Микология и фитопатология, 2023, T. 57, № 1, стр. 3-24
Juniper-Associated Wood-Inhabiting Basidiomycota in East European Boreal Forests (Republic of Belarus, European Russia)
O. N. Ezhov 1, *, D. B. Belomesyatseva 2, **, V. A. Dudka 3, ***, E. O. Yurchenko 4, ****, Yu. R. Khimich 5, *****, S. V. Volobuev 3, ******, A. V. Ruokolainen 6, *******, E. F. Malysheva 3, ********, D. A. Kosolapov 7, *********, I. V. Zmitrovich 3, **********
1 N. Laverov Federal Center for Integrated Arctic Research of the Ural Branch of the Russian Academy of Sciences
163000 Arkhangelsk, Russia
2 V.F. Kuprevich Institute of Experimental Botany of the National Academy of Sciences of Belarus
220072 Minsk, Belarus
3 Komarov Botanical Institute of the Russian Academy of Sciences
197376 St. Petersburg, Russia
4 Institute of Forest Sciences, Białystok University of Technology
15-351 Białystok, Poland
5 Institute of North Industrial Ecology Problems, Kola Science Center of the Russian Academy of Sciences
184209 Apatity, Russia
6 Forest Research Institute of the Karelian Research Center, Russian Academy of Sciences
185910 Petrozavodsk, Russia
7 Institute of Biology of Komi Scientific Center of the Ural Branch of the Russian Academy of Sciences
167000 Syktyvkar, Russia
* E-mail: olegezhik@gmail.com
** E-mail: dasha_belom@yahoo.com
*** E-mail: dudkavasiliy.a@gmail.com
**** E-mail: eugene.o.yurchenko@gmail.com
***** E-mail: ukhim@inbox.ru
****** E-mail: sergvolobuev@mail.ru
******* E-mail: aruokolainen@mail.ru
******** E-mail: ef.malysheva@gmail.com
********* E-mail: kosolapov@ib.komisc.ru
********** E-mail: iv_zmitrovich@mail.ru
Поступила в редакцию 13.06.2022
После доработки 25.08.2022
Принята к публикации 4.11.2022
- EDN: HMUJHI
- DOI: 10.31857/S002636482301004X
Аннотация
The paper is devoted to ecological and biodiversity studies on basidiomycetes associated with common juniper (Juniperus communis) from subtaiga zone of Belarus as well as several taiga regions of the European part of Russia, namely, the Leningrad Region, the Republic of Karelia, the Komi Republic, the Arkhangelsk Region, the Murmansk Region. The taiga zone represents an ecological-coenotic optimum of common juniper and the widest spectrum of its fungal consorts is expected to be revealed. A total of 96 species of wood-inhabiting basidiomycetes, both from dead and living wood, were recorded in association with J. communis in the boreal regions of Belarus and the European part of Russia. These species belong to the Pucciniomycetes (Pucciniales, 3 species), Dacrymycetes (Dacrymycetales, 1 species), and Agaricomycetes (Agaricales, Atheliales, Auriculariales, Boletales, Cantharellales, Gloeophyllales, Gomphales, Hymenochaetales, Polyporales, Russulales, Thelephorales, Trechisporales, 92 species). An annotated species list is given, including an expanded substratum and ecology datasets. The ecological and geographical preferences of these fungi and the strength of their connection with juniper were analyzed by thermal map, clusterization, and Sankey diagram methods. The various types of wood decomposition by juniper-associated basidiomycetes were discussed. The global distribution pattern of the species was analyzed. It was concluded that the biota of basidiomycetes associated with juniper in Eastern European taiga forests is a heterogeneous and heterochronous formation, where it is possible to distinguish clearly a florogenetically ancient core of the species specialized on juniper, exhibiting in one or other way biotrophic properties, and several taiga sets of species (suites) connected with pine trees and their litter as well as with spruce-pine-leaved taiga mosaics and boreo-nemoral mosaics typical of the Northern Hemisphere.
INTRODUCTION
The genus Juniperus is an example of ecological specialization of initially subtropical Cupressaceae in cold (high altitude and boreal) climates. The common juniper (Juniperus communis) is a Holarctic species (recorded as an invasive species in Libya, South Africa, Argentina, Australia, and New Zealand) with the highest abundance on fairly drained soils in boreal forests. It is an evergreen tree or shrub with egg-shaped or conical crown, and gray-brown longitudinally scaly bark. The needles are dense, 1–1.5 cm long and 0.7–1.5 mm wide, shallowly grooved at the top, brilliantly green below with a blunt keel, elongated into a thorny point at the end. This plant is anemophilous, mesophilic, microthermal, oligotrophic, and represents rather stable asseсtator. Over a large extent of its range, the common juniper grows predominantly in the undergrowth of coniferous (Pinus sylvestris, Picea abies, P. obovata, Larix sibirica) and also in birch and birch-aspen derived forests. The communities with its dominance are very rare, as, for example, in alvars on limestone along the Baltic Sea or in forest-tundra valleys. In boreal forests this species grows mostly in lichen, Vaccinium myrtillus or V. vitis-idaea types of forests, although it is recorded in flow-moistened spruce forests too, reaching 18 m in height. On dry and stony soils in communities of sparsely standing larch on plateaus of Siberia as well as in tundra light forests it grows as a shrub, reaching 1–1.5 m in height. At the southern boundary of its range, J. communis is included in the undergrowth of steppe insular oligotrophic pine forests (Sokolov, Svyazeva, 1965; Lammers et al., 2016).
The juniper’s mycobiota attracted the attention of researchers since a long time (Holm, Holm, 1977; Belomesyatseva, 2004; Volobuev, Ivanushenko, 2020) because it is a species with very strong wood, dense needles, and a very peculiar niche, which occupied by only some fungi from the total mycobiome of communities with juniper.
Concerning basidiomycetes, yet Linnaeus (1753) described Gymnosporangium teliostage on juniper as Tremella juniperina, whereas Persoon (1794) described the same species as Puccinia juniperi. Subsequently, the juniper as a substratum for fungi attracted attention of Fries (1828), who described from this substratum such species as Thelephora juniperina and Th. laevigata. Karsten (1876) described from juniper such species as Corticium juniperi (later under the name Xerocarpus juniperi; Karsten, 1881); the same researcher noted 22 species of basidiomycetes for this host. Bourdot and Gal-zin described for the first time from juniper such taxa as Corticium serum var. juniperi (Bourdot, Galzin, 1911; current name is Lyomyces juniper) and Peniophora glebulosa subsp. juniperina [Bourdot, Galzin, 1913; current name is Tubulicrinis sororius (Bourdot et Galzin) Oberw.].
There is a number of classical works devoted to the rust genus Gymnosporangium (Arthur, 1934; Dietel, 1888; Sydow, Sydow, 1924; Transhel, 1939). This genus is a kind of exception among other rust fungi because coniferous plants usually serve as a reservoir for its teliostage, while most of other rust fungi infecting coniferous species (the genera Cronartium, Coleosporium, Calyptospora, Hyalopsora, Melampsora, Melampsorella, Pucciniastrum, Uredinopsis) develop on coniferous hosts their aecia and spermogonia. Another exception is Chrysomyxa, which produce its telia on Picea).
Crowell (1940) summarized the data on the localities of Gymnosporangium species, Sávulescu (1953) published a picture of the distribution of Gymnosporangium species in Europe. The origin and taxonomy of the genus Gymnosporangium were discussed by Leppik (1956), who considered the correlation between evolution of host groups and formation of sections within the genus, and this process was analyzed in time and ecological-geographical aspects.
Parmasto (1968) mentioned 15 species which develop on Juniperus communis. In particular from juniper he described a new species, Atheloderma mirabile. Scandinavian mycologists, primarily J. Eriksson and his students, made a great contribution to the study of aphyllophoroid fungi on J. communis. In the beginning period of his studies, Eriksson described an interesting species, Peniophora junipericola, specialized on this genus (Eriksson, 1950). In the book series “The Corticiaceae of North Europe” (Eriksson, Ryvarden, 1973, 1975, 1976; Eriksson et al., 1978, 1981, 1984; Hjortstam et al., 1987, 1988), Eriksson and co-authors cited many finds from this plant, including Amylostereum laevigatum, Lyomyces juniperi, Peniophora junipericola.
Several species of corticioid fungi were described on juniper in the Netherlands and Germany by B. de Vries, for example, Hyphoderma cryptocallimon and Trechispora kavinioides (Vries, 1987). The finds of fungi confined to Juniperus communis were listed in special papers by Bernicchia (2000), and Sell and Kotiranta (2011).
Annotated checklists of basidiomycetes, published in recent decades, include some species indicated on common juniper as a substratum. However, special studies of this host in the optimum zone of the species, similar to that Bernicchia did for southern species of Juniperus, began relatively recently starting from the territory of the Republic of Belarus (Belomesyatseva, 2002, 2004). The present paper complements this data with new finds of basidiomycetes on J. communis from the subtaiga part of Belarus as well as several taiga regions of the European part of Russia, namely, the Leningrad Region, the Republic of Karelia, the Komi Republic, the Arkhangelsk Region, the Murmansk Region. The taiga zone represents an ecological-coenotic optimum of common juniper (at least this follows from the documented records of this species in GBIF database, 2022) and the widest spectrum of its fungal consorts is expected to be observed. Having identified the list of basidiomycetes associated with living and dead wood of common juniper, we intended to analyze ecological and geographical preferences of these fungal species and to assess the strength of their connection with common juniper as substratum. We assumed that this information would shed a light on the issues of ecological specialization and substratum adaptation of wood-inhabiting Basidiomycota.
MATERIAL AND METHODS
Study area. The studies were carried out in the following vegetation bands in eastern Europe (the borders of zones and subzones in Russia follow the maps by Ogureeva (1999).
Subtaiga. Belarus: Vitebsk Region, Grodno Region, Minsk Region. The border between boreal and nemoral biomes in Belarus is accepted by us as the boundary between Pre-Polesie and Polesie physiographic provinces (Martsin-kevich et al., 2001).
Southern taiga, middle taiga near the oceanic sector of the taiga zone. Leningrad Region: Vyborg, Vsevolozhsk, Lodeynopolsky, Podporozhsky districts.
Middle taiga. Arkhangelsk region: Shenkursk, Vel’sky, Kotlas, Plesetsk, Nyandoma districts. The Republic of Karelia: Kondopoga, Lakhdenpokhya, Pitkyaranta, Prionezhsky, Pudozh, Sortavala districts, Kostomuksha town. The Republic of Komi: Knyazhpogostsky district (Lyali village).
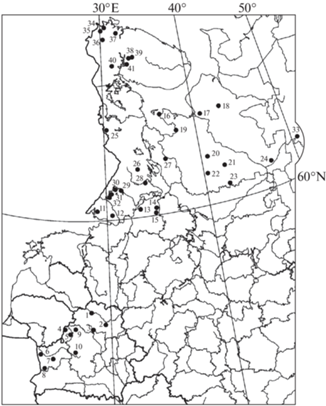
Northern taiga. Arkhangelsk region: Onega, Primorsky, Pinega districts. Murmansk Region: Pechenga, Kola, Kovdor districts, Apatity Town, Khibiny Mountains (Fig. 1).
Fig. 1.
Study area. Belarus: Vitebsk Region: 1 – Myory district, 2 – Beshankovichy district, 3 – Lepel district, Grodno Region: 4 – Astravets district, 5 – Smarhon district, 6 – Hrodna district, 7 – Masty district, 8 – Svislach district, Minsk Region: 9 – Myadzel district, 10 – Stoubtsy, Uzda, and Dzyarzhynsk districts. Russia: Leningrad Region: 11 – Vyborg district, 12 – Vsevolozhsk district, 13 – Lodeyno-polsky district, 14, 15 – Podporozhsky district; Arkhangelsk region: 16, 17 – Primorsky district, 18 – Pinega district; 19 – Onega district, 20 – Plesetsk district, 21 – Shenkursk district, 22 – Nyandoma district, 23 – Velsk district, 24 – Kotlas district; the Republic of Karelia: 25 – Kostomuksha town, 26 – Kondopoga district, 27 – Pudozh district, 28 – Prionezhye district, 29 – Pitkyarantsk district, 30 – Sorta-vala district, 31, 32 – Lakhdenpokhya district; the Republic of Komi: 33 – Knyazhpogostsky district; Murmansk Region: 34, 35, 36 – Pechenga district, 37 – Kola district, 38, 39 – Khibiny Mountains, 40 – Kovdor district, 41 – Apa-tity Town.
Extrazonal locatity in mountain taiga belt. Karachayevo-Circassian Republic: Teberda State Biosphere Reserve.
Sampling. When collecting the material, mostly the method of conventional floristic route studies was used, with more detailed searches within the comunities with old or numerous juniper plants. Additionally, the methods of radial routes proposed by Tolmachev (1974) was used with the concentration of routes near the base camp and sparse routes of reconnaissance character at the periphery of the site. Living and dead trunks, branches and stumps of juniper in different types of forests (spruce, pine, aspen, birch, mixed stands) were examined. Particular attention was paid to the areas with favourable conditions for the development of fungi growing on roots and litter. Well recognized in field conditions and widespread species were mostly not collected and information about them was recorded in field notebooks.
The collected material is deposited in the Komarov Botanical Institute Herbarium (LE), N. Laverov Federal Center for Integrated Arctic Research Herbarium (AR), Karelian Research Center RAS Herbarium (PTZ), Herbarium of Institute of North Industrial Ecology Problems, Kola Science Center, Russian Academy of Sciences (INEP), and Herbarium of V.F. Kupre-vich Institute of Experimental Botany, National Academy of Sciences of Belarus (MSK).
Data analysis. Identification of the collected material was carried out using light microscopes MBS-10, Axio Imager.A1, Nikon Eclipse E200, and Olympus BX-51 under magnification up to ×1000. Generative elements and mycelia of the fungi were prepared in 3–5% KOH water solution – for dissolving part of glucans and softening plectenchymes, Melzer’s reagent – for identifying the amyloid and dextrinoid reaction of glucan structures; 0.1% solution of blue toluidine dye in lactophenol (“cotton-blue”) was also used – for identification of cyanophilic reaction of the walls of spores and hyphae (Zmitrovich, 2008). Some microscopic studies of specimens were carried out at the Center for collective use of scientific equipment “Cellular and molecular technologies of studying plants and fungi” (Komarov Botanical Institute, Russian Academy of Sciences, St. Petersburg).
The collected specimens of wood-inhabiting fungi were prepared for storage in herbarium in accordance with recommendations by Ryvarden and Gilbertson (1993). Material identification was carried out using key-books and monographic treatments of the corresponding taxa (Eriksson, Ryvarden, 1973, 1975, 1976; Eriksson et al., 1978, 1981, 1984; Jülich, Stalpers, 1980; Hjortstam et al., 1987, 1988; Ryvarden, Gilbertson, 1993, 1994; Kõljalg, 1996; Hansen, Knudsen, 1997; Ryvarden, Melo, 2014, 2017) as well as by comparative analysis with specimens available in herbaria LE, AR, PTZ, INEP.
The taxonomic status of species is given in accordance with the nomenclatural database Index Fungorum (2022). Higher taxa of basidiomycetes are given according to Kirk et al. (2008), the trophic status – according to Zmitrovich et al. (2015). Latin nomenclature of plant communities is given according to dominant classification, widely accepted in East European geobotanical tradition (Sukachev, 1931; Tsinzerling, 1932; Lashchenkova, 1954; Vasilevich, Bibikova, 2010; Kucherov, 2014). The communities called Pinetum pleuroziosum are included in this paper in Pinetum hylocomiosum type.
The cluster analysis was performed using R 3.3.3 programming language (R Core, 2022) in RStudio 1.0.136 software environment (RStudio Team, 2022). For this, a matrix of Euclidean distances built on non-standardized data was used, constructed using Vegan package (Oksanen et al., 2018). The clustering of the obtained matrix was performed by Ward’s method, followed by visualization of the obtained data using APE package (Paradis et al., 2004) in RStudio.
The graphs were performed using the packages Ggplot2 (Wickham, 2009), Scales (Scales, 2022), Ggpubr (RPackages, 2022) as well as the online program Sankey Diagram Generator v. 1.2 (Sankey, 2022).
RESULTS
A total of 96 species of wood-inhabiting basidiomycetes were identified in association with Juniperus communis in the boreal regions of Belarus and the European part of Russia (Table 1). These species belong to the Pucciniomycetes (Pucciniales, 3 species), Dacrymycetes (Dacrymycetales, 1 species), and Agaricomycetes (Aga-ricales, Atheliales, Auriculariales, Boletales, Cantharellales, Gloeophyllales, Gomphales, Hymenochaetales, Polyporales, Russulales, Thelephorales, Trechisporales, 92 species; fig. 2). The richest orders are Hymenochaetales, representing 25% of the considered species diversity, and Thelephorales (23.9%). The orders Polyporales (11.4%), Atheliales (10.4%), and Russulales (8.4%) are quite representative. The share of the remaining orders in the structure of considered fungi diversity is small and varies from 1.1% (Auriculariales, Dacrymycetales, Gloeophyllales, Gomphales) to 3.1% (Pucciniales, Trechisporales).
Table 1.
Overview of juniper-associated wood-inhabiting basidiomycetes in East European boreal forests (Republic of Belarus, European Russia)
| Species | Regional distribution | Herbarium vouchers | Trophic status | Type of rot | Substrate spectrum1 | Coenotic confinement | Distribution pattern |
|---|---|---|---|---|---|---|---|
| Agaricomycetes Agaricales |
|||||||
| Cylindrobasidium evolvens (Fr.) Jülich | Russia (Arkhangelsk Region) | AR 3039 | S | II | AP, AH, BV, BP, CV, CS, LX, PAV, PT, RI, SS, SA, TC, UP | Pinetum cladinosum | bipolar |
| Radulomyces confluens (Fr.) M.P. Christ. | “ ” | AR 2520 | S | II | AI, BP, CA, CS, EC, HR, PAV, QR, SC, SR | Pinetum cladinosum | circumglobal |
| R. rickii (Bres.) M.P. Christ. | “ ” | AR 3045 | S | II | BP, HR, SC | Pinetum cladinosum | bipolar |
| Atheliales | |||||||
| Amphinema byssoides (Pers.) J. Erikss. | Belarus (Minsk, Vitebsk Regions), Russia (Arkhangelsk Region, Murmansk Region, Republic of Karelia) | AR 1618, 1884, 2647, 2708, INEP 2079, MSK 4358, 4791, 5677a, 5678, 5679a, b, 5680, 8058, PTZ 2217 | S, EM | I | AI, BPD, BP, LS, PA, PS, PT, SC, LT | Piceetum hylocomiosum, Pinetum cladinosum, P. hylocomiosum, P. myrtillosum, P. vacciniosum, Parvo-Betuletum myrtilloso-hylocomiosum | “ ” |
| Athelia alnicola (Bourdot et Galzin) Jülich | Belarus (Grodno Region) | MSK 4100 | S | I | AI, AG, PS | Pinetum hylocomiosum | Euro-American |
| A. epiphylla Pers. | Belarus (Minsk) | MSK 5676a–c | B, S | I | AS, AI, AG, BPD, BP, LD, LS, PA, PO, PS, PT, TC, LT | Pinetum vacciniosum | bipolar |
| Hypochnella violacea Auersw. ex J. Schröt. | Russia (Arkhangelsk Region) | AR 2990 | S | I? | AI, BP, LS, PA, SA, LT | Pinetum cladinosum | “ ” |
| Leptosporomyces fuscostratus (Burt) Hjortstam | “ ” | AR 1510 | S | I | LS, PO | Pinetum myrtillosum | Holarctic |
| L. galzinii (Bourdot) Jülich | Belarus (Minsk Region), Russia (Arkhangelsk Region) | MSK 12000, 5176, 8004, AR 2646 | S | I | BP, FF, LS, PS, PA, LT | “ ” | bipolar |
| Piloderma bicolor (Peck) Jülich | Russia (Arkhangelsk Region, Republic of Karelia) | AR 2509, PTZ 2522 | S, EM | I | BP, PA, PS, PT, SC, | Pinetum cladinosum | Holarctic |
| P. byssinum (P. Karst.) Jülich | Belarus (Minsk Region, Vitebsk Region), Russia (Republic of Karelia) | AR 2507, AR 2784, MSK 4790, 8056, PTZ sine no. | S, EM | I | AI, BP, PA, PT, QR, TC, LT | Pinetum cladinosum, P. hylocomiosum | bipolar |
| P. lanatum (Jülich) J. Erikss. et Hjortstam | Russia (Murmansk Region) | INEP 2087, LE 314092 | S | I | PS, LT | Pinetum myrtillosum | Euro-American |
| Tylospora asterophora (Bonord.) Donk | Belarus (Vitebsk Region) | MSK 4920 | S, EM | I | PA, PS, LT | “ ” | “ ” |
| Auriculariales | |||||||
| Basidiodendron caesiocinereum (Höhn. et Litsch.) Luck-Allen | “ ” | MSK sine no. | S | I | PA | “ ” | bipolar |
| Boletales | |||||||
| Coniophora arida (Fr.) P. Karst. | Russia (Arkhangelsk Region, Republic of Karelia) | AR 2898, 3028, 3054, PTZ 2527 | S | III | AS, AI, BP, CS, HR, LS, PA, PS, PT, SC | Pinetum cladinosum, P. myrtillosum | “ ” |
| C. olivacea (Fr.) P. Karst. | Russia (Arkhangelsk Region) | AR 2380, 2788, 3061, 3101 | S | III | AI, BP, AS, LS, PF, PA, PS, PT, PC | “ ” | circumglobal |
| Cantharellales | |||||||
| Ceratobasidium cornigerum (Bourdot) D.P. Rogers | Belarus (Minsk Region) | MSK 5674, 5677a | S | I | PS, LT | Pinetum hylocomiosum, P. myrtillosum | bipolar |
| Botryobasidium candicans J. Erikss. | Belarus (Vitebsk Region) | MSK 4491, 4507 | S | I | AS, PA, PS, PT, QR, LT | Pinetum myrtillosum | circumglobal |
| B. conspersum J. Erikss. | Russia (Arkhangelsk Region) | AR 2449, PTZ 2112 | S | I | AI, BP, PS, PT, LT | “ ” | bipolar |
| B. isabellinum (Fr.) D.P. Rogers | Russia (Arkhangelsk Region, Republic of Karelia) | AR 2693, 3069, PTZ sine no | S | I | AS, AI, BP, LS, PA, PSB, PS, PT, SC, SA, LT | Pinetum cladinosum, P. myrtillosum | Holarctic |
| Sistotrema alboluteum (Bourdot et Galzin) Bondartsev et Singer | Russia (Arkhangelsk Region) | AR 2785 | S | I | SC | Pinetum myrtillosum | “ ” |
| Gloeophyllales | |||||||
| Gloeophyllum sepiarium (Wulfen) P. Karst. | “ ” | AR 2310 | S | III | AI, BP, AS, LS, PO, PS, PT, SC | Pinetum cladinosum | circumglobal |
| Gomphales | |||||||
| Ramaricium albo-ochraceum (Bres.) Jülich | Belarus (Vitebsk Region) | MSK 4744 | S | I | PA, LT | Pinetum hylocomiosum | Euro-American |
| Hymenochaetales | |||||||
| Asterodon ferruginosus Pat. | Russia (Arkhangelsk Region) | AR sine no. | S | II | LS, PO, PS, BSP, PT, SA | “ ” | Holarctic |
| Fibricium rude (P. Karst.) Jülich | “ ” | AR 3007 | S | II | PO, PS, RR | Pinetum myrtillosum | “ ” |
| Globulicium hiemale (Laurila) Hjortstam | “ ” | AR 1208, PTZ 1707 | S | II? | PS | “ ” | Euro-American |
| Hymenochaete cinnamomea (Pers.) Bres. | “ ” | AR 385 | S | II | AI, BP, AS, PO, PS, PT, SC | Pinetum myrtillosum | circumglobal |
| H. fuliginosa (Pers.) Lév. | Russia (Arkhangelsk Region, Republic of Karelia, Murmansk Region) | AR 2339, INEP 2371 | S | II | LS, LX, PAV, PO, PS | Pinetum cladinosum, Cladinetum empetroso-nano-betulosum | Holarctic |
| Hymenochaetopsis tabacina (Sowerby) S.H. He et Jiao Yang | Russia (Arkhangelsk Region) | AR 1931, 2378 | S | II | AS, AI, BP, LS, PAV, PO, PT, QR, SC, SA | Pinetum cladinosum | circumglobal |
| Kneiffiella cineracea (Bourdot et Galzin) Julich et Stalpers | Russia (Komi Republic, Republic of Karelia) | SYK sine no., PTZ 2520 | S | II | PS | Pinetum hylocomiosum | European |
| Kneiffiella cf. floccosa (Bourdot et Galzin) Julich et Stalpers | Belarus (Grodno Region) | MSK 4103 | S | II | PA, PS | “ ” | bipolar |
| K. subalutacea (P. Karst.) Julich et Stalpers | Russia (Arkhangelsk Region) | AR sine no. | S | II | AS AI, LS, PO, PS, PT | Pinetum myrtillosum | “ ” |
| Kurtia argillacea (Bres.) Karasiński | Russia (Murmansk Region) | INEP 2082, LE 314086 | S | II | AI, BP, PA, PT | Pinetum myrtillosum, Parvo-Betuletum myrtilloso-hylocomiosum | circumglobal |
| Lyomyces crustosus (Pers.) P. Karst. | Russia (Arkhangelsk Region) | AR 2385, 2775, 3195 | S | II | AI, BSP, PAV, PT, SSP, SA, LT | Pinetum cladinosum, P. hylocomiosum | “ ” |
| L. juniperi (Bourdot et Galzin) Riebesehl et Langer | “ ” | AR 3102 | S | II | – | Pinetum myrtillosum | bipolar* |
| L. sambuci (Pers.) P. Karst. | “ ” | AR 2787 | S | II | AN, BP, PAV, PT, SR, SA, TC, LT | “ ” | circumglobal |
| Oxyporus millavensis (Bourdot et Galzin) Ryvarden et Melo | “ ” | AR 2384, PTZ 2113, 2242 | S | II | – | Pinetum cladinosum | European |
| Peniophorella pallida (Bres.) K.H. Larss. | Russia (Murmansk Region) | LE 314089 | S | II | AI, PA | Pinetum myrtillosum | Holarctic |
| P. praetermissa (P. Karst.) K.H. Larss. | Russia (Arkhangelsk Region, Murmansk Region) | AR 1929, INEP 2083, LE 314090 | S | II | AS, AI, BP, PO, PT, SA | Pinetum myrtillosum, Parvo-Betuletum myrtilloso-hylocomiosum | circumglobal |
| Phellinus viticola (Schwein.) Donk | Russia (Arkhangelsk Region, Republic of Karelia) | AR 655, AR 1861, PTZ 2220, 2356 | S | II | AS, LS, PO, PS | Pinetum cladinosum, P. myrtillosum | “ ” |
| Resinicium bicolor (Alb. et Schwein.) Parmasto | “ ” | AR 1280, 2399, 2776, 3205, 3221, LE 295827, PTZ 2519 | B, S | II | AS, BP, LS, LX, PAV, PO, PS, PT, PC, QR, SC | “ ” | “ ” |
| R. furfuraceum (Bres.) Parmasto | Russia (Arkhangelsk Region, Leningrad Region, Republic of Karelia) | AR 2778, 3219, 3220, LE 203463, PTZ 2331 | S | II | LS, PO, PS | Pinetum myrtillosum, P. vacciniosum | Holarctic |
| Tubulicrinis glebulosus (Fr.) Donk | Russia (Arkhangelsk Region) | AR 3193 | S | III | AI, LS, PSB, PS, PAV, PT, SC | Pinetum cladinosum | bipolar |
| T. medius (Bourdot et Galzin) Oberw. | “ ” | AR 2381 | S | III | BP, PS | Pinetum myrtillosum | European |
| T. subulatus (Bourdot et Galzin) Donk | Russia (Republic of Karelia), Belarus (Vitebsk Region) | PTZ 2516, MSK 4919 | S | III | PA, PS, LT | “ ” | bipolar |
| Xylodon asperus (Fr.) Hjortstam et Ryvarden | Russia (Leningrad Region) | LE 206648 | S | II | BP, PA, PS, PT, LT | “ ” | Holarctic |
| X. brevisetus (P. Karst.) Hjortstam et Ryvarden | Russia (Leningrad Region, Republic of Karelia) | LE 202066, PTZ 2521 | S | II | AI, BP, PA, PS, PT, LT | Pinetum vacciniosum | bipolar |
| Polyporales | |||||||
| Ceraceomyces eludens K.H. Larss. | Russia (Arkhangelsk Region) | AR 2738 | S | II | PS | Pinetum myrtillosum | Eurasian |
| C. serpens (Tode) Ginns | Russia (Arkhangelsk Region, Republic of Karelia) | AR 1448, 2379, 3003, 3112, PTZ 2312 | S | II | AI, BP, LS, PO, PS, PT, SC, SA, LT | “ ” | bipolar |
| Hyphoderma sibiricum (Parmasto) J. Erikss. et Å. Strid | Russia (Arkhangelsk Region, Murmansk Region) | AR 3206, INEP 2378 | S | II | PO | “ ” | circumglobal |
| Incrustoporia papyracea (A. David) Zmitr. | Russia (Arkhangelsk Region) | AR 2721 | S | II | AS, PO, PS | Pinetum cladinosum | European |
| Phanerochaete laevis (Fr.) J. Erikss. et Ryvarden | Russia (Arkhangelsk Region, Murmansk Region) | AR 2733, LE 314091 | S | II | AS, AI, BP, PO, PS, PT, QR, SA | Pinetum myrtillosum | bipolar |
| Ph. sanguinea (Fr.) Pouzar | Russia (Murmansk Region, Republic of Karelia) | INEP 2086, PTZ 2219 | S | II | BP, PS, PA, PT | “ ” | Holarctic |
| Ph. sordida (P. Karst.) J. Erikss. et Ryvarden | Russia (Arkhangelsk Region, Murmansk Region) | AR 2340, AR 3024, INEP 2085 | S | II | AS, BP, LS, PO, PSB, PS, PT, SA | Pinetum cladinosum, P. myrtillosum, Parvo-Betuletum myrtilloso-hylocomiosum | circumglobal |
| Postia caesia (Schrad.) P. Karst. | Russia (Republic of Karelia) | PTZ 2517 | S | III | AS, PA, PS | Pinetum myrtillosum | bipolar |
| Phlebia segregata (Bourdot et Galzin) Parmasto | “ ” | PTZ 1739 | S | II | PA, PS | Piceetum myrtillosum | European |
| Steccherinum fimbriatum (Pers.) J. Erikss. | Russia (Arkhangelsk Region, Republic of Karelia) | AR 2365, 3194, PTZ 2032, PTZ sine no. | S | II | BP, LS, LX, PO, PT, SC, SA | Pinetum myrtillosum | bipolar |
| Xenasmatella vaga (Fr.) Stalpers | Belarus (Minsk Region, Vitebsk Region), Russia (Arkhangelsk Region, Republic of Karelia) | AR 2991, MSK 4512, 5604a, b, PTZ 2221 | S | II | AS, AI, BP, LS, PA, PO, PS, PT, QR, TC, LT | Pinetum cladinosum, P. myrtillosum | bipolar |
| Russulales | |||||||
| Aleurodiscus lividocaeruleus (P. Karst.) Lemke | Russia (Republic of Karelia) | PTZ 2387 | S | II | AS, PO | Pinetum myrtillosum | Euro-American |
| Amylostereum laevigatum (Fr.) Boidin | Belarus (Grodno Region, Vitebsk Region), Russia (Arkhangelsk Region, Leningrad Region, Pskov Region, Republic of Karelia) | AR 2672, MSK 4099, 4491, LE 37554, 311512, 213847, 242229, 203872, 234500, 210181, 242328, 235666, 242106, PTZ 1726, 2357, 2387 | PS | II | PS | “ ” | “ ” |
| Asterostroma laxum Bres. | Russia (Archangelsk Region) | AR 3089 | S | II | PS | “ ” | Euro-American |
| Gloeocystidiellum convolvens (P. Karst.) Donk | “ ” | AR 327 | S | II | AI, BP, LS, LX, PO, PT, SC | “ ” | Holarctic |
| Gloiothele citrina (Pers.) Ginns et G.W. Freeman | Belarus (Vitebsk Region), Russia (Arkhangelsk Region, Murmansk Region) | INEP 2080, MSK 4492, AR 2448, 2450, PTZ 2111 | S | II | AI, BP, LS, PS, PA, PO, PS, PT, SC, SA, LT | Pinetum myrtillosum, Betuleto-Piceetum myrtilloso-herbosum | “ ” |
| Metulodontia nivea (P. Karst.) Parmasto | Russia (Archangelsk Region) | AR 2709 | S | II | BP, PO, PT | Pinetum cladinosum | bipolar |
| Peniophora incarnata (Pers.) P. Karst. | Russia (Murmansk Region) | INEP 2087, LE 314087 | S | II | AI, AG, BP, BPD, PT | Pinetum myrtillosum | circumglobal |
| P. junipericola J. Erikss. | Russia (Archangelsk Region) | AR 1943, 2311, 2734, LE 287558 | PS | II | – | Pinetum cladinosum | European |
| Thelephorales | |||||||
| Odontia fibrosa (Berk. et M.A. Curtis) Kõljalg | Russia (Murmansk Region) | LE 314087 | S, EM | II | PS | Pinetum myrtillosum | circumglobal |
| Pseudotomentella mucidula (P. Karst.) Svrček | Russia (Archangelsk Region) | AR 1860 | S, EM | II | LS | “ ” | Eurasian |
| P. nigra (Höhn. et Litsch.) Svrček | Russia (Archangelsk Region, Murmansk Region) | AR 3192, INEP 2078 | S, EM | II | PS, SC | Pinetum myrtillosum, Betuleto-Piceetum myrtilloso-herbosum | Holarctic |
| P. tristis (P. Karst.) M.J. Larsen | Belarus (Minsk Region, Vitebsk Region), Russia (Archangelsk Region) | AR 2695, MSK 5673, 8058, 4358 | S, EM | II | AS, BP, PS, PA, PT, SC, LT | Pinetum vacciniosum | “ ” |
| Thelephora atra Weinm. | Belarus (Vitebsk Region) | MSK 4452 | S, EM | II | PA, PS, LT | Pinetum myrtillosum | Euro-American |
| Th. ellisii (Sacc.) Zmitr., Shchepin, Volobuev et Myasnikov | Russia (Archangelsk Region) | AR 2397, 2649, PTZ 2209 | S, EM | II | AI, BP, LS, PT, SC, SA, TC | Pinetum cladinosum, P. myrtillosum | circumglobal |
| Th. terrestris Ehrh. | Belarus (Grodno Region) | MSK 8047a | S, EM | II | BP, BN, PS, LT | Pinetum hylocomiosum | “ ” |
| Th. terrestris f. radiosa (P. Karst.) Zmitr. | Russia (Arkhangelsk Region, Murmansk Region, Republic of Karelia) | AR 3096, LE 314098, PTZ sine no. | S, EM | II | AI, BP, LS, PO, PS, PT, SA | Pinetum myrtillosum | “ ” |
| Th. cf. wakefieldiae Zmitr., Shchepin, Volobuev et Myasnikov | Belarus (Vitebsk Region) | MSK 4358 | S, EM | II | AI, BP, PA, PO, PS, PT | Pinetum vacciniosum | “ ” |
| Tomentella badia (Link) Stalpers | Belarus (Vitebsk Region), Russia (Republic of Karelia) | MSK 4091, 4108, PTZ 2302 | S, EM | II | PA, PS, LT | Pinetum hylocomiosum, P. myrtillosum | Holarctic |
| T. bryophila (Pers.) M.J. Larsen | Russia (Archangelsk Region, Murmansk Region, Republic of Karelia) | AR 1886, 2519, 2648, INEP 2081, 2084, LE 314093, 314094, PTZ 2518 | S, EM | II | AS, AI, BP, IR, LS, PO, PS, PT, SC, SA, LT | Pinetum myrtillosum, Parvo-Betuletum myrtilloso-hylocomiosum | “ ” |
| T. cinerascens (P. Karst.) Höhn. et Litsch. | Russia (Archangelsk Region, Murmansk Region) | AR 1885, LE 314095 | S, EM | II | BP, LS, PO, PS, PT, SC, SA | Pinetum myrtillosum | Eurasian |
| T. cinereoumbrina (Bres.) Stalpers | “ ” | AR 2364, 2412, 2451, 3025, AE 3031, PTZ 2033, LE 314096 | S, EM | II | AI, BP, PS, SC | Pinetum cladinosum | European |
| T. fuscocinerea (Pers.) Donk | Russia (Archangelsk Region) | AR 1444, 2398 | S, EM | II | PT, SC | Pinetum cladinosum, P. myrtillosum | Holarctic |
| T. galzinii Bourdot | “ ” | LE 295895 | S, EM | II | PS | Pinetum myrtillosum | Euro-American |
| T. griseoumbrina Litsch. | Russia (Archangelsk Region) | AR 3197 | S, EM | II | AI, PS, PT | “ ” | Holarctic |
| T. lapida (Pers.) Stalpers | Belarus (Minsk Region, Vitebsk Region), Russia (Arkhangelsk Region, Murmansk Region) | AR 3044, INEP 2077, LE 257458, 314097, MSK 5677a, 5678, 8005, 4790 | S, EM | II | AI, BP, PA, PS, LT | Pinetum cladinosum, P. hylocomiosum, Betuleto-Piceetum myrtilloso-herbosum | “ ” |
| T. lilacinogrisea Wakef. | Russia (Arkhangelsk Region) | AR 3196 | S, EM | II | AI, BP, PO, LT | Pinetum myrtillosum | Holarctic |
| T. punicea (Alb. et Schwein.) J. Schröt. | “ ” | AR 2338, PTZ 2240 | S, EM | II | PA, PS | Pinetum cladinosum | bipolar |
| T. stuposa (Link) Stalpers | “ ” | AR 2777, 3218 | S, EM | II | AI, BP, LS, PO, PS, PT | Pinetum cladinosum, P. myrtillosum | circumglobal |
| T. subclavigera Litsch | Russia (Republic of Karelia) | PTZ sine no. | S, EM | II | PA | Pinetum myrtillosum | Holarctic |
| T. terrestris (Berk. et Broome) M.J. Larsen | “ ” | PTZ 2316 | S, EM | II | PS | “ ” | bipolar |
| Tomentellopsis echinospora (Ellis) Hjortstam | Russia (Arkhangelsk Region) | AR 2696 | S, EM | II | PS, SC, SA | “ ” | Holarctic |
| Trechisporales | |||||||
| Sistotremastrum suecicum Litsch. ex J. Erikss. | “ ” | AR 2396 | S | I | AS, LS, PO, PS | Pinetum cladinosum | “ ” |
| Trechispora hymenocystis (Berk. et Broome) K.H. Larss. | Russia (Murmansk Region) | INEP 1711 | S | I | PA | Pinetum empetroso-cladinosum | “ ” |
| T. kavinioides B. de Vries | Russia (Archangelsk Region) | AR 2786 | S | I | PO | Pinetum myrtillosum | European |
| Dacrymycetes Dacrymycetales |
|||||||
| Dacrymyces minor Peck | Russia (Karachayevo-Circassian Republic) | LE 253862 | S | III | JS, BSP | Betuleto-Piceetum myrtilloso-herbosum | Holarctic |
| Pucciniomycetes Pucciniales |
|||||||
| Gymnosporangium clavariiforme (Wulfen) DC. | Belarus (Grodno Region, Minsk Region, Vitebsk Region), Russia (Leningrad Region, Murmansk Region) | MSK 8011, 8114, 8171, LE 75311, (Transhel, 1936) | B | AO, CS, CO, PC, SA | Pinetum hylocomiosum | bipolar | |
| G. cornutum Arthur ex F. Kern | Belarus (Grodno Region, Vitebsk Region) | MSK 8156, 8157, 8170 | B | AO, MD, MS, SA | Pinetum cladinosum, P. hylocomiosum, P. myrtillosum | Euro-American | |
| G. tremelloides R. Hartig | Belarus (Vitebsk Region), Russia (Leningrad Region, Murmansk Region) | MSK 8113, 8155, LE 74335, 76044 | B | CO, MD, MS, PC | Pinetum myrtillosum | Holarctic | |
Note. Trophic groups: S – saprotrophs; S EM – saprotrophic species on forest litter and small wood debris capable to form ectomycorrhiza; B – biotrophs; PS – pathogenic saprotrophs; B S – saprotrophic fungi capable to biotrophic nutrition on epixylic algae and protonemata mats. Types of rot: I – ancestral soft rot with prevalence of hydrolases, II – white rot with involvement of laccases and peroxidases, III – brown rot with oxidation by Fenton mechanism. Substrata: AG – Alnus glutinosa, AH – Aesculus hippocastanum, AI – Alnus incana, AN – Acer negundo, AO – Amelanchier ovalis, AP – Acer platanoides, AS – Abies sibirica, BN – Betula nana, BP – B. pubescens, BPD – B. pendula, BSP – Betula spp., BV – Berberis vulgaris, CA – Caragana arborescens, CO – Cydonia oblonga, CS – Crataegus sanguinea, CV – Cerasus vulgaris, EC – Elaeagnus commutata, FF – Fomes fomentarius, HR – Hippophaе rhamnoides, IR – Inocutis rheades, JS – Juniperus sabina, LD – Larix dahurica, LS – L. sibirica, LT – litter, LX – Lonicera xylosteum, MD – Malus domestica, MS – M. sylvestris, PA – Picea abies, PAV – Padus avium, PC – Pyrus communis, PF – Phellinidium ferrugineofuscus, PO – Picea obovata, PS – Pinus sylvestris, PSB – P. sibirica, PT – Populus tremula, QR – Quercus robur, RI – Rubus idaeus, RR – Ribes rubrum, SA – Sorbus aucuparia, SC – Salix caprea, SR – Sambucus racemosa, SSP – Salix spp., TC – Tilia cordata, UP – Ulmus parvifolia. *In Southern Hemisphere, three occurences of Lyomyces juniperi were recorded on some islands of Indian Ocean nearly to the Madagascar on the Tropic of Capricorn (GBIF, 2022).@1Aecidial stages of Pucciniales are associated to hosts distributed within intrazonal plant communities (Cydonia, Crataegus, etc.).
Fig. 2.
Taxonomic structure of juniper-associated basidiomycetes biota of the taiga regions of Belarus and the European part of Russia.
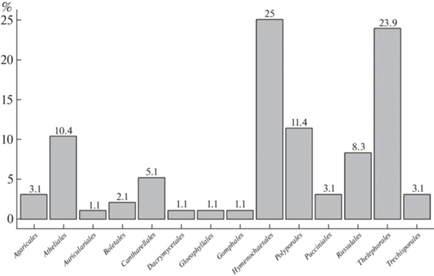
In terms of their trophic status (Fig. 3), most of the juniper-associated basidiomycetes identified (65.6%) are saprotrophs, decomposing lignocellulosic components of the wood of dead trunks. Essentially saprobic group of pathogenic saprotrophs (2.1%) also develops on living trunks. This group is adapted to develop under conditions of functioning xylem. At the same time, a part of saprotrophs colonizing wood debris or the part of a trunk submerged in forest litter, can form ectomycorrhizae with both juniper and adjacent alien rhizospheres (28.1%). Moreover, the teliostage of several species of true biotrophic fungi of the order Pucciniales (3.1%) was revealed. Several saprotrophic species associated with colonization of small tree remnants exhibit biotrophic properties with respect to aerophytic algae and mosses protonemata (1.1%).
Fig. 3.
Trophic structure of juniper-associated basidiomycetes biota of the taiga regions of Belarus and the European part of Russia (classification of trophic groups according to: Zmitrovich et al., 2015): S – saprotrophs; S EM – saprotrophic species on forest litter and small wood debris capable to forming of ectomycorrhizas; B – biotrophs; PS – pathogenic saprotrophs; B S – saprotrophic fungi capable to biotrophic nutrition on epixylic algae and protonemata mats.
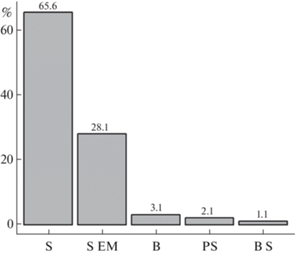
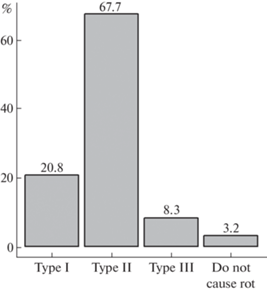
As it was shown by Nagy et al. (2015), there are three main types of rot caused by basidiomycetes: I – ancestral soft rot with the prevalence of hydrolases, II – white rot, III – brown rot with oxidation by Fenton mechanism. Most of the studied fungi species (69.9%) cause white rot, the ancestral soft rot and brown rot are less common (21.5 and 8.6%, respectively). Among the studied fungi, 3 species (Gymnosporangium clavariiforme, G. cornutum, G. tremelloides; Pucciniales) do not cause rot, but feed by establishing a parasite-host interface (Fig. 4).
Fig. 4.
Distribution of juniper-associated fungi from this study on types of caused rot (I – ancestral soft rot, II – white rot, III – brown rot).
As can be seen from table 1, there are not too many species of basidiomycetes strictly specialized on juniper. Of these, Lyomyces juniperi, Oxyporus millavensis, Peniophora junipericola, and the teliostages of Gymnosporangium should be mentioned (while aecio- and uredostadia of the latter are associated with members of the Rosaceae family: Amelanchier ovalis, Cydonia oblonga, Malus spp., Pyrus communis). A group of species, highly specialized on juniper, includes also Amylostereum laevigatum, although this fungus occasionally can be found also on Pinus sylvestris.
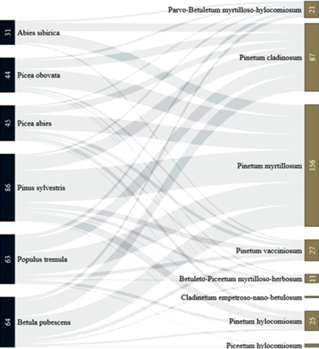
Most species found on juniper have a fairly wide spectrum of substrata, even within the taiga zone. Figure 5 shows the so-called “thermal map” of the distribution of the identified species by host genera, from which it can be seen that most of the fungi found on Juniperus are also occur on such tree genera as Alnus, Betula, Picea, Pinus, Populus, less often on Abies and Salix. On the remaining genera of plants, basidiomycetes from the present work are found sporadically.
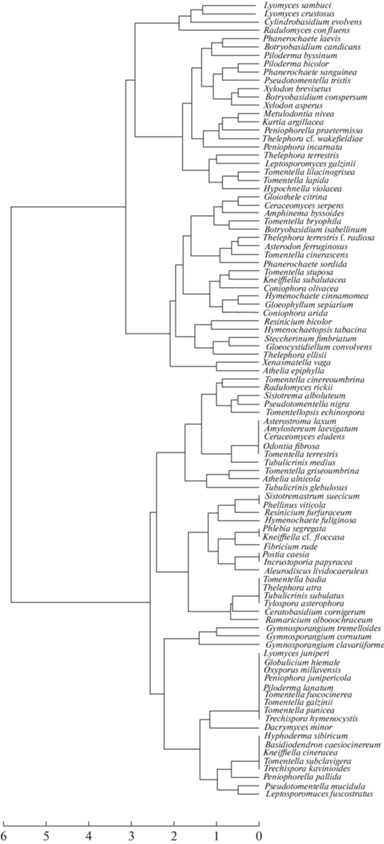
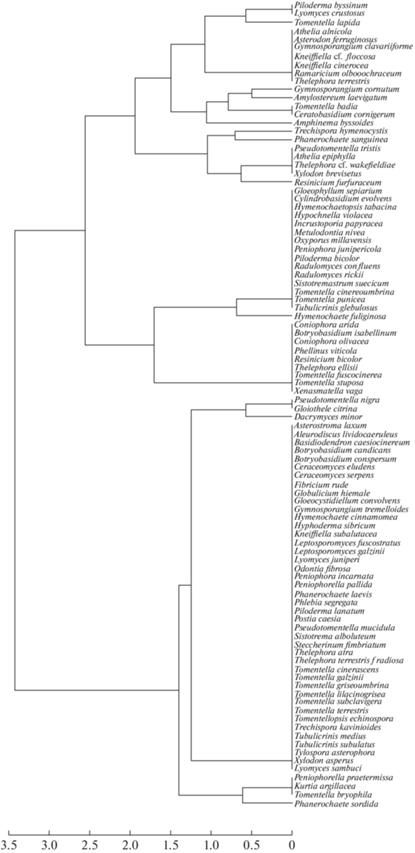
Fig. 6 shows the similarity of species composition of juniper-inhabiting basidiomycetes from our list in association with the genera of host plants in environment of Eastern European taiga forests. According with this analysis, the core of juniper-associated mycobiota consists of Basidiodendron caesiocinereum, Globulicium hiemale, Gymnosporangium tremelloides, G. cornutum, G. clavariiforme, Kneiffiella cineracea, Leptosporomyces fuscostratus, Lyomyces juniperi, Peniophora junipericola, Peniophorella pallida, Piloderma lanatum, Pseudotomentella mucidula, Tomentella fuscocinerea, T. galzinii, and Trechispora kavinioides.
Fig. 6.
Dendrogram of similarity for species composition of juniper-associated basidiomycetes, recorded for various genera of host plants in Eastern European taiga forests (scale bar shows Euclidean distance).
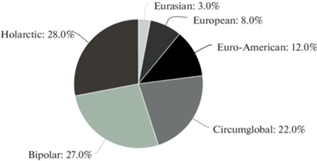
According to their patterns of global distribution (Fig. 7), basidiomycetes associated with juniper in Eastern European taiga forests were grouped as Holarctic, i.e., common in non-tropical areas of the Northern Hemisphere (27 species, 28%), bipolar, i.e., common in temperate regions of both hemispheres with pronounced disjunction in tropical belt (26 species, 27%), circumglobal, i.e., widespread everywhere, including tropical climates (21 species, 22%), Euro-American, i.e. distributed in Europe and North America (11 species, 12%), European, i.e., common in Europe, sometimes irradiating a little east of the Urals (8 species, 8%), Eurasian, i.e., common in temperate Eurasia (3 species, 3%).
Fig. 7.
Distribution of juniper-associated basidiomycetes from this study by global distribution patterns according to GBIF data (2022).
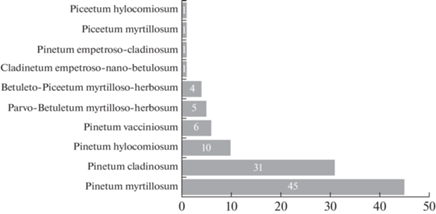
The ecological-phytocoenotic spectrum of basidiomycetes associated with common juniper in taiga Eastern European forests is presented In the Fig. 8. As it can be seen, most of species (45) are associated with Pinetum myrtillosum, then follow Pinetum cladinosum (31 species), Pinetum hylocomiosum (10 species), Pinetum vacciniosum (6 species), Parvo-Betuletum myrtilloso-herbosum (5 species), and Betuleto-Piceetum myrtilloso-herbosum (4 species). In sub-virgin spruce forests Piceetum hylocomiosum/P. myrtillosum (where common juniper is extremely rare) as well as in north taiga communities with Empetrum – Pinetum empetroso-cladinosum and Cladinetum empetroso-nano-betulosum these fungi are represented by 1 species in each type.
Fig. 8.
Distribution of species of juniper-associated fungi over the phytocoenoses types of Eastern European taiga forests.
In the Fig. 9 it is possible to trace the correspondence between coenotic gravitation of basidiomycetes and their general geographical distribution pattern. The largest number of Holarctic species is confined to Pinetum myrtillosum, the circumglobal species are evenly distributed in the wet (Pinetum myrtillosum), dry (Pinetum cladinosum), and cryotic (Parvo-Betuletum myrtilloso-hylocomiosum) types. Bipolar, European, and Euro-American species also have fairly diverse coenotic spectra.
Fig. 9.
Sankey diagram of relationships of coenotic preferences of fungi with their distribution pattern. The coenotic preferences of fungi is shown in the left column, the relation of individual coenotic preferences to the general geographical distribution of fungi is shown in the right column. For groupings containing one or two species, the numbers are not indicated.
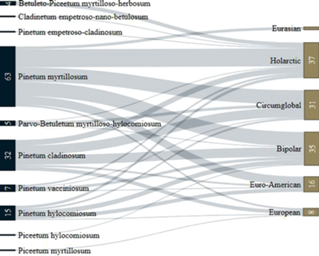
In the Fig. 10, the correspondence between the spectrum of substrata and the coenotic preferences of juniper-associated basidiomycetes can be traced. It can be seen that many species are able to colonize the main stand-formers of the taiga zone in a wide range of ecological and coenotic conditions.
Fig. 10.
Sankey diagram of relationships of the substrate (the main taiga stand-formers) and the coenotic preferences of juniper-associated basidiomycetes. In the left column is the number of species of basidiomycetes confined to tree species, in the right column – the number of fungi having a certain coenotic affinity for the above species of trees. For groupings containing one ot two species, the numbers are not indicated.
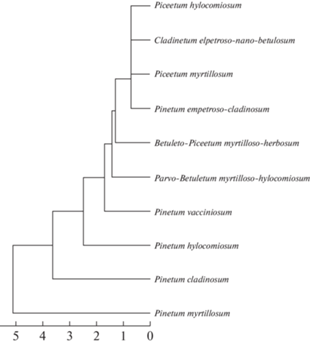
In the Figure 11, the similarity between species of juniper-associated basidiomycetes in their ecological-phytocoenotic preferences is shown. There are two main clusters corresponding to dry (Pinetum cladinosum: Gloeophyllum sepiarium – Tubulicrinis glebulosus) and moderately wet conditions (Pinetum myrtillosum: Asterostroma laxum – Lyomyces sambuci).
Fig. 11.
Dendrogram of similarity of juniper-associated basidiomycete species by ecological-phytocoenotic preferences (scale bar shows Euclidean distance).
The Fig. 12 shows the similarity of phytocoenoses of Eastern European taiga forests by species composition of juniper-associated basidiomycetes. Oligospecies communities form a single cluster, whereas multiple species communities are differentiated in order of increasing differences in species composition in the following series: Pinetum vacciniosum – Pinetum hylocomiosum – Pinetum cladinosum – Pinetum myrtillosum.
DISCUSSION
The present study significantly enlarges our knowledge of the species composition of basidiomycetes associated with juniper. More than 90 species have been observed in total, that constitutes about 1/5 of the wood-inhabiting basidiomycetes diversity of the taiga zone. The main orders of the classes Agaricomycetes, Dacrymycetes, and Pucciniomycetes are presented on the substratum in question. It is noteworthy that there are no representatives of Tremellomycetes, quite commonly associated in the taiga zone with fungi that colonize other conifers (for example, spruce and pine). The reason may be related to clear specialization of Tremellomycetes to certain species of xylotrophic Russulales, which, in turn, also show a tendency to substratum specialization. A rather high proportion of Thelephorales associated with juniper attracts attention, too. These are mainly debris-inhabiting species that can develop in litter and are quite widespread in pine forests, developing in extremely poor (oligotrophic) soil conditions, where Juniperus communis mainly grows.
Common uniper is characterized by significantly dense wood with relatively small diameter and height of senile shoots, so its destruction occurs after a fairly long decay in standing state, where it harbours most of saprotrophic basidiomycetes. Pathogenic saprotrophs, such as Amylostereum laevigatum, begin this process. The parts of the stem that come into contact with forest floor are colonized by unspecialized saprotrophs and saprotrophic ectomycorrhiza-forming species from the genera Thelephora and Tomentella (Zmitrovich et al., 2018). The bark is colonized by group of saprotrophs with biotrophic activity, capable of absorbing the metabolites produced by aerophytic algae (Athelia epiphylla, Resinicium bicolor, possibly also Lyomyces juniperi). True biotrophic species (Gymnosporangium spp.), attacking cambium cells, cause hypertrophy and hyperplasia of shoots. Like pathogenic biotrophs, true biotrophs develop chronically, but the results of their development can be detachment of bark and partial necrosis of stems, which are then colonized by a wide range of saprotrophs.
Like in the taiga mycobiotas as a whole, white rot species are predominating (69.9%) among xylotrophs on juniper, although the percentage of species capable of causing brown rot is also quite high (21.5%), which is typical for conifers (Zmitrovich et al. 2007; Arefyev 2010). It should be noted, however, that the brown rot of juniper proceeds inactively and the integrity of the trunks and branches is maintained for some time; usually old plants penetrated by deep corrosive rot collapse from fracture in the area of root collar, while in wet forest types they remain standing, somewhat retained by moss carpets or Lerchenfeldia flexuosa curtains. The group of fungi that cause ancestral soft rot (8.6%) is also fairly representative – these are mainly species colonizing small wood debris associated with common juniper phytosphere.
Specialized juniper species gravitate to various parts of this plant: cambial zone (Gymnosporangium ssp.), bark (Lyomyces juniperi, Oxyporus millavensis, Peniophora junipericola) as well as sapwood (Amylostereum laevigatum). The majority of juniper-associated species (Fig. 5) are widely represented on the main forest-forming arboreous plant genera of the taiga zone, as Picea, Pinus, Abies, Larix, Populus, Betula, Alnus, Sorbus, Salix as well as on species introduced or irradiant from the broadleaf forest zone (Quercus robur, Tilia cordata, and their satellites).
Juniperus communis is a Holarctic species (recorded as an invasive in some tropicl and Southern Hemisphere areas) (GBIF, 2022).
It seems therefore quite natural that Holarctic (28.1%) and bipolar (27.1%) species play an important role in the considered fungal list and that circumglobal species complexes have a significant presence as well. Basidiomycetes having more fine differentiation of their distribution areas within the Holarctic are also present in the composition of juniper-associated mycobiota. At the same time, there seems to be a natural representation of Eurasian species (3.1%), which optimum area is generally shifted to the cryo-xerophilic zones.
The ecological and phytocoenotic optimum of J. communis in the taiga belt is pine forests (at least this follows from the documented records of this species in GBIF database, 2022) in oligotrophic sandy soil conditions, having the dynamics after occasional fires, and lacked spruce renewal. This type corresponds to Pinetum cladinosum and pioneering communities preceding this type on the burnt areas. However, in the pine communities of Pinetum myrtillosum and Pinetum hylocomiosum types, which are the long-lived successive stages of Piceetum myrtillosum, J. communis plays a rather prominent role in the structure of the phytocenoses till the stage of spruce and pine trees crown closure. Most species of basidiomycetes (45) were noted on common juniper in Pinetum myrtillosum. These are sufficiently moist forests, the hydrothermal regime of which is suitable for the development of many fungal species. Together with Pinetum hylocomiosum (10 species), they give almost half of the species list of juniper-associated basidiomycetes identified in the taiga zone. The drier types are Pinetum cladinosum (31 species), Pinetum vacciniosum (6 species), Parvo-Betuletum myrtilloso-herbosum (5 species), Betuleto-Piceetum myrtilloso-herbosum (4 species), Pinetum empetroso-cladinosum (1 species), and Cladinetum empetroso-nano-betulosum (1 species), which add fewer species, but they are more characteristic of the portrait of the juniper-associated mycobiota, corresponding to fungal associations such as Bankero-Amyloporietum and Suillo bovinis-Amylostereum laevigatidis (Zmitrovich, 2011). Thus, Peniophora junipericola is found on juniper predominantly in open pine forests, and only on shores of sea or large lakes (Sell et al., 2011; Ezhov, Zmitrovich, 2017).
Correspondence between coenotic preferences of basidiomycete species and their general geographical distribution (Fig. 8) shows that the greatest number of Holarctic species is confined to Pinetum myrtillosum, circumglobal species are distributed uniformly in wet (Pinetum myrtillosum), dry (Pinetum cladinosum), and cryotic (Parvo-Betuletum myrtilloso-hylocomiosum) types, whereas bipolar, European, and Euro-American species have fairly diverse coenotic spectra. There is no doubt that dry and cryotic highland habitats involving pine and juniper are peculiar guides of taiga species complexes to warmer pine and juniper communities in the Southern Hemisphere.
The revealed relationships between a spectrum of substrata and a coenotic preferences of juniper-associated basidiomycetes show that most of the basidiomycetes can colonize the main stand-formers of the taiga zone in a wide range of ecological-coenotic conditions, that is, they have a wide environmental plasticity. At the same time, there are two main complexes among juniper-associated basidiomycetes (Fig. 10), according to their ecological-phytocoenotic preferences, corresponding to: 1) dry conditions (Pinetum cladinosum: Gloeophyllum sepiarium – Tubulicrinis glebulosus), which in terms of coenogeography represents the binding complex with those of the Southern Hemisphere pine forests, and 2) moderately humid conditions (Pinetum myrtillosum: Asterostroma laxum – Lyomyces sambuci), which in terms of coenogeography represents the core of the taiga juniper-associated mycobiota.
The similarity of phytocoenoses of Eastern European taiga forests in respect of species composition of juniper-associated basidiomycetes (Fig. 12) reflects a kind of “degenerate” species composition of open communities with the participation of juniper, the similarity of which is determined by few dominant basidiomycetes. The Pinetum myrtillosum – Pinetum cladinosum – Pinetum hylocomiosum – Pinetum vacciniosum series does not reflect any gradient of vegetation conditions, but rather the fact that most numerous groups of basidiomycetes are characteristic of Pinetum myrtillosum and Pinetum cladinosum, where the juniper is most abundant and its consortium is most developed.
It is necessary to note that our conclusions are based on boreal juniper-associated fungal diversity studies and in the case of addition of data from the Eastern Europe regions located to the center and east, where vast thickets of juniper develop on limestones in the south boreal and hemiboreal forests, then the results will change.
CONCLUSION
In general, we can conclude that the biota of basidiomycetes associated with juniper in Eastern European taiga forests is a heterogeneous and heterochronous formation, where it is possible to distinguish clearly a florogenetically ancient core of the species specialized on juniper, exhibiting in one way or other biotrophic properties, and several taiga sets of species (suites) connected with pine trees and their litter as well as with spruce-pine-leaved taiga mosaics and boreo-nemoral mosaics, typical to the Northern Hemisphere.
The work of O.N. Ezhov was carried out in framework of State Task “Study of patterns of space-time changes the forest ecosystems in the subarctic territories of the European North of Russia” (project no. 122011400384-2). The work of V.A. Dudka, S.V. Volobuev, E.F. Malysheva, and I.V. Zmitrovich was carried out within the framework of the State Task to the Komarov Botanical Institute of the Russian Academy of Sciences (project no. 122011900033-4) and was partly supported by Russian Foundation for Basic Research (RFBR). The work of Yu.R. Khimich was carried out within the framework of the State Task (project no. FMEZ-2022-0021) of the Institute of the Industrial Ecology Problems of the North of the Kola Science Center of RAS and partly within the project of RFBR (grant no. 18-05-00398 A). The work of A.V. Ruokolainen was carried out under state assignment to the Forest Research Institute of the Karelian Research Center of the Russian Academy of Sciences (FRI KarRC RAS). The research of D.A. Kosolapov was carried out in accordance of the state assignment of the Institute of Biology of the Komi Scientific Center, Ural Branch of the Russian Academy of Sciences (project no. 122040600026-9).
Список литературы
Arefyev S.P. System analysis of biota of wood-destroying fungi. Nauka, Novosibirsk, 2010 (in Russ.).
Arthur J.Ch. Manual of the rusts of United States and Canada. Purdue Research Foundation, Lafayette, 1934.
Belomesyatseva D.B. The fungi in the consortium of common juniper in Belarus. Mycena. 2002. V. 2 (1). P. 4–16.
Belomesyatseva D.B. Mycobiota of juniper consortium in Belarus. Pravo i ekonomika, Minsk, 2004 (in Russ.).
Bernicchia A. Wood-inhabiting aphyllophoraceous fungi on Juniperus spp. in Italy. Mycotaxon. 2000. V. 75. P. 241–256.
Bourdot H., Galzin A. Hyménomycètes de France III. Corticiés: Corticium, Epithele, Asterostromella. Bull. Trim. Soc. Mycol. France. 1911. V. 27 (2). P. 223–226.
Bourdot H., Galzin A. Hyménomycètes de France IV. Corticiés: Vuilleminia, Aleurodiscus, Dendrothele, Gloeocystidium, Peniophora. Bull. Trim. Soc. Mycol. France. 1913. V. 28. P. 349–409.
Crowell I.H. The local distribution of the genus Gymnosporangium. Can. J. Res. 1940. V. 18. P. 469–488.
Dietel P. Über einige auf Compositen vorkommende Rostpilze. Über das Vorkommen von zweierlei Teleutosporen bei der Gattung Gymnosporangium. Hedwigia. 1888. V. 27. P. 303–304.
Eriksson J. Peniophora Cke sect. Coloratae Bourd. et Galz. A taxonomical study. Symb. Bot. Upsal. 1950. V. 10 (5). P. 1–76.
Eriksson J., Hjortstam K., Ryvarden L. The Corticiaceae of North Europe / With drawings by J. Eriksson. V. 5: Mycoaciella – Phanerochaete. Fungiflora, Oslo, 1978.
Eriksson J., Hjortstam K., Ryvarden L. The Corticiaceae of North Europe / With drawings by J. Eriksson. V. 6: Phlebia–Sarcodontia. Fungiflora, Oslo, 1981.
Eriksson J., Hjortstam K., Ryvarden L. The Corticiaceae of North Europe / With drawings by J. Eriksson. V. 7: Schizopora–Suillosporium. Fungiflora, Oslo, 1984.
Eriksson J., Ryvarden L. The Corticiaceae of North Europe / With drawings by J. Eriksson. V. 2: Aleurodiscus–Confertobasidium. Fungiflora, Oslo, 1973.
Eriksson J., Ryvarden L. The Corticiaceae of North Europe / With drawings by J. Eriksson. V. 3: Coronicium–Hypoderma. Fungiflora, Oslo, 1975.
Eriksson J., Ryvarden L. The Corticiaceae of North Europe / With drawings by J. Eriksson. V. 4: Hyphodermella–Mycoacia. Fungiflora, Oslo, 1976.
Ezhov O.N., Zmitrovich I.V. Lignotrophic basidiomycetes of pioneering microsites of the taiga forests of the White Sea Region. Byulleten Moskovskogo obshchestva ispytateley prirody. Biologiya. 2017. V. 122 (6). P. 44–50.
Fries E.M. Elenchus fungorum, sistens commentarium in Systema mycologicum V. 1. Mauritius, Gryphiswaldiae, 1828.
GBIF. Global Biodiversity Information Facility. 2022. https://www.gbif.org. Accessed 22.06.022.
Hansen L., Knudsen H. (eds). Nordic macromycetes. V. 3: heterobasidioid, aphyllophoroid and gastromycetoid basidiomycetes. Copenhagen, Nordsvamp, 1997.
Hjortstam K., Larsson K.-H., Ryvarden L. The Corticiaceae of North Europe. V. 1: Introduction and keys. Fungiflora, Oslo, 1987.
Hjortstam K., Larsson K.-H., Ryvarden L. The Corticiaceae of North Europe / With drawings by J. Eriksson. V. 8: Phlebiella; Thanatephorus–Ypsilonidium. Fungiflora, Oslo, 1988.
Holm K., Holm L. Nordic junipericolous ascomycetes. Symb. Bot. Upsal. 1977. V. 21. P. 1–70.
Index Fungorum. CABI Database. 2022. http://www.indexfungorum.org. Accessed 22.06.2022.
Jülich W., Stalpers J.A. The resupinate non-poroid Aphyllophorales of the temperate Northern Hemisphere. North-Holland Publishing Company, Amsterdam, Oxford, N.Y., 1980.
Karsten P.A. Mycologia Fennica. Pars tertia. Basidiomycetes. Bidr. Känned. Finl. Natur och Folk 1876. V. 25. P. 1–377.
Karsten P.A. Enumeratio Boletinearum, Polyporearum, Hydnearum, Thelephorearum et Clavariarum Fennicarum systemate novo dispositarum. Rev. Mycol. 1881. V. 3 (9). P. 16–19.
Kirk P.M., Cannon P.F., David J.C. et al. Ainsworth and Bisby’s Dictionary of the Fungi. 9th edition. Oxford Univ. Press, N.Y. etc., 2001.
Kõljalg U. Tomentella (Basidiomycota) and related genera in temperate Eurasia. Fungiflora, Oslo, 1996.
Kucherov I.B. Green-mosses (blueberry) pine forests of the middle and northern taiga of European Russia: a review of coenotic diversity. Trudy Karelskogo nauchnogo tsentra RAN. Seriya Biogeografiya 2014. V. 2. P. 14–26 (in Russ.).
Lammers H., van Hooff H., Boudewijns T. et al. Jeneverbes, een bikkel! Coolia. 2016. V. 59 (1). P. 1–10.
Lashchenkova A.N. Pine forests. In: Productive forces of the Komi ASSR: 126–156. Publishing House of the Academy of Sciences of the USSR, Moscow, 1954 (in Russ.).
Leppik E. Some viewpoints on the phylogeny of rust fungi. 2. Gymnosporangium. Mycologia. 1956. V. 48 (5). P. 637–654.
Linnaeus C. Species plantarum, exhibentes plantas rite cognitas ad genera relatas, cum differentiis specificis, nominibus trivialibus, synonymis selectis, locis natalibus, secundum systema sexuale digestas. V. 2. Holmie, Laurentii. Salvil., 1753.
Martsinkevich G.I., Klitsunova N.K., Schastnaya I.I. et al. Physiographic division of Belarus in European digital system of division. Vestnik Belorusskogo gosudarstvennogo universiteta. Seriya 2. 2001. V. 1. P. 85–90 (in Russ.).
Nagy L.G., Riley R., Tritt A. et al. Comparative genomics of early-diverging mushroom-forming fungi provides insights into the origins of lignocellulose decay capabilities. Molec. Biol. Evol. 2015. V. 334. P. 959–970. https://doi.org/10.1093/molbev/msv337
Ogureeva G.N. (ed.) Zones and types of zonality of the vegetation of Russia and adjacent territories (Series of the maps of nature for high school). Ekor, Moscow, 1999 (in Russ.).
Oksanen J., Blanchet F.G., Friendly M. et al. Vegan: community ecology package. R package version 2.5-1. 2018. https://CRAN.R-project.org/package/vegan. Accessed 09.09.2022.
Paradis E., Claude J., Strimmer K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics. 2004. V. 20. 289–290. https://doi.org/10.1093/bioinformatics/btg412
Parmasto E. Conspectus systematis Corticiacearum. Institutum zoologicum et botanicum Academiae scientiarum R.P.S.S, Tartu, 1968.
Persoon Ch.H. Neuer Versuch einer systematischen Eintheilung der Schwamme. Neues Mag. Bot. 1794. V. 1. P. 63–128.
R Core. Team R: A language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. 2022. http://www.R-project.org. Accessed 11.09.2022.
R Packages. 2022. Available from https://rpkgs.datanovia.com. Accessed 11.09.2022.
RStudio Team. RStudio: Integrated Development for R. RStudio, Inc., Boston. 2022. Available from http://www.rstudio.com/Saleh-Rastin. Accessed 11.09.2022.
Ryvarden L., Gilbertson R.L. European Polypores, Pt 1. Abortiporus–Lindtneria. Synopsis Fung. V. 6. Oslo, Fungiflora, 1993.
Ryvarden L., Gilbertson R.L. European Polypores, Pt 2. Meripilus–Tyromyces. Synopsis Fung. V. 7. Oslo, Fungiflora, 1994.
Ryvarden L., Melo I. Poroid fungi of Europe / with photos by T. Niemelä and drawings by I. Melo and T. Niemelä. Synopsis Fung. V. 31. Fungiflora, Oslo, 2014.
Ryvarden L., Melo I. Poroid fungi of Europe / with photos by T. Niemelä and drawings by I. Melo and T. Niemelä. Synopsis Fung. V. 37. Fungiflora, Oslo, 2017.
Sankey Diagram Generator. 2022. http://sankey-diagram-generator.acquireprocure.com. Accessed 11.09.2022.
Sávulescu T. Monografia Uredinalelor din Republica Populara Romana. 2. Editura Academiei Republici Populare Romane, Bucuresti, 1953.
Scales 1.0.0. 2022. https://scales.r-lib.org. Accessed 11.09.2022.
Sell I., Kotiranta H. Diversity and distribution of aphyllophoroid fungi growing on common juniper (Juniperus communis L.) in Estonia. Folia Cryptogamica Estonica. 2011. V. 48. P. 73–84.
Sell I., Kotiranta H., Kaart T. Habitat requirements of Peniophora junipericola (Basidiomycota, Russulales). Ann. Bot. Fennici. 2011. V. 48. P. 232–236. https://doi.org/10.5735/085.048.0303
Sokolov S.Ya., Svyazeva O.A. Geography of trees of USSR. Nauka, Moscow, Leningrad, 1965 (in Russ.).
Sukachev V.N. Guide to the study of forest types. 3rd ed. Moscow, Selkhozgiz, 1931 (in Russ.).
Sydow P., Sydow H. Monographia Uredinearum. I–IV. Leipzig, Borntraeger, 1902–1924.
Tolmachev A.I. Introductory plant geography. Leningrad, 1974 (in Russ.).
Transhel V.G. Outline of rust fungi of USSR. Acad. Sci. USSR publ. house, Moscow, Leningrad, 1939.
Tsinzerling Yu.D. Geography of the vegetation cover of the northwest of the European part of the USSR. Publishing house of the Academy of Sciences of the USSR, Leningrad, 1932 (in Russ.).
Vasilevich V.I., Bibikova T.V. Lichen and lichen-green-moss pine forests of Eastern Europe. Botanicheskiy zhurnal. 2010. V. 95 (5). P. 601–617 (in Russ.).
Volobuev S.V., Ivanushenko Yu.Yu. Aphyllophoroid fungi (Basidiomycota) on juniper on the Gunib Plateau, inner-mountain Dagestan. Czech Mycology. 2020. V. 72 (1). P. 83–93.
Vries B.W.L. Some new corticioid fungi. Mycotaxon. 1987. V. 28 (1). P. 77–90.
Wickham H. Ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag, N.Y., 2009.
Zmitrovich I.V. Definitorium fungorum Rossicum. Aphyllophorales. 3. Atheliaceae et Amylocorticiaceae. Nauka, St. Petersburg, 2008 (in Russ.).
Zmitrovich I.V. The middle taiga of the Karelian Isthmus: zonal, intrazonal and extrazonal phenomena. Vestnik ekologii, lesovedeniya i landshaftovedeniya. 2011. № 12. P. 54–76 (in Russ.).
Zmitrovich I.V., Psurtseva N.V., Belova N.V. Evolutionary-taxonomic aspects of the search and study of lignin-destroying fungi – active producers of oxidative enzymes. Mikologiya i fitopatologiya 2007. V. 41 (1). P. 57–78 (in Russ.).
Zmitrovich I.V., Shchepin O.N., Malysheva V.F. et al. Basidiome reduction in litter-inhabiting Thelephorales in boreal forest environments: morphological and molecular evidence. Current Res. Environm. Appl. Mycol. 2018. V. 8 (3). P. 360–371. https://doi.org/10.5943/cream/8/3/7
Zmitrovich I.V., Wasser S.P., Ţura D. Wood-inhabiting fungi. In: Fungi from different substrates / J.K. Misra etc. (eds). CRC Press, Taylor and Francis group, N.Y., 2015. P. 17–74.
Арефьев С.П. (Arefyev) Системный анализ биоты дереворазрушающих грибов. Новосибирск: Наука, 2010. 407 с.
Беломесяцева Д.Б. (Belomesyatseva) Микобиота в консорции можжевельника в Беларуси. Минск: Право и экономика, 2004. 235 с.
Василевич В.И., Бибикова Т.В. (Vasilevich, Bibikova) Лишайниковые и лишайниково-зеленомошные сосняки Восточной Европы // Ботанический журнал. 2010. Т. 95. № 5. С. 601–617.
Змитрович И.В. (Zmitrovich) Определитель грибов России. Порядок афиллофоровые; Вып. 3. Семейства ателиевые и амилокортициевые. СПб.: Товарищество научных изданий КМК, 2008. 278 с.
Змитрович И.В. (Zmitrovich) Средняя тайга Карельского перешейка: зональные, интразональные и экстразональные явления // Вестник экологии, лесоведения и ландшафтоведения. 2011. № 12. С. 54–76.
Змитрович И.В., Псурцева Н.В., Белова Н.В. (Zmitrovich et al.) Эволюционно-таксономические аспекты поиска и изучения лигнинразрушающих грибов – активных продуцентов окислительных ферментов // Микология и фитопатология. 2007. Т. 41. № 1. С. 57–78.
Кучеров И.Б. (Kucherov) Зеленомошные (черничные) сосняки средней и северной тайги Европейской России: обзор ценотического разнообразия // Тр. Карельского научного центра РАН. Серия Биогеография 2014. Т. 2. С. 14–26.
Лащенкова А.Н. (Lashchenkova) Сосновые леса // Производительные силы Коми АССР. М.: Изд-во АН СССР, 1954. С. 126–156.
Марцинкевич Г.И., Клицунова Н.К., Счастная И.И. и др. (Martsinkevich et al.) Физико-географическое районирование Беларуси в европейской десятичной системе // Вестник Белорусского государственного университета. Серия 2. 2001. Т. 1. С. 85–90.
Огуреева Г.Н. (ред.) (Ogureeva) Зоны и типы поясности растительности России и сопредельных территорий. Москва: Экор, 1999.
Соколов С.Я., Связева О.А. (Sokolov, Svyazeva) География древесных растений СССР. М.; Л.: Наука, 1965. 265 с.
Сукачев В.Н. (Sukachev) Руководство к исследованию типов леса. 3-е изд. М.: Сельхозгиз, 1931. 328 с.
Толмачев А.И. (Tolmachev) Введение в географию растений. Л.: ЛГУ, 1974. 244 с.
Траншель В.Г. Обзор ржавчинных грибов СССР. М.; Л.: Изд-во АН СССР, 1939. 426 с.
Цинзерлинг Ю.Д. (Tsinzerling) География растительного покрова Cеверо-Запада Европейской части СССР. Л.: Изд-во АН СССР, 1932. 328 с.
Дополнительные материалы отсутствуют.
Инструменты
Микология и фитопатология