Микология и фитопатология, 2021, T. 55, № 6, стр. 387-395
An Ultrastructural Study of Opportunistic Fungal Pathogen Rhizomucor pusillus (Mucoromycetes)
Yu. L. Avdeenko 1, *, A. E. Vishnyakov 2, **, A. N. Ivanova 2, 3, ***, A. E. Taraskina 1, ****, G. A. Chilina 1, *****, I. A. Bosak 1, ******, T. S. Bogomolova 1, *******, S. A. Karpov 1, 2, 4, ********
1 Kashkin Research Institute of Medical Mycology of North-Western State Medical University named after I.I. Mechnikov
St. Petersburg, Russia
2 Saint Petersburg State University
St. Petersburg, Russia
3 Komarov Botanical Institute of the Russian Academy of Sciences
St. Petersburg, Russia
4 Zoological Institute of the Russian Academy of Sciences
St. Petersburg, Russia
* E-mail: yurii.avdeenko@szgmu.ru
** E-mail: vishnyakov@hotmail.com
*** E-mail: alyx@bk.ru
**** E-mail: anastasiya.taraskina@szgmu.ru
***** E-mail: galina.chilina@szgmu.ru
****** E-mail: ilya.bosak@szgmu.ru
******* E-mail: tatiyana.bogomolova@szgmu.ru
******** E-mail: sakarpov4@gmail.com
Поступила в редакцию 15.05.2021
После доработки 20.05.2021
Принята к публикации 24.05.2021
Аннотация
Mucoromycetes are well-known cosmopolitan molds. Recently, these fungi have become increasingly important due to their ability to cause life-threatening infections in immunocompromised persons. This paper is the first one to study the ultrastructure of hyphae and sporangiospores in Rhizomucor pusillus, using the strain RCPF 1845. Species identification was done by morphological analysis and confirmed by DNA sequencing of the large subunit ribosomal RNA gene. For a more comprehensive analysis, transmission electron microscopy was carried out with specimens, fixed by chemical and cryofixation with high pressure freezing procedure. High pressure cryofixation preserved microtubules in the cytoplasm and nucleoplasm better, although traditional chemical fixation using paraform allowed to get the acceptable images of sporangiospores. We described the structure of nuclei, cell walls and septa, mitochondria and autophagic vacuoles. New data have been obtained on the vesicular transport through the hyphal wall.
INTRODUCTION
Mucoromycetes are ubiquitous saprotrophic fungi that can cause life-threatening human infections, primarily in patients with diabetes mellitus and immunocompromised people (Jeong et al., 2019; Klimko et al., 2019; Singh et al., 2021). The number of invasive infections caused by mucoromycetes (mucormycosis) has increased significantly in recent years, including COVID-19 patients. The Global burden of invasive mucormycosis is estimated as ~5000 cases in patients with leukemia and around 35 000 cases related to malignancy and bone marrow transplantation (https://gaffi.org). There are many mucoromycete-associated infections in India and Southeast Asia (Singh et al., 2021). Most of the strains isolated from such diseases belong to the genera Rhizopus, Mucor, Lichtheimia, and Rhizomucor (Maurer et al., 2019; de Hoog et al., 2020).
Rhizomucor pusillus accounts for 20–25% of 100 cases culture proven mucormycosis in Russian Federation during 2002–2020 years (Khostelidi et al., 2019, 2020). This fungus has a world-wide distribution and is associated with soil, rotten fruits and vegetables and compost heaps. It is a thermophilic species with wide temperature growth range: minimum at 20–27°C, optimum at 35–55°C (Kidd et al., 2016). The cultures are grey to greyish brown, 2–3 mm high. Stolons and rhizoids are poorly developed. Sporangiophores brownish, 8–15 µm wide, with apical branches each terminating with a sporangium, always with a septum below the sporangium. Sporangia are dark-coloured, globose (40–100 µm in diameter), each possessing an oval or pear-shaped columella (20–30 µm), without apophysis. Sporangiospores hyaline, smooth-walled, globose to subglobose, 3–4 µm in diameter (Kidd et al., 2016; de Hoog et al., 2020).
The analysis of pathogenous mucoromycetes includes strain isolation, collection and study using light and electron microscopy as well as methods of molecular biology. In the North-Western State Medical University named after I.I. Mechnikov, the Russian Collection of Pathogenic Fungi (RCPF) is kept. The studies of the collection strains isolated from patients with mucormycosis were carried out in several directions, including morphological studies by histological and electron microscopic methods and the studies of molecular diversity of medically relevant fungi. To address the issues, connected with fungal virulence, a number of animal models were developed. The series of resulting publications includes publications on several strains being stored in the RCPF, by Stepanova et al. (2012), Mikhaylova and Polishchuk (2012), Vasilyeva et al. (2013, 2019a, 2019b), Pchelin et al. (2020).
Here we provide the data on ultrastructure of hyphae and sporangiospores of Rhizomucor pusillus (Lindt) Schipper strain RCPF 1845 fixed for TEM by traditional chemical (CF) and cryofixation with high pressure freeze (HPF) procedure for a more comprehensive comparative analysis.
MATERIALS AND METHODS
Fungal strain. The fungal strain Rh. pusillus RCPF 1845 was isolated in November 2017 from a sample of bronchoalveolar lavage in Saint Petersburg, Russia. To obtain material for research, the culture was inoculated on solid nutrient media, Sabouraud agar or wort agar (Research Center of Pharmacotherapy, Russia) and incubated at 37°C for 3 days to get both the young mycelium and the sporangia.
Species identification was performed by phenotypic analysis with the use of Atlas of Pathogenic Fungi (de Hoog et al., 2020) and confirmed by DNA sequencing of partial ribosomal large subunit RNA gene (LSU) and partial translation elongation factor 1 alpha (TEF1α) gene. Amplification of LSU locus was carried out with the primers NL1 (5'-GCATATCAATAAGCGGAGGAAAAG-3') and NL4 (5'-GGTCCGTGTTTCAAGACGG-3') (O’Donnell, 1993). A thermocycler was programmed for initial 5 min denaturation at 95°C, followed by 34 cycles of denaturation for 30 s at 95°C, annealing 30 s at 56°C and extension for 1 min at 72°C. The final elongation step lasted for 10 min at 72°C. The locus TEF1α was amplified with the primers Al33_F1 (5'-GAYTTCATCAAGAACATGAT-3') and Al33_R2 (5'-GACGTTGAADCCRACRTTGTC-3') (Robert et al., 2011). The amplification program included initial denaturation step at 95°C for 5 min, 40 cycles of denaturation at 94°C for 30 s, annealing at 48°C for 45 s, and elongation at 72°C for 60 s and a final elongation step at 72°C for 7 min. The resulting PCR products were sequenced as described earlier (Pchelin et al., 2020). The obtained nucleotide sequence of D1 and D2 domains of the locus LSU was deposited in NCBI Nucleotide database under the accession MZ401142 and the sequence of TEF1α locus was deposited under the accession MZ420270.
Phylogenetic analysis. The alignment of LSU locus sequences contained sequences of species, found to cause human infections in St. Petersburg (unpublished data) as well as closely related species. We also included two LSU sequences of local strains from an earlier study (Mikhailova, Polischouk, 2012). An unrooted Maximum Likelihood phylogenetic tree was calculated with the use of PhyML 3.1 software (Guindon et al., 2010) as implemented in SeaView 5.0.4 package (Gouy et al., 2010). The substitution model was GTR + Γ with 12 across site variation rates. The stability of branches was assessed by performing 100 bootstrap resamples.
Electron microscopy. Fungal material was prepared for electron microscopy using chemical fixation or high pressure freezing and freeze substitution according commonly used protocols (Kuo, 2014). Chemical fixation (CF) was performed in a mixture of 3% glutaraldehyde (EMS, USA) and 4% paraformaldehyde (final concentrations) in 0.1М cacodylate buffer pH 7.2–7.4 for 4 hours at room temperature. After rinsing in 0.2M cacodylate buffer twice for 20 min, the material was post fixed in 1% OsO4, 0.1 М cacodylate buffer at 4°С overnight. After dehydration in alcohol series and propylene-oxide the samples were embedded in LV Resin (Agar).
For cryofixation, the pieces of mycelium were high pressure frozen (HPF) using HPM100 (Leica Microsystems) in aluminum carriers 100 µm deep and 6 mm in diameter, filled with 1-hexadecene (Sigma Aldrich). After freezing, the samples were warmed in substitution medium containing dry acetone, 1% OsO4 (EMS, USA), 0.2% uranyl acetate (SPI, USA) and 2% methanol from –100 to 0°С during 42 hours in freeze substitution (FS) machine AFS2 (Leica Microsystems). The samples were washed three times with cold acetone at 0°C for 1h and warmed to room temperature. Infiltration and embedding in Epon EmBed 812 resin (EMS, USA) was subsequently performed at room temperature. Sections were observed using transmission electron microscope Jeol JEM 1400 equipped with side camera Veleta (Olympus).
RESULTS AND DISCUSSION
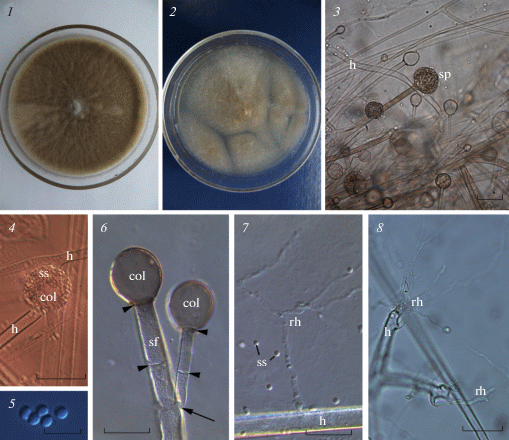
Species identification. Seven-days old fungus colony on Sabouraud agar developed pronounced aerial mycelium, was felt-like and colored greyish brown (Fig. 1, 1). The reverse side of the colony was smooth and colorless. On wort agar medium, the colony was white-gray-brown, felt-like, with smooth and colorless reverse side (Fig. 1, 2). The hyphae were 6–12 µm thick and pauciseptate, colorless. Small and rare rhizoids emerged from both the lateral side and the tip of hyphae (Fig. 1, 7, 8). The sporangiophores were smooth, randomly branching, brown, 5–10 µm thick, extending from aerial hyphae or stolons. Each sporangiophore branch bore a multispored terminal brown sporangium (Fig. 1, 3, 4, 6). The mature sporangia were spherical and measured 30–50 µm in diam., separated from sporangiophore with septa (Fig. 1, 3, 4, 6). The columellae were round or pyriform, 13–25 µm in diam., without apophysis (Fig. 1, 4, 6). The sporangiospores were smooth-walled, rounded, greenish-brown in color, 2.5–4 µm in diam. (Fig. 1, 5). This set of traits indicated that the isolated RCPF 1845 belonged to the species Rh. pusillus (de Hoog et al., 2020).
Fig. 1.
General view of the Rhizomucor pusillus strain RCPF 1845 on the Sabouraud agar (1), wort-agar (2), sporangiophore/mycelium structure (3), sporangium (4), sporangiospores (5), branching (arrow) septate (arrowheads) sporangiophore with columellae (6), lateral (7) and terminal (8) rhizoids. 3, 8 – bright field, 4–7 – DIC. Scale bars: 3, 8 – 30 µm, 4 – 50 µm, 5 – 10 µm, 6, 7 – 20 µm.
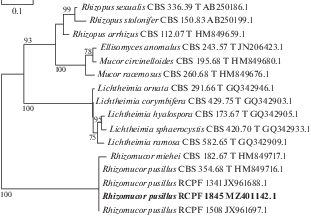
To confirm this morpho-biological identification, we sequenced the LSU locus of the strain RCPF 1845 and built a phylogenetic tree of mucoromycetes (Fig. 2). The LSU sequence of this strain was identical to the sequences of two previously characterized local strains of Rh. pusillus and with the sequence of the type strain CBS 354.68 (Fig. 2). The results of phylogenetic analysis demonstrated that the genotype of studied strain does not differ from other local and type Rh. pusillus strains. Therefore, our results are expected to be applicable to the Rh. pusillus population as a whole.
Fig. 2.
ML phylogenetic tree based on partial 28S rRNA gene (domains D1 and D2) analysis of locally encountered clinical mucoralean species. Studied strain is given in bold. T – type strain. Branch support values ≥75% are shown.
Ultrastructure of hyphae. We studied sections of young growing hyphae without aging and degrading areas. The hyphae contained nuclei, numerous mitochondria with lamellar cristae, rare ER cisterns, and vesicles of various diameters. The same cell components were found in hyphae fixed both chemically and with HPF.
The cell wall was electron-dense and appeared rather homogeneous with either method of fixation (Fig. 3, 1–11). Hyphal wall was apparently formed by one layer, in which it is difficult to establish heterogeneity with both methods of fixation. The septae were rarely found in growing hyphae. In one case, the formation of a septum was noted to isolate the area containing reserve nutrients: lipid globules of different sizes are concentrated in one of the compartments (Fig. 3, 3). On the section, it is noticeable (indicated by arrows) that the septum wall was formed by the thickening of the inner layer of the cell wall of each cytoplasmic portion.
Fig. 3.
Ultrastructure of hyphae of the Rhizomucor pusillus strain RCPF 1845: 1 – cross section of a thick hypha containing several nuclei (n), mitochondria (m), and vesicles (v); 2 – longitudinal section of young hypha with two nuclei, dark vesicles (dv) at the anterior end (to the right). Insert: microtubular profile at the cell periphery (arrow); 3 – segregation of cytoplasm containing lipid globules. Note the septa formation from both sides of cytoplasm (arrows); 4 – cell wall and mitochondrion structure at higher magnification; 5 – mitochondrion with plate-like cristae (cr) cut at the different planes; 6 – multivesicular body and a vesicle at the cell periphery; 7 – nucleus with euchromatine and eccentric nucleolus (nu), and mitochondria; 8 – myelin-like structure (my) at the cell periphery; 9–10 – two putative stages of exocytosis preceding intrawall vesicle formation shown on fragment 11; arrowheads on fragment 9 show the membrane cistern around portion of cytoplasm under the hyphal wall; arrows on fragment 10 show small hypha wall protrusions around a portion of cytoplasm in the periplasmic space; 11 – intrawall vesicle (arrow); 2, 11 – HPF, others – CF. Scale bars: 1–3 – 1 µm, 4, 7, 8 – 500 nm, 5–6, 9–11 – 200 nm. Abbreviations: av – autophagous vacuole, col – columella, cr – cristae in mitochondrion, cw – columellar wall, dm – degraded mitochondrion, dv – dark vesicles, er – endoplasmic reticulum, h – hypha, hw – hyphal wall, il – inner layer of sporangiospore wall, is – inter sporangiospore space, l – lipid globule, n – nucleus, nu – nucleolus, m – mitochondrion, mv – multivesicular body, my – myelin-like structure, ol – outer layer of sporangiospore wall, pm – plasma membrane, rh – rhizoids, sf – sporangiophore, sp – sporangium, ss – sporangiospore, sw – sporangial wall, v – vesicle, va – vacuole.
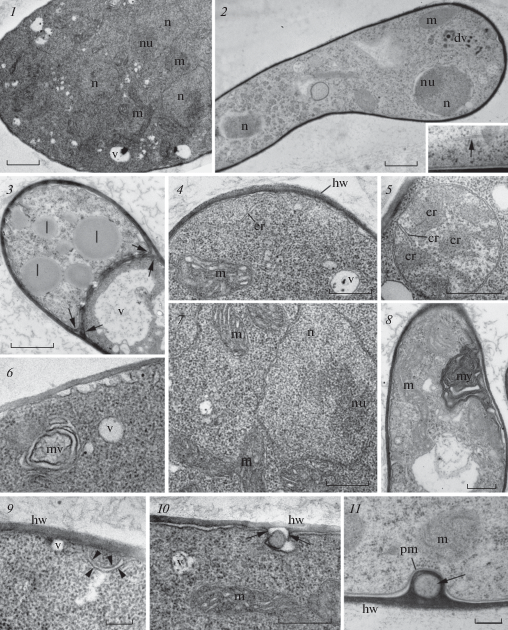
The main part of the cytoplasm was homogeneous and contained a large number of ribosomes (Fig. 3, 1, 2, 4, 5, 7). The cytoplasm after HPF fixation was not as dense and homogeneous as after CF, and the cytoplasmic microtubules (Fig. 3, 2, insertion) were better visible than after CF.
Mitochondria profiles were usually 300–500 nm long, although branching larger ones measured up to 1 µm were also found (Fig. 3, 2, 4, 5–7, 9, 10). After the traditional method of fixation (CF), the mitochondrial matrix was darker, and the cristae were slightly swollen in general, although they retained their lamellar shape. At the same time, CF method better preserved a space between inner and outer membranes and the shape of the cristae, which were clearly visible on longitudinal and transverse sections (Fig. 3, 5). The HPF fixation gave unstable results for mitochondrial cristae and matrix (Fig. 3, 2, 11; 5, 4).
The nuclei in growing hyphae were usually distanced from each other (Fig. 3, 2). Only in the thickening of the hyphae we could see several closely located nuclei surrounded by mitochondria (Fig. 3, 1). Heterochromatin was not detected in the mycelium nuclei, a large eccentric nucleolus was clearly visible (Fig. 3, 1, 2, 7).
Vacuoles of various sizes and shapes were abundant in old hyphae. In young hyphae they were less frequent and usually small (Fig. 3, 2, 4–7). The microbodies covered with single membrane were not found in the hyphae, but they present in immature sporangiospores (see below). Being the thermophilic fungus, Rhizomucor pusillus might have the microbodies containing electron dense crystalline inclusions, like ones found in Thermomyces lanuginosus and Th. stellatus (Ellis, 1981), but it has not. In Thermomyces species the microbodies with crystalline inclusions appeared in hyphae at 52°C, but were absent at the same species grown at 40°C. Probably, the temperature optimum at 37°C for Rhizomucor pusillus hyphal growth is too low for crystalline formation in microbodies. At the same time, Rh. pusillus was able to produce special reservoirs for lipid storage separated with septa (Fig. 3, 3). This kind of lipid storage structures are characteristic of thermophilic mucoromycetes.
On the periphery of some hyphae, the so-called myelinous and multivesicular bodies, which are tangles of membranes, were found (Fig. 3, 6, 8). Both types of the bodies are commonly found in fungal cytoplasm, but their function is still unknown (Kamzolkina et al., 2014).
Vesicular transport. Our study revealed many cases of single vesicles with either translucent content or containing some particles, and multivesiculated bodies as well. They were found near the plasma membrane of hyphae (Fig. 3, 1, 4, 6, 9, 10). Many authors have shown, that typical eukaryotic vesicular transport takes place in the fungal hyphae and serves for exocytosis of macromolecules of proteins, lipids, polysaccharides and pigments (summarized by Rodrigues et al., 2011 and Rodrigues, Casadevall, 2018). However, it remains unknown how the relatively large vesicles cross the cell wall that has too small pores (~5.8 nm) in the cell wall to reach the extracellular space. Nevertheless, a possibility of such transfer has been recently shown using cryofixation for TEM for Candida albicans and Cryptococcus neoformans with liposomes containing amphotericin B (Walker et al., 2018). The authors demonstrated that the liposomes with diameter of 60 to 80 nm remained intact during transit through the fungal cell wall to deliver amphotericin B from outside to the plasma membrane (Walker et al., 2018).
Alternative way of big cytoplasmic portion transfer through the hyphal wall can be proposed on the base of our current data on the exocytosis of Rhizomucor pusillus. An unusual invagination of the plasma membrane (Fig. 3, 9 arrowheads) isolating cytoplasmic portion at the cell periphery may represent the first stage of exocytosis. At the next stage a drop of cytoplasm extruded from the vesicle into periplasmic space (Fig. 3, 10) and small protrusions of the hyphal wall appear around it (Fig. 3, 10 arrows). We suggest, that the hyphal wall overgrowth this drop of cytoplasm, which was then totally enclosed by hyphal wall as seen at Fig. 3, 11. While the main cell content become isolated by the hyphal wall, the exocytosis may occur by degradation of the outer portion of hyphal wall. Perhaps, these three images might represent consecutive stages of vesicular transport through the hyphal wall, but such interpretation of ultrastructural images needs confirmation with cytochemical methods.
Ultrastructure of sporangium and sporangiospores. The longitudinal sections of chemically fixed immature sporangia (Fig. 4, 1–3) clearly showed the sporangiospores on the surface of the columellae. The sporangia were covered with a sporangial wall, formed by external thickening of the sporangiophore wall (Fig. 4, 3). Sporangial wall was homogeneous and smooth, obviously composed of one layer and detached from the immature sporangium contents. Three layered structure like in Ellisomyces anomalus (Beakes, Campos-Takaki, 1984) was not revealed by TEM in chemically fixed specimens.
Fig. 4.
Sporangiophore structure of the Rhizomucor pusillus strain RCPF 1845: 1 – longitudinal section through the sporangium on the sporangiophore; 2, 3 – portion of sporangium (2) and sporangiophore (3) at higher magnification, showing the columellar and sporangial wall formation; 4 – two-layered wall (sw) structure of five neighbor intrasporangial sporangiospores probably at different stages of development. Scale bars: 1 – 5 µm, 2, 3 – 2 µm, 4 – 200 nm. CF for all images. For abbreviations, see legend to fig. 3.
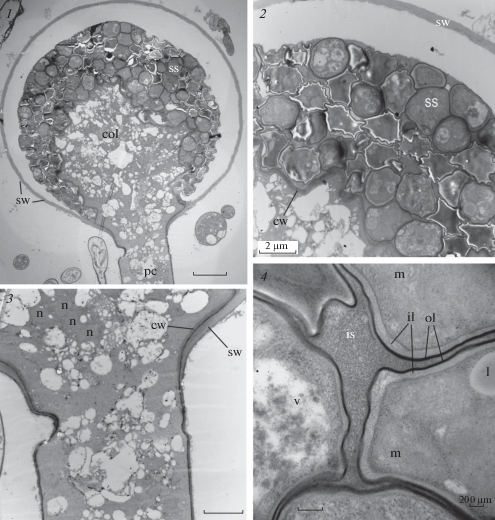
The internal thickening of the stem wall produces the wall of columella. At the base of the sporangium, both the future walls are separated by a narrow electron-dense layer, which later becomes the outer layer of the columella wall (Fig. 4, 2–3). The content of the columella is represented by highly vacuolated cytoplasm, containing numerous nuclei (Fig. 4, 3). Later during sporangial maturation these nuclei with surrounding cytoplasm segregate from columella and become the nuclei of sporangiospores. Sporangiospores at different stages of maturation lay outside the columella membrane in the extracellular matrix. They were covered with a two-layer wall similar in structure to the wall of columella: a thick moderately dense layer is covered with an electron-dense thin layer (Fig. 4, 4). Apparently, sporangiospores inherit this wall as a result of segregation along the sporogenesis. Intrasporangial spores contain nucleus with an eccentric nucleolus, relatively large mitochondria, lipid globules, and vacuoles with indefinite contents (Fig. 5, 1, 2). Released sporangiospores are globular with a smooth surface and thick (up to 170 nm) walls. The wall is composed of two layers: a thick loose layer of moderate density adjacent to the plasma membrane, and external thin electron-dense layer (Fig. 5, 4). Two-layered sporangiospore wall has been reported for the majority of investigated Mucoromycetes (Jeffries, Young, 1984).
Fig. 5.
Structure of Rhizomucor pusillus strain RCPF 1845 intrasporangial (1, 2) and released (3, 4) sporangiospores; 1, 2 – CF; 3, 4 – HPF. For abbreviations, see legend to fig. 3. Scale bar – 500 nm for all figures.
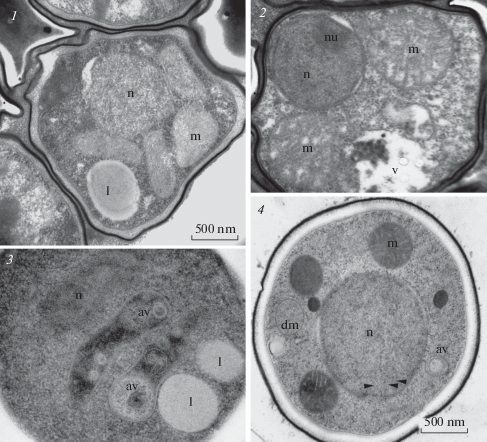
The sporangiospores of Rhizomucor pusillus contained one nucleus in the center of the sporangiospore with several small mitochondria around it. No Golgi apparatus was found, ER cisternae were rare in the cytoplasm (Fig. 5, 3, 4). In the nucleoplasm, unordered microtubules were clearly visible (Fig. 5, 4 arrowheads), which might be at the stage of disassembly after completed mitosis. Other mucoromycetes, such as Ellisomyces anomalus, also have only one nucleus with a central nucleolus and euchromatin (Beakes, Campos-Takaki, 1984; Tansey et al., 1984). The larger sporangiospores of Rhizopus arrhizus, Rh. stolonifer and Rh. sexualis contained several nuclei and they also do not contain heterochromatin (Hawker, Abbott, 1963; Necas et al., 1963; Buckley et al., 1968; Hess, Weber, 1973). The nucleolus was also present, which indicates active processes in the sporangiospore cell.
In addition to these cellular structures in sections of cryofixed released sporangiospores we found two membrane limited vesicles with homogenous content (Fig. 5, 4), which correspond to inactive mitochondria with degraded cristae. Besides this, one or two unusual elongated vacuoles with heterogeneous contents (Fig. 5, 3, 4) have been found. They are delimited with single membrane and contain vesicles of different dimension and shape, sometimes the membranes, and rather dense matrix. Probably, these vesicles represented autophagic vacuoles utilizing the degraded mitochondria, what is common for the fungal cells (Liu et al., 2016).
This ultrastructural study of mycelium, sporangium and sporangiospores of the Rhizomucor pusillus strain RCPF 1845 revealed the same main structures as in other mucoromycetes. Using two different methods of fixation we have shown some rarely described phenomena like microtubules in sporangiospore nuclei, autophagic vacuoles, or the intrawall cytoplasmic inclusions, which can be interpreted as a stage of exocytosis. A deeper analysis of ultrastructure is needed for different fungi, besides the model objects.
Support for this work was provided by Russian Ministry of Health grant N 056-00056-1900 (morphological and molecular study, data analysis), and Russian Science Foundation, grant N 21-74-20089 (comparison of CF and HPF electron microscopic methods, manuscript writing). High pressure freezing, freeze substitution and TEM were performed in Research Resource Center “The development for molecular and cell technologies”, St. Petersburg State University. We thank the anonymous reviewer for his valuable comments on the manuscript.
Список литературы
Beakes G.W., Campos-Takaki G.M. Sporangiole ultrastructure in Ellisomyces anomalus (Mucorales, Thamnidiaceae). Trans. Br. Mycol. Soc. 1984. V. 83. P. 607–613.
Buckley P.M., Sommer N.F., Matsumoto T.T. Ultrastructural details in germinating sporangiospores of Rhizopus stolonifer and Rhizopus arrhizus. J. Bacteriol. 1968. V. 95. P. 2365–2373.
de Hoog G.S., Guarro J., Gené J. et al. Atlas of Clinical Fungi, 4th edition. Hilversum, 2020.
Ellis D.H. Ultrastructure of thermophilic fungi IV. Conidial ontogeny in Thermomyces. Trans. Br. mycol. Soc. 1981. V. 77. P. 229–241.
Gouy M., Guindon S., Gascuel O. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 2010. V. 27. P. 221–224.
Guindon S., Dufayard J.F., Lefort V. et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 2010. V. 59. P. 307–321.
Hawker L.E., Abbott P.McV. An electron microscope study of maturation and germination of sporangiospores of two species of Rhizopus. J. Gen. Microbiol. 1963. V. 32. P. 295–298.
Hess W.M., Weber D.J. Ultrastructure of dormant and germinated sporangiospores of Rhizopus arrhizus. Protoplasma. 1973. V. 77. P. 15–33.
Jeffries P., Young T.W.K. Sporangiospore structure and germination in Dimargaritales. Trans. Br. Mycol. Soc. 1984. V. 83. P. 223–232.
Jeong W., Keighley C., Wolfe R. et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin. Microbiol. Infect. 2019. V. 25. P. 26–34. https://doi.org/10.1016/j.cmi.2018.07.011
Kamzolkina O.V., Mageika I.S., Shtaer O.V. et al. Endomembrane system of fungi: traditional and modern conceptions. Tsitologia. 2014. V. 56. P. 549–561 (in Russ.).
Khostelidi S.N., Borzova Y.V., Volkova A.G. et al. Mucormycosis in children: results of prospective study in Saint Petersburg, Russia. Problemi medicinskoy mikologii. 2019. V. 21. № 1. P. 7–10 (in Russ.).
Khostelidi S.N., Shadrivova O.V., Borzova Y.V. et al. Clinical and laboratory features of mucormycosis in adults. Problemi medicinskoy mikologii. 2020. V. 22. № 2. P. 22–28 (in Russ.)
Kidd S., Halliday C.L., Alexiou H. et al. Descriptions of medical fungi. David Ellis, Adelaide, 2016.
Klimko N., Khostelidi S., Shadrivova O. et al. Contrasts between mucormycosis and aspergillosis in oncohematological patients. Med. Mycol. 2019. V. 1. Suppl. P. S138–S144. https://doi.org/10.1093/mmy/myy116
Kuo J. Electron microscopy methods and protocols. 3rd edition. Humana Press, N.Y., 2014. https://doi.org/10.1007/978-1-62703-776-1
Liu X.H., Xu F., Snyder J.H. et al. Autophagy in plant pathogenic fungi. Semin. Cell Dev. Biol. 2016. V. 57. P. 128–137.
Maurer E., Hoertnagl C., Lackner M. et al. Galleria mellonella as a model system to study virulence potential of mucormycetes and evaluation of antifungal treatment. Med. Mycol. 2019. V. 57. P. 351–362.
Mikhaylova Y.V., Polishchuk A.G. Molecular identification of zygomycetes from Russian collection of pathogenic fungi based on fungal ribosomal DNA sequence data. Probl. Med. Mikol. 2012. V. 14. P. 59–63 (in Russ.).
Necas O., Havelkova M., Soudek D. Submicroscopic morphology of Rhizopus nigricans. Folia Microbiol. (Prague). 1963. V. 8. P. 290–292.
O’Donnell K. Fusarium and its near relatives. In: Reynolds DR, Taylor JW (eds). The Fungal Holomorph: Mitotic, Meiotic and Pleomorphic Speciation in Fungal Systematics. Wallingford, UK: CAB International, 1993, pp. 225–233.
Pchelin I.M., Mochalov Y.V., Azarov D.V. et al. Genotyping of Russian isolates of fungal pathogen Trichophyton rubrum, based on simple sequence repeat and single nucleotide polymorphism. Mycoses. 2020. V. 63. P. 1244–1254. https://doi.org/10.1111/myc.13162
Robert V., Szöke S., Eberhardt U. et al. The quest for a general and reliable fungal DNA barcode. The Open and Applied Informatics Journal. 2011. V. 5. P. 45–61.
Rodrigues M.L., Casadevall A. A two-way road: novel roles for fungal extracellular vesicles. Molecular Microbiology. 2018. V. 110. P. 11–15. https://doi.org/10.1111/mmi.14095
Rodrigues M.L., Nosanchuk J.D., Schrank A. et al. Vesicular transport systems in Fungi. Future Microbiol. 2011. V. 6. P. 1371–1381.
Singh A.K., Singh R., Joshi S.R. et al. Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India. Diabetes Metab. Syndr. 2021. V. 21. https://doi.org/10.1016/j.dsx.2021.05.019
Stepanova A.A., Khostelidi S.N., Araviyskiy R.A. et al. Electron-microscopic investigations of Lichtheimia corymbifera in vivo and in vitro. Probl. Med. Mikol. 2012. V. 14 (4). P. 55–61 (in Russ.).
Tansey M.R., Kamel S.N., Shamsai R. The number of nuclei in sporangiospores of Rhizomucor species: taxonomic and biological significance. Mycologia. 1984. V. 76. P. 1089–1094.
Vasilyeva N.V., Stepanova A.A., Bogomolova T.S. et al. Cytological investigation of Lichtheimia corymbifera in vitro. Probl. Med. Mikol. 2019a. V. 21 (3). P. 3–8 (in Russ.).
Vasilyeva N.V., Stepanova A.A., Bogomolova T.S. et al. Cytological study of the causative agent of mucormycosis Lichtheimia corymbifera in vivo. Probl. Med. Mikol. 2019b. V. 21 (4). P. 3–7 (in Russ.).
Walker L., Sood P., Lenardon M.D. et al. The viscoelastic properties of the fungal cell wall allow traffic of AmBisome as intact liposome vesicles. MBio. 2018. V. 9. e02383-17. https://doi.org/ .02383-17.https://doi.org/10.1128/mBio
Васильева Н.В., Степанова А.А., Богомолова Т.С. и др. (Vasilyeva et al.) Цитологическое изучение Lichtheimia corymbifera in vitro // Проблемы медицинской микологии. 2019a. Т. 21. № 3. С. 3–8.
Васильева Н.В., Степанова А.А., Богомолова Т.С. и др. (Vasilyeva et al.) Цитологическое изучение Lichtheimia corymbifera in vivo // Проблемы медицинской микологии. 2019b. Т. 21. № 4. С. 3–7.
Камзолкина О.В., Мажейка И.С., Штаер О.В. и др. (Kamzolkina et al.) Эндомембранная система у грибов: классическое и современное представления // Цитология. 2014. Т. 56. № 8. С. 549–561.
Михайлова Ю.В., Полищук А.Г. (Mikhaylova, Polishchuk) Молекулярная идентификация представителей Zygomycetes из Российской коллекции патогенных грибов по нуклеотидным последовательностям рДНК // Проблемы медицинской микологии. 2012. Т. 14. № 3. С. 59–63.
Степанова А.А., Хостелиди С.Н., Аравийский Р.А. и др. (Stepanova et al.) Электронно-микроскопическое исследование Lichtheimia spp. in vivo и in vitro // Проблемы медицинской микологии. 2012. Т. 14. С. 55–61.
Хостелиди С.Н., Борзова Ю.В., Волкова А.Г. и др. (Khostelidi et al.) Мукормикоз у детей: результаты проспективного исследования в Санкт-Петербурге // Проблемы медицинской микологии. 2019. Т. 21. № 1. С. 7–10.
Хостелиди С.Н., Шадривова О.В., Борзова Ю.В. и др. (Khostelidi et al.) Клинико-лабораторные особенности мукормикоза у взрослых // Проблемы медицинской микологии. 2020. Т. 22. № 2. С. 22–28.
Дополнительные материалы отсутствуют.
Инструменты
Микология и фитопатология


