Микология и фитопатология, 2021, T. 55, № 5, стр. 340-352
Species richness of Agaricomycetes on hedge vines in Ekaterinburg сity (Russia)
A. G. Shiryaev 1, *, I. V. Zmitrovich 2, **, O. S. Shiryaeva 1, ***
1 Institute of Plant and Animal Ecology of Ural Branch of the Russian Academy of Sciences
620144 Ekaterinburg, Russia
2 Komarov Botanical Institute of the Russian Academy of Sciences
197376 St. Petersburg, Russia
* E-mail: anton.g.shiryaev@gmail.com
** E-mail: iv_zmitrovich@mail.ru
*** E-mail: olga.s.shiryaeva@gmail.com
Поступила в редакцию 11.03.2021
После доработки 14.04.2021
Принята к публикации 24.05.2021
Аннотация
Species composition of basidiomycetous macrofungi (class Agaricomycetes) associated with vines in Ekaterinburg Сity environment was revealed and characterized for the first time. Over a hundred-year history of the study of the macrofungal diversity on this type of substrate was analyzed. A total of 108 species were identified during field and herbarium observations, among them 15 species (Cerioporus rangiferinus, Crepidotus subverrucisporus, Crustomyces expallens, Flammulina fennae, F. rossica, Gloeohypochnicium analogum, Hohenbuehelia grisea, Hydnophlebia chrysorhiza, Mycoacia uda, Pholiota limonella, Ph. tuberculosa, Pluteus podospileus, Radulomyces rickii, Stecherinum bourdotii, Tomentella olivascens) represent a first record for Sverdlovsk Region. One species, Loweomyces wynneae, was collected in the middle of the 20th century and has not been found in Sverdlovsk Region for more than half a century. Agaricomycetous macrofungi were found on 25 species of vines. The greatest number of fungi was found on Magnolia-Vine and Variegated-Leaf Hardy Kiwi (38 species on each), North American Virginia Creeper and Amur Grape Vine (36 species on each). The richest Agaricomycetes group is aphyllophoroid fungi (78.5% of the total number of species), whereas for agaricoids, gasteroids and heterobasidiomycetous fungi in the range 2.8–13.9%. The most widespread species, Typhula micans, was found on 16 species of the studied vines. Also, rather common species were Xylodon sambuci, Cylindrobasidium evolvens, Irpex lacteus, Peniophora cinerea, and Bjerkandera adusta. Forty four fungal species (40.7%) collected on one vine species only. In trophic mode, the group of xylosaprotrophs predominates, including those associated with dead stems (74.1%), whereas litter-destroyers contain 17.6%. Also, there are some parasitic (11.1%) and ectomycorrhizal (Pseudotomentella, Sebacina, Tomentella) species (4.6%).
INTRODUCTION
In the last two decades, the rate of invasion of alien organisms, i.e. the appearance of an increasing number of alien species per unit of area, has been significantly increased (Wagner et al., 2021). In Russia, this process is most clearly manifested in the maritime climate of the Far East, on the coast of the Black and Baltic Seas (The most dangerous.., 2018). Favorable climatic conditions, intensive green building in urbanized areas with a large-scale acquisition and import of various plant material, a large flow of tourists and international trade objects at the end of the 20th century and the beginning of the 21st century led to the appearance of dozens of invasive animal, plant and fungal species in the maritime regions (Desprez-Loustau et al., 2009; Biological invasions.., 2010; Motiejunaite et al., 2017; Morozova, Zhmylev, 2020). Recorded invasions of some alien fungi, e.g. Amphibian chytrid (Batrachochytrium dendrobatidis Longcore, Pessier et Nichols), Alder rust (Melampsoridium hiratsukanum S. Ito ex Hiratsuka), Dutch elm disease (Ophiostoma novo-ulmi Brasier) became resonant events of the Russian national scale (The most dangerous.., 2018). Similar processes are increasingly revealed in the continental climate of the Urals and Siberia (Hoshino et al., 2004; Shiryaev, 2009; Arefyev, Kazantseva, 2016; Shiryaeva, 2018; Tomoshevich, 2019).
Sverdlovsk Region (Province, Oblast) is one of mycologically long-term best-studied regions of the Urals and Russia as a whole (Demidova, 1960; Stepanova, 1971; Stepanova, Sirko, 1977; Shiryaev, 2008; Shiryaev et al., 2010, 2012; Shiryaeva, 2015). A number of macrofungal species have been identified here for the first time in the past 20–60 years, which is probably due to the increased participation of alien woody plants in a local flora (Stepanova-Kartavenko, 1967; Shiryaev, 2009; Shiryaeva, 2018). In the greenhouses of Eka-terinburg city, such East Asian species as Physalacria orientalis (Kobayasi) Berthier, Ph. cryptomeriae Berthier et Rogerson were found on the wood of Japanese Redwood [Cryptomeria japonica (L.) D. Don] and Japanese Bigleaf Magnolia [Magnolia obovata Thunb.] (Shiryaev, 2007), as well as East Asian fungi Clavulina ornatipes (Peck) Corner and Clavulinopsis aurantiocinnabarina (Schw.) Corner were collected on the soil under these trees (Shiryaev et al., 2010). In the open ground of parks, the East Asian poroid Leucophellinus irpicoides (Bondartsev ex Pilát) Bondartsev et Singer was collected on the wood of Manchurian Lime [Tilia mandshurica Rupr. et Maxim.] (Volobuev et al., 2021). On wood of Limber Honeysuckle (Lonicera orientalis L.) was found the corticoid fungus Peniophora versicolor (Bres.) Sacc. et P. Syd. widespread in the subtropical Eurasian climates (Yurchenko, 2010). Only on alien Sea Buckthorn (Hippophae rhamnoides L.), the poroid Phellinus hippophaeicola H. Jahn, specialized to this shrub species, was recorded (Shiryaev et al., 2010). All of the aforementioned species were found in Sverdlovsk Region mainly within the botanical gardens and parks, exclusively on substrates alien to the region. On the other hand, on such wild running invasive species as North-American Boxelder Maple (Acer negundo L.) and Middle-Asian Domesticated Apple (Malus domestica Borh.), some wide spread species of aphyllophoroid and agaricoid macrofungi were revealed (Ushakova, 2004; Shiryaev, 2009; Shiryaev et al., 2010; Shiryaeva, 2018; Shiryaeva, Palamarchuk, 2019). Alien herbaceous plants also take a significant contribution to the formation of diversity of the urban mycobiota (Karelina, 2017).
The macrofungi associated with such a plant life form as lianas (vines)11 have never been the topic of mycological research in Ekaterinburg and the Urals as a whole till now. This is probably due to the fact that these plants do not represent sufficient economic and resource value. In a native environment of region, there are only two species of low-growing vines – hops (Humulus lupulus L.) and Siberian Clematis (Atragene sibirica L.), whereas large woody lianas are absent in the native flora of the Urals. At the same time, the species spectrum of alien vines species in the urban flora of Ekaterinburg includes more than 60 species (Dorofeeva, 2018). In terms of the number of species, East Asian (and North American) vines significantly exceed European ones, despite the fact that the distance from Ekaterinburg to the northern border of the natural range of the Common Grape Vine (Vitis vinifera L.) in the Caucasus is about 1800 km, and about 5000 km to the Far East where Variegated-Leaf Hardy Kiwi [Actinidia kolomikta (Maxim. et Rupr.) Maxim.], Magnolia-Vine [Schizandra chinensis (Turcz.) Baill.], and Amur Grape [Vitis amurensis Rupr.] are distributed.
In Ekaterinburg Сity green building, the Magnolia-Vine (S. chinensis) and Variegated-Leaf Hardy Kiwi (A. kolomikta) are becoming more common from year to year. The demand for these species is also growing as the medicinal and food raw materials (Fedorov, 1965). The Common Grape Vine (Vitis vinifera) and Amur Grape (V. amurensis) are also widely used in modern folk and scientific medicine. Due to the warming climate, they are becoming more widespread in the Urals as a food plant: the yield of grapes is growing, and the border of wine production is shifting farther north every year (Nemytov, 2016). What species of macrofungi colonize the vines far from their natural area, in the harsh continental boreal climate environment?
The objectives of the present work were: 1) to establish the species composition of the agaricomycetous macrofungi (aphyllophoroid, agaricoid, gasteroid, and heterobasidiomycetous fungi) associated with vines in Ekaterinburg city environment; 2) to determine the level of fungal species richness on certain vines species; 3) to reveal the spectrum of life forms and trophic groups of the fungi in question.
MATERIALS AND METHODS
Ekaterinburg Сity is situated in the south boreal subzone, straight on the border of Europe and Asia. This area covers 468 km2, whereas the population reaches 1.5 million people. The average annual temperature over the past ten years has varied in the range of 2.8–3.5°С and the amount of precipitation consists 480–560 mm per year (Fick, Hijmans, 2017). The climate is continental with rather characteristic sharp variability of weather conditions and well-defined seasons. The average annual temperature in July is 19.5°С, the maximum is 39.6°С. The average annual temperature in January is –14.3°С, and the minimum is –46.7°С.
The history of vines in Ekaterinburg Сity can be traced back to the 18–19th centuries when the city developed as the administrative center of the mining industry in the Urals and Siberia. Here was the control center of the vast network of Demidov metallurgical mills, which was the main supplier of steel and gold in the Russian Empire. Wealthy plant managers visited Saint Petersburg and Moscow, went abroad, and on their return tried to equip their houses and gardens in the European manner. As ornamental crops in the gardens were grown southern species of flowers, and sometimes lianas. For example, the Common Grape Vine (V. vinifera) was brought from the Caucasus and Crimea. At the beginning of the 20th century, vines that were probably at least 50 years old were growing on several manors in the city center. In the 1940–1950s, the center of Ekaterinburg (Sverdlovsk) was actively changing: old estates were demolished, and multi-story buildings were built in their place. All grapes in the orchards were destroyed. It should be noted that already in the 18–19th centuries, the Garden Cucumber (Cucumis sativus L.) and Field Pumpkin (Cucurbita pepo L.) were grown in open ground and greenhouses, and at the end of the 19th century, as weeds, e.g. Climbing Nightshade (Solanum dulcamara L.) and Bindweed (Convolvulus arvensis L.) (Tretyakova, Kulikov, 2013). Among alien herbaceous vines in the current urban flora, the most common are Hedge False Bindweed [Calystegia sepium (L.) R. Br.], and Ground Virgins Bower (Clematis recta L.).
Native vines (hops and Siberian Clematis) have been decorative elements in urban landscape since the 19th century. The oldest plantings of these plants have been preserved in old cemeteries that have been functioning since the 19th century. Large thickets of hops develop at the Ivanovskoye cemetery (where the writer P.P. Bazhov is buried), which has existed since 1810. Centennial thickets of hops and the Siberian Clematis are also preserved at the Mikhailovsky cemetery, founded in 1865.
Currently, the alien woody vines grow in the parks of Ekaterinburg, along the walls of various buildings. The most common are North American Virginia Creeper [Parthenocissus quinquefolia (L.) Michx.], Magnolia-Vine [Schizandra chinensis], Variegated-Leaf Hardy Kiwi [Actinidia kolomikta], Amur grape [Vitis amurensis], Yellow Honeysuckle [Lonicera prolifera (Kirchn.) Rehder]. Common Grapes (V. vinifera), Chinese Bittersweet (Celastrus orbiculatus Thunb.), Regel’s Threewingnut (Tripterygium regelii Sprague et Takeda), Climbing Hydrangea (Hydrangea petiolaris Siebold et Zucc.), European Ivy (Hedera helix L.) are common in the gardens. Within the aforementioned species, the Common Grape Vine and European Ivy are most often found indoors. All the presented East Asian, North American, and European species of tree lianas were introduced into the urban flora in the 1950–1970s.
The first macromycete specimen, Inonotus hispidus, was collected in Ekaterinburg from a Common Grave Vine in 1923. The hundred-year history of fungal research on lianas can be divided into three periods (the names of fungal collectors are given): 1) 1921–1950: European lianas in old gardens, estates and cemeteries (Z.A. Demidova, A.I. Vanin, F.A. Solovjev, A.S. Kazansky); 2) 1951–1990: the destruction of old estates and the importation of vines from the Far East and North America, the development of the vines in the Botanical Gardens (N.T. Stepanova-Kartavenko, L.K. Kazantseva, A.V. Sirko, L.M. Mezentseva, E.A. Shurova); 3) 1991–2020: mature vines in the Botanical Gardens and Parks (A.G. Shiryaev, N.V. Ushakova, K.A. Fefelov, E.V. Bryndina, and O.S. Shiryaeva).
In the present work, we study macrofungi that develop only on the stems of vines, their leaves, and dead parts. The following fungal representatives were excluded from the work: 1) forming the basidiomata on the soil; 2) found in greenhouses only.
RESULTS
The following annotated list contains the names of fungal species given according to the Index Fungorum nomenclatural database (2021), whereas the plant names are given according to the Plant List nomenclatural database (2021). The fungal species are arranged in alphabetical order. The species annotation is given in the following sequence: species name; life form (Aph – aphyllophoroid, Ag – agaricoid, Ga – gasteroid, He – heterobasidiomycetous, Cla – clavarioid, Cor – corticioid, Por – poroid); trophic mode (Par – parasite, Sap – saprotroph, Myc – mycorrhiza-former), saprotrophic fungi differentiated as (W) wood-destroyers, and (L) litter-decomposers; the total number of vines species on which the fungus has been identified; habitats (O – open, G – inside the glasshouse); date of collection; abbreviation of names of collectors and identifiers.
When describing the specimens, the following abbreviation of localities were used: Botanical Garden RAS – Botanical Garden of the Ural Branch of the Russian Academy of Sciences; Central Arboretum – Central Arboretum on 8 Marta str., 37A; Arboretum on Pervomayskaya – Arboretum on Pervomayskaya str., 87; Zoo – the Ekaterinburg citizen Zoo; Vigorov Garden – Professor Vigorov medicinal plant garden; Mamin-Sibiryak house – Garden of the Mamin-Sibiryak house museum; Kalinin Machine plant – Garden of Kalinin Machine-building plant; points with single finds are described without abbreviations. The names of vines are abbreviated as following: Am – Aristolochia manshuriensis, Ak – Actinidia kolomikta, As – Atragene sibirica, Ca – Convolvulus arvensis, Co – Celastrus orbiculatus, Cr – Clematis recta, Cs – Calystegia sepium, Ds – Dioscorea caucasica, Hh – Hedera helix, Hl – Humulus lupulus, Hp – Hydrangea petiolaris, Lc – Lonicera caprifolium, Lp – L. prolifera, Md – Menispermum dauricum, Pq – Parthenocissus quinquefolia, Sc – Schizandra chinensis, Tr – Tripterygium regelii, Va – Vitis amurensis, Vac – Vitis acerifolia, Vv – V. vinifera. The names of collectors and identifiers are abbreviated as following: AR – R. Almanaite, BE – E.V. Bryndina, DL – L.M. Dorofeeva, DZ – Z.A. Demidova, FK – K.A. Fefelov, KA – A.S. Kazanskiy, KL – L.K. Kazantseva, ML – L.M. Mezentseva; SA – A.G. Shiryaev, SAV – A.V. Sirko, SN – N.T. Stepanova-Kartavenko, SO – O.S. Shiryaeva, SF – F.A. Solovyev, SY – Y.A. Shurova, UN – N.V. Ushakova, VS – S.I. Vanin, ZI – I.V. Zmitrovich.
All the collections are kept in the fungarium of the Institute of Plant and Animal Ecology, Ekaterinburg [SVER (F)]. The duplicates of some specimens were submitted to Mycological herbarium of the Komarov Botanical Institute RAS, Saint Petersburg (LE). The species new for Sverdlovsk Region are marked with asterisk.
Aleurodiscus cerussatus (Bres.) Höhn. et Litsch. – Aph-Cor, SapW; 2 [O: Vigorov Garden, dead stem Sc, 29.07.1969, Velnov/SA, SVER(F)96270; Botanical Garden RAS, dead stem Sc, 02.08.1977, SN/UN, SVER(F)96269; ibid., dead stem Va, 15.06.2020, SA/ZI, SVER(F)96183].
Amphinema byssoides (Pers.) J. Erikss. – Aph-Cor, SapW; 1 [O: Vigorov Garden, dead stem Sc, 22.09.1977, SY/SA, SVER(F)96321; Botanical Garden RAS, dead branch Sc, 18.09.2020, SA/ZI, SVER(F)96207].
Amyloporia xantha (Fr.) Bondartsev et Singer – Aph-Por, SapW; 1 [O: Mamin-Sibiryak house, dead root Vv, 08.1948, SF/SF, SVER(F)96239; Tolmachova str., partially burnt dead vine base Vv, 14.08.1952, Кotov I.Е./SN, SVER(F)96238].
Antrodiella serpula (P. Karst.) Spirin et Niemelä – Aph-Por, SapW; 1 [O: Central Arboretum, dead stem Pq, 29.08.1977, SN/SN, SVER(F)96401; Botanical Garden RAS, dead stem Pq, 25.09.2020, SA/ZI, SVER(F)96400].
A. onychoides (Egeland) Niemelä – Aph-Por, SapW; 1 [O: Botanical Garden RAS, dead stem Tr, 20.09.2007, SA/AR, SVER(F)96427].
A. romellii (Donk) Niemelä – Aph-Por, SapW; 1 [O: Botanical Garden RAS, dead stem Sc, 17.09.2020, SA/ZI, SVER(F)96237].
Apioperdon pyriforme (Schaeff.) Vizzini – Gas, SapW,L; 3 [O: Tolmachova str., fallen dead stems and leaves Vv, 17.09.1952, SN/SA, SVER(F)96322; Vigorov Garden, dead stem Sc, 29.07.1969, Velnov/SA, SVER(F)96325; Kalinin Machine plant, dead stems and leaves Hp, 30.08.1973, SAV/SAV, SVER(F)96323; Botanical Garden RAS, dead stems and leaves Sc, 02.10.2015, SA/SA, SVER(F)96324].
Armillaria borealis Marxm. et Korhonen – Ag, Par; 1 [O: Botanical Garden RAS, root Pq, 04.09.2020, Minogina E./SO, SVER(F)96236].
Athelia bombacina (Link) Pers. – Aph-Cor, SapW; 2 [O: Kalinin Machine plant, dead stems and leaves Hp, 30.08.1973, SAV/SAV, SVER(F)96426; Botanical Garden RAS, dead root Va, 17.09.2020, SA/ZI, SVER(F)96198].
A. decipiens (Höhn. et Litsch.) J. Erikss. – Aph-Cor, SapW; 4 [O: Ivanovskoe cemetery, roots Hl, 02.09.1963, SN/UN, SVER(F)96472; Mikhailovskoe cemetery, roots Hl, 04.09.1971, KL/KL, SVER(F)96471; Botanical Garden RAS, dead stems Ak, FK/NU, 23.09.2005 SVER(F)96473; ibid., dead stems Sc, 18.09.2020, SA/ZI, SVER(F)96474; G: ibid., dead stems Hh, 28.06.2005, SA/NU, SVER(F)96470].
A. rolfsii (Curzi) C.C. Tu et Kimbr. – Aph-Cor; Sap/Par; 2 [О: Botanical Garden RAS, roots Vv, 30.08.1968, SN/SN, SVER(F)96319; G: ibid., alive roots Hh, UN/AR, 30.06.2004, SVER(F)96320].
A. salicum Pers. – Aph-Cor, SapL; 1 [O: Botanical Garden RAS, fallen dead fruits Am, 10.10.2020, DL/ZI, SVER(F)986483].
Bjerkandera adusta (Willd.) P. Karst. Aph-Por, SapW; 9 [O: Tolmachova str., dead stem Vv, 26.08.1944, VS/VS, SVER(F)96530; Botanical Garden RAS, dead stem Va, 30.07.1974, KL/KL, SVER(F)96533; Kalinin Machine plant, dead stem Hp, 30.08.1973 SAV/SAV, SVER(F)96534; Botanical Garden RAS, dead stem Ak, 21.09.2020, SA/ZI, SVER(F)96531; ibid., dead stem Sc, 23.09.2020, SA/ZI, SVER(F)96536; ibid., dead stem Pq, 12.07.2020, SA/SA, SVER(F)96537; ibid., dead stem Co, 01.08.2020, SA/SA, SVER(F)96529; ibid., dead stem Dc, 10.10.2020, SA/ZI, SVER(F)96535; G: ibid., root collar Hh, 15.06.2005, SA/UN, SVER(F)96532].
Botryobasidium vagum (Berk. et M.A. Curtis) D.P. Rogers – Aph-Cor, SapW; 2 [O: Vigorov Garden, dead stem Sc, 19.09.1979, SY/UN, SVER(F)96398; Botanical Garden RAS, dead stems Va, 18.09.2020, SA/ZI, SVER(F)96399; ibid., dead stems Sc, 18.09.2020, SA/ZI, SVER(F)96397].
Byssomerulius corium (Pers.) Parmasto – Aph-Cor, SapW; 1 [O: Central Arboretum, dead stem Pq, 30.08.1976, SAV/SAV, SVER(F)96235; Botanical Garden RAS, dead stem Pq, 17.07.2020, SA/ZI, SVER(F)96234].
Ceratobasidium cornigerum (Bourdot) D.P. Rogers – Aph-Cor; Par/Sap; 3 [O: Tolmachova str., roots and fruits Vv, 12.08.1973, KL/SN, SVER(F)96497; Botanical Garden RAS, dead stem Co, 04.09.2003, UN/ZI, SVER(F)96498; G: ibid., roots Vv, 07.07.2003, FK/UN, SVER(F)96496; ibid., roots and fallen fruits Va, 29.06.2020 SA/SA, SVER(F)96499].
Cerioporus scutellatus (Schwein.) Zmitr. – Aph-Por, SapW; 2 [O: Botanical Garden RAS, dead stem Ak, 17.09.2020, SA/SA, SVER(F)96271; ibid., dead stem Va, SA/ZI, 17.09.2020, SVER(F)96272].
*C. rangiferinus (Bolton) Zmitr., Volobuev, I. Parmasto et Bondartseva – Aph-Por, SapW; 1 [O: Botanical Garden RAS, dead roots and stem Pq, 29.06.2020, SA/ZI, SVER(F) 97317].
Cerrena unicolor (Bull.) Murrill – Aph-Por, SapW; 2 [O: Kalinin Machine plant, dead stem Hp, SN/SN, 12.08.1977, SVER(F)96394; Botanical Garden RAS, dead stem Tr, 07.09.1993, SY/UN, SVER(F)96393; ibid., dead stem Tr, 10.08.2003, UN/UN, SVER(F)96396; ibid., dead stem Tr, 02.10.2018, SA/SA, SVER(F)96395].
Coniophora puteana (Schumach.) P. Karst. – Aph-Cor; SapW; 2 [O: Kuibysheva str., dead base of Vv, 30.07.1953, SN/UN, SVER(F)96402; Kalinin Machine plant, roots and stem Hp, 05.10.1975, SAV/SN, SVER(F)96403].
Crepidotus mollis (Schaeff.) Staude – Ag, SapW; 2 [O: Botanical Garden RAS, dead stem Sc, 28.09.2020, DL/SO, SVER(F)96268; G: ibid., dead stem Hh, SA/SO, 10.06.2008, SVER(F)96267].
*C. subverrucisporus Pilát – Agar, SapW; 2 [O: Botanical Garden RAS, dead stem Sc, 18.09.2020, SA/SO, SVER(F)96476; ibid., dead stem Ak, 21.09.2020, SA/SO, SVER(F)96475].
Crucibulum laeve (Huds.) Kambly – Gas, SapW; 7 [O: Mikhailovskoe cemetery, dead stems Hl, As, 02.09.1956, SN/SA, SVER(F)96328; Vigorov Garden, dead stem Sc, 29.07.1969, Velnov/SA, SVER(F)96330; Botanical Garden RAS, dead stems Pq, Ak, 08.08.1978, SN/SA, SVER(F)96329, 96558, respectively; ibid., dead stems Cr, 30.09.2005, UN/SA, SVER(F)96327; G: ibid., dead stems and leaves Hh, 21.07.2014, Semkin A./SA, SVER(F)96326].
*Crustomyces expallens (Bres.) Hjortstam – Aph-Cor, SapW; 1 [O: Botanical Garden RAS, dead stem Co, 04.10.2019, SA/ZI, SVER(F)96233].
C. subabruptus (Bourdot et Galzin) Jülich – Aph-Cor, SapW; 1 [O: Tolmachova str., dead stems Vv, 07.1953, SN/UN, SVER(F)96318].
Cylindrobasidium evolvens (Fr.) Jülich – Aph-Cor; SapW; 9 [O: Tolmachova str., dead stems Vv, 17.08.1944, SF/VS, SVER(F)96418; Vigorov Garden, dead stem Sc, 29.07.1969, Velnov/SA, SVER(F)96421; Mamin-Sibiryak house, dead stems Pq, 5.09.1976, SN/SN, SVER(F)96425; Botanical Garden RAS, dead stems Ak, BE/UN, 15.09.1998, SVER(F)96422; ibid., dead stems Hh, 18.06.2001, SA/UN, SVER(F)96416; Zoo, dead stems Lp, 05.09.1984, ML/UN, SVER(F)96423; Botanical Garden RAS, dead stems Tr, 02.10.2018, SA/ZI SVER(F)96420; ibid., dead stems Co, 04.08.2019, SA/ZI, SVER(F)96424; ibid., dead stems Sc, 04.08.2019, SA/ZI, SVER(F)96415; ibid., dead stems Va, 21.09.2020, SA/ZI, SVER(F)96417; ibid., dead stems Ak, 21.09.2020, SA/ZI, SVER(F)96419].
Dacrymyces chrysospermus Berk. et M.A. Curtis – He, SapW; 1 [O: Botanical Garden RAS, dead stem Va, 29.08.1975, SAV/AR, SVER(F)96265; ibid., dead stem Va, 17.09.2020, SA/SA, SVER(F)96266].
Erythricium laetum (P. Karst.) J. Erikss. et Hjortstam – Aph-Cor, SapW; 1 [O: Botanical Garden RAS, dead stems Ak, SA/ZI, 21.09.2020, SVER(F)96469].
Exidia nigricans (With.) P. Roberts – He, SapW; 1 [O: Botanical Garden RAS, dead stem Sc, 02.09.1977, SN/SAV, SVER(F)96405; ibid., dead stem Sc, 18.09.2020, SA/ZI, SVER(F)96404].
E. repanda Fr. – He, SapW; 1 [O: Botanical Garden RAS, dead stem Ak, 21.09.2020, SA/ZI, SVER(F)96208].
*Flammulina fennae Bas – Ag, SapW; 2 [O: Arboretum on Pervomayskaya, stem Vv, 10.1954, SN/SO, SVER(F)96391; Botanical Garden RAS, stem Pq, 11.10.2010, FK/SO, SVER(F)96392].
*F. rossica Redhead et R.H. Petersen – Ag, SapW; 1 [O: Botanical Garden RAS, stem Hh, 08.08.2020, DL/SO, SVER(F)96192].
Fomitiporia punctata (P. Karst.) Murrill – Aph-Por, Par; 1 [O: Botanical Garden RAS, alive stem Vv, 30.08.2018, SA/SA, SVER(F)96211].
Gloeocystidiellum convolvens (P. Karst.) Donk – Aph-Cor, SapW; 2 [O: Kalinin Machine plant, roots and stem Hp, 05.10.1975, SAV/SN, SVER(F)96240; Botanical Garden RAS, dead stems Tr, 04.10.2019, SA/ZI SVER(F)96241].
Gloeocystidiellum porosum (Berk. et M.A. Curtis) Donk – Aph-Cor, SapW; 1 [O: Literaturny Kvartal, dead stem Pq, 29.08.1969, SN/UN, SVER(F)96315; Central Arboretum, dead stem Pq, 26.08.1977, KL/UN, SVER(F)96317; Botanical Garden RAS, dead stems Pq, 17.09.2020, SA/ZI, SVER(F)96316].
*Gloeohypochnicium analogum (Bourdot et Galzin) Hjortstam – Aph-Cor, SapW; 1 [O: Botanical Garden RAS, dead stems Ak, 21.09.2020, SA/ZI, SVER(F)96199].
Gloeoporus pannocinctus (Romell) J. Erikss. – Aph-Por, SapW; 1 [O: Botanical Garden RAS, dead stem Sc, 18.09.2020, SA/SA, SVER(F)96331].
Irpex lacteus (Fr.) Fr. – Aph-Por, SapW/Par; 9 [O: Tolmachova str., dead stem Vv, 10.08.1948, SF/SF, SVER(F)96461; Kalinin Machine plant, dead stem Hp, 09.1959, SN/SN SVER(F)96465; Botanical Garden RAS, dead stem Va, SAV/KL, 30.07.1972, SVER(F)96459; Zoo, dead stem Pq, 05.09.1984 ML/UN, SVER(F)96466; Botanical Garden RAS, dead stem Аk, 17.09.1999, UN/UN, SVER(F)96458; ibid., dead stem Co, 29.06.2000, SA/ZI, SVER(F)96467; ibid., dead stem Tr, 29.07.2013, SA/SA, SVER(F)96462; Vigorov Garden, dead stem Sc, 29.07.2013, SA/SA, SVER(F)96468; Central Arboretum, frost crack Va, 30.06.2016, SA/SA, SVER(F)96464; Botanical Garden RAS, dead stem Ak, 21.09.2020, SA/ZI, SVER(F)96457; ibid., dead stem Pq, 22.09.2020, SA/ZI, SVER(F)96460; ibid., dead stem Lp, 22.09.2020, SA/SA, SVER(F)96463].
Heteroradulum deglubens (Berk. et Broome) Spirin et Malysheva – He, SapW; 1 [O: Botanical Garden RAS, dead stem Ak, SA/ZI, 21.09.2020, SVER(F)96186].
*Hohenbuehelia grisea (Peck) Singer – Ag, SapW; 2 [O: Botanical Garden RAS, dead stems Sc, 18.09.2020, SA/SO, SVER(F)96407; ibid., dead stem Ak, 21.09.2020, SA/SO, SVER(F)96406].
Hohenbuehelia petaloides (Bull.) Schulzer – Ag, SapL; 1 [O: Botanical Garden RAS, dead leaves Ak, 18.09.2020, DL/SO, SVER(F)96201].
*Hydnophlebia chrysorhiza (Eaton) Parmasto – Aph-Cor, SapW; 1 [O: Botanical Garden RAS, dead stems Sc, 23.09.2020, DL/ZI, SVER(F)96314].
Hydnoporia tabacina (Sowerby) Spirin, Miettinen et K.H. Larss. – Aph-Cor, SapW; 4 [O: Tolmachova str., dead stems Vv, 08.1935, KA/DZ, SVER(F)96430; Kalinin Machine plant, dead stems Hp, 12.08.1977, SN/UN, SVER(F)96431; Botanical Garden RAS, dead stems Sc, 21.06.2020, SA/ZI, SVER(F)96429; ibid., dead stems Va, 19.07.2020, SA/ZI, SVER(F)96428].
Hymenochaete cinnamomea (Pers.) Bres. – Aph-Cor, SapW; 1 [O: Botanical Garden RAS, dead stems Va, 12.09.2020, DL/ZI, SVER(F)96477].
Hyphoderma setigerum (Fr.) Donk – Aph-Cor, SapW; 2 [O: Botanical Garden RAS, dead stem Ak, 10.08.1974, NS/NS, SVER(F)96332; ibid., dead stem Ak, 02.06.2020, SA/ZI SVER(F)96334; ibid., dead stem Sc, 02.06.2020, SA/ZI, SVER(F)96333].
Hyphodontia barba-jovis (Bull.) J. Erikss. – Aph-Cor, SapW; 2 [O: Literaturny Kvartal, dead stem Pq, 29.08.1969, SN/UN, SVER(F)96188; Botanical Garden RAS, dead stem Pq, 01.09.1973, SN/KL, SVER(F)96190; ibid., dead stem Tr, 15.07.2020, SA/ZI, SVER(F)96189].
Hypochnicium lundellii (Bourdot) J. Erikss. – Aph-Cor, SapW; 1 [O: Vigorov Garden, dead stem Sc, 19.09.1979, SY/UN, SVER(F)96216; Botanical Garden RAS, dead stem Sc, 18.09.2020, SA/ZI, SVER(F)96215].
Inonotus hispidus (Bull.) P. Karst. – Aph-Por, Par; 2 [O: Kolobovskaya (Tolmachova) str., alive stem Vv, 16.08.1923, Perunina N.V./DZ, SVER(F)96312; Botanical Garden RAS, alive stem Pq, 16.09.2019, SA/SA, SVER(F) 96313].
Lentinus arcularius (Batsch) Zmitr. – Aph-Por, SapW; 5 [O: Botanical Garden RAS, dead stem Sc, 7.09.1971, KL/KL, SVER(F)96409; Kalinin Machine plant, dead stem Hp, 12.08.1977, SN/SN, SVER(F)96410; Botanical Garden RAS, dead stem Ak, 03.09.2004, UN/UN, SVER(F)96408; Central Arboretum, dead stem Pq, 18.06.2020, SA/ZI, SVER(F)96411; Botanical Garden RAS, dead stem Tr, 17.07.2020, SA/SA SVER(F)96412].
L. substrictus (Bolton) Zmitr. et Kovalenko – Aph-Por, SapW; 3 [O: Botanical Garden RAS, dead stem Pq, 21.08.1975, KL/KL, SVER(F)96454; ibid., dead stem Ak, 21.09.2019, SA/ZI, SVER(F)96456; ibid., dead stem Co, 02.10.2020, SA/SA, SVER(F)96455].
Lilaceophlebia cf. ochraceofulva (Bourdot et Galzin) Spirin et Zmitr. – Aph-Cor, SapW; 1 [O: Botanical Garden RAS, dead stem Va, DL/ZI, 20.08.2020, SVER(F)96335].
Loweomyces wynneae (Berk. et Broome) Jülich – Aph-Por, SapW; 1 [O: Tolmachova str., dead stem Vv, 29.08.1944, VS/VS, SVER(F)96232].
Litschaurella clematitis (B. et Galz.) Eriks. et Ryv. – Aph-Cor, SapL; 1 [O: Botanical Garden RAS, dead stem Cr, 04.09.2003, UN/ZI, SVER(F)96263; ibid., dead stem Cr, 17.09.2020, SA/ZI, SVER(F)96264].
Lyomyces crustosus (Pers.) P. Karst. – Aph-Cor; SapW; 3 [O: Ivanovskoe cemetery, dead stems Hl, 27.08.1952, SN/SN, SVER(F)96262; ibid., dead stems Hl, 10.09.1967, KL/UN, SVER(F)96258; Mikhailovskoe cemetery, 03.09.1974, SAV/SAV, SVER(F)96260; Botanical Garden RAS, dead stems Tr, 4.10.2019, SA/ZI, SVER(F)96259; G: ibid., dead stem Hh, 14.06.2003 SA/AR, SVER(F)96261].
L. erastii (Saaren. et Kotir.) Hjortstam et Ryvarden – Aph-Cor, SapW; 1 [O: Botanical Garden RAS, dead stem Ak, 21.09.2020, SA/ZI, SVER(F)96188].
Marasmius epiphyllus (Pers.) Fr. – Ag, SapL; 2 [O: Botanical Garden RAS, dead leaves Sc, 26.08.2020, DL/SO, SVER(F)96414; ibid., dead leaves Ak, 17.09.2020, SA/SO, SVER(F)96413].
Merismodes anomala (Pers.) Singer – Ag, SapW; 3 [O: Botanical Garden RAS, dead stems Ak, 01.08.1973, SAV/ZI, SVER(F)96308; ibid., dead stems Sc, 14.08.1984, ML/ZI, SVER(F)96311; Mamin-Sibiryak house, dead stems Pq, 20.08.2009, SA/ZI, SVER(F)96310; Botanical Garden RAS, dead stems Ak, 21.09.2020, SA/ZI SVER(F)96309].
Mycetinis scorodonius (Fr.) A.W. Wilson et Desjardin – Ag, SapW; 2 [O: Botanical Garden RAS, dead root Va, 17.09.2020, SA/SO, SVER(F)96242; ibid., dead stems Sc, 18.09.2020, SA/SO, SVER(F)96243].
*Mycoacia uda (Fr.) Donk – Aph-Cor, SapW; 1 [O: Botanical Garden RAS, dead stem Co, 11.09.2020, SA/ZI, SVER(F)96200].
Peniophora cinerea (Pers.) Cooke – Aph-Cor; SapW; 8 [O: Archiereiskaya (Chapaeva) str., dead stem Vv, 09.1928, DZ/DZ, SVER(F)96384; Mikhailovskoe cemetery, dead stem Hl, 02.09.1938, KA/DZ, SVER(F)96381; Literaturny Kvartal, dead stem Vv, 24.08.1952, SF/SF, SVER(F)96383; Ivanovskoe cemetery, dead stem As, 10.09.1966, SAV/SAV, SVER(F)96386; Mamin-Sibiryak house, dead stem Pq, 07.09.1972, KL/KL, SVER(F)96388; Botanical Garden RAS, dead stem Ak, 03.09.1997, BE/ZI, SVER(F)96387; ibid., dead stem Ak, 20.09.2005, UN/ZI, SVER(F)96390; ibid., dead base of stems Cr, 20.09.2009, SA/AR, SVER(F)96389; ibid., dead stem Va, 13.09.2020, SA/ZI, SVER(F)96380; ibid., dead stem Tr, 25.09.2020, SA/ZI, SVER(F)96382; ibid., dead stem Pq, 17.04.2020, SA/ZI, SVER(F)96385].
P. incarnata (Pers.) P. Karst. – Aph-Cor, SapW; 1 [O: Botanical Garden RAS, dead stem Ak, 21.09.2020, SA/ZI, SVER(F)96217].
P. limitata (Chaillet ex Fr.) Cooke – Aph-Cor, SapW; 3 [O: Vigorov Garden, dead stem Sc, 19.09.1979, SY/UN, SVER(F)96276; Botanical Garden RAS, dead stem Co, 25.09.2003, UN/UN, SVER(F)96273; ibid., dead stem Sc, 18.09.2020, SA/ZI, SVER(F)96274; ibid., dead stem Tr, 25.09.2020, DL/ZI, SVER(F)96275].
P. lycii (Pers.) Höhn. et Litsch. – Aph-Cor; SapW; 4 [O: Botanical Garden RAS, dead stem Pq, 30.09.1975, KL/UN, SVER(F)96197; ibid., dead stem Co, 19.09.2002, FK/UN, SVER(F)96196; ibid., dead stem Sc, SA/ZI, 20.09.2020, SVER(F)96194; G: ibid., dead stem Hh, 20.06.2003, SA/AR, SVER(F)96195].
P. nuda (Fr.) Bres. – Aph-Cor; SapW; 2 [O: Literaturny Kvartal, dead stem Pq, 29.08.1978, KL/UN, SVER(F)96337; Zoo, dead stem Lp, 05.09.1984, ML/UN, SVER(F)96336].
Phellinopsis conchata (Pers.) Y.C. Dai – Aph-Por, Par; 1 [O: Literaturny Kvartal, alive stem Vv, 09.1954, Pachomova/SN, SVER(F)96306; Botanical Garden RAS, alive stem Vv, 22.09.2019, SA/ZI, SVER(F)96307].
Pholiota flammans (Batsch) P. Kumm. – Ag, SapW; 1 [O: Botanical Garden RAS, root Pq, 17.09.2004, UN/SO, SVER(F)96218].
*Ph. limonella (Peck) Sacc. – Ag, SapW/Par; 1 [O: Botanical Garden RAS, root Va, 05.09.2020, Minogina E./SO, SVER(F)96231].
*Ph. tuberculosa (Schaeff.) P. Kumm. – Ag, SapW; 1 [O: Botanical Garden RAS, stem Vac, 30.08.2020, Minogina E./SO, SVER(F)96209; ibid., stem Va, 17.09.2004, UN/SO, SVER(F)96210].
Pleurotus pulmonarius (Fr.) Quél. – Ag, SapW/Par; 1 [O: Botanical Garden RAS, alive root Va, 14.05.2020, SA/SO, SVER(F)96244; ibid., dead root and stem Va, 05.09.2020, Minogina E./SO, SVER(F)96245].
Plicaturopsis crispa (Pers.) D.A. Reid – Aph-Cor, SapW; 1 [O: Botanical Garden RAS, dead stem Va, 17.09.1979, SN/UN, SVER(F)96452; ibid., dead stem Va, 17.09.2020, SA/ZI, SVER(F)96453].
*Pluteus podospileus Sacc. et Cub. – Ag, SapW; 2 [O: Botanical Garden RAS, fallen dead stems and leaves Ak, 17.09.2020, SA/SO, SVER(F)96187; ibid., G: dead stem Hh, 01.09.2020, Minogina E./SO, SVER(F)96560].
Pseudotomentella tristis (P. Karst.) M.J. Larsen – Aph-Cor, SapW; 1 [O: Botanical Garden RAS, dead roots Va, 17.09.2020, SA/ZI, SVER(F)96230].
Pterulicium gracile (Desm. et Berk.) Leal-Dutra, Dentinger et G.W. Griff. – Aph-Cla, SapL; 11 [O: Ivanovskoe cemetery, dead stems and leaves Hl, 27.08.1956, SN/SA, SVER(F)96371; Mikhailovskoe cemetery, dead leaves As, 28.09.1965, SN/SN, SVER(F)96368; Botanical garden RAS, dead leaves Hl, 01.10.1972, KL/KL, SVER(F)96372; Arboretum on Pervomayskaya, dead stems Cs, 21.08.1974, SAV/SAV, SVER(F)96376; Botanical Garden RAS, dead leaves Ak, 09.08.1985, ML/SA, SVER(F)96367; Zoo, dead leaves Lp, 30.08.1994, ML/SA, SVER(F)96379; Vigorov Garden, dead stems and leaves Sc, 13.08.2004, SY/SA, SVER(F)96377; Botanical Garden RAS, dead stems and leaves Hl, 10.10.2004, SA/SA, SVER(F)96375; ibid., dead stems and leaves Co, 29.08.2019, SA/SA, SVER(F)96369; ibid., dead leaves Pq, 29.07.2020, SA/SA, SVER(F)96370; ibid., dead stems Cr, 03.10.2020, SA/SA, SVER(F)96373; ibid., dead stems Dc, 03.10.2020, SA/SA, SVER(F)96378; Mikhailovskoe cemetery, dead leaves Ca, 04.10.2020, SA/SA, SVER(F)96374].
*Radulomyces rickii (Bres.) M.P. Christ. – Aph-Cor, SapW; 3 [O: Botanical Garden RAS, dead stem Va, 14.08.2003, UN/ZI, SVER(F)96340; ibid., dead stem Va, 18.09.2009, SA/ZI, SVER(F)96342; ibid., dead stem Ak, 11.09.2011, SA/ZI, SVER(F)96345; Arboretum on Pervomayskaya, dead stem Va, 27.07.2012, FK/ZI, SVER(F)96341; Botanical Garden RAS, dead stem Va, 29.08.2017, SA/ZI, SVER(F)96343; ibid., dead stem Ak, 21.09.2020, SA/ZI, SVER(F)96344; ibid., dead stem Sc, 17.09.2020, SA/ZI, SVER(F)96339; Central Arboretum, dead stem Va, 28.08.2020, SA/ZI, SVER(F)96338].
Resinicium bicolor (Alb. et Schwein.) Parmasto – Aph-Cor, SapW; 1 [O: Botanical Garden RAS, dead stem Va, 02.09.1974, SN/SN, SVER(F)96229; ibid., dead stem Va, 19.09.2019, SA/ZI, SVER(F)96228].
Rhizoctonia solani J.G. Kühn – Aph-Cor; Par; 2 [O: Shirokaya rechka area, meadow, alive roots and stem of Cucurbita pepo, 20.08.2003, SY/UN, SVER(F)96254; G: Shirokaya rechka area, private garden, roots and fruits of Cucumis sativus, 16.06.2008, SY/AR, SVER(F)96253].
Sebacina incrustans (Pers.) Tul. et C. Tul. – He; Myc; 2 [O: Botanical Garden RAS, alive root Ak, 28.08.1975, KL/KL, SVER(F)28004; ibid., alive and dead roots Va, 11.10.2019, SA/SA, SVER(F)96206].
Schizophyllum commune Fr. – Aph-Por, SapW/Par; 7 [O: Mamin-Sibiryak house, frost crack alive Vv, 01.09.1944, VS/VS, SVER(F)96352; Botanical Garden RAS, alive Va, 20.06.1978, SN/SN, SVER(F)96350; Central Arboretum, dead stem Pq, 30.07.1999, UN/UN, SVER(F)96348; Botanical Garden RAS, dead stem Co, 23.09.2020, SA/ZI, SVER(F)96351; ibid., dead stem Tr, 11.09.2020, AS/IZ, SVER(F)96347; ibid., alive stem Sc, 29.06.2019, SA/SA, SVER(F)96346; ibid., dead stem Ak, 15.09.2019, SA/SA, SVER(F)96349].
Schizopora flavipora (Berk. et M.A. Curtis ex Cooke) Ryvarden – Aph-Por, SapW; 3 [O: Botanical Garden RAS, dead stem Vv, 23.08.1971, KL/ZI, SVER(F)96305; ibid., dead stem Pq, UN/ZI, 20.09.2004, SVER(F)96304; ibid., dead stem Tr, 20.08.2020, SA/ZI, SVER(F)96303].
Sistotrema brinkmannii (Bres.) J. Erikss. – Aph-Cor, SapW; 2 [O: Botanical Garden RAS, dead stem Tr, 02.10.1979, SN/UN, SVER(F)96279; ibid., dead stem Va, 28.09.2003, UN/UN, SVER(F)96277; G: ibid., dead roots and stem Va, 25.09.2003, UN/ZI, SVER(F)96278].
Sistotremastrum niveocremeum (Höhn. et Litsch.) J. Erikss. – Aph-Cor, SapW; 1 [Vigorov Garden, dead stem Sc, 19.09.1979, SN/UN, SVER(F)96184; Botanical Garden RAS, dead stems Sc, 17.09.2020, SA/SA, SVER(F)96185].
Sphaerobolus stellatus Tode – Gas, SapW; 4 [O: Literaturny Kvartal, dead stem Pq, 29.08.1969, SN/SA, SVER(F)96202; Arboretum on Pervomayskaya, dead stems Va, 08.1976, Ipatov L.F./SA, SVER(F)96205; Botanical Gadren RAS, dead stems and leaves Tr, 27.09.1999, NU/SA, SVER(F)96204; ibid., dead stems and leaves Pq, Sc, 18.09.2020, SA/SA, SVER(F)96203, 96559, respectively].
*Steccherinum bourdotii Saliba et A. David – Aph-Por, SapW; 4 [O: Botanical Garden RAS, dead stem Va, 21.09.1973, SN/ZI, SVER(F)96365; ibid., dead stem Lp, 10.09.2001, SA/ZI, SVER(F)96363; ibid., dead stem Аk, 10.08.2018, SA/ZI, SVER(F)96362; ibid., dead stem Co, 18.09.2020, SA/ZI, SVER(F)96364; ibid., dead stem Тr, 07.10.2020, SA/ZI, SVER(F)96366].
S. fimbriatum (Pers.) J. Erikss. – Aph-Por, SapW; 1 [O: Vigorov Garden, dead stem Sc, 22.09.1977, SY/SA, SVER(F)96219; Botanical Garden RAS, dead stem Sc, 18.09.2020, SA/ZI, SVER(F)96220].
S. ochraceum (Pers. ex J.F. Gmel.) Gray — Aph-Por, SapW; 4 [O: Mamin-Sibiryak house, dead stem Pq, 07.09.1972, KL/UN, SVER(F)96479; Botanical Garden RAS, dead stem Аk, 02.06.2002, SA/ZI, SVER(F)96481; Central Arboretum, dead stem Pq, 29.07.2001, UN/ZI, SVER(F)96480; Botanical Garden RAS, dead stem Sc, 17.09.2020, SA/ZI, SVER(F)96478; ibid., dead stem Co, 3.10.2015, SA/ZI, SVER(F)96482].
Stereum hirsutum (Willd.) Pers. – Aph-Cor, SapW/Par; 2 [O: Botanical Garden RAS, frost crack Va, 31.08.1988, ML/UN, SVER(F)96226; ibid., dead stem Tr, SA/UN, 09.09.2005, SVER(F)96227].
Subulicystidium longisporum (Pat.) Parmasto – Aph-Cor, SapWW; 4 [O: Literaturny Kvartal, dead stem Vv, 09.1948, SF/SF, SVER(F)96432; Ivanovskoye cemetery, dead stem Hl, 05.09.1964, KL/KL, SVER(F)96434; Botanical Garden RAS, dead stems Hl, 2.10.1975, SAV/SN, SVER(F)96433; ibid., dead stem Tr, 28.09.2009, SA/AR, SVER(F)96436; ibid., dead stem Va, 4.10.2019, SA/ZI, SVER(F)96435].
Tomentella bryophila (Pers.) M.J. Larsen – Aph-Cor, Myc; 2 [O: Tolmachova str., dead root Vv, 08.1946, SF/SF, SVER(F)96248; Central Arboretum, dead roots and stem Vv, 21.08.1979, SY/AR, SVER(F)96247; Botanical Garden RAS, dead roots Hh, 30.08.2005, UN/UN, SVER(F)96246].
T. cinerascens Höhn. et Litsch. – Aph-Cor, Myc; 4 [O: Mamin-Sibiryak house, dead stem Pq, 07.09.1972, KL/KL, SVER(F)96355; Botanical Garden RAS, base of dead Va, 05.09.1998, UN/UN, SVER(F)96357; ibid., dead stem Co, 24.08.2004, UN/UN, SVER(F)96353; ibid., fallen branches Tr, 29.09.2009, FK/SA, SVER(F)96356; ibid., dead stem Pq, 27.09.2020, SA/ZI, SVER(F)96354].
*T. olivascens (Berk. et M.A. Curtis) Bourdot et Galzin – Aph-Cor, Myc; 1 [O: Botanical Garden RAS, dead roots Va, 16.09.2010, FK/AR, SVER(F)96280].
Trametes ochracea (Pers.) Gilb. et Ryvarden – Aph-Por, SapW; 5 [O: Kolobovskaya (Tolmachova) str., dead roots Vv, 17.09.1929, DZ/DZ, SVER(F)96440; Literaturny Kvartal, dead stem Vv, 08.1935, KA/DZ, SVER(F)96443; Central Arboretum, dead stem Pq, 07.08.1976, SAV/SAV, SVER(F)96438; Botanical Garden RAS, dead root Tr, 25.09.2004, UN/UN, SVER(F)96442; ibid., dead stem Ak, 23.09.2020, SA/ZI, SVER(F)96439; G: ibid., dead root Hh, 10.06.2000, Semkin A./SA, SVER(F)96437; Central Arboretum, dead root Vv, 7.05.2011, Lapteva A.N./ZI, SVER(F)96441].
Typhula crassipes Fuckel [incl. T. anceps P. Karst., T. corallina Quél.] – Aph-Cla, SapL; 13 [O: Mikhailovskoe cemetery, dead leaves and stems Hl, As, 02.09.1952, SF/SA, SVER(F)96487; Tolmachova str., dead leaves Va, 22.08.1953, SN/SN, SVER(F)96486; Kalinin Machine plant, dead leaves Hp, 02.10.1969, SN/SN, SVER(F)96488; Vigorov Garden, dead leaves and stems Sc, 11.09.1973, Trunov/KL, SVER(F)96489; Central Arboretum, dead leaves and stems Pq, 30.08.1979 SN/SA, SVER(F)96491; Zoo, dead stems Lp, 17.08.1982, ML/SA, SVER(F)96494; Mamin-Sibiryak house, dead stems Ca, 28.09.1998, SA/SA, SVER(F)96490; Botanical Garden RAS, dead stems Cr, 26.09.2002 UN/SA, SVER(F)96495; ibid., dead stems Cs, 22.09.2008 SA/SA, SVER(F)96493; ibid., dead stems and leaves Co, 27.09.2015, SA/SA, SVER(F)96484; ibid., dead leaves and stems Ak, 21.09.2020, SA/SA, SVER(F)96492; ibid., dead leaves and stems Md, 21.09.2020, SA/SA. SVER(F)96485].
T. culmigena (Mont. et Fr.) Berthier – Aph-Cla, SapL; 12 [O: Mikhailovskoe cemetery, dead stems Hl, As, 02.09.1962, SN/SA, SVER(F)96543; Mamin-Sibiryak house, dead stems Ca, 10.09.1972, NS/SA, SVER(F)96544; Tolmachova str., dead leaves Vv, 03.09.1952, NS/SA, SVER(F)96539; Vigorov Garden, dead leaves Sc, NS/SA, 03.08.1978, SVER(F)96545; Zoo, dead leaves and stems Cs, 05.09.1984, ML/SA, SVER(F)96542; Central Arboretum, Pq, 05.09.1998, FK/SA, SVER(F)96548; Botanical Garden RAS, dead stems and leaves Ak, 03.09.2006, SA/SA, SVER(F)96546; ibid., dead leaves Va, 09.09.2015, SA/SA, SVER(F)96540; ibid., dead leaves Co, 11.07.2020, SA/SA, SVER(F)96538; ibid., dead leaves Dc, 17.09.2020, SA/SA, SVER(F)96547; ibid., dead stems Cr, 03.10.2020, SA/SA, SVER(F)96541].
T. erythropus (Pers.) Fr. – Aph-Cla, SapL; 7 [O: Botanical Garden RAS, dead leaves Cr, 21.09.1968, KL/KL, SVER(F)96447; ibid., dead leaves Hh, 09.1978, Deryagina/SN, SVER(F)96446; Vigorov Garden, dead leaves, Sc, 21.09.1982, SY/SA, SVER(F)96450; Zoo, dead leaves Lp, SY/SA, 05.09.1984, SVER(F)96449; Mikhailovskoe cemetery, dead stems and leaves Cs, 11.07.1999, SA/SA, SVER(F)96451; Botanical Garden RAS, dead stem Tr, 17.09.2002, UN/SA, SVER(F)96444; ibid., dead leaves Co, 21.09.2020, SA/SA, SVER(F)96448; dead stems and leaves Cr, 03.10.2020, SA/SA, SVER(F)96445].
T. ishikariensis S. Imai – Aph-Cla, Par; 1 [O: Ivanovskoe cemetery, alive root Hl, 04.06.1977, SN/SA, SVER(F)96221].
T. lutescens Boud. – Aph-Cla, SapL; 2 [O: Mikhailovskoe cemetery, dead leaves As, 05.10.2004, SA/SA, SVER(F)96250; Botanical Garden RAS, dead leaves and stems Hl, SA/SA, 18.10.2018, SVER(F)96249].
T. micans (Pers.) Berthier – Aph-Cla, SapL; 16 [O: Ivanovskoe cemetery, dead stems and leaves Hl, As, 03.09.1951, SN/SN, SVER(F)96514, 96515, respectively; Tolmachova str., dead leaves Vv, 22.08.1955, SN/SN, SVER(F)96517; Chapaeva str., dead leaves Ca, 19.09.1968, SN/SN, SVER(F)96518; Kalinin Machine plant, dead leaves Hp, 30.08.1973, SAV/SAV, SVER(F)96521; Zoo, dead leaves Pq, 17.08.1982; ML/SA, SVER(F)96516; Botanical Garden RAS, dead stems and leaves Tg, 02.10.1999, SA/SA, SVER(F)96522; ibid., dead stems and leaves Co, 30.07.2008, SA/SA., SVER(F)96513; ibid., dead stems and leaves Cr, Cs, Ak, Va, Sc, Md, 17.09.2020, SA/SA, SVER(F)96523-96528, respectively; ibid., dead stems Lp, 25.09.2020, SA/SA, SVER(F)96520; G: ibid., dead stem Hh, 06.11.2020, SA/SA, SVER(F)96519].
T. juncea (Alb. et Schwein.) P. Karst. – Aph-Cla, SapL; 10 [O: Ivanovskoe cemetery, dead leaves and stems Hl, 11.09.1929, DZ/DZ, SVER(F)96549; Kalinin Machine plant, dead leaves Hp, 15.09.1959, NS/NS, SVER(F)96553; Arboretum on Pervomayskaya, dead leaves and stems Pq, 02.09.1966, KL/KL, SVER(F)96550; Botanical Garden RAS, dead stems and leaves Cr, 1973 SAV/SAV, SVER(F)96554; Vigorov Garden, dead leaves and stems Sc, 21.09.1982, SY/SA, SVER(F)96555; Zoo, dead leaves and stems Lp, 05.09.1984 ML/SA, SVER(F)96556; Botanical Garden RAS, dead leaves and stems Ak, 30.09.1999, SA/SA, SVER(F)96552; ibid., dead leaves and stems Tr, 30.09.2003, UN/UN, SVER(F)96557; ibid., dead stems and leaves Dc, 21.09.2020, SA/SA, SVER(F)96551].
T. setipes (Grev.) Berthier [incl. T. gyrans (Batsch) Fr.] – Aph-Cla, SapL; 12 [O: Ivanovskoe cemetery, dead leaves Hl, 27.08.1972, SN/SA, SVER(F)96501; Tolmachova str., dead leaves Ca, 03.09.1963, SN/SN, SVER(F)96503; Vigorov Garden, dead leaves Sc, 21.09.1982, SY/SA, SVER(F)96504; Mikhailovskoe cemetery, dead leaves As, 02.09.1974, SAV/SA, SVER(F)96505; Botanical Garden RAS, dead stem Co, 17.09.1999, UN/SA, SVER(F)96502; ibid., dead leaves Tr, 30.06.2016, SA/SA, SVER(F)96500; ibid., dead leaves Ak, Cs, Cr, Dc, Md, Pq, Va, 17.09.2020, SA/SA, SVER(F)96506-96512, respectively].
T. sphaeroidea Remsberg – Aph-Cla, SapW; 2 [O: Botanical Garden RAS, dead stems Co, 30.06.2016, SA/SA, SVER(F)96358; ibid., dead stems Tr, 21.09.2020, SA/SA, SVER(F)96359].
T. trifolii Rostr. – Aph-Cla, SapL; 3 [O: Botanical Garden RAS, dead stems and leaves Hl, 08.08.1978, SN/SA, SVER(F)96212; ibid., dead leaves Dc, 17.09.2020, SA/SA, SVER(F)96193; ibid., dead stems and leaves Hl, 29.09.2020, SA/SA, SVER(F)96214; ibid., dead leaves and stems Cr, SA/SA 03.10.2020, SVER(F)96213].
T. viticola (Peck) Berthier – Aph-Cla, SapL; 3 [O: Botanical Garden RAS, dead leaves Pq, 11.06.2008, SA/SA, SVER(F)96284; ibid., dead leaves Pq, Va, 17.09.2018, SA/SA, SVER(F)96283, 96282, respectively; ibid., dead leaves Vv, 03.10.2020, SA/SA, SVER(F)96281].
Tulasnella eichleriana Bres. – Aph-Cor, SapW; 1 [O: Botanical Garden RAS, dead stem Ak, 15.09.1979, SN/UN, SVER(F)96223; ibid., dead stem Ak, 21.09.2020, SA/ZI, SVER(F)96222].
Xylodon asper (Fr.) Hjortstam et Ryvarden – Aph-Cor, SapW; 2 [O: Kalinin Machine plant, dead stem Hp, SN/UN, 12.08.1977, SVER(F)96361; Botanical Garden RAS, dead stem Va, 17.09.2020, SA/ZI, SVER(F)96360].
X. brevisetus (P. Karst.) Hjortstam et Ryvarden – Aph-Cor, SapW; 2 [O: Botanical Garden RAS, dead stem Sc, 07.08.1967, SN/ZI, SVER(F)96257; Kalinin Machine plant, roots and stem Sc, 05.10.1975, SAV/SN, SVER(F)96255; Botanical Garden RAS,, dead stem Ak, 21.09.2020, SA/ZI, SVER(F)96256].
X. detriticus (Bourdot) K.H. Larss., Viner et Spirin – Aph-Cor, SapW; 2 [O: Botanical Garden RAS, dead stems Pq, 08.08.1978, SN/UN, SVER(F)96225; ibid., dead stem Co, 04.10.2019, SA/ZI, SVER(F)96224].
X. rimosissimus (Peck) Hjortstam et Ryvarden – Aph-Cor, SapW; 2 [O: Vigorov Garden, dead stem Sc, 08.1969, Zueva/KL, SVER(F)96252; Botanical Garden RAS, dead stem Sc, 25.08.1972, SN/SN, SVER(F)96253; ibid., dead stem Ak 21.09.2020, SA/ZI, SVER(F)96251].
X. sambuci (Pers.) Ţura, Zmitr., Wasser et Spirin — Aph-Cor; SapW; 13 [O: Tolmachova str., dead stem Vv, 09.1939, KA/DZ, SVER(F)96302; Mikhailovskoe cemetery, dead stem Hl, 23.09.1944, KA/SF, SVER(F)96293; Mamin-Sibiryak house, dead stem Vv, 19.08.1951, SN/SN, SVER(F)96294; ibid., dead stem Pq, 12.09.1968, SAV/KL, SVER(F)96285; Vigorov Garden, dead stem Ak, 1.09.1973, SN/SN, SVER(F)96286; Zoo, dead stem Lp, 17.08.1982 ML/ZI, SVER(F)96287; Central Arboretum, dead stem Pq, 14.09.1999, BE/UN, SVER(F)96299; Botanical Garden RAS, dead stem Md, 17.08.2004, FK/UN, SVER(F)96295; ibid., dead stem Va, 08.09.2015, SA/SA, SVER(F)96298; ibid., dead stems Tr, 22.09.2018, SA/ZI, SVER(F)96297; ibid., dead stem Ak, 08.08.2018, SA/ZI, SVER(F)96296; ibid., dead stem Sc, 14.09.2018, SA/ZI, SVER(F)96290; ibid., dead stem Sc, 18.09.2019, SA/ZI, SVER(F)96291; ibid., dead stem Ak, 21.09.2020, SA/ZI, SVER(F)96292; ibid., dead stem Vac, 29.06.2020, SA/ZI, SVER(F)96289; ibid., dead stem Ampelopsis grandulosa, 17.04.2020, SA/ZI, SVER(F)96288; Mamina-Sibiryak house, dead stem Pq, 28.06.2020, SA/ZI SVER(F)96300; G: Botanical Garden RAS, dead stem Hh, 3.07.2009, SA/AR, SVER(F)96301].
A total of 108 species of agaricomycetous macrofungi were recorded on vines in Ekaterinburg City during century long investigations. This is a fairly large number for such a small area. For comparison, the species composition of macrofungi on lianas is well studied in the Meditteranean (Senn-Irlet, 1995; Bernicchia, Gorjon, 2010; Ryvarden, Melo, 2014), however, these regional and local lists do not exceed 20–30 species (Bernicchia, 2001; Checklist., 2005; Fischer, 2006; Isikov, 2009; Ţura et al., 2010; Fischer, Gonzalez, 2015; Sarkina, Mironova, 2015; Karadelev et al., 2018).
As a new for Sverdlovsk Region we list 15 species, such as Cerioporus rangiferinus, Crepidotus subverrucisporus, Crustomyces expallens, Flammulina fennae, F. rossica, Gloeohypochnicium analogum, Hohenbuehelia grisea, Hydnophlebia chrysorhiza, Mycoacia uda, Pholiota limonella, Ph. tuberculosa, Pluteus podospileus, Radulomyces rickii, Stecherinum bourdotii, and Tomentella olivascens. Such species as Loweomyces wynneae was not found in Sverdlovsk Region for over 50 years (Shiryaev et al., 2010). It was collected in 1944 on Vitis vinifera in Ekaterinburg City as well as in 1954 and 1959 years in hemiboreal forests on wood of native Norway Maple (Acer platanoides L.). This circumstance allows us to consider it in the “regionally extinct” (RE) status (IUCN, 2001; Shiryaev et al., 2010). Besides, noteworthy are the second finds in Sverdlovsk Region of such rare species as Antrodiella onychoides, Lyomyces erastii, Peniophora lycii, and Lilaceophlebia cf. ochraceofulva.
During the 1960–1970s, in Ekaterinburg City a total of 6 specimens of Athelia rolfsii were revealed on the roots of grapes, potatoes, onions, etc. (Stepanova, 1971). These specimens, which we studied in the 2000s, were sterile, and, therefore, the fungus was transferred to the status of “species whose findings are doubtful or not confirmed by herbarium specimens” (Shiryaev et al., 2010). Recently, we revealed the specimen collected in 2004 at Hedera helix roots, with demonstrated a sufficient number of developed basidia and basidiospores.
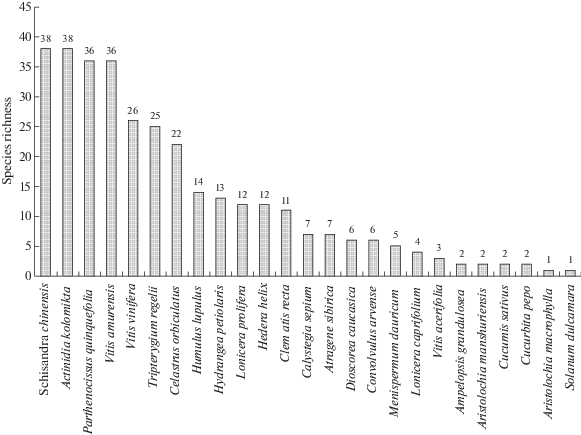
The agaricomycetous macrofungi were found on 25 species of vines (Fig. 1). The largest number of fungal species was found on the most common vines of 30 years old or more. On Schizandra chinensis and Actinidia kolomikta were collected 38 species each, on Parthenocissus quinquefolia and Vitis amurense – 36 species. On V. vinifera a total of 26 species of macrofungi have been identified, although till now this liana doesn’t occupy large areas, but it is characterized by the longest research history (it was sole alien woody liana in the city in the 1920–1950s). All the oldest finds of macrofungi (Inonotus hispidus, Peniophora cinerea, Trametes ochracea, Typhula juncea) made in the 1920s were found on the Common Grape. On the richest herbaceous vine, the hops, a total 14 species of agaricomycetous macrofungi were identified.
The morphogroup of aphyllophoroid fungi is the richest within revealed ones. This includes 85 species, i.e. 78.5% of total species richness of all species collected on vines (Table 1). The agaricoid, heterobasidiomycetous and gasteroid macrofungi include no more than 2.8–13.9% if revealed species diversity. The corticioids consist the richest subgroup within the aphyllophoroid fungi (51 species/47.2%), then follow the poroids and clavarioids (22 and 12 species, respectively). Some others subgroups of the aphyllophoroid fungi (as cantharelloids, thelephoroids, hericioids) were not found on vines in Ekaterinburg City.
Table 1.
Species richness and trophic modes of agaricomycetous macrofungi on vines (number of species/%)
| Fungal group | Species richness | Saprotrophs | Pathogens | Mycorrhiza-formers | |
|---|---|---|---|---|---|
| wood | litter | ||||
| Aphyllophoroid | 85/78.7 | 61/56.5 | 15/13.9 | 9/8.3 | 4/3.7 |
| Corticioid | 51/47.2 | 41/37.9 | 4/3.7 | 4/3.7 | 4/3.7 |
| Poroid | 22/20.4 | 19/17.5 | 0 | 4/3.7 | 0 |
| Clavarioid | 12/11.1 | 1/0.9 | 10/9.2 | 1/0.9 | 0 |
| Agaricoid | 15/13.9 | 12/11.1 | 3/2.8 | 3/2.8 | 0 |
| Gasteroid | 3/2.8 | 3/2.8 | 1/0.9 | 0 | 0 |
| Heterobasidiomycetous | 5/4.6 | 4/3.7 | 0 | 0 | 1/0.9 |
| In total | 108/100 | 80/74.1 | 19/17.6 | 12/11.1 | 5/4.6 |
The aphyllophoroid species Typhula micans was found in the largest vines species number (16 host species), then follow T. setipes (14), Xylodon sambuci (13), Typhula crassipes (13), T. culmigena (12), T. juncea (10), Pterulicium gracile (11), Bjerkandera adusta (9), Irpex lacteus (9), Peniophora cinerea (9), Cylindrobasidium evolvens (8), Typhula erythropus (7), Schizophyllum commune (7). These species form its basidiomata on woody and herbaceous vines, with an exception of Bjerkandera adusta, Cylindrobasidium evolvens, Irpex lacteus, Schizophyllum commune which infest only woody vines. Also the rich fungal taxa associated with woody vines are Lentinus arcularius (5 host species), Peniophora nuda (5), and Trametes ochracea (5). Much fewer agaricomycetous macrofungi were found on herbaceous vines alone (e.g., Typhula trifolii infests three species of herbaceous vines). Only on one vine species were collected of 44 species of fungi in question (40.7%).
In the respect of trophic modes, 95 species of fungi revealed (87.9%) are obligate or facultative saprotrophs (80 species are wood-destroyers and 19 ones – litter saprotrophs), 12 species can be characterized as obligate or facultative pathogens on woody vines, causing necrosis, stem rot, or developing on frost cracks (Armillaria borealis, Athelia rolfsii, Ceratobasidium cornigerum, Fomitoporia punctata, Inonotus hispidus, Phelli-nopsis conchata, Pholiota limonella, Pleurotus pulmonarius, Stereum hirsutum, Rhizoctonia solani). Some of them cause well-known diseases of grapes (Jayawardena et al., 2018) e.g. esca, stem rot, and root rot. On the herbaceous Humulus lupulus, the pathogenic fungus Typhula ishikariensis was identified, which causes a dangerous disease “root rot”, or “snow mold” (Tkachenko, 2017). There are 5 species of mycorrhiza-formers (Pseudotomentella, Sebacina, Tomentella). They form ectomycorrhiza with both deciduous and coniferous trees, whereas their basidiomata are formed on wood debris and dead parts of vines.
CONCLUSION
The species richness of the agaricomycetous macrofungi associated with cultivated vines in the Ekaterinburg city and in the Urals as a whole is studied by us for the first time. For the area of 468 km2 in a boreal continental climate, the species richness revealed to be unexpectedly large (108 species) if compare with the regional and national lists of territories located on the northern border of the woody lianas ranges.
Two-thirds of new species revealed were mainly of European, East Asian, or tropical distribution range. Such morphogroup as aphyllophoroid fungi takes up to 80% of the species diversity revealed.
Cultivated hedge vines have accumulated many local widespread litter species, widely distributed in forest and grasslands litter. This is evidenced by large numbers of native litter species identified on the vines. The species of xylosaprotrophs, which normally occur on wood debris in forest litter, are regularly observed on dead parts of the vines. Colonization of variously lignified and even herbaceous shoots of lianas by wood debris/litter saprotrophs seems to be quite natural phenomenon, reminiscent that all herbaceous plants contain hydro-phenyl lignin in varying amounts (Manskaya, Kodina, 1975).
The revealed mycobiota is dominated by multisubstrate species, common on deciduous trees and as well as on herbaceous-deciduous litter. There were no species that prefer the conifer wood.
Woody vines are reliably richer than herbaceous ones. The richest vine is Schizandra chinensis, Actinidia kolomikta, Parthenocissus quinquefolia and Vitis amurensis, which occupies the largest squares in the city. Typhula micans is the most common fungus, found on woody and herbaceous vines. It is possible that a reason for the large number of fungi species revealed is the high species richness of vines and the high selective effort (compared to natural conditions) for a local area.
In Ekaterinburg City, some species have been identified, known as traditional pathogens of lianas in the world, causing significant economic damage to viticulture. In the Urals, all these are common and widespread species (Armillaria, Fomitoporia, Phellinopsis, Pleurotus, Pholiota, Stereum representatives) growing on deciduous trees. Finds of these species of fungi on vines are still rare, probably due to the scattered distribution of the substrate. The list of pathogenic species of fungi and their number is increasing due to climate warming and an increase in the age of vines in the region. Thus, pathogenic species of fungi are already waiting for the expansion of the viticulture zone up to the Urals.
The work of A.G. Shiryaev and O.S. Shiryaeva was supported by the State Research Task N АААА-А19-119031890084-6. The work of I.V. Zmitrovich was supported by the State Research Task N AAAA-A19-119020890079-6.
Список литературы
Arefyev S.P., Kazantseva M.N. Changes in structure of wood-inhabiting aphyllophoroid fungi communities in system of complex environmental monitoring of Tyumen. Mikologiya i fitopatologiya. 2016. V. 50 (1). P. 5–13 (in Russ.).
Bernicchia A. A checklist of corticioid, polyporoid and clavarioid fungi (Basidiomycotina) from the Emilia-Romagna region, Italy. Sydowia. 2001. V. 53 (1). P. 1–33.
Bernicchia A., Gorjon S.P. Corticiaceae s.l. Fungi Europaei V. 12. Edizioni Candusso, 2010.
Biological invasions in Poland. V. 1. W. Szafer Institute of Botany of the Polish Academy of Sciences, Krakow, 2010.
Checklist of Italian Fungi. Basidiomycota. Coordinated by S. Onofri. Sassari, 2005.
Demidova Z.A. A brief overview of research on mycology and phytopathology in the Urals. Proc. Institute of Biol. UF AS USSR. V. 15. Sverdlovsk, 1960. P. 5–16 (in Russ.).
Desprez-Loustau M.-L. Alien fungi of Europe. In: Handbook of alien species in Europe. Springer, 2009. P. 15–28.
Dorofeeva L.M. Vines in the Botanical Garden of Urals Branch of the Russian Academy of Sciences. Proc. of Conference “Ecology and geography of plants and plant communities”. Ural Federal Univ. Ekaterinburg, 2018. P. 244–247 (in Russ.).
Fedorov A.A. Plant resources of the USSR for the national economy and medicine. Rastitelnye resursy. 1965. V. 1 (1). P. 5–18 (in Russ.).
Fick S.E., Hijmans R.J. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int. J. Clim. 2017. V. 37 (12). P. 4302–4315.
Fischer M. Biodiversity and geographic distribution of basidiomycetes causing esca-associated white rot in grapevine: a worldwide perspective. Phytopathol. Mediterr. 2006. V. 45. P. 30–42.
Fischer M., Gonzalez V. An annotated checklist of European basidiomycetes related to white rot of grapevine (Vitis vinifera). Phytopathol. Mediterr. 2015. V. 54 (2). P. 281–298.
Hoshino T., Tkachenko O., Kiriaki M. et al. Winter damage caused by Typhula ishikariensis biological species I on conifer seedlings and hop roots collected in the Volga-Ural regions of Russia. Can. J. Plant Pathol. 2004. V. 26 (3). P. 391–396.
Index Fungorum. CABI Bioscience, 2021. http://www.indexfungorum.org. Accessed 14.02.2021.
Isikov V.P. Fungi on trees and bushes of Crimea. Systematic catalogue. Simferopol, 2009 (in Russ.).
IUCN Red List categories and criteria, version 3.1. IUCN, Cambridge etc., 2001.
Jayawardena R.S., Purahong W., Zhang W. et al. Biodiversity of fungi on Vitis vinifera L. revealed by traditional and high-resolution culture-independent approaches. Fungal Diversity. 2018. V. 90. P. 1–84.
Karadelev M., Rusevska K., Kost G. et al. Checklist of macrofungal species from the phylum Basidiomycota of the Republic of Macedonia. Acta Musei Macedonici Scient. Natur. 2018. V. 21. P. 23–112.
Karelina E.D. First report about powdery mildew in Ekaterinburg. Vestnik Instituta biologii Komi nauchnogo tsentra RAN. 2017. N 2. C. 15–19 (in Russ.).
Manskaya S.M., Kodina L.A. Geochemistry of lignin. Nauka, Moscow, 1975 (in Russ.).
Morozova O.V., Zhmylev P.Yu. Taxonomic differentiation and functional homogenization of flora in the middle part of European Russia as a result of naturalization of alien species. Vestnik Sankt-Peterburgskogo universiteta. V. 65 (2). P. 284–302 (in Russ.).
Motiejunaite J., Markovskaja S., Kutorga E. et al. Alien fungi in Lithuania: List of species, current status and trophic structure. Botanica Lithuanica. 2017. V. 23 (2). P. 139–152.
Nemytov A.Yu. Shatilov grapes. Chelyabinsk Printing House, Chelyabinsk, 2016 (in Russ.).
Ryvarden L., Melo I. Poroid fungi of Europe. Synopsis Fungorum 31. Fungiflora, 2014.
Sarkina I.S., Mironova L.P. Annotated list of Basydiomycetes and Ascomycetes macrofungi in the Karadag Nature Reserve. Uchenye zapiski zapovednika “Mys Martyan”. 2015. V. 6. P. 297–327.
Senn-Irlet B. The genus Crepidotus (Fr.) Staude in Europe. Persoonia. 1995. V. 16 (1). P. 1–80.
Shiryaev A.G. Two rare and interesting species of genus Physalacria in the Urals. Novosti sistematiki nizshikh rasteniy. 2007. V. 41. C. 167–173 (in Russ.).
Shiryaev A.G. Clavarioid fungi of the anthropogenic territories of the Urals. Vestnik ekologii, lesovedeniya i landshavtovedeniya. 2008. N 8. P. 80–91 (in Russ.).
Shiryaev A.G. Changes in mycobiota of Ural-and-Siberian region under global warming and anthropogenic impact. Vestnik ekologii, lesovedeniya i landshavtovedeniya. 2009. № 9. C. 37–47 (in Russ.).
Shiryaev A.G., Kotiranta H., Mukhin V.A. et al. Aphyllophoroid fungi of Sverdlovsk Region, Russia. Biodiversity, distribution, ecology and the IUCN threat categories. Goshchitskiy publ., Ekaterinburg, 2010.
Shiryaev A.G., Mukhin V.A., Kotiranta H. et al. Biodiversity of aphyllophoroid fungi of the Urals. In: Biological diversity of the Urals’s plant world and adjacent territories. IPAE, Ekaterinburg, 2012. P. 311–313 (in Russ.).
Shiryaeva O.S. History of investigations and species richness of agaricoid Basidiomycetes in Sverdlovsk region. Vestnik Orenburgskogo gosudarstvennogo pedagogicheskogo universiteta. 2015. № 4 (16). C. 49–58 (in Russ.).
Shiryaeva O.S. New records of agaricoid fungi from Sverdlovsk Region, Russia. Botanica. 2018. V. 24 (2). P. 150–161.
Shiryaeva O.S., Palamarchuk M.A. New data on agaricoid fungi (Basidiomycota) of the Urals. Novosti sistematiki nizshikh rasteniy. 2019. V. 53 (1). P. 89–106 (in Russ.).
Stepanova N.T. Ecogeographical analysis of aphyllophoroid fungi of the Urals. Dis. … Dr. Sci. Biol. Institute UFAS USSR, Sverdlovsk, 1971 (in Russ.).
Stepanova-Kartavenko N.T. Aphyllophoralean fungi of the Urals. UFAS USSR, Sverdlovsk, 1967 (in Russ.).
Stepanova N.T., Sirko A.V. To the flora of agaricoid fungi and gastromycetes of the Urals. In: Mycological research in the Urals. UF AN USSR, Sverdlovsk, 1977. P. 51–106 (in Russ.).
The most dangerous invasive species of Russia (TOP-100). KMK, Moscow, 2018 (in Russ.).
The Plant List. 2021. http://www.theplantlist.org. Accessed 18.02.2021.
Tkachenko O.B. Snow molds (history of the study, pathogens, their biological characteristics). RAS, Moscow, 2017 (in Russ.).
Tomoshevich M.A. Interrelation between alien and native foliar fungal pathogens and woody plants in Siberia. Contemporary Problems of Ecology. 2019. V. 12 (6). P. 642–657.
Tretyakova A.S., Kulikov P.V. Adventitious component of the flora of Sverdlovsk Region: dynamics of the species. Vestnik Udmurtskogo universiteta. 2013. V. 4. P. 184–188 (in Russ.).
Ţura D., Zmitrovich I.V., Wasser S.P. et al. Checklist of Hymenomycetes (Aphyllophorales s.l.) and Heterobasidiomycetes in Israel. Mycobiology. 2010. V. 38 (4). P. 256–273.
Ushakova N.V. Special features of polypore biota of Ekaterinburg city. Proc. VIII conf. of young botanists in St. Petersburg. SPbSUGT, St. Petersburg, 2004, p. 75.
Volobuev S.V., Bolshakov S.V., Shiryaev A.G. et al. New species for regional mycobiotas of Russia. 6. Report 2021. Mikologiya i fitopatologiya. 2021. V. 55 (6) (in press.).
Wagner V., Večeřa M., Jiménez-Alfaro B. et al. Alien plant invasion hotspots and invasion debt in European woodlands. J. Veg. Sci. 2021. V. 32. e13014.
Yurchenko E. The genus Peniophora (Basidiomycota) of Eastern Europe. Morphology, taxonomy, ecology and distribution. Belorusskaya nauka, Minsk, 2010.
Арефьев С.П., Казанцева М.Н. (Arefyev, Kazantseva) Изменение структуры ксилотрофных афиллофороидных грибов в системе комплексного мониторинга г. Тюмени // Микология и фитопатология. 2016. Т. 50. № 1. С. 5–13.
Демидова З.А. (Demidova) Краткий обзор исследований по микологии и фитопатологии на Урале // Тр. Ин-та биол. УФ АН СССР. Вып. 15. Свердловск, 1960. С. 5–16.
Дорофеева Л.М. (Dorofeeva) Лианы в Ботаническом саду УрО РАН. Тр. конф. “Экология и география растений и растительных сообществ”. Екатеринбург: УрФУ, 2018. С. 244–247.
Исиков В.П. (Isikov) Грибы на деревьях и кустарниках Крыма. Систематический каталог. Симферополь: ИТ Ариал, 2009. 297 с.
Карелина Е.Д. (Karelina) Первое сообщение о мучнисторосяных грибах города Екатеринбурга // Вестник ИБ Коми НЦ УрО РАН. 2017. № 2. С. 15–19.
Манская С.М., Кодина Л.А. (Manskaya, Kodina) Геохимия лигнина. М.: Наука, 1975. 229 с.
Морозова О.В., Жмылев П.Ю. (Morozova, Zhmylev) Таксономическая дифференциация и функциональная гомогенизация флор Средней России в результате натурализации чужеродных видов // Вест. Санкт-Петербургского ун-та. Сер. Науки о Земле. 2020. Т. 65(2). С. 284–302.
Немытов А.Ю. (Nemytov) Виноград Шатилова. Челябинск: Челябинский дом печати, 2016. 96 с.
Самые опасные инвазионные виды России (ТОП-100) (The most dangerous). Тов. научных изд. КМК, 2018. 688 с.
Саркина И.C., Миронова Л.П. (Sarkina, Mironova) Аннотированный список базидиальных и сумчатых макромицетов Карадагского природного заповедника // Научные записки заповедника “Мыс Мартьян”. 2015. Вып. 6. С. 297–327.
Степанова Н.Т. (Stepanova) Эколого-географическая характеристика афиллофоровых грибов Урала. Дис. … докт. биол. наук. Свердловск: ИБ УФ НЦ РАН, 1971. 821 с.
Степанова Н.Т., Сирко А.В. (Stepanova, Sirko) К флоре агариковых грибов и гастромицетов Урала // Микологические исследования на Урале. Свердловск: УНЦ АН СССР, 1977. С. 51–106.
Степанова-Картавенко Н.Т. (Stepanova-Kartavenko) Афиллофоровые грибы Урала. Свердловск: УФАН СССР, 1967. 296 с.
Ткаченко О.Б. (Tkachenko) Снежные плесени (история изучения, возбудители, их биологические особенности). М.: РАН, 2017. 72 с.
Третьякова А.С., Куликов П.В. (Tretyakova, Kulikov) Адвентивный компонент флоры Свердловской области: динамика видового состава // Вестн. Удмурт. ун-та. Биология. Науки о Земле. 2013. Вып. 4. С. 184–188.
Ушакова Н.В. (Ushakova) Специфика структуры биоты трутовых грибов города Екатеринбург. Тр. VIII конф. мол. ботаников в С.-Петербурге. СПб., СПбГЭТУ, 2004. С. 75.
Федоров А.А. (Fedorov) Растительные ресурсы СССР для народного хозяйства и медицины // Растительные ресурсы. 1965. Т. 1. Вып. 1. С. 5–18.
Ширяев А.Г. (Shiryaev) Два редких и интересных вида рода Physalacria на Урале // Новости систематики низших растений. 2007. Т. 41. С. 167–173.
Ширяев А.Г. (Shiryaev) Изменения микобиоты Урало-Сибирского региона в условиях глобального потепления и антропогенного воздействия // Вестник экологии, лесоведения и ландшафтоведения. 2009. № 9. С. 37–47.
Ширяев А.Г. (Shiryaev) Клавариоидные грибы антропогенных территорий Урала // Вестник экологии, лесоведения и ландшафтоведения. 2008. № 8. С. 80–91.
Ширяев А.Г., Мухин В.А., Котиранта Х. и др. (Shiryaev et al.) Биоразнообразие афиллофоровых грибов Урала. В сборнике конф. “Биологическое разнообразие растительного мира Урала и сопредельных территорий”. Екатеринбург, ИЭРиЖ УрО РАН, 2012. С. 311–313.
Ширяева О.C. (Shiryaeva) История изучения и видовое богатство агарикоидных базидиомицетов Свердловской области // Вестник Оренбургского государственного педагогического университета. 2015. № 4 (16). С. 49–58.
Ширяева О.С., Паламарчук М.А. (Shiryaeva, Palamarchuk) Новые сведения об агарикоидных грибах (Basidiomycota) Урала // Новости систематики низших растений. 2019. Т. 53. Ч. 1. P. 89–106.
Дополнительные материалы отсутствуют.
Инструменты
Микология и фитопатология