Молекулярная биология, 2020, T. 54, № 6, стр. 939-954
Клеточная заместительная терапия при болезни Паркинсона – история развития и перспективы использования в клинической практике
Н. В. Католикова a, b, *, А. Б. Малашичева b, c, Р. Р. Гайнетдинов a, d
a Институт трансляционной биомедицины Санкт-Петербургского государственного университета
199034 Санкт-Петербург, Россия
b Институт цитологии Российской академии наук
194064 Санкт-Петербург, Россия
c Национальный медицинский исследовательский центр им. В.А. Алмазова Министерства здравоохранения Российской Федерации
197341 Санкт-Петербург, Россия
d Клиника высоких медицинских технологий им. Н.И. Пирогова Санкт-Петербургского государственного университета
199034 Санкт-Петербург, Россия
* E-mail: nkatolikova@yandex.ru
Поступила в редакцию 23.04.2020
После доработки 16.06.2020
Принята к публикации 25.06.2020
Аннотация
Болезнь Паркинсона – широко распространенное нейродегенеративное заболевание, которое характеризуется гибелью дофаминергических нейронов в черной субстанции среднего мозга. Клинически болезнь проявляется тремором, брадикинезией, ригидностью мышц и другими моторными и не моторными симптомами, которые в конечном итоге приводят к инвалидизации пациентов. На сегодняшний день существуют только симптоматические варианты лечения болезни Паркинсона, поэтому поиск новых подходов рассматривается как одно из важнейших направлений терапии этого заболевания. Идея о возможности применения клеточной заместительной терапии, основанная на локальном характере и специфичности повреждения конкретного типа нейронов при болезни Паркинсона, появилась еще в 1970-х годах. Выбор источника клеток, метода и места их введения, показания к проведению данной операции и особенности ведения пациентов прошли длительный путь развития. К настоящему моменту эффективность клеточной заместительной терапии подтверждена целым рядом исследований на модельных животных. Уже начаты клинические испытания, в ближайшее время планируется проведение еще нескольких. В представленном обзоре описаны основные предпосылки к применению клеточной заместительной терапии при болезни Паркинсона, этапы развития этого метода и клинические испытания, начавшиеся в последние несколько лет.
БОЛЕЗНЬ ПАРКИНСОНА
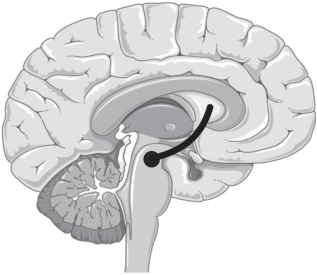
Болезнь Паркинсона (БП) – распространенное нейродегенеративное заболевание, частота которого, по данным Американской ассоциации БП, составляет 1–2% среди людей старше 60 лет и 3–5% у лиц старше 85 лет (https://www.apdaparkinson.org). Основной клинической триадой болезни являются тремор, ригидность и брадикинезия. Помимо этого, у больных наблюдаются апатия, когнитивная дисфункция, снижение артериального давления, сексуальные проблемы, нарушения мочеиспускания и другие проявления, которые в значительной степени влияют на качество жизни [1]. Основной причиной заболевания считается гибель дофаминергических нейронов черной субстанции среднего мозга (рис. 1), приводящая к значимому снижению уровня дофамина в полосатом теле.
Рис. 1.
Нигростриарный путь, представленный в основном аксонами дофаминергических нейронов, соединяющий черную субстанцию (substantia nigra) среднего мозга и полосатое тело (стриатум).
Основной препарат, применяемый в настоящее время при БП – леводопа (L-DOPA), предшественник дофамина. В организме леводопа метаболизируется декарбоксилазой ароматических аминокислот (L-AADC) до дофамина, что приводит к восстановлению уровня дофамина в стриатуме. К сожалению, данный вариант лечения имеет много недостатков. Так, постепенно развивается нечувствительность к препарату, что делает необходимым увеличение дозы препарата. Кроме того, на фоне приема леводопы возникают побочные эффекты, один из которых – ярко выраженные леводопа-индуцированные дискинезии, возникающие либо сразу после приема препарата, либо при снижении его концентрации в крови. Симптоматическими являются и другие варианты медикаментозного лечения, в том числе агонистами дофаминергических рецепторов, антихолинергическими препаратами, ингибиторами катехол-О-метилтрансферазы (COMT) и моноаминоксидазы (MAO), антагонистами аденозиновых рецепторов и другими, которые также только частично снижают проявления болезни. Хирургический метод лечения – глубокая стимуляция мозга (deep brain stimulation (DBS)) – показан только при тяжелых моторных нарушениях и имеет много ограничений. Поэтому поиск новых подходов к лечению БП представляется одним из важнейших направлений.
Одним из новых подходов, потенциально эффективных при нейродегенеративных заболеваниях, стала клеточная заместительная терапия.
МЕТОД КЛЕТОЧНОЙ ЗАМЕСТИТЕЛЬНОЙ ТЕРАПИИ: ИСТОРИЯ ПОЯВЛЕНИЯ И РАЗВИТИЯ
Модели болезни Паркинсона
Интерес к возможности использования клеточной заместительной терапии при БП появился еще в конце 1970-х годов одновременно с разработкой первых животных моделей, в которых гибель дофаминергических нейронов индуцировали введением токсинов [2]. На сегодняшний день чаще всего применяют две классические модели БП на животных: 1) 6-гидроксидофаминовую (6-OHDA model), основанную на уни- или билатеральном введении 6-гидроксидофамина (6-hydroxydopamine, 6-OHDA) в стриатум, нигростриарный путь или черную субстанцию; и 2) модель с введением протоксина МФТП (1-метил-4-фенил-1,2,3,6-тетрагидропиридин, MPTP model).
При введении в мозг 6-OHDA попадает селективно в дофаминергические нейроны, поскольку он проникает в клетки при помощи обратного захвата дофамина. На грызунах чаще всего используется унилатеральное введение 6-OHDA, при котором происходит гибель дофаминергических нейронов только с стороны введения токсина. При введении животным веществ, стимулирующих дофаминергическую систему, таких как амфетамин или апоморфин, появляется характерная моторная асимметрия, которая выражается в усиленном вращении в одну сторону [3].
6-OHDA-модель позволяет не только быстро и достаточно точно определить степень гибели дофаминергических нейронов, но и наблюдать за динамикой процесса на фоне проводимого лечения. Появление 6-OHDA-модели значительно расширило возможности изучения БП и разработки новых вариантов лечения [4].
Первые трансплантации нейронов пациентам с болезнью Паркинсона
К моменту публикации первых работ с описанием 6-OHDA-модели уже были проведены эксперименты, подтверждавшие возможность трансплантации нейронов в мозг [5, 6]. В работах, опубликованных в конце 70-х–начале 80-х гг. [7, 8], источником трансплантируемых нейронов служили графты, полученные из вентральной части среднего мозга плода (ВЧС графты, fetal ventral midbrain graft, fVM). Сначала графты подсаживали целиком в полость, которую формировали хирургическим путем. Но вскоре стало понятно, что этот подход не очень эффективен и достаточно травматичен, поэтому потребовался способ создания суспензии клеток и ее введения в мозг [8, 9].
Результаты, полученные с использованием стереотаксического введения диссоциированной суспензии клеток, оказались достаточно обнадеживающими [10]. Удалось показать, что ВЧС графты крысы, трансплантированные в стриатум модельных 6-OHDA-крыс, выживают, прорастают в стриатум и снижают моторную асимметрию, индуцированную введением амфетамина. Кроме того, трансплантация ВЧС графтов даже в латеральные желудочки головного мозга крысы [11] приводила к прорастанию графтов в стриатум и снижению вращения, вызванного введением амфетамина.
Первые попытки применения клеточной заместительной терапии при БП были сделаны в 1982–1985 гг. в Швеции, но они не были связаны с ВЧС графтами. В каждом из двух проведенных исследований двум пациентам с БП в структуры базальных ганглиев головного мозга трансплантировали фрагменты собственных надпочечников [12, 13]. Этот подход опирался на ранее проведенные на животных исследования, которые показали, что введение ткани, вырабатывающей катехоламины, оказывает положительный эффект, но у людей ожидаемый результат не был получен. Однако в этих исследованиях разработаны более аккуратные тесты для оценки малых и локальных изменений моторных функций, что позволило более точно проследить динамику изменений. Кроме того, определены наиболее подходящие точки для введения клеток, показана наибольшая эффективность при трансплантации суспензии клеток в путамен.
Первые работы по введению клеточной суспензии ВЧС человека провели на 6-OHDA-моделях крыс [14, 15]. Суспензию клеток ВЧС человека получали из абортивного материал (стадия развития 6–9 недель). Помимо подтверждения эффективности данного метода с использованием клеток человека выявили маркеры, позволяющие наиболее эффективно выделять из эмбрионального мозга клетки, содержащие дофамин.
Первые клинические испытания
Исходя из результатов предыдущих исследований [14], в марте 1986 года Шведское общество медицины (Swedish Society of Medicine (SLS)) одобрило протокол клинических испытаний с использованием ВЧС графтов человека при БП. Одной из важных составляющих этого протокола стала разработка методов оценки состояния имплантированных нейронов с использованием позитронно-эмиссионной томографии (ПЭТ) с 18F-фтор-DOPA. Первые две операции по этому протоколу выполнены в конце 1987 года. Двум пациентам ввели суспензии ВЧС человека, полученные в каждом случае от четырех эмбрионов. Клетки вводили унилатерально в две зоны в путамен и в одну зону в области головы хвостатого ядра [16]. После публикации данных, полученных в ходе первых операций, были прооперированы еще два пациента, но с использованием несколько измененного протокола: увеличением количества точек введения клеточной суспензии (до трех в путамен) и уменьшением диаметра канюли, с помощью которой вводили клетки. Показано, что клетки ВЧС выживают в мозге взрослого человека и снижают клинические проявления БП [17]. У одного из пациентов эффект был настолько выражен, что это позволило отменить терапию леводопа, которую он получал до операции; при этом необходимость вернуться к поддерживающим дозам леводопа на фоне прогрессирования симптомов появилась только через 6 лет. Исследование мозга этого пациента методом ПЭТ с 11С-раклопридом, проведенное через 9 лет после операции, показало, что введенные клетки все еще функционировали, и спонтанный, и индуцированный выброс дофамина на стороне введения был восстановлен до нормального уровня, в то время как на противоположной стороне выброс дофамина составил примерно 10% от нормальных значений [18].
Один из пациентов с наиболее выраженным эффектом от операции умер естественной смертью через 24 г. после трансплантации. Гистологическое исследование его мозга показало, что графты выжили во всех точках введения и успешно встроились в окружающую систему. Вокруг графтов не выявлено какой-либо иммунной реакции, однако 11–12% клеток графтов содержали тельца Леви с альфа-синуклеином и убиквитином, что указывало на постепенное распространение патологического процесса и на клетки графтов [19]. Помимо этого, в лимбической системе и неокортексе обнаружены тельца Леви и атрофические изменения. В последние годы жизни пациент страдал выраженной деменцией, а эффект от введения графтов практически исчез через 14 лет после операции.
После успешных операций, проведенных в 1989 году в Лунде, по этому протоколу прооперировали еще 18 пациентов. К сожалению, результат операций не был стабильным – у некоторых пациентов наблюдалось значительное улучшение состояния, однако у других не удалось добиться ожидаемого результата.
В середине 90-х годов под эгидой Национального Института Здоровья США (National Institute of Health (NIH)) были проведены два новых клинических испытания с использованием ВЧС графтов человека. Одно – на базе больницы Университета Колорадо (University of Colorado Hospital (UCH), Colorado), в другом, мультицентровом, участвовали Школа медицины горы Синай (Mount Sinai School of Medicine, New York), Пресвитерианский медицинский центр Раш (Rush Presbyterian Medical Center, Chicago), Университет Южной Флориды (University of South Florida, Tampa, Florida) и Тихоокеанский центр исследования БП Университета Британской Колумбии (Pacific Parkinson’s Research Centre, University of British Columbia, Vancouver) [20, 21]. В исследованиях участвовали 40 и 34 пациента соответственно. Протокол был выполнен по принципу двойных слепых исследований с отрицательным контролем в виде шрама. Несмотря на ожидания, в этих исследованиях не удалось добиться значительного улучшения состояния. Кроме того, у части пациентов, которым выполнили трансплантацию, появилась выраженная дискинезия, получившая название “дискинезия, индуцированная графтом” (graft induced dyskinesia (GID)). Эти результаты привели к серьезному снижению интереса к разработке клеточной заместительной терапии БП. На сегодняшний день, когда уже виден результат проведенных работ, мнение ученых о причинах неудачной трансплантации сводится к техническим недоработкам: не отработаны полноценно протоколы выделения и культивирования клеток. Оставались открытыми вопросы техники, а также точки введения клеток, трансплантацию выполняли пациентам с крайне тяжелым течением БП, часть из которых уже подверглась другому варианту хирургического лечения БП, а именно, глубокой стимуляции мозга. Иммуносупрессивная терапия после оперативного вмешательства была недостаточно продолжительной, не были полностью отработаны сроки проведения контрольных исследований. Но к моменту окончания исследований интерес к разработке клеточной заместительной терапии БП на основе ВЧС резко упал.
В последующие несколько лет были сделаны новые попытки использования для клеточной заместительной терапии различных тканей, содержащих дофамин, в том числе аутологичных каротидных телец [22], пигментного эпителия сетчатки [23]. Но в перечисленных исследованиях не выявлен какой-либо эффект от введения указанных выше типов клеток – графты не оказывали значительного эффекта на клинические проявления болезни, не обнаружено статистически значимых отличий между опытной и контрольной группами при оценке по шкалам проявления болезни, а также по качеству жизни пациентов.
TRANSEURO
В середине 2000-х, оценив результаты уже проведенных исследований [24], несколько групп ученых приняли решение о необходимости объединения усилий. Проведена серия встреч и обсуждений, во время которых проанализировали все факторы, способные влиять на эффект трансплантационной терапии. Показано, что помимо уже перечисленных факторов, важное значение для эффективности трансплантационной терапии при БП имеет возраст пациентов. Более ранний возраст и более раннюю стадию болезни, при которой еще не произошло значительного снижения количества связей с вентральным стриатумом, расценивали как более благоприятные и перспективные для проведения операции. Важное значение отведено ответу на терапию леводопа – чем лучше пациент реагировал на начало терапии, тем более хорошие результаты можно было ожидать от клеточной заместительной терапии. Важной особенностью также признали клинические особенности течения заболевания – клеточная заместительная терапия потенциально более эффективна при выраженных моторных нарушениях и может быть почти неэффективной при когнитивных изменениях.
На основе этих встреч в 2006 г. был разработан протокол для нового Европейского открытого слепого исследования по применению клеточной заместительной терапии при спорадической БП, который получил название TRANSEURO [25]. Эти исследования продолжаются до сих пор и в них принимают участие Больница Адденбрука (Кембридж, Великобритания), Имперский колледж (Лондон, Великобритания), Национальная неврологическая и нейрохирургическая больница (Лондон), Университет Кардиффа (Кардифф, Великобритания), Университетская больница Сконе (Лунд, Швеция), Фрайбургский университет (Фрайбург, Германия), при участии Государственной больницы Парижа (Франция). В 2010 г. в качестве источника клеток для исследования были выбраны ВЧС графты человека, поскольку в то время именно их считали наиболее перспективным источником клеток.
Протокол TRANSUERO разделен на несколько временны́х отрезков: 1) с 2010 по 2013 г. происходил отбор пациентов, которые потенциально могли участвовать в исследовании; 2) с 2015 по 2018 г. проведены все операции по трансплантации; 3) окончательные результаты исследования должны быть опубликованы в 2021 г. Однако предварительные результаты и подробные протоколы этого исследования уже опубликованы, что может иметь важное значение для новых клинических испытаний, которые уже начались или будут начаты в ближайшее время.
Определены критерии включения/не включения пациентов в исследование, основной целью которых было максимальное снижение риска побочных эффектов от процедуры трансплантации и максимальная вероятность получения положительного результата от хирургического вмешательства по сравнению с другими методами. Критерии включения/не включения пациентов в TRANSEURO приведены в табл. 1 [25].
Таблица 1.
Критерии включения и не включения пациентов в исследования TRANSEURO*
| Критерии включения пациентов в исследования | Критерии не включения пациентов в исследования |
|---|---|
| • Диагноз БП поставлен в соответствии с критериями Queen Square Brain Bank (QSBB) | • Атипичный паркинсонизм, включая паттерны 18F-DOPA на ПЭТ, соответствующие этому |
| • Длительность заболевания ≥2 и ≤13 лет | • Значения по краткой шкале оценки психического состояния (Mini-mental State Examination, (MMSE)) <24 (<26 для тех, кому будет проведена трансплантация) или свидетельство деменции с использованием критериев диагностического и статистического руководства по психическим расстройствам IV издания (Diagnostic and Statistical Manual of mental disorders IV (DSM-IV)) |
| • Возраст пациентов ≥30 и ≤68 лет во время трансплантации | • Отсутствие возможности аккуратно и точно скопировать два взаимосвязанных пятиугольника и семантическая вербальная беглость <20 в течение 90 с |
| • Стадия 2 или меньше по шкале Hoehn, Yahr на фоне приема лекарств | • Серьезное сопутствующее соматическое или психическое заболевание, включая депрессию и психоз |
| • Без медикаментозной терапии или только стандартная терапия БП | • Любое заболевание, требующее терапии нейролептиками |
| • Без значимой леводопа-индуцированной дискинезии | • Значительная индуцированная леводопа-дискинезия (>2 для любой части тела по шкале оценки аномальных непроизвольных движений (Abnormal Involuntary Movement Scale (AIMS)) |
| • Значительное улучшение ≥33% по III части унифицированной шкалы оценки БП Международного общества расстройств движений (UPDRS) в ответ на острое введение леводопа при переходе с безмедикаментозного этапа на медикаментозный | • Предшествующее нейрохирургическое вмешательство |
| • Наличие сигнала 18F-DOPA в вентральном стриатуме | • Невозможность проведения МРТ |
| • Клинически незначимый ответ на прием леводопа | |
| • Любое противопоказание к иммуносупрессивной терапии | |
| • Прием антикоагулянтов | |
| • Пациенты, использующие преимущественно левую руку (левши) |
Разработаны стандартные операционные процедуры (СОП) подготовки клеток для трансплантации. ВЧС выделяли из абортивного материала (от 6 до 8 нед. беременности). Диссекцию и подготовку клеток проводили по стандартам надлежащей производственной практики (good manufacturing practice (GMP)) (в Великобритании) или просто в стерильных условиях (в Швеции). Стандарты надлежащей производственной практики – это свод требований к организации производства и контролю качества лекарственных средств для медицинского и ветеринарного применения. Клетки могут сохраняться в условиях in vitro не более 4 дней. Выживаемость клеток перед проведением хирургического вмешательства должна превышать 80%. Критическим фактором является количество трансплантируемых клеток. Как показано ранее [19, 26], минимально достаточное количество клеток, которое нужно ввести в путамен с одной стороны, равно 100 000 – для получения этого количества клеток необходимы как минимум три эмбриона.
Оперативное вмешательство проводили отдельно на каждой стороне мозга. Интервал между вмешательствами варьировал от 4 до 35 нед. Протокол разработан на основе данных по наилучшей приживаемости введенных клеток [19, 27]. Процедуру выполняли под общей анестезией. Под стереотаксическим контролем в одном полушарии формировали пять трактов для введения клеток: два в прекомиссуральную область, три – в посткомиссуральную область путамена. По ходу каждого тракта сделано восемь точек с введением по 2.5 мкл суспензии клеток в каждой. Общий объем введения – 100 мкл (около 100 000 клеток). Клетки вводили при помощи инструмента Rehcrona, специально разработанного с этой целью в университете Лунда в 1980–1990-е годы.
Сопутствующая иммуносупрессивная терапия, разработанная на основе предыдущего опыта, включала три препарата и продолжалась в течение 12 мес. Помимо иммуносупрессивной терапии и стандартных препаратов, снижающих ее побочные эффекты (омепразол и алендроновая кислота), пациенты после операции получали курс антибиотикотерапии.
На протяжении всего исследования, т.е. во время включения пациентов в группу, непосредственно перед трансплантацией и в точке, выбранной для оценки результатов, проводили тесты для оценки стадии болезни (перечислены выше в критериях включения/не включения), МРТ и ПЭТ-сканирование с использованием двух меток – 18F-DOPA (для оценки содержания дофамина), 11С-PE2I (для оценки экспрессии переносчика дофамина (DAT)) и 11С-DASB (для оценки количества серотонинергических нейронов). Последнее проводили только после трансплантации, поскольку одной из возможных причин индуцированных графтом дискинезий, наблюдаемых во время первых клинических испытаний с использованием ВЧС графтов, считаются именно серотонинергические нейроны, которые могут содержаться в графте. Поскольку введенные клетки постепенно встраиваются в существующую систему, и этот процесс занимает от 3 до 5 лет [27], первой точкой для оценки эффективности трансплантации выбрали 36 мес.
К моменту написания нашего обзора клинические испытания TRANSEURO еще не закончились, но уже сейчас можно утверждать, что они стали важным этапом разработки клеточной заместительной терапии при БП. В эти исследования первоначально включили 150 пациентов, но в итоге в течение трехлетнего периода, когда проводились трансплантации, подсадку графтов выполнили только 21 раз (т.е. 10 пациентам операция выполнена с двух сторон, а один пациент прооперирован унилатерально). Столь низкое число операций связано в основном со сложностями получения материала для графтов. И это главная причина, из-за которой ВЧС графты не будут использоваться для лечения пациентов с БП. Однако результаты предыдущих исследований [27] и данные TRANSEURO показывают, что экзогенные дофаминергические нейроны могут выживать, встраиваться и снижать клинические проявления БП, поэтому необходимо искать другой источник материала для клеточной заместительной терапии. Кроме того, во время этих исследований были тщательно продуманы протоколы по отбору пациентов. И все пациенты, выбранные для данного исследования, смогут принять участие в следующих испытаниях (см. ниже). Также показано, что ПЭТ-сканирование с меткой 11C-PE2I имеет большую чувствительность и прогностическую значимость для выявления моторных нарушений, чем сканирование с меткой 18F-DOPA [25]. Постоянное наблюдение за пациентами позволило оценить динамику прогрессирования болезни в течение 10 лет. Также разработали рекомендации по протоколам иммуносупрессивной терапии, хотя необходимо подчеркнуть, что в эти протоколы могут быть внесены изменения, поскольку спектр антигенов, представленных на поверхности ВЧС графтов, может значительно отличаться от антигенов на поверхности нейронов, полученных из плюрипотентных клеток.
ПЛЮРИПОТЕНТНЫЕ СТВОЛОВЫЕ КЛЕТКИ
Плюрипотентные стволовые клетки (ПСК) могут использоваться для получения любого типа клеток. Культуры плюрипотентных клеток человека в настоящий момент представлены: 1) эмбриональными стволовыми клетками человека (ЭСК), которые получают из эмбрионов на стадии бластоцисты [28, 29]; 2) индуцированными ПСК (иПСК), полученными путем репрограммирования соматических клеток [30, 31]. Первые иПСК получены из фибробластов кожи [30]. Для получения иПСК чаще всего используют мононуклеары периферической крови или пуповинную кровь [32]. ПСК могут дифференцироваться в клетки всех трех типов зародышевых листков – энтодерму, мезодерму, эктодерму.
Первые работы по нейрональной дифференцировке ПСК выполнены на линиях мышей [33, 34]. Оказалось, что в условиях in vitro эти клетки могут дифференцироваться в зрелые нейроны и глиальные клетки. Как показано чуть позже, ЭСК человека, как и ЭСК мыши, могут дифференцироваться в нейрональном направлении [35, 36].
Первые протоколы по дифференцировке ЭСК основаны на спонтанной дифференцировке в условиях длительного культивирования культуры с повышенной плотностью клеток [37]. Такие условия приводили к формированию смешанной культуры, состоящей из предшественников различных типов клеток. Дальнейшее выделение и культивирование нейроэктодермальных предшественников позволяло получить зрелые нейроны, синтезирующие белок 2, ассоциированный с микротрубочками (MAP2), β3-тубулин, синаптофизин и белок нейрофиламентов 160 кДа, а также продуцирующие глутамат и содержащие ключевые ферменты синтеза γ-аминомасляной кислоты. Другим вариантом дифференцировки стало использование химически модифицированной среды с добавлением таких факторов, как активин А, морфогенетические белки кости-2, -4, -6, -7 (BMP-2, -4, -6, -7), фолистатин [38]. Кроме того, для получения нейрональных культур из ЭСК использовали индукцию ретиноевой кислотой [34].
Одним из классических протоколов дифференцировки ЭСК в нейрональном направлении в условиях монослоя стал так называемый протокол Смита [39]. Дифференцировка ЭСК мыши в этом случае проходит в условиях адгезии на чашках, покрытых желатином.
Еще один подход к дифференцировке ЭСК – их рассев в неадегезивных условиях, что приводит к формированию эмбриоидных телец [34]. Эмбриоидные тельца могут дать начало производным всех трех зародышевых листков [40], однако можно влиять на направление их дифференцировки. Несмотря на эффективность дифференцировки клеток через формирование эмбриоидных телец, одну из основных проблем использования данного метода представляет их полиморфность и, как следствие, неоднородность получаемой культуры клеток. С целью решения этой проблемы разработан метод культивирования эмбриоидных телец в висячих каплях [41]. Этот метод позволяет предотвратить слипание нескольких эмбриоидных телец в единый конгломерат, что обеспечивает его поддержание в более униморфном состоянии. Но даже униморфные эмбриоидные тельца имеют свои особенности, так как клетки в них находятся в разном положении и разной доступности для питательных веществ и ростовых факторов, так что получаемая в результате дифференцировки клеточная культура в любом случае неоднородна по своему составу.
В 2009 году опубликован протокол нейрональной индукции, основанный на ингибировании BMP и TGF-β [42]. Метод, описанный в этом протоколе, получил название двойное ингибирование SMAD (dual SMAD inhibition), поскольку сигнальные каскады от BMP и TGF-β идут через систему белков SMAD. Изначально участие ингибиторов BMP в нейрональном развитии показали в экспериментах на лягушках [43–45]. Позже рекомбинантный белок Noggin использовали в нескольких протоколах нейрональной дифференцировки ЭСК человека [46]. Сигнальные молекулы BMP и TGF-β принадлежат к суперсемейству белков TGF-β и имеют свои специфические рецепторы. Выделяют два типа рецепторов семейства TGF-β: I и II. Все они представляют собой рецепторные серин-треонин-специфичные протеинкиназы. У человека выделены семь рецепторов типа I и пять рецепторов типа II. Активация этих рецепторов приводит к формированию комплексов белков SMAD, которые затем транслоцируются в ядро и регулируют экспрессию генов. Классическим ингибитором сигналов BMP является Noggin. Выделен ингибитор TGF-β – SB431542. Показано, что ингибирование сигнализации SMAD сочетанием Noggin и SB431542 крайне эффективно для нейрональной дифференцировки ЭСК человека [42]. Согласно [42], более 80% клеток на 7-й день дифференцировки накапливали Pax6 (ранний маркер эктодермальной дифференцировки). Помимо запуска нейрональной дифференцировки и супрессии генов плюрипотентности, таких как Nanog, Noggin препятствует дифференцировке в сторону энтодермы за счет ингибирования Sox17, а SB431542 ингибирует Brachyury (маркер мезодермальной дифференцировки) [42]. Таким образом, двойное ингибирование SMAD крайне эффективно для дифференцировки в нейрональном направлении и по гомогенности результата превосходит такие методы дифференцировки, как протокол Смита или протоколы с использованием эмбриоидных телец. В настоящий момент двойное ингибирование SMAD применяют в большинстве протоколов получения нейронов.
Процесс дифференцировки клеток регулируется очень сложно. Важную роль в нейрональной дифференцировке в направлении дофаминергических предшественников играют Sonic hedghog (SHH) [47, 48], сигнальный каскад Wnt [49], белки семейства FGF [47] и TGF-β [46, 50], а также сигнальный каскад Notch [51]. Выявлено участие таких факторов, как Lmx1b, Еngrailed-1 (En1), Nurr1 и Pitx3 [52–54], которые экспрессируются на разных этапах развития дофаминергических нейронов. Однако эти факторы участвуют в поздних этапах эмбрионального развития дофаминергических нейронов, поэтому в условиях in vitro они способствуют именно созреванию, а не спецификации дофаминергических нейронов [55].
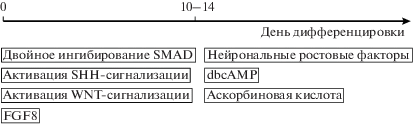
Современные методы получения дофаминергических нейронов из плюрипотентных клеток включают несколько стадий, которые могут несколько различаться в разных протоколах. Но общим принципом всегда остается первоначальная нейрональная дифференцировка на основе ингибирования сигнальных каскадов BMP и TFG/Activin/Nodal со спецификацией в вентральном направлении за счет активации SHH-сигнализации и в каудальном направлении путем воздействия на WNT-сигналы и активацию FGF, с дальнейшим созреванием полученных нейрональных прогениторов в присутствии нейрональных факторов роста BDNF и GDNF (рис. 2).
Разработка относительно быстрых и эффективных способов получения зрелых дофаминергических нейронов и их предшественников из плюрипотентных клеток привела к проведению расширенных доклинических испытаний, разработке протоколов, соответствующих стандартам GMP, масштабному производству клеток и началу новых клинических испытаний [56].
ПРЯМОЕ РЕПРОГРАММИРОВАНИЕ
Открытие репрограммирования и получение иПСК [30] стимулировало изучение возможностей изменения дифференцированного состояния клеток. В 2010 г. опубликован метод прямого репрограммирования [57], который дает возможность получать дифференцированные клетки одного типа из клеток других типов без стадии плюрипотентности. Одними из первых были разработаны протоколы прямого репрограммирования для получения дофаминергических нейронов [58, 59]. Полученные таким образом нейроны мыши обладают всеми свойствами взрослых дофаминергических нейронов: имеют сходные морфологические характеристики, синтезируют специфичные белки (TH, VMAT, Pitx3), обладают электрической активностью, способны образовывать синапсы, а также продуцировать дофамин и выделять его в синаптическую щель [60, 61]. Они способны эффективно встраиваться в структуры головного мозга модельных животных и снижать клинические проявления БП.
Несмотря на первоначальный интерес, метод прямого репрограммирования применяется не очень широко. Причиной этого стали, во-первых, изначально низкая эффективность этого метода, что в дальнейшем удалось исправить, а, во-вторых, сложность воспроизведения in vivo результатов, полученных в условиях in vitro. Учитывая все сказанное, в настоящее время предпочтение отдается развитию методов дифференцировки для создания пула клеток, которые могут использоваться в клеточной заместительной терапии. Однако метод прямого репрограммирования имеет свои преимущества, в частности, не содержит стадии плюрипотентности клеток, что делает его интересным для дальнейших исследований и разработок.
СОВРЕМЕННЫЕ КЛИНИЧЕСКИЕ ИСПЫТАНИЯ
Общая схема применения плюрипотентных клеток для клеточной заместительной терапии БП представлена на рис. 3.
Рис. 3.
Применение плюрипотентных стволовых клеток для заместительной клеточной терапии болезни Паркинсона.
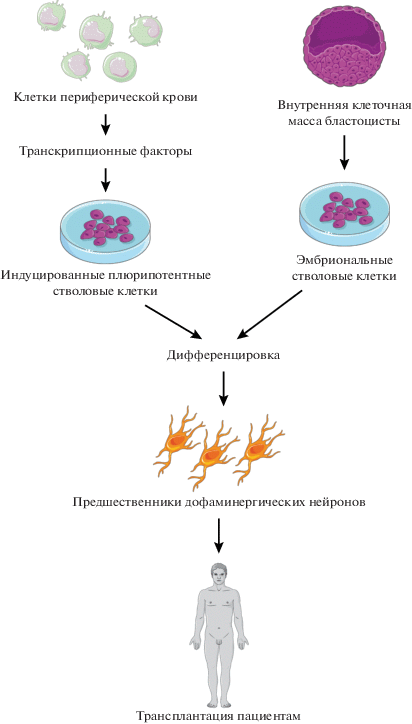
Первые клинические испытания, в которых использовали плюрипотентные клетки и их дифференцированные производные, начались в 2016 г. International Stem Cell Corporation (ISCO) в Мельбурне (ClinicalTrials.gov NCT02452723). Для заместительной терапии в них использовали партеногенетические нейрональные стволовые клетки человека (клеточная линия ISC-hpNSC), которые вводили билатерально в хвостатое ядро, путамен и черную субстанцию. Партеногенетические стволовые клетки человека, по своим характеристикам очень похожие на ЭСК, получают путем химической индукции неоплодотворенной яйцеклетки [62]. Они способны дифференцироваться в зрелые дофаминергические нейроны или их предшественники, эффективно снижают проявления БП, смоделированной на грызунах и приматах [63–65]. Согласно опубликованному протоколу в этих испытаниях, начатых в 2016 г., приняли участие 12 пациентов, а последние данные собраны в июне 2020 г.
Следующие клинические испытания (ClinicalTrials.gov NCT03119636) были начаты в Чжэнчжоу, Китай в 2017 году [66, 67] под руководством Qi Zhou. В этом исследовании для трансплантации должны были использовать нейрональные прогениторы, полученные из ЭСК человека, которые планировалось ввести 50 пациентам. Эти клинические испытания были начаты на основании неопубликованных данных [68]. Однако уже после начала испытаний по результатам, полученным еще на доклиническом этапе, опубликованы две статьи [69, 70]. Окончание данного исследования запланировано на декабрь 2020.
Крайне важные клинические испытания с использованием нейрональных прогениторов, полученных из иПСК, начались в Киото (Япония) в 2018 г. иПСК получают из соматических клеток за счет индукции факторов плюрипотентности. Сначала с этой целью использовали фибробласты [30], а в настоящее время чаще всего применяют клетки периферической крови. Линии иПСК, используемые в данном исследовании, предоставлены Банком иПСК для регенеративной медицины Центра по исследованию и применению иПСК (iPS Cell Stock for Regenerative Medicine CiRA, Kyoto, Japan) [71]. В 2015 году Центр разрешил использовать созданные и сохраненные в нем клеточные линии [32]. В этом центре хранятся линии иПСК, соответствующие стандартам клинического использования, полученные из мононуклеаров периферической крови и пуповинной крови доноров, гомозиготных по антигенам системы HLA – HLA-A, HLA-B и HLA-DR, но большая часть линий гомозиготна также по HLA-C, HLA-DQ и HLA-DP [32]. Как показано на приматах, использование клеток и производных клеток, гомозиготных по системе HLA, значительно снижает воспаление в зоне введения и улучшает приживаемость трансплантата при применении клеточной заместительной терапии [72].
В данном исследовании примут участие семь пациентов, которым билатерально в путамен введут предшественники дофаминергических нейронов, полученные из описанных выше линий иПСК. Протокол дифференцировки иПСК, описанный Doi [73], включает две стадии: 1) нейрональную индукцию за счет двойного ингибирования SMAD в сочетании с активаторами SHH, Wnt-сигнализации и FGF8 для индукцию дифференцировки в сторону среднего мозга, в конце этого этапа (12-й день) клетки сортируют; 2) созревание нейрональных прогениторов в виде эмбриоидных телец в присутствии ретиноевой кислоты, dbcAMP и факторов роста GDNF и BDNF. Важная особенность этого протокола – сортировка клеток по маркеру пластинки дна CORIN в конце первой стадии дифференцировки [73]. Сортировка позволяет получить клетки, менее склонные к пролиферации, что снижает риск малигнизации трансплантата. Помимо этого, сортировка обогащает культуру клетками FoxaA2+ и TH+ (маркеры предшественников и дофаминергических нейронов) [73].
Этот протокол уже использовали в доклинических исследованиях на приматах [72, 74], и он показал свою эффективность. Основная цель этих клинических исследований – определение безопасности и эффективности протокола для людей, а также отработка протокола иммуносупрессивной терапии. Первый пациент в этих клинических испытаниях прооперирован в ноябре 2018 г., а окончательные результаты исследования ожидаются к 2023 году.
В ближайшее время планируется еще несколько клинических исследований. Работа Научного консорциума стволовых клеток штата Нью-Йорк (New York State Stem Cell Science Consortia, New York, USA) основана на использовании производных ЭСК линии H9 (WiCell Stem Cell Bank) [75]. Протокол дифференцировки также начинается с двойного ингибирования SMAD и активации факторов, характерных для пластинки дна и среднего мозга, с последующим созреванием в присутствии нейрональных факторов роста, dbAMP, аскорбиновой кислоты и TGF-β [76]. Подготовка к клиническим исследованиям включала выбор новых линий плюрипотентных клеток, соответствующих стандартам FDA (Управление по контролю за продуктами и лекарствами, США), обогащение полученной культуры клеток дофамин-ергическими нейронами с фенотипом А9 при помощи сортировки клеток, а также разработку протоколов криозаморозки клеток и стандартов качества для банка клеток.
В университете Лунда (Швеция) под руководством Malin Parmar уже в 2012 году разработаны эффективные и хорошо воспроизводимые протоколы получения дофаминергических нейронов [77, 78]. Свои первые работы они выполняли на линии H9 (Wicells), но позже продублировали их на линии RC17 (RoslinCT, Эдинбург Великобритания), которая соответствует стандартам GMP. Подготовка к проведению клинических испытаний потребовала смены практически всех реагентов и отказа от стадии эмбриоидных телец. Крайне важным оказался момент смены подложки, на которой растут клетки, чтобы протокол был четко стандартизирован и соответствовал стандартам GMP. Протокол занимает 16 дней и начинается с 12 млн. клеток. В результате получают 14 флаконов Т175, что соответствует 500 дозам. Часть клеток расходуется на проведение различных тестов и анализов. Клетки проверяют на соответствие, проводят функциональные тесты и пробные трансплантации лабораторным животным для оценки их онкогенного потенциала, безопасности, а также эффективности при трансплантации. Кроме того, клетки кариотипируют, часть клеток сохраняют в качестве контроля. Полный цикл проверки клеток перед проведением клинических испытаний занимает приблизительно 1–1.5 г.
В клинические испытания, которые начнутся в ближайшее время, приглашены пациенты, уже вовлеченные в исследование TRANSEURO, которым из-за нехватки материала не была проведена трансплантация. Это позволит сравнить эффективность нескольких подходов – трансплантации фетальных тканей, трансплантации клеток, полученных из плюрипотентных линий, и эффективность медикаментозной терапии, доступной на данный момент, поскольку часть пациентов, отобранных для участия в исследовании TRANSEURO, не сможет получить трансплантацию, так как за время, прошедшее с начала исследования, болезнь настолько прогрессировала, что им уже была начата терапия леводопа.
GLOBAL FORCE PD
Перевод клеточной заместительной терапии в клиническую практику – это сложный и многоступенчатый процесс, который нуждается не только в разработке протоколов получения клеток, их проверки и проведения доклинических исследований, но включает в себя юридические и этические аспекты. Поэтому в 2014 г. несколько групп ученых, которые уже принимали участие в исследованиях TRANSEURO и Neurostemcellrepair, a также ученые из Memorial Sloan Kettering Cancer Center (MSKCC), California Institute for Regenerative Medicine (CIRM) и Center for iPS Cell Research and Application (CiRA), занимающиеся разработкой протоколов для клеточной заместительной терапии БП, решили объединить свои усилия. На основании этого решения создан консорциум G-Force PD (http://www.gforce-pd.com).
В состав G-Force PD вошли также благотворительные организации: Фонд Майкл Джей Фокса (Michael J Fox Foundation (MJFF)) и Фонд болезни Паркинсона (Parkinson’s Disease Foundation (PDF)), а также Проект, финансируемый Советом по медицинским исследованиям (Medical Research Council (MRC)), Великобритания [79].
Основные вопросы, которые поставили перед собой участники G-Force PD – это, во-первых, анализ доклинических данных, позволяющий сделать вывод о безопасности и эффективности клеток и клеточных линий, из которых они получены. Важно, что современные протоколы получения нейрональных предшественников начинаются с плюрипотентных клеток, а их использование в клинике должно строго регулироваться. Во-вторых, важным моментом является разработка процесса производства клеток, удовлетворяющих стандарту GMP, а также их криозаморозка и хранение, что не должно менять качество клеточных линий и нарушать стандарты. Кроме того, клеточные линии должны быть стабильными и сохранять свои свойства в течение всего времени, которое требуется на их подготовку. Использование клеточной заместительной терапии поднимает также много этических вопросов, связанных с использованием материалов, полученных от других пациентов и из клеток эмбрионов. Поэтому консорциум считает очень важным активное взаимодействие с обществом и разработку образовательных программ в сфере клеточных технологий, делающих их более прозрачными и понятными для населения. Необходимо учитывать также, что базовые исследования, которые легли в основу методики клеточной заместительной терапии БП, проведены в академических институтах, но в дальнейшем они должны быть коммерциализированы.
ЗАКЛЮЧЕНИЕ
С момента первых попыток использования экзогенных клеток и тканей при БП прошло более 40 лет. Результаты первых экспериментов в этой области, связанных с применением фетальных тканей, очень неоднозначны, наблюдалось также появление побочных эффектов. Но при этом у части пациентов отмечен ярко выраженный положительный эффект, позволивший на несколько лет полностью отказаться от стандартной терапии, и это стало очень обнадеживающим фактом. В дальнейшем были решены многие проблемы, которые существовали на первых этапах, в том числе выбор оптимального источника клеток, позволяющего получать стабильные линии клеток в больших масштабах. Проанализированы количества клеток, необходимые для трансплантации, отработаны протоколы и определены оптимальные точки введения, выработаны критерии эффективности заместительной клеточной терапии, проведены доклинические исследования на модельных животных, подтверждающие эффективность данного подхода.
Переход от работы в условиях исследовательской лаборатории к масштабному производству клеточного продукта, соответствующего стандартам GMP, потребовал пересмотра всех отработанных ранее протоколов, использования продуктов, соответствующих стандартам GMP, разработки эффективных и безопасных протоколов криозаморозки, стандартизации методов контроля за клетками на всех этапах производства. Крайне важным стал выбор начальной линии плюрипотентных клеток. Во-первых, хотя линии и являются плюрипотентными, они несколько по-разному отвечают на процесс дифференцировки в определенном направлении, что требует отработки протокола под конкретную линию. Во-вторых, по формальным причинам, так как линии получены в разное время и порой регулируются разными стандартами. Важное значение для разработки клеточной заместительной терапии имеет этическая оценка метода [80], необходимо внесение изменений в законодательство разных стран, что сделает возможным клиническое использование данного вида лечения.
Многие из описанных выше вопросов уже решены, однако на многие еще нет ответа. Клеточная заместительная терапия будет иметь свои ограничения, несмотря на то, насколько положительными окажутся результаты проводимых сейчас клинических испытаний, поскольку она может использоваться только для коррекции моторных проявлений БП и не будет эффективной при патологических изменениях за пределами черной субстанции. Помимо этого, как уже видно из отдаленных результатов первых экспериментов, восстановление пула клеток и уровня дофамина за счет введения экзогенных клеток не останавливает прогрессирование заболевания, и патологический процесс со временем может распространиться и на нововведенные клетки. Таким образом, заместительная клеточная терапия – это только симптоматический метод, что не отменяет как его потенциальное положительное влияние на качество жизни пациентов, так и необходимость дальнейшего поиска методов патогенетической терапии.
Количество клинических исследований, которые уже проводятся или должны начаться в самое ближайшее время, достаточно велико, что обусловлено положительными результатами широких доклинических исследований, проведенных разными группами ученых. Таким образом, мы можем надеяться, что у клеточной заместительной терапии БП есть шанс занять в обозримом будущем свое место среди подходов к лечению данного заболевания наравне с медикаментозной терапией и хирургическими методами.
Исследование выполнено при финансовой поддержке РФФИ в рамках научного проекта № 19-14-50566.
Настоящая статья не содержит каких-либо исследований с участием людей или животных в качестве объектов исследований.
Авторы заявляют об отсутствии конфликта интересов.
Список литературы
Иллариошкин С.Н., Левина О.С. (2017) Руководство по диагностике и лечению болезни Паркинсона. Москва: ООО “ИПК Парето-Принт”, 336 с.
Ungerstedt U. (1968) 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur. J. Pharmacol. 5(1), 107–110. https://doi.org/10.1016/0014-2999(68)90164-7
Ungerstedt U., Arbuthnott G.W. (1970) 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur. J. Pharmacol. 24(3), 485–493. https://doi.org/10.1016/0006-8993(70)90187-3
Björklund A., Lindvall O. (2017) Replacing dopamine neurons in Parkinson’s disease: how did it happen? J. Parkinson’s Disease. 7(s1), S23–S33. https://doi.org/10.3233/JPD-179002
Olson L., Seiger A. (1972) Brain tissue transplanted to the anterior chamber of the eye. 1. Fluorescence histochemistry of immature catecholamine and 5-hydroxytryptamine neurons reinnervating the rat iris. Z. Zellforsch. Mikrosk. Anat. 135(2), 175–194. https://doi.org/10.1007/bf00315125
Das G.D., Altman J. (1971) Transplanted precursors of nerve cells: their fate in the cerebellums of young rats. Science. 173(3997), 637–638. https://doi.org/10.1126/science.173.3997.637
Bjorklund A., Stenevi U. (1979) Reconstruction of the nigrostriatal dopamine pathway by intracerebral nigral transplants. Bram Res. 177(3), 555–560. https://doi.org/10.1016/0006-8993(79)90472-4
Björklund A., Gage F.H., Stenevi U., Dunnett S.B. (1983) Intracerebral grafting of neuronal cell suspensions. VI. Survival and growth of intrahippocampal implants of septal cell suspensions. Acta Physiol. Scand. Suppl. 522, 49–58. PMID: 6586055
Brundin P., Isacson O., Björklund A. (1985) Monitoring of cell viability in suspensions of embryonic CNS tissue and its use as a criterion for intracerebral graft survival. Brain Res. 331(2), 251–259. https://doi.org/10.1016/0006-8993(85)91550-1
Björklund A., Schmidt R.H., Stenevi U. (1980) Functional reinnervation of the neostriatum in the adult rat by use of intraparenchymal grafting of dissociated cell suspensions from the substantia nigra. Cell Tissue Res. 212(1), 39–45. https://doi.org/10.1007/BF00234031
Perlow M.J., Freed W.J., Hoffer B.J., Seiger A., Olson L., Wyatt R.J. (1979) Brain grafts reduce motor abnormalities produced by destruction of nigrostriatal dopamine system. Science. 204(4393), 643–647. https://doi.org/10.1126/science.571147
Backlund E.O., Granberg P.O., Hamberger B., Knutsson E., Martensson A., Sedvall G., Seiger A., Olson L. (1985) Transplantation of adrenal medullary tissue to striatum in parkinsonism. First clinical trials. J. Neurosurg. 62, 169–173. https://doi.org/10.3171/jns.1985.62.2.0169
Lindvall O., Backlund E.O., Farde L., Sedvall G., Freedman R., Hoffer B., Nobin A., Seiger A., Olson L. (1987) Transplantation in Parkinson’s disease: two cases of adrenal medullary grafts to the putamen. Ann. Neurol. 22(4), 457–468. https://doi.org/10.1002/ana.410220403
Brundin P., Nilsson O.G., Strecker R.E., Lindvall O., Astedt B., Bjorklund A. (1986) Experimental brain research behavioural effects of human fetal dopamine neurons grafted in a rat model of Parkinson’s disease. Exp. Brain Res. 65(1), 235–240. https://doi.org/10.1007/BF00243848
Brederlau A., Correia A.S., Anisimov S.V., Elmi M., Paul G., Roybon L., Morizane A., Bergquist F., Riebe I., Nannmark U., Carta M., Hanse E., Takahashi J., Sasai Y., Funa K., Brundin P., Eriksson P.S., Li J.Y. (2006) Transplantation of human embryonic stem cell-derived cells to a rat model of Parkinson’s disease: effect of in vitro differentiation on graft survival and teratoma formation. Stem Cells. 24(6), 1433–1440. https://doi.org/10.1634/stemcells.2005-0393
Lindvall O., Rehncrona S., Brundin P., Gustavii B., Astedt B., Widner H., Lindholm T., Björklund A., Leenders K.L., Rothwell J.C., Frackowiak R., Marsden D., Johnels B., Steg G., Freedman R., Hoffer B.J., Seiger A., Bygdeman M., Strömberg I., Olson L. (1989) Human fetal dopamine neurons grafted into the striatum in two patients with severe Parkinson’s disease. a detailed account of methodology and a 6-month follow-up. Ach. Neurol. 46(6), 615–631. https://doi.org/10.1001/archneur.1989.00520420033021
Lindvall O., Brundin P., Widner H., Rehncrona S., Gustavii B., Frackowiak R., Leenders K.L., Sawle G., Rothwell J.C., Marsden C.D., Bjorklund A. (1990) Grafts of fetal dopamine neurons survive and improve motor function in Parkinson’s disease. Science. 247(4942), 574–577. https://doi.org/10.1126/science.2105529
Piccini P., Brooks D.J., Björklund A., Gunn R.N., Grasby P.M., Rimoldi O., Brundin P., Hagell P., Rehncrona S., Widner H., Lindvall O. (1999) Dopamine release from nigral transplants visualized in vivo in a Parkinson’s patient. Nat. Neurosci. 2(12), 1137–1140. https://doi.org/10.1038/16060
Li W., Englund E., Widner H., Mattsson B., van Wes-ten D., Lätt J., Rehncrona S., Brundin P., Björklund A., Lindvall O., Li J.-Y. (2016) Extensive graft-derived dopaminergic innervation is maintained 24 years after transplantation in the degenerating parkinsonian brain. Proc. Natl. Acad. Sci. USA. 113(23), 6544–6549. https://doi.org/10.1073/pnas.1605245113
Freed C.R., Greene P.E., Breeze R.E., Tsai W.Y., DuMouchel W., Kao R., Dillon S., Winfield H., Culver S., Trojanowski J.Q., Eidelberg D., Fahn S. (2001) Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N. Engl. J. Med. 344(10), 710–719. https://doi.org/10.1056/NEJM200103083441002
Olanow C.W., Goetz C.G., Kordower J.H., Stoessl A.J., Sossi V., Brin M.F., Shannon K.M., Nauert G.M., Perl D.P., Godbold J., Freeman T.B. (2003) A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann. Neurol. 54(3), 403–414. https://doi.org/10.1002/ana.10720
Mínguez-Castellanos A., Escamilla-Sevilla F., Hotton G.R., Toledo-Aral J.J., Ngel Ortega-Moreno A., Méndez-Ferrer S., Martín-Linares J.M., Katati M.J., Mir P., Villadiego J., Meersmans M., Pérez-García M., Brooks D.J., Arjona V., López J., López-Barneo J. (2007) Carotid body autotransplantation in Parkinson disease: a clinical and positron emission tomography study. J. Neurol. Neurosurg. Psychiatry. 78, 825–831. https://doi.org/10.1136/jnnp.2006.106021
Gross R.E., Watts R.L., Hauser R.A., Bakay R.A., Reichmann H., von Kummer R., Ondo W.G., Reissig E., Eisner W., Steiner-Schulze H., Siedentop H., Fichte K., Hong W., Cornfeldt M., Beebe K., Sandbrink R. (2011) Spheramine investigational group. Intrastriatal transplantation of microcarrier-bound human retinal pigment epithelial cells versus sham surgery in patients with advanced Parkinson’s disease: a double-blind, randomised, controlled trial. Lancet Neurol. 10(6), 509–519. PMID: 2156557.https://doi.org/10.1016/S1474-4422(11)70097-7
Lindvall O., Björklund A. (2004) Cell therapy in Parkinson’s disease. NeuroRx: J. Am. Soc. Exp. NeuroTherap. 1(4), 382–393. https://doi.org/10.1602/neurorx.1.4.382
Barker R.A., Farrell K., Guzman N.V., He X., Lazic S.E., Moore S., Morris R., Tyers P., Wijeyekoon R., Daft D., Hewitt S., Dayal V., Foltynie T., Kefalopoulou Z., Mahlknecht P., Lao-Kaim N. P., Piccini P., Bjartmarz H., Björklund A., Winkler C. (2019) Designing stem-cell-based dopamine cell replacement trials for Parkinson’s disease. Nat. Med. 25(7), 1045–1053. https://doi.org/10.1038/s41591-019-0507-2
Kordower J.H., Rosenstein J.M., Collier T.J., Levey A.E., Mufson E.J., Freeman T.B., Olanow C.W., Burke M.A., Chen E.-Y., Li M., Martel L. (1996) Functional fetal nigral grafts in a patient with Parkinson’s disease: chemoanatomic, ultrastructural, and metabolic studies. J. Comp. Neurol. 370(2), 203–230. https://doi.org/10.1002/(SICI)1096-9861(19960624)370: 2<203::AID-CNE6>3.0.CO;2-6
Kefalopoulou Z., Politis M., Piccini P., Mencacci N., Bhatia K., Jahanshahi M., Widner H., Rehncrona S., Brundin P., Björklund A., Lindvall O., Limousin P., Quinn N., Foltynie T. (2014) Long-term clinical outcome of fetal cell transplantation for Parkinson disease: two case reports. JAMA Neurol. 71(1), 83–87. https://doi.org/10.1001/jamaneurol.2013.4749
Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V. S., Jones J.M. (1998) Embryonic stem cell lines derived from human blastocysts. Science. 282(5391), 1145–1147. https://doi.org/10.1126/science.282.5391.1145
Thomson J.A., Odorico J.S. (2000) Human embryonic stem cell and embryonic germ cell lines. Trends Biotechnol. 18(2), 53–57. PMID: https://doi.org/10.1016/s0167-7799(99)01410-9
Takahashi K., Yamanaka S. (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 126(4), 663–676. https://doi.org/10.1016/j.cell.2006.07.024
Park I.H., Zhao R., West J.A., Yabuuchi A., Huo H., Ince T.A., Lerou P.H., Lensch M.W., Daley G.Q. (2008) Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 45(7175), 141–146. https://doi.org/10.1038/nature06534
Umekage M., Sato Y., Takasu N. (2019) Overview: an iPS cell stock at CiRA. Inflammation Regeneration. 39(1). https://doi.org/10.1186/s41232-019-0106-0
Okabe S., Forsberg-Nilsson K., Spiro A.C., Segal M., McKay R.D. (1996) Development of neuronal precursor cells and functional postmitotic neurons from embryonic stem cells in vitro. Mech. Dev. 59(1), 89–102. https://doi.org/10.1016/0925-4773(96)00572-2
Bain G., Kitchens D., Yao M., Huettner J.E., Gottlieb D.I. (1995) Embryonic stem cells express neuronal properties in vitro. Dev. Biol. 168(2), 342–357. https://doi.org/10.1006/dbio.1995.1085
Reubinoff B.E., Itsykson P., Turetsky T., Pera M.F., Reinhartz E., Itzik A., Ben-Hur T. (2001) Neural progenitors from human embryonic stem cells. Nat. Biotechnol. 19(12), 1134–1140. https://doi.org/10.1038/nbt1201-1134
Zhang S.C., Wernig M., Duncan I.D., Brüstle O., Thomson J.A. (2001) In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol. 19(12), 1129–1133. https://doi.org/10.1038/nbt1201-1129
Reubinoff B.E., Pera M.F., Fong C.Y., Trounson A., Bongso A. (2000) Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat. Biotechnol. 18(4), 399–404. https://doi.org/10.1038/74447
Wiles M.V., Johansson B.M. (1999) Embryonic stem cell development in a chemically defined medium. Exp. Cell Res. 247(1), 241–248. PMID: 10047466https://doi.org/10.1006/excr.1998.4353
Ying Q.-L., Smith A.G. (2003) Defined conditions for neural commitment and differentiation. Meth. Enzymol. 365, 327–341. https://doi.org/10.1016/s0076-6879(03)65023-8
Itskovitz-Eldor J., Schuldiner M., Karsenti D., Eden A., Yanuka O., Amit M., Soreq H., Benvenisty N. (2000) Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol. Med. 6(2), 88–95. http://www.ncbi. nlm.nih.gov/pubmed/10859025.
Keller G.M. (1995) In vitro differentiation of embryonic stem cells. Curr. Opin. Cell Biol. 7(6), 862–869. https://doi.org/10.1016/0955-0674(95)80071-9
Chambers S.M., Fasano C.A., Papapetrou E.P., Tomishima M., Sadelain M., Studer L. (2009) Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 27(3), 275–280. https://doi.org/10.1038/nbt.1529
Sasai Y., Lu B., Steinbeisser H., Geissert D., Gont L.K., De Robertis E.M. (1994) Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 79(5), 779–790. https://doi.org/10.1016/0092-8674(94)90068-x
Hemmati-Brivanlou A., Kelly O.G., Melton D.A. (1994) Follistatin, an antagonist of activin, is expressed in the spemann organizer and displays direct neuralizing activity. Cell. 77(2), 283–295. https://doi.org/10.1016/0092-8674(94)90320-4
Smith W.C., Harland R.M. (1992) Expression cloning of noggin, a new dorsalizing factor localized to the spemann organizer in Xenopus embryos. Cell. 70, 829–840. https://doi.org/10.1016/0092-8674(92)90316-5
Smith J.R., Vallier L., Lupo G., Alexander M., Harris W.A., Pedersen R.A. (2008) Inhibition of activin/nodal signaling promotes specification of human embryonic stem cells into neuroectoderm. Dev. Biol. 313(1), 107–117. https://doi.org/10.1016/j.ydbio.2007.10.003
Ye W., Shimamura K., Rubenstein J.L., Hynes M.A., Rosenthal A. (1998) FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell. 93(5), 755–766. https://doi.org/10.1016/s0092-8674(00)81437-3
Maye P., Becker S., Siemen H., Thorne J., Byrd N., Carpentino J., Grabel L. (2004) Hedgehog signaling is required for the differentiation of ES cells into neurectoderm. Dev. Biol. 265(1), 276–290. https://doi.org/10.1016/j.ydbio.2003.09.027
Davidson K.C., Jamshidi P., Daly R., Hearn M.T.W., Pera M.F., Dottori M. (2007) Wnt3a regulates survival, expansion, and maintenance of neural progenitors derived from human embryonic stem cells. Mol. Cell. Neurosci. 36(3), 408–415. https://doi.org/10.1016/j.mcn.2007.07.013
Rao B.M., Zandstra P.W. (2005) Culture development for human embryonic stem cell propagation: molecular aspects and challenges. Curr. Opin. Biotechnol. 16(5), 568–576. https://doi.org/10.1016/j.copbio.2005.08.001
Hitoshi S., Alexson T., Tropepe V., Donoviel D., Elia A.J., Nye J.S., Conlon R.A., Mak T.W., Bernstein A., van der Kooy D. (2002) Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes. Dev. 16(7), 846–858. https://doi.org/10.1101/gad.975202
Smidt M.P., van Schaick H.S., Lanctôt C., Tremblay J.J., Cox J.J., van der Kleij A.A., Wolterink G., Drouin J., Burbach J.P. (1997) A homeodomain gene Ptx3 has highly restricted brain expression in mesencephalic dopaminergic neurons. Proc. Natl. Acad. Sci. USA. 94(24), 13305–13310. https://doi.org/10.1073/pnas.94.24.13305
Saucedo-Cardenas O., Quintana-Hau J.D., Le W.D., Smidt M.P., Cox J.J., De Mayo F., Burbach J.P.H., Conneely O.M. (1998) Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc. Natl. Acad. Sci. USA. 95(7), 4013–4018. https://doi.org/10.1073/pnas.95.7.4013
Simon H.H., Saueressig H., Wurst W., Goulding M.D., O’Leary D.D.M. (2001). Fate of midbrain dopaminergic neurons controlled by the engrailed genes. J. Neurosci. 21(9), 3126–3134. https://doi.org/10.1523/jneurosci.21-09-03126.2001
Yan Y., Yang D., Zarnowska E.D., Du Z., Werbel B., Valliere C., Pearce R.A., Thomson J.A., Zhang S.-C. (2005) Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells (Dayton, Ohio). 23(6), 781–790. https://doi.org/10.1634/stemcells.2004-0365
Parmar M., Grealish S., Henchcliffe C. (2020) The future of stem cell therapies for Parkinson disease. Nat. Rev. Neurosci. 21(2), 103–115. https://doi.org/10.1038/s41583-019-0257-7
Vierbuchen T., Ostermeier A., Pang Z.P., Kokubu Y., Südhof T.C., Wernig M. (2010) Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 463(7284), 1035–1041. https://doi.org/10.1038/nature08797
Kim S.M., Kim J.W., Kwak T.H., Park S.W., Kim K.P., Park H., Lim K.T., Kang K., Kim J., Yang J.H., Han H., Lee I., Hyun J.K., Bae Y.M., Schöler H.R., Lee H.T., Han D.W. (2016) Generation of integration-free induced neural stem cells from mouse fibroblasts. Biol. Chem. 291(27), 14199–14212. https://doi.org/10.1074/jbc.M115.713578
Caiazzo M., Dell’Anno M.T., Dvoretskova E., Lazarevic D., Taverna S., Leo D., Sotnikova T.D., Menegon A., Roncaglia P., Colciago G., Russo G., Carninci P., Pezzoli G., Gainetdinov R.R., Gustincich S., Dityatev A., Broccoli V. (2011) Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 476(7359), 224–227. https://doi.org/10.1038/nature10284
Colasante G., Lignani G., Rubio A., Medrihan L., Yekhlef L., Sessa A., Massimino L., Giannelli S.G., Sacchetti S., Caiazzo M., Leo D., Alexopoulou D., Dell’Anno M.T., Ciabatti E., Orlando M., Studer M., Dahl A., Gainetdinov R.R., Taverna S., Benfenati F., Broccoli V. (2015) Rapid conversion of fibroblasts into functional forebrain GABAergic interneurons by direct genetic reprogramming. Cell Stem Cell. 17(6), 719–734. https://doi.org/10.1016/j.stem.2015.09.002
Dell’Anno M.T., Caiazzo M., Leo D., Dvoretskova E., Medrihan L., Colasante G., Giannelli S., Theka I., Russo G., Mus L., Pezzoli G., Gainetdinov R.R., Benfenati F., Taverna S., Dityatev A., Broccoli V. (2014) Remote control of induced dopaminergic neurons in parkinsonian rats. J. Clin. Invest. 124(7), 3215–3229. https://doi.org/10.1172/JCI74664
Revazova E.S., Turovets N.A., Kochetkova O.D., Kindarova L.B., Kuzmichev L.N., Janus J.D., Pryzhkova M.V. (2007) Patient-specific stem cell lines derived from human parthenogenetic blastocysts. Cloning Stem Cells. 9(3), 432–449. https://doi.org/10.1089/clo.2007.0033
Garitaonandia I., Gonzalez, R., Christiansen-Weber T., Abramihina, T., Poustovoitov, M., Noskov A., Sherman G., Semechkin A., Snyder E., Kern R. (2016) Neural stem cell tumorigenicity and biodistribution assessment for Phase I clinical trial in Parkinson’s disease. Sci. Repts. 6, 34478 https://doi.org/10.1038/srep34478
Garitaonandia I., Gonzalez R., Sherman G., Semechkin A., Evans A., Kern R. (2018) Novel approach to stem cell therapy in Parkinson’s disease. Stem Cells Dev. 27(14), 951–957. https://doi.org/10.1089/scd.2018.0001
Gonzalez R., Garitaonandia I., Poustovoitov M., Abramihina T., McEntire C., Culp B., Attwood J., Noskov A., Christiansen-Weber T., Khater M., Mora-Castilla S., To C., Crain A., Sherman G., Semechkin A., Laurent L.C., Elsworth J.D., Sladek J., Snyder E.Y., Kern R.A. (2016) Neural stem cells derived from human parthenogenetic stem cells engraft and promote recovery in a nonhuman primate model of Parkinson’s disease. Cell Transplantation. 25(11), 1945–1966. https://doi.org/10.3727/096368916X691682
Takahashi J. (2018) Stem cells and regenerative medicine for neural repair. Curr. Opin. Biotechnol. 52, 102–108. https://doi.org/10.1016/j.copbio.2018.03.006
Лебедева О.С., Лагарькова М.А. (2018) Плюрипотентные стволовые клетки для моделирования и клеточной терапии болезни Паркинсона. Биохимия. 83(9), 1046–1056. https://doi.org/10.1134/S0006297918090067
Cyranoski D. (2017) Trials of embryonic stem cells to launch in China. Nature. 546(7656), 15–16. https://doi.org/10.1038/546015a
Gu Q., Wang J., Wang L., Liu Z.-X., Zhu W.-W., Tan Y.-Q., Han W.-F., Wu J., Feng C.-J., Fang J.-H., Liu L., Wang L., Li W., Zhao X.-Y., Hu B.-Y., Hao J., Zhou Q. (2017) Accreditation of biosafe clinical-grade human embryonic stem cells according to chinese regulations. Stem Cell Repts. 9(1), 366–380. https://doi.org/10.1016/J.STEMCR.2017.04.017
Wang Y.-K., Zhu W.-W., Wu M.-H., Wu Y.-H., Liu Z.-X., Liang L.-M., Sheng C., Hao J., Wang L., Li W., Zhou Q., Hu B.-Y. (2018) Human clinical-grade parthenogenetic ESC-derived dopaminergic neurons recover locomotive defects of nonhuman primate models of Parkinson’s disease. Stem Cell Repts. 11(1), 171–182. https://doi.org/10.1016/j.stemcr.2018.05.010
Takahashi J. (2020) Preclinical evaluation of patient-derived cells shows promise for Parkinson’s disease. J. Clin. Invest. https://doi.org/10.1172/JCI134031
Morizane A., Glasser M.F., Ogasawara K., Doi D., Hayashi T., Onoe H., Doi H., Itoh Y., Takara S., Kikuchi T., Yamasaki E., Shiina T., Mawatari A., Takahashi J., Ishigaki H., Mizuma H., Okita K., Yamanaka S. (2017) MHC matching improves engraftment of iPSC-derived neurons in non-human primates. Nat. Commun. 8(1), 1–12. https://doi.org/10.1038/s41467-017-00926-5
Doi D., Samata B., Katsukawa M., Kikuchi T., Morizane A., Ono Y., Sekiguchi K., Nakagawa M., Parmar M., Takahashi J. (2014) Isolation of human induced pluripotent stem cell-derived dopaminergic progenitors by cell sorting for successful transplantation. Stem Cell Repts. 2(3), 337–350. https://doi.org/10.1016/j.stemcr.2014.01.013
Kikuchi T., Morizane A., Doi D., Magotani H., Onoe H., Hayashi T., Mizuma H., Takara S., Takahashi R., Inoue H., Morita S., Yamamoto M., Okita K., Nakagawa M., Parmar M., Takahashi J. (2017) Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature. 548(7669), 592–596. https://doi.org/10.1038/nature23664
Studer L. (2017) Strategies for bringing stem cell-derived dopamine neurons to the clinic—The NYSTEM trial. Progress Brain Res. 230, 191–212. https://doi.org/10.1016/bs.pbr.2017.02.008
Kriks S., Shim J.-W., Piao J., Ganat Y.M., Wakeman D.R., Xie Z., Carrillo-Reid L., Auyeung G., Antonacci C., Buch A., Yang L., Beal M.F., Surmeier D.J., Kordo-wer J.H., Tabar V., Studer L. (2011) Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 480(7378), 547–551. https://doi.org/10.1038/nature10648
Kirkeby A., Grealish S., Wolf D.A., Nelander J., Wood J., Lundblad M., Lindvall O., Parmar M. (2012) Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Repts. 1(6), 703–714. https://doi.org/10.1016/j.celrep.2012.04.009
Kirkeby A., Nolbrant S., Tiklova K., Heuer A., Kee N., Cardoso T., Ottosson D.R., Lelos M.J., Rifes P., Dunnett S.B., Grealish S., Perlmann T., Parmar M. (2017) Predictive markers guide differentiation to improve graft outcome in clinical translation of hESC-based therapy for Parkinson’s disease. Cell Stem Cell. 20(1), 135–148. https://doi.org/10.1016/j.stem.2016.09.004
Barker R.A., Studer L., Cattaneo E., Takahashi J., G-Force PD consortium (2015) G-Force PD: a global initiative in coordinating stem cell-based dopamine treatments for Parkinson’s disease. NP. J. Parkinsons. Dis. 1, 15017. https://doi.org/10.1038/npjparkd.2015.17
Schweitzer J., Song B., Herrington T., Park T., Lee N., Ko S., Jeon J., Cha Y., Kim K., Li Q., Henchcliffe C., Kaplitt M., Neff C., Rapalino O., Seo H., Lee I., Kim J., Kim T., Petsko G., Ritz J., Cohen B., Kong S., Leblanc P., Carter B., Kim K. (2020) Personalized iPSC-derived dopamine progenitor cells for Parkinson’s disease. N. Engl. J. Med. 382, 1926–1932. https://doi.org/10.1056/NEJMoa1915872
Дополнительные материалы отсутствуют.
Инструменты
Молекулярная биология