Молекулярная биология, 2022, T. 56, № 4, стр. 564-573
Связанная с GTP форма Rab3D способствует росту липидных капель в адипоцитах
T. Wang a, b, M. J. Jin a, b, L. K. Li a, b, *
a MOE Key Laboratory of Bioinformatics and Tsinghua-Peking Center for Life Sciences
100084 Beijing, China
b Advanced Innovation Center for Structural Biology, School of Life Sciences, Tsinghua University
100190 Beijing, China
* E-mail: liangkui826@mail.tsinghua.edu.cn
Поступила в редакцию 11.10.2021
После доработки 27.12.2021
Принята к публикации 27.12.2021
- EDN: PEQCHX
- DOI: 10.31857/S0026898422040140
Аннотация
GTPaзы Rab ‒ главные регуляторы мембранного трафика. Профилирование протеома выявило десятки белков Rab, ассоциированных с липидными каплями (LD), но установлены функции лишь некоторых из них. Белок Cidec, которым обогащены участки контактов LD‒LD, опосредует слияние и рост LD. Нами изучена роль Rab3D в хранении липидов в адипоцитах. Подтверждено, что уровень транскриптов Rab3D в адипоцитах выше, чем у других членов семейства Rab3; различия были наиболее выраженными в белой жировой ткани. Более того, нами показано, что Rab3D способствует дозозависимому росту LD в преадипоцитах 3T3-L1 независимо от опосредованного Cidec слияния LD. Наконец, подтверждено, что GTP-связанная форма Rab3D способствует росту LD; в ходе дифференцировки адипоцитов эта форма транслоцируется в LD из других везикул. Напротив, Rab3D-GDP остается в цитоплазме и не влияет на размеры LD. Получены данные, доказывающие участие Rab3D в контролируемом образовании крупных LD в адипоцитах. Можно заключить, что Rab3D ‒ это новый регулятор LD, свойства которого отличаются от свойств идентифицированных ранее белков Rab18 и Rab8a, ассоциированных с LD.
ВВЕДЕНИЕ
Липидные капли (LD) ‒ высокодинамичные субклеточные органеллы, обнаруженные у разных организмов ‒ от дрожжей до млекопитающих [1]. LD состоят из нейтрального липидного ядра, окруженного монослоем фосфолипидов и ассоциированных белков [2, 3]. LD претерпевают динамические изменения, включающие биогенез, слияние/рост и деградацию [4‒6]. Биогенез LD инициируется de novo в эндоплазматическом ретикулуме (ER) [7, 8]. Рост индивидуальных LD достигается несколькими путями. Во-первых, LD приобретают нейтральные липиды из ER. Во-вторых, LD могут синтезироваться in situ поверхностными белками LD, такими как DGAT2 [9]. Наконец, рост LD может определяться их слиянием, опосредуемым CIDE. Cайты контактов LD‒LD обогащены индуцируемым клеточной смертью эффекторным белком, подобным фактору 45 фрагментации ДНК (CIDE), который участвует в слиянии и росте LD посредством переноса липидов от мелких LD к более крупным [10‒12]. Белки CIDE включают Cidea, Cideb и Cidec [13‒17]: Cidea экспрессируется преимущественно в бурой жировой ткани (BAT) [18, 19], Cideb – в печени [20‒22], а Cidec почти исключительно в белой жировой ткани (WAT) [23‒25]. Катаболизм LD включает гидролиз и/или аутофагию. Гидролиз LD опосредуется клеточными липазами, которые катализируют расщепление триацилглицерина и его метаболитов. Аутофагия LD это процесс деградации, в котором LD поглощаются аутофагосомой, которая затем сливается с лизосомой с образованием аутолизосомы [3, 26, 27].
Малые GTPaзы Rab играют важные роли в транспорте везикул и динамике мембран [28‒31]. Активность белков Rab регулируется специфичными активирующими GTPазы белками (GAP), факторами обмена гуаниновых нуклеотидов (GEF), нижележащими эффекторами. GDP-связанный Rab трансформируется в GTP-форму под действием мембраноспецифичного GEF, тогда как GTP-связанная форма Rab превращается в Rab-GDP под действием GAP [32, 33]. Большинство белков Rab активны в GTP форме и взаимодействуют с широким кругом эффекторов [34]. Особенности функционирования белков Rab (циклическое чередование включенных “on” и выключенных “off” состояний) ведет лишь к временному взаимодействию с эффекторами, которое сложно фиксировать.
К настоящему времени идентифицированы многие ассоциированные с LD Rab GTPaзы [35‒37]. Однако лишь некоторые из них верифицированы функционально. Среди них наиболее хорошо охарактеризован ассоциированный с LD белок Rab18, который способствует росту LD за счет привязки ER к LD [38‒41]. Rab8a служит активатором опосредуемого Cidec слияния LD в адипоцитах [42]. Мутанты Rab32 с потерей функции снижают запасы липидов, нарушая аутофагию у Drosophila [43]. Истощение Rab40c уменьшает размеры LD, тогда как сверхэкспрессия Rab40c индуцирует кластеризацию LD [44]. Rab7 незаменим для деградации LD в гепатоцитах путем липофагии [45]. Получены также косвенные свидетельства того, что Rab1 и 5 взаимодействуют с LD [46‒48]. Подсемейство Rab3 включает четыре гомологичных белка ‒ Rab3A, Rab3B, Rab3C и Rab3D [49]. Rab3A, Rab3B и Rab3C экспрессируются преимущественно в головном мозге и нейроэндокринных клетках и локализуются исключительно в синаптических везикулах [50]. Тем не менее, Rab3D сначала идентифицировали в адипоцитах и показали постепенное повышение его экспрессии по мере дифференцировки адипоцитов [51, 52]. Белок Rab3D обильно представлен в нескольких других не нейронных тканях, включая поджелудочную и околоушную железу [52‒56].
В настоящей работе определены уровни экспрессии четырех представителей семейства Rab3 в адипозных тканях, проанализировано действие Rab3D на рост LD. Кроме того, нами проведен анализ субклеточной локализации Rab3D в пре- и зрелых адипоцитах 3T3-L1. Полученные нами данные указывают на важную роль Rab3D в развитии адипоцитов.
ЭКСПЕРИМЕНТАЛЬНАЯ ЧАСТЬ
Животные, культуры клеток и временная трансфекция. Mышей, использованных в работе, содержали в контролируемых условиях (температура 22°C, 12-часовой цикл день/ночь) со свободным доступом к воде и стандартному сбалансированному корму для грызунов. Опыты проводили в виварии Университета Tsinghua (Пекин, КНР). Проведение опытов одобрено Комитетом по обращению с животными университета Tsinghua.
Преадипоциты 3T3-L1 (ATCC, Manassas, США) культивировали в среде Игла, модифицированной Дульбекко (DMEM), с 10% фетальной сыворотки крупного рогатого скота (FBS) и антибиотиками (100 Ед./мл пенициллина и 100 Ед./мл стрептомицина) при 37°C в камерах с 5% CO2. Линия клеток Hela со стабильной экспрессией Cidec получена в подарок от Feng-Jung Chen (Fudan University, КНР).
Зрелые адипоциты 3T3-L1 получали из преадипоцитов ранних генераций согласно протоколу ATCCl. Через 48 ч после достижения 100%-ной плотности среду заменяли индукционной средой (к исходной среде добавляли 1 мкг/мл инсулина, 1 мкМ розиглитазона, 1 мкM дексаметазона и 0.5 мкM метилизобутирилксантина) и инкубировали в течение 48 ч, а затем заменяли поддерживающей средой (содержащей только 1 мкг/мл инсулина) и продолжали индуцировать в течение 6‒8 дней, с заменой среды каждые 2 дня.
Транзиентную трансфекцию клеток HeLa плазмидами проводили, используя Lipofectamine 3000 (“Invitrogen”, США) согласно инструкции производителя. Электропорацию преадипоцитов 3T3-L1 и зрелых адипоцитов выполняли, используя систему Amaxa Nucleofector II (“Lonza”, Швейцария), согласно инструкции производителя. Образование LD в преадипоцитах 3T3-L1 и клетках Hela индуцировали, добавляя к среде 200 мкM олеиновой кислоты (OA) (в комплексе с 1%-ным бычьим сывороточным альбумином, свободным от жирных кислот (BSA), в дважды дистиллированной H2O) и выдерживали в течение 18 ч вместе с красителем LD Bodipy-C12.
Экстракция РНК и количественная ПЦР в реальном времени (qRT-PCR). qRT-PCR проводили, как описано ранее [57]. Сначала из клеток одной чашки диаметром 6 см с использованием реагента TRIzol выделяли суммарную РНК (1.0 мл) (“Invitrogen”). Затем проводили синтез первой цепи кДНК с 1 мкг суммарной РНК с использованием набора Superscript Reversal Kit (“Invitrogen”) по протоколу производителя. qRT-PCR проводили с SYBR Green Master Mix (“Applied Biosystems”, США) на приборе ABI 7500 fluorescent quantitative PCR. Использовали следующие условия ПЦР: 95°C, 30 с (1 цикл); затем 95°C, 5 с и 60°C, 30 с (40 циклов), в конце проводили анализ кривой плавления. В качестве внутреннего контроля выбраны праймеры на β-актин. Относительный уровень экспрессии генов рассчитывали методом 2–ΔΔCT. Нуклеотидные последовательности праймеров показаны в табл. 1 .
Tаблица 1. Праймеры для qRT-PCR, использованные в работе
| Праймер | Нуклеотидная последовательность (5′ → 3′) |
|---|---|
| β -Actin | ACACTGTGCCCATCTACGAG |
| CAGCACTGTGTTGGCATAGAG | |
| Rab3a | CTCCAGCCTTTGTCAGCACCGT |
| CAACTGACAACTGTGGACGGGAT | |
| Rab3b | CCTCCTTCCTTTTCCGCTATG |
| TCACACGCTTCTCATGGCG | |
| Rab3c | CTGTTCCGTTATGCCGACGAT |
| CCCCTCGATAGTAGGCTGTG | |
| Rab3d | ATGGCATCCGCTAGTGAGC |
| TCGGTAGACCGTCTTGACCTT |
Конструирование плазмид. кДНК, кодирующую Rab3D мыши, клонировали в векторе экспрессии для клеток млекопитающих pEGFP-C1 (“Clontech”, США). Мутанты Rab3D с блокированными сайтами связывания GTP и GDP (Q81L или N135I) синтезировали путем сайт-направленного мутагенеза pEGFP-Rab3D, как описано ранее [58]. Полноразмерную кДНК, кодирующую перилипин (Plin1), субклонировали в вектор pmCherry-N1 (“Clontech”), как описано ранее [59]. Все полученные конструкции верифицированы с помощью секвенирования ДНК (см. рис. S1, S2 Приложения, см. Дополнительные материалы на сайте http://www.molecbio.ru/downloads/2022/4/ supp_Wang_rus.pdf).
Флуоресцентный имиджинг. Клетки культивировали на покровных стеклах, промывали 3 раза PBS, фиксировали 4%-ным параформальдегидом в течение 20 мин. Проводили еще один раунд из трех промывок, а затем клетки окрашивали Bodipy493/503 (разведение 1 : 200) в течение 30 мин и Hoechest 33258 (1 : 10 000) в течение 10 мин. Изображения получали на конфокальном микроскопе сверхвысокого разрешения Nikon A1 при ×100 увеличении с иммерсионным маслом. Диаметры LD измеряли, используя программу ImageJ и анализировали с помощью программы Graphpad Prism 5.0.
Анализ иммуноблотов. Вестерн-блотинг проводили, как описано ранее [57]. Экспериментальные образцы лизировали в буфере RIPA и денатурировали при 95°C в течение 10 мин. Затем образцы белков разделяли с помощью электрофореза в 10%-ном SDS-ПААГ и переносили на PVDF-мембрану. Мембрану блокировали в свежеприготовленном растворе PBST, содержащем 5% обезжиренного сухого молока (Yili), в течение 1 ч и инкубировали с антителом к GFP (1 : 1000) (“Santa Cruz Biotechnology”, sc-8334, США) при 4°C. После этого мембрану отмывали PBST (3 раза по 5 мин) и инкубировали с меченными пероксидазой хрена вторичными антителами при комнатной температуре в течение 1 ч. Наконец, после еще трех отмывок полосы визуализировали с помощью Western Lightning Plus-ECL (“PerkinElmer”, США). В качестве контроля использовали IgG к β-актину мыши (“Sigma-Aldrich”, A5441, США). Антитела морской свинки к перилипину 1 (20R-pp004) получали от “Fitzgerald Industries” (США).
Статистика. Результаты по меньшей мере трех независимых опытов анализировали и представляли графически с использованием Graphpad Prism 5.0. Результаты опытов представляли как среднее значение ± стандартное отклонение. Для сравнения значимости различий между группами использовали t-тест, *p < 0.05, **p < 0.01, ***p < 0.001.
РЕЗУЛЬТАТЫ ИССЛЕДОВАНИЯ
Rab3D активно экспрессируется в WAT
Для определения паттернов экспрессии четырех членов подсемейства Rab3 в разных жировых тканях экстрагировали РНК из четырех типов тканей мыши, а именно из белого жира гонад (gWAT), подкожного белого жира (sWAT), белого жира брыжейки (mWAT) и бурого жира (BAT). Методом qRT-PCR во всех четырех жировых тканях выявлен наиболее высокий уровень экспрессии мРНК Rab3d, затем следует Rab3a, тогда как транскрипты Rab3b и Rab3c в белом жире слабо экспрессируются (рис. 1). Кроме того, транскрипты Rad3d экспрессируются на одинаковом уровне во всех трех типах WAT и немного менее активно в BAT, но различия были незначимыми (P > 0.05). Таким образом, представитель подсемейства Rab ‒ Rab3d, в наибольшей степени ассоциирован с WAT. Поэтому дальнейшая работа была сфокусирована преимущественно на изучении функции белка Rab3d (рис. 1).
Рис. 1.
Паттерны экспрессии разных генов Rab3 в жировых тканях мыши. qRT-PCR-анализ транскриптов Rab3a–Rab3d в разных тканях. В качестве референсного образца использовали транскрипт Rab3a из образца gWAT, а уровень мРНК β-актина в качестве внутреннего контроля. Представлены значения среднего ± стандартное отклонение, вычисленные из результатов трех независимых экспериментов. ns ‒ статистически незначимо.
Rab3D способствует росту LD в преадипоцитах 3T3-L1
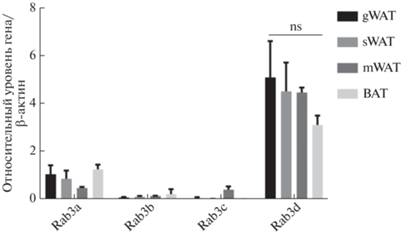
Для мониторинга динамики роста LD под действием ОА (индуктор накопления LD), преадипоциты 3T3-L1 в шестилуночном планшете транзиентно трансфицировали pEGFP-C1-Rab3D или плазмидой pEGFP-C1 без вставки (1 мкг/лунку). Показано, что белок Rab3D локализован преимущественно вокруг LD (рис. 2a). Кроме того, мы систематически определяли размеры самых крупных LD в клетках дикого типа или в клетках, сверхэкспрессирующих Rab3D, после обработки OA. В контрольных клетках большинство LD имели диаметр около 1 мкм, тогда как средний диаметр LD в группе Rab3D был почти в 2 раза больше, чем в контроле (Р < 0.05) (рис. 2б). С увеличением концентрации трансфицируемой ДНК Rab3d происходит дальнейшее увеличение соответствующих LD (рис. 2в, г). Это свидетельствует о дозозависимой стимуляции роста LD в преадипоцитах 3T3-L1 под действием Rab3D.
Рис. 2.
Сверхэкспрессия Rab3D в преадипоцитах 3T3-L1 и субклеточная локализация Rab3D в преадипоцитах 3T3-L1 (a). Диаметры самых крупных LD в каждой клетке (среднее ± SD; ≥ 80 в каждой группе; ***P < 0.001) (б). Диаметры самых крупных LD в преадипоцитах 3T3-L1, сверхэкспрессирующих различные концентрации Rab3D (среднее ± SD; n ≥ 100 в каждой группе) (в). Вестерн-блот, показывающий уровни белка. В качестве контроля нагрузки использовали β-актин (г). NC – контрольные клетки.
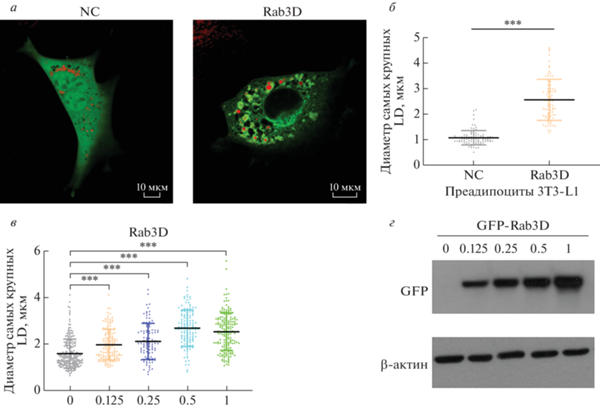
LD-стимулирующая активность Rab3D не зависит от опосредованного Cidec слияния LD
Поскольку Rab3D более активно экспрессируется в WAT, чем в BAT, и способен стимулировать рост LD, мы предположили, что он может участвовать в опосредуемом Cidec слиянии и росте LD. Чтобы исключить влияние других компонентов, мы ввели Rab3D в клетки Hela дикого типа или в стабильные линии клеток, сверхэкспрессирующих Cidec. Для индукции образования LD клетки Hela каждой группы стимулировали также обработкой OA. В отличие от преадипоцитов 3T3-L1, в клетках Hela Rab3D локализовался преимущественно в участках контактов с LD, а добавление белка Cidec не влияло на его локализацию (рис. 3a, в). Согласно количественным данным, Rab3D не регулирует размер LD в клетках Hela дикого типа (рис. 3б). В клетках Hela со стабильной экспрессией Cidec этот белок стимулировал образование более крупных LD, средний диаметр которых был почти в 4 раза больше, чем в клетках дикого типа, что свидетельствует о функционировании процессов слияния и роста LD в этой стабильной клеточной линии Hela. Добавление Rab3D, к сожалению, не влияет на размер LD (рис. 3г). Кроме того, опыты по коиммунопреципитации подтвердили, что взаимодействие между Rab3D и Cidec (данные не приведены). Эти данные свидетельствуют о том, что Rab3D стимулирует образование крупных LD не с помощью опосредуемого Cidec слияния LD, а с использованием других неизвестных процессов.
Рис. 3.
Сверхэкспрессия Rab3D в клетках Hela и субклеточная локализация Rab3D в клетках Hela дикого типа или в клетках Hela со стабильной сверхэкспрессией Cidec (a, в). Диаметры наиболее крупных LD в клетках (б, г). Приведено среднее значение ± стандартное отклонение; n ≥ 60 для каждой группы; ns – незначимое различие. NC – контрольные клетки.
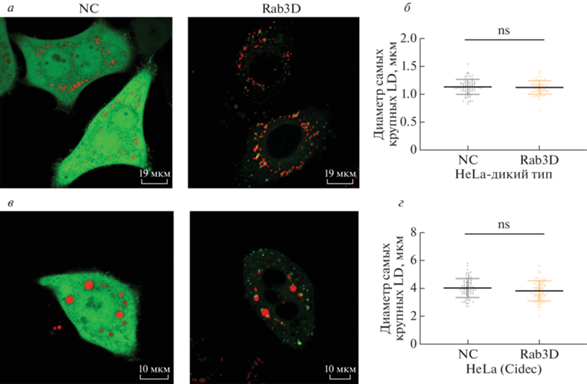
GTP-связанный Rab3D действует как активатор роста LD в преадипоцитах 3T3-L1
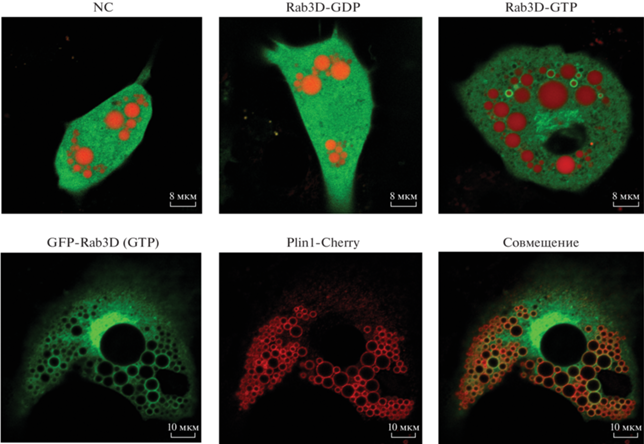
Исходя из свойств белков Rab (цикличное чередование “активного” GTP-связанного и “неактивного” GDP-связанного состояния), мы использовали сайт-направленный мутагенез для Rab3D в GDP- или GTP-связанной форме. Ранее было показано, что замена глутамина (Q) в положении 81 на лейцин (L) приводит к конформации Rab3D-GTP, тогда как замещение аспарагина (N) в положении 135 изолейцином (I) приводит к стабилизации мутанта Rab3D-GDP. Поэтому мы сконструировали специфические мутанты Rab3D и экспрессировали их в виде белков, слитых с зеленым флуоресцентным белком (EGFP). Показано, что Rab3D-GDP локализован в цитоплазме, тогда как Rab3D-GTP находится преимущественно в других везикулах, но не в структурах LD (рис. 4a). Это подтверждено путем котрансфекции с перилипином 1 (plin1) – специфическим маркером поверхности LD. Rab3D-GTP, коэкспрессируемый с plin1-cherry, не колокализуется с plin1 (рис. 4б). В целом это указывает на различия в субклеточной локализации разных форм Rab3D; неожиданно оказалось, что в преадипоцитах 3Т3-L1 активная форма Rab3D-GTP локализуется не в LD, а в других мембранных структурах. Далее мы определили размеры LD в преадипоцитах 3T3-L1, сверхэкспрессирующих специфические мутанты Rab3D. Как видно из рис. 4в, в клетках с GTP-связанным мутантом Rab3D LD имеют такой же диаметр, как в клетках с Rab3D дикого типа (P > > 0.05), тогда как размеры LD в группе Rab3D-GDP были значительно меньше, чем в первых двух группах (P < 0.001). Эти данные показывают, что именно форма Rab3D-GTP действует как активатор роста LD в преадипоцитах 3T3-L1.
Рис. 4.
Субклеточная локализация мутантов Rab3D в преадипоцитах 3T3-L1. a, б – Преадипоциты 3T3-L1 трансфицированные различными формами GFP-Rab3D (зеленые сигналы) или котрансфицированые plin1 (красные сигналы). LD окрашивали BodipyC12 (красный). Все опыты повторяли 3 раза. в – Измерение диаметров самых крупных LD в клетках (среднее ± SD; n ≥ 50 в каждой группе; P < 0.001***; ns, отсутствие значимых различий). NC – контрольные клетки.
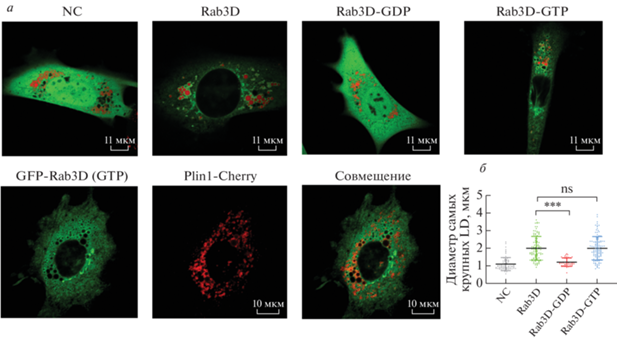
В зрелых адипоцитах 3T3-L1 GTP-связанная форма Rab3D частично транслоцируется в LD
Поскольку в ходе дифференцировки адипоцитов уровень белка Rab3D постепенно повышается, мы проанализировали субклеточную локализацию мутантов Rab3D в зрелых адипоцитах 3T3-L1. Оказалось, что Rab3D-GDP более равномерно распределен в цитоплазме, чем в контрольной группе. Интересно, что Rab3D-GTP сильно концентрируется вокруг некоторого числа LD, образуя в зрелых адипоцитах относительно равномерную кольцевую структуру. Более того, в зрелых адипоцитах 3T3-L1, котрансфицированных Rab3D-GTP и белком plin1 выявлена определенная степень колокализации Rab3D-GTP с мембранной системой LD, в которой локализуется plin1 (рис. 5). Одним словом, это показывает, что GTP-связанная форма Rab3D частично локализуется в LD, тогда как Rab3D-GDP остается в цитоплазме зрелых адипоцитов 3T3-L1.
Рис. 5.
Субклеточная локализация мутантов Rab3D в дифференцированных клетках 3t3-l1. зрелые клетки 3t3-l1 трансфицировали только мутантами gfp-rab3d (зеленые сигналы) или котрансфицировали с plin1 (красные сигналы). ld окрашивали bodipyc12 (красный). все опыты повторяли 3 раза. NC – контрольные клетки.
ОБСУЖДЕНИЕ РЕЗУЛЬТАТОВ
Мы определяли уровни экспрессии мРНК четырех белков семейства Rab3 в жировых тканях. Кроме того, с помощью сайт-направленного мутагенеза мы сконструировали Rab3D в разных состояниях и проанализировали их субклеточную локализацию в адипоцитах 3T3-L1 до и после дифференцировки и изучили их регуляцию при росте адипоцитов.
Во-первых, мы подтвердили, что из четырех членов семейства Rab3 самый высокий уровень экспрессии на уровне мРНК имеет Rab3D. Ранее в адипоцитах идентифицировали Rab3D, экспрессия которого постепенно увеличивалась в ходе дифференцировки адипоцитов. Однако функциональные исследования до настоящего времени не проводили. Более высокий уровень Rab3D в WAT подразумевает, что эта форма является критически важным регулятором метаболизма липидов. WAT сохраняет избыток энергии в форме триглицеридов в крупных “однокамерных” LD, тогда как запасенная в BAT энергия рассеивается в виде тепла; ВАТ обычно содержит “многокамерные” LD и обогащена митохондриями, кровеносными сосудами и нервами [60]. Более того, подтверждено, что Rab3D может стимулировать увеличение LD. И в WAT, и в BAT существуют три пути роста LD, которые наиболее сильно различаются процессами слияния LD, опосредуемыми белками CIDE. Так Cidea, один из трех членов семейства CIDE, экспрессируется преимущественно в BAT, тогда как Cidec ‒ почти исключительно в WAT [11, 61]. Поэтому мы предположили, что Rab3D может играть роль в опосредуемом Cidec слиянии LD. Однако введение Rab3D в клетки Hela, стабильно экспрессирующие Cidec, не влияло на размеры LD. Кроме того, не выявлено прямого взаимодействия между Rab3D и Cidec. Это показывает, что другие неизвестные компоненты, специфичные для адипоцитов, но не Cidec, могут участвовать в этом процессе. Для идентификации белков-мишеней Rab3D, участвующих в контроле увеличения размеров LD, необходимы дальнейшие исследования. Аномальную локализацию Rab3D и утрату его функционирования в контроле роста LD в клетках Hela дикого типа можно объяснить отсутствием специфических эффекторов и вспомогательных молекул, таких как в клетках 3T3-L1.
Кроме того, мы проверили субклеточную локализацию Rab3D. Показано, что в преадипоцитах 3T3-L1 Rab3D-GTP локализуется на поверхности других везикул, а не LD. В дифференцированных адипоцитах, однако, GTP-форма частично находится в LD, формируя равномерную кольцевую структуру. Такая локализация, по-видимому, важна для функционирования Rab3D, так как GDP-форма Rab3D в ходе дифференцировки остается в цитоплазме. Одним из объяснений может быть то, что в этих двух моделях Rab3D-GTP способен участвовать в разных процессах. Кроме того, GTP-связанная форма Rab3D транслоцируется в LD из других везикул, и эта ассоциация зависит от других, еще не известных компонентов, образующихся в ходе дифференцировки адипоцитов. Последняя гипотеза более приемлема, поскольку Rab3D-GTP может повышать образование крупных LD в преадипоцитах 3T3-L1. Это согласуется с представлениями о том, что GTP-связанные Rab активно стимулируют мембранный трафик, тогда как GTPaзы в GDP-связанной форме редко играют позитивную регуляторную роль. Изначально идентифицированный в адипоцитах белок Rab3D экспрессируется и в других секреторных гранулах и действует как позитивный модулятор экзоцитоза [53, 56, 62‒64]. Несмотря на то, что в протеомных исследованиях обнаружены десятки белков Rab, ассоциированных с LD, только несколько из них, как сообщалось, активно участвуют в образовании LD, включая Rab 1, 5, 7, 8a, 18, 32 и 40c. В число этих белков входит Rab32 ‒ уникальный представитель этого семейства, не локализованный в LD. Rab32 действует на лизосомы и аутофагосомы, влияя на аутофагию, а следовательно, и на запасание липидов. Таким образом, для выяснения того, являются ли везикулы, в которых локализован Rab3D, лизосомами или какими-то другими органеллами, и каким образом Rab3D транслоцируются из этих везикул в LD, необходимы дальнейшие исследования.
Все процедуры, выполненные в данной работе, соответствуют этическим стандартам институционального комитета по исследовательской этике и Хельсинкской декларации 1964 года и ее последующим изменениям или сопоставимым нормам этики
Авторы заявляют об отсутствии конфликта интересов.
T.W. и L.K.L. предложили идею и разработали схему эксперимента, T.W. и M.J.J. провели эксперименты, T.W. проанализировал и интерпретировал данные, а также написал статью.
Список литературы
Rosen E.D., Spiegelman B.M. (2014) What we talk about when we talk about fat. Cell. 156(1‒2), 20‒44.
Fujimoto T., Parton R.G. (2011) Not just fat: the structure and function of the lipid droplet. Cold Spring. Harb. Perspect. Biol. 3(3). a004838
Olzmann J.A., Carvalho P. (2019) Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 20(3), 137‒155.
Martin S., Parton R.G. (2006) Lipid droplets: a unified view of a dynamic organelle. Nat. Rev. Mol. Cell Biol. 7(5), 373‒378.
Farese R.V., Jr., Walther T.C. (2009) Lipid droplets finally get a little R-E-S-P-E-C-T. Cell. 139(5), 855‒860.
Thiam A.R., Farese R.V., Jr., Walther T.C. (2013) The biophysics and cell biology of lipid droplets. Nat. Rev. Mol. Cell Biol. 14(12), 775‒786.
Wilfling F., Haas J.T., Walther T.C., Farese R.V., Jr. (2014) Lipid droplet biogenesis. Curr. Opin. Cell Biol. 29, 39‒45.
Long A.P., Manneschmidt A.K., VerBrugge B., Dortch M.R., Minkin S.C., Prater K.E., Biggerstaff J.P., Dunlap J.R., Dalhaimer P. (2012) Lipid droplet de novo formation and fission are linked to the cell cycle in fission yeast. Traffic. 13(5), 705‒714.
Krahmer N., Guo Y., Farese R.V., Jr., Walther T.C. (2009) SnapShot: lipid droplets. Cell. 139(5), 1024‒U1192.
Gong J., Sun Z., Wu L., Xu W., Schieber N., Xu D., Shui G., Yang H., Parton R.G., Li P. (2011) Fsp27 promotes lipid droplet growth by lipid exchange and transfer at lipid droplet contact sites. J. Cell Biol. 195(6), 953‒963.
Wu L.Z., Zhou L.K., Chen C., Gong J.Y., Xu L., Ye J., Li D., Li P. (2014) Cidea controls lipid droplet fusion and lipid storage in brown and white adipose tissue. Sci. China-Life Sci. 57(1), 107‒116.
Murphy S., Martin S., Parton R.G. (2010) Quantitative analysis of lipid droplet fusion: inefficient steady state fusion but rapid stimulation by chemical fusogens. PLoS One. 5(12), e15030.
Gong J., Sun Z., Li P. (2009) CIDE proteins and metabolic disorders. Curr. Opin. Lipidology. 20(2), 121‒126.
Inohara N., Koseki T., Chen S., Wu X.Y., Nunez G. (1998) CIDE, a novel family of cell death activators with homology to the 45 kDa subunit of the DNA fragmentation factor. EMBO J. 17(9), 2526‒2533.
Puri V., Konda S., Ranjit S., Aouadi M., Chawla A., Chouinard M., Chakladar A., Czech M.P. (2007) Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. J. Biol. Chem. 282(47), 34213‒34218.
Puri V., Ranjit S., Konda S., Nicoloro S.M.C., Straubhaar J., Chawla A., Chouinard M., Lin C., Burkart A., Corvera S., Perugini R.A., Czech M.P. (2008) Cidea is associated with lipid droplets and insulin sensitivity in humans. Proc. Natl. Acad. Sci. USA. 105(22), 7833–7838.
Jambunathan S., Yin J., Khan W., Tamori Y., Puri V. (2011) FSP27 promotes lipid droplet clustering and then fusion to regulate triglyceride accumulation. PLoS One. 6(12), e28614.
Lin S.C., Li P. (2004) CIDE-A, a novel link between brown adipose tissue and obesity. Trends Mol. Med. 10(9), 434‒439.
Wu C., Zhang Y., Sun Z., Li P. (2008) Molecular evolution of Cide family proteins: novel domain formation in early vertebrates and the subsequent divergence. BMC Evol. Biol. 8, 159.
Li J.Z., Lei Y., Wang Y., Zhang Y., Ye J., Xia X., Pan X., Li P. (2010) Control of cholesterol biosynthesis, uptake and storage in hepatocytes by Cideb. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids. 1801(5), 577‒586.
Li J.Z., Ye J., Xue B., Qi J., Zhang J., Zhou Z., Li Q., Wen Z., Li P. (2007) Cideb regulates diet-induced obesity, liver steatosis, and insulin sensitivity by controlling lipogenesis and fatty acid oxidation. Diabetes. 56(10), 2523‒2532.
Singaravelu R., Lyn R.K., Srinivasan P., Delcorde J., Steenbergen R.H., Tyrrell D.L., Pezacki J.P. (2013) Human serum activates CIDEB-mediated lipid droplet enlargement in hepatoma cells. Biochem. Biophys. Res. Commun. 441(2), 447‒452.
Danesch U., Hoeck W., Ringold G.M. (1992) Cloning and transcriptional regulation of a novel adipocyte-specific gene, Fsp27 ‒ CAAT-enhancer-binding protein (c/EBP) and c/EBP-like proteins interact with sequences required for differentiation-dependent expression. J. Biol. Chem. 267(10), 7185‒7193.
Li D., Zhang Y., Xu L., Zhou L., Wang Y., Xue B., Wen Z., Li P., Sang J. (2010). Regulation of gene expression by FSP27 in white and brown adipose tissue. BMC Genomics. 11, 446.
Nishino N., Tamori Y., Tateya S., Kawaguchi T., Shibakusa T., Mizunoya W., Inoue K., Kitazawa R., Kitazawa S., Matsuki Y., Hiramatsu R., Masubuchi S., Omachi A., Kimura K., Saito M., Amo T., Ohta S., Yamaguchi T., Osumi T., Cheng J., Fujimoto T., Nakao H., Nakao K., Aiba A., Okamura H., Fushiki T., Kasuga M. (2008) FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. J. Clin. Invest. 118(8), 2808‒2821.
Walther T.C., Farese R.V., Jr. (2012) Lipid droplets and cellular lipid metabolism. Annu. Rev. Biochem. 81, 687‒714.
Ducharme N.A., Bickel P.E. (2008) Lipid droplets in lipogenesis and lipolysis. Endocrinology. 149(3), 942‒949.
Pereira-Leal J.B., Seabra M.C. (2001) Evolution of the Rab family of small GTP-binding proteins. J. Mol. Biol. 313(4), 889‒901.
Seabra M.C., Mules E.H., Hume A.N. (2002) Rab GTPases, intracellular traffic and disease. Trends Mol. Med. 8(1), 23‒30.
Vonmollard G.F., Stahl B., Li C., Sudhof T.C., Jahn R. (1994) Rab proteins in regulated exocytosis. Trends Biochem. Sci. 19(4), 164‒168.
Mizuno-Yamasaki E., Rivera-Molina F., Novick P. (2012) GTPase networks in membrane traffic. Annu. Rev. Biochem. 81, 637‒659.
Pfeffer S., Aivazian D. (2004) Targeting RAB GTPases to distinct membrane compartments. Nat. Rev. Mol. Cell Biol. 5(11), 886‒896.
Seabra M.C., Wasmeier C. (2004) Controlling the location and activation of Rab GTPases. Curr. Opin. Cell Biol. 16(4), 451‒457.
Gillingham A.K., Sinka R., Torres I.L., Lilley K.S., Munro S. (2014) Toward a comprehensive map of the effectors of rab GTPases. Dev. Cell. 31(3), 358‒373.
Bersuker K., Peterson C.W.H., To M., Sahl S.J., Savi-khin V., Grossman E.A., Nomura D.K., Olzmann J.A. (2018) A proximity labeling strategy provides insights into the composition and dynamics of lipid droplet proteomes. Dev. Cell. 44(1), 97‒112. e117.
Krahmer N., Hilger M., Kory N., Wilfling F., Stoehr G., Mann M., Farese R.V., Jr., Walther T.C. (2013) Protein correlation profiles identify lipid droplet proteins with high confidence. Mol. Cell Proteomics. 12(5), 1115‒1126.
Turro S., Ingelmo-Torres M., Estanyol J.M., Tebar F., Fernandez M.A., Albor C.V., Gaus K., Grewal T., Enrich C., Pol A. (2006) Identification and characterization of associated with lipid droplet protein 1: a novel membrane-associated protein that resides on hepatic lipid droplets. Traffic. 7(9), 1254‒1269.
Xu D., Li Y., Wu L., Li Y., Zhao D., Yu J., Huang T., Ferguson C., Parton R.G., Yang H., Li P. (2018) Rab18 promotes lipid droplet (LD) growth by tethering the ER to LDs through SNARE and NRZ interactions. J. Cell Biol. 217(3), 975‒995.
Martin S., Driessen K., Nixon S.J., Zerial M., Parton R.G. (2005) Regulated localization of Rab18 to lipid droplets: effects of lipolytic stimulation and inhibition of lipid droplet catabolism. J. Biol. Chem. 280(51), 42325–42335.
Ozeki S., Cheng J., Tauchi-Sato K., Hatano N., Taniguchi H., Fujimoto T. (2005) Rab18 localizes to lipid droplets and induces their close apposition to the endoplasmic reticulum-derived membrane. J. Cell Sci. 118(Pt 12), 2601‒2611.
Pulido M.R., Diaz-Ruiz A., Jimenez-Gomez Y., Garcia-Navarro S., Gracia-Navarro F., Tinahones F., Lopez-Miranda J., Fruhbeck G., Vazquez-Martinez R., Malagon M.M. (2011) Rab18 dynamics in adipocytes in relation to lipogenesis, lipolysis and obesity. PLoS One. 6(7), e22931.
Wu L., Xu D., Zhou L., Xie B., Yu L., Yang H., Huang L., Ye J., Deng H., Yuan Y.A., Chen S., Li P. (2014) Rab8a-AS160-MSS4 regulatory circuit controls lipid droplet fusion and growth. Dev. Cell. 30(4), 378‒393.
Wang C., Liu Z., Huang X. (2012) Rab32 is important for autophagy and lipid storage in Drosophila. PLoS One. 7(2), e32086.
Tan R., Wang W., Wang S., Wang Z., Sun L., He W., Fan R., Zhou Y., Xu X., Hong W., Wang T. (2013) Small GTPase Rab40c associates with lipid droplets and modulates the biogenesis of lipid droplets. PLoS One. 8(4), e63213.
Schroeder B., Schulze R.J., Weller S.G., Sletten A.C., Casey C.A., McNiven M.A. (2015) The small GTPase Rab7 as a central regulator of hepatocellular lipophagy. Hepatology. 61(6), 1896‒1907.
Li C., Yu S.S. (2016) Rab proteins as regulators of lipid droplet formation and lipolysis. Cell Biol. Int. 40(10), 1026‒1032.
Liu P., Bartz R., Zehmer J.K., Ying Y.S., Zhu M., Serrero G., Anderson R.G. (2007). Rab-regulated interaction of early endosomes with lipid droplets. Biochim. Biophys. Acta. 1773(6), 784‒793.
Nevo-Yassaf I., Yaffe Y., Asher M., Ravid O., Eizenberg S., Henis Y.I., Nahmias Y., Hirschberg K., Sklan E.H. (2012) Role for TBC1D20 and Rab1 in hepatitis C virus replication via interaction with lipid droplet-bound nonstructural protein 5A. J. Virol. 86(12), 6491‒6502.
Yamaguchi K., Tanaka M., Mizoguchi A., Hirata Y., Ishizaki H., Kaneko K., Miyoshi J., Takai Y. (2002) A GDP/GTP exchange protein for the Rab3 small G protein family up-regulates a postdocking step of synaptic exocytosis in central synapses. Proc. Natl. Acad. Sci. USA. 99(22), 14536‒14541.
Schluter O.M., Schmitz F., Jahn R., Rosenmund C., Sudhof T.C. (2004) A complete genetic analysis of neuronal Rab3 function. J. Neurosci. 24(29), 6629‒6637.
Schluter O.M., Khvotchev M., Jahn R., Sudhof T.C. (2002) Localization versus function of Rab3 proteins ‒ Evidence for a common regulatory role in controlling fusion. J. Biol. Chem. 277(43), 40919‒40929.
Valentijn J.A., Sengupta D., Gumkowski F.D., Tang L.H., Konieczko E.M., Jamieson J.D. (1996) Rab3D localizes to secretory granules in rat pancreatic acinar cells. Eur. J. Cell Biol. 70(1), 33‒41.
Valentijn J.A., van Weeren L., Ultee A., Koster A.J. (2007) Novel localization of Rab3D in rat intestinal goblet cells and Brunner’s gland acinar cells suggests a role in early Golgi trafficking. Am. J. Physiol. Gastrointest. Liver Physiol. 293(1), G165‒177.
Chen X., Edwards J.A., Logsdon C.D., Ernst S.A., Williams J.A. (2002) Dominant negative Rab3D inhibits amylase release from mouse pancreatic acini. J. Biol. Chem. 277(20), 18002‒18009.
Chen X., Ernst S.A., Williams J.A. (2003) Dominant negative Rab3D mutants reduce GTP-bound endogenous Rab3D in pancreatic acini. J. Biol. Chem. 278(50), 50053‒50060.
Riedel D., Antonin W., Fernandez-Chacon R., Alvarez de Toledo G., Jo T., Geppert M., Valentijn J.A., Valentijn K., Jamieson J.D., Sudhof T.C., Jahn R. (2002) Rab3D is not required for exocrine exocytosis but for maintenance of normally sized secretory granules. Mol. Cell Biol. 22(18), 6487‒6497.
Wang T., Meng J., Li L., Zhang G. (2016) Characterization of CgHIFalpha-like, a novel bHLH-PAS transcription factor family member, and its role under hypoxia stress in the pacific oyster Crassostrea gigas. PLoS One. 11(11), e0166057.
Iezzi M., Escher G., Meda P., Charollais A., Baldini G., Darchen F., Wollheim C.B., Regazzi R. (1999) Subcellular distribution and function of Rab3A, B, C, and D isoforms in insulin-secreting cells. Mol. Endocrinol. 13(2), 202‒212.
Sun Z., Gong J., Wu H., Xu W., Wu L., Xu D., Gao J., Wu J.W., Yang H., Yang M., Li P. (2013) Perilipin1 promotes unilocular lipid droplet formation through the activation of Fsp27 in adipocytes. Nat. Commun. 4, 1594.
Saely C.H., Geiger K., Drexel H. (2012) Brown versus white adipose tissue: a mini-review. Gerontology. 58(1), 15‒23.
Xu X., Park J.-G., So J.-S., Lee A.-H. (2015) Transcriptional activation of Fsp27 by the liver-enriched transcription factor CREBH promotes lipid droplet growth and hepatic steatosis. Hepatology (Baltimore, Md). 61(3), 857‒869.
Zhu S., Chim S.M., Cheng T., Ang E., Ng B., Lim B., Chen K., Qiu H., Tickner J., Xu H., Pavlos N., Xu J. (2016) Calmodulin interacts with Rab3D and modulates osteoclastic bone resorption. Sci. Rep. 6, 37963.
Pavlos N.J., Xu J., Riedel D., Yeoh J.S., Teitelbaum S.L., Papadimitriou J.M., Jahn R., Ross F.P., Zheng M.H. (2005) Rab3D regulates a novel vesicular trafficking pathway that is required for osteoclastic bone resorption. Mol. Cell Biol. 25(12), 5253‒5269.
van Weeren L., de Graaff A.M., Jamieson J.D., Batenburg J.J., Valentijn J.A. (2004). Rab3D and actin reveal distinct lamellar body subpopulations in alveolar epithelial type II cells. Am. J. Respir. Cell Mol. Bio. 30(3), 288‒295.
Дополнительные материалы
- скачать ESM_1.tiff
- S1 Fig. Rab3D mutants’ DNA sequences alignment.
S2 Fig. Rab3D mutants’ protein sequences alignment.
Инструменты
Молекулярная биология


