Записки Российского минералогического общества, 2020, T. 149, № 3, стр. 1-17
Arsmirandite, Na18Cu12Fe3+O8(AsO4)8Cl5, and lehmannite, Na18Cu12TiO8(AsO4)8FCl5, new mineral species from fumarole exhalations of the Tolbachik volcano, Kamchatka, Russia
I. V. Pekov 1, *, S. N. Britvin 2, 3, V. O. Yapaskurt 1, N. N. Koshlyakova 1, Yu. S. Polekhovsky 2, J. Göttlicher 4, N. V. Chukanov 5, M. F. Vigasina 1, S. V. Krivovichev 2, 3, A. G. Turchkova 1, E. G. Sidorov 6
1 Faculty of Geology, Moscow State University
119991 Moscow, Vorobievy Gory, Russia
2 Institute of Earth Sciences, Saint Petersburg State University
199034 Saint Petersburg, University emb., 7/9, Russia
3 Kola Science Center RAS
184209 Apatity, Fersman st., 14, Russia
4 Karlsruhe Institute of Technology, Institute for Synchrotron Radiation
D-76344 Eggenstein-Leopoldshafen, Hermann-von-Helmholtz-Platz 1, Germany
5 Institute of Problems of Chemical Physics RAS
142432 Moscow region, Chernogolovka, Russia
6 Institute of Volcanology and Seismology, Far Eastern Branch RAS
683006 Petropavlovsk-Kamchatsky, Piip Boulevard, 9, Russia
* E-mail: igorpekov@mail.ru
Поступила в редакцию 8.04.2020
После доработки 8.04.2020
Принята к публикации 16.04.2020
Аннотация
Two closely related new minerals arsmirandite Na18Cu12Fe3+O8(AsO4)8Cl5 and lehmannite Na18Cu12Ti4+O8(AsO4)8FCl5 were discovered in sublimates of the Arsenatnaya fumarole at the Second scoria cone of the Northern Breakthrough of the Great Tolbachik Fissure Eruption, Tolbachik volcano, Kamchatka, Russia. They are associated with one another and with hematite, sanidine, sylvite, halite, tenorite, cassiterite, rutile, and various arsenates and sulfates. Arsmirandite and lehmannite are visually indistinguishable and occur as equant crystals up to 20 × 20 × 30 μm3, typically combined in thin crusts up to 2 × 3 cm. The minerals are dark greyish green to olive-greenish black and have strong vitreous lustre. Dcalc = 3.715 (arsmirandite) and 3.676 (lehmannite) g cm–3. The empirical formula of arsmirandite is (Na17.06K0.51Pb0.08Ca0.06)Σ17.71(Cu11.73Mg0.11Zn0.08Mn0.01)Σ12.93(${\text{Fe}}_{{0.92}}^{{3 + }}$ Ti0.10Al0.02)Σ1.04(As7.91S0.08P0.03Si0.02V0.01)Σ8.05O40.23Cl4.77. The empirical formula of lehmannite is (Na17.92K0.18Ca0.24)Σ18.34(Cu11.59${\text{Fe}}_{{0.21}}^{{3 + }}$)Σ11.80(Ti0.85Sn0.11)Σ0.96 (As7.74S0.14P0.09Si0.03)Σ8O40.10F0.75Cl5.42. Both minerals are monoclinic, space group C2/m, Z = 2. Unit-cell parameters (arsmirandite/lehmannite) are: a = = 10.742(2)/10.8236(15), b = 21.019(3)/21.1077(17), c = 11.787(2)/11.8561(11) Å, β = = 117.06(3)/117.195(8)°, and V = 2370.0(7)/2409.2(5) Å3. The crystal structures of arsmirandite and lehmannite were solved by means of single-crystal X-ray diffraction analysis. The minerals have two unique structural features: (1) they contain Fe3+ and Ti4+ in cubic coordination for arsmirandite and lehmannite, respectively; (2) their structures are built up by packing of unusual nanoscale (~1.5 nm across) clusters with the composition {[MCu12O8](AsO4)8} (M = Fe3+ in arsmirandite and Ti4+ in lehmannite). Each nanocluster contains (MO8) cube surrounded by twelve flat squares (CuO4) linked with eight (AsO4) tetrahedra. Sodium and halogen atoms are located in between the nanoclusters. The name arsmirandite reflects the presence of arsenic and the unusual crystal structure (from the Latin mirandus, marvellous). Lehmannite is named in honour of the outstanding German and Russian mineralogist and geologist Johann Gottlob Lehmann (1719–1767).
INTRODUCTION
Fumarole fields related to the Tolbachik volcano at Kamchatka is a unique natural phenomenon, the present-day world record-holder in both number of new mineral species discovered within one geological object and the general diversity of minerals formed in volcanic fumaroles. Tolbachik fumaroles belong to the oxidizing type and the overwhelming majority of minerals formed here are oxocompounds (oxysalts and oxides) and halides (chlorides and fluorides). One of the most remarkable mineralogical features of the Tolbachik fumarole exhalations is the great diversity of arsenate minerals: more than fifty arsenates are discovered here including forty-two (!) new mineral species. All arsenates in Tolbachik fumaroles crystallized under high temperatures (>350–400 °C) and are hence hydrogen-free. The fumarolic arsenate mineralization was recently reviewed by Pekov et al. (2018). The majority of Tolbachik arsenates are concentrated in the sublimates of the active Arsenatnaya fumarole located at the apical part of the Second scoria cone of the Northern Breakthrough of the Great Tolbachik Fissure Eruption 1975–1976 (GTFE). This scoria cone, located 18 km south of the Ploskiy Tolbachik volcano in the central part of Kamchatka Peninsula (Far-Eastern Region, Russia), is a monogenetic volcano about 300 m high and approximately 0.1 km3 in volume formed in 1975 (Fedotov, Markhinin, 1983). Now, forty-five years after the GTFE, its fumarole fields are still active: numerous gas vents with temperatures up to 490 °C were observed by us in 2012–2018.
In the present paper we characterize two structurally related new minerals from the Arsenatnaya fumarole, arsmirandite Na18Cu12Fe3+O8(AsO4)8Cl5 (Cyrillic: арсмирандит) and lehmannite Na18Cu12TiO8(AsO4)8FCl5 (Cyrillic: леманнит). The name arsmirandite reflects the presence of arsenic and the very unusual crystal structure, exceptional for a natural compound (from the Latin mirandus, marvellous). Lehmannite is named in honour of the outstanding German and Russian mineralogist and geologist Johann Gottlob Lehmann (1719–1767), Academician of the Royal Prussian Academy of Sciences (1754) and the Imperial Russian Academy of Sciences (1761). Since 1761, he worked in St. Petersburg. J.G. Lehmann is, in particular, the author of the original description of crocoite (“red lead ore”, 1766), the first new mineral species discovered in Russia11 (Vernadsky, 1911).
Both new minerals and their names have been approved by the IMA Commission on New Minerals, Nomenclature and Classification (arsmirandite: IMA2014–081; lehmannite: IMA2017–057a). The type specimens are deposited in the systematic collection of the Fersman Mineralogical Museum of the Russian Academy of Sciences, Moscow, at the catalogue numbers 94 623 (arsmirandite) and 96 255 (lehmannite). The preliminary report on arsmirandite and lehmannite, focusing on the unique features of their crystal structures was recently published by Britvin et al. (2020).
OCCURRENCE AND GENERAL APPEARANCE
The general description of the Arsenatnaya fumarole was given by Pekov et al. (2014a, 2018). Specimens containing both new minerals were collected by us from different areas of Arsenatnaya. First samples were found in 2012, but only in 2014 and 2015 we collected the material that gave crystals suitable for the crystal-structure determination of arsmirandite and lehmannite, respectively.
The temperatures measured using a chromel-alumel thermocouple at the time of collecting in pockets with arsmirandite and lehmannite were 360–450 °C. It seems that both new minerals were deposited from fumarolic gases at temperatures higher than 450 °C. Arsmirandite and lehmannite are constituents of fumarolic incrustations consisting of arsenates, sulfates, oxides, chlorides, and silicates. They are associated with one another and with hematite, sanidine (As-bearing variety), sylvite, halite, tenorite, cassiterite, rutile, pseudobrookite, johillerite, bradaczekite, hatertite, magnesiohatertite, arsenatrotitanite, melanarsite, dmisokolovite, shchurovskyite, pharmazincite, katiarsite, lammerite, lammerite-β, urusovite, alarsite, ericlaxmanite, kozyrevskite, yurmarinite, popovite, tilasite, svabite, durangite, anhydrite, aphthitalite, langbeinite, calciolangbeinite, steklite, arcanite, palmierite, dolerophanite, euchlorine, wulffite, alumoklyuchevskite, klyuchevskite, krasheninnikovite, vanthoffite, fluoborite, gahnite (Cu-bearing variety), corundum, and fluorophlogopite.
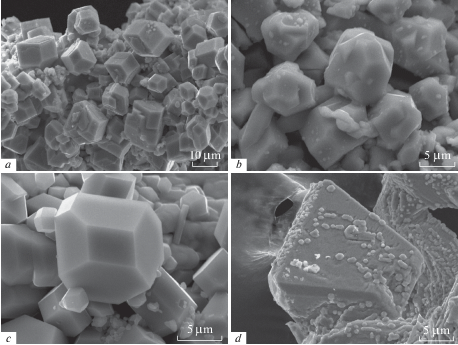
Arsmirandite and lehmannite are visually indistinguishable. Both minerals typically occur as solid or, more commonly, interrupted crusts consisting of well-shaped or coarse crystals (Fig. 1, a–c), which overgrow basalt scoria and crusts of As-bearing sanidine, hematite or johillerite. Areas covered by interrupted crusts of the new minerals may reach 2 × 3 cm2. The crusts are usually not thicker than 50 μm, rarely up to 0.5 mm thick. Open-work clusters of arsmirandite and lehmannite crystals occur in cavities. Separate crystals of both new minerals are typically not larger than 5 μm across but some crystals are up to 20 × 20 × 30 μm3 in size. Crystals are equant, thick tabular or short prismatic with pyramid-like terminations; they are probably shaped by faces of pinacoids and orthorhombic prisms (symmetry class 2/m) that typically give the combination looking as a rhombic dodecahedron or cuboctahedron, distorted or almost perfect (Fig. 1, a, c); some crystals are more complex in shape. Cyclic interpenetration twins morphologically very similar to the twins well-known for the zeolites of the phillipsite series are common for arsmirandite (Fig. 1, b). Epitactic overgrowths of tiny lehmannite crystals on much bigger crystals of arsenatrotitanite, an arsenate with the idealized formula NaTiO(AsO4) and a titanite-type structure (Pekov et al., 2019), have been observed (Fig. 1, d). Lehmannite also forms open-work pseudomorphs after bunches of board- or sword-shaped crystals of an unidentified mineral. The fine-grained, earthy aggregates, sometimes intimately intergrown with As-bearing sanidine or Na-bearing sylvite, are typical for both new minerals.
Fig. 1.
Morphology of crystals and aggregates of arsmirandite and lehmannite: a – crystal crust of arsmirandite, b – interpenetration twins of arsmirandite, c – lehmannite crystals, d – tiny crystals of lehmannite resembling rhombic dodecahedra epitactically overgrowing crystals of arsenatrotitanite. SEM (SE) images. Рис. 1. Морфология кристаллов и агрегатов арсмирандита и леманнита: a – корочка кристаллов арсмирандита, b – двойники прорастания арсмирандита, c – кристаллы леманнита, d – мелкие кристаллы леманнита, по форме напоминающие ромбододекаэдр, эпитаксически нарастают на кристаллы арсенатротитанита. РЭМ-фотографии во вторичных электронах.
PHYSICAL PROPERTIES AND OPTICAL DATA
Arsmirandite and lehmannite are dark greyish green with an olive hue to olive-greenish black in separate crystals and green or greyish green to olive-drab in earthy aggregates. The crystals have strong vitreous lustre. Both minerals are megascopically almost opaque but thin sections are translucent, dark green. Streak is greyish green with an olive hue. The minerals are brittle. Cleavage or parting is not observed. The fracture is uneven (observed under the scanning electron microscope). The mean micro-indentation hardness (VHN) of lehmannite is 416, range is 339–537 kg mm–2 (load 100 g). Mohs’ hardness was not measured directly because of the small size of crystals of both minerals, the value estimated for lehmannite from the micro-indentation hardness is ca. 4½. Density could not be measured because of the small size of crystals and porous character of aggregates. Density values calculated using the empirical formulae are 3.715 and 3.676 g cm–3 for arsmirandite and lehmannite, respectively.
Under the microscope in reflected light, both minerals are dark grey, pleochroism was not observed. Bireflectance is very weak, ΔR = 0.25% and 0.8% for arsmirandite and lehmannite, respectively (589 nm). Anisotropism is very weak. Weak brown internal reflections were observed for lehmannite. The reflectance values for both minerals measured in air by means of the MSF-21 microspectrophotometer (LOMO, Russia) using the SiC standard (Zeiss, No. 545) are given in Table 1.
Table 1.
Reflectance data of arsmirandite and lehmannite Таблица 1. Коэффициенты отражения арсмирандита и леманнита
| λ, nm | Arsmirandite | Lehmannite | λ, nm | Arsmirandite | Lehmannite | ||||
|---|---|---|---|---|---|---|---|---|---|
| Rmin, % | Rmax, % | Rmin, % | Rmax, % | Rmin, % | Rmax, % | Rmin, % | Rmax, % | ||
| 400 | 7.2 | 7.5 | 8.3 | 8.9 | 560 | 6.9 | 7.1 | 7.9 | 8.5 |
| 420 | 7.4 | 7.7 | 8.2 | 8.9 | 580 | 6.8 | 7.1 | 7.8 | 8.5 |
| 440 | 7.4 | 7.7 | 8.2 | 8.8 | 589 | 6.8 | 7.1 | 7.6 | 8.4 |
| 460 | 7.3 | 7.6 | 8.1 | 8.7 | 600 | 6.8 | 7.0 | 7.7 | 8.4 |
| 470 | 7.3 | 7.6 | 8.1 | 8.7 | 620 | 6.7 | 7.0 | 7.7 | 8.4 |
| 480 | 7.2 | 7.5 | 8.1 | 8.7 | 640 | 6.7 | 7.0 | 7.6 | 8.3 |
| 500 | 7.1 | 7.4 | 8.0 | 8.7 | 650 | 6.7 | 7.0 | 7.6 | 8.3 |
| 520 | 7.0 | 7.3 | 8.0 | 8.6 | 660 | 6.7 | 6.9 | 7.6 | 8.3 |
| 540 | 6.9 | 7.2 | 7.9 | 8.6 | 680 | 6.7 | 6.9 | 7.5 | 8.2 |
| 546 | 6.9 | 7.2 | 7.9 | 8.5 | 700 | 6.7 | 6.9 | 7.5 | 8.1 |
RAMAN SPECTROSCOPY
The Raman spectrum of arsmirandite (Fig. 2, a) was obtained on a randomly oriented crystal using an EnSpectr R532 instrument (Dept. of Mineralogy, Moscow State University) with a green laser (532 nm) at room temperature. The output power of the laser beam was about 3.5 mW. The spectrum was processed using the EnSpectr expert mode program in the range from 100 to 4000 cm–1 with the use of a holographic diffraction grating with 1800 lines per cm and a resolution of 6 cm–1. The diameter of the focal spot on the sample was about 10 μm. The backscattered Raman signal was collected with 40x objective; signal acquisition time for a single scan of the spectral range was 1500 ms and the signal was averaged over 10 scans.
Fig. 2.
The Raman spectrum of arsmirandite (a) and the IR spectrum of lehmannite (b). Рис. 2. КР-спектр арсмирандита (a) и ИК-спектр леманнита (b).
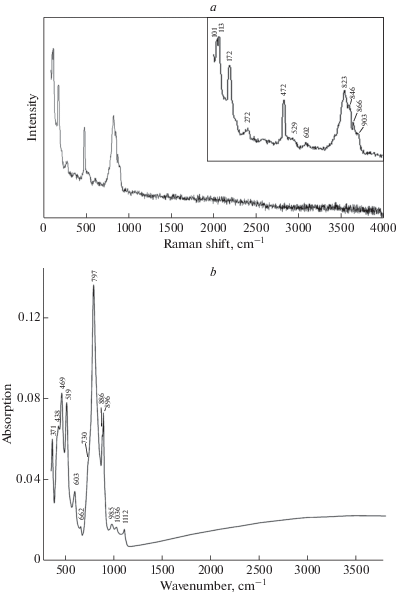
The bands at 823, 846, 866 and 903 cm–1 correspond to As5+–O stretching vibrations of ${\text{AsO}}_{4}^{{3 - }}$ anions. The presence of numerous bands in this region reflects the presence of two non-equivalent, distorted AsO4 tetrahedra, in line with the structural data (see below). The assignment of the bands in the range from 400 to 700 cm–1 is discussed below, in the section Infrared Spectroscopy. Bands with frequencies lower than 400 cm–1 correspond to lattice modes involving bending vibrations of AsO4 tetrahedra and MO8 polyhedra, as well as Na–Cl stretching vibrations. The absence of bands with frequencies higher than 950 cm–1 in the Raman spectrum of arsmirandite indicates the absence of groups with O–H, C–H, C–O, N–H, N–O and B–O bonds.
INFRARED SPECTROSCOPY
Infrared (IR) absorption spectrum of lehmannite (Fig. 2, b) was obtained using an ALPHA FTIR spectrometer (Bruker Optics) at a resolution of 4 cm–1 and 16 scans. Preliminarily, the sample was powdered, mixed with anhydrous KBr and pelletized. The IR spectrum of an analogous pellet of pure KBr was used as a reference.
The splitting of the bands of As–O stretching vibrations in the range 700–900 cm–1 reflects the presence of two nonequivalent distorted AsO4 tetrahedra in the crystal structure of lehmannite. The weak bands at 1112 and 1036 cm–1 correspond to asymmetric stretching vibrations of SO4 and PO4 tetrahedra that are present in trace amounts in this mineral. The band of banding vibrations of AsO4 tetrahedra is observed at 371 cm–1.
By analogy with ericlaxmanite and yaroshevskite (Siidra et al., 2020), the bands in the range 430–480 cm–1 and at 603 cm–1 correspond to Cu–O stretching vibrations in which a major part of energy is concentrated on the long and the shortest Cu–O bonds, respectively. Analogous Raman bands are observed at 472 and 602 cm–1.
According to XANES spectroscopy data (see below), titanium in lehmannite is tetravalent. The IR spectrum is in agreement with this conclusion. There is a correlation between the lengths of Ti4+–O bonds and wavenumbers of corresponding stretching vibrations (Chukanov, Chervonnyi, 2016). In particular, stretching vibrations involving four long bonds of the Ti4+O5 tetragonal pyramid (lamprophyllite-group minerals, fresnoite, natisite, paranatisite) with the mean Ti–O distances from 1.92 to 2.00 Å are observed in the range 549–627 cm–1 whereas the wavenumber of the band related to stretching vibrations of the shortest Ti–O bond of the Ti4+O5 polyhedron (with the Ti–O distance 1.63–1.69 Å) is about 860 cm–1. The range of Ti–O stretching vibrations of isolated TiO6 octahedra (in which mean Ti–O distances vary from 1.96 to 2.00 Å) is 540–570 cm–1.
In lehmannite, the Ti–O distances are equal to 2.170 and 2.184 Å. Consequently, one should expect a significantly lower frequency of Ti–O stretching vibrations in this mineral. Based on this consideration, the band at 519 cm–1 in the IR spectrum of lehmannite is tentatively assigned to Ti–O stretching vibrations. This assumption is in a good agreement with the fact that the analogous Raman band of arsmirandite observed at 529 cm–1 has a very low intensity as compared of the intensity of the band of Cu–O stretching vibrations at 472 cm–1.
The weak IR band at 985 cm–1 may be related to a combination mode of Cu2–O8–Ti or Cu2–O8–Ti because the sum of wavenumbers of corresponding fundamentals (469 and 519 cm–1) is close to 985 cm–1.
The IR spectrum of lehmannite is unique and can be used for its identification. The absence of bands with the frequencies higher than 1120 cm–1 in the IR spectrum of lehmannite indicates the absence of groups with O–H, C–H, C–O, N–H, N–O and B–O bonds.
XANES SPECTROSCOPY
A unique cubic coordination of titanium in lehmannite and unusually long Ti–O distances (see below) required the direct determination of Ti oxidation state. The latter was carried out using X-ray Absorption Near Edge Structure (XANES) spectroscopy. A sample of lehmannite was grinded in an agate mortar and the powder has been glued on a Kapton tape. Spectra have been measured in fluorescence mode at the SUL-X beamline of the ANKA synchrotron ring (Karlsruhe Institute of Technology) on powdered crystals with a focused 27 pole wiggler beam of about 200 µm (hor.) × 100 µm (vert.) at sample position using a Si(111) double crystal monochromator. TiKα fluorescence emission intensities were recorded with a 7 element Si(Li) solid state detector (RAYSPEC, former Gresham) and divided by the incoming signal intensity using an ADC ionization chamber. Ten sample spectra have been measured to achieve good signal-to-noise ratio. Energy step of 0.3 eV has been chosen across the Ti K-edge to resolve spectral features. Spectra were merged and further processed with pre- and post-edge background subtraction and normalization of edge jump of µd 1 using the Athena program of the IFFEFIT software package (Ravel, Newville, 2005).
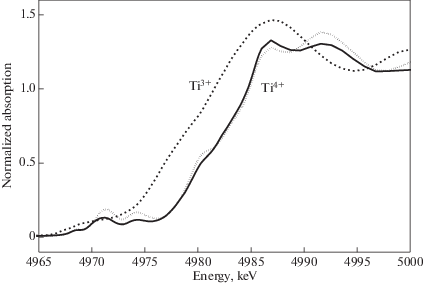
Ti K XANES spectra of Ti2O3 as reference for Ti(III) and two modifications of TiO2 (rutile and brookite), FeTiO3 (ilmenite) and CaTiOSiO4 (titanite) as references for Ti(IV) were measured in transmission using ionization chambers from company ADC. Energy has been calibrated with a Ti foil to 4966 eV (1st maximum of the 1st derivative). As the obtained data show, Ti in lehmannite is tetravalent. It is proven by the coincidence of the edge position of the rising flank of the sample Ti K XANES spectrum with a series of the spectra of the above-listed Ti(IV) reference samples (rutile, brookite, titanite, and ilmenite), in contrast to the Ti(III) reference spectrum (Ti2O3). The match with the Ti(IV) edge position is shown in Fig. 3 in comparison with the rutile reference spectrum. The edge position of the Ti(IV) reference rutile and of the sample is located at about 3.5 eV above the edge position of the Ti(III) reference (Ti2O3) at normalized absorption of µd 0.8 (Fig. 3).
Fig. 3.
The Ti K XANES spectrum of lehmannite (solid line) compared to the spectra of Ti2O3 as reference for Ti(III) (dashed line) and of rutile TiO2 as reference for Ti(IV) (dotted line). Рис. 3. Спектр XANES Ti K леманнита (сплошная линия) в сравнении с аналогичными спектрами эталонов: Ti2O3 для Ti(III) (штриховая линия) и рутила TiO2 для Ti(IV) (пунктирная линия).
CHEMICAL COMPOSITION
The chemical data for arsmirandite and lehmannite were obtained using a Jeol JSM-6480LV scanning electron microscope equipped with an INCA-Wave 500 wavelength-dispersive spectrometer (Laboratory of Analytical Techniques of High Spatial Resolution, Dept. of Petrology, Moscow State University), with an acceleration voltage of 20 kV, a beam current of 20 nA, and a 3 μm beam diameter. The chemical composition of both minerals and standards used are given in Table 2. Contents of other elements with atomic numbers higher than carbon are below detection limits.
Table 2.
Chemical composition (wt %) of arsmirandite and lehmannite Таблица 2. Химический состав (мас. %) арсмирандита и леманнита
| Constituent | Arsmirandite | Lehmannite | Probe standard | ||||
|---|---|---|---|---|---|---|---|
| mean (5 anal.) |
range | SD | mean (8 anal.) |
range | SD | ||
| Na2O | 20.04 | 19.53–20.61 | 0.43 | 20.62 | 19.87–22.91 | 1.03 | Lorenzenite |
| K2O | 0.91 | 0.79–1.05 | 0.10 | 0.31 | 0.25–0.36 | 0.03 | Orthoclase |
| CaO | 0.12 | 0.09–0.16 | 0.03 | 0.51 | 0.09–0.72 | 0.18 | CaWO4 |
| PbO | 0.67 | 0.59–0.72 | 0.05 | – | PbTe | ||
| MgO | 0.17 | 0.06–0.24 | 0.07 | – | Diopside | ||
| MnO | 0.03 | 0.00–0.06 | 0.02 | – | Mn | ||
| CuO | 35.37 | 34.77–35.89 | 0.46 | 34.24 | 32.75–35.14 | 0.76 | CuFeS2 |
| ZnO | 0.25 | 0.18–0.36 | 0.07 | – | ZnS | ||
| Al2O3 | 0.03 | 0.00–0.06 | 0.02 | – | Al2O3 | ||
| Fe2O3 | 2.79 | 2.38–3.07 | 0.27 | 0.63 | 0.37–0.88 | 0.20 | CuFeS2 |
| TiO2 | 0.29 | 0.19–0.40 | 0.08 | 2.53 | 1.96–3.70 | 0.53 | Ilmenite |
| SnO2 | – | 0.62 | 0.12–1.37 | 0.38 | SnS | ||
| SiO2 | 0.05 | 0.00–0.10 | 0.04 | 0.06 | 0.02–0.10 | 0.03 | Diopside |
| P2O5 | 0.07 | 0.05–0.11 | 0.02 | 0.23 | 0.13–0.32 | 0.06 | GaP |
| V2O5 | 0.04 | 0.00–0.09 | 0.03 | – | V | ||
| As2O5 | 34.46 | 34.20–34.75 | 0.24 | 33.04 | 31.84–34.28 | 0.72 | FeAsS |
| SO3 | 0.25 | 0.17–0.37 | 0.09 | 0.43 | 0.00–2.67 | 0.43 | ZnS |
| F | – | 0.53 | 0.48–0.60 | 0.05 | MgF2 | ||
| Cl | 6.41 | 6.21–6.49 | 0.11 | 7.13 | 6.94–7.58 | 0.19 | NaCl |
| –O=(F,Cl) | –1.45 | –1.83 | |||||
| Total | 100.50 | 99.05 | |||||
The empirical formula of arsmirandite, calculated on the basis of 45 anions (O+Cl) pfu, is (Na17.06K0.51Pb0.08Ca0.06)Σ17.71(Cu11.73Mg0.11Zn0.08Mn0.01)Σ12.93(${\text{Fe}}_{{0.92}}^{{3 + }}$Ti0.10Al0.02)Σ1.04 (As7.91S0.08 P0.03Si0.02V0.01)Σ8.05O40.23Cl4.77. The simplified formula is Na18Cu12Fe3+O8(AsO4)8Cl5, which requires Na2O 21.06, CuO 36.03, Fe2O3 3.01, As2O5 34.71, Cl 6.69, −O=Cl −1.51, total 100 wt %.
The empirical formula of lehmannite, calculated on the basis of the sum of tetrahedrally coordinated components (As + P + S + Si) = 8 apfu, is (Na17.92K0.18Ca0.24)Σ18.34(Cu11.59${\text{Fe}}_{{0.21}}^{{3 + }}$)Σ11.80 (Ti0.85Sn0.11)Σ0.96(As7.74S0.14P0.09Si0.03)Σ8O40.10F0.75Cl5.42. The anionic basis of the formula calculation was not used in this case, because of the presence of vacancies at the Cl and F sites (see below). The simplified formula is Na18Cu12Ti4+O8(AsO4)8FCl5, which requires Na2O 20.97, CuO 35.90, TiO2 3.00, As2O5 34.56, F 0.71, Cl 6.66, –O=(F,Cl) –1.80, total 100 wt %.
X-RAY CRYSTALLOGRAPHY
Powder X-ray diffraction data of arsmirandite and lehmannite (Tables 3 and 4, respectively) were collected with a Rigaku R-AXIS Rapid II diffractometer equipped with a cylindrical image plate detector (radius 127.4 mm) using Debye-Scherrer geometry, CoKα radiation (rotating anode with VariMAX microfocus optics), 40 kV, 15 mA and an exposure time of 10 min. Data were integrated using the software package osc2tab (Britvin et al., 2017). Parameters of monoclinic unit cells calculated from the powder data are as follows: arsmirandite: a = 10.76(2), b = = 21.13(1), c = 11.76(2) Å, β = 117.0(1)° and V = 2403(5) Å3; lehmannite: a = 10.83(1), b = = 21.117(2), c = 11.86(1) Å, β = 117.36(7)° and V = 2409(4) Å3.
Table 3.
Powder X-ray diffraction data of arsmirandite Таблица 3. Результаты расчета порошковой рентгенограммы арсмирандита
| Iobs | dobs | Icalc* | dcalc | h k l |
|---|---|---|---|---|
| 79 | 10.58 | 57, 64 | 10.568, 10.565 | 001, 020 |
| 100 | 8.74 | 94, 100 | 8.734, 8.730 | 110, 11-1 |
| 9 | 7.475 | 20 | 7.471 | 021 |
| 7 | 5.683 | 1, 1, 1 | 5.679, 5.676, 5.676 | 111, 13-1, 11-2 |
| 46 | 5.381 | 98 | 5.381 | 20-1 |
| 80 | 5.288 | 86, 73 | 5.284, 5.282 | 002, 040 |
| 1 | 4.783 | 1 | 4.795 | 22-1 |
| 3 | 4.730 | 5, 6 | 4.726, 4.725 | 022, 041 |
| 14 | 3.868 | 5, 10, 6, 3 | 3.869, 3.867, 3.867, 3.867 | 112, 150, 11-3, 15-1 |
| 33 | 3.770 | 18, 31, 18 | 3.771, 3.769, 3.769 | 201, 24-1, 20-3 |
| 19 | 3.737 | 26 | 3.736 | 042 |
| 16 | 3.550 | 8, 11, 7, 9 | 3.552, 3.551, 3.550, 3.549 | 221, 240, 24-2, 22-3 |
| 22 | 3.525 | 24, 30 | 2.523, 2.522 | 003, 060 |
| 4 | 3.435 | 4, 3, 1, 4 | 3.435, 3.434, 3.434, 3.434 | 132, 151, 13-3, 15-2 |
| 11 | 3.343 | 13, 16 | 3.342, 3.341 | 023, 061 |
| 27 | 3.161 | 20, 18, 20, 20 | 3.162, 3.161, 3.160, 3.160 | 310, 33-1, 33-2, 31-3 |
| 14 | 2.946 | 9, 22, 9 | 2.948, 2.946, 2.946 | 202, 26-1, 20-4 |
| 15 | 2.932 | 15, 22 | 2.931, 2.930 | 043, 062 |
| 9 | 2.914 | 9, 9 | 2.912, 2.910 | 330, 33-3 |
| 8 | 2.882 | 3, 9, 3, 5, 4, 2 | 2.880, 2.880, 2.879, 2.879, 2.879, 2.879 | 113, 152, 11-4, 170, 15-3, 17-1 |
| 28 | 2.693 | 76, 6, 4, 4, 4 | 2.690, 2.688, 2.687, 2.687, 2.687 | 40-2, 133, 171, 13-4, 17-2 |
| 30 | 2.643 | 54, 58 | 2.642, 2.641 | 004, 080 |
| 4 | 2.609 | 1, 2, 1 | 2.608, 2.607, 2.607 | 40-1, 42-2, 40-3 |
| 74 | 2.574 | 64, 69, 68, 77 | 2.574, 2.574, 2.573, 2.573 | 242, 261, 24-4, 26-3 |
| 18 | 2.551 | 16, 14, 13, 18 | 2.550, 2.550, 2.550, 2.550 | 331, 350, 35-3, 33-4 |
| 2 | 2.397 | 2, 4, 2 | 2.398, 2.397, 2.397 | 400, 44-2, 40-4 |
| 3 | 2.367 | 2, 5, 2, 4, 5 | 2.372, 2.371, 2.363, 2.362 | 203, 28-1, 20-5, 044, 082 |
| 5 | 2.315 | 1, 1, 1, 2 | 2.315, 2.314, 2.313, 2.313 | 223, 280, 22-5, 28-2 |
| 18 | 2.297 | 33, 36 | 2.297, 2.296 | 351, 35-4 |
| 9 | 2.281 | 5, 5, 4, 6 | 2.281, 2.281, 2.280, 2.280 | 114, 11-5, 190, 19-1 |
| 7 | 2.261 | 6, 7 | 2.261, 2.260 | 262, 26-4 |
| 3 | 2.183 | 5, 4 | 2.184, 2.183 | 440, 44-4 |
| 3 | 2.164 | 2, 1, 1, 2 | 2.164, 2.163, 2.163, 2.163 | 243, 281, 24-5, 28-3 |
| 3 | 2.074 | 3, 3 | 2.072, 2.072 | 025, 0.10.1 |
| 3 | 2.049 | 3, 3, 3, 3 | 2.049, 2.049, 2.048, 2.048 | 51-1, 53-2, 53-3, 51-4 |
| 6 | 1.975 | 5, 5 | 1.976, 1.975 | 53-1, 53-4 |
| 3 | 1.967 | 4, 1, 4, 7, 1, 1 | 1.968, 1.967, 1.967, 1.967, 1.967, 1.967 | 204, 282, 20-6, 2.10.-1, 26-5, 28-4 |
| 24 | 1.885 | 29, 58, 28, 1 | 1.886, 1.885, 1.884, 1.884 | 402, 48-2, 40-6, 115 |
| 12 | 1.868 | 48 | 1.868 | 084 |
| 11 | 1.850 | 2, 1, 1, 2 | 1.851, 1.851, 1.850, 1.850 | 530, 55-1, 55-4, 53-5 |
| 2 | 1.812 | 2, 2 | 1.812, 1.812 | 065, 0.10.3 |
| 3 | 1.775 | 2, 1, 2, 1 | 1.776, 1.775, 1.775, 1.775 | 442, 480, 48-4, 44-6 |
| 2 | 1.765 | 2, 3 | 1.765, 1.764 | 283, 28-5 |
| 5 | 1.642 | 2, 2, 2, 2, 4, 5 | 1.642, 1.642, 1.641, 1.641, 1.641, 1.641 | 423, 4.10.-1, 4.10-3, 42-7, 194, 19-5 |
| 10 | 1.583 | 11, 11, 11, 11 | 1.583, 1.582, 1.582, 1.582 | 512, 59-2, 59-3, 51-7 |
| 7 | 1.533 | 6, 8, 5, 4, 5, 6 | 1.535, 1.534, 1.530, 1.530, 1.529, 1.529 | 482, 48-6, 640, 66-1, 66-5, 64-6 |
| 7 | 1.513 | 4, 4, 3, 4, 3, 4, 3 | 1.516, 1.515, 1.512, 1.512, 1.512, 1.511, 1.510 | 571, 57-6, 265, 2.12.2, 26-7, 2.12.-4, 007 |
| 8 | 1.496 | 2, 2, 2, 2, 23 | 1.498, 1.498, 1.498, 1.498, 1.494 | 71-2, 73-3, 73-4, 71-5, 0.10.5 |
| 4 | 1.477 | 2, 2, 2, 2 | 1.477, 1.477, 1.477, 1.477 | 374, 3.11.2, 37-7, 3.11-5 |
| 5 | 1.469 | 4, 4 | 1.469, 1.469 | 73-2, 73-5 |
| 5 | 1.431 | 5, 5, 5, 5 | 1.431, 1.430, 1.430, 1.430 | 513, 5.11-2, 5.11.-3, 51-8 |
| 5 | 1.416 | 6, 5, 5, 5 | 1.416, 1.415, 1.415, 1.415 | 73-1, 75-2, 75-5, 73-6 |
| 16 | 1.402 | 21, 20, 21, 18 | 1.402, 1.401, 1.401, 1.401 | 246, 24-8, 2.14.1, 2.14.-3 |
| 4 | 1.367 | 6, 1, 5 | 1.367, 1.367, 1.367 | 75-1, 60-8, 75-6 |
| 4 | 1.345 | 4, 2, 2, 2, 1 | 1.345, 1.345, 1.345, 1.345, 1.344 | 80-4, 730, 77-2, 77-5, 73-7 |
Table 4.
Powder X-ray diffraction data of lehmannite Таблица 4. Результаты расчета порошковой рентгенограммы леманнита
| Iobs | dobs | Icalc* | dcalc | h k l |
|---|---|---|---|---|
| 65 | 10.52 | 62, 63 | 10.553, 10.545 | 020, 001 |
| 100 | 8.74 | 99, 100 | 8.763, 8.759 | 11-1, 110 |
| 11 | 7.44 | 26 | 7.460 | 021 |
| 4 | 5.644 | 0.5, 0.5, 0.5, 0.5 | 5.682, 5.681, 5.680, 5.680 | 13-1, 130, 11-2, 111 |
| 36 | 5.419 | 95 | 5.412 | 20-1 |
| 74 | 5.273 | 76, 77 | 5.277, 5,272 | 040, 002 |
| 1 | 4.826 | 0.5, 1, 0.5 | 4.816, 4.816, 4.814 | 20-2, 22-1, 200 |
| 2 | 4.708 | 2, 2 | 4.719, 4.717 | 041, 022 |
| 3 | 4.382 | 2, 2 | 4.381, 4.380 | 22-2, 220 |
| 10 | 3.861 | 6, 6, 6, 5 | 3.866, 3.866, 3.865, 3.863 | 15-1, 150, 11-3, 112 |
| 37 | 3.772 | 33, 16, 17 | 3.778, 3.778, 3.775 | 24-1, 20-3, 201 |
| 13 | 3.726 | 33 | 3.730 | 042 |
| 13 | 3.552 | 7, 7, 7, 7 | 3.557, 3.557, 3.556, 3.555 | 24-2, 22-3, 240, 221 |
| 21 | 3.511 | 24, 24 | 3.518, 3.515 | 060, 003 |
| 4 | 3.430 | 2, 2, 2, 2 | 3.433, 3.432, 3.432, 3.431 | 15-2, 151, 13-3, 132 |
| 11 | 3.331 | 12, 13 | 3.337, 3.335 | 061, 023 |
| 28 | 3.178 | 32, 16, 16 | 3.174, 3.174, 3.173 | 31-3, 33-1, 310 |
| 5 | 3.071 | 2, 2 | 3.072, 3.070 | 24-3, 241 |
| 20 | 2.947 | 18, 9, 9 | 2.950, 2.949, 2.947 | 26-1, 20-4, 202 |
| 24 | 2.927 | 18, 18, 12, 12 | 2.926, 2.926, 2.921, 2.920 | 062, 043, 33-3, 330 |
| 11 | 2.876 | 2, 2, 6, 6, 3, 2 | 2.878, 2.878, 2.877, 2.876, 2.876, 2.875 | 17-1, 170, 15-3, 152, 11-4, 113 |
| 22 | 2.712 | 72 | 2.706 | 40-2 |
| 18 | 2.687 | 5, 5, 5, 5 | 2.685, 2.684, 2.684, 2.683 | 17-2, 171, 13-4, 133 |
| 43 | 2.636 | 55, 57 | 2.638, 2.636 | 080, 004 |
| 98 | 2.573 | 68, 69, 68, 70 | 2.575, 2.574, 2.574, 2.573 | 26-3, 24-4, 261, 242 |
| 32 | 2.553 | 19, 19, 19, 19 | 2.556, 2.555, 2.555, 2.554 | 35-3, 33-4, 350, 331 |
| 5 | 2.373 | 4, 2, 2 | 2.372, 2.371, 2.370 | 28-1, 20-5, 203 |
| 3 | 2.358 | 4, 4 | 2.360, 2.358 | 082, 044 |
| 30 | 2.301 | 38 | 2.300 | 35-4 |
| 5 | 2.277 | 5, 5, 5, 5 | 2.279, 2.279, 2.277, 2.277 | 19-1, 190, 11-5, 114 |
| 5 | 2.260 | 8, 8 | 2.260, 2.259 | 26-4, 262 |
| 4 | 2.201 | 5, 5 | 2.191, 2.190 | 44-4, 440 |
| 2 | 2.162 | 1, 1, 1, 1 | 2.163, 2.163, 2.162, 2.162 | 28-3, 281, 24-5, 243 |
| 1 | 2.144 | 0.5, 0.5 | 2.142, 2.142 | 51-3, 51-2 |
| 6 | 2.068 | 4, 5 | 2.070, 2.068 | 0.10.1, 025 |
| 4 | 1.981 | 5, 5 | 1.985, 1.985 | 53-4, 53-1 |
| 8 | 1.965 | 7, 4, 4 | 1.966, 1.966, 1.965 | 2.10.-1, 20-6, 204 |
| 33 | 1.889 | 56, 28, 28 | 1.889, 1.889, 1.888 | 48-2, 40-6, 402 |
| 15 | 1.864 | 55 | 1.865 | 084 |
| 11 | 1.842 | 6, 7, 7, 7 | 1.843, 1.842, 1.842, 1.841 | 2.10.-3, 2.10.1, 24-6, 244 |
| 4 | 1.779 | 2, 2, 2, 2 | 1.779, 1.778, 1.778, 1.777 | 48-4, 44-6, 62-3, 442 |
| 2 | 1.762 | 3, 3 | 1.763, 1.763 | 28-5, 283 |
| 3 | 1.646 | 1, 1, 0.5, 0.5, 0.5, 0.5 | 1.648, 1.647, 1.647, 1.647, 1.646, 1.646 | 0.10.4, 085, 39-5, 37-6, 392, 373 |
| 2 | 1.623 | 2, 2 | 1.624, 1.624 | 64-5, 641 |
| 12 | 1.586 | 13, 25, 0.5, 12 | 1.586, 1.586, 1.586, 1.585 | 59-3, 59-2, 443, 512 |
| 6 | 1.540 | 7, 11, 6, 7, 6 | 1.536, 1.536, 1.535, 1.535, 1.535 | 48-6, 66-5, 66-1, 482, 640 |
| 4 | 1.510 | 4, 4, 4, 4 | 1.511, 1.510, 1.510, 1.510 | 2.12.-4, 2.12.2, 26-7, 265 |
| 7 | 1.491 | 1, 32, 1 | 1.493, 1.492, 1.491 | 0.14.1, 0.10.5, 027 |
| 2 | 1.478 | 2, 4, 2, 2, 4, 2 | 1.477, 1.477, 1.477, 1.477, 1.476, 1.475 | 3.11.-5, 73-5, 37-7, 3.11.2, 73-2, 374 |
| 4 | 1.451 | 5, 2, 2, 5, 2, 2, 5 | 1.452, 1.452, 1.451, 1.450, 1.449, 1.449, 1.449 | 2.14.-1, 20-8, 206, 0.14.2, 3.13.-3, 3.13.0, 047 |
| 11 | 1.435 | 3, 3, 1, 1 | 1.438, 1.438, 1.438, 1.438 | 2.10.-6, 2.10.4, 22-8, 226 |
| 6 | 1.407 | 2, 0.5, 0.5, 2, 2, 0.5, 0.5, 2 | 1.407, 1.407, 1.407, 1.407, 1.407, 1.406, 1.406, 1.406 | 5.11.-4, 59-6, 57-7, 5.11.-1, 53-8, 591, 572, 533 |
| 18 | 1.401 | 26, 26, 27, 27 | 1.400, 1.400, 1.400, 1.399 | 2.14.-3, 2.14.1, 24-8, 246 |
| 2 | 1.377 | 6, 6 | 1.373, 1.373 | 75-6, 75-1 |
| 2 | 1.351 | 4, 0.5, 0.5, 1, 1, 2, 2 | 1.353, 1.351, 1.351, 1.350, 1.350, 1.350, 1.350 | 80-4, 0.12.5, 0.10.6, 73-7, 77-5, 77-2, 730 |
Single-crystal X-ray studies of both new minerals were carried out using a Bruker Smart Kappa Apex DUO diffractometer equipped with an APEXII CCD detector. The crystal structures of arsmirandite and lehmannite were solved by direct methods and refined with the use of SHELX-97 software package (Sheldrick, 2008) to R = 0.051 and 0.046, respectively. The detailed structure information for arsmirandite and lehmannite, including coordinates and displacement parameters of atoms and interatomic bond lengths, was reported by Britvin et al. (2020).
DISCUSSION
Crystal Structure
The crystal structures of arsmirandite and lehmannite have been described in detail by Britvin et al. (2020) as based upon unusual nanoscale (~1.5 nm across) clusters with the composition {[MCu12O8](AsO4)8} (species-defining M = Fe3+ in arsmirandite and Ti4+ in lehmannite) as shown in Fig. 4. The nanocluster contains a basic pseudo-cubic unit represented by a nearly perfect (MO8) cube (the angular deviations from perfect cube are ±0.07° for arsmirandite and ±0.62° for lehmannite) surrounded by twelve (CuO4) planar squares. The (CuO4) polyhedra are corner-linked to eight (AsO4) tetrahedra. The Na+ cations and halogen anions are located in between the nanoclusters.
Fig. 4.
The structure of the arsenate nanoclusters in arsmirandite and lehmannite shown in ellipsoidal and polyhedral representations (exemplified by arsmirandite): a – cubic coordination of M1 site; b, c – 13-nuclear metal-oxide core; d, e – the whole clusters. Displacement ellipsoids are shown at the 50% probability level. Рис. 4. Строение арсенатных нанокластеров в арсмирандите и леманните, показанное на примере арсмирандита с помощью эллипсоидов (c, e) и в полиэдрическом представлении (a, b, d): a – кубическая координация позиции M1; b, c – 13-ядерная оксидная центральная часть кластера; d, e – кластер целиком.
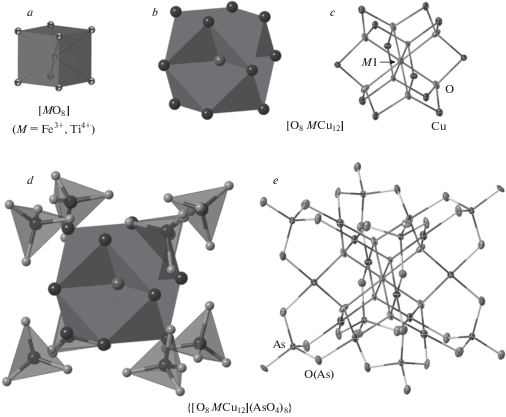
The most peculiar feature of the discussed structures is the presence of Fe3+ (arsmirandite) or Ti4+ (lehmannite) in a cubic coordination (Fig. 4), which is enforced by the topology of the clusters. The eightfold coordination is untypical for both Fe3+ and Ti4+ and had so far been unknown for minerals, though reported for several synthetic compounds (Britvin et al., 2020). We note that, for Ti4+, there is an analogy with Zr4+, which shows high (>6) coordination numbers, e.g. in baddeleyite ZrO2. The tetravalent state of Ti in lehmannite and the trivalent state of Fe in both new minerals is in agreement with the extremely oxidizing conditions of mineral deposition in the Arsenatnaya fumarole (Pekov et al., 2014a, 2018), where the majority of elements demonstrate only their highest oxidation states known in nature: S6+, Mo6+, As5+, V5+, Fe3+, etc.
Description of the crystal structures of arsmirandite and lehmannite in terms of anion-centred tetrahedra (Krivovichev et al., 2013) is enlightening. The O8 and O10 atoms do not belong to the arsenate group and are coordinated by three Cu2+ and one M (Fe3+ or Ti4+) cations each (Fig. 4). As such, OCu3M tetrahedra linked by sharing common edges constitute the cationic [O8MCu12] core of the {[MCu12O8](AsO4)8} nanoclusters (Fig. 4). The core is surrounded by eight AsO4 tetrahedra that are in the face-to-face orientation relative to the OCu3M tetrahedra (Fig. 4). The metal-oxide [O8MCu12] core of the nanocluster can be considered as a fluorite derivative and as such has been observed in several synthetic compounds (Krivovichev et al., 2013).
As reported by Britvin et al. (2020), the crystal structures of arsmirandite and lehmannite can be described as a periodic incorporation of metal-oxide nanoclusters into NaCl matrix, which explains a pseudo-tetragonal symmetry of both minerals (the NaCl matrix is tetragonally compressed). The transformation matrix from true monoclinic (C2/m) to pseudo-tetragonal (I4/mmm) setting is (−1/2 −1/2 −1) (−1/2 −1/2 −1) (1 0 0). The lattice parameters of pseudo-tetragonal cell for arsmirandite are: a = 14.854 and c = 10.742 Å. The relationships between the monoclinic and pseudo-tetragonal unit cells are very similar to those known for the aluminosilicate zeolites of the phillipsite series (Steinfink, 1962; Rinaldi et al., 1974). This similarity, albeit solely geometric, may explain the similarity of pseudo-tetragonal twins of arsmirandite (Fig. 1, b) to the well-known twins of the phillipsite-group minerals.
Relationship of arsmirandite and lehmannite to one another and with other minerals
The difference between arsmirandite and lehmannite is related to the nature of the M cation. Since Fe3+ and Ti4+ possess different charges, substitution of trivalent iron for tetravalent titanium requires corresponding compensation of the charge balance as described by Britvin et al. (2020). The main formal substitution scheme responsible for the transition from arsmirandite to lehmannite is: Fe3+ + □0 → Ti4+ + F–. Thus, the idealized, end-member formula of lehmannite contains 46 anions instead of 45 in arsmirandite (Table 5).
Table 5.
Comparative data for arsmirandite and lehmannite* Таблица 5. Сравнительная характеристика арсмирандита и леманнита*
|
Mineral |
Arsmirandite |
Lehmannite |
|
Idealized formula |
Na18Cu12Fe3+O8(AsO4)8Cl5 |
Na18Cu12Ti4+O8(AsO4)8FCl5 |
|
Crystal system |
Monoclinic |
|
|
Space group |
C2/m |
|
|
a, Å |
10.742 (2) |
10.8236 (15) |
|
b, Å |
21.019 (3) |
21.1077 (17) |
|
c, Å |
11.787 (2) |
11.8561 (11) |
|
β, ° |
117.06 (3) |
117.195 (8) |
|
V, Å3 |
2370.0 (7) |
2409.2 (5) |
|
Z |
2 |
2 |
|
Dcalc, g cm–3 |
3.715 |
3.676 |
|
Strongest reflections of the powder X-ray diffraction pattern: d, Å – I, % |
10.58 – 79 |
10.52 – 65 |
|
8.74 – 100 |
8.74 – 100 |
|
|
5.381 – 46 |
5.419 – 36 |
|
|
5.288 – 80 |
5.273 – 74 |
|
|
3.770 – 33 |
3.772 – 37 |
|
|
2.693 – 28 |
2.636 – 43 |
|
|
2.643 – 30 |
2.573 – 98 |
|
|
2.574 – 74 |
1.889 – 33 |
|
The topology of the metal-oxide-arsenate clusters in the two minerals is similar to that observed in polyoxopalladates [see Britvin et al. (2020) for references]. Lehmannite is the third natural hydrogen-free arsenate with the species-defining Ti, after katiarsite, KTiO(AsO4) (Pekov et al., 2016), and arsenatrotitanite, NaTiO(AsO4) (Pekov et al., 2019). All three minerals were found in high-temperature sublimates in the same Arsenatnaya fumarole. Braithwaiteite, ideally NaCu5(Ti4+Sb5+)O2(AsO4)4[AsO3(OH)]2 · 8H2O, demonstrates some similarity to lehmannite in terms of the chemical composition, but it is a highly hydrated arsenate formed in oxidation zone of a sulfide copper deposit (Paar et al., 2009).
From the chemical point of view, both arsmirandite and lehmannite are polyoxocuprates and belong to the emerging class of minerals containing polyoxometalate clusters (Krivovichev, 2020). As a rule, polyoxometalate minerals crystallize from low-temperature aqueous environments, mostly in oxidation zones of mineral deposits. For instance, bouazzerite, Bi6(Mg,Co)11Fe14(AsO4)18O12(OH)4(H2O)86 (Brugger et al., 2007), and whitecapsite, ${{{\text{H}}}_{{16}}}{\text{Sb}}_{6}^{{3 + }}{\text{Fe}}_{5}^{{2 + }}{\text{Fe}}_{{14}}^{{3 + }}$(AsO4)18O16(H2O)120 (Pekov et al., 2014b), which are both based upon heptanuclear metal-oxide core arsenate nanoclusters, have been discovered in oxidation zones, where they crystallized from low-temperature solutions. In contrast, arsmirandite and lehmannite are the first and the only known minerals that contain polyoxometalate clusters and form in anhydrous high-temperature fumarolic environments.
Список литературы
Britvin S.N., Dolivo-Dobrovolsky D.V., Krzhizhanovskaya M.G. Software for processing the X-ray powder diffraction data obtained from the curved image plate detector of Rigaku RAXIS Rapid II diffractometer. Zapiski RMO (Proc. Russian Miner. Soc.). 2017. Vol. 146. N 3. P. 104–107 (in Russian).
Britvin S.N., Pekov I.V., Yapaskurt V.O., Koshlyakova N.N., Göttlicher J., Krivovichev S.V., Turchkova A.G., Sidorov E.G. Polyoxometalate chemistry at volcanoes: discovery of a novel class of polyoxocuprate nanoclusters in fumarolic minerals. Sci. Rep. 2020. Vol. 10. Paper 6345.
Brooke H.J., Miller W.H. An Elementary Introduction to Mineralogy by the late William Phillips. New Edition with extensive alterations and additions. Longman, Brown, Green and Longmans, London, 1852.
Brugger J., Meisser N., Krivovichev S., Armbruster T., Favreau G. Mineralogy and crystal structure of bouazzerite from Bou Azzer, Anti-Atlas, Morocco: Bi-As-Fe nanoclusters containing Fe3+ in trigonal prismatic coordination. Amer. Miner. 2007. Vol. 92. P. 1630–1639.
Chukanov N.V., Chervonnyi A.D. Infrared Spectroscopy of Minerals and Related Compounds. Springer Verlag, Cham, 2016. 1109 pp.
Fedotov S.A., Markhinin Y.K., eds. The Great Tolbachik Fissure Eruption. Cambridge University Press, New York, 1983. 341 pp.
Krivovichev S.V. Polyoxometalate clusters in minerals: review and complexity analysis. Acta Crystallogr. 2020. Vol. B76 (in press).
Krivovichev S.V., Mentré O., Siidra O.I., Colmont M., Filatov S.K. Anion-centered tetrahedra in inorganic compounds. Chem. Rev. 2013. Vol. 113. P. 6459–6535.
Paar W.H., Cooper M.A., Hawthorne F.C., Moffatt E., Gunther M.E., Roberts A.C., Dunn P.J. Braithwaiteite, NaCu5(TiSb)2O2(AsO4)[AsO3(OH)]2 ⋅ 8H2O, a new mineral species from Laurani, Bolivia. Canad. Miner. 2009. Vol. 47. P. 947–952.
Pekov I.V., Zubkova N.V., Yapaskurt V.O., Belakovskiy D.I., Lykova I.S., Vigasina M.F., Sidorov E.G., Pushcharovsky D.Yu. New arsenate minerals from the Arsenatnaya fumarole, Tolbachik volcano, Kamchatka, Russia. I. Yurmarinite, Na7(Fe3+,Mg,Cu)4(AsO4)6. Miner. Mag. 2014a. Vol. 78. P. 905–917.
Pekov I.V., Zubkova N.V., Göttlicher J., Yapaskurt V.O., Chukanov N.V., Lykova I.S., Belakovskiy D.I., Jensen, M.C., Leising J.F., Nikischer A.J., Pushcharovsky D.Yu. Whitecapsite, a new hydrous iron and trivalent antimony arsenate mineral from the White Caps mine, Nevada, USA. Eur. J. Miner. 2014b. Vol. 26. P. 577–587.
Pekov I.V., Yapaskurt V.O., Britvin S.N., Zubkova N.V., Vigasina M.F., Sidorov E.G. New arsenate minerals from the Arsenatnaya fumarole, Tolbachik volcano, Kamchatka, Russia. V. Katiarsite, KTiO(AsO4). Miner. Mag. 2016. Vol. 80. P. 639–646.
Pekov I.V., Koshlyakova N.N., Zubkova N.V., Lykova I.S., Britvin S.N., Yapaskurt V.O., Agakhanov A.A., Shchipalkina N.V., Turchkova A.G., Sidorov E.G. Fumarolic arsenates – a special type of arsenic mineralization. Eur. J. Miner. 2018. Vol. 30. P. 305–322.
Pekov I.V., Zubkova N.V., Agakhanov A.A., Belakovskiy D.I., Vigasina M.F., Yapaskurt V.O., Sidorov E.G., Britvin S.N., Pushcharovsky D.Yu. New arsenate minerals from the Arsenatnaya fumarole, Tolbachik volcano, Kamchatka, Russia. IX. Arsenatrotitanite, NaTiO(AsO4). Miner. Mag. 2019. Vol. 83. P. 453–458.
Ravel B., Newville M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchr. Rad. 2005. Vol. 12. P. 537–541.
Rinaldi R., Pluth J.J., Smith J.V. Zeolites of the phillipsite family. Refinement of the crystal structures of phillipsite and harmotome. Acta Crystallogr. 1974. Vol. B30. P. 2426–2433.
Shannon R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976. Vol. A32. P. 751–767.
Sheldrick G.M. A short history of SHELX. Acta Crystallogr. 2008. Vol. A64. P. 112–122.
Siidra O.I., Vladimirova V.A., Tsirlin A.A., Chukanov N.V., Ugolkov V.L. Cu9O2(VO4)4Cl2, a first copper oxychloride vanadate: mineralogically inspired synthesis and magnetic behaviour. Inorg. Chem. 2020. Vol. 59. P. 2136–2143.
Steinfink H. The crystal structure of the zeolite, phillipsite. Acta Crystallogr. 1962. Vol. 15. P. 644–651.
Vernadsky V.I. On the discovery of crocoite. Lomonosovskii Sbornik. Saint Petersburg, 1911. P. 345–354 (in Russian).
Дополнительные материалы отсутствуют.
Инструменты
Записки Российского минералогического общества


