Записки Российского минералогического общества, 2020, T. 149, № 6, стр. 110-121
Pb10Al10Si8O41: Lead aluminosilicate with the narsarsukite-related structure
1 Nanomaterials Research Centre, Kola Science Centre RAS
184209 Apatity, Fersman st., 14, Russia
2 Saint Petersburg State University
199034 Saint Petersburg, University Emb., 7/9, Russia
* E-mail: s.krivovichev@ksc.ru
Поступила в редакцию 2.10.2020
После доработки 5.10.2020
Принята к публикации 7.10.2020
Аннотация
The crystal structure of Pb10Al10Si8O41 (orthorhombic, Fddd, a = 16.2940(17), b = 21.666(2), c = 22.655(2) Å, V = 7997.8(13) Å3, Z = 8) had been solved by direct methods and refined to R1 = 0.042 on the basis of 1393 unique observed reflections. The crystal structure of Pb10Al10Si8O41 contains three symmetrically independent Pb atoms, each having distorted coordination environment due to the stereoactivity of the 6s2 lone electron pair. There are four tetrahedrally coordinated sites in the structure, T1–T4, with the average ❬T–O❭ bond lengths varying from 1.66 to 1.69 Å. The site occupancies for these sites were assigned to Si0.5Al0.5. There is one additional Al site that has the fivefold trigonal bipyramidal (TBP) coordination with the O9 site half-occupied. The crystal structure is based upon tubular tetrahedral chains, [T4O10], parallel to the a axis and linked into a three-dimensional framework via Pb atoms and dimers of corner-sharing AlO5 trigonal bipyramids located in between the chains. The comparison between the experimental X-ray powder diffraction pattern for Pb4Al4Si3O16 (ICDD PDF #32-0505) and theoretical one calculated on the basis of the crystal-structure data shows remarkable similarities, which provides strong evidence in favor of the identity of Pb10Al10Si8O41 with the “Pb4Al4Si3O16” compound reported previously. The crystal-chemical formula of Pb10Al10Si8O41 can be written as Pb10Al2O[(Si2Al2)O10]4 that emphasizes the composition of the tetrahedral chains with disordered distribution of Si and Al over tetrahedral sites. The structure type of the title compound is new for inorganic compounds and may be viewed as a hybrid of the structure types of Pb6O[(Si6Al2)O20] and narsarsukite. The unit cell of the title compound corresponds to the 2 × 2 × 2 (eightfold) supercell relative to both narsarsukite and Pb6O[(Si6Al2)O20]. The information-based structural complexity parameters are higher than those of the parent structure types with the total structural information per cell (590.100 bits) about 6.2–6.5 times larger.
1. INTRODUCTION
Lead aluminosilicates represent technologically and mineralogically important group of mineral phases that occur in complex sinters and slugs produced during lead and zinc smelting and are considered as possible matrices for immobilization of Pb (Lu, Shih, 2011, 2012, 2015; Lu et al., 2013; Yang et al., 2020). The “Pb4Al4Si3O16” phase was found on Roman coins of the III–IVth centuries, where it most probably formed as a constituent of the patinas the patinas (Mata et al., 2010). Only one Pb aluminosilicate mineral without additional cations (except for H) or anions is known to date, rongibbsite, Pb2(Si4Al)O11(OH), that is based upon a zeolite-like microporous interrupted tetrahedral framework (Yang et al., 2013).
The PbO–Al2O3–SiO2 system was first investigated by Geller and Bunting (1943), who reported formation in this system of three ternary compounds: Pb8Al2Si4O19, Pb4Al2Si2O11, and Pb6Al2Si6O21. The existence of these compounds was confirmed by Mylyanych et al. (1999) during their studies of crystallization of Pb-containing aluminosilicate glasses. Haensel et al. (1976) described another ternary compound, Pb4Al4Si3O16, and reported its X-ray diffraction pattern (ICDD PDF #32-0505). In the detailed study of phase equilibria in the PbO–Al2O3–SiO2 system, Chen et al. (2001) found eleven different ternary crystalline phases: Pb8Al2Si4O19, Pb4Al2Si2O11, Pb6Al2Si6O21, Pb4Al4Si3O16, PbAl2Si2O8, Pb3Al10SiO20, Pb4Al4SiO12, Pb4Al4Si5O20, Pb5Al2Si10O28, Pb12Al2Si17O49, and Pb12Al2Si20O55. Dörsam et al. (2008) reported the high-pressure and high-temperature (2 GPa, 650 °C) hydrothermal synthesis of Pb2Al2Si2O9 that was described as an Al analogue of kentrolite, Pb2Mn2Si2O9, and melanotekite, Pb2Fe2Si2O9. The high-pressure phase Pb0.8Al1.6Si2.4O8 with a hollandite-type structure was studied by Downs et al. (1995).
However, despite such a rich and outstanding chemical diversity of Pb aluminosilicates, the crystal-structures are known for four of them only: PbAl2Si2O8 (the Pb analogue of feldspar; Benna et al., 1996, 1999; Tribaudino et al., 1998; Curetti et al., 2015), Pb2Al2Si2O9 (Dörsam et al., 2008), Pb6Al2Si6O21 (Siidra et al., 2009), and Pb0.8Al1.6Si2.4O8 (Downs et al., 1995).
Herein we report on the crystal structure of Pb10Al10Si8O41, a lead aluminosilicate, which is most probably identical to the phase previously reported as Pb4Al4Si3O16.
2. EXPERIMENTAL
Several tiny colorless transparent crystals of Pb10Al10Si8O41 were obtained as a by-product during our experiments in the PbO–SiO2 system (Siidra et al., 2009). The mixture of PbO and SiO2 in the 1 : 1 ratio was ground, mixed in an agate mortar and placed into an alumina crucible that was heated to 800 °C and kept at that temperature for 1 hour. The crucible was then cooled to room temperature at the rate of 2.5 °C/min. The main product was a vitreous matrix with few tiny crystals of the title compound grown on the border between the matrix and the crucible walls.
One of the crystals was placed onto a Bruker SMART three-circle diffractometer equipped with an APEX CCD area detector. More than a hemisphere of the diffraction data was collected by using the exposure time of 45 s per frame. The absorption correction was done using the SADABS program. The crystal structure was solved and refined by means of the SHELX program package (Sheldrick et al., 2015). The crystal data and experimental parameters of the X-ray diffraction experiment are given in Table 1, atom coordinates, site-occupancies and displacement parameters are provided in Table 2. Table 3 contains selected interatomic distances for the crystal structure.
Table 1.
Crystal parameters, data collection and structure refinement parameters for Pb10Al10Si8O41Таблица 1. Кристаллографические данные и экспериментальные параметры для Pb10Al10Si8O41
| Crystal Data | |
| Formula | Pb10Al10Si8O41 |
| Crystal size, mm | 0.12 × 0.05 × 0.02 |
| Crystal system | Orthorhombic |
| Space group | Fddd |
| a, Å | 16.2940(17) |
| b, Å | 21.666(2) |
| c, Å | 22.655(2) |
| V, Å3 | 7997.8(13) |
| Z | 8 |
| Dx, g/cm3 | 5.738 |
| Data Collection | |
| Instrument | Bruker Smart CCD |
| Radiation | MoKα |
| 2Θ range (degrees) | 3.6–56.60 |
| Total collected reflections | 11933 |
| Unique reflections | 2459 |
| Unique observed |Fo| ≥ 4σF | 1393 |
| Rint | 0.108 |
| hkl range | −17→21; −26→28; −25→30 |
| Refinement | |
| R1 (|Fo| ≥ 4σF) | 0.042 |
| wR2 | 0.092 |
| S | 0.849 |
Table 2.
Fractional atomic coordinates, site occupancies (Q), and displacement parameters (Å2) for Pb10Al10Si8O41Таблица 2. Координаты атомов, заселенности позиций (Q) и параметры смещения атомов (Å2) для кристаллической структуры Pb10Al10Si8O41
| Atom | Q | x | y | z | Ueq | U11 | U22 | U33 | U23 | U13 | U12 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pb1 | Pb | 0.12939(4) | 0.21467(2) | 0.03831(2) | 0.01455(17) | 0.0171(3) | 0.0128(3) | 0.0137(3) | 0.0025(2) | –0.0009(2) | –0.0003(3) |
| Pb2 | Pb | 0.29758(5) | 1/8 | 1/8 | 0.0161(2) | 0.0151(4) | 0.0157(4) | 0.0176(4) | 0.0009(4) | 0 | 0 |
| Pb3 | Pb | 0.36696(6) | 0.28634(4) | 0.27293(4) | 0.0438(3) | 0.0514(6) | 0.0372(5) | 0.0428(5) | 0.0056(3) | –0.0016(4) | –0.0126(5) |
| T1 | Si0.5Al0.5 | 0.2661(3) | 0.28505(19) | 0.1270(2) | 0.0176(9) | 0.020(2) | 0.021(2) | 0.012(2) | 0.001(3) | –0.0005(19) | –0.0098(17) |
| T2 | Si0.5Al0.5 | 0.2709(3) | 0.1162(2) | –0.0265(2) | 0.0115(11) | 0.013(2) | 0.011(3) | 0.011(2) | 0.0010(19) | 0.0020(16) | –0.0028(18) |
| T3 | Si0.5Al0.5 | 0.0339(3) | 0.3879(2) | 0.0273(2) | 0.0182(13) | 0.020(2) | 0.015(4) | 0.019(2) | 0.005(2) | 0.0065(19) | 0.0023(19) |
| T4 | Si0.5Al0.5 | 0.4633(3) | 0.26643(19) | 0.1258(3) | 0.0159(9) | 0.024(2) | 0.0126(19) | 0.011(2) | 0.000(2) | 0.001(2) | –0.0005(18) |
| Al | Al | 0.4738(5) | 3/8 | 3/8 | 0.051(3) | 0.020(4) | 0.034(5) | 0.101(8) | 0.018(6) | 0 | 0 |
| O1 | O | 0.2134(6) | 0.1226(7) | 0.0360(4) | 0.018(2) | 0.020(5) | 0.015(5) | 0.021(5) | –0.001(6) | 0.006(4) | –0.005(6) |
| O2 | O | 0.2137(6) | 0.2184(4) | 0.1259(5) | 0.021(2) | 0.021(6) | 0.024(6) | 0.018(5) | –0.001(6) | 0.006(5) | 0.000(4) |
| O3 | O | 0.2491(8) | 0.3197(6) | 0.0637(6) | 0.032(3) | 0.048(9) | 0.028(8) | 0.019(7) | –0.004(6) | –0.007(6) | –0.002(6) |
| O4 | O | 0.4679(8) | 0.3649(6) | 0.2803(6) | 0.042(4) | 0.028(7) | 0.019(9) | 0.079(11) | 0.011(7) | 0.011(6) | –0.003(6) |
| O5 | O | 0.2422(8) | 0.1970(6) | 0.3125(6) | 0.029(3) | 0.046(8) | 0.019(7) | 0.020(7) | –0.007(6) | 0.002(6) | 0.015(6) |
| O6 | O | 0.3679(7) | 0.1088(5) | –0.0053(6) | 0.030(3) | 0.017(6) | 0.038(7) | 0.036(7) | –0.017(6) | 0.007(5) | 0.003(5) |
| O7 | O | 0.4881(8) | 0.2049(7) | 0.3142(6) | 0.037(4) | 0.043(9) | 0.030(8) | 0.039(9) | 0.009(7) | –0.015(6) | 0.001(7) |
| O8 | O | 0.0206(11) | 0.3244(7) | 0.0669(7) | 0.058(5) | 0.098(14) | 0.030(9) | 0.046(11) | 0.027(8) | 0.038(9) | 0.015(9) |
| O9 | O | 3/8 | 0.3390(14) | 3/8 | 0.022(7) | 0.009(14) | 0.041(18) | 0.016(15) | 0 | –0.002(14) | 0 |
| O10 | O | 0.4852(18) | 0.1913(8) | 0.1149(7) | 0.126(11) | 0.33(3) | 0.040(10) | 0.008(9) | 0.002(7) | 0.000(13) | 0.065(15) |
| O11 | O | 0.3654(9) | 0.2714(10) | 0.1393(8) | 0.092(7) | 0.028(9) | 0.162(19) | 0.084(13) | 0.064(13) | –0.004(8) | 0.006(10) |
Table 3.
Selected interatomic distances (Å) for Pb10Al10Si8O41Таблица 3. Избранные межатомные расстояния (Å) для Pb10Al10Si8O41
| Pb1–O2 | 2.416(12) | T1–O3 | 1.642(14) |
| Pb1–O1 | 2.420(14) | T1–O8 | 1.643(14) |
| Pb1–O1 | 2.422(13) | T1–O11 | 1.668(15) |
| Pb1–O2 | 2.467(11) | T1–O2 | 1.677(10) |
| Pb1–O8 | 3.035(15) | ❬T1–O❭ | 1.66 |
| Pb1–O5 | 3.047(13) | ||
| Pb1–O3 | 3.052(13) | T2–O3 | 1.658(14) |
| Pb1–O3 | 3.133(14) | T2–O6 | 1.659(12) |
| ❬Pb1–O❭ | 2.75 | T2–O5 | 1.662(13) |
| T2–O1 | 1.702(10) | ||
| Pb2–O2 | 2.442(10) 2x | ❬T2–O❭ | 1.67 |
| Pb2–O1 | 2.439(10) 2x | ||
| Pb2–O6 | 3.185(12) 2x | T3–O8 | 1.656(14) |
| Pb2–O11 | 3.37(2) 2x | T3–O6 | 1.678(12) |
| Pb2–O10 | 3.38(3) 2x | T3–O4 | 1.693(14) |
| ❬Pb2–O❭ | 2.96 | T3–O7 | 1.713(15) |
| ❬T3–O❭ | 1.69 | ||
| Pb3–O4 | 2.374(13) | ||
| Pb3–O4 | 2.553(14) | T4–O11 | 1.628(16) |
| Pb3–O9 | 2.582(13) | T4–O10 | 1.685(16) |
| Pb3–O7 | 2.808(13) | T4–O7 | 1.693(15) |
| Pb3–O5 | 2.947(14) | T4–O5 | 1.703(13) |
| Pb3–O10 | 3.03(2) | ❬T4–O❭ | 1.68 |
| Pb3–O11 | 3.045(19) | ||
| Pb3–O7 | 3.084(15) | Al–O10 | 1.600(16) 2x |
| Pb3–O8 | 3.14(2) | Al–O9 | 1.789(15) 2x |
| Pb3–O6 | 3.169(11) | Al–O9 | 2.158(15) |
| ❬Pb3–O❭ | 2.87 | ❬Al–O❭ | 1.79 |
3. RESULTS
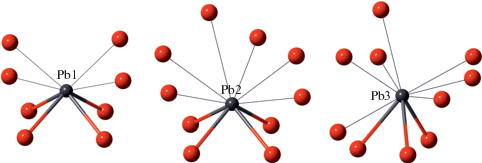
The crystal structure of Pb10Al10Si8O41 contains three symmetrically independent Pb atoms, each having distorted coordination environment due to the stereoactivity of the 6s2 lone electron pair. The Pb1 and Pb2 atoms form four Pb–O bonds shorter than 2.5 Å, arranged at the corners of the base of PbO4 square pyramid with Pb atom at the apical corner (Fig. 1). The similar coordination is observed in the crystal structure of litharge, PbO. However, in the title compound the PbO4 square pyramid is complemented by four and six long Pb–O bonds (Pb–O > 3.0 Å) for the Pb1 and Pb2 atoms, respectively. The Pb3 atoms is coordinated by three O atoms with the Pb–O bonds shorter than 2.6 Å and seven Pb–O bonds with the bond lengths in the range 2.8–3.2 Å.
Fig. 1.
Coordination of Pb2+ cations in the crystal structure of Pb10Al10Si8O41. Pb and O atoms are shown as black and red, respectively. The Pb–O bonds longer than 2.6 Å are shown as black lines. Рис. 1. Координация катионов Pb2+ в кристаллической структуре Pb10Al10Si8O41. Атомы Pb и O изображены черным и красным, соответственно. Связи Pb–O с длиной выше 2.6 Å показаны черными линиями.
There are four tetrahedrally coordinated sites in the structure, T1–T4, with the average 〈T–O〉 bond lengths varying from 1.66 to 1.69 Å. These bonds are longer than the ideal Si–O bonds and shorter than the ideal Al–O bonds for Si and Al in tetrahedral coordinations, which points out that the T1–T4 sites are statistically occupied by Si and Al. In order to fulfill the stoichiometry requirements, the site occupancies for these sites were fixed at Si0.5Al0.5. There is one additional Al site that has a fivefold trigonal bipyramidal (TBP) coordination with the O9 site half-occupied and split over two symmetrically equivalent positions located at 1.56 Å from one another (see below). The TBP coordination is typical for Al (Gorelova et al., 2019).
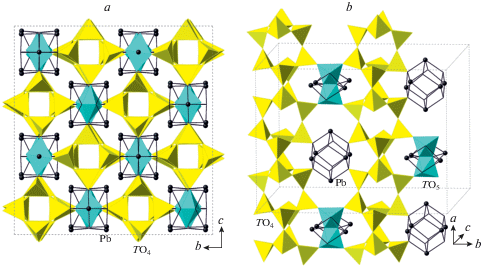
The crystal structure is based upon tubular tetrahedral chains, [T4O10], running parallel to the a axis and formed by T1–T4 atoms coordinated by O (Fig. 2a). The chains have a square section and belong to the family of tubular silicate anions reviewed by Rozhdestvesnkaya and Krivovichev (2011). The chains are linked into framework via Pb atoms and dimers of corner-sharing AlO5 trigonal bipyramids located in between the chains.
Fig. 2.
The crystal structure of Pb10Al10Si8O41 projected along [100] (a) and its slice along the (001) plane showing linkage of aluminosilicate tetrahedral chains via Pb2+ cations and dimers of (AlO5) trigonal bipyramids (b). Рис. 2. Кристаллическая структура Pb10Al10Si8O41 в проекции вдоль [100] (a) и ее срез по плоскости (001), показывающий связь алюмосиликатных тетраэдрических цепочек через катионы Pb2+ и димеры тригональных бипирамид (AlO5) (b).
4. DISCUSSION
The Pb10Al10Si8O41 stoichiometry has not been observed previously for the PbO–Al2O3–SiO2 system (see Introduction), but possesses some similarity to the Pb4Al4Si3O16 stoichiometry first reported by Haensel et al. (1976). The Pb : Al : Si ratios for the Pb10Al10Si8O41 and Pb4Al4Si3O16 compositions are equal to 5 : 5 : 4 and 4 : 4 : 3, or 1 : 1 : 0.80 and 1 : 1 : 0.75, respectively, which are rather close to each other and can potentially be mixed up in the chemical analyses. The comparison between the experimental X-ray powder diffraction pattern for Pb4Al4Si3O16 (ICDD PDF #32-0505; Haensel et al., 1976) and theoretical one calculated on the basis of crystal-structure data reported herein show remarkable similarities, except for the reflection with d = 4.36 Å versus the calculated value of 4.27 Å (in our opinion, a misprint cannot be excluded). The closeness of the two diffraction patterns provides strong evidence in favor of the identity of Pb10Al10Si8O41 studied by us with the Pb4Al4Si3O16 compound reported previously.
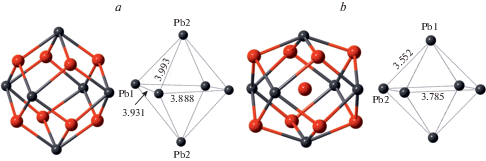
According to the crystal-structure study, the crystal-chemical formula of Pb10Al10Si8O41 can be written as Pb10Al2O[(Si2Al2)O10]4 that emphasizes the composition of tetrahedral chains with disordered distribution of Si and Al over tetrahedral sites. The crystal structure of the title compound is related to that of Pb6O[(Si6Al2)O20] (Siidra et al., 2009), which is based upon the tubular [(Si6Al2)O20]10– chains with the same topology as observed in Pb10Al10Si8O41 (Fig. 3a). However, the linkage of the chains is different and is provided by the oxocentered (Pb6O)10+ units located in between the chains (Fig. 3b). In fact, each of these units forms a core of the larger {(Pb6${\text{O}}_{8}^{T}$)Oa} units, where OT and Oa notations correspond to the O atoms bonded to T atoms and additional O atoms, respectively. The (Pb6${\text{O}}_{8}^{{{\text{Si}}}}$) configuration has the shape of a rhombododecahedron with Pb atoms at the tetravalent corners and O atoms at the trivalent corners. The similar units are present in the title compound as well. Figure 4 compares geometries of the (Pb6${\text{O}}_{8}^{{{\text{Si}}}}$) units in Pb10Al2O[(Si2Al2)O10]4 and Pb6O[(Si6Al2)O20] (Figs. 4a and b, respectively). It can be seen that the units in the latter compound are more compressed, most likely due to the presence of the additional Oa atom at the center of the (Pb6) octahedron. The Pb…Pb distances in the octahedron are in the range 3.89–3.99 Å in the title compound (with “empty” (Pb6) octahedron) and 3.55–3.79 Å in Pb6O[(Si6Al2)O20] (with the (Pb6) octahedron centered by the Oa atom). The occurrence of the same structural units in similar structure types with occupied and non-occupied polyhedral cavities has been observed previously in inorganic structures with lone-electron-pair cations [e.g., the “empty” (Pb6) octahedron in Pb21[Si7O22]2[Si4O13] (Siidra et al., 2014) versus (ClPb6) octahedron in hyttsjöite, Pb18Ba2Ca5${\text{Mn}}_{2}^{{2 + }}{\text{Fe}}_{2}^{{3 + }}$Si30O90Cl · 6H2O (Grew et al., 1996)]. It should be noted that, in the title compound, only Pb1 and Pb2 atoms participate in the formation of (Pb6) octahedra, whereas the Pb3 atom is associated with the dimers of (AlO5) trigonal bipyramids.
Fig. 3.
The crystal structure of Pb6O[(Si6Al2)O20] projected along [001] (a) and the linkage of aluminosilicate tetrahedral chains to the (OPb6) units (b). Рис. 3. Кристаллическая структура Pb6O[(Si6Al2)O20] в проекции вдоль [001] (a) и объединение алюмосиликатных тетраэдрических цепочек с группами (OPb6) (b).
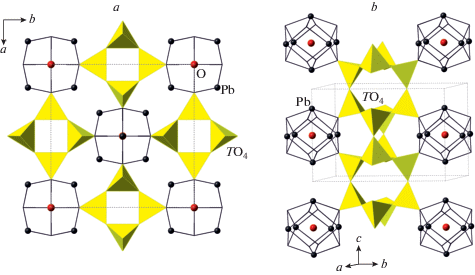
Fig. 4.
Rhombododecahedral Pb–O units in the crystal structures of Pb10Al10Si8O41 (a) and Pb6O[(Si6Al2)O20] (b) and the geometrical parameters of Pb6 octahedral motifs. See text for details. Рис. 4. Ромбододекаэдрические Pb–O единицы в кристаллических структурах Pb10Al10Si8O41 (a) и Pb6O[(Si6Al2)O20] (b) и геометрические параметры октаэдров Pb6. См. пояснения в тексте.
The crystal structure of Pb10Al10Si8O41 is also similar to that of narsarsukite, Na2TiO[Si4O10] (Pyatenko, Pudovkina, 1960; Peacor, Buerger, 1962), and isotypic compound K2ScF[Si4O10] (Kolitsch, Tillmanns, 2004). Both structures are based upon tubular chains of four-membered rings, which are interlinked by the chains of octahedra running parallel to the silicate chains. Figure 5 shows general features of the crystal structure of K2ScF[Si4O10].
Fig. 5.
The crystal structure of K2ScF[Si4O10] projected along [001] (a) and the linkage of octahedral and tetrahedral chains through sharing common corners (b). Рис. 5. Кристаллическая структура K2ScF[Si4O10] в проекции вдоль [001] (a) и объединение тетраэдрических и октаэдрических цепочек через общие вершины (b).
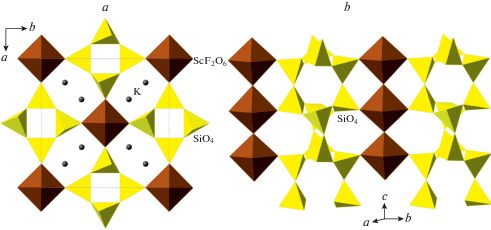
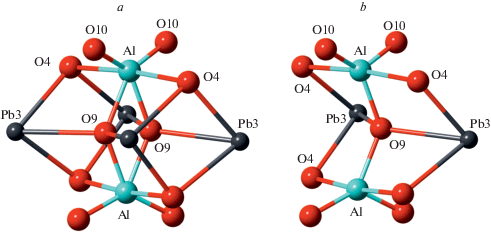
The structure type of the title compound is new for inorganic compounds and may be viewed as a hybrid of the structure types of Pb6O[(Si6Al2)O20] and narsarsukite. On one hand, it can be obtained from narsarsukite by replacing each second pair of corner-sharing (TiO6) octahedra by (Pb6) octahedron (as a consequence, the sixfold coordination of Ti transforms into fivefold coordination of Al). On the other hand, the crystal structure of Pb10Al10Si8O41 can be obtained from that of Pb6O[(Si6Al2)O20] by the replacement of each second (Pb6O) octahedron by the TBP dimer surrounded by four Pb3 atoms. The disordered and ordered configurations of the dimer are shown in Fig. 6. The O9 site that is shared between two adjacent (AlO5) units is half-occupied so that the averaged configuration (Fig. 6a) is the overlap of two ordered configurations (Fig. 6b). As a result, the atoms involved in the disordered moiety (especially Al, O9 and O10) possess strongly elongated displacement parameters, indicating that the observed Al–O bond lengths are strongly distorted due to the libration effects.
Fig. 6.
The disordered (a) and ordered (b) configurations of trigonal bipyramidal dimers in the crystal structure of Pb10Al10Si8O41. Рис. 6. Разупорядоченная (a) и упорядоченная (b) конфигурации тригонально-бипирамидальных димеров в кристаллической структуре Pb10Al10Si8O41.
The structure type of Pb10Al10Si8O41, being the ordered hybrid version of the narsarsukite and Pb6O[(Si6Al2)O20] structure types, can be considered as a superstructure of both of them. The analysis of the unit-cell parameters for the three structure types (Table 5) shows that the unit cell of the title compound corresponds to the 2 × 2 × 2 (eightfold) supercell relative to both narsarsukite and Pb6O[(Si6Al2)O20]. The information-based structural complexity parameters (Krivovichev, 2013) of Pb10Al10Si8O41 (Table 5) are therefore higher than those of the parent structure types with the total structural information per cell (590.100 bits) about 6.2–6.5 times larger. This agrees well to the previous observations on structural complexity for the minerals with the “structure-superstructure” relations (Krivovichev et al., 2019).
Table 4.
Comparison of experimental X-ray powder diffraction pattern for Pb4Al4Si3O16 (ICDD PDF #32-0505) and theoretical* X-ray powder pattern for Pb10Al10Si8O41Таблица 4. Сравнение экспериментальных порошковых данных для Pb4Al4Si3O16 (ICDD PDF #32-0505) и теоретической* рентгенограммы для Pb10Al10Si8O41
| ICDD PDF #32-0505 | Calculated for Pb10Al10Si8O41 | |||
|---|---|---|---|---|
| d, Å | I/I0 | d, Å | I/I0 | hkl |
| 7.78 | 30 | 7.83 | 60 | 022 |
| 6.57 | 16 | 6.61 | 17 | 202 |
| 6.53 | 12 | 113 | ||
| 6.48 | 18 | 6.51 | 26 | 220 |
| 5.63 | 16 | 5.66 | 14 | 004 |
| 5.41 | 7 | 5.42 | 7 | 211 |
| 5.13 | 6 | 5.13 | 8 | 311 |
| 4.36 | 20 | 4.27 (?) | 15 | 115 |
| 4.18 | 11 | 4.19 | 16 | 242 |
| 4.06 | 7 | 4.07 | 10 | 400 |
| 3.90 | 11 | 3.91 | 11 | 044 |
| 3.75 | 9 | 3.76 | 13 | 333 |
| 3.41 | 70 | 3.43 | 100 | 206 |
| 3.29 | 100 | 3.31 | 50 | 404 |
| 3.30 | 99 | 260 | ||
| 3.25 | 40 | 3.26 | 39 | 440 |
| 3.08 | 10 | 3.09 | 8 | 353 |
| 2.883 | 13 | 2.90 | 14 | 137 |
| 2.843 | 25 | 2.85 | 25 | 264 |
| 2.816 | 25 | 2.823 | 19 | 444 |
| 2.748 | 13 | 2.758 | 6 | 317 |
| 2.671 | 20 | 2.683 | 26 | 426 |
| 2.623 | 40 | 2.628 | 23 | 462 |
| 2.601 | 60 | 2.610 | 49 | 066 |
| 2.318 | 45 | 2.313 | 2 | 375 |
| 2.258 | 6 | 2.258 | 1 | 555 |
| 2.255 | 2 | 480 | ||
| 2.207 | 20 | 2.205 | 3 | 606 |
| 2.192 | 25 | 2.197 | 22 | 466 |
| 2.171 | 20 | 2.170 | 8 | 660 |
| 2.143 | 7 | 2.149 | 2 | 268 |
| 2.122 | 8 | 2.128 | 2 | 0.10.2 |
| 2.089 | 11 | 2.094 | 9 | 2.10.0 |
| 2.031 | 45 | 2.042 | 24 | 646 |
| 2.027 | 27 | 664 | ||
Table 5.
Crystallographic data and complexity parameters for narsarsukite-related compounds* Таблица 5. Кристаллографические данные и параметры структурной сложности для нарсарсукитоподобных соединений*
| Chemical formula | Mineral name | Sp. gr. | a, Å | b, Å | c, Å | Reference | IG, bits/atom | IG,total, bits/cell |
|---|---|---|---|---|---|---|---|---|
| Na2TiO[Si4O10] | Narsarsukite | I4/m | 10.727 | = a | 7.948 | Peacor, Buerger, 1962 | 2.670 | 96.117 |
| K2ScF[Si4O10] | – | I4/m | 10.207 | = a | 8.166 | Kolitsch, Tillmanns, 2004 | 2.670 | 96.117 |
| Rb2ScF[Si4O10] | – | I4/m | 11.262 | = a | 8.305 | Kahlenberg et al., 2014 | 2.670 | 96.117 |
| Pb6O[(Si6Al2)O20] | – | I4/mmm | 11.716 | = a | 8.044 | Siidra et al., 2009 | 2.558 | 89.525 |
| Pb10Al2O[(Si2Al2)O10]4 | – | Fddd | 16.294 | 21.666 | 22.655 | This work | 4.215 | 590.100 |
The author is grateful to Igor Pekov for the useful remarks on the manuscript. The reported study was funded by the Russian Science Foundation, project number 19-17-00038.
Список литературы
Benna P., Tribaudino M., Bruno E. The structure of ordered and disordered lead feldspar PbAl2Si2O8. Amer. Miner. 1996. Vol. 81. P. 1337–1343.
Benna P., Tribaudino M., Bruno E. High-temperature in situ structural investigation on lead feldspar. Amer. Miner. 1999. Vol. 84. P. 120–129.
Chen S., Zhao B., Hayes P. C., Jak E. Experimental study of phase equilibria in the PbO–Al2O3–SiO2 system. Metall. Mater. Trans. B. Process. Metall. Mater. Process. Sci. 2001. Vol. 32. P. 997–1005.
Curetti N., Benna P., Bruno E. High-pressure equation of state and phase transition in PbAl2Si2O8 feldspar. Amer. Miner. 2015. P. 100. P. 1568–1577.
Dörsam G., Liebscher A., Wunder B., Franz G. Crystal structures of synthetic melanotekite (Pb2Fe2Si2O9), kentrolite (Pb2Mn2Si2O9), and the aluminum analogue (Pb2Al2Si2O9). Amer. Miner. 2008. Vol. 93. P. 573–583.
Downs R.T., Hazen R.M., Finger L.W., Gasparik T. Crystal chemistry of lead aluminosilicate hollandite: a new high-pressure synthetic phase with octahedral Si. Amer. Miner. 1995. Vol. 80. P. 937–940.
Geller R.F., Bunting E.N. Report on the systems lead oxide–alumina and lead oxide–alumina–silica. Res. Paper RP1564. Part J. Res. Nat. Bur. Stand. 1943. Vol. 31. P. 255–270.
Gorelova L.A., Pakhomova A.S., Krivovichev S.V., Dubrovinsky L.S., Kasatkin A.V. High pressure phase transitions of paracelsian BaAl2Si2O8. Sci. Rep. 2019. Vol. 9. P. 12652.
Grew E.S., Peacor D.R., Rouse R.C., Yates M.C., Su S.-C., Marquez N. Hyttsjöite, a new, complex layered plumbosilicate with unique tetrahedral sheets from Långban, Sweden. Amer. Miner. 1996. Vol. 81. P. 743–753.
Haensel S., Willgallis A., Heyer H. Verbindungsbildung in den Systemen PbO–SiO2, PbO–Al2O3–SiO2 und PbO–Ga2O3–SiO2. Glastechn. Ber. 1976. Bd. 9. S. 207–210.
Kahlenberg V., Manninger T., Perfler L., Toebbens D. M. One-pot occurrence of two polymorphs of Rb2Sc(Si4O10)F and their structural, spectroscopic and computational characterization. J. Solid State Chem. 2014. Vol. 220. P. 79–90.
Kolitsch U., Tillmanns E. The structural relation between the new synthetic silicate K2ScFSi4O10 and narsarsukite, Na2(Ti,Fe3+)(O,F)Si4O10. Eur. J. Miner. 2004. Vol. 16. P. 143–149.
Krivovichev S.V. Structural complexity of minerals: information storage and processing in the mineral world. Miner. Mag. 2013. Vol. 77. P. 275–326.
Krivovichev S.V., Panikorovskii T.L., Zolotarev A.A., Bocharov V.N., Kasatkin A.V., Škoda R. Jahn-Teller distortion and cation ordering: the crystal structure of paratooite-(La), a superstructure of carbocernaite. Minerals. 2019. Vol. 9. P. 370.
Lu X., Shih K. Phase transformation and its role in stabilizing simulated lead-laden sludge in aluminum-rich ceramics. Water Res. 2011. Vol. 45. P. 5123–5129.
Lu X., Shih K. Metal stabilization mechanism of incorporating lead-bearing sludge in kaolinite-based ceramics. Chemosphere. 2012. Vol. 86. P. 817–821.
Lu X., Shih K. Formation of lead-aluminate ceramics: Reaction mechanisms in immobilizing the simulated lead sludge. Chemosphere. 2015. Vol. 138. P. 156–163.
Lu X., Shih K., Cheng H. Lead glass-ceramics produced from the beneficial use of waterworks sludge. Water Res. 2013. Vol. 47. P. 1353–1360.
Momma K., Izumi F. VESTA-3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011. Vol. 44. P. 1272–1276.
Mylyanych A.O., Sheredko M.A., Melnyk S.K. Study of glass structures and crystalline phases in the PbO–Al2O3–SiO2 system. J. Anal. Atom. Spectrom. 1999. Vol. 14. P. 513–521.
Peacor D.R., Buerger M.J. The determination and refinement of the structure of narsarsukite, Na2TiOSi4O10. Amer. Miner. 1962. Vol. 47. P. 539–556.
Pyatenko Y.A., Pudovkina Z.V. Crystal structure of narsarsukite. Sov. Phys. Crystallogr. 1960. Vol. 5. P. 540–548.
Rozhdestvenskaya I.V., Krivovichev S.V. Tubular chains in the structures of natural and synthetic silicates. Crystallogr. Rep. 2011. Vol. 56. P. 1007–1018.
Sheldrick G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015. Vol. C71. P. 3–8.
Siidra O.I., Krivovichev S.V., Depmeier W. Crystal structure of Pb6O[(Si6Al2)O20]. Glass Phys. Chem. 2009. Vol. 35. P. 406-410.
Siidra O.I., Zenko D.S., Krivovichev S.V. Structural complexity of lead silicates: Crystal structure of Pb21[Si7O22]2[Si4O13] and its comparison to hyttsjöite. Amer. Miner. 2014. Vol. 99. P. 817–823.
Tribaudino M., Benna P., Bruno E. Structural variations induced by thermal treatment in lead feldspar PbAl2Si2O8. Amer. Miner. 1998. Vol. 83. P. 159–166.
Yang H., Downs R.T., Evans S.H., Jenkins R.A., Bloch E.M. Rongibbsite, Pb2(Si4Al)O11(OH), a new zeolitic aluminosilicate mineral with an interrupted framework from Maricopa County, Arizona, U.S.A. Amer. Miner. 2013. Vol. 98. P. 236–241.
Yang J., Lu X., Liu Y., Wang F., Chao Y. Transformation of hazardous lead into aluminosilicate ceramics: structure evolution and lead leaching. Environ. Sci. Pollut. Res. 2020. Vol. 27. P. 10 404–10 414.
Дополнительные материалы отсутствуют.
Инструменты
Записки Российского минералогического общества


