. on chlorophyll fluorescence in garden strawberry. Sibirskiy vestnik selskokhozyaistvennoy nauki. 2019. V. 49 (2). P. 94–102 (in Russ.).
Avazkhodjaev M.Kh., Zeltzer S.S., Nuritdinova H., Raviprakashi G.D. Phytoalexins as a factor in Wilt Resistance of Cotton. In: Handbook of phytoalexin metabolism and action. Marcel Dekker Inc., N.Y. etc., 1995, pp. 129−160.
Babar M.A., Saleem M., Hina A. et al. Chlorophyll as biomarker for early disease diagnosis. Laser Physics. 2018. V. 28 (6). P. 158–163.
Caldero’n R., Lucena C., Trapero-Casas J.L. et al. Soil temperature determines the reaction of olive cultivars to Verticillium dahliae pathotypes. Plos One. 2014. V. 9 (10). P. 1–16. https://doi.org/10.1371/journal.pone.0110664
Cristhian C.C.A., Sandra G.C., Herman R.D. Physiological, biochemical and chlorophyll fluorescence parameters of Physalis peruviana L. seedlings exposed to different short-term waterlogging periods and Fusarium wilt infection. Agronomy. 2019. V. 9 (5). P. 213–219.
Fang Y., Ramasamy R.P. Current and prospective methods for plant disease detection. Review. Biosensors. 2015. V. 5 (3). P. 537–561.
Ibragimov A.P. Molecular genetics features of cotton resistance to wilt. Tashkent, FAN, 1978. 99 p.
Israeli V.P., Shklyar S.N., Beltyukova K.I. et al. A guide for studying bacterial plant diseases. General issues. Kolos, Moscow, 1968. 343 p. (in Russ.).
Korneev D.Yu. Information possibilities of the method of inducing fluorescence of chlorophyll. Kiev, Alterpres, 2002. 188 p.
Kshirsagar A., Reid A.J., McColl S.M., Saunders V.A., Whalley A.J.S., Evans E.H. The effect of fungal metabolites on leaves as detected by chlorophyll fluorescence. New Phytol. 2001. V. 151 (2). P. 451–457.
Mandal K., Saravanan R., Maiti S., Kothari I.L. Effect of downy mildew disease on photosynthesis and chlorophyll fluorescence in Plantago ovata Forsk. J. Plant Diseases Protection. 2009. V. 116 (4). P. 164–168.
Martinez-Ferri E., Zumaquero A., Ariza M.T. et al. Nondestructive detection of white root rot disease in avocado root-stocks by leaf chlorophyll fluorescence. Plant Diseases. 2016. V.100 (1). P. 49–58.
Nesterenko T.V., Sidko F.Ya. On the quantitative description of the slow induction of chlorophyll fluorescence in the ontogenesis of leaves of higher plants. Fiziologiya rasteniy. 1993. V. 40 (1). P. 10–15 (in Russ.).
Pascual I., Azcona I., Morales F. et al. Photosynthetic response of pepper plants to wilt induced by Verticillium dahliae and soil water deficit. J. Plant Physiol. 2010. V. 167 (9). P. 701–708.
Pavlovskaya N.E., Tukhtaeva G.M., Khojaev A.S. On the quantitative change in pigments and photo- chemical activity of cotton chloroplasts under the influence of the fungus Verticillium dahliae. Uzbekskiy biologicheskiy zhurnal. 1973. N 2. P. 26–28 (in Russ.).
Pikulenko M.M., Bulychev A.A. Using the parameters of fluorescence and the generation of electric potentials in the membranes of plant cells to assess the state of biological objects. Bulleten MOIP. Ser. Biol. 2007. V. 112 (1). P. 80–84 (in Russ.).
Posudin Yu.I., Godlevska O.O., Zaloilo I.A. et al. Application of portable fluorometer for estimation of plant tolerance to abiotic factors. Int. Agrophysics. 2010. V. 24 (4). P. 363–368.
Ptushenko V.V., Ptushenko O.S., Tikhonov A.N. Induction of chlorophyll fluorescence, chlorophyll content and leaf color characteristics as indicators of the aging of the photosynthetic apparatus in woody plants. Biokhimiya. 2014. V. 79 (3). P. 260–272 (in Russ.).
Raimondi V., Cecchi G., Lognolli D. et al. The fluorescence lidar technique for the remote sensing of photoautotrophic biodeteriogens in the outdoor cultural heritage: A decade of in situ experiments. Int. Biodeteriorat. Biodegradation. 2009. V. 63 (7). P. 823–835. https://doi.org/10.1016/j.ibiod.2009.03.006
Romanov V.A., Galeluka I.B., Sakharan E.V. Portable fluorimeter and features of its application. Sensornaya elektronika i mikroskopicheskie tekhnologii. 2010. V. 1 (7). P. 146–152 (in Russ.).
Rubin B.A., Voronkov L.A., Perova I.A. et al. Changes in the pigment composition of cotton leaves with Verticillium wilt disease. Biologicheskie nauki. 1974. N 9. P. 57–63 (in Russ.).
Veselovsky V.A, Veselova T.V. Luminescence of plants. Theoretical and practical aspects. Nauka, Moscow, 1990 (in Russ.).
Vorobyov V.N., Nevmerzhitskaya Yu.Yu., Khusnetdinova L.Z. et al. Practical works on plant physiology. Kazan, 2013 (in Russ.).
Voronkov L.A., Perova I.A., Shvyreva V.V. The effect of Verticillium wilt infection on the structure and functions of the photosynthetic apparatus of cotton. In: Pathological physiology and plant immunity. Moscow, 1976, pp. 172–188. (in Russ.).
Zhurbitsky Z.I. Theory and practice of the vegetative method. Moscow, Nauka, 1968. 268 p. (in Russ.).
Zlatev Z.S., Yordanov I.T. Effects of soil drought on photosynthesis and chlorophyll fluorescence in bean plants. Bulg. J. Plant Physiol. 2004. V. 30. P. 3–18.
Агишев В.С., Хусаинов И.А., Зиновьев А.В. и др. (Agishev et al.) Исследование cпектральных и временных характеристик люминесценции высших растений при возбуждении лазерным излучением с различными энергетическими и временными параметрами // Узбекский биологический журнал. 2002. № 5–6. С. 80–83.
Акиншина Н.Г., Рашидова Д.К., Азизов А.А. (Akinshina et al.) Капсулирование семян препаратами хитозана и его производных восстанавливает фотосинтез у растений хлопчатника (Gossypium L., 1753) на фоне вилта // Сельскохозяйственная биология. 2016. Т. 51. № 5. С. 696–704.
Алейников A.Ф., Минеев В.В. (Aleynikov, Mineev) Влияние гриба Ramularia tulasnei Sacc. на флуоресценцию хлорофилла садовой клубники // Сибирский вестник сельскохозяйственной науки. 2019. Т. 49. № 2. Р. 94–102.
Ахмеджанов И.Г., Агишев В.С., Джолдасова К.Б. и др. (Akhmedzhanov et al.) Применение портативного флуориметра для исследования влияния водного дефицита на характеристики замедленной флуоресценции листьев хлопчатника // Доклады Академии наук Республики Узбекистан. 2013. № 3. С. 58–60.
Веселовский В.А., Веселова Т.В. (Veselovskiy, Veselova) Люминесценция растений. Теоретические и практические аспекты. М.: Наука, 1990. 176 с.
Воробьев В.Н., Невмержитская Ю.Ю., Хушнетдинова Л.З. и др. (Vorobyov et al.) Практикум по физиологии растений. Казань, 2013. 168 с.
Воронков Л.А., Перова И.А., Швырева В.В. (Voronkov et al.) Влияние заражения вертициллезным вилтом на структуру и функции фотосинтетического аппарата хлопчатника // Патологическая физиология и иммунитет растений. МГУ, 1976. С. 172–188.
Журбицкий З.И. (Zhurbitskiy) Теория и практика вегетационного метода. М.: Наука, 1968. 268 с.
Ибрагимов А.П. (Ibragimov) Молекулярно-генетические особенности устойчивости хлопчатника к вилту. Ташкент: ФАН, 1978. 99 с.
Израильский В.П., Шкляр С.Н., Бельтюкова К.И. и др. (Israilskiy et al.) Руководство для изучения бактериальных болезней растений. Общие вопросы. Москва: Колос, 1968. 343 с.
Корнеев Д.Ю. (Korneev) Информационные возможности метода индукции флуоресценции хлорофилла. Киев: Альтерпрес, 2002. 188 с.
Нестеренко Т.В., Сидько Ф.Я. (Nesterenko, Sidko) О количественном описании медленной индукции флуоресценции хлорофилла в онтогенезе листьев высших растений // Физиология растений. 1993. Т. 40. № 1. С. 10−15.
Павловская Н.Е., Тухтаева Г.М., Ходжаев А.С. (Pavlovskaya et al.) О количественном изменении пигментов и фотохимической активности хлоропластов хлопчатника под влиянием гриба V. dahliae // Узбекский биологический журнал. 1973. № 2. С. 26–28.
Пикуленко М.М., Булычев А.А. (Pikulenko, Bulychev) Использование параметров флуоресценции и генерации электрических потенциалов в мембранах растительных клеток для оценки состояния биологических объектов // Бюл. МОИП. Отд. биол. 2007. Т. 112. № 1. С. 80–84.
Птушенко В.В., Птушенко О.С.,Тихонов А.Н. (Ptushenko et al.) Индукция флуоресценции хлорофилла, содержание хлорофилла и характеристики цветности листьев как показатели старения фотосинтетического аппарата у древесных растений // Биохимия. 2014. Т. 79. № 3. С. 260–272.
Романов В.А., Галелюка И.Б., Сахаран Е.В. (Romanov et al.) Портативный флуориметр и особенности его применения // Сенсорная электроника и микроскопические технологии. 2010. Т. 1. № 7. С. 146–152.
Рубин Б.А., Воронков Л.А., Перова И.А. и др. (Rubin et al.) Изменение пигментного состава листьев хлопчатника при заболевании вертициллезным вилтом // Биологические науки. 1974. № 9. С. 57–63.
Дополнительные материалы отсутствуют.
Инструменты
Микология и фитопатология


