Микология и фитопатология, 2020, T. 54, № 5, стр. 384-388
MICROMYCETES ROSSICAE: СHOROLOGICAL AND TAXONOMICAL NOTES. 2. MELAMPSORA ARCTICA (PUCCINIALES, BASIDIOMYCOTA) – UREDINIOSPORE VARIABILITY IN SPECIMENS FROM EUROPEAN AND SIBERIAN ARCTIC
I. V. Zmitrovich 1, *, V. A. Dudka 1, **
1 Komarov Botanical Institute of the Russian Academy of Sciences
197376 St. Petersburg, Russia
* E-mail: iv_zmitrovich@mail.ru
** E-mail: dudkavasiliy.a@gmail.com
Поступила в редакцию 15.04.2020
После доработки 5.05.2020
Принята к публикации 11.05.2020
Аннотация
The rust fungus Melampsora arctica was described by Rostrup from Greenland in 1888. The uredinium and telium stages of this fungus were confined to polar Salix spp. In 1899, the spermagonium and aecium stages associated with this species were revealed on Saxifraga spp. During the XX century, M. arctica has been consistently recorded in all regions of the Arctic (Alaska, Canada, Greenland, Iceland, Spitsbergen, continental Fennoscandia, the European and Siberian sectors of the Russian Arctic, and the Russian Far East). However, by the beginning with XXI century, there was a tendency to include this species (in the status of a specialized form) in the synonymy of M. epitea, a species with wider distribution range, covering southern mountain regions of Eurasia. Molecular studies by Chinese research group (2017) showed an independent species status of M. arctica, that raised a question of need to further testing the homogeneity of the species within a circumpolar belt. In 2019, the herbarium of the Komarov Botanical Institute of the Russian Academy of Sciences was replenished with additional material from West Spitsbergen Island, where the fungus (stages II, III) was confined to Salix polaris leaves. A comparative morphometry study of this specimen vs. specimens from the Siberian sector of the Arctic (Taimyr Peninsula) was carried out. The analysis showed a significant overlap of the ureiniospore variability spectra for representatives of Melampsora arctica in Europe and Siberia (p = 0.8 > 0.05), and a significant difference was revealed between the spore width in Taimyr specimen and that of recently collected specimen from West Spitsbergen Island. The former is characterized by almost globose (on average) urediniospores and more pronounced exosporium ornamentation, whilst the paraphyses in this specimen are sufficiently longer. The other specimen from Taimyr had also visually wider urediniospores (vs. European material), but in this case the differences were not statistically confirmed. In the future, it is planned to verify revealed fine differences by molecular methods.
The present notice continues a series devoted to rare and interesting species of micromycetes of various regions of Russia that cause rust and other leaf spots (Zmitrovich et al., 2020).
The genus Melampsora Castagne (Melampsoraceae, Pucciniales, Pucciniomycetes, Basidiomycota) is presented by more than 100 species of rust fungi, prevalent in the Northern Hemisphere. The genus is characterized by spermatogonia of type 2, group I (subepidermal) or type 3, group I (subcuticular) (Hiratsuka, Hiratsuka, 1980) and subepidermal aecia of the Caeoma-type with rudimentary subinvisible peridium. Aeciospores of representatives of this genus are as a rule warty and lying in chains. Subepidermal uredinia of Uredo-type, with numerous club-shaped or capitate paraphyses and normally rapidly disappearing peridium. Urediniospores stalked, finely ornamented; their germination pores are scattered or arranged within two zones. Telia develop subepidermally, less often subcuticularly. Teliospores mostly 1(2)-celled, arranged into 1–2-layered dense palisade, pigmented, giving rise an external basidia (Arthur, 1934, 1962; Gäumann, 1959; Azbukina, 2015).
This genus includes both macrocyclic monoecious or heteroecious species as well as microcyclic ones (Pei, 2005). Some species of the are widely distributed over Russia, as Melampsora abietis-populi S. Imai, M. caprearum (DC.) Thüm., M. euphorbiae (C. Schub.) Castagne, M. hypericorum (DC.) G. Winter in Rabenh., M. larici-pentandrae Kleb., M. larici-populina Kleb., M. magnusiana Wagner ex Kleb., M. paradoxa Dietel et Holw., and M. populnea (Pers.) P. Karst. The host range of the genus representatives is rather wide and includes both coniferous and deciduous trees or herbaceous plants, whilst the uredinia and telia of macrocyclic species often associated with leaves of various species of the Salicaceae family (Kuprevich, Transhel, 1957; Pei, 2005; Azbukina, 2015).
In July 2019, an arctoalpine representative of the genus, M. arctica Rostr., was found by I.Yu. Kirtsideli at the Russian base of the Spitsbergen archipelago, in the valley of the river Grendalen. Uredinia of this fungus abundantly covered the Salix polaris leaves.
The lectotype of this species was confined to S. groenlandica (Rostrup, 1888), but in the pre-molecular period its taxonomy was rather unstable. Bagyanaryana (2005) formally described this species as forma specialis of Melampsora epitea (Kunze et J.C. Schmidt) Thüm., although the tendency to combine both species existed earlier (Smith, Blanchette, 2004; Azbukina, 2005). As a linneon which included also M. alpina (Arthur) Juel (1894), the species was indicated as arctoalpine and circumpolar – distributed through Alaska, Canada, Greenland, Iceland, Spitsbergen and the continental polar Fennoscandia. In the Russian sector of the Arctic, it was indicated for the Murmansk and Arkhangelsk regions, the Komi Republic, the Tyumen Region, Yakutia, Chukotka, the Magadan and Kamchatka regions, the Sakhalin Region, the Primorsky Territory, as a subalpic also for the Altai Republic and Ciscaucasia mountains (Karatygin et al., 1999; Azbukina, 2005, 2015).
However, a molecular revision of the Salix-associated Melampsora species of the Asian Holarctic sector showed an independent species status of M. alpina and M. epitea (Zhao et al., 2017). Since M. epitea has a more southern distribution pattern than M. arctica, a reasonable question about possible finer differentiation of the last one has arisen to date.
Despite of extensive reports on M. arctica in a literature, in the herbarium of the Komarov Botanical Institute of the Russian Academy of Sciences only three specimens of this species (all from the Taimyr Peninsula) were found. These specimens are represented by uredinium stage, confined to the leaves of polar willows. The purpose of this notice is a comparative morphological analysis of specimen from Spitsbergen and the Taimyr Peninsula material, with special emphasis on urediniospore morphometry and aim to detect the absence or presence of European vs. Asian material differentiation.
The find was made on the West Spitsbergen island, in Grendalen valley (Jule 2019). The locality coordinates are 78′01″345 N, 14′22″988 E (coll. I. Yu. Kirtsideli). According to the Barentsburg meteorological observatory, the average annual value of total solar radiation in this area is 528 804 000 calories/m2, the average duration of sunshine is 886 hours. On the latitude of Barentsburg from April 19 to August 24, stays a polar day, from October 28 to February 15 – a polar night. The average annual temperature of the warmest month (July) is 8°С. The coldest month is February with a temperature ca. –18°С. On average, 563 mm of precipitation falls annually, which mainly falls in January – February. The territory is located in the permafrost zone, the depth of summer thawing is 98–190 cm, depending on the nature of the vegetation cover. Climatic features determine a short growing season (40–70 days), the duration of which is determined by the time of snow melting in local habitats (Osokin, Sosnovskiy, 2008).
The leaves of living plants were herbarized according to standard reccommendations (Geltman, 1995). Dried shoots were viewed using the MBS-3 binocular stereoscopic microscope. The micromorphological analysis of the basidiomata was carried out using an Axio Scope A1 light microscope at the Laboratory of Systematics and Geography of the Fungi (BIN RAS). Micro-preparations for general hyphal morphology study were prepared using a 5% KOH solution. Such media as Melzer’s reagent, Congo Red, and 5% NH4OH solution were used to testing of thickened wall structures (spore surface sculpture). The urediniospores measurements were carried out into the distilled water. The material collected is loaned in the Mycological Herbarium of the Komarov Botanical Institute of the Russian Academy of Sciences (LE F).
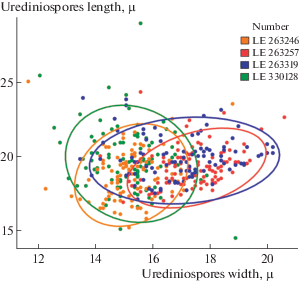
For each specimen, a total of 100 uredinospores were measured in length and width. The mean, minimum and maximum values as well as the standard deviation were calculated. Statistical estimation of the data was performed using the programming language R 3.3.3 (R Core Team, 2012) in the software environment RStudio 1.0.136 (RStudio Team, 2017). To measure the overall variability of the data, a Multiple Linear Regression analysis was used. The graph was visualized using the “ggplot2” package (Wickham, 2009).
To study a significance of differences in the variability of urediniospores within a species, an analysis of multiple linear regression was used. The data obtained was standardized by logarithmization of log(x). After constructing the linear model, a check was performed for multicollinearity: M = 1/1 – R2, Cooke distance, the remainder of the predicted values, and the quantile graph of the residuals (Parmasto et al., 1987).
Below follow the diagnosis of the species (stages II, III) based on morphological analysis and the results of comparative morphometry of the urediniospores in specimens from European vs. Siberian sectors of the Arctic.
Melampsora arctica Rostr., Meddr. Grønland, Biosc. 3: 535, 1888; Uredo rostrupiana Arthur, N. Amer. Fl. 7 (2): 100, 1907; ?Melampsora alpina Juel, Öfvers. K. Svensk. Vetensk.-Akad. Förhandl. 51 (8): 417, 1894. – Fig. 1.
Fig. 1.
Melampsora arctica (LE 330128): a – general view of infested Salix polaris leaves; b–c – uredinia spots collection on leaves underside; d – urediniospore outlines drawing without visualization of ornamented exosporium; e – paraphyses drawing; f – paraphyses under light microscope; g–h – urediniospores under light microscope (fine ornamentation is visible between external and internal contours of the spore wall). Scale bars – 20 μm. Photo and drawings by V.A. Dudka.

Uredinia minute, amphigenous, mainly hypophyllous, scattered or aggregated, 0.2–1.1 mm in diam., subepidermal, erumpent, pulverulent, bright orange-yellow. Urediniospores 14.5–28.9 × 11.7–20.6 μm, broadly ellipsoid to subglobose, wall to 2 mm thick, finely echinulate (inside a smooth contour under light microscope)11. Paraphyses numerous, clavate to capitate, 30–65 × 13.5–30 μm long, wall 4–6.5 μm thick.
Telia minute, amphigenous, scattered, 0.2–0.7 mm in diam., often fusing, reddish-brown, subepidermal (non-erumpent). Teliospores prismatic, rarely club-like, 20–48 × 8–17 μm, with pale golden-brown contents; their wall reaches 1.5 μm thick.
Material examined: Spitsbergen, Salix polaris (LE 330128); Taymyr peninsula, S. polaris (LE 263246); Taymyr peninsula, S. polaris (LE 263257); Taymyr peninsula, S. arctica (LE 263319).
Spermogonia (0) and aecia (I) of this species develop on Saxifaga spp. (Jacky, 1899) and were not found by us. According to literature descriptions, spermogonia amphigenous, scattered or in groups, subepidermal, 150–160 μm wide, 90–130 μm high; aecia minute, caeomoid, amphigenous, mainly hypophyllous, 0.3–0.5 mm, subepidermal, erumpent, pulverulent; aeciospores 15–26 × 15–21 μm, globose, ovoid, rarely angular, wall 2–3 μm thick, finely verrucose (Bagyanarayana, 2005).
The most allocable morphological differences of the species in question from M. reticulatae A. Blytt, the fungus, also reported from polar willows, are reduced to the wall thickness of the urediniospores – up to 2 μm in M. arctica and 2.5–6 μm in M. reticulatae (limits of variation of the urediniospores of this species are 15–30 × 11–25 μm; see Azbukina, 2005). Thus, the variability spectra of urediniospores of both species sufficiently overlap. The sizes of urediniospores given in the above diagnosis were presented as a result of their morphometric study (for detailed picture, see Table 1).
Table 1.
Urediniospores morphometry on Melampsora arctica specimens
| Specimens studied | Lmean | Wmean | Lmin | Wmin | Lmax | Wmax | LSD | WSD |
|---|---|---|---|---|---|---|---|---|
| LE 330128 | 19.66 | 15.26 | 14.50 | 12.10 | 28.90 | 18.90 | 2.07 | 1.12 |
| LE 263246 | 18.79 | 15.29 | 15.20 | 11.70 | 25.00 | 18.80 | 1.67 | 1.01 |
| LE 263257 | 19.32 | 17.51 | 16.90 | 14.20 | 24.30 | 20.60 | 1.33 | 1.13 |
| LE 263319 | 19.86 | 17.03 | 17.10 | 13.50 | 23.90 | 20.20 | 1.40 | 1.52 |
In general, the analysis showed a significant overlap of the variability spectra of urediniospores in representatives of M. arctica in Europe and Siberia (p = 0.8 > 0.05) as well as the fact that these indicators (i.e., the length and width of urediniospores) have described less than 10% all variability (Multiple R-squared: ≈ 0.10). This indicates that exist some additional factors affecting the variability of urediniospores and they aren’t taken into account in our statistical model. However, the p-value in a studied model showed a significant difference between the spore width in one Taimyr specimen (LE 263319) (p = 0.048 < 0.05) and that of the Spitsbergen specimen (LE 330128) (Fig. 2). A subglobose form of urediniospores and their larger ornamentation spines are really characteristic of this specimen, whilst its paraphyses are sufficiently longer. The other Taimyr specimen (LE 263257) had a seemingly rounder shape of urediniospores, too, but the p-value of the urediniospore width (p = 0.068 > 0.005) didn’t confirm a significant difference.
It is obvious that in order to obtain a more reliable view to the nature of spore variability, it is necessary to take into account also some other parameters: together with urediniospore parameters (such as spore wall thickness, spines length). Also, it is necessary to include some external factors (habitat environments, the climate of macroregion) and to expand selection sites in order to accommodation some more geographic patterns. In fundamental monograph by Parmasto et al. (1987) a lot of examples of intraspecific variability of spores in various representatives of basidiomycetes and peronosporalean fungi was considered, although it should be noted that this monograph was completed in a pre-molecular period, therefore a range of linneons under consideration was consequently splitted basing on molecular phylogenetic studies. We hope that some fine deviations of the Taimyr material on M. arctica would be sufficient basis for its further molecular taxonomic testing.
The authors are very grateful to Dr. Sci. I.Yu. Kirtsideli for M. arctica sample provision. The work was carried out using technique of the Center “Cellular and Molecular Technologies for Studying Plants and Fungi” at Komarov Botanical Institute of the Russian Academy of Sciences. Laboratory work was supported by the RFBR grant (N 19-04-00024 A) and the State Research Task № AAAA-A19-119020890079-6.
Список литературы
Arthur J.Ch. Manual of the rusts of United States and Canada. Lafayatte, 1934.
Arthur J.Ch. Manual of the rusts of United States and Canada. Reprint by G.B. Cummins. Hafner, N.Y., 1962.
Azbukina Z.M. (Azbukina) Rust fungi. (Lower plants, fungi and bryophytes of the Russian Far East. Fungi. V. 5). Dalnauka, Vladivostok, 2005 (in Russ.).
Azbukina Z.M. Keybook to the fungi of Russia. Order Pucciniales. 1. Families Pucciniastraceae, Cronartiaceae, Coleosporiaceae, Melampsoraceae, Phakopsoraceae, Chaconiaceae, Mikronegeriaceae. Dalnauka, Vladivostok, 2015 (in Russ.).
Bagyanarayana G. The species of Melampsora on Salix (Salicaceae). In: M.H. Pei, A.R. McCracken (eds). Rust diseases of willow and poplar. CABI Publishing, Wallingford, 2005, pp. 29–50.
Gäumann E. Die Rostpilze Mitteleuropas. Bern, 1959.
Geltman D.V. (ed.). The Herbarium handbook. Kew, 1995 (in Russ.).
Hiratsuka Y., Hiratsuka N. Morphology of spermogonia and taxonomy of rust fungi. Rep. Tottori Mycol. Institute (Jap.). 1980. № 18. P. 257–268.
Jacky E. Untersuchungen über Einige Schweizerische Rostpilze. Ber. Schw. Bot. Ges. 1899. V. 9. P. 49.
Juel H.O. Mykologische Beiträge I. Öfversigt af Konglelige Vetenskaps-Akademiens Förhandlingar. 1894. V. 51 (8). P. 409–418.
Karatygin I.V., Nezdoyminogo E.L., Novozhilov Yu.K. et al. (Karatygin et al.) Russian Arctic Fungi. Publishing House of the St. Petersburg Chemical Pharmaceutical Academy, SPb., 1999 (in Russ.).
Kuprevich V.F., Transhel V.G. Keybook to the rusts of USSR. 1. Family Melampsoraceae. Flora sporovykh rasteniy SSSR. Nauka, Moscow, Leningrad, 1957 (in Russ.).
Osokin N.I., Sosnovsky A.V. The influence of climate change on the thermal regime of permafrost on the Svalbard archipelago. In: The nature of the shelf and archipelagos of the European Arctic. Moscow, 2008, pp. 280–284 (in Russ.).
Parmasto E., Parmasto I., Möls T. Variation of basidiospores in Hymenomycetes and its significance to their taxonomy. Bibltheca Mycol. 1987. V. 115. P. 9–168.
Pei M.H. A brief review of Melampsora rusts on Salix. In: M.H. Pei, A.R. McCracken (eds). Rust diseases of willow and poplar. CABI Publishing, Wallingford, 2005, pp. 11–28.
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2012. http://www.R-project.org/.
Rostrup E. Mykologiske meddeleser. Meddl. Bot. Forening. 1888. V. 2. P. 84–93.
RStudio Team. RStudio: Integrated Development for R. RStudio, Inc., Boston, 2017. https://rstudio.com/.
Smith J.A., Blanchette R.A. Molecular and morphological characterization of the willow rust fungus, Melampsora epitea, from arctic and temperate hosts in North America. Mycologia. 2004. V. 96 (6). P. 1330–1338.
Wickham H. Ggplot2: Elegant graphics for data analysis. Springer-Verlag, N.Y., 2009.
Zhao P., Kakishima M., Wang Q. et al. Resolving the Melampsora epitea complex. Mycologia. 2017. V. 109 (3). P. 397–401. https://doi.org/10.1080/00275514.2017.1326791
Zmitrovich I.V., Dudka V.I., Shevchuk S.V. Micromycetes Rossicae: Chorological and taxonomical notes. 1. Chrysomyxa succinea (Pucciniales, Basidiomycota) – new find for Saint Petersburg, European Russia. Mikologiya i fitopatologiya. 2020. V. 54 (4). P. 305–308. https://doi.org/10.31857/S0026364820040133
Азбукина З.М. (Azbukina) Ржавчинные грибы. (Низшие растения, грибы и мохообразные Дальнего Востока России. Грибы; т. 5). Владивосток: Дальнаука, 2005. 616 с.
Азбукина З.М. (Azbukina) Определитель грибов России. Порядок ржавчинные. 1. Семейства пукциниастровые, кронарциевые, мелампсоровые, факопсоровые, чакониевые, микронегериевые. Владивосток: Дальнаука, 2015. 281 с.
Гельтман Д.В. (Geltman) (ред.) Гербарное дело. Справочное руководство. Русское издание. Кью: Королевские ботанические сады, 1995. 342 с.
Каратыгин И.В., Нездойминого Э.Л., Новожилов Ю.К. и др. (Karatygin et al.) Грибы Российской Арктики. СПб.: Изд-во Санкт-Петербургской химико-фармацевтической академии, 1999. 212 с.
Купревич В.Ф., Траншель В.Г. (Kuprevich, Transhel) Ржавчинные грибы. 1. Сем. Melampsoraceae // Флора споровых растений СССР. Т. 4. М.; Л.: Наука, 1957. 419 с.
Осокин Н.И., Сосновский А.В. (Osokin, Sosnovskiy) Влияние климатических изменений на термический режим многолетнемерзлых пород на архипелаге Шпицберген // Природа шельфа и архипелагов европейской Арктики: матер. межд. научн. конф. (Мурманск, 9–11 ноября 2008 г.). М.: Гeoc, 2008. С. 280–284.
Дополнительные материалы отсутствуют.
Инструменты
Микология и фитопатология