Микология и фитопатология, 2022, T. 56, № 1, стр. 16-21
The First Record of Suillus Glandulosus (Suillaceae) in Russia from Magadan Region
E. A. Zvyagina 1, *, N. A. Sazanova 2, **
1 Lomonosov Moscow State University
119991 Moscow, Russia
2 Institute of Biological Problems of the North of the Far East Branch of the Russian Academy of Sciences
685000 Magadan, Russia
* E-mail: mycena@yandex.ru
** E-mail: nsazanova_mag@mail.ru
Поступила в редакцию 21.03.2021
После доработки 15.05.2021
Принята к публикации 22.05.2021
- EDN: FRSSNS
- DOI: 10.31857/S0026364822010147
Аннотация
A North American species Suillus glandulosus was found for the first time for Russia. Specimens were collected in August 1990, 2013, and 2018 in Larix cajanderi-dominated communities in the continental and coastal areas of Magadan Region. A high similarity of ITS sequences and morphological characters of Asian and American samples was shown. A morphological description, photographs of basidiomata, spores and cystidia are given. Considerable disjunction of the northern part of the species range and its association with possible Beringian refugia of vegetation during the Quaternary glaciation are discussed. Specimens of Suillus glandulosus were found in clusters with Gomphidius borealis. An occurrence of the three-way assotiation of Suillus glandulosus, Gomphidius borealis and Larix cajanderi is recorded for the first time in Asia.
INTRODUCTION
We collected the specimens with red-brown slimy and viscid basidiomata several times since 1990 in different locations in Magadan Region. The correspondence to the description in the work of Pomerleau and Smith (1962) and similarity to the specimens from the territory of Canada [MQ17065-QFB29573 and ANT123–QFB28750 (BOLD Systems)] suggested that we are dealing with Suillus glandulosus (Peck) Singer.
According to Pomerleau and Smith (1962), Smith and Thiers (1971), as well as datasets of the iBOL (International.., 2016), EMBL-EBI (European.., 2019), CMMF (Archambault, 2019), F (Grant, Konrat, 2019), DUKE (Duke University.., 2020) and WTU (University.., 2021) mycological collections, the species range covers mostly Northeastern America. Some specimens (UWBM 109120, EMBL-EBI KX213742) were collected in Alaska (GBIF, 2021). Only a few finds of this species are known in Asia (Ding, Wen, 2003; Nagasawa, Sato, 2016). Phylogenetic analysis shows a high similarity of the specimens collected in Magadan Region with American ones. In this report we present data on the first records of S. glandulosus in Russia, give a description of the collected specimens, discuss the distribution of S. glandulosus and its mycorrhizal association.
MATERIAL AND METHODS
Specimens were photographed in situ, examined for taste and smell, dried at temperatures below 50°C. Specimens are stored in the herbarium of the Institute of Biological Problems of the North, Far East Branch, Russian Academy of Sciences (MAG). A part of the sequenced specimen of Suillus glandulosus (MAG 5110, GenBank MW672504) and the specimen of Gomphidius borealis from the cluster (MAG 5072) were inserted into the herbarium of the Komarov Botanical Institute under the numbers LE 312670 and LE 312671 correspondingly. Macroscopic descriptions were based on the study of both fresh and dried materials as well as on photographs. Microstructures were observed at ×400 and at ×1000 in squash preparations in 5% KOH, Congo Red, and Melzer’s reagent. Up to 30 basidiospores, 10 cystidia, and 10 terminal elements of pileipellis per specimen were measured to obtain descriptive statistics. Measurements were made in ToupView V.3.7 (ToupTek Photonics) calibrated by OMP object-micrometre (LOMO). Dimensions are given as (abs min) average min – average max (abs max), Q = average min – average max quotient (length/width ratio).
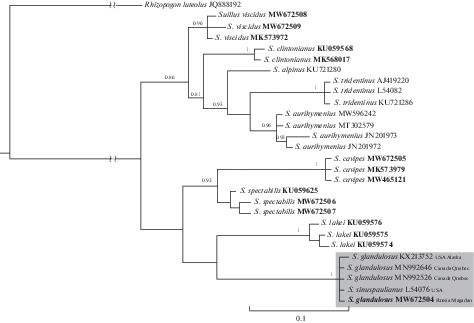
PCR ITS products were obtained without DNA extraction using the standard protocol of Thermo Scientific Phire Tissue Direct PCR Master Mix kit and amplification with ITS1-F and ITS4-B primers (Gardes, Bruns, 1993). Amplified products were sequenced using BigDyeH Terminator 3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, California). The sequences were assembled in CodonCode Aligner V.9.0.1 (CodonCode Corporation) and manually interpreted to correct the ambiguous bases. 28 ITS sequences, including 14 newly generated and 14 retrieved from GenBank database, were used in the phylogenetic analysis focused on Suillus glandulosus and closely related species. Rhizopogon luteolus was chosen as an outgroup. Genbank accession numbers of sequences are indicated in the phylogenetic tree (Fig. 1) after the species names and in the section “Specimens examined”. Sequences were first aligned in MAFFT (Katoh et al., 2019), then the alignment was manually optimized. Ambiguous alignment areas were removed manually. The alignment containing a total of 455 bases with gaps was used for phylogenetic analysis. ITS rDNA phylogenetic tree (Fig. 1) was obtained using MrBayes v. 3.2.1 (Ronquist et al., 2012) under GTR + I + G model with 3 M generations and a sampling frequency of every 100th generation. Posterior probability values exceeding 0.80 are indicated on the branches. The sequence alignment and phylogenetic tree of this project are available under TreeBase study TB2:S27843. Phylogenetic differences were measured using the Hamming dissimilarity in UGENE v.37 (Okonechnikov et al., 2012) on ITS sequence alignment (478 nucleotides including alignment gaps) of morphologicaly similar species: Suillus glandulosus, S. spectabilis and S. tridentinus.
Fig. 1.
ITS rDNA phylogenetic tree obtained with MrBayes v. 3.2.1 under GTR + I + G model for 3 M generations. The GenBank accession numbers are indicated after species names. Newly generated sequences are marked in bold. Posterior probability values greater than 0.8 are indicated on the branches. Scale bar = 0.1 indicates expected substitution per site. The tree is rooted with Rhizopogon luteolus.
RESULTS
According to the tree in Fig. 1, ITS sequence MW672504 of the specimen MAG5110 belongs to the well supported S. glandulosus clade. The Hamming distance between the Magadan and North American samples is less than 1% or 1–2 nucleotide substitutions per 478 alignment positions. Basidiomata in the photos of the sequenced North American S. glandulosus specimens (MQ17065-QFB29573 and ANT123–QFB28750) and those of S. glandulosus specimens from Magadan Region have similar macromorphological features. Hamming distance to the most morphologically similar species S. spectabilis and S. tridentinus is 6–7 and 9–10% (31–33 and 43–47 nucleotide substitutions). Below we present a description of the S. glandulosus specimens collected in Magadan Region.
Suillus glandulosus (Peck) Singer, Lilloa 22: 657, 1951 (“1949”).
Basionym: Boletinus glandulosus Peck, Bull. New York State Mus. 131: 34, 1909.
Synonymy: Fuscoboletinus glandulosus (Peck) Pomerleau et Smith, Brittonia 14: 162, 1962.
Iconography: Pomerleau and Smith [1962: 162, pls. 3, 4 (upper figure) as F. glandulosus]; Snell and Dick (1970: 26, pl. 75, Fig. 5).
Mature basidiomata boletoid. Pileus 4–8 (10) cm diam, convex, flattening with age, margin floccose-appendiculate with thin glutinous flakes. Surface copiously viscid when wet, glabrous, brick-red, slightly cracking in dry weather with translucent spots of yellow flesh. Pileus cuticle thin, easy to remove. Pores angular, large, 1 mm diam or more, variable in size, yellow when young and yellow-brown to brown when mature, tubes concolour or a bit lighter. Hymenophore boletinoid, subdecurrent to decurrent. Context fleshy, golden-yellow. Stipe cylindrical slightly tapering towards the base, central, 1–1.5 cm broad, 3.5–6 (8) cm long, reticulate and golden-yellow above the ring, whitish and covered with thin brick-red scurf under the ring. Ring thin, double, brick-red mucous in upper part and whitish membranous in lower part. Context fleshy, yellow. Flesh of the cap and stipe after heat treatment becomes from lilac-pink to raspberry colour. Taste indistinct or slightly sour; odour pleasant, mushroom.
Basidiospores (7.7) 8.1–11.0 (11.5) × (3.1) 3.4–4.6 (5.0) μm, Q = 2.1–3.0, narrowly ellipsoid, slightly tapered at the distal end, inequilateral in profile, moderately thick-walled, honey-yellow or bright yellow-brown in KOH, smooth. Basidia (20.1) 21.6–29.3 (31.5) × (4.5) 5.3–7.4 (8.5) μm, clavate to subclavate, hyaline or with yellowish brown context in KOH, 4-spore. Cystidia (34.5) 38.1–72.3 (80.8) × (3.8) 4.2–7.3 (8.5) μm, cylindric, rounded in apex, with brown context in KOH or sometimes hyaline, arranged in fascicles. Pileipellis ixocutis, consisting of septate hyphae (37) 43.9–101 (119) × (3.8) 4.3–8.9 (10.5) μm, hialine.
Habitat and Distribution: the species was recorded in the continental (upper reaches of the Kolyma River) and coastal (within 50 km from the sea) parts of Magadan Region. It grows in mixed forests dominated by Larix cajanderi, preferring sparse areas with lingonberry-lichen cover and sandy-pebble soil. Often grows in groups with other species of Suillus (S. aurihymenius, S. cavipes, S. grevillei, S. spectabilis, etc.) and in clusters with Gomphidius borealis O.K. Mill., Aime et Peintner (Fig. 2, d). Fruiting in late summer. Edible mushroom, harvested by local people.
Fig. 2.
Suillus glandulosus basidiomata (a–c) coll. MAG 5110; fused fruiting bodies of the S. glandulosus and Gomphidius borealis coll. MAG 5072 (d), cystidia and spores in KOH (e, f). Scale bar = 10 µm.

Specimens examined: Magadan Region, Srednekanskiy District, Seymchan village vicinities, 62.8339° N, 152.4313° E, in mixed Larix cajanderi-dominated forest with Betula platyphylla, Salix spp., 28 08 2018, coll. N.A. Sazanova, det. E.A. Zvyagina (MAG 5110, dupl. LE 312670, ITS GenBank MW672504); ibid., Larix-domitated forest with Betula platyphylla, 28 08 2018, coll. et det. N.A. Sazanova (MAG 5114); ibid., 62.9821° N, 152.3193 E°, mixed forest with Larix cajanderi, Pinus pumila, Betula platyphylla, B. middendorffii, and Vaccinium vitis-idaea-lichen cover, 08 08 2013, coll. S.A. Yarysheva, det. N.A. Sazanova (MAG 4591); ibid., 62.9804° N, 152.3199E°, Larix-domitated forest, growing in clusters with Gomphidius borealis, 29 08 2018, N.A. Sazanova (MAG 5072, dupl. LE 312671); Olskiy District, vicinity of Chistoe Lake, 59.5768° N, 151.8264°E, scattered Larix cajanderi forest with Pinus pumila and Vaccinium vitis-idaea-lichen cover, on sandy-pebble soil, abundant, 14 08 1990, coll. et det. N. A. Sazanova (MAG 1326).
Notes: Brick-red to reddish-brown mucous pileus and stipe surfaces, yellow flesh, golden yellow decurrent hymenophore with big pores, membranous transparent partial veil, and mucous ring are characteristic features of Suillus glandulosus. In the field, basidiomata of S. glandulosus superficially resemble those of S. aurihymenius. The latter is distinguished by a dull yellow-orange flesh, orange tint of hymenophore, a fibrous ring, and the absence of mucus and bright brown plaque on a pileus surface. S. spectabilis, which also has a brownish-red mucous pileus and stipe, golden-yellow flesh and a mucous ring, is characterized by a scaly pileus surface. S. clintonianus is also similar in appearance to S. glandulosus, but has smaller pores (less than 1 mm) and a thick cherry-coloured cuticle that is difficult to remove.
DISCUSSION
Brown mucous pileus, decurrent hymenophore and mucous yellow-brown annulate stipe of the specimens from the Magadan Region correspond to original descriptions of Boletinus glandulosus (Peck, 1909). Pomerleau and Smith (1962) studied the type specimen and gave the following description of microstructures: spores 3.5–4.5 × 8.6–11.5 μm, cystidia are numerous fasciculate, with brown incrustation, and deep purple in Melzer’s reagent. The microstructures of the Magadan specimens fully correspond to the description and sizes of microstructures of the type and other specimens given by Pomerleau and Smith (1962).
Morphological and genetic similarity of Magadan and North American specimens identified as Suillus glandulosus confirms the correct identification. However, molecular analysis of type specimens is needed in order to prove the correct interpretation of concept of this species described by Peck. Due to the fact that holotype is older than 100 years, it would be important to select molecularly reactable epitype from the type locality.
S. glandulosus has a disjunctive range, which is typical for Beringian species. It is widespread mainly in the northeast of America, where it occurs in groups and is quite abundant. This species firstly was described from the territory of New Brunswick in Eastern Canada (Peck, 1909). Later, individual finds were made in Alaska (GBIF, 2021). It is difficult to determine the Asian border of the range. No more finds were recorded in Northern Asia, except for Magadan Region and Hokkaido (Nagasawa, Sato, 2016). Occurrences of the species in Southeast Asia are reported (Bi et al., 1993; Ding, Wen, 2003). However, we did not find ITS sequences and photos of finds or stored specimens from Southeast Asia in the available public databases. At present, the range of the species appears to be composed of isolated locations, the northern part of which corresponds to the refugia in the Western and Eastern Beringia, which existed in the Quaternary glaciations (Abbott, Brochmann, 2003; Anderson et al., 2010; Brubaker et al., 2005; Binney et al., 2009).
The question of the mycorrhizal host of this species remains controversial. In the original description of C.H. Peck (1909) there is no direct indication of the mycorrhizal symbiont. Later works reported that this species grows under conifers, mainly under Abies balsamea, Thuja occidentalis, Tsuga canadensis (Pomerleau, Smith 1962; Smith, Thiers, 1971). Smith and Tiers (1971) also indicated that it is not growing under larch as a distinguishing feature of this species. The study of the mycorrhizal roots of Picea did not confirm the presence of mycorrhiza with Suillus glandulosus (Nguyen at al., 2016). Nguyen et al. (2016) suggested that larch is the main host, while spruce and fir may be secondary symbionts. The known habitats of S. glandulosus on the territory of Magadan Region are located in 700 km to the east from the modern border of the Picea obovata distribution (from the Verkhoyansk ridge) and 350 km north and 100 km south-west of the relict spruce site of Magadan Region (“Yamskiy spruce island”, middle reaches of the Yama River). In the forests where we collected the specimens, only two species of conifers Larix cajanderi and Pinus pumila are growing (Fig. 3). Specimens of Suillus glandulosus were found in clusters with Gomphidius borealis (Fig. 2, d). According to Miller (2003) Chroogomphus and Gomphidius exhibit strong specificity for the plant hosts at the generic level. Due to the fact that Gomphidius mostly forms mycorrhiza with conifers subfamilies Piceoideae, Laricoideae, and Abietoideae, we assume that in this case there is a triple association between G. borealis, Suillus glandulosus, and Larix cajanderi. The molecular and morphological evidence of a three-way interaction has been previously shown for Suillus bovinus, Gomphidius roseus and Pinus sylvestris (Ollson et al., 2000). In North America Gomphidius borealis is associated with Suillus glandulosus and Abies balsamea, Picea glauca and Larix laricina (Aime, Voitk, 2014). Gomphidius borealis is not widely known in Asia, despite the fact that it was described from Yakutia (Miller et al., 2002). Our report on this species is the first for the Far East. G. borealis is very similar at first glance to G. glutinosus. The first is distinguished by a pinkish-beige pileus, a pinkish-beige tint between the gills, a fibrillose veil and a light orange-yellow base of the stem, blackening on handling. Due to the similarity of the two species, it will be useful to review Gomphidius specimens from other Asian reports (Nagasawa, Sato, 2016) to understand the spectrum of species associated with Suillus glandulosus.
Fig. 3.
Specimen’s locality in mixed (Larix cajanderi, Pinus pumila, Betula platyphylla, B. middendorffii) Vaccinium vitis-idaea-lichen forest, Seymchan Village vicinities, Magadan Region, Russia (coll. MAG 4591).

Elena A. Zvyagina’s work is supported by a grant from the Russian Foundation for Basic Research (RFBR) grant N 20-04-00349. The research of Nina A. Sazanova was carried out within the frame of government assignments for Institute of Biological Problems of the North FEB RAS (project АААА-А17-117122590002-0 “Inventory and classification of taxonomic and spatial diversity of plants and plant communities of the Far East North of Russia”). We thank Svetlana A. Yarysheva for the help in field work.
Список литературы
Abbott R.J., Brochmann C. History and evolution of the arctic flora: in the footsteps of Eric Hulten. Mol. Ecol. 2003. V. 12 (2). P. 299–313.
Aime M.C., Voitk A. Gomphidius in Newfoundland and Labrador with a redescription of Gomphidius borealis. Omphalina. 2014. V. 5 (3). P. 3–10.
Anderson P.M., Lozhkin A.V., Solomatkina T.B. et al. Paleoclimatic implications of glacial and postglacial refugia for Pinus pumila in western Beringia. Quat. Res. 2010. V. 73 (2). P. 269–276.
Archambault R. Cercle des mycologues de Montréal Funga-rium (CMMF). Version 11.6. Université de Montréal Biodiversity Centre. 2019. Occurrence dataset https://doi.org/ accessed via GBIF.org on 2021-02-18.https://doi.org/10.5886/jcq7t9e9
Bi Z., Zheng G., Taihui L. The macrofungus flora of China’s Guangdong Province. Chinese University Press, 1993.
Binney H.A., Willis K.J., Edwards M.E. et al. The distribution of late-Quaternary woody taxa in northern Eurasia: evidence from a new macrofossil database. Quaternary Science Reviews. 2009. V. 28 (23–24). P. 2445–2464.
Brubaker L.B., Anderson P.M., Edwards M.E. et al. Beringia as a glacial refugium for boreal trees and shrubs: new perspectives from mapped pollen data. J. Biogeography. 2005. V. 32 (5). P. 833–848.
Ding M.R., Wen H.A. Species of Suillus in China. Mycosystema. 2003. V. 22. P. 182–190.
Duke University, herbarium fungal collection. 2020. Occurrence dataset accessed via GBIF.org on 2021-02-18.https://doi.org/10.15468/vxptfo
European nucleotide nrchive (EMBL-EBI). Geographically tagged INSDC sequences. 2019. Occurrence dataset https://doi.org/10.15468/cndomv accessed via GBIF.org on 2021-02-18.10.15468/cndomv
Gardes M., Bruns T.D. ITS primers with enhanced specifity for Basidiomycetes: application to identification of mycorrhizae and rusts. Mol. Ecol. 1993. V. 2. P. 113–118.
GBIF Occurrence Download https://doi.org/10.15468/dl.245nte
Grant S., von Konrat M. Field museum of natural history (botany) fungi collection. Version 4.9. Field Museum. 2019. Occurrence dataset https://doi.org/10.15468 /x2hjnp accessed via GBIF.org on 2021-02-18.https://doi.org/10.15468/x2hjnp
Katoh K., Rozewicki J., Yamada K.D. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics. 2019. V. 20 (4). P. 1160–1166. https://doi.org/10.1093/bib/bbx108
Miller O.K. The Gomphidiaceae revisited: a worldwide perspective. Mycologia. 2003. V. 395 (1). P. 176–183. https://doi.org/10.1080/15572536.2004.11833147
Miller O.K., Aime M.C., Camacho F.G. et al. Two New Species of Gomphidius from the Western United States and Eastern Siberia. Mycologia. 2002. V. 94 (6). P. 1044–1050.
Nagasawa E., Sato S. Suillus glandulosus, a bolete species new to Japan. Rep. Tottori Mycol. Inst. 2016. V. 46. P. 39–42.
Nguyen N.H., Vellinga E.C., Bruns T.D. et al. Phylogenetic assessment of global Suillus ITS sequences supports morphologically defined species and reveals synonymous and undescribed taxa. Mycologia. 2016. V. 108(6). P. 1216–1228. https://doi.org/10.3852/16-106
Okonechnikov K., Golosova O., Fursov M. et the UGENE team. Unipro UGENE: a unified bioinformatics toolkit. Bioinformatics. 2012. V. 28. P. 1166–1167. https://doi.org/10.1093/bioinformatics/bts091
Olsson P.A., Münzenberger B., Mahmood Sh. et al. Molecular and anatomical evidence for a three-way association between Pinus sylvestris and the ectomycorrhizal fungi Suillus bovinus and Gomphidius roseus. Mycol. Res. 2000. V. 104 (11). P. 1372–1378.
Peck C.H. Report of the State Botanist. 1908. Bull. N.Y. State Museum. 1909. V. 131. P. 1–202.
Pomerleau R., Smith A.H. Fuscoboletinus, a new genus of the Boletales. Brittonia. 1962. V. 14. P. 156–171.
Ronquist F., Teslenko M., van der Mark P. et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012. V. 61 (3). P. 539–542. https://doi.org/10.1093/sysbio/sys029
Smith A.H., Thiers H.D. The boletes of Michigan. Michigan, 1971.
Snell W.H., Dick E.A. The boleti of northearstern North America. J. Cramer, Lehre, 1970.
The International barcode of Life consortium. International barcode of Life project (iBOL). 2016. Occurrence dataset https://doi.org/ accessed via GBIF.org on 2021-02-18.https://doi.org/10.15468/inygc6
University of Washington Burke museum. Mycological collection – University of Washington Herbarium (WTU). Occurrence dataset accessed via GBIF.org on 2021-02-18.https://doi.org/10.15468/ajny5r
Дополнительные материалы отсутствуют.
Инструменты
Микология и фитопатология


