Микология и фитопатология, 2021, T. 55, № 5, стр. 311-317
A Contribution to the Aphyllophoroid Funga (Basidiomycota) of the Tlyaratinsky Protected Area (Dagestan, Russia)
1 Komarov Botanical Institute of the Russian Academy of Sciences
197376 Saint Petersburg, Russia
* E-mail: sergvolobuev@binran.ru
Поступила в редакцию 12.02.2021
После доработки 19.04.2021
Принята к публикации 24.05.2021
Аннотация
New data on 69 species of aphyllophoroid fungi registered in the Tlyaratinsky Federal State Nature Sanctuary (Russia, Dagestan, North-Eastern Caucasus) is presented including 67 species of Agaricomycetes and two species of Dacrymycetes. Sixty-one species are reported from the Tlyaratinsky Zakaznik for the first time, 36 species are new to Dagestan. Amylocorticium suaveolens, Antrodia macra, Ceriporia viridans, Ceriporiopsis resinascens, Coniophora fusispora, Dendrocorticium polygonioides, Trechispora laevis, and T. stevensonii are revealed from the Northern Caucasus for the first time. An annotated list of species with detailed information on substrata, habitats, localities, and numbers of specimens deposited in the Mycological Herbarium of the Komarov Botanical Institute RAS (LE) is provided. A total of 98 species of aphyllophoroid fungi, including four heterobasidioid species (Auricularia auricula-judae, Calocera cornea, C. viscosa, Tulasnella violea) are known to date for the Tlyaratinsky Protected Area.
INTRODUCTION
Mountain ecosystems are unique and host an over-proportioned share of global biodiversity and support an estimated one-third of terrestrial biological diversity (Spehn et al., 2011). Montane habitats are acknowledged biodiversity hot spots, but the intensity of inventory studies and the completeness of biodiversity examination for different groups of biota are extremely uneven. The territory of Dagestan located in the Eastern part of the North Caucasus could be taken as an example of such unevenness. About 3400 species of vascular plants and 850 species of lichens are known in the Republic of Dagestan (Murtazaliev, 2016; Ismailov et al., 2019). At the same time, until now the wood-inhabi-ting basidial macrofungi, with their sufficiently highly visible fruiting bodies, have been poorly investigated in the montane forest ecosystems of the North-Eastern Caucasus. First of all, the protected areas that ensure the conservation of biodiversity in mountain regions need an attention in respect of inventory studies (Tishkov, Belonovskaya, 2012).
One of these territories is the Tlyaratinskiy Zakaznik, or the Tlyaratinsky Federal State Nature Sanctuary (IUCN Category IV), located in the Republic of Dagestan (Russia) along the eastern part of the Greater Caucasus Range at the border with Georgia and Azerbaijan. The only publication on the poroid and corticioid fungi of the Zakaznik is known to date. It reports 37 species revealed in the field survey in May 2017 (Viner, 2017). This study aims to increase the knowledge on the species diversity and ecology of aphyllophoroid fungi in the protected area.
MATERIALS AND METHODS
Study area. The Tlyaratinsky Zakaznik is located in Tlyaratinsky District and occupies 835 km2 in the upper reaches of the Avarskoe Koisu River (the Dzhurmut River Basin). The territory covers the northern slopes of the Greater Caucasus Range and the southwestern spurs of the Nukatl Range. The altitude range of the protected area is between 1500 and 3932 m a.s.l. The Zakaznik borders on the south with the Lagodekhi Nature Reserve of Georgia and the Zakatala Nature Reserve of Azerbaijan. The average sum of precipitation is about 800 mm per year. The average annual relative humidity is 65–75% (Akaev et al., 1996; Yarovenko et al., 2004). The Zakaznik has a well-defined altitudinal gradient, from top to bottom: a nival belt with glaciers and snowfields, alpine and subalpine meadows, scrubby birch woodlands, coniferous, mixed and deciduous mountain forests, meadows and bushes, stony slopes with xerophytic vegetation (Ismailov, 2017). The Tlyaratinsky Zakaznik flora includes more than 600 species of vascular plants with 60 species of trees and shrubs (Yarovenko et al., 2004). Coniferous (pine) forests are formed by Pinus kochiana and distributed at about 1900–2200 m a.s.l. Mixed forests with Pinus kochiana, Betula litwinowii, B. pendula, and B. raddeana are spread at about the altitude of 1800–1900 m as well as a bit above the belt of pine forests. Deciduous forests are presented by stands with Quercus macranthera (on the southern exposition slopes), Populus tremula, and Betula spp. at about 1700–1800 m.
Sampling. Basidiocarps of aphyllophoroid fungi were collected by the author at different types of forests during a route survey of the protected area in the beginning of September 2020. Various woody substrata such as fallen trunks and branches, dry attached branches, stumps, living trees and shrubs, were mainly examined. The geographical coordinates and altitudes of studied localities were measured by the Garmin 64st GPS-navigator. The following numbers are corresponding to the investigated localities and date of collection.
September 4, 2020: t1 – 41.94677° N, 46.53877° E, 1951 m a.s.l.; t2 – 41.94740° N, 46.53669° E, 1997 m a.s.l.; t3 – 41.94716° N, 46.53601° E, 2025 m a.s.l.;
September 5, 2020: t4 – 41.94745° N, 46.53192° E, 2134 m a.s.l.; t5 – 41.94469° N, 46.53288° E, 2186 m a.s.l.; t6 – 41.94387° N, 46.53752° E, 2133 m a.s.l.;
September 6, 2020: t7 – 41.97405° N, 46.50333° E, 1802 m a.s.l.; t8 – 41.97456° N, 46.50285° E, 1829 m a.s.l.; t9 – 41.97572° N, 46.50230° E, 1861 m a.s.l.; t10 – 41.97505° N, 46.50291° E, 1868 m a.s.l.;
September 7, 2020: t11 – 42.01050° N, 46.50235° E, 1745 m a.s.l.; t12 – 42.01244° N, 46.50680° E, 1754 m a.s.l.; t13 – 41.97574° N, 46.49365° E, 1930 m a.s.l.; t14 – 41.97485° N, 46.49325° E, 1952 m a.s.l.
Identification and examination of specimens. Microscopic identification of herbarized specimens were performed using a AxioScope A1 microscope, a LOMO Mikmed-6 microscope with a standard set of chemicals (5% KOH, Melzer’s reagent, 0.1% Cotton Blue) based on key monographs on poroid and corticioid fungi (Kõljalg, 1996; Bernicchia, Gorjón, 2010, 2020; Ryvarden, Melo, 2017) as well as some modern taxonomy articles. Data on the fungal species distribution is based on available publications and according to the updated database on Agaricomycetes diversity (Bolshakov et al., 2017). Voucher specimens are deposited in the Mycological Herbarium of the Komarov Botanical Institute RAS, St. Petersburg (LE).
RESULTS
As a result of mycological survey of Tlyaratinsky Zakaznik forests 69 species of aphyllophoroid fungi (Basidiomycota) have been registered. The list of species revealed is provided with data on localities, occupied substrata, types of habitats and herbarium numbers of specimens examined. The nomenclature of fungal taxa follows the Index Fungorum (2021). The species new to the Republic of Dagestan are marked with “!” and new to the Northern Caucasus – with “!!”. An asterisk (*) shows the species recorded for the territory of the Tlyaratinsky Zakaznik for the first time.
Agaricales
*Mucronella calva (Alb. et Schwein.) Fr. – t1: on fallen trunk of Pinus kochiana (d = 25 cm) in blueberry-herbal pine-dominated forest (LE F-334503).
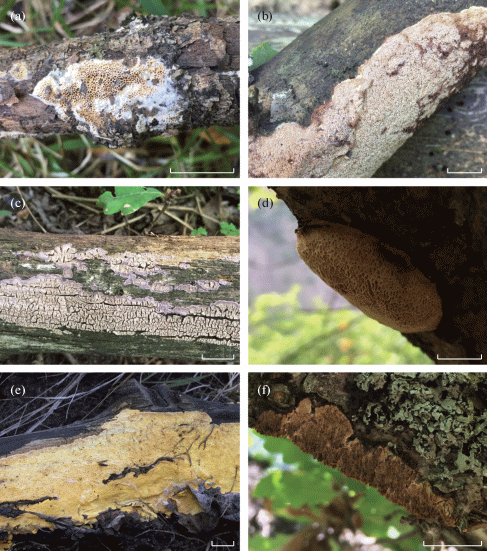
Radulomyces molaris (Chaillet ex Fr.) M.P. Christ. – t7, t8, t9: on dry dead branches of Quercus macranthera in herb-rich oak-dominated forest (LE F-334536). Fig. 1 (F).
Fig. 1.
Basidiocarps of aphyllophoroid fungi species revealed: a – Antrodia macra, b – Ceriporia bresadolae, c – Dendrocorticium polygonioides, d – Dichomitus campestris, e – Perenniporia tenuis var. pulchella, f – Radulomyces molaris. Scale bar – 1 cm.
*Schizophyllum commune Fr. – t14: on fallen trunk (d = = 15 cm) of Populus tremula in herb-rich birch forest with aspen and pine.
Amylocorticiales
!!Amylocorticium suaveolens Parmasto – t5: on fallen trunk of Pinus kochiana (d = 20 cm) in fern-herbal pine-dominated forest with birch (LE F-334517).
!Plicatura crispa (Pers.) Rea – t9: on fallen branches of Quercus macranthera in herb-rich oak-dominated forest with aspen (LE F-334544).
Atheliales
*Amphinema byssoides (Pers.) J. Erikss. – t6: on fallen trunk of Pinus kochiana (d = 40 cm) in herb-rich pine-do-minated forest (LE F-334523).
!Athelia decipiens (Höhn. et Litsch.) J. Erikss. – t9: on fallen trunk of Populus tremula (d = 30 cm) in herb-rich oak-dominated forest with aspen (LE F-334547).
Auriculariales
*Auricularia auricula-judae (Bull.) Quél. – t9: on fallen branches of Quercus macranthera in herb-rich oak-dominated forest with aspen (LE F-334543).
Boletales
!!Coniophora fusispora (Cooke et Ellis) Cooke – t6: on fallen trunk of Pinus kochiana (d = 40 cm) in herb-rich pine-dominated forest (LE F-334524).
*C. puteana (Schumach.) P. Karst. – t14: on fallen trunk of Populus tremula (d = 15 cm) in herb-rich birch forest with aspen and pine (LE F-334563).
Cantharellales
Botryobasidium candicans J. Erikss. – t6: on fallen trunk of Pinus kochiana (d = 35 cm) in herb-mosses pine-dominated forest (LE F-334526).
*B. isabellinum (Fr.) D.P. Rogers – t1, t14: on fallen branches of Pinus kochiana (d = 5 cm) in blueberry-herbal pine-dominated forest (LE F-334499) and on fallen branches of Populus tremula (d = 10 cm) in herb-rich birch forest with aspen and pine (LE F-334571).
B. subcoronatum (Höhn. et Litsch.) Donk – t6: on fallen trunk of Pinus kochiana (d = 35 cm) in herb-mosses pine-dominated forest (LE F-334529).
Corticiales
!Corticium roseum Pers. – t12, t14: on dry dead branches of Salix caprea in floodplain mixed forest (LE F-334559) and on fallen trunk (d = 15 cm) and fallen branches (d = 10 cm) of Populus tremula in herb-rich birch forest with aspen and pine (LE F-334573).
!!Dendrocorticium polygonioides (P. Karst.) M.J. Larsen et Gilb. – t9: on fallen branches of Quercus macranthera (d = 5 cm) in herb-rich oak-dominated forest with aspen (LE F-334550). Fig. 1 (C).
!Vuilleminia comedens (Nees) Maire – t7: on dry dead branches of Quercus macranthera in herb-rich oak-dominated forest (LE F-334533).
Gloeophyllales
!Gloeophyllum trabeum (Pers.) Murrill – t14: on fallen trunk of Pinus kochiana (d = 10 cm) in herb-rich birch forest with aspen and pine (LE F-334562).
Hymenochaetales
!Hydnoporia tabacina (Sowerby) Spirin, Miettinen et K.H. Larss. – t6: on dead standing tree of Betula raddeana (d = 10 cm) in herb-rich birch forest (LE F-334518).
!Kurtia argillacea (Bres.) Karasiński – t1, t6: on fallen branches (d = 5 cm) and fallen trunk (d = 35 cm) of Pinus kochiana in blueberry-herbal pine-dominated forest (LE F-334501) and in herb-mosses pine-dominated forest (LE F-334530).
*Lyomyces crustosus (Pers.) P. Karst. – t4: on dry dead branches of Pinus kochiana (d = 1 cm) in herb-rich pine-dominated forest (LE F-334516).
Peniophorella praetermissa (P. Karst.) K.H. Larss. – t6, t9: on fallen trunks of Pinus kochiana (d = 40 cm) and Populus tremula in herb-rich pine-dominated forest (LE F-334525) and in herb-rich oak-dominated forest with aspen (LE F-334546).
*Phellinus nigricans (Fr.) P. Karst. – t6: on dead standing tree of Betula raddeana (d = 10 cm) in herb-rich birch forest (LE F-334519).
*Ph. tremulae (Bondartsev) Bondartsev et P.N. Borisov – t9, t13, t14: on dead standing trees (d = 20 cm) and fallen trunk (d = 30 cm) of Populus tremula in herb-rich oak-dominated forest with aspen, in herb-rich aspen forest, in herb-rich birch forest with aspen and pine (LE F-334569).
!Porodaedalea pini (Brot.) Murrill – t5: on living tree of Pinus kochiana (d = 80 cm) in fern-herbal pine-dominated forest with birch (LE F-334576).
!Trichaptum fuscoviolaceum (Ehrenb.) Ryvarden – t6: on fallen trunk of Pinus kochiana (d = 35 cm) in herb-mosses pine-dominated forest (LE F-334528).
!Xylodon asperus (Fr.) Hjortstam et Ryvarden – t11: on fallen trunk of Quercus macranthera (d = 50 cm) in floodplain mixed forest (LE F-334553).
Polyporales
!!Antrodia macra (Sommerf.) Niemelä – t12, t14: on fallen and dry branches of Salix caprea in floodplain mixed forest (LE F-334558) and on fallen branches of Populus tremula (d = 10 cm) in herb-rich birch forest with aspen and pine (LE F-334570). Fig. 1 (A).
*Ceriporia bresadolae (Bourdot et Galzin) Donk – t1: on fallen branches of Pinus kochiana (d = 5 cm) in blueberry-herbal pine-dominated forest (LE F-334505). Fig. 1 (B).
!!C. viridans (Berk. et Broome) Donk – t9: on fallen trunk of Populus tremula (d = 30 cm) and on fallen branches of Quercus macranthera (d = 5 cm) in herb-rich oak-dominated forest with aspen (LE F-334548, LE F-334549).
!!Ceriporiopsis resinascens (Romell) Domański – t11: on fallen trunk of Quercus macranthera (d = 50 cm) in floodplain mixed forest (LE F-334556).
*Cerrena unicolor (Bull.) Murrill – t2, t7, t10: on fallen branches of Prunus padus, on dead standing tree of Quercus macranthera and at the base of living tree of Carpinus betulus in herb-mosses pine-dominated forest, in herb-rich oak-dominated forest and in herb-rich hornbeam forest with oak (LE F-334551).
*Daedaleopsis confragosa (Bolton) J. Schröt. – t11: at the base of living tree of Salix sp. in floodplain mixed forest (LE F-334554).
!Dichomitus campestris (Quél.) Domański et Orlicz – t7, t9: on dry dead branches of Quercus macranthera in herb-rich oak-dominated forest (LE F-334532). Fig. 1 (D).
*Efibula tuberculata (P. Karst.) Zmitr. et Spirin – t8: on fallen trunk of Quercus macranthera in herb-rich oak-dominated forest (LE F-334537).
*Fomitopsis betulina (Bull.) B.K. Cui, M.L. Han et Y.C. Dai – t14: on fallen branches of Betula sp. (d = 15 cm) in herb-rich birch forest with aspen and pine.
!Hyphoderma mutatum (Peck) Donk – t13: on fallen trunk of Populus tremula (d = 15 cm) in herb-rich aspen forest (LE F-334561).
H. setigerum (Fr.) Donk – t9: on fallen branches of Populus tremula (LE F-334540) and Quercus macranthera (LE F-334545) in herb-rich oak-dominated forest with aspen.
Irpex lacteus (Fr.) Fr. – t9, t14: on dry dead branches of Quercus macranthera and on fallen branches of Populus tremula (d = 10 cm) in herb-rich oak-dominated forest with aspen (LE F-334538) and in herb-rich birch forest with aspen and pine (LE F-334574).
Meruliopsis taxicola (Pers.) Bondartsev – t4: on dry dead branches of Pinus kochiana (d = 20 cm) in herb-rich pine-dominated forest (LE F-334515).
!Perenniporia tenuis (Schwein.) Ryvarden – t14: on fallen trunk (d = 15 cm) and fallen branches (d = 10 cm) of Populus tremula in herb-rich birch forest with aspen and pine (LE F-334564, LE F-334566). Fig. 1 (E).
*Phanerochaete laevis (Fr.) J. Erikss. et Ryvarden – t1: on fallen branches of Pinus kochiana (d = 3 cm) in herb-mosses pine-dominated forest (LE F-334507).
*Ph. sordida (P. Karst.) J. Erikss. et Ryvarden – t6: on fallen branches of Pinus kochiana (d = 6 cm) in herb-mosses pine-dominated forest (LE F-334531).
*Ph. velutina (DC.) P. Karst. – t4: on fallen branches of Pinus kochiana (d = 7 cm) in herb-rich pine-dominated forest (LE F-334512).
Skeletocutis amorpha (Fr.) Kotl. et Pouzar – t1, t6: on fallen trunks of Pinus kochiana (d = 25–40 cm) in herb-rich and blueberry-herbal pine-dominated forests (LE F-334504).
*Trametes betulina (L.) Pilát – t11: on living tree of Betula sp. in floodplain mixed forest (LE F-334555).
*T. hirsuta (Wulfen) Lloyd – t2, t9: on fallen branches of Quercus macranthera and Sorbus sp. in herb-rich oak-dominated forest with aspen and in herb-mosses pine-dominated forest (LE F-334508).
*T. ochracea (Pers.) Gilb. et Ryvarden – t13, t14: on fallen trunk (d = 15 cm) and dead standing tree (d = 20 cm) of Populus tremula in herb-rich aspen forest and in herb-rich birch forest with aspen and pine.
*T. pubescens (Schumach.) Pilát – t11: at the base of Betula sp. tree (d = 10 cm) in floodplain mixed forest (LE F-334557).
*T. versicolor (L.) Lloyd – t2, t3: on fallen trunk of Betula raddeana (d = 5 cm), on fallen branches of Prunus padus and Sorbus sp. in herb-mosses and herb-rich pine-dominated forest (LE F-334509).
Russulales
!Megalocystidium luridum (Bres.) Jülich – t2: on fallen branches of Prunus padus in herb-mosses pine-dominated forest (LE F-334511).
!Peniophora incarnata (Pers.) P. Karst. – t7, t9: on dry dead branches of Quercus macranthera in herb-rich oak-dominated forest (LE F-334534).
*P. laeta (Fr.) Donk – t10: on fallen branches of Carpinus betulus (d = 1 cm) in herb-rich hornbeam forest with oak (LE F-334552).
!P. pini (Schleich. ex DC.) Boidin – t4: on fallen branches of Pinus kochiana (d = 10 cm) in herb-rich pine-dominated forest (LE F-334514).
!P. quercina (Pers.) Cooke – t9: on dry dead branches of Quercus macranthera in herb-rich oak-dominated forest with aspen (LE F-334542).
!P. rufa (Fr.) Boidin – t9, t13: on fallen branches and fallen trunk (d = 15 cm) of Populus tremula in herb-rich oak-dominated forest with aspen and in herb-rich aspen forest (LE F-334539, LE F-334560).
!P. violaceolivida (Sommerf.) Massee – t9: on fallen branches of Populus tremula in herb-rich oak-dominated forest with aspen (LE F-334541).
!Stereum gausapatum (Fr.) Fr. – t7, t9: on dry dead branches and at the base of alive Quercus macranthera in herb-rich oak-dominated forest (LE F-334535).
*S. hirsutum (Willd.) Pers. – t6, t9: on fallen branches of Betula raddeana (d = 1 cm), Populus tremula and on dry dead branches of Quercus macranthera in herb-rich birch forest and in herb-rich oak-dominated forest with aspen (LE F-334522).
!S. rugosum Pers. – t2, t5: on fallen branches of Prunus padus and at the base of Betula raddeana (d = 50 cm) in herb-mosses pine-dominated forest and in fern-herbal pine-dominated forest with birch (LE F-334510).
!S. sanguinolentum (Alb. et Schwein.) Fr. – t1: on fallen branches of Pinus kochiana (d = 5 cm) in blueberry-herbal pine-dominated forest (LE F-334502).
!Xenasmatella vaga (Fr.) Stalpers – t4, t6: on fallen branches (d = 7 cm) and fallen trunks (d = 15–35 cm) of Pinus kochiana in herb-rich and herb-mosses pine-dominated forests (LE F-334513).
Thelephorales
!Tomentella sublilacina (Ellis et Holw.) Wakef. – t14: on fallen trunk of Populus tremula (d = 15 cm) in herb-rich birch forest with aspen and pine (LE F-334565).
*T. subtestacea Bourdot et Galzin – t14: on fallen branches of Populus tremula (d = 10 cm) in herb-rich birch forest with aspen and pine (LE F-334567).
Trechisporales
!Porpomyces mucidus (Pers.) Jülich – t6: on old destroyed stump of Pinus kochiana in herb-rich birch forest (LE F-334521).
!Trechispora farinacea (Pers.) Liberta – t1: on fallen branches of Pinus kochiana (d = 5 cm) in blueberry-herbal pine-dominated forest (LE F-334506).
!!T. laevis K.H. Larss. – t6: on fallen trunk of Pinus kochiana (d = 35 cm) in herb-mosses pine-dominated forest (LE F-334527).
!!T. stevensonii (Berk. et Broome) K.H. Larss. – t14: on fallen branches of Populus tremula (d = 10 cm) in herb-rich birch forest with aspen and pine (LE F-334568, LE F-334575).
Dacrymycetales
!Calocera cornea (Batsch) Fr. – t1, t14: on fallen branches of Pinus kochiana (d = 5 cm) and Populus tremula (d = 10 cm) in blueberry-herbal pine-dominated forest and in herb-rich birch forest with aspen and pine (LE F-334500, LE F-334572).
!C. viscosa (Pers.) Fr. – t6: on buried dead wood at the base of Betula raddeana in herb-rich birch forest (LE F-334520).
DISCUSSION
A total of 69 fungal species from 45 genera and 14 orders of Basidiomycota are listed. Most of the species are Agaricomycetes (67) and only two species belong to Dacrymycetes. The most species-rich orders are Polyporales (23 species), Russulales (12), and Hymenochaetales (9), that reflects the conventional taxonomical structure of aphyllophoroid fungi in temperate forest zone at the order level. The maximum species richness has been noted for the genera Peniophora (6), Trametes (5), Stereum (4). Apparently, this diversity underpinned by the simultaneous presence of diverse woody substrata, namely plant genera, at the studied area. In particular, there were revealed four host-specific species of Peniophora namely P. laeta (Carpinus betulus), P. pini (Pinus spp.), P. quercina (Quercus spp.), P. rufa (Populus tremula). At the same time, most of aphyllophoroid fungi have no strict substrate requirements within the groups of deciduous- or coniferous-inhabiting species. Besides four Peniophora species named above, Ceriporia bresadolae (Fig. 1, B), Porodaedalea pini, Trichaptum fuscoviolaceum (on pine), Fomitopsis betulina (on birch), Phellinus tremulae (on aspen), Stereum gausapatum (on oak) are stenotrophic.
The remarkable record of Phanerochaete velutina from fallen branches of Pinus kochiana together with verified records of the species from the Russian Far East (Spirin et al., 2017) confirmed the ability of this fungus to inhabitat both angiosperm and coniferous substrata. This fungal ecological guild distinguished as pantotrophs (Volobuev, 2015) is presented currently by Botryobasidium isabellinum, Calocera cornea, and Peniophorella praetermissa, but further investigations undoubtedly will complement this list.
Among the species revealed 61 are registered from the Tlyaratinsky Zakaznik for the first time, including 35 species are new to Dagestan with eight species found on the territory of the Northern Caucasus for the first time. The latter ones are Amylocorticium suaveolens, Antrodia macra (Fig. 1, A), Ceriporia viridans, Ceriporiopsis resinascens, Coniophora fusispora, Dendrocorticium polygonioides (Fig. 1, C), Trechispora laevis, and T. stevensonii. Some of them are worthy of brief discussion.
T. laevis and T. stevensonii seem to be the least known species in Russia and the Caucasus. To date in Russia T. laevis is reported only from northern Siberia (Yamalo-Nenets Autonomous Okrug) and the Urals (Sverdlovsk Oblast) (Kotiranta, Penzina, 1998; Shiryaev et al., 2010). T. stevensonii has been revealed in four regions of the north-west and the center of the European Russia (Kotkova, 2014; Ezhov et al., 2019) as well as from Armenia, Iran and Turkey in the Caucasus (Ghobad-Nejhad et al., 2009).
Amylocorticium suaveolens is characterized by yellowish-ochre resupinate pronouncedly scented basidiocarps which develop on coniferous dead wood. At the moment this species is known from nine regions of the north and the center of the European Russia (Zmitrovich, 2002; Volobuev, 2015) and the Urals (Shiryaev et al., 2010).
Coniophora fusispora is uncommon species differing by rather thin, pellicular brown-coloured basidiocarps and fusiform basidiospores. The species grows usually on gymnosperm (Pinaceae) wood, but also was reported from deciduous, and it has been represented by single records only in eight Russian regions, viz. the north-west of the European part, the Urals, Siberia and the Russian Far East (Bondartseva, Parmasto, 1986; Lositskaya et al., 1999; Shiryaev et al., 2010; Stavishenko, 2011).
Two angiosperm-dwelling species, Dendrocorticium polygonioides and Perenniporia tenuis, have already been found in the Caucasus (Ghobad-Nejhad et al., 2009), but require a special attention regarding to their conservation value. Dendrocorticium polygonioides has been proposed as indicator species for undisturbed oak-dominanted forests (Spirin, 2002). Our record on Quercus macranthera fallen branches from the Tlyaratinsky Zakaznik is the second one for the Caucasus after the Iranian collection also observed on decorticated fallen branch of Quercus sp. (Ghobad-Nejhad et al., 2008). According to Eriksson and Ryvarden (1976) this species prefers dry open localities and inhabits different deciduous substrata, e.g. Salix, Populus, Betula, Fagus, Fraxinus, Quercus, Rosa, etc. In total 17 species (24.6%) in the studied protected area are noted to be associated with Q. macranthera wood that highlights the significant contribution of oak-dominated forests spread solely along the southern exposition slopes to conservation of suitable habitats for aphyllophoroid fungi in the Tlyaratinsky Zakaznik.
Perenniporia tenuis is widely distributed in forest zone of Eurasia and Northern America (Ryvarden, Melo, 2017) but the species is everywhere a rare one. Perenniporia tenuis var. pulchella (Schwein.) Gilb. et Ryvarden (Fig. 1, E) with bright yellow pore surface of resupinate basidiocarps has been revealed on fallen trunk and branches of Populus tremula. The species has no strict host specificity (aspen, oak, and other deciduous trees) but requires humid old-growth forests and forest ecosystems free from any anthropogenic activity. The population of Perenniporia tenuis in the Tlyaratinsky Zakaznik is recommended for further monitoring.
CONCLUSION
Being as an obligate component of forest ecosystems, aphyllophoroid fungi are involved in dead wood degradation as well as they form ectomycorrhizal symbiosis with trees and shrubs and thus contribute to the productivity of ecosystems. An assessment of ecosystem functions for any group of biota, including fungi, starts with the species diversity inventory in particular area conditions. In this study, new data on 69 species of aphyllophoroid fungi (Basidiomycota), their substrata and habitats, has been obtained for the Tlyaratinsky Zakaznik which is one of the state nature protected areas at federal level. According to these results coupled with published data, a total of 98 species of aphyllophoroid fungi, including four heterobasidiomycetous (Auricularia auricula-judae, Calocera cornea, C. viscosa, Tulasnella violea) are known currently for this area. To date, eight species of aphyllophoroid fungi have been registered for the Northern Caucasus only from the Tlyaratinsky Protected Area.
The author is grateful to Dr. Aziz B. Ismailov (Mountain Botanical Garden, the Dagestan Federal Research Center of RAS, Makhachkala) and to the administration of the “Dagestansky” State Nature Reserve for the organization of fieldwork. This study was supported financially by the Russian Science Foundation (RSF project N 19-77-00085).
Список литературы
Akaev B.A., Ataev Z.V., Gadzhiev B.S. et al. Physical geography of Dagestan. School, Moscow, 1996 (in Russ.).
Bernicchia A., Gorjón S.P. Corticiaceae s. l. Fungi Europaei. Vol. 12. Edizioni Candusso, Alassio, 2010.
Bernicchia A., Gorjón S.P. Polypores of the Mediterranean Region. Romar, Segrate, 2020.
Bolshakov S.Yu., Volobuev S.V., Ezhov O.N. et al. Checklist of aphyllophoroid fungi of the European part of Russia: the first results. In: Dyakov Yu.T., Sergeev Yu.V. (eds.) Current mycology in Russia. Vol. 6. National Academy of mycology, Moscow, 2017. P. 120–122 (in Russ.).
Bondartseva M.A., Parmasto E. Clavis diagnostica fungorum URSS. Ordo Aphyllophorales. Fasc. 1. Familiae Hymenochaetaceae, Lachnocladiaceae, Coniophoraceae, Schizophyllaceae. Nauka, Leningrad, 1986 (in Russ.).
Eriksson J., Ryvarden L. The Corticiaceae of North Europe. V. 4: Hyphodermella–Mycoacia. Fungiflora, Oslo, 1976.
Ezhov O.N., Zmitrovich I.V., Ruokolainen A.V. New data on aphyllophoroid fungi and some other groups of macromycetes of the Solovetsky Archipalago. Transactions of KarRC RAS. 2019. V. 1. P. 85–92 (in Russ.). https://doi.org/10.17076/bg849
Ghobad-Nejhad M., Hallenberg N., Kotiranta H. Additions to the corticioids of the Caucasus from NW Iran. Mycotaxon. 2008. V. 105 (3). P. 269–293.
Ghobad-Nejhad M., Hallenberg N., Parmasto E. et al. A first annotated checklist of corticioid and polypore basidiomycetes of the Caucasus region. Mycologia Balcanica. 2009. V. 6 (3). P. 123–168. https://doi.org/10.5281/zenodo.2550071
Index Fungorum. CABI Bioscience, 2021. http://www.indexfungorum.org. Accessed 30.01.2021.
Ismailov A.B. A contribution to the lichen flora of Tlyaratinskiy Protected Area (East Caucasus, Dagestan, Russia). Novosti sistematiki nizschikh rasteniy. 2017. V. 51. P. 178–190. https://doi.org/10.31111/nsnr/2017.51.178
Ismailov A.B., Urbanavichus G.P., Vondrak J. New lichenized fungi for Russia from Dagestan (East Caucasus). Folia Cryptog. Estonica. 2019. V. 56. P. 7–10. https://doi.org/10.12697/fce.2019.56.02
Kotiranta H., Penzina T. Notes on the North Ural Aphyllophorales (Basidiomycetes). In: V.A. Mukhin, H. Knudsen (eds). Arctic and Alpine Mycology 5: Proceedings of the Fifth International Symposium on Arcto-Alpine Mycology (Labytnangi, Russia, August 15–27, 1996). Ekaterinburg, 1998. P. 67–81.
Kotkova V.M. Rare and new for the territory of St. Petersburg species of aphyllophoraceous fungi (Basidiomycota). Novosti sistematiki nizschikh rasteniy. 2014. V. 48. P. 146–151 (in Russ.) https://doi.org/10.31111/nsnr/2014.48.146
Kõljalg U. Tomentella (Basidiomycota) and related genera in temperate Eurasia. Synopsis Fungorum. V. 9. Fungiflora, Oslo, 1996.
Lositskaya V.M., Bondartseva M.A., Krutov V.I. Aphyllophoraceous fungi as indicators of the pine stands status in the Kostomuksha City industrial zone (Karelia). Mikologiya i fitopatologiya. 1999. V. 33 (5). P. 331–337 (in Russ.).
Murtazaliev R.A. Analysis of species distribution in the Dagestan flora. Botanicheskiy zhurnal. 2016. V. 101 (9). P. 1056–1074 (in Russ.).
Ryvarden L., Melo I. Poroid fungi of Europe. Second revised edition. Synopsis Fungorum. Vol. 37. Fungiflora, Oslo, 2017.
Shiryaev A.G., Kotiranta H., Mukhin V.A. et al. Aphyllophoroid fungi of Sverdlovsk Region, Russia: biodiversity, distribution, ecology and the IUCN threat categories. Goshchitskiy Publisher, Ekaterinburg, 2010.
Spehn E.M., Rudmann-Maurer K., Körner C. Mountain biodiversity. Plant Ecology and Diversity. 2011. V. 4 (4). P. 301–302. https://doi.org/10.1080/17550874.2012.698660
Spirin V.A. Aphyllophoroid macromycetes in oak forests of Nizhny Novgorod Region. Mikologiya i fitopatologiya. 2002. V. 36 (2). P. 43–52 (in Russ.).
Spirin V.A., Volobuev S.V., Okun M.V. et al. What is the type species of Phanerochaete (Polyporales, Basidiomycota)? Mycol. Progress. 2017. V.16 (2). P. 171–183. https://doi.org/10.1007/s11557-016-1267-8
Stavishenko I.V. Aphyllophoraceous fungi of the Nature Reserve “Malaya Sosva” (Western Siberia). Mikologiya i fitopatologiya. 2011. V. 45 (2). P. 142–157 (in Russ.).
Tishkov A., Belonovskaya E. Mountain natural biodiversity conservation in Russia. Geography, environment, sustainability. 2012. V. 5 (2). P. 51–67. https://doi.org/10.24057/2071-9388-2012-5-2-51-67
Viner I.A. New records of polypores and corticioid fungi in Dagestan. In: Proceedings of “Dagestanskiy” State Nature Reserve. V. 13. Makhachkala, 2017. P. 13–19 (in Russ.).
Volobuev S.V. Aphyllophoroid fungi of the Oryol Region: Taxonomical composition, distribution, ecology. Lan, Moscow, SPb., 2015 (in Russ.).
Yarovenko Yu.A., Murtazaliev R.A., Ilyina E.V. Reserved places of Dagestan. Raduga-1, Makhachkala, 2004 (in Russ.).
Zmitrovich I.V. The genus Amylocorticium Pouzar in Russia. Novosti sistematiki nizschikh rasteniy. 2002. V. 36. P. 31–35 (in Russ.).
Акаев Б.А., Атаев З.В., Гаджиев Б.С. и др. (Akaev et al.) Физическая география Дагестана. М.: Школа, 1996. 380 с.
Большаков С.Ю., Волобуев С.В., Ежов О.Н. и др. (Bolshakov et al.) Чек-лист афиллофороидных грибов европейской части России: первые результаты // Современная микология в России. Том 6 / Ред. Ю.Т. Дьяков, Ю.В. Сергеев. М.: Нац. акад. микол., 2017. С. 120–122.
Бондарцева М.А., Пармасто Э.Х. (Bondartseva, Parmasto) Определитель грибов СССР. Порядок афиллофоровые; Вып. 1. Семейства гименохетовые, лахнокладиевые, кониофоровые, щелелистниковые / Отв. ред. М.В. Горленко. Л.: Наука, 1986. 192 с.
Винер И.А. (Viner) Новые находки трутовых и кортициоидных грибов в Дагестане // Труды государственного природного заповедника “Дагестанский”. Вып. 13. Махачкала: Алеф, 2017. С. 13–19.
Волобуев С.В. (Volobuev) Афиллофороидные грибы Орловской области: Таксономический состав, распространение, экология. СПб.; М.: Лань, 2015. 304 с.
Ежов О.Н., Змитрович И.В., Руоколайнен А.В. (Ezhov et al.) Новые данные об афиллофоровых грибах и некоторых других группах макромицетов Соловецкого архипелага // Труды Карельского научного центра РАН. 2019. № 1. С. 85–92.
Змитрович И.В. (Zmitrovich) Род Amylocorticium Pouzar в России // Новости систематики низших растений. 2002. Т. 36. С. 31–35.
Коткова В.М. (Kotkova) Редкие и новые для территории Санкт-Петербурга виды афиллофоровых грибов (Basidiomycota) // Новости систематики низших растений. 2014. Т. 48. С. 146–151.
Лосицкая В.М., Бондарцева М.А., Крутов В.И. (Lositskaya et al.) Афиллофоровые грибы как индикаторы состояния сосновых древостоев промышленной зоны города Костомукши (Карелия) // Микология и фитопатология. 1999. Т. 33. № 5. С. 331–337.
Муртазалиев Р.А. (Murtazaliev) Анализ распределения видов флоры Дагестана // Ботанический журнал. 2016. Т. 101. № 9. С. 1056–1074.
Спирин В.А. (Spirin) Афиллофороидные макромицеты дубрав Нижегородской области // Микология и фитопатология. 2002. Т. 36. № 2. С. 43–52.
Ставишенко И.В. (Stavishenko) Афиллофоровые грибы заповедника “Малая Сосьва” (Западная Сибирь) // Микология и фитопатология. 2011. Т. 45. № 2. С. 142–157.
Яровенко Ю.А., Муртазалиев Р.А., Ильина Е.В. (Yarovenko et al.) Заповедные места Дагестана. Махачкала: Радуга-1, 2004. 96 с.
Дополнительные материалы отсутствуют.
Инструменты
Микология и фитопатология


