Записки Российского минералогического общества, 2020, T. 149, № 4, стр. 67-77
Philoxenite, (K,Na,Pb)4(Na,Ca)2(Mg,Cu)3(${\text{Fe}}_{{0.5}}^{{3 + }}$Al0.5)(SO4)8, a new mineral from fumarole exhalations of the Tolbachik volcano, Kamchatka, Russia
I. V. Pekov 1, *, A. A. Agakhanov 2, N. V. Zubkova 1, D. I. Belakovskiy 2, M. F. Vigasina 1, S. N. Britvin 3, 4, A. G. Turchkova 1, E. G. Sidorov 5
1 Faculty of Geology, Moscow State University
119991 Moscow, Vorobievy Gory, Russia
2 Fersman Mineralogical Museum RAS
119071 Moscow, Leninsky pr., 18-2, Russia
3 Institute of Earth Sciences, Saint Petersburg State University
199034 Saint Petersburg, University emb., 7/9, Russia
4 Kola Science Center RAS
184200 Apatity, Fersman st., 14, Russia
d Institute of Volcanology and Seismology, Far Eastern Branch RAS
683006 Petropavlovsk-Kamchatsky, Piip Boulevard, 9, Russia
* E-mail: igorpekov@mail.ru
Поступила в редакцию 18.05.2020
После доработки 18.05.2020
Принята к публикации 17.06.2020
Аннотация
A new mineral philoxenite (K,Na,Pb)4(Na,Ca)2(Mg,Cu)3(${\text{Fe}}_{{0.5}}^{{3 + }}$Al0.5)(SO4)8 is found in sublimates of the Yadovitaya fumarole at the Second scoria cone of the Northern Breakthrough of the Great Tolbachik Fissure Eruption, Tolbachik volcano, Kamchatka, Russia. It is associated with euchlorine, langbeinite, hematite, tenorite, piypite, alumoklyuchevskite, dolerophanite, vergasovaite, cupromolybdite, ziesite, and yaroshevskite. Philoxenite forms isolated oblique-angled, pseudotrigonal or polygonal tabular crystals up to 0.3 × 0.6 mm. The mineral is transparent, colourless to very pale yellowish with a vitreous lustre. Philoxenite is brittle. Cleavage was not observed, the fracture is uneven. Dcalc = 3.03 g cm–3. Philoxenite is optically biaxial (–), α = 1.562(2), β = 1.572(2), γ = 1.580(2), 2Vmeas = 85(5)° (λ = 589 nm). The Raman spectrum is given. The chemical composition (wt %, electron-microprobe data) is: Na2O 4.67, K2O 13.34, Rb2O 0.13, CaO 2.84, PbO 4.54, MgO 6.37, MnO 0.20, CuO 5.40, ZnO 1.48, Al2O3 3.40, Fe2O3 3.29, SO3 54.62, total 100.28. The empirical formula calculated on the basis of 32 O apfu is (K3.30Na1.76Ca0.59Pb0.24Rb0.02)Σ5.91(Mg1.84Cu0.79Al0.78${\text{Fe}}_{{0.48}}^{{3 + }}$Zn0.21Mn0.03)Σ4.13S7.96O32. Philoxenite is triclinic, space group P-1, a = 8.8410(3), b = 8.9971(3), c = 16.1861(5) Å, α = = 91.927(3), β = 94.516(3), γ = 90.118(3)°, V = 1282.77(7) Å3, and Z = 2. The strongest reflections of the X-ray powder pattern [(d,Å(I)(hkl)] are: 5.70(18)(111); 4.030(24)(004); 3.146(100)(–220); 3.136(72)(220); 2.965(36)(–1–15), 2.912(35)(–115), 2.834(36)(0–32, 1–15), and 2.784(42)(115, 032). The crystal structure of philoxenite is unique. It is based on a novel-type microporous heteropolyhedral framework built by SO4 tetrahedra and MO6 octahedra (M = Mg, Cu2+, Zn, Fe3+ and Al). The name philoxenite is derived from the ancient Greek words φίλος, a friend, and ξένος, a guest, in allusion to the complex cationic composition of the mineral and the presence of significant amounts of admixtures at nine of eleven independent cationic positions in its crystal structure.
INTRODUCTION
Tolbachik volcano at Kamchatka is an outstanding geological object in different aspects. One of them is the mineralogy of the fumarolic formation: at present day, Tolbachik can rightfully be called the object number 1 in the world in diversity, originality and knowledge degree of minerals formed in oxidizing-type fumaroles. Almost 350 minerals are reliably known in fumarole systems at Tolbachik and majority of them are of exhalation origin; more than 120 new mineral species have been discovered in Tolbachik fumaroles (Pekov et al., 2020). Sulfates are the most numerous minerals in Tolbachik fumaroles: sixty valid mineral species belonging to this chemical class are known here in high-temperature assemblages. Sulfates are the main constituents in sublimates of the majority of Tolbachik fumaroles. Among the most abundant sulfate minerals there are langbeinite, anhydrite, aphthitalite-group members, euchlorine and some others.
This article is devoted to a new sulfate mineral philoxenite (Cyrillic: филоксенит) (K,Na,Pb)4(Na,Ca)2(Mg,Cu)3(${\text{Fe}}_{{0.5}}^{{3 + }}$Al0.5)(SO4)8 from fumarolic exhalations of Tolbachik. So complicated crystal chemistry, reflected even in the simplified formula, caused the choice of the name for this mineral species: it is derived from the ancient Greek words φίλος, a friend, and ξένος, a guest, in allusion to the complex cationic composition of the mineral and the presence of significant amounts of admixtures at nine of eleven independent cationic positions in its crystal structure.
The new mineral and its name have been approved by the IMA Commission on New Minerals, Nomenclature and Classification (IMA2015–108). The type specimen (part of holotype) is deposited in the systematic collection of the Fersman Mineralogical Museum of the Russian Academy of Sciences, Moscow with the catalogue number 95283.
OCCURRENCE AND GENERAL APPEARANCE
Philoxenite originates from the Yadovitaya ('Poisonous') fumarole located at the apical part of the Second scoria cone of the Northern Breakthrough of the Great Tolbachik Fissure Eruption 1975–1976 (NB GTFE), Tolbachik volcano, Kamchatka Peninsula, Far-Eastern Region, Russia (55°41' N 160°14' E, 1200 m asl). The Second scoria cone of the NB GTFE is a monogenetic volcano about 300 m high and 0.1 km3 in volume formed in 1975 (Fedotov, Markhinin, 1983). Many active fumaroles occur at this scoria cone even at present day, after more than forty years after the eruption.
Yadovitaya is one of the famous Tolbachik fumaroles, especially in the mineralogical aspect being, in particular, the type locality of twenty-seven (!) valid mineral species. It remains active and hot from 1975 and has been characterized in several papers. The most detailed data were reported by L.P. Vergasova and S.K. Filatov (2016). The gases of the Yadovitaya fumarole are escaping through a cave about 1.5 m wide and 2 m deep with the walls covered by thick incrustations of different sublimate minerals, mainly sulfates. Euchlorine, piypite, langbeinite or metathénardite are prevailing in the different parts of the cave. Arsenate crusts with lammerite as the main component occur in some areas.
Philoxenite is one of the rarest minerals in Yadovitaya: only several crystals are found. All these crystals are present in the single specimen collected by us in July 2015 from the moderately hot zone of the fumarole. The temperature measured using chromel-alumel thermocouple in this zone during collecting was ca 250°C. We think that philoxenite was deposited directly from gaseous phase as volcanic sublimate or was formed as a result of the interaction between fumarolic gases and host basalt scoria (probable source of low-volatile Mg, Ca and Al) at temperatures not less than 250–300 °C.
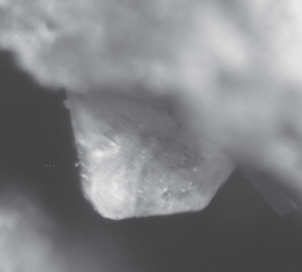
Philoxenite forms isolated well-shaped tabular crystals up to 0.6 mm across and up to 0.3 mm thick. The crystals are oblique-angled, pseudotrigonal or polygonal. They occur in cavities within euchlorine crusts (Fig. 1) overgrowing basalt scoria altered by fumarolic gases. Other associated minerals are langbeinite, hematite, tenorite, piypite, alumoklyuchevskite, dolerophanite, vergasovaite, cupromolybdite, ziesite, and yaroshevskite.
Fig. 1.
Tabular crystal of philoxenite on euchlorine. FOV width: 0.7 mm. Photo: I.V. Pekov and A.V. Kasatkin. Рис. 1. Таблитчатый кристалл филоксенита на эвхлорине. Ширина поля снимка 0.7 мм. Фотография: И.В. Пеков и А.В. Касаткин.
PHYSICAL PROPERTIES AND OPTICAL DATA
Philoxenite is colourless to very pale yellowish, transparent, with a vitreous lustre and a white streak. The mineral is non-fluorescent in ultraviolet rays. Philoxenite is brittle with uneven fracture and Mohs’ hardness of ca 3. No cleavage or parting was observed. Density was not measured because of paucity of material; the density calculated using the empirical formula and unit-cell volume obtained from single-crystal data is 3.027 g cm–3.
In plane-polarized transmitted light, philoxenite is colourless and non-pleochroic. It is optically biaxial (–), α = 1.562(2), β = 1.572(2), γ = 1.580(2) (589 nm), 2Vmeas = 85(5)° and 2Vcalc = 83°. Dispersion of optical axes is very strong, r > v.
RAMAN SPECTROSCOPY
The Raman spectrum of philoxenite (Fig. 2a) was recorded using an EnSpectr R532 spectrometer with an Ar+ laser (532 nm) at room temperature. The output power of the laser beam was about 20 mW. The spectrum was processed using the EnSpectr expert mode program in the range from 100 to 4000 cm–1 with the use of a holographic diffraction grating with 1800 lines cm–1 and a resolution equal to 6 cm–1. The diameter of the focal spot on the sample was about 10 μm. Raman spectrum was acquired on a polycrystalline (powdered) sample.
Fig. 2.
Raman spectra of philoxenite (a, with enlarged low-frequency region) and langbeinite (b), both from the Yadovitaya fumarole, Tolbachik volcano, Kamchatka, Russia. Рис. 2. КР-спектры филоксенита (a, с увеличенным фрагментом, показывающим низкочастотную область) и лангбейнита (b). Оба образца происходят из фумаролы Ядовитой, вулкан Толбачик, Камчатка.
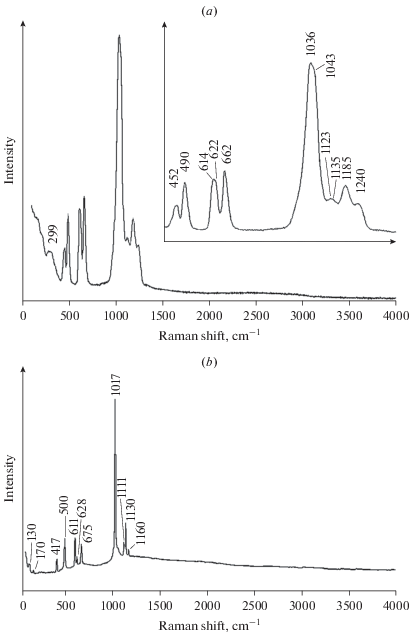
The bands in the Raman spectrum of philoxenite and their assignments, according to K. Nakamoto (1986), are (cm–1, s – strong band): 1240, 1185, 1135, 1123 [F2(ν3)-type stretching vibrations of ${\text{SO}}_{4}^{{2 - }}$], 1043s, 1036s [A1(ν1) symmetric stretching vibrations of ${\text{SO}}_{4}^{{2 - }}$], 662, 622, 614 [F2(ν4) bending vibrations of ${\text{SO}}_{4}^{{2 - }}$], 490 and 452 [E(ν2) bending vibrations of ${\text{SO}}_{4}^{{2 - }}$].
The absence of bands with frequencies higher than 1250 cm–1 indicates the absence of groups with O–H, C–H, C–O, N–H and N–O bonds in philoxenite.
The Raman spectrum of the cubic (P213) sulfate langbeinite K2Mg2(SO4)3 remotely related to philoxenite, recorded under the same conditions, is shown in Fig. 2b. It has some common features with the spectrum of philoxenite, however, the latter contains more bands, including distinct splitting of the strongest band near 1040 cm–1, definitely due to lower symmetry of philoxenite and the presence of eleven differently occupied sites of metal cations in its crystal structure (see below) whereas the structure of langbeinite contains four such sites (Mereiter, 1979).
CHEMICAL DATA
The chemical composition of philoxenite was determined using a Jeol 733 electron microprobe instrument operated in WDS mode with an accelerating voltage of 20 kV, a beam current of 20 nA, and a beam diameter of 3 μm. The chemical composition (average of five spot analyses) is given in Table 2. Contents of other elements with atomic numbers higher than that of carbon are below detection limits.
Table 1.
Comparative data for philoxenite and langbeinite Таблица 1. Сравнительная характеристика филоксенита и лангбейнита
| Mineral | Philoxenite | Langbeinite* |
|---|---|---|
| Formula | (K,Na,Pb)4(Na,Ca)2 (Mg,Cu)3(Fe3+0.5Al0.5)(SO4)8 |
K2Mg2(SO4)3 |
| Crystal system Space group |
Triclinic P-1 |
Cubic P213 |
| a, Å b, Å c, Å α, ° β, ° γ, ° |
8.8410(3) 8.9971(3) 16.1861(5) 91.927(3) 94.516(3) 90.118(3) |
9.92 |
| V, Å3 | 1282.77(7) | 976 |
| Z | 2 | 4 |
| Strong lines of the powder X-ray diffraction pattern: d, Å – I, % |
5.70 – 18 4.030 – 24 3.146 – 100 3.136 – 72 2.965 – 36 2.912 – 35 2.834 – 36 2.784 – 42 |
4.05 – 25 3.137 – 100 2.992 – 16 2.753 – 16 2.651 – 35 2.405 – 12 1.946 – 10 1.609 – 12 |
| Optical data | Biaxial (–), α = 1.562, β = 1.572, γ = 1.580, 2Vmeas = 80° | Isotropic, n = 1.533–1.535 |
| Density, g·cm–3 | 3.03 (calc) | 2.77–2.83 |
| Source | this work | Mereiter, 1979; Anthony et al., 2003 |
Table 2.
Chemical composition (wt %) of philoxenite Таблица 2. Химический состав (мас. %) филоксенита
| Constituent | Mean | Range | Standard deviation | Probe standard |
|---|---|---|---|---|
| Na2O | 4.67 | 4.06–5.19 | 0.42 | Albite |
| K2O | 13.34 | 13.21–13.50 | 0.12 | Microcline |
| Rb2O | 0.13 | 0.00–0.35 | 0.13 | Rb2Nb4O11 |
| CaO | 2.84 | 2.71–2.99 | 0.11 | Wollastonite |
| PbO | 4.54 | 4.30–4.97 | 0.27 | PbTiO3 |
| MgO | 6.37 | 5.87–6.65 | 0.33 | Chromite |
| MnO | 0.20 | 0.00–0.42 | 0.19 | Mn |
| CuO | 5.40 | 5.13–5.62 | 0.18 | Cu |
| ZnO | 1.48 | 1.38–1.60 | 0.10 | ZnS |
| Al2O3 | 3.40 | 3.15–3.79 | 0.25 | Al2O3 |
| Fe2O3* | 3.29 | 2.94–3.80 | 0.37 | Magnetite |
| SO3 | 54.62 | 53.85–54.95 | 0.47 | ZnS |
| Total | 100.28 |
The empirical formula calculated on the basis of 32 O atoms per formula unit is K3.30Na1.76Ca0.59Pb0.24Rb0.02Mg1.84Cu0.79Al0.78${\text{Fe}}_{{0.48}}^{{3 + }}$Zn0.21Mn0.03S7.96O32. It can be also written in the following form, with large A cations and medium-sized M cations grouped taking into consideration the structure data (see below): A(K3.30Na1.76Ca0.59Pb0.24Rb0.02)Σ5.91M(Mg1.84Cu0.79Al0.78${\text{Fe}}_{{0.48}}^{{3 + }}$Zn0.21Mn0.03)Σ4.13S7.96O32.
The Gladstone-Dale compatibility index [1 – (Kp/Kc)] value (Mandarino, 1981) for philoxenite is –0.003, superior.
At room temperature philoxenite hydrolyses (becomes dull and soft in half an hour) in H2O and further slowly dissolves.
X-RAY CRYSTALLOGRAPHY, CRYSTAL STUCTURE DATA AND SIMPLIFIED FORMULA
Powder X-ray diffraction (XRD) data of philoxenite (Table 3) were collected with a Rigaku R-AXIS Rapid II single-crystal diffractometer equipped with a cylindrical image plate detector (r = 127.4 mm) using Debye-Scherrer geometry, CoKα radiation (rotating anode with VariMAX microfocus optics), 40 kV, 15 mA and an exposure time of 10 min. Angular resolution of the detector is 0.045 2Θ (pixel size 0.1 mm). The data were integrated using the software package osc2tab (Britvin et al., 2017). The powder XRD diagram of philoxenite is unique and can be used as good diagnostic tool for the mineral. Parameters of the triclinic unit cell calculated from powder data are: a = 8.84(4), b = 9.00(2), c = 16.20(3) Å, α = 91.94(3), β = = 94.51(2), γ = 90.08(3)°, V = 1284(1) Å3, and Z = 2.
Table 3.
Powder X-ray diffraction data of philoxenite Таблица 3. Результаты расчета порошковой рентгенограммы филоксенита
| Iobs | dobs, Å | Icalc* | dcalc, Å ** | h k l |
|---|---|---|---|---|
| 3 | 8.01 | 2, 2 | 8.063, 8.004 | 002, –101 |
| 1 | 7.49 | 1.5 | 7.489 | 101 |
| 9 | 5.99 | 8 | 6.016 | –1–11 |
| 4 | 5.93 | 5 | 5.942 | –111 |
| 12 | 5.79 | 11 | 5.811 | 1–11 |
| 18 | 5.70 | 22 | 5.700 | 111 |
| 1 | 4.254 | 1 | 4.255 | –1–13 |
| 1 | 4.148 | 0.5, 0.5 | 4.168, 4.159 | 201, –113 |
| 24 | 4.030 | 19, 1 | 4.032, 4.027 | 004, 1–13 |
| 6 | 3.981 | 6 | 3.985 | 0–22 |
| 8 | 3.872 | 8 | 3.871 | 022 |
| 4 | 3.815 | 4 | 3.822 | 121 |
| 8 | 3.740 | 9 | 3.745 | 202 |
| 11 | 3.475 | 14 | 3.481 | 2–12 |
| 15 | 3.433 | 18 | 3.433 | 212 |
| 3 | 3.308 | 5 | 3.314 | –1–23 |
| 4 | 3.231 | 2, 0.5 | 3.276, 3.225 | 114, 005 |
| 9 | 3.205 | 11 | 3.207 | 1–23 |
| 100 | 3.146 | 100 | 3.155 | –220 |
| 72 | 3.136 | 75, 3 | 3.140, 3.129 | 220, –2–21 |
| 12 | 3.107 | 13 | 3.111 | 123 |
| 2 | 3.050 | 1, 0.5 | 3.053, 3.037 | 0–24, 221 |
| 36 | 2.965 | 2, 54 | 2.971, 2.967 | –222, –1–15 |
| 35 | 2.912 | 41, 4 | 2.911, 2.909 | –115, –214 |
| 36 | 2.834 | 10, 1, 31, 3 | 2.841, 2.836, 2.835, 2.834 | 0–32, 1–24, 1–15, 130 |
| 42 | 2.784 | 3, 7, 4, 24, 11 | 2.789, 2.788, 2.786, 2.780, 2.779 | 310, –3–11, –311, 115, 032 |
| 3 | 2.710 | 1, 3, 0.5 | 2.709, 2.708, 2.706 | 311, 214, –205 |
| 11 | 2.668 | 15 | 2.668 | –303 |
| 5 | 2.565 | 2, 4 | 2.568, 2.567 | –1–33, –3–13 |
| 5 | 2.550 | 1, 3 | 2.552, 2.549 | 016, –313 |
| 4 | 2.521 | 3, 3 | 2.525, 2.519 | –224, 1–33 |
| 4 | 2.509 | 0.5, 0.5, 2 | 2.510, 2.507, 2.505 | 205, 1–25, –133 |
| 2 | 2.476 | 1, 1 | 2.484, 2.473 | –230, 230 |
| 4 | 2.451 | 1, 0.5, 3 | 2.457, 2.454, 2.449 | –3–21, 320, 133 |
| 5 | 2.417 | 5, 0.5, 0.5 | 2.419, 2.418, 2.414 | 3–21, 3–13, –2–32 |
| 4 | 2.395 | 2, 1 | 2.396, 2.393 | 321, 313 |
| 1 | 2.286 | 1.5 | 2.285 | –216 |
| 3 | 2.188 | 2, 1 | 2.187, 2.184 | 107, –315 |
| 2 | 2.167 | 1, 2 | 2.169, 2.165 | 2–16, 225 |
| 5 | 2.140 | 1, 2, 4 | 2.142, 2.139, 2.139 | 1–17, 1–35, 216 |
| 3 | 2.128 | 5 | 2.127 | –2–26 |
| 4 | 2.077 | 6, 3, 2 | 2.080, 2.077, 2.071 | –226, 3–31, 135 |
| 7 | 2.055 | 1, 5, 7 | 2.055, 2.055, 2.055 | –1–27, 331, –1–43 |
| 4 | 2.034 | 6, 0.5, 1 | 2.036, 2.034, 2.030 | 4–12, –2–35, 1–43 |
| 12 | 2.016 | 7, 2, 7 | 2.016, 2.014, 2.011 | 008, 2–26, –143 |
| 4 | 1.993 | 4 | 1.993 | 1–27 |
| 6 | 1.957 | 4, 3 | 1.958, 1.957 | –4–14, –4–22 |
| 7 | 1.948 | 2, 4, 1, 0.5, 6 | 1.950, 1.948, 1.945, 1.945, 1.941 | –422, –414, 2–17, 4–13, 127 |
| 1 | 1.901 | 1, 2 | 1.902, 1.900 | 1–18, 333 |
| 1 | 1.862 | 1 | 1.861 | –2–18 |
| 2 | 1.840 | 1, 0.5 | 1.840, 1.840 | 4–14, 3–34 |
| 6 | 1.824 | 2, 12 | 1.826, 1.824 | 414, –3–35 |
| 7 | 1.790 | 1, 12 | 1.792, 1.789 | 2–44, –335 |
| 6 | 1.758 | 2, 5, 0.5 | 1.761, 1.757, 1.756 | –1–19, 1–51, 341 |
| 5 | 1.741 | 1.5, 4 | 1.746, 1.741 | 244, 151 |
| 5 | 1.738 | 1, 1, 5, 2 | 1.739, 1.736, 1.736, 1.735 | –119, 218, 3–35, –511 |
| 2 | 1.717 | 2 | 1.716 | 424 |
| 2 | 1.706 | 1.5, 3 | 1.709, 1.703 | 5–11, 511 |
| 3 | 1.699 | 1, 3 | 1.696, 1.695 | 046, 335 |
| 1 | 1.646 | 0.5, 1.5, 1 | 1.647, 1.644, 1.644 | 038, 3–27, –5–21 |
| 1 | 1.622 | 1, 0.5 | 1.623, 1.621 | 425, –2–38 |
| 2 | 1.615 | 0.5, 0.5, 1, 1 | 1.615, 1.615, 1.613, 1.612 | 5–13, –3–37, 327, –246 |
| 1 | 1.603 | 1.5 | 1.603 | 2–46 |
| 8 | 1.578 | 1, 1, 1, 11 | 1.579, 1.578, 1.578, 1.577 | 522, 0.1.10, –238, –440 |
| 5 | 1.571 | 1, 4, 1 | 1.573, 1.570, 1.570 | –515, 440, –3–19 |
| 4 | 1.557 | 0.5, 1, 2, 1, 4, 1 | 1.558, 1.555, 1.555, 1.555, 1.555, 1.554 | –442, 246, –1–39, –319, –2.0.10, 4–26 |
| 5 | 1.535 | 1, 4, 2, 1, 1, 2 | 1.536, 1.535, 1.534, 1.533, 1.533, 1.532 | –155, 0.–2.10, –3–51, 1.1.10, –4–18, –351 |
| 2 | 1.523 | 1, 1.5, 1 | 1.523, 1.522, 1.521 | –531, –5–31, –418 |
| 2 | 1.515 | 0.5, 2, 0.5 | 1.516, 1.514, 1.514 | 530, –436, –5–25 |
| 2 | 1.505 | 0.5, 1, 2 | 1.504, 1.502, 1.502 | –4–44, –525, 0.2.10 |
| 2 | 1.484 | 1, 1 | 1.485, 1.483 | –444, –2.–2.10 |
| 2 | 1.480 | 0.5, 3 | 1.479, 1.477 | 309, 2.0.10 |
| 2 | 1.467 | 0.5, 3 | 1.470, 1.466 | –161, –602 |
| 1 | 1.433 | 1 | 1.433 | 1–63 |
| 2 | 1.420 | 0.5, 0.5, 1, 0.5 | 1.421, 1.418, 1.418, 1.418 | –260, –2–61, –3–39, 2–56 |
| 2 | 1.406 | 1, 1, 1, 1 | 1.407, 1.406, 1.405, 1.405 | 163, 612, –4–46, –535 |
| 1 | 1.393 | 1 | 1.393 | –5–27 |
Single-crystal XRD studies were carried out using an Xcalibur S CCD diffractometer (MoKα radiation). Philoxenite is triclinic, space group P-1. The unit-cell parameters obtained from single-crystal XRD data are given in Table 1.
The crystal structure of philoxenite was solved by direct methods based on single-crystal XRD data and refined to R = 0.0567 on the basis of 5367 independent reflections with I > > 2σ(I). The crystal structure of the new mineral is unique. It is characterized and discussed in separate paper (Zubkova et al., 2020) and here we report only major structural features of philoxenite.
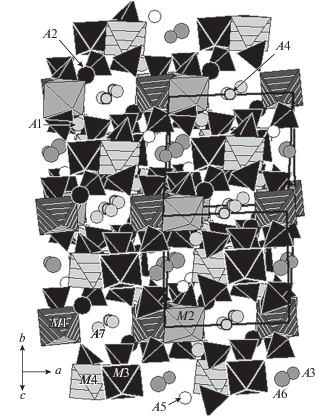
The crystal structure of this mineral (Fig. 3) contains eleven crystallographically nonequivalent positions of metal cations Me: seven A sites of large cations (K, Na, Ca and Pb2+) and four octahedrally coordinated M sites of medium-sized cations (Mg, Cu2+, Zn, Fe3+ and Al). Isolated MO6 octahedra are connected via isolated from each other SO4 tetrahedra forming a novel-type of a heteropolyhedral (tetrahedral-octahedral) three-dimensional framework. This framework is interrupted: one oxygen vertex of each SO4 tetrahedron is not shared with MO6 octahedron. Large A cations are located in the tunnels of this microporous M–S–O framework.
Fig. 3.
The crystal structure of philoxenite. The unit cell is outlined. Рис. 3. Кристаллическая структура филоксенита. Показана элементарная ячейка.
The structural formula of philoxenite is (Z = 1):
We prefer to present the content of the M(3) site in the simplified formula of the mineral as (${\text{Fe}}_{{0.5}}^{{3 + }}$Al0.5) but not as (Fe3+,Al) because the contents of Fe and Al in this site are almost equal and, therefore, even slight their variations in chemical analyses result insignificant prevailing of Fe or Al in M(3). This could not be, in our mind, a reason of the formal division of philoxenite to two separate mineral species, with Fe > Al and with Al > Fe in the M(3) site. In this approach we are guided by the rule formulated in the IMA report devoted to solid solutions in mineral nomenclature: “if the known composition embrace to 50% mark, but do not appear to extend to either end member, only one name should apply to the compositional range. However, the compositional range should be taken into account; if it is very small, then only one name should be given, but if it is large, consideration may be given to two names” (Nickel, 1992). In philoxenite, the compositional range is very small and embraces the 50% mark between Al- and Fe-dominant compositions of the content the M(3) site.
Complicated scheme of cation substitutions, including heterovalent ones, prevents to write a correct charge-balanced formula idealized to only species-defining (i.e., prevailing in different structure positions) chemical constituents. The simplified formula considering all the above-discussed data is (K,Na,Pb)4(Na, Ca)2(Mg, Cu)3(${\text{Fe}}_{{0.5}}^{{3 + }}$Al0.5)(SO4)8.
Philoxenite demonstrates rare example of a natural sulfate with the microporous heteropolyhedral three-dimensional framework, even interrupted one.
Philoxenite is remotely related to langbeinite K2Mg2(SO4)3. The general motifs of arrangement of Me cations in these minerals are similar (Zubkova et al., 2020) that results in some common features in their powder X-ray diffraction patterns (in part of strong reflections) and Raman spectra (Table 1; Fig. 2). However, philoxenite and langbeinite, occurring in the same mineral association in the Yadovitaya fumarole, significantly differ in majority of essential characteristics (Table 1).
Список литературы
Anthony J.W., Bideaux R.A., Bladh K.W., Nichols, M.C. Handbook of Mineralogy. Vol. Borates, Carbonates, Sulfates. Mineral Data Publishing: Tucson, 2003. 813 p.
Britvin S.N., Dolivo-Dobrovolsky D.V., Krzhizhanovskaya M.G. Software for processing the X-ray powder diffraction data obtained from the curved image plate detector of Rigaku RAXIS Rapid II diffractometer. Zapiski RMO (Proc. Russian Miner. Soc.). 2017. Pt. 146. N 3. P. 104–107 (in Russian).
Fedotov S.A., Markhinin Y.K., eds. The Great Tolbachik Fissure Eruption. Cambridge University Press, New York, 1983. 341 p.
Mandarino J.A. The Gladstone–Dale relationship: Part IV. The compatibility concept and its application. Canad. Miner. 1981. Vol. 19. P. 441–450.
Mereiter K. Refinement of the crystal structure of langbeinite, K2Mg2(SO4)3. Neues Jahrb. Miner, Monatshefte. 1979. P. 182–188.
Nakamoto K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. John Wiley & Sons: New York, 1986. 484 p.
Nickel E.H. Solid solutions in mineral nomenclature. Canad. Miner. 1992. Vol. 30. P. 231–234.
Pekov I.V., Agakhanov A.A., Zubkova N.V., Koshlyakova N.N., Shchipalkina N.V., Sandalov F.D., Yapaskurt V.O., Turchkova A.G., Sidorov E.G. Oxidizing-type fumaroles of the Tolbachik volcano, a mineralogical and geochemical unique. Russian Geol. Geophys. 2020. Vol. 61. N 5–6. P. 675–688.
Vergasova L.P., Filatov S.K. A study of volcanogenic exhalation mineralization. J. Volcan. Seismol. 2016. Vol. 10. N 2. P. 71–85.
Zubkova N.V., Pekov I.V., Agakhanov A.A., Pushcharovsky D.Yu. A novel-type microporous heteropolyhedral framework in crystal structure of the natural sulfate philoxenite. Crystallogr. Rep. 2020 (in press).
Дополнительные материалы отсутствуют.
Инструменты
Записки Российского минералогического общества


