Микология и фитопатология, 2023, T. 57, № 4, стр. 247-254
Amanita phalloides in Northwest European Russia
L. B. Kalinina 1, 2, *, S. V. Volobuev 1, **, A. A. Khovpachev 3, ***, D. A. Tomchin 4, ****, E. A. Palomozhnykh 1, *****, S. Yu. Bolshakov 1, ******, N. V. Shakhova 1, *******, E. S. Popov 1, ********
1 Komarov Botanical Institute of the Russian Academy of Sciences
197022 St. Petersburg, Russia
2 Polistovsky State Nature Reserve
182840 Bezhanitsy, Russia
3 Kirov Military Medical Academy
194044 St. Petersburg, Russia
4 Control of Complex Systems Laboratory, Institute for Problems in Mechanical Engineering of the Russian Academy of Sciences
199178 St. Petersburg, Russia
* E-mail: lkalinina@binran.ru
** E-mail: sergvolobuev@binran.ru
*** E-mail: khovpachev@gmail.com
**** E-mail: dtomchin@yandex.ru
***** E-mail: epalomozhnykh@binran.ru
****** E-mail: sbolshakov@binran.ru
******* E-mail: nshakhova@binran.ru
******** E-mail: epopov@binran.ru
Поступила в редакцию 1.02.2023
После доработки 17.04.2023
Принята к публикации 7.05.2023
- EDN: VUVGFH
- DOI: 10.31857/S0026364823040049
Аннотация
The article reviews available data on the “death cap” (Amanita phalloides) occurrences in the North-West of the European Russia (Leningrad, Novgorod, Pskov Oblasts and St. Petersburg City). The literature data are analyzed, the review of ecological preferences of the species in the studied area is carried out. It has been suggested that the species can be both native and imported and was introduced with broad-leaved tree seedlings during the foundation of numerous manor parks in the XVIII–XIX centuries. Molecular-genetic analysis was carried out, showing that ITS sequences of A. phalloides samples from Leningrad Oblast form a common clade with the sequences of collections from Central Russia as well as from Northern and Central Europe.
INTRODUCTION
Amanita phalloides (Vaill. ex Fr.) Link is an iconic species, “death cap” that is responsible for numerous human poisonings. It was assumed earlier, that the species is rare or even absent in the Northwestern part of European Russia. During the last twenty years, new findings of the species on the territory were made by both mycologists and amateurs. Present paper summarizes all existing collections of the species to highlight the presence of this deadly poisonous species in the region.
MATERIALS AND METHODS
Sampling and identification. For the literature review information from database of agaricoid fungi distribution was used (Bolshakov et al., 2021). For the existing specimens we checked Mycological Herbarium of the Komarov Botanical Institute RAS (LE). During survey of the mycobiota of Northwest Russia, several new collections of the species were obtained and new localities were revealed. Due to characteristic appearance, identification was performed in the field.
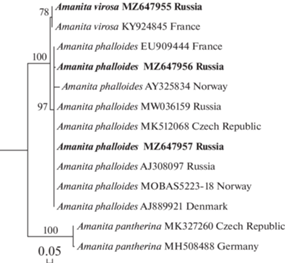
DNA techniques and phylogenetic analysis. DNA was extracted from small pieces of dried basidiomata using the FitoSORB DNA extraction kit (Syntol, Russia) according to the manufacturer’s instructions. PCR reactions were performed in 20 μL of reaction mixtures containing 10 μL of iQ Supermix (BioRad), 0.2 μL of each PCR primer, 4.6 μL of deionized H2O, and 5 μL of template DNA. The ribosomal ITS1–5.8S–ITS2 region was amplified with the primers ITS1F/ITS4B (Gardes, Bruns, 1993). PCR products were visualized using agarose gel electrophoresis and GelRed staining, and subsequently purified with the Fermentas PCR Purification Kit (Thermo Fisher Scientific, Lithuania). Purified PCR products were sequenced on an ABI model 3500 Genetic Analyzer (Applied Biosystems, USA). Raw data were edited and assembled using Molecular Evolutionary Genetics Analysis Version 6.0 (MEGA6) software (Tamura et al., 2012). Newly generated sequences were deposited in the GenBank. Additionally, 10 ITS sequences were retrieved from GenBank and the BOLD Systems (Ratnasingham, Hebert, 2007) (Table 1). Sequences were aligned with the MAFFT version 7 web tool (Katoh et al., 2019) using the E-INS-1 option. Maximum Likelihood (ML) analysis was performed in the IQ-TREE Web Server (Trifinopoulos et al., 2016) with 1000 ultrafast bootstrap replicates.
Table 1.
Sequences and strains of Amanita species included in molecular analysis
| Species | GenBank accession number | ID (specimen number, strain) | Country | References |
|---|---|---|---|---|
| Amanita pantherina | MK327260 | RET 403-8 | Czech Republic | –** |
| MH508488 | MB-102863 | Germany | Cui at al. (2018) | |
| A. phalloides | AJ308097 | FVORO-0023 | Russia | – |
| AY325834 | O Gulden 49/94, Norway | Norway | – | |
| EU909444 | Am.pha.PV02.1 | France | Pringle et al. (2009) | |
| MK512068 | A54 | Czech Republic | – | |
| MW036159 | LE-BIN 4016 (strain) | Russia | – | |
| MZ647956 | Khovpachev-A3b | Russia | current study | |
| MZ647957 | LE 332058 | Russia | current study | |
| NOBAS5223-18* | O-F-21495 | Norway | – | |
| AJ889921 | KF02-19 | Denmark | – | |
| A. virosa | MZ647955 | Khovpachev-A1b | Russia | current study |
| KY924845 | RET 291-3 | France | – |
RESULTS
Literature review
XIX–XX centuries. In 1828 Weinmann published the list of fungi that were found in Pavlovsk manor (Weinmann, 1828). He mentioned “Agaricus phalloides pileo flavo” with reference to Fries. Fries in his Systema Mycologicum (Fries, 1821) accepted broad concept of the species and cited five “infraspecific” taxa differing in pileus color. Iconography given to the form “b. pileo flavo” depicts rather Amanita citrina Pers. in modern concept (Fig. 1). As Weinmann followed Fries, it can be assumed that he probably found not A. phalloides, but A. citrina. Later, in subsequent summarizing work on flora of “agro Petropolitano” (Weinmann, 1837) he also mentioned five forms, simultaneously citing iconography. Fungi depicted on illustrations cited fit quite well the modern concept of A. phalloides (Fig. 2). No precise localities are given, but it is known that Weinmann mostly worked in Tsarskoye Selo (suburban area of modern St. Petersburg) and Gatchina (modern Leningrad Oblast). To our knowledge, no specimen of A. phalloides collected by Weinmann exists. In 1892 and 1894 Thesleff found the species during his investigations on the territory of modern Vyborgsky District (Thesleff, 1920) with mention “ekskog” (oak forests). According to the list of localities, he visited oak forests in three localities: around Vyborg Bay (“kring Viborgska viken i Viborgs socken”), Vesennii Island (“Luuri-holmen i St Johannes socken”) and Malyi Beryozovyi Island (“holmen Vasikkasaari i Finska viken, Björkö socken”). Thus, up to 2000 there were only literature data based on century-old observations of the species from the territory of North-Western Russia with no specimens deposited in collections.
Fig. 1.
Original descriptions of taxa associated with Amanita phalloides concept: a – “forms” of Agaricus phalloides from Systema Mycologicum (Fries, 1821). We collect here iconography ascribed to “b. pil. flavo” (encased in rectangle); b – illustration of Agaricus citrinus from Schaeffer (1762); c – illustration from Nees von Esenbeck (1817); d – description of Amanita citrina by Persoon (1801), there is also reference to Schaeffers’ plate; e – illustration of Agaricus verrucosus from Curtis (1787); f – description of Agaricus mappa from Willdenow, 1787; g – page from Weinmann (1828).

Fig. 2.
Original descriptions of taxa associated with Amanita phalloides and illustration of the species in modern concept: a – “varieties” of Agaricus phalloides from Weinmann (1837); b – plate from Vaillant (1727) that was chosen by Weinmann as main “variety”; c – illustration to variety “c. pileo pallide-viridi”, A. virescens from Vahl (1799); d – to the variety “a. pileo albo”, A. vernalis from Bolton (1788); e – to the “b. pileo flavo”, A. bulbosus from Bulliard (1798); f – to the “d. pileo olvaceo-viridi” and “e. pileo fuscescente”, A. bulbosus from Bulliard (1798); g – basidiomata of Amanita phalloides exposed during mushroom exhibition in St. Petersburg in 2018; h – A. phalloides in situ.

New findings (2000 – nowadays). First data on A. phalloides findings from the territory of St. Petersburg confirmed by specimens belongs to the 2010s when the species was found in Primorsky and Kurortny districts of St. Petersburg. Since 2011, death cap basidiomata collected from the territory of St. Petersburg and Leningrad Oblast were regularly exposed during the annual autumn mushroom exhibition organized by St. Petersburg Mycological Society (Fig. 2, g). In 2018 the species was included in Red Data Book of St. Petersburg (Arslanov, 2018). In 2018, 2020, and 2021 authors of present paper found new localities of the species in Leningrad Oblast (Kingiseppsky and Luzhsky districts).
In Pskov Oblast the species is included in the Red Data Book (Sudnitsyna, 2014) as protected on the territory of Sebezhsky National Park with reference to Kovalenko et al. (2003). Nevertheless, in the latter paper the species is not mentioned and its’ presence in the protected area is not confirmed. Later it was found in Loknyansky district (Popov et al., 2013), Polistovsky Nature Reserve (Kalinina, 2021) and in the Pushkin Museum-Reserve (Morozova et al., 2022). Noteworthy, that in 2021 large population of A. phalloides was firstly detected in particular site of a forest regularly inspected since 1995.
No information on A. phalloides from Novgorod Oblast is known so far.
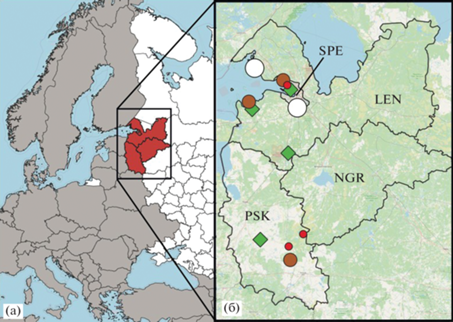
Amanita phalloides in the North-West of European Russia. Here we provide list of known occurrences of the species arranged in chronological order (Fig. 3).
Fig. 3.
Occurrences of Amanita phalloides in the Northwestern European Russia: a – North-West of European Russia (in brown) on the map of Russia; b – Leningrad (LEN), Novgorod (NGR), Pskov (PSK) Oblasts and the city of St. Petersburg (SPE). The white circles represent data from XIX century, brown dots – personal observations, red dots – published data not confirmed by specimens kept in LE and green diamonds represent localities of specimens kept in LE.
St. Petersburg and Leningrad Oblast: Pavlovsk – ?A. citrina; no locality given (Weinmann, 1837).
St. Petersburg: Primorsky district, vicinity of former station “Morskaya”, pine forest, coll. and det. S.N. Arslanov, 30.09.2016, LE 311893, LE 311894; Kurortny district (Arslanov, 2018); Primorsky district, Reserve “Severnoe Poberezhye Nevskoy Guby” (Morozova et al., 2020); Kurortny district, vicinity of Zelenogorsk, 05.09.2020 (A. A. Khovpachev, pers. observ.).
Leningrad Oblast: Vyborgsky District (Thesleff, 1920); Kingiseppsky district, vicinities of Velikino settlement, in oak valley (remnants of manor park) and coniferous-broadleaved forest, coll. and det. D.A. Tomchin, 14.09.2018, LE F-332058, LE 315730; Luzhsky district, Shalovo-Perechitsky protected area, mixed forest with Quercus robur, Tilia cordata and Corylus avellana in undergrowth, coll. and det. D.A. Tomchin, 25.07.2020, LE 312591; Kingissepky district, vicinity of Vistino village, 25.08.2020 (A.A. Khovpachev, pers. observ.); Kingissepky district, Kotelsky protected area, vicinity of Glubokoe lake, mixed forest with Quercus robur, 26.08.2020 (L.Yu. Semyonova, pers. observ.); Kingissepky district, Kurgalsky protected area, mixed forest with Tilia cordata and Corylus avellana in undergrowth, 12.09.2020 (D.A. Tomchin, pers. observ.); Luzhsky district, Cheremenetsky protected area, mixed forest with Corylus avellana in undergrowth, 26.08.2021 (D.A. Tomchin, pers. observ.).
Pskov Oblast: Loknyansky district, vicinity of Bashovo, mixed forest, under oak, 10.08.1998 (Popov et al., 2013); Sebezhsky district – doubtful (Sudnitsyna, 2014); Bezhanitsky district, Polistovsky State Nature Reserve, 17.08.2017 (Kalinina, 2021); Loknyansky district, Polistovsky State Nature Reserve, 04.09.2017 (Kalinina, 2021); Loknyanskiy district, vicinity of Skraby village, under oak, 20.08.2017 (E.S. Popov, pers. observ.); Pushkinogorsky district, museum “Mikhailovskoye”, Mikhaylovskoye manor, on the grass under lime and oaks, coll. and det. O.V. Morozova, 11.09.2018, LE 315731 (Morozova et al., 2022); Loknyansky district, vicinity of Maloye Koskovo village, forest with Quercus robur and Corylus avellana in undergrowth, 14.09.2019 (L.B. Kalinina, pers. observ.); Loknyansky district, Bashovo, forest dominated by Alnus incana with admixture of Quercus robur and Fraxinus excelsior and numerous Corylus avellana in undergrowth, under hazel, 21.08.2021 (E.S. Popov, pers. observ.); Loknyansky district, vicinity of Ko-shnevo village, mixed forest, under oaks, 30.08.2021 (E.S. Popov, pers. observ.).
Phylogenetic analysis. Our findings can be considered as conspecific with specimens from Central Russia, Northern and Western Europe as a common clade is formed on the phylogenetic tree derived from ITS nrDNA sequences (Fig. 4).
DISCUSSION
А. phalloides, a conspicuous and attractive mushroom, is responsible for numerous human poisonings due to presence of amatoxins (Wieland, 1968; Wienland, Faulstich, 1991; Gurevich, Zhurkovich, 1995; Khovpachev et al., 2020) and is considered as one of the most feared fungi (Hyde et al., 2018). The species is native to Europe and widespread. In mycological literature of the XX century, the species was reported also from Asia (Imazeki, Hongo, 1987; Teng, 1996), but recent studies showed that Asian lethal amanitas represent several distinct taxa endemic to East Asia (Zhang et al., 2010; Cui et al., 2018). Outside Europe it is known as invasive from North America (Pringl et al., 2009; Wolfe et al., 2010) and from Australia (Trim et al., 1999). The species is easily exported with its symbionts (Tulloss, 2023), and there is evidence that the species is able to host shift (Berch et al., 2016). Regarding distribution in Russia, the species is common and widespread in the nemoral zone in the European part (Bolshakov et al., 2021). The most frequent habitats are pine and pine-birch forests, oak and coniferous-broadleaved ones are less frequent. In addition, the species is drawn to anthropogenic habitats such as aspen forests, birch stands with grasses, pine plantings (Kiyashko, 2015).
Habitats of our findings can be divided into two groups – old artificial stands with Quercus robur and deciduous or coniferous-broadleaved forest with Quercus robur, Tilia cordata and/or Corylus avellana in undergrowth. Probably, in the artificial stands the species was brought with tree seedlings used for numerous manor parks foundation in XVIII–XIX century. Noteworthy, that during the long-termed survey of genus Amanita in Cyprus, A. phalloides was found only from a Corylus avellana plantation and was absent from native habitats (Loizides et al., 2018). The second group of habitats is presented by deciduous or coniferous-broadleaved forests with Quercus robur, Tilia cordata and/or Corylus avellana in undergrowth. These habitats are not very common and readily accessible, so thorough mycological studies have not been done until recently. Possibly, in such habitats species is native but is not observed. In oak forest situated on the north-west of Izhora Upland Leningrad Oblast (protected area “Oak forests near Velkota village”), Amanita phalloides was not found despite the regular surveys since 2012. Interestingly, that in 2021 large population of A. phalloides was firstly detected in particular site of a forest (Bashovo, Pskov Oblast) regularly inspected since 1995.
The authors are very grateful to O.V. Morozova (BIN RAS, Saint Petersburg) and L.Yu. Semyonova for kindly providing information about the findings of the species. The work was supported by the institutional research project of the Komarov Botanical Institute [“Herbarium collections of the BIN RAS (history, conservation, research and replenishment)”, № 122011900032-7] using the equipment of the Core Facility Centre “Cell and Molecular Technologies in Plant Science” at the Komarov Botanical Institute, RAS (St. Petersburg, Russia).
Список литературы
Arslanov S.N. Amanita phalloides (Vaill. ex Fr.) Link. In: D.V. Geltman (ed.). Red data book of St. Petersburg. Diton, St. Petersburg, 2018. P. 58 (in Russ.).
Berch S.M., Kroeger P., Finston T. The death cap mushroom (Amanita phalloides) moves to a native tree in Victoria, British Columbia. Botany. 2016. V. 95 (4). P. 435–440. https://doi.org/10.1139/cjb-2016-0183
Bolshakov S., Kalinina L., Palomozhnykh E. et al. Agaricoid and boletoid fungi of Russia: the modern country-scale checklist of scientific names based on literature data. Biological Communications. 2021. V. 66 (4). P. 316–325. https://doi.org/10.21638/spbu03.2021.404
Bolton J. An History of Fungusses, growing about Halifax. Huddersfield, 1788.
Bulliard J.B.F. Herbier de la France, ou Collection Complette des Plantes Indigènes de ce Royaume; avec leurs Détails Anatomiques, leurs Propriétés, et leurs Usages en Médecine. Paris, 1780–1798.
Cui Y.-Y., Cai Q., Tang L.-P. et al. The family Amanitaceae: molecular phylogeny, higher-rank taxonomy and the species in China. Fungal Diversity. 2018. V. 91 (1). P. 5–230. https://doi.org/10.1007/s13225-018-0405-9
Curtis W. Flora Londinensis. London, 1775–1798.
Fries E. Systema mycologicum, sistens fungorum ordines, genera et species, hucusque cognitas, quas ad normam naturalis determinavit. Volumen I. Lund, Ex Officina Berlingiana, 1821..
Gardes M., Bruns T.D. ITS primers with enhanced specificity for basidiomycetes – application to the identification of mycorrhizae and rusts. Molec. Ecol. 1993. V. 2 (2). P. 113–118. https://doi.org/10.1111/j.1365-294x.1993.tb00005.x
Gurevich L.S., Zhurkovich I.K. Toxins of some species of the genus Amanita Pers. Mikologiya i fitopatologiya. 1995. V. 29 (1). P. 41–50 (in Russ.).
Hyde K.D., Al-Hatmi A.M.S., Andersen B. et al. The world’s ten most feared fungi. Fungal Diversity. 2018. V. 93 (1). P. 161–194. https://doi.org/10.1007/s13225-018-0413-9
Imazeki R., Hongo T. Colored illustrations of mushrooms of Japan. V. 1. Hoikusha Publishing Co. Ltd., Osaka, 1987.
Kalinina L.B. Agaricoid fungi of Polistovsky Nature Reserve (annotated check-list). Tovarishhestvo nauchnyh izdaniy KMK, Moscow, 2021 (in Russ.).
Katoh K., Rozewicki J., Yamada K.D. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics. 2019. V. 20. P. 1160–1166. https://doi.org/10.1093/bib/bbx108
Khovpachev A.A., Basharin V.A., Chepur S.V. et al. Actual concepts of higher fungi’s toxins: cyclic peptides. Uspekhi sovremennoy biologii. 2020. V. 140 (6). P. 584–600 (in Russ.). https://doi.org/10.31857/S0042132420060058
Kiyashko A.A. A word about toadstools. Planeta gribov. 2015. V. 8. P. 20–25 (in Russ.).
Kovalenko A.E., Kolmakov P.Yu., Morozova O.V. et al. Macromycetes of the Sebezhsky National Park. Mikologiya i fitopatologiya. 2003. V. 37 (5). P. 37–48 (in Russ.).
Loizides M., Bellanger J.-M., Yiangou Y. et al. Preliminary phylogenetic investigations into the genus Amanita (Agaricales) in Cyprus, with a review of previous records and poisoning incidents. Doc. Mycol. 2018. V. 37. P. 201–218.
Morozova O.V., Popov E.S., Kotkova V.M. et al. Fungi of the Pushkinsky Reserve. St. Petersburg, 2022 (in Russ.).
Morozova O.V., Smirnov L.E., Krivosheev S.V. et al. Agaricoid and gasteroid basidiomycetes. In: E.A. Volkova, G.A. Isachenko, V.N. Khramtsov (eds). Nature of the Reserve “Severnoe Poberezhye Nevskoy Guby”. St. Petersburg, 2020. P. 123–138 (in Russ.).
Nees von Esenbeck C.G. Das System der Pilze und Schwämme. Stahelschen Buchhandlung, Würzburg, 1817.
Persoon C.H. Synopsis methodica fungorum. Göttingen, 1801.
Popov E.S., Kovalenko A.E., Gapienko O.S. et al. Mycobiota of the Belarus-Valday Lakeland. Tovarishhestvo nauchnyh izdaniy KMK, Moscow, St. Petersburg, 2013 (in Russ.).
Pringle A., Adams R.I., Cross H.B. et al. The ectomycorrhizal fungus Amanita phalloides was introduced and is expanding its range on the west coast of North America. Molec. Ecol. 2009. V. 18 (5). P. 817–833. https://doi.org/10.1111/j.1365-294X.2008.04030.x
Ratnasingham S., Hebert P.D.N. BOLD: The Barcode of Life Data System (www.barcodinglife.org). Molec. Ecology Notes. 2007. V. 7. P. 355–364. https://doi.org/10.1111/j.1471-8286.2006.01678.x
Schaeffer J.C. Fungorum, qvi in Bavaria et Palatinatu circa Ratisbonam nascuntur. V. 1. Typis Zunkelianis, Ratisbonae, 1762.
Sudnitsyna D.N. Amanita phalloides. In: Red Data book of Pskov Region. Pskov, 2014. P. 251 (in Russ.).
Tamura K., Stecher G., Peterson D. et al. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution. 2012. V. 30 (12). P. 2725–2729. https://doi.org/10.1093/molbev/mst197
Teng S.C. Fungi of China. Mycotaxon Ltd, Ithaca, 1996.
Thesleff A. Studier öfver basidsvampfloran i sydöstra Finland med hänsyn till dess sammansättning, fysiognomi, fenologi och ekologi. Bidrag till Kännedom av Finlands Natur och Folk. 1920. V. 79 (1). P. 1–140.
Trifinopoulos J., Nguyen L.T., von Haeseler A. et al. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Research. 2016. V. 44. P. W232–W235. https://doi.org/10.1093/nar/gkw256
Trim G.M., McKeown R.V., Couteur D.G.L. et al. Poisoning by Amanita phalloides (“deathcap”) mushrooms in the Australian Capital Territory. Medical J. Australia. 1999. V. 171 (5). P. 247–249. https://doi.org/10.5694/j.1326-5377.1999.tb123631.x
Tulloss R.E., Possiel L. Amanita phalloides. Amanitaceae studies. http://www.amanitaceae.org/?Amanita%20phalloides. Accessed 31.01.2023.
Vahl M. Flora Danica. V. 7, fasc. 21. 1799.
Vaillant S. Botanicon Parisiense. Jean et Herman Verbeek et Balthazar Lakeman, Leiden, Amsterdam, 1727.
Weinmann J.A. Enumeratio Fungorum in agro Pawlowskiensi praecipue crescentium. In: Sylloge plantarum novarum itemque minus cognitarum. A praestantissimis botanicis adhuc viventibus collecta et a Societate regia botanica Ratisbonensi edita. 1828. V. 2. P. 82–118.
Weinmann J.A. Enumeratio stirpium in agro Petropolitano sponte crescentium, secundum systema sexuale linneanum composita. Petropoli, 1837.
Wieland T. Poisonous principles of mushrooms of the genus Amanita. Science. 1968. V. 159 (3812). P. 946–952. https://doi.org/10.1126/science.159.3818.946
Wieland T., Faulstich H. Fifty years of amanitin. Experientia. 1999. V. 47 (11). P. 1186–1193. https://doi.org/10.1007/BF01918382
Willdenow C.L. Florae berolinensis prodromus secundum systema Linneanum ab illustr. viro ac Eq. C.P. Thunbergio emendatum conscriptus, Berlin, 1787.
Wolfe B.E., Richard F., Cross H.B. et al. Distribution and abundance of the introduced ectomycorrhizal fungus Amanita phalloides in North America. New Phytologist. 2010. V. 185 (3). P. 803–816. https://doi.org/10.1111/j.1469-8137.2009.03097.x
Zhang P., Chen Z.H., Xiao B. et al. Lethal amanitas of East Asia characterized by morphological and molecular data. Fungal Diversity. 2010. V. 42 (1). P. 119–133. https://doi.org/10.1007/s13225-010-0018-4
Арсланов С.Н. (Arslanov) Бледная поганка Amanita phalloides (Vaill. ex Fr.) Link. // Красная книга Санкт-Петербурга / ред. Д.В. Гельтман. СПб.: Дитон, 2018. С. 58.
Гуревич Л.С., Журкович И.К. (Gurevich, Zhurkovich) Токсины некоторых видов рода Amanita Pers. // Микология и фитопатология. 1995. Т. 29. № 1. С. 41–50.
Калинина Л.Б. (Kalinina) Агарикоидные грибы Полистовского заповедника (Аннотированный список видов). Москва: Товарищество научных изданий КМК, 2021. 58 с.
Ховпачев А.А., Башарин В.А., Чепур С.В. и др. (Khovpachev et al.) Современные представления о токсинах высших грибов: циклические пептиды // Успехи современной биологии. 2020. Т. 140. № 6. С. 584–600.
Кияшко А.А. (Kiyashko) Слово о поганках // Планета грибов. 2015. Т. 8. С. 20–25.
Коваленко А.Е., Колмаков П.Ю., Морозова О.В. и др. (Kovalenko et al.) Макромицеты национального парка “Себежский” // Микология и фитопатология. 2003. Т. 37. № 5. С. 37–48.
Морозова О.В., Попов Е.С., Коткова В.М. и др. (Morozova et al.) Грибы Пушкинского Заповедника. СПб.: Марафон, 2022. 128 с.
Морозова О.В., Смирнов Л.Э., Кривошеев С.В. и др. (Morozova et al.) Агарикоидные и гастероидные базидиомицеты // Е.А. Волкова, Г.А. Исаченко, В.Н. Храмцов (ред.). Природа заказника “Северное побережье Невской губы”. СПб., 2020. С. 123–138.
Попов Е.С., Коваленко А.Е., Гапиенко О.С. и др. (Popov et al.) Микобиота Белорусско-Валдайского поозерья. М.; СПб.: Товарищество научных изданий КМК, 2013. 399 с.
Судницына Д.Н. (Sudnitsyna) Бледная поганка // Красная книга Псковской области. Псков, 2014. С. 251.
Дополнительные материалы отсутствуют.
Инструменты
Микология и фитопатология