Растительные ресурсы, 2020, T. 56, № 3, стр. 280-288
Biologically Active Compounds and Biological Activity of Physalis alkekengi (Solanaceae)
1 Institute of Botany of Azerbaijan National Academy of Sciences
Baku, Azerbaijan
* E-mail: eldar_novruzov@yahoo.co.uk
Поступила в редакцию 16.07.2019
После доработки 20.11.2019
Принята к публикации 18.03.2020
Аннотация
Physalis alkekengi L. was well known as a medicinal plant in Ancient China. Fruits, calyx, and plant roots have been used to treat a number of ailments, including sore throat, cough, eczema, hepatitis, and tumours. In the Unani system of medicine, Kaknaj, the dried fruits of P. alkekengi are valued primarily as a source of vitamin C and used as a diuretic, antiseptic and sedative. Modern medical research has shown that P. alkekengi is effective in therapy of the disorders of immune system, thyroid hormones, liver enzymes, reproductive sex hormones, against cancer. The plant owes its beneficial properties to the presence of biologically active compounds, such as alkaloids, flavonoids, steroids, vitamin C, etc. The purpose of this study is to summarize literature data on the results of experimental studies of the component composition and biological activity of P. alkekengi. The results of the chemical study of P. alkekengi showes that fresh fruits, calyx, roots and leaves are rich in various biologically active substances such as flavonoids, steroids, alkaloids, etc. Chemical composition of various organs of P. alkekengi as well as antimycobacterial, immunomodulating, antitumour, anti-inflammatory and other properties of groups of substances and individual compounds isolated from P. alkekengi extracts were studied. The studied literature data allows to consider P. alkekengi as a promising source of substances for medicinal products.
The genus Physalis L. from the Solanaceae L. family has from 75 to 120 species in the world flora, widely distributed throughout South and North America, as well as in the temperate regions of Europe and Asia [1–4]. In Azerbaijan, the genus is represented by 2 species. One of them is wild-growing – Physalis alkekengi L. is widely distributed in almost all botanical and geographical regions of Azerbaijan, the second is Physalis ixocarpa Brot ex Hornem. found only in culture [3]. Among the species belonging to the genus Physalis L., P. alkekengi. is the most popular food, ornamental and medicinal plant.
P. alkekengi was well known as a medicinal plant in Ancient China. Fruits, calyx, and plant roots have been used to treat a number of ailments, including sore throat, cough, eczema, hepatitis, and tumours [2]. In the Unani system of medicine, Kaknaj, the dried fruits of Physalis alkekengi are valued primarily as a source of vitamin C and used as a diuretic, antiseptic and sedative remedies [1]. For centuries, infusion of fruits has been used as an abortive and contraceptive remedy in Iranian traditional medicine [4]. Modern medical research has shown that P. alkekengi is effective in therapy of the disorders of immune system, thyroid hormones, liver enzymes, reproductive sex hormones, against cancer. The plant owes its beneficial properties to the presence of biologically active compounds, such as alkaloids, flavonoids, steroids, vitamin C, etc. [5]. The juice form P. alkekengi fruit has an antiseptic effect, therefore it is used externally to treat septic wounds and ulcers [6].
The purpose of this study is to summarize literature data on the results of experimental studies of the component composition and biological activity of P. alkekengi.
BIOLOGICALLY ACTIVE COMPONUNDS OF PHYSALIS ALKEKENGI L.
About nineteen flavonoids have been isolated and identified from various parts of the P. alkekengi in the form of flavones and flavonols. Isolated flavonoids include luteolin and their glycosides, quercetin derivatives and kampferol glycosides, which are widely distributed in medicinal plants [7–13]. In addition, four flavone analogs, apigenin-O-β-D-glucopyranoside, diosmetin-O-β-D-diglucopyranoside, chrysoriol and its glucopyranoside, as well as three flavonol analogs, 3', 4' dimethoxymyritsetin, ombine and 5.4', 5'-trihydroxy-7,3'-dimethoxyflavonol were obtained from sepals [8–10, 14, 15). These isolated flavonoids were best known for their antioxidant and anti-inflammatory properties [16–18].
As a result of a study conducted by Prokopenko and co-authors, 46 lipophilic compounds were found in the aboveground part of P. alkekengi, of which 29 compounds were identified and estimated, including organic acids, phytosterols, hydrocarbons, terpene compounds, etc. Among identified compounds, palmitic and linolenic acids were found [6].
Linoleic acid has a high biological activity and is not produced in the human body. Owing to the high content of linoleic acid, the aboveground parts of P. alkekengi are used as raw material for the production of dietary supplements.
In early studies it was reported that only 10 carotenoids are present in the fruits and calyx of P. alkekengi [19–21]. The study of 2017 allowed to isolate 69 different carotenoids and carotenoid esters in the fruits and calyx of P. alkekengi, of which 45 were identified. Zeaxanthin esters with various fatty acids were the most common carotenoids, accounting for 51–63% of the total carotenoids. The fruits and calyx of plant have been reported to be rich sources of xanthophylls, mainly zeaxanthin esters (6.39 mg zeaxanthin/100 g fresh fruit, 54.89 mg zeaxanthin/100 g fresh sepal), followed by β-cryptoxanthin esters (no data on fruits, 3.92 mg/g of dry sepal). Esters of carotenoids accounted for 94–96% of the total amount of carotenoids in P. alkekengi the fruits. Zeaxanthin esters (56–63% of the total amount of carotenoids) were the most common in the fruit, followed by β-cryptoxanthin (18–24%), anterxanthine (4–6%) and violaxanthin esters (2–4%). Small amount of lutein, lutoxanthin, mutatoxanthin, neoxanthin, auroxanthin and zeinoxanthin esters were identified. In addition to carotenoids, a small amount of chlorophyll b, chlorophyll a and pheophytin was also identified in P. alkekengi fruits [22]. According to the content as the sum of carotenoids and xanthophylls, the fruits of P. alkekengi exceeds fruits of all other plants rich in carotenoids.
Pintea and others showed that P. alkekengi calyx contain zeaxanthin and beta-cryptoxanthin esters, which are widely used as nutritional supplements [20].
Physalins have been extensively studied over the past 20 years and are reported to have anti-mycobacterial, immunomodulatory, anti-tumour, and anti-inflammatory effects [23, 24].
Physalins are the main steroid components of P. alkekengi and their rare 13,14-seco-16,24-cycloergostane skeletons were first established by x-ray structural analysis [25–27]. Physalin A, isolated from the leaves of P. alkekengi in 1969, was the first steroid of this group [28]. Subsequently, a series of physalins with a complex and diverse structure were obtained from various species of the genus Physalis. So far, phytochemical studies of P. alkekengi have led to isolation of fifty physalins, most of which were first found while studying this species. The structural diversity of physalins is determinde by cyclization, changes in the degree of unsaturation, and changes in the ring substitution structure. For example, W, X, Y, and Z physalins, as well as 3-O-methylphysaline X and isophysalin G, are 3-hydroxyl and/or 3-methoxy-substituted physalins, while fizalin C and 5α, 6β-dihydroxyphysaline R are components 5,6-dihydroxyl group [24]. Physalin P was the first neofizalin, isolated from the aboveground part of P. alkekengi in 1993 [29]. In 2013, a group of Chinese researches isolated three new physicals from P. alkekengi sepals and named them physalin III, physalin IV, 3-O-methylphysalin X [30]. In 2016, another 6 new physalins 7β-methoxylisophysalin B, 7β-methoxyphysaline C, fizalin V, fizalin VI, fizalin VII, isophiline I [31] were determined.
The chemical study of P. alkekengi (Table 1) show that fresh fruits, as well as other parts (calyx, roots, leaves) are rich in various biologically active substances (flavonoids, steroids, alkaloids, etc.).
Table 1.
Chemical compounds isolated from various parts of P. alkekengi L.
| Chemical group | Chemical component | Structure | Part of the plant | References |
|---|---|---|---|---|
| Flavonoids | Luteolin | 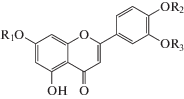 R1=R2=R3=H
R1=R2=R3=H
|
Calyx | [8–10, 13] |
| Luteolin-7-O-β-D- glucopyranoside | R1=Glu; R2=R3=H | Calyx | [7–10, 13] | |
| Luteolin-4'-O-β-D- glucopyranoside | R1=R3=H; R2=Glu | Calyx | [7, 8] | |
| Luteolin-7,4'-di-O-β-D- glucopyranoside | R1=R2=Glu; R3=H | Calyx | [8] | |
| Luteolin-7,3'-di-O-β-D- glucopyranoside | R1=R3=Glu; R2=H | Calyx | [7, 8] | |
| 3',7-Dimethylquercetin |  R1=R2=CH3; R3=R4=H
R1=R2=CH3; R3=R4=H
|
Calyx | [12] | |
| 3',4',7-Trimethylquercetin | R1=R2=R3=CH3; R4=H | Calyx | [12] | |
| 3',4'-Dimethylquercetin | R1=R4=H; R2=R3=CH3 | Calyx | [11] | |
| Quercetin-3-O-β-D- glucopyranoside | R1=R2=R3=H; R4=Glu | Calyx | [7, 8] | |
| Quercetin-3,7-di-O-β-D- glucopyranoside | R1=R4=Glu; R2=R3=H | Calyx | [7, 8] | |
| Kaempferol-3-O-β-D- Glucose |  R1=H; R2=Glu
R1=H; R2=Glu
|
Calyx | [11] | |
| Steroids | Physalin A |  |
Calyx | [8, 9, 11, 13, 14, 28, 31, 46–48] |
| Physalin B |  |
Calyx | [8, 9, 11, 12, 14, 31, 46, 47] | |
| Physalin D |  |
Calyx | [8, 10, 13, 14, 31, 47, 49] | |
| Physalin O |  |
Calyx | [8, 9, 13, 31, 50] | |
| Physalin F |  |
Calyx | [8, 11, 31] | |
| Physalin G |  |
Calyx | [14, 31] | |
| Physalin L |  |
Calyx | [8, 9, 13, 23, 31, 46, 47] | |
| Alkaloids | Calystegin A5 |  R1=R2=H
R1=R2=H
|
Roots | [14] |
| Calystegin B1 |  R=OH
R=OH
|
Roots | [14] | |
| Calystegin B2 |  R1=OH; R2=H
R1=OH; R2=H
|
Roots | [51] | |
| Organic acids | Syringic acid | 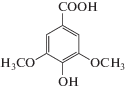 |
Calyx | [52] |
| 1,5-Dimethyl citrate |  |
Calyx | [53] | |
| 5-Hydroxymethylfuroic acid |  |
Calyx | [53] |
ANTIMICROBIAL AND ANTIFUNGAL ACTIVITY
Species of the genus Physalis, including Physalis alkekengi, are rich sources of physalins, steroids of the cycloergosiderated class, common only in this genus. Chemical composition of various organs of P. alkekengi as well as antimycobacterial, immunomodulating, antitumour, anti-inflammatory and other properties of groups of substances and individual compounds isolated from P. alkekengi extracts were studied.
Eight phenylpropanoids, consisting of four phenylpropanoic acid derivatives and four lignans, were isolated from P. alkekengi var. franchetii calyx. The isolated phenylpropanoic acid derivatives are chemical compounds, including ferulic acid methyl ester, 3-caffeoylquinic acid and syringalide, which express antibacterial, antiviral, and anti-inflammatory properties. All isolated lignans possess a tetrahydrofuran-type skeleton and exist in the form of glycosides containing syringaresinol, (+) – pinoresinol-di-O-β-D-glucopyranoside and (+) – medioresinol-di-O-β-D-glucopyranoside [14, 15].
Numerous studies of the antimicrobial and antifungal activities of P. alkekengi extracts have been conducted. Thus, the methanol extract from the aboveground parts and the dichloromethane extract from the P. alkekengi calyx were tested against five gram-positive and five gram-negative bacteria and five Candida species. According to the results of the experiments, the methanol extract showed moderate antifungal activity with minimum inhibitory concentration (MIC) from 128 to 512 μg/ml, dichloromethane extract and fizalin D had low antifungal activity with MIC from 256 to 512 μg/ml [32]. Chlorogenic acid isolated from the aerial parts of P. alkekengi showed high antibacterial activity against Staphylococcus aureus, Streptococcus pneumoniae, Bacillus subtilis, Escherichia coli and Shigella dysenteriae with MIC values ranging from 20 to 80 µg/ml. Physalins B, D, F and G showed an antimalarial effect against Plasmodium falciparum in vitro with IR 50 values ranging from 2.2 to 55 μM, where physalin F showed the highest effect (IC50 = 2.2 μM and LC50 = 13.3 μM) [33].
Six physalins isolated from the plant: 7β-methoxylisophysalin B, 7β-methoxyphysalin C, physalin V, physalin VI, physalin VII, isophysalin I were tested for antibacterial activity and cytotoxicity against human HL-60, SMMC-7721, A-549, MCF-7 and SW-480. The results showed that these compounds exhibit strong cytotoxicity (IC50 < 5 μM) in vitro. Antibacterial analysis showed that physalins exhibit high antibacterial activity against Bacillus subtilis and Escherichia coli [31]. Aqueous, ethanolic, and methanol fruit extracts were used against 3 types of fungi: Microsporum canis, Candida albicans, Trichophyton mentagrophytes. The aqueous extract had a limited spectrum of antifungal effect compared to the other two extracts. Ethanol extract had the strongest effect at MIC = 15.62 against all fungi tested [34].
ANTITUMOUR ACTIVITY
Several authors [35–38] provide information on the antitumour activity of P. alkekengi. Taken together, physalins, flavonoids and phenylpropanoids may be considered as the main antitumour substances. Physalins A and B, being representative compounds for the genus Physalis, were tested for their antitumor properties using cell analysis. [35, 38]. When studying physalin B, its antiproliferative effect was established. Physalin B was particularly active against human melanoma A375 and A2058 cells (IC50 <4.6 μg/ml) and had lower cytotoxicity on H9c2 rat cells and T/G HA-VSMC and CCD-966SK human cells. These results have shown that these two physalins A and B may be promising therapeutic agents for the treatment of cancer, especially melanoma.
The antitumour properties of the flavonoids isolated from P. alkekengi, including luteolin, quercetin and their analogues, have been thoroughly investigated [39, 40]. It has been found that these flavonoids can induce apoptosis of cancer cells, stop the cancer cell cycle and inhibit metastasis and angiogenesis.
ANTI-DIABETIC ACTIVITY
Several studies [41, 44] have found that P. alkekengi has the potential to reduce serum glucose concentration in alloxan-induced diabetic rats. The reduced glucose levels and activity may be associated with chemical compounds contained in fruits, mainly physalin, flavonoids, citric acid and vitamin C.
Antidiabetic activity of ferulic and chlorogenic acids isolated from P. alkekengi was studied. Using various diabetes models in vivo, it was found that these two compounds can reduce blood glucose levels, stimulate insulin secretion, improve glucose tolerance and insulin resistance, inhibit lipid absorption and stimulate lipid metabolism in vivo [42, 43].
Asano et al. established the antidiabetic potential of polysaccharides (calystegines) isolated from P. alkekengi fruits. Administration of isolated polysaccharides (50 and 100 mg/kg) reduced the blood glucosse level. Calystegines are a group of structural analogues of glucose and galactose, and, thus, they can block the process of carbohydrate metabolism by competitive inhibition of key glucosidases and galactosidases. It was found that сalystegin B2 isolated from P. alkekengi is a potent competitive inhibitor of almond β-glucosidase (Ki = 1.2 μM) and cofee bean α-galactosidase (Ki = = 0.86 μM) [45].
Despite using Physalis alkekengi for medical purposes, the reasons for its high biological activity have not been fully identified. The analysis of literature data allows to consider P. alkekengi as a promising source of substances for medicinal products.
Список литературы
Flora of China. 1978. Chinese Academy of Sciences. Science press, Beijing, China. Vol. 67. P. 50.
Martinez M. 1998. Revision of Physalis section Epeteiorhiza (Solanaceae). – Anales Inst. Biol. Univ. Nac. Auton. Mexico, Bot. 69(2): 71–117. http://www.ibiologia.unam.mx/BIBLIO68/fulltext/art-bota/692(1)bota.pdf.
Maggie W.P.S.M. 2005. Untangling Physalis (Solanaceae) from the Physaloids: A Two-Gene Phylogeny of the Physalinae. – Systematic Botany. 30(1): 216–230. https://doi.org/10.1600/0363644053661841
Wei J.L., Hu X.R., Yang J.J., Yang, W.C. 2012. Identification of Single-Copy Orthologous Genes between Physalis and Solanum lycopersicum and Analysis of Genetic Diversity in Physalis Using Molecular Markers. – PLoS One. 7(11): e50164. https://doi.org/10.1371/journal.pone.0050164
Flora of Azerbaijan. 1961. The Solanaceae. Volume 7: 400.
Prokopenko Yu.S., Georgiyants V.A., Mishchenko V.A. 2014. Lipophilic compounds of the aerial part of Physalis alkekengi. – Chem. Nat. Compd. 50(6): 1094–1095. https://doi.org/10.1007/s10600-014-1167-6
Qiu L., Jiang Z., Liu H., Chen L., Qu G., Qiu F. 2007. Flavonoid glycosides of the calyx Physalis. – Journal of Shenyang Pharm. Uni. 24(12): 744–747. (In Chinese)
Qiu L., Zhao F., Jiang Z.-H., Chen L.-X., Zhao Q., Liu H.X., Yao X.S., Qiu F. 2008. Steroids and flavonoids from Physalis alkekengi var. franchetii and their inhibitory effects on nitric oxide production. – J. Nat. Prod. 71(4): 642–646. https://doi.org/10.1021/np700713r
Xu D., Wang B., Zhou L., Kang T. 2009. Chemical constituents of Physalis alkekengi var. franchetii. – Zhong Cao Yao (Chinese Traditional and Herbal Drugs). 2: 175–178. (In Chinese)
Cai Q., Liu Y., Ma Z. 2009. Isolation and identification of chemical constituents from the fruit and calyx of Physalis alkekenegi L. var. franchetii (Mast.) Makino. – Shenyang Yao Ke Da Xue Xue Bao (Journal of Shenyang Pharmaceutical University). 10: 807–810, 817. (In Chinese)
Xing F., Jiang J. 2013. Chemical constituents in the calyx of Physalisalkekengivar. franchetii. – Xian Dai Yao Wu Yu Lin Chuang (Pharmaceutical and Clinical Research). 4: 344–346. (In Chinese)
Han A.R., Shim I., Seo E.K. 2015. Chemical constituents of Physalis alkekengi var. franchetii. – Chem. Nat. Compd. 51(6): 1160–1161. https://doi.org/10.1007/s10600-015-1517-z
Lin F., Wang J. 2011. Chemical constituents of physalins from dried calyx of Physalis alkekengi var. franchetii. – Xiandai Yaowu Yu Linchuang (Drugs and Clinic). 26(6): 469–472. http://www.tiprpress.com/xdywlc/article/abstract/201106012 (In Chinese)
Chen L.X., Xia G.Y., Liu Q.Y., Xie Y.Y., Qiu F. 2014. Chemical constituents from the calyces of Physalis alkekengi var. franchetii. Biochem. – Syst. Ecol. 54: 31–35. https://doi.org/10.1016/j.bse.2013.12.030
Shu Z., Xu B., Xing N., Li X., Wang Q., Yang B., Kuang H. 2014. Chemical constituents of Physalis Calyx Seu Fructus. – Zhong Guo Shi Yan Fang Ji Xue Za Zhi (Chinese Journal of Experimental Traditional Medical Formulae). 21: 99–102. (In Chinese)
Birt D.F., Hendrich S., Wang W. 2001. Dietary agents in cancer prevention: flavonoids and isoflavonoids. – Pharmacol. Ther. 90: 157–177. https://doi.org/10.1016/S0163-7258(01)00137-1
Nijveldt R.J., Van Nood E., Van Hoorn D.E., Boelen, P.G., Van Norren K., Van Leeuwen P.A. 2001. Flavonoids: a review of probable mechanisms of action and potential applications. – Am. J. Clin. Nutr. 74(2): 418–425. https://doi.org/10.1093/ajcn/74.4.418
Yao L.H., Jiang Y., Shi J., Tomas-Barberan F., Datta N., Singanusong R., Chen S. 2004. Flavonoids in food and their health benefits. – Plant Foods Hum. Nutr. 59(3): 113–122. https://doi.org/10.1007/s11130-004-0049-7
Weller P., Breithaupt D.E. 2003. Identification and quantification of zeaxanthin esters in plants using liquid chromatography-mass spectrometry. – J. Agric. Food Chem. 51(24): 7044–7049. https://doi.org/10.1021/jf034803s
Pintea A., Varga A., Stepnowski P., Socaciu C., Culea M., Diehl H. 2005. Chromatographic analysis of carotenol fatty acid esters in Physalis alkekengi and Hippophae rhamnoides. – Phytochem. Anal. 16(3): 188–195. https://doi.org/10.1002/pca.844
Deineka V.I., Sorokopudov V.N., Deineka L.A., Tretyakov M.Y., Fesenko V.V. 2008. Studies of Physalis alkekengi L. fruits as a source of xanthophylls. – Pharm. Chem. J. 42(2): 87–88. https://doi.org/10.1007/s11094-008-0065-2
Wen X., Hempel J., Schweiggert R.M., Ni Y., Carle R. 2017. Carotenoids and Carotenoid Esters of Red and Yellow Physalis (Physalis alkekengi L. and P. pubescens L.) Fruits and Calyces. – J. Agric. Food Chem. 65(30): 6140–6151. https://doi.org/10.1021/acs.jafc.7b02514
Kawai M., Matsuura T., Kyuno S., Matsuki H., Takenaka M., Katsuoka T., Butsugan Y., Saito K. 1987. A new physalin from Physalis alkekengi. Structure of physalin L. – Phytochemistry. 26(12): 3313–3317. https://doi.org/10.1016/S0031-9422(00)82495-4
Li X., Zhang C., Wu D., Tang L., Cao X., Xin Y. 2012. In vitro effects on intestinal bacterium of physalins from Physalis alkekengi var. francheti. – Fitoterapia 83(8), 1460–1465. https://doi.org/10.1016/j.fitote.2012.08.011
Pharmacopoeia of People’s Republic of China. 2010. Volume 1. Chemical Industry Press. P. 337–338.
Wu H.Y., Suo Q.L., Li J. 2007. Research and Development on Physalis alkekengi of Pharmacology Activity in Clinical Function Plant. – Neimenggu Shiyou Huagong (Inner Mongolia Petrochemical Industry). 2: 5–8. (In Chinese)
Zhang N., Bie Z.M., Qing W.J., Zhang Y., Ge H.J., Shu C., Shi H.G., Ma H.B. 2008. The chemical composition and physiological effect of Physalis alkekengi L. var. franchetii (Mast.) Makino. – Journal of Jilin Medical College. 2: 104–107. (In Chinese)
Matsuura T., Kawai M., Nakashima R., Butsugan Y. 1969. Bitter principles of Physalis alkekengi var francheti: structure of physalin A. – Tetrahedron Lett. 10(14): 1083–1086. https://doi.org/10.1016/S0040-4039(01)97745-7
Kawai M., Matsumoto A., Makino B., Mori H., Ogura T., Butsugan Y., Ogawa K., Hayashi M. 1993. The Structure of Physalin P, a Neophysalin from Physalis alkekengi. – Bull. Chem. Soc. Jpn. 66 (4): 1299–1300. https://doi.org/10.1246/bcsj.66.1299
Xu W., Chen J., Liu J. et al. 2013. Three new physalins from Physalis alkekengi var. franchetii. – Nat. Prod. Bioprospect. 3(3): 103–106. https://doi.org/10.1007/s13659-013-0021-z
Yang Y.K., Xie S.D., Xu W.X., Nian Y., Liu X.L., Peng X.R., Ding Z.T., Qiu M.H. 2016. Six new physalins from Physalis alkekengi var. franchetii and their cytotoxicity and antibacterial activity. – Fitoterapia. 112: 144–152. https://doi.org/10.1016/j.fitote.2016.05.010
Helvacı S., Kökdil G., Kawai M., Duran N., Duran G., Güvenç A. 2010. Antimicrobial activity of the extracts and physalin D from Physalis alkekengi and evaluation of antioxidant potential of physalin D. – Pharm. Biol. 48(2): 142–150. https://doi.org/10.3109/13880200903062606
Sa M.S., de Menezes M.N., Krettli A.U., Ribeiro I.M., Tomassini T.C., dos Santos R.R., de Azevedo Jr W.F., Soares M.B. 2011. Antimalarial activity of physalins B, D, F, and G. J. – Nat. Prod. 74(10): 2269–2272. https://doi.org/10.1021/np200260f
Torabzadeh P., Panahi P. 2011. Evaluation of Antifungal Activity of Physalis alkekengi L. Extracts on Microsporum canis, Candida albicans, Trichophyton mentagrophytes and Nocardia asteroids. – American-Eurasian J. Agric. & Environ. Sci. 11 (6): 863–866. https://www.idosi.org/aejaes/jaes11(6)11/16.pdf
Vandenberghe I., Créancier L., Vispé S., Annereau J.P., Barret J.M., Pouny I., Samson A., Aussagues Y., Massiot G., Ausseil F., Bailly C., Kruczynski A. 2008. Physalin B, a novel inhibitor of the ubiquitin-proteasome pathway, triggers NOXA-associated apoptosis. Biochem. – Pharmacol. 76(4): 453–462. https://doi.org/10.1016/j.bcp.2008.05.031
Kang N., Jian J.F., Cao S.J., ZhangQ., Mao Y.W., Huang Y.Y., Peng Y.F., Qiu F., Gao X.M. 2016. Physalin A induces G2/M phase cell cycle arrest in human non-small cell lung cancer cells: involvement of the p38 MAPK/ROS pathway. – Mol. Cell. Biochem. 415: 145–155. https://doi.org/10.1007/s11010-016-2686-1
Zhu F., Dai C., Fu Y., Loo J.F., Xia D., Gao S.P., Ma Z., Chen Z. 2016. Physalin A exerts anti-tumor activity in non-small cell lung cancer cell lines by suppressing JAK/STAT3 signaling. – Oncotarget. 7: 9462–9476. https://doi.org/10.18632/oncotarget.7051
He H., Zang L.-H., Feng Y.-S., Chen L.-X., Kang N., Tashiro S.-I., Onodera S., Qui F., Ikejima T. 2013. Physalin A induces apoptosis via p53-Noxa-mediated ROS generation, and autophagy plays a protective role against apoptosis through p38-NF-κB survival pathway in A375-S2 cells. – Journal of Ethnopharmacology. 148(2): 544–555. https://doi.org/10.1016/j.jep.2013.04.051
Lin Y., Shi R., Wang X., Shen H.M. 2008. Luteolin, a flavonoid with potential for cancer prevention and therapy. – Curr. Cancer Drug Targets. 8(7): 634–646. https://doi.org/10.2174/156800908786241050
Murakami A., Ashida H., Terao J. 2008. Multitargeted cancer prevention by quercetin. – Cancer Lett. 269(2): 315–325. https://doi.org/10.1016/j.canlet.2008.03.046
Sanchooli N. 2011. Antidiabetic properties of Physalis alkekengi extract in alloxan-induced diabetic rats. – Res. J. Pharm., Biol. Chem. Sci. 2(3): 168. https://www.rjpbcs.com/pdf/2011_2(3)/21.pdf
Meng S., Cao J., Feng Q., Peng J., Hu Y. 2013. Role of chlorogenic acid on regulating glucose and lipids metabolism: a review. Evid. Based Complement. – Alternat. Med. Special Issue. Article ID 801457. https://doi.org/10.1155/2013/801457
Mancuso C., Santangelo R. 2014. Ferulic acid: pharmacological and toxicological aspects. – Food Chem. Toxicol. 65: 185–195. https://doi.org/10.1016/j.fct.2013.12.024
Mirheydar H. 2003. Herbal information: usage of plants in prevention and treatment of disease. Islamic Culture Publishing Center, Tehran. P. 39–42.
Asano N., Kato A., Miyauchi M., Kizu H., Tomimori T., Matsui K., Nash R.J., Molyneux, R.J. 1997. Specific α‑Galactosidase Inhibitors, N-Methylcalystegines Structure/Activity Relationships of Calystegines from Lycium chinense. Eur. J. Biochem., 248(2): 296-303. https://doi.org/10.1111/j.1432-1033.1997.00296.x
Li J., Li J., Li D. 2002. Studies on the chemical constituents of Physalis alkekengil L.var. francheti (Mast.) Makino (II). – Zhong Cao Yao (Chinese Traditional and Herbal Drugs). 9: 788–789. (In Chinese)
Li X., Zhao J., Yang M., Liu Y., Li Z., Li R., Li X., Li N., Xu Q., Khan I. A., Yang S. 2014. Physalins and withanolides from the fruits of Physalis alkekengi L. var. franchetii (Mast.) Makino and the inhibitory activities against human tumor cells. – Phytochem. Lett. 10: 95–100. https://doi.org/10.1016/j.phytol.2014.08.004
Sunayama R., Kuroyanagi M., Umehara K., Ueno A. 1993. Physalin and neophysalins from Physalis alkekengi var francheti and their differentiation inducing activity. – Phytochemistry. 34(2):529–533. https://doi.org/10.1016/0031-9422(93)80040-Y
Li K., Diao Y., Wang M., Yang S., Zhang H., Huang S., Yang H. 2010. Chemical constituents of the fruits of Physalis alkekengi L. var. franchetii (Mast.) Makino. – Chinese Journal of Organic Chemistry. 30(1): 128–131. (In Chinese)
Kawai M., Ogura T., Butsugan Y., Taga T., Hayashi M. 1991. Benzilic acid rearrangement-type reaction of physalins to neophysalins. Structural revision of one of the dehydration products of physalin A. – Tetrahedron. 47(12-13): 2103–2110. https://doi.org/10.1016/S0040-4020(01)96121-6
Basey K., McGaw B.A., Woolley J.G. 1992. Phygrine, an alkaloid from Physalis species. – Phytochemistry. 31(12): 4173–4176. https://doi.org/10.1016/0031-9422(92)80437-J
Asano N., Kato A., Oseki K., Kizu H., Matsui K. 1995. Calystegins of Physalis alkekengi var. francheti (Solanaceae). – Eur. J. Biochem. 229(2): 369–376. https://doi.org/10.1111/j.1432-1033.1995.0369k.x
Asano N., Kato A., Kizu H., Matsui K. 1996. 1β-amino-2α,3β,5β-trihydroxycycloheptane from Physalis alkekengi var. francheti. – Phytochemistry. 42(3): 719–721. https://doi.org/10.1016/0031-9422(96)00034-9
Дополнительные материалы отсутствуют.
Инструменты
Растительные ресурсы


