Успехи физиологических наук, 2023, T. 54, № 3, стр. 3-24
Иммунная функция лимфатической системы
Г. И. Лобов *
Федеральное государственное бюджетное учреждение науки Институт физиологии им. И.П. Павлова РАН, лаборатория сердечно-сосудистой и лимфатической систем
199034 Санкт-Петербург, Россия
* E-mail: LobovGI@infran.ru
Поступила в редакцию 22.03.2023
После доработки 29.03.2023
Принята к публикации 01.04.2023
- EDN: OXKSLU
- DOI: 10.31857/S0301179823030049
Аннотация
Лимфатическая система играет определяющую роль в иммунитете, выходящую далеко за рамки простого транспорта иммунных клеток и антигенов. Эндотелиальные клетки в различных отделах этой сосудистой сети высоко специализированы для выполнения различных специфических функций. Лимфатические капилляры экспрессируют хемокины и молекулы адгезии, которые в тканях способствуют привлечению и трансмиграции иммунных клеток. Сигнальные молекулы, продуцируемые эндотелиальными клетками лимфатических капилляров при воспалении, модулируют в лимфатических узлах миграцию лимфоцитов через венулы с высоким эндотелием из крови в паренхиму лимфатических узлов. Лимфатические сосуды обеспечивают активный регулируемый транспорт иммунных клеток и антигенов в лимфатические узлы. В лимфатических узлах с их сложной структурой, организованной стромальными клетками, создаются оптимальные условия для контактов антигенпрезентирующих клеток с лимфоцитами. Различные субпопуляции лимфатических эндотелиальных клеток лимфатических узлов выполняют специфические функции в соответствии с локализацией в лимфатическом узле и способствуют как врожденному, так и приобретенному иммунному ответу посредством презентации антигена, ремоделирования лимфатического узла и регуляции входа и выхода лейкоцитов.
ВВЕДЕНИЕ
Лимфатическая система представляет собой разветвленную сеть лимфатических сосудов (ЛС), пронизывающих практически все органы и ткани, и вторичных лимфоидных органов – лимфатических узлов (ЛУ), включенных в сеть ЛС. Длительное время интерес к лимфатической сосудистой сети был невелик, отчасти по причине ее малозаметности. Кроме того, определенную роль сыграл тот факт, что ранее известные патологические изменения в лимфатической системе не представляли угрозу для жизни, в отличие от заболеваний кровеносной системы. ЛС традиционно рассматривались как довольно инертная дренажная система, которая просто пассивно транспортирует жидкость. Однако в последнее время становится все более очевидным, что лимфатическая система активно поддерживает жидкостный гомеостаз, и – это всего лишь одна из функций лимфатической сосудистой системы. В последнее десятилетие все большее внимание уделяется иммунной функции лимфатической системы, показано, что эта система играет решающую роль в иммунитете, выходящую далеко за рамки простого обеспечения каналов для перемещения лейкоцитов и антигенов. Появляется все больше данных, свидетельствующих о важной роли ЛС в регуляции и формировании адаптивного иммунитета и иммунного надзора в целом [57]. В последние годы становится все более очевидным, что ЛС очень динамичны и играют гораздо более активную роль в воспалительных и иммунных процессах, чем представлялось ранее. Воспаление тканей вызывает быстрое стимул-специфическое усиление продукции хемокинов и молекул адгезии в лимфатических эндотелиальных клетках (ЛЭК) и пролиферативное расширение лимфатической сети в воспаленной ткани и дренирующих ЛУ. ЛЭК в различных отделах лимфатической сосудистой сети значительно отличаются и высоко специализированы для выполнения иммунных ролей, основанных на их местонахождении [42].
Первый этап иммунного процесса начинается в интерстициальном пространстве тканей с миграции антигенпрезентирующих дендритных клеток (ДК) и лимфоцитов в направлении лимфатических капилляров (ЛК) и уже на этом этапе проявляется важная роль ЛС. Направление перемещения иммунных клеток определяется гаптотактическим (иммобилизованным) градиентом хемокинов и адгезивных молекул, продуцируемых ЛЭК ЛК [16]. Помимо этого, ЛК имеют уникальные межклеточные соединения, предназначенные для эффективного поглощения жидкости и макромолекул, эти же соединения используются для избирательного переноса специфических субпопуляций иммунных клеток. Следующий этап – активная трансмиграция иммунных клеток из тканей в ЛК осуществляется по градиенту хемокинов, продуцируемых ЛЭК. Затем следует внутринтерстициальное пространстворосветное ползание ДК и лимфоцитов по люминальной поверхности ЛК, также регулируемое хемокинами. В дальнейшем иммунные клетки поступают в ЛС и в составе лимфы транспортируются в дренирующие ЛУ [104].
Помимо этого, в ответ на события в периферических тканях, такие как воспаление или инфекция, растворимые факторы из ЛЭК оказывают “дистанционное управление” иммунным процессом, модулируя в ЛУ миграцию лимфоцитов через стенку венул с высоким эндотелием из крови в паренхиму ЛУ [16]. Эти иммунные центры высокоорганизованы и расположены в критических точках, в них сходятся потоки лимфы и крови, что позволяет максимально увеличить вероятность встречи между ДК и родственными лимфоцитами [92]. Разные субпопуляции ЛЭК демонстрируют различия в экспрессии генов, связанных со специфическими функциями и локализацией в ЛУ, способствуя как врожденному, так и приобретенному иммунному ответу посредством презентации антигена, ремоделирования ЛУ и регуляции входа и выхода лейкоцитов.
С улучшением в последнее десятилетие наших знаний о механизмах, посредством которых иммунный ответ инициируется во время острой инфекции или воспаления, мы стали лучше понимать роль лимфатической системы в этом процессе и то, как она контролирует каждый этап иммунного ответа. Тем не менее, все еще остается большое количество вопросов без ответа, особенно вопросы о том, как мы можем использовать многообразные возможности лимфатической системы для регуляции воспалительных процессов и лечения новообразований.
Основная задача обзора – продемонстрировать функции различных отделов лимфатической системы в инициации и развитии иммунного ответа. Многие иммунные процессы, осуществляемые с участием дендритных клеток, Т- и В-лимфоцитов, в значительной степени выходят за рамки данного обзора.
ИНТЕРСТИЦИАЛЬНОЕ ПРОСТРАНСТВО
В основе любого органа лежит рыхлая соединительная ткань, которая, с одной стороны, формирует каркас органа, а с другой – образует интерстициальное пространство между клетками паренхимы [95]. Интерстициальное пространство состоит в основном из коллагена I, III и V и эластина, которые механически сшиты и перепутаны, образуя сложную трехмерную сеть. Между волокнами коллагена и эластина располагается основное вещество, сформированное из протеогликанов, гликопротеинов и гиалуронана, часть которого связана с коллагеном и эластином, а другая часть находится в свободной форме. Эти крупные полимерные молекулы синтезируются фибробластами и высвобождаются в интерстициальное пространство [22]. На скорость синтеза крупных молекул интерстициального пространства влияют местные условия и гормональные факторы. Фибробласты также выделяют множество ферментов, которые непрерывно разрушают компоненты матрикса, так что полная замена молекул внеклеточного матрикса происходит примерно каждые 50 дней [22]. Полимерные молекулы образуют множество связей и активно взаимодействуют с клетками соединительной ткани (фибробластами, адипоцитами, макрофагами, ДК) и клетками паренхимы органов посредством связывания с рецепторами клеточных мембран. Помимо выполнения важнейшей функции каркаса, экстраклеточный матрикс обеспечивает маршруты транспортировки питательных веществ и продуктов жизнедеятельности, а также определяет физические транспортные свойства ткани (гидравлическую проводимость и податливость) [109, 118]. Интерстициальное пространство представляет собой не просто пассивную систему каналов для потока жидкости и растворенных веществ, но функционирует как высокодинамичная и сложная структура, физические свойства которой оказывают глубокое влияние на обмен жидкости и растворенных веществ и поведение клеток тканей. Физико-химические свойства экстраклеточного матрикса в значительной степени обусловлены поведением молекул гликозаминогликанов, из которых важнейшее значение имеет гиалуронан [18].
Гелеобразные свойства интерстиция существенно ограничивают наличие свободной воды, но при этом в интерстициальном пространстве существуют “ручейки” свободной жидкости [51]. Потоки этой свободной жидкости, образующейся в результате капиллярной фильтрации, обеспечивают транспорт белка и других растворенных веществ из крови к интерстициальным и паренхиматозным клеткам. Интерстициальная жидкость представляет собой в основном ультрафильтрат плазмы с концентрацией белка 50–60% от его концентрации в плазме крови и электролитами, состав и концентрация которых близки к составу плазмы, и является важнейшим компонентом интерстициального пространства [18]. Интерстициальная жидкость непрерывно образуется в тканях в соответствии с законом Старлинга, заключающегося в том, что: 1) жидкость выходит их сосудов микроциркуляторного русла под действием градиента гидростатического давления и 2) осмотический градиент, создаваемый макромолекулами плазмы, противодействует потере жидкости из сосудов [72]. С течением времени в закон Старлинга были внесены некоторые изменения, дополнившие и уточнившие его. В частности, было установлено, что роль фильтра выполняют не межэндотелиальные щели, как утверждалось ранее, а эндотелиальный гликокаликс, слой гликопротеинов, секретируемых эндотелием, и являющийся своеобразным “молекулярным ситом” со средним радиусом пор 3 нм [83]. Через такой фильтр относительно свободно проходят мелкие макромолекулы, в частности альбумин, а перенос более крупных макромолекул, присутствующих в плазме, ограничен из-за их большого размера и отрицательного заряда. Второе важное дополнение, внесенное в принцип Старлинга в последние годы, заключается в том, что реабсорбция жидкости в кровеносных капиллярах оказалась незначительной и не может объяснить удаление значительных объемов жидкости из интерстициального пространства тканей, эту функцию в первую очередь выполняют ЛС [72].
Фильтрация белков из плазмы в интерстиций осуществляется по градиенту концентрации, но вместе с тем здесь функционируют и активные механизмы, в частности – везикулярный транспорт [72]. Полагают, что часть альбумина и некоторые другие макромолекулы транспортируются через эндотелиальную клетку в дискретных мембраносвязанных везикулах, называемых кавеолами. Помимо белков плазмы, в интерстициальной жидкости представлен также протеом, образующийся в результате метаболической активности паренхиматозных клеток (протеины и пептиды, синтезированные клетками паренхимы, продукты тканевого метаболизма и катаболизма), специфический для каждой ткани, а также клеточный детрит, апоптозные клетки и циркулирующие иммунные клетки [101, 126].
В ряде случаев в процесс обмена жидкости на уровне микроциркуляторного русла, помимо сил Старлинга, могут подключаться и другие механизмы, способные изменять проницаемость стенки микрососудов [85]. С наибольшей эффективностью подобные механизмы функционируют при воспалении, когда под действием провоспалительных медиаторов значительно увеличивается проницаемость стенки капилляров и венул, что позволяет создать условия для выхода в интерстиций крупных эффекторных молекул, принимающих участие в неспецифических и специфических защитных реакциях, таких как комплемент и иммуноглобулины [100].
Объем интерстициальной жидкости в физиологических условиях поддерживается довольно постоянным на уровне ~20% массы тела [134]. Это среднее значение, различия между тканями очень значительны (70% в коже и всего 10% в скелетных мышцах) [18]. Интерстициальная жидкость, являющаяся средой для транспорта питательных веществ, метаболитов и сигнальных молекул между клетками и капиллярами, представляет собой также буферный объем жидкости, за счет которого сглаживаются колебания объема плазмы и объема клеток паренхимы. Важным показателем состояния ткани является давление интерстициальной жидкости, которое тесно связано с объемом интерстициальной жидкости, трансэндотелиальным потоком, интерстициальным потоком и потоком лимфы [95]. Измерения давления интерстициальной жидкости с использованием метода перфорированной капсулы показали, что в большинстве мягких тканей оно обычно слегка отрицательное (от –4 до 0 мм рт. ст.) [134]. Однако, даже очень незначительная дополнительная фильтрация жидкости в тканевые пространства повышает давление интерстициальной жидкости, что, в свою очередь, увеличивает скорость перемещения интерстициальной жидкости от кровеносных капилляров к лимфатическим и повышает лимфообразование. Увеличенный поток лимфы приводит к восстановлению давления интерстициальной жидкости благодаря двум механизмам: 1) прямому удалению жидкости из интерстициального пространства и 2) удалению белка из интерстиция, тем самым уменьшая коллоидно-осмотическое давление интерстициальной жидкости и обеспечивая более эффективный осмос жидкости из интерстициального пространства обратно в кровеносные капилляры.
Поток жидкости в интерстиции проходит не только через трехмерный ансамбль молекул внеклеточного матрикса, но и вокруг интерстициальных клеток, таких как фибробласты, внесосудистые иммунные клетки и адипоциты, а также паренхиматозных клеток, составляющих ткань или орган [111]. Этот медленный поток жидкости в тканях от кровеносных капилляров к лимфатическим способствует эффективному снабжению клеток внутри этих тканей питательными веществами и сигнальными молекулами, включая гормоны и хемокины. Хемокины (chemotactic cytokine) представляют собой семейство небольших белков, секретируемых разными клетками, которые передают сигналы через хемокиновые рецепторы, связанные с G-белком на поверхности клеток, в первую очередь – лейкоцитов.
Хотя состав интерстициальной жидкости и скорость ее потока лишь иногда обсуждаются в области иммунологии, в реальности они оказывают важное влияние на иммунитет. В частности, эти факторы изменяют скорость перемещения в интерстиции иммунных клеток, в первую очередь дендритных клеток, которые являются первичными посредниками в формировании адаптивных иммунных ответов.
Дендритные клетки (ДК) образуются в костном мозге в результате лимфомиелоидного кроветворения и образуют важный интерфейс между врожденным восприятием патогенов и активацией адаптивного иммунитета [33]. Они в значительных количествах выявляются в коже и слизистых оболочках. ДК представляют собой профессиональные антигенпрезентирующие клетки, обладающие уникальной способностью индуцировать активацию и дифференцировку наивных Т-клеток. Не менее важной является их способность индуцировать и поддерживать иммунную толерантность в гомеостатических условиях. Принято выделять резидентные ДК с тремя разновидностями (плазмоцитоидные ДК, миелоидные ДК1 и миелоидные ДК2) и ДК, образующиеся при воспалении из моноцитов. Первые являются врожденными иммунными клетками, способными распознавать и реагировать на сигналы, связанные с патогенами и опасностями, запуская острую воспалительную реакцию. Их основополагающая роль в адаптивном иммунитете заключается в обработке внеклеточных и внутриклеточных белков и представлении антигенов в контексте молекул главного комплекса гистосовместимости (major histocompatibility complex, MHC) для праймирования наивных Т-клеток. ДК, происходящие из моноцитов, принято называть “воспалительными ДК”, поскольку они появляются при некоторых особых видах воспаления (экзема, псориаз) [33].
Наиболее важным регулятором миграции ДК в интерстициальном пространстве является хемокиновый рецептор CCR7. Стимулы патогенного происхождения вызывают процесс созревания ДК, во время которого они снижают свою эндоцитарную активность и активируют гены, участвующие в презентации антигена и активации Т-клеток, такие как молекулы МНС, костимулирующие молекулы и цитокины [122]. Также после воздействия сигналов опасности, незрелые ДК проходят сложную программу дифференцировки, в рамках которой они активируют экспрессию хемокинового рецептора CCR7. CCR7 имеет решающее значение для направления ДК к ЛУ, его экспрессия и функция важны для адекватного адаптивного иммунного ответа [40]. Следует также отметить, что кроме важнейшей функции хемоаттракции, CCR7 регулирует цитоархитектуру, эндоцитоз, выживаемость, скорость миграции, адгезию и дифференцировку ДК [130]. Различные функции, контролируемые CCR7, способствуют повышению эффективности ДК в иммунной системе и лучшему адаптивному иммунному ответу.
Чтобы мигрирующие иммунные клетки эффективно перемещались из тканей в ЛК, они должны иметь какие-то ориентиры и уметь находить пути к точкам входа в ЛК. Миграция ДК в лимфатическую систему в основном происходит в CCL21-экспрессирующих ЛК, которые, в отличие от ЛС, имеют сильно фенестрированную базальную мембрану и “пуговичные” соединения эндотелиальных клеток. ЛЭК постоянно синтезируют хемокин CCL21 – лиганд CCR7 [125]. Количественная визуализация позволила установить, что в ЛЭК существует депо CCL21, который диффундирует в окружающее интерстициальное пространство, в итоге в интерстиции создается гаптотактический градиент хемокина CCL21 с максимальной концентрацией вблизи стенки ЛК. Диффундирующий от ЛЭК CCL21 в интерстиции иммобилизуется на гепарансульфатах благодаря электростатическим взаимодействиям между хемокином и гепарансульфатами, ограничивая тем самым свободную диффузию хемокина [93, 108]. Установлено, что существующий в тканях градиент CCL21 эффективен на расстоянии до 90 мкм и этот градиент CCL21 хорошо адаптирован к распределению ЛК в коже. Расчеты показывают, что 92% объема межклеточного пространства находится в пределах 90 мкм от ближайшего ЛК, т.е. ДК, находящиеся в интерстициальном пространстве, всегда подвержены влиянию градиента CCL21 от одного или нескольких ЛК [52]. ДК, контактировавшие с патогенами, способны ощущать CCL21 и мигрировать вдоль этого локального градиента, чтобы приблизиться к ЛК [14, 93, 94].
Количество мигрирующих к ЛК ДК резко увеличивается (до 20 раз) во время инфекции или при воспалении, а также в ответ на бактериальные эндотоксины и провоспалительные цитокины, такие как IL-1 и TNF-α. Столь значительное увеличение числа ДК, рекрутируемых в ЛК при воспалении, происходит за счет нескольких механизмов: 1) при воспалении резко увеличивается проницаемость кровеносных капилляров и возрастает количество фильтрата, под действием протеаз происходит деградация полимеров интерстиция, гель интерстициального пространства разжижается, в результате чего возрастает поток интерстициальной жидкости от кровеносных капилляров к ЛК; 2) хотя CCL21 в ЛЭК экспрессируется конститутивно, при воспалении его производство заметно увеличивается, т.е. CCL21 является одновременно как конститутивным, так и индуцируемым и имеет черты как гомеостатического, так и воспалительного хемокина [61]; 3) в физиологических условиях перемещение ДК не зависит от процессов адгезии, в то время как при воспалении наблюдается активация ЛЭК, что приводит к экспрессии ключевых рецепторов адгезии ICAM-1 и VCAM-1, т.е. трансмиграция ДК, опосредованная CCL21, в значительной степени становится еще и интегрин-зависимой [62].
Наряду с CCL21, который является важнейшим фактором миграции ДК, на процесс миграции оказывают влияние и ряд других молекул. В частности, миграцию ДК в ЛК регулируют медиаторы воспаления, показано, что IL-1β и TNF-α способствуют ускорению движения ДК в сторону ЛК [138]. Простагландин E2 увеличивает экспрессию CCR7 на ДК, тем самым усиливая миграцию ДК в соответствии с градиентом CCL21.
Помимо ДК, в тканях находятся и другие клетки, относящиеся к подмножеству иммунных: моноциты, макрофаги, Т- и В-лимфоциты и нейтрофилы.
Моноциты представляют собой циркулирующие лейкоциты, которые фагоцитируют и убивают бактерии и грибки, а также регулируют активность других иммунных клеток посредством высвобождения цитокинов. Они могут дифференцироваться в ДК и макрофаги [47]. В нескольких исследованиях показано, что моноциты выходят из тканей через ЛК и транспортируют антиген к дренирующим ЛУ [58]. Оказавшись там, моноциты могут презентировать антигены и индуцировать пролиферацию антиген-специфических CD4+ и CD8+ Т-клеток. Считается, что CCR7 может быть важным фактором, способствующим миграции моноцитов [71].
Т- и В-лимфоциты. Т-клетки сосредоточены в эпителиальных и субэпителиальных тканях кожи и слизистых оболочек, где они действуют как первая линия защиты от патогенов. Важнейшей функцией CD8+ Т-лимфоцитов является уничтожение инфицированных клеток, в то время как CD4+ хелперные Т-клетки секретируют цитокины и регулируют функцию других иммунных клеток [133]. Установлено, что как при гомеостатическом состоянии, так и при воспалении большинство мигрирующих Т-клеток составляют эффекторные Т-лимфоциты [120]. Миграция Т-лимфоцитов играет важнейшую роль в иммунном надзоре и разрешении воспаления. Т-лимфоциты, подобно ДК, для миграции к ЛК и далее к ЛУ используют CCR7. Миграцию Т-клеток в ЛС также опосредует сфингозин-1-фосфат (S1P) [26].
Что касается В-клеток, то эксперименты с канюлированием ЛС у овец и опыты с фотоконверсией на мышах показали, что В-клетки также для миграции из тканей в дренирующие ЛУ используют ЛК [44]. Механизмы этой миграции слабо изучены, однако есть данные, что при хроническом воспалении выход В-клеток требует наличия CCR7 [26].
Нейтрофилы являются первыми иммунными клетками, рекрутируемыми в очаги воспаления, где они уничтожают патогены и высвобождают медиаторы, которые привлекают другие лейкоциты [115]. Значительная часть нейтрофилов погибает в очагах воспаления. В то же время имеются данные, полученные методом прижизненной визуализации, что нейтрофилы могут проникать в очагах воспаления в тканевые ЛК и мигрировать к дренирующим ЛУ [17]. Следует отметить, что значение и степень лимфатической миграции нейтрофилов изучены не полностью. Важно то, что нейтрофилы могут транспортировать антигены и микроорганизмы из очага инфекции в ЛУ [8]. И, поскольку они являются первой подгруппой врожденных иммунных клеток, которые попадают в ЛУ из воспаленных тканей и часто несут микробы и их фрагменты, миграция нейтрофилов по ЛС может оказывать значительное влияние на последующий адаптивный иммунный ответ [8].
Как ДК и другие иммунные клетки интерпретируют градиенты хемокинов и как они связывают направленное восприятие с поляризацией и стойким хемотаксисом, остается в значительной степени неясным, однако данные, полученные в разных лабораториях, приводят к однозначному выводу: лимфатическая система оказывает выраженное влияние на иммунные реакции не только в структурах самой лимфатической системы, но и за ее пределами. В частности, ЛС формируют в интерстициальном пространстве тканей градиент хемокина CCL21, продуцируют и другие хемокины, и тем самым активно привлекают и направляют ДК, моноциты, Т- и В-лимфоциты в ЛК как в физиологических условиях, так и при воспалении.
ЛИМФАТИЧЕСКИЕ КАПИЛЛЯРЫ
Подобно многим биологическим системам, которые поглощают и транспортируют жидкости к определенному центру, сети ЛС имеют преимущественно фрактальную геометрическую организацию. Подобный тип распределения позволяет мельчайшим дистальным сосудам покрывать в тканях большую площадь и обеспечивать эффективное поглощение жидкости. В разных источниках эти сосуды называют начальными ЛС, терминальными ЛС или лимфатическими капиллярами [3]. В данном обзоре мы будем использовать термин “лимфатические капилляры” (ЛК) поскольку он полностью отражает функцию этих мелких сосудов. Диаметр ЛК значительно варьирует в зависимости от ткани и вида животного. Минимальными размерами (10–25 мкм) отличаются млечные сосуды (ЛК) внутри ворсинок кишечника крысы, максимальными (до 450 мкм) – ЛК в крыльях летучих мышей [105]. ЛК располагаются в непосредственной близости от кровеносных сосудов МЦР, их стенка представляет собой один слой ЛЭК, расположенных на прерывистой базальной мембране. В разных тканях ЛК имеют разный вид: слепо начинающиеся округлые образования (мешочки) (рис. 1) или сети взаимосвязанных сосудов. Независимо от вида, ЛК впадают в лимфатические посткапилляры и отделяются от них клапаном, образованным тонкой пластинкой из коллагена и покрывающих ее эндотелиальных клеток [105]. Лимфатические посткапилляры сливаются во все более крупные собирающие ЛС, которые транспортируют лимфу в грудной и правый лимфатические протоки, впадающие в подключичные вены.
Рис. 1.
Начальные лимфатические сосуды в микроциркуляторном русле: лимфатические капилляры, лимфатический посткапилляр и первый лимфангион. ГМК – гладкомышечные клетки.
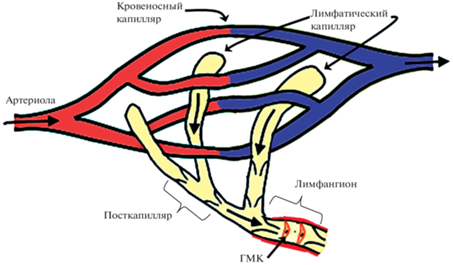
Большинство исследователей считают, что ЛК способны неизбирательно поглощать интерстициальное содержимое. Неселективное поглощение молекул из интерстициального пространства имеет решающее значение для обеспечения доступа разнообразных чужеродных антигенов к дренирующим ЛУ и подтверждается в различных исследованиях при введении в паренхиму тканей разных красителей и соединений, которые показывают неограниченный доступ к дренирующим ЛУ. В то же время имеются данные и о существовании дополнительных селективных механизмов для проникновения определенных молекул в ЛК [36].
Значительная часть аблюминальной поверхности ЛЭК прикреплена к окружающим коллагеновым волокнам с помощью многочисленных тонких (10–12 нм) нитей, называемых “якорными филаментами” (рис. 2). Эти филаменты представлены как в виде отдельных волокон, так и в виде пучков, простирающихся на значительные расстояния в прилегающую соединительную ткань. Они обеспечивают прочное прикрепление стенки ЛК к прилегающим коллагеновым волокнам и клеткам соединительной ткани. Считается, что якорные филаменты предотвращают коллапс ЛК в условиях высокого интерстициального давления и важны для связи между ЛК и окружающим матриксом. Эти филаменты, с одной стороны, помогают поддерживать форму и проходимость ЛК в среде, где быстро меняются градиенты давления, а с другой – позволяют обнаруживать локальные силы в окружающей ткани и могут служить для передачи сигналов из интерстиция клеткам ЛК [19].
Рис. 2.
Строение стенки лимфатического капилляра. Некоторые участки эндотелиальных клеток прикреплены друг к другу и фиксированы к окружающему матриксу “якорными филаментами”, другие не прикреплены и формируют “первичные лимфатические клапаны”. Свободные части эндотелиальных клеток могут отодвигаться внутрь капилляра при повышении давления интерстициальной жидкости и пропускать жидкость в просвет лимфатического капилляра (слева). При повышении давления в капилляре первичные клапаны закрываются и не пропускают лимфу в интерстициальное пространство (справа).
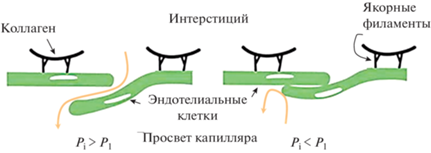
Помимо этих фокальных прикреплений, ЛЭК также имеют участки мембраны, не прикрепленные к соседним ЛЭК и окружающему матриксу. При описании контактов ЛЭК ЛК часто используют термин “пуговичные соединения”, подчеркивая тем самым, что ЛЭК соединены только в отдельных местах, а остальные участки клеточных мембран ЛЭК не фиксированы и могут отодвигаться, формируя “первичные” лимфатические клапаны с размером отверстий 0.5–1 мкм, через которые в ЛК входят интерстициальная жидкость и мигрирующие лейкоциты [19]. Подобная организация стенки ЛК делает их способными “ощущать” изменения интерстициального давления. При повышении давления в интерстиции части клетки, будучи свободными, легко отклоняются в просвет ЛК, открывая таким образом “первичные” лимфатические клапаны, чтобы обеспечить поступление жидкости в ЛК. Уменьшение интерстициального давления и инверсия градиента давления приводит к закрытию клапанов. В результате интерстициальная жидкость, поступившая в ЛК, не может выйти в интерстиций и поступает в лимфатический посткапилляр (и с этого момента называется лимфой). Таким образом, ЛК, используя внешние силы, осуществляют перемещение лимфы в посткапилляры и ЛС.
Как уже указывалось выше, основным механизмом, обеспечивающем переход интерстициальной жидкости в ЛК, является градиент гидростатического давления между интерстициальным пространством и просветом ЛК, который подвержен частым изменениям, как по величине, так и по направлению. Изменения величины интерстициального давления часто связаны с осуществлением органами своих функций (сокращение и расслабление сердца, дыхательные движения, которые приводят к колебаниям давления не только в грудной, но и в брюшной полости, перистальтика кишечника, ритмические сокращения скелетных мышц и др.). Кроме этого, различные химические (гистамин, тромбин и др.) и физические (гипертермия) раздражители приводят к изменению формы ЛЭК и их барьерной функции [24]. Также было показано, что при гипергидратации тканей (что часто бывает при воспалении) усиленный трансмуральный поток интерстициальной жидкости через стенку ЛК активирует образование в ЛЭК аквапорина-2, что может повышать проницаемость ЛЭК для оптимизации дренажа тканей [84].
Недавно также были получены данные, свидетельствующие об активном участии ЛЭК в формировании лимфы. Так, при исследовании млечных сосудов кишечника в цитоплазме ЛЭК было обнаружено большое количество липидсодержащих везикул, которые перемещались в базально-апикальном направлении, что свидетельствует об участии активного транспорта в образовании лимфы [36]. В другой работе при исследовании ЛК кожи уха мыши после введения в кожу альбумина в цитоплазме ЛЭК было обнаружено большое количество экзогенного альбумина. Было показано, что поглощение альбумина происходило как кавеолами, так и везикулами, покрытыми клатрином [121]. Таким образом, в ЛК наряду с парацеллюлярным транспортом веществ, в основе которого лежат колебания интерстициального давления, функционирует и трансцеллюлярный транспорт: ЛЭК поглощают растворенные вещества из интерстициального пространства с помощью механизмов везикулярного транспорта. Все большее количество данных свидетельствует, что трансцеллюлярный транспорт жидкости и растворенных веществ, опосредованный внутриклеточными везикулами, является важным активным компонентом лимфообразования, т.е. образование лимфы в тканях может активно регулироваться ЛЭК ЛК [121].
Наличие “пуговичных” соединений между ЛЭК и способность первичных лимфатических клапанов к открыванию–закрыванию позволяет ЛК не только захватывать жидкость, но и рекрутировать иммунные клетки: активированные CCR7-позитивные ДК и Т-лимфоциты, а также моноциты, макрофаги и В-клетки, хотя и в меньшем количестве [105, 113]. В исследованиях на коже уха мыши было показано, что миграция ДК в тканях происходит случайным образом до тех пор, пока клетки не достигнут расстояния ~90 мкм от ЛК, после чего их миграция становится направленной и постоянной [133]. Поступление ДК в ЛК длительное время считали пассивным процессом, в основном управляемым потоком. Однако недавние исследования, демонстрирующие участие ряда молекул, экспрессируемых LEC, в миграции ДК к ЛУ, указывают на молекулярную регуляцию процесса проникновения [79]. За последние 15 лет было выявлено несколько медиаторов миграции ДК в ЛК, тем не менее, общепризнанно, что основной движущей силой интерстициальной миграции и трансмиграции ДК в состоянии покоя и воспаления является хемотаксис, создаваемый в первую очередь хемокином CCL21, секретируемым ЛЭК, и его рецептором на ДК, связанным с G-белком CCR7. Эта направленность движения обусловлена, как уже было описано ранее, гаптотактическим градиентом хемокина CCL21, зафиксированным на коллагене и гепарансульфатах в окружающем интерстициальном матриксе. Миграция ДК происходит за счет “амебоидного движения”, при котором CCL21 направляет перемещение ДК через трехмерный коллагеновый матрикс [87].
Анализ изображений с интервальной съемкой, проведенный в последние годы, предоставил новые важные сведения о процессе миграции ДК из тканей в ЛУ. В частности, эти исследования показали, что перемещение ДК из тканей в ЛУ происходит в несколько этапов, на каждом из которых проявляется действие градиента CCL21. Первый этап – это перемещение CCR7-экспрессирующих ДК к ЛК по иммобилизованному в интерстиции перилимфатическому градиенту CCL21 [133]. Необходимо отметить, что максимальная концентрация CCL21 создается на люминальной поверхности ЛЭК, которая при приближении ДК к ЛК способствует их переносу в просвет ЛК – трансмиграции ДК через стенку ЛК [99]. Размеры отверстий в первичных лимфатических клапанах (<3 мкм) и базальная мембрана с небольшими фенестрами представляют собой определенный барьер для ДК, что вынуждает их изменять свою форму при прохождении через порталы [91]. Было отмечено, что мигрирующие ДК вступают в физический контакт с базальной мембраной и расширяют отверстия в базальной мембране до ~2 мкм [112]. После пересечения базальной мембраны мигрирующие ДК сталкиваются с ЛЭК и проходят через первичные клапаны в просвет ЛК [91]. Визуализация этого процесса с высоким разрешением в эксплантатах уха мыши показала, что проникновение ДК сопровождается деформацией как ДК, так и ЛЭК и изгибом участков мембраны ЛЭК внутрь ЛК. Установлено, что процесс взаимодействия ДК с ЛЭК и трансмиграции через стенку ЛК довольно длительный и занимает от 30 до 60 мин [91].
Оказавшись внутри ЛК, ДК длительное время активно ползают по внутренней поверхности стенок ЛК в разных направлениях (полунаправленное патрулирование), но преимущественно в сторону собирающих ЛС [112]. Расчеты показывают, что скорость тока лимфы в ЛК (5–8 мкм/мин) слишком низкая для поддержания пассивного перемещения ДК и Т-клеток в просвете ЛК. Эти клетки вместо лимфотока используют существующий на люминальной поверхности эндотелиоцитов хемотаксический градиент CCL21 [87]. Таким образом, был раскрыт еще один механизм, с помощью которого CCL21 способствует транспортировке ДК: он направляет внутрилимфатическую миграцию ДК из ЛК в ЛС и тем самым способствует более эффективному продвижению ДК по лимфатической сети [112]. Пассивный транспорт ДК с потоком лимфы наблюдается только в нижележащих собирающих ЛС, в которых пиковые скорости лимфотока достигают несколько миллиметров в минуту, т.е. на три порядка выше по сравнению с ЛК [37].
Синтез CCL21 заметно активируется в ЛЭК в ответ на воспаление, в результате чего хемокин накапливается во внутриклеточных запасных везикулах, готовых к секреции, особенно на базолатеральной поверхности эндотелия, где мигрируют лейкоциты [21]. Повышенная экспрессия в LEC CCL21 способствует усилению миграции ДК в ЛК. Помимо CCL21, LEC синтезируют множество других хемокинов, которые являются хемотаксическими для Т-клеток, ДК и моноцитов и нейтрофилов, экспрессирующих рецепторы, связанные с G-белком. Как и в случае с CCL21, все они активируются при воздействии на эндотелий воспалительных цитокинов или других воспалительных стимулов [65, 124]. Если в физиологических условиях ДК могут проникать в ЛК и мигрировать в ЛУ независимо от интегринов, то при воспалении в миграции ДК в ЛУ участвуют молекулы клеточной адгезии ICAM-1 и VCAM-1 [69, 128].
Несомненно, что и многие другие молекулы адгезии, расположенные внутри и вокруг пуговичных соединений ЛЭК, способствуют трансмиграции ДК. Имеются подтверждения, полученные в ходе различных исследований in vitro и in vivo, что маннозный рецептор, ALCAM (CD166), L1CAM, 4-1BB (CD137), CD99 и CD31 (PECAM-1) вовлечены в этот процесс [116]. Однако точная функциональная роль, которую играет каждый из этих рецепторов, и то, как они индивидуально управляются в процессе трансмиграции ДК, еще предстоит выяснить.
Пройдя через стенку ЛК, мигрирующие ДК попадают в просвет сосуда и продолжают дальнейшее движение к дренирующим ЛУ. ДК перемещаются в ЛК со скоростью, лишь незначительно превышающей скорость их движения в интерстициальном пространстве. Полагают, что некоторое увеличение скорости перемещения ДК в ЛК связано с тем, что, в отличие от интерстиция, в просвете ЛК клеткам не нужно протискиваться через плотный внеклеточный матрикс [87]. Прижизненная визуализиция показала, что ДК мигрируют в ЛК даже медленнее, чем сама лимфа, и что большинство трансмигрировавших ДК не переносятся пассивным потоком, а скорее ползут по люминальной поверхности ЛК [112]. Скорость и направление внутрипросветного ползания ДК не зависят от изменений лимфотока, ползание продолжается даже в его отсутствие [98].
Было установлено, что ползание ДК вызывается хемотаксисом, ДК перемещаются в соответствии с физическими градиентами CCL21, секвестрированном на поверхности ЛЭК, о чем свидетельствуют данные конфокальной и иммунной электронно-микроскопической визуализации ЛС дермы мыши [99]. Получается, что в ЛК поток лимфы не перемещает клетки за счет физического движения, а создает хемотаксические градиенты, в соответствии с которыми ДК ползают по ЛК. Необходимая сила для ползания обеспечивается интегрином β2 и ICAM-1 на люминальной поверхности эндотелия, экспрессия которого значительно повышается при воспалении [87]. Также имеются данные, что такое неупорядоченное ползание регулируется взаимодействиями между расположенным на люминальной поверхности ЛЭК LYVE-1 и CD44, заякоренным на гликокаликсе ДК [63].
CCL21 является хемокином, управляющим входом в ЛК не только ДК, но и входом T-клеток, в частности популяцией CD4+ и CD8+ T-лимфоцитов, которые выходят из кровеносных капилляров для патрулирования воспаленных тканей. Как правило, почти все Т-клетки, которые мигрируют в афферентную лимфу, экспрессируют CCR7 и хемотаксически реагируют на CCL21 [34]. Это группа мигрирующих CCR7+ T-клеток, которые в конечном итоге могут повторно попадать в кровь через грудной проток, в отличие от других CCR7– Т-лимфоцитов, которые остаются резидентными в тканях в качестве часовых. Для мигрирующих Т-клеток так же, как и для ДК, характерно полунаправленное ползание по внутренней поверхности ЛК. Отмечается, что скорость внутрипросветного ползания Т-клеток в стационарном состоянии составляет около 4 мкм/мин и заметно увеличивается (до 12 мкм/мин) в ЛК воспаленной кожи мышей. Увеличение скорости ползания Т-клеток поддерживается интегрин-опосредованными адгезивными взаимодействиями с ICAM-1 на внутренней поверхности эндотелия [57, 117]. Достигнув первых сокращающихся ЛС, Т-клетки начинают катиться со скоростью, достигающей 300 мкм/мин. В более крупных ЛС мигрирующие лейкоциты сталкиваются со значительным увеличением скорости тока лимфы (>1 мм/мин), что, вероятно, делает внутрипросветное ползание излишним, и они перемещаются с потоком лимфы. Переход к потоковому движению позволяет лейкоцитам ускоряться в сотни и тысячи раз и является ключевым моментом для своевременного поступления лейкоцитов в ЛУ [12].
Имеются данные, что нейтрофилы также используют механизм хемотаксиса CCL21 для проникновения в ЛК. Кроме этого, трансмиграция нейтрофилов регулируется с помощью CXCL8 на ЛЭК и его рецепторов CXCR1/2 на нейтрофилах [90]. При исследовании воспаленной кожи у мышей, популяция нейтрофилов, мигрирующих в ЛК, была идентифицирована исключительно как CCR7+, и при ингибировании CCL21 трансмиграция нейтрофилов практически прекращалась [21]. Нейтрофилы также ползают в просвете ЛК в основном вниз по течению к лимфатическим коллекторам и с примерно такими же скоростями (в среднем 6–13 мкм/мин), что и ДК и Т-клетки. Как и в случае с последними, было показано, что миграция нейтрофилов определяется гаптотактическим градиентом CCL21, секвестрированном на внутренней поверхности ЛК в направлении тока лимфы, силами, опосредованными интегрином β2 и ICAM-1 [57].
В экспериментах с визуализацией ЛК было установлено, что ДК и T-клетки, мигрирующие в дренирующие ЛУ, проводят много часов, ползая и задерживаясь в ЛК [99]. В это время клетки находятся в тесном контакте с эндотелием, что позволяет лейкоцитам и ЛЭК “общаться” и влиять друг на друга. Полученные недавно данные показывают, что периоды ползания ДК по поверхности ЛК сменяются остановками, порой довольно продолжительными [87]. Подобные остановки ДК в просвете ЛК представляются крайне важными, особенно в свете недавних сообщений о том, что ДК останавливаются во время такой миграции с целью формирования долгоживущих MHC-зависимых взаимодействий с антиген-специфическими Т-клетками [63]. Ранее сообщалось, что в ЛК людей были обнаружены клеточные агрегаты (кластеры), содержащие ДК и CD4+ T-клетки [131]. Поскольку кластеризация и скопление Т-клеток являются характерными фазами активации Т-клеток в ЛУ, авторы исследования полагают, что в ЛК Т-клетки в составе кластеров могут взаимодействовать с ДК. Высказывается предположение, что на поверхности ЛЭК в контакты с ДК вступают как CD4+ эффекторные Т-клетки, так и регуляторные Т-клетки, которые в интерстициальном пространстве не встретились с родственными антигенами. Авторы также наблюдали Т-клетки, которые выходили из ЛК обратно в окружающие ткани, количественная оценка показала, что в течение периода визуализации (45 мин) в общей сложности от 5 до 10% Т-клеток вышли из ЛК в интерстициальное пространство. Полагают, что контакт с родственным антигеном внутри ЛК позволяет Т-клетке активироваться и “получить указание выйти из ЛК обратно в окружающие ткани и продолжить поиски антигена”. Такое поведение способствует ускорению иммунологического надзора, поскольку эти клетки будут перемещаться по короткому пути, а не рециркулировать через ЛС, дренирующие ЛУ и кровь. При этом не было обнаружено ни одной ДК, выходящей из ЛК в интерстициальное пространство [46].
ЛЭК обладают несколькими механизмами регуляции взаимодействия ДК и Т-клеток. ЛЭК способны модулировать иммунный ответ как посредством прямого взаимодействия с иммунными клетками, включая ДК и Т-клетки, так и посредством секреции хемокинов и цитокинов. В физиологических условиях прямые взаимодействия ЛЭК и T-клеток приводят к усилению апоптоза CD4+ T-клеток и анергии и дисфункции CD8+ Т-клеток, что способствует толерантности. ЛЭК также активируют молекулы адгезии, молекулы MHC и другие факторы, которые помогают поддерживать популяции регуляторных Т-клеток и Т-клеток памяти и ингибируют созревание ДК. Во время воспаления ЛЭК могут продуцировать такой фактор, как NO, который ингибирует пролиферацию Т-клеток. После разрешения воспаления ЛЭК могут сохранять антигены в течение длительных периодов времени (явление, называемое архивированием антигенов) и передавать их ДК, тем самым способствуя поддержанию иммунологической памяти. Высказывается мнение, что ЛЭК в ЛК могут выполнять такие же иммуномодулирующие функции, как и в дренирующих ЛУ [56].
Таким образом, в ЛК осуществляются адаптивные взаимодействия ЛЭК, ДК и T-клеток. ЛЭК ЛК способны различными способами модулировать иммунные функции: с одной стороны, они инициируют и облегчают адаптивные иммунные реакции, а с другой – устраняют воспаление, подавляют иммунитет или способствуют толерантности путем продукции различных иммуносупрессивных факторов, таких, например, как TGF-β и iNOS [29].
ЛИМФАТИЧЕСКИЕ СОСУДЫ
ЛК переходят в посткапилляры, а последние – в ЛС, часто называемые лимфатическими коллекторами или собирательными сосудами. Эти сосуды собирают лимфу из ЛК и транспортируют ее вниз по течению к ЛУ. В отличие от стенки ЛК, имеющей прерывистую базальную мембрану и ЛЭК, которые неплотно соприкасаются друг с другом и формируют “пуговичные соединения”, пропускающие инерстициальную жидкость в ЛК, стенка ЛС имеет непрерывную базальную мембрану, поверх которой располагается слой гладкомышечных клеток (ГМК). Еще одно важное различие между этими двумя элементами лимфатического сосудистого русла имеется на уровне межклеточных соединений, ЛЭК ЛС формируют плотные контакты из соединительных молекул адгезии (CD31, VE-кадгерин и др.) по всему периметру (“застежки-молнии”). В физиологических условиях наличие подобных соединений и непрерывная базальная мембрана делают стенку ЛС непроницаемой для клеток и крупных молекул [107].
ЛС образуют сеть взаимосвязанных иерархических каналов, так что в итоге существуют магистрали, по которым в основном осуществляется лимфоток, и коллатерали, по которым ток лимфы в физиологических условиях незначителен или отсутствует. Основная функция ЛС заключается в транспорте лимфы в дренирующие ЛУ и, в конечном итоге, за пределы ЛУ в эфферентные ЛС. ЛС имеют внутрипросветные двустворчатые клапаны, которые разделяют ЛС на отдельные сегменты – лимфангионы, сокращающиеся, как правило, автономно и независимо от соседних лимфангионов (рис. 3) [4]. Клапаны предотвращают отток лимфы обратно к тканям после каждого цикла сокращения лимфангиона. Клапаны открываются и закрываются в зависимости от величин давления по обе стороны от створок клапана. Когда давление в дистальном лимфангионе выше, чем в проксимальном, створки открываются и лимфа перемещается в проксимальный лимфангион, а при изменении направления градиента давления створки клапана закрываются, что делает невозможным возврат лимфы в дистальный лимфангион [3].
Рис. 3.
Строение лимфатического сосуда. Сегмент лимфатического сосуда от одного клапанного участка до другого называется лимфангионом. ГМК – гладкомышечная клетка, ЛЭК – лимфатическая эндотелиальная клетка. Зеленые стрелки показывают направление силы, развиваемой ГМК лимфангиона при сокращении. Синяя стрелка показывает направление лимфотока при сокращении ГМК дистального лимфангиона.
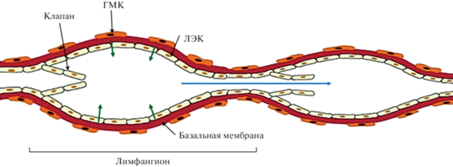
В стенке крупных ЛС выделяют три слоя ГМК, которые являются высокоспециализированными и уникальными, они экспрессируют смесь изоформ миозинов сердечных, скелетных и сосудистых гладких мышц, которые делают их способными не только изменять тонус, но и быстро сокращаться [5, 103]. При электронно-микроскопическом исследовании стенки ЛС хорошо видны многочисленные плотные контакты между ГМК, объединяющие их в пределах лимфангиона в структурно-функциональную единицу. В цитоплазме ГМК большое количество митохондрий, что свидетельствует о способности ГМК генерировать значительное количество энергии [2]. В пределах лимфангиона имеется группа ГМК, которые выполняют функцию пейсмекера, не только генерирующего потенциалы действия, но и активно реагирующего на величину трансмурального давления [74]. Благодаря наличию плотных контактов, ГМК лимфангиона, подобно кардиомиоцитам в сердце, сокращаются синхронно и способны развивать значительную силу: в эксперименте при окклюзии одиночные лимфангионы развивали давление до 60 см водн. ст. [3, 5]. ЛС фактически представляют собой цепочки лимфатических микросердец (лимфангионов), которые перекачивают лимфу на значительное расстояние, вплоть до впадения основного лимфатического коллектора организма – грудного протока в крупные вены шеи.
Литературные данные свидетельствуют, что собирательные ЛС не участвуют в иммунных реакциях. Если рассматривать ЛС с позиций иммунологии, то они являются всего лишь транспортными магистралями, обеспечивающими связь тканей с иммунной системой посредством переноса ДК, лимфоцитов, антигенов и цитокинов [132]. Характер перемещения ДК при переходе их из ЛК в ЛС изменяется принципиально. Покадровая визуализация показывает, что если в ЛК иммунные клетки активно ползают по стенке ЛК, медленно перемещаясь к выходу из ЛК, то при переходе в первые сегменты собирательных ЛС ДК и лимфоциты отделяются от ЛЭК и увлекаются потоком лимфы, переходя от активного движения к пассивному за счет сокращений лимфангионов. Скорость перемещения иммунных клеток существенно возрастает и в физиологических условиях достигает сотен микрометров и даже нескольких миллиметров (в крупных ЛС) в секунду [68]. Таким образом, переход ДК и лимфоцитов к пассивному перемещению в ЛС представляет собой решающий шаг, необходимый для своевременного прибытия клеток в дренирующие ЛУ.
В воспаленных тканях скорость лимфотока изменяется. Повышение проницаемости кровеносных капилляров и венул, наблюдающееся при воспалении, приводит к образованию значительных объемов лимфы, что сопровождается быстрым расширением ЛК и ЛС. При этом ауторегуляторные механизмы, характерные для ЛС [5], способствуют увеличению амплитуды сокращений ГМК лимфангионов, увеличению их систолического объема и, как следствие, увеличению лимфотока. Небольшое расширение ЛС способствует улучшению оттока избытка интерстициальной жидкости, в то же время чрезмерное растяжение может привести к нарушению транспорта жидкости и замедлению разрешения отека и воспаления. Имеются данные, что выраженное воспаление часто сопровождается урежением частоты и уменьшением амплитуды сокращений лимфангионов [1, 6]. Основными эффекторами, приводящими к нарушению прокачки лимфы, являются некоторые цитокины, реализующие свое действие посредством экспрессии индуцибельной NO-синтазы [13, 23]. Наблюдаемое в подобных случаях снижение лимфатического оттока приводит к накоплению в тканях медиаторов воспаления, которые в норме выводятся с лимфой и влияют на перенос иммунных клеток в ЛУ. Это может нарушать иммунные регулирующие пути, которые зависят от связи между тканью и ЛУ [123].
При воспалении макрофаги в тканях продуцируют VEGF-C, который стимулирует лимфангиогенез в ЛК и гипертрофию собирающих ЛС. Это облегчает иммунный ответ за счет мобилизации дендритных клеток и увеличения способности ЛС транспортировать бóльшие объемы лимфы и, следовательно, способствует восстановлению тканевого гомеостаза и разрешению воспаления [82]. ЛС реагируют на воспаление не только гипертрофией, но и изменением проницаемости стенки ЛС. Классическим примером подобного ремоделирования являются изменения ЛС кишечника при болезни Крона. Стенка кишечника человека содержит четыре отдельных лимфатических капиллярных русла, которые берут начало в различных анатомических пространствах и дренируют их [94]. Эти лимфатические капиллярные сети реабсорбируют множество критических антигенов, а млечные сосуды ворсинок переносят пищевой жир, упакованный в хиломикроны. В итоге брыжеечные ЛС и ЛУ подвергаются периодическим высоким жировым нагрузкам, в которых помимо жирных кислот пищевого происхождения содержатся и липиды, синтезированные бактериями кишечника. Еще в первых описаниях болезни Крона отмечалось, что болезнь характеризуется выраженным лимфангитом. Позднее было установлено, при болезни Крона в собирательных ЛС образуются третичные лимфоидные структуры, которые препятствуют току лимфы к дренирующим ЛУ. В результате – мигрирующие ДК не могут попасть в дренирующие ЛУ. Они накапливаются в афферентных ЛС и вместе с хиломикронами выходят через стенку ЛС, которые, в результате хронического воспаления, становятся негерметичными, в окружающий ЛС жир. Выходящие за пределы ЛС ДК и хиломикроны стимулируют воспалительный процесс в окружающих тканях, что дополнительно увеличивает проницаемость стенки ЛС, формируя, таким образом, порочный круг [110].
В физиологических условиях трансмиграция иммунных клеток через стенку собирательных ЛС не происходит. Однако недавние данные свидетельствуют, что повышенная проницаемость стенки коллекторных ЛС при воспалении создает условия для проникновения ДК из тканей в просвет ЛС. Методом покадровой визуализации было показано, что в воспаленной ткани ДК проникают в собирательные ЛС кожи, минуя этап медленного активного ползания в ЛК [15]. Исследования миграции in vivo показали, что проникновение ДК в ЛС при воспалении сокращает общее время, необходимое им для миграции из периферической ткани в ЛУ. Основной молекулой, обеспечивающей повышение проницаемости стенки ЛС, является VCAM-1, который при воспалении экспрессируется в ЛЭК ЛС в значительно большей степени по сравнению с ЛЭК ЛК [15]. При воспалении в процесс привлечения и переноса ДК через стенку ЛС вносят свой вклад также некоторые хемокины. Было показано, что два из них – а именно, CXCL12 и CX3CL1, при воспалении регулируют перемещение ДК к дренирующим ЛУ. При воспалении выраженная в ЛЭК ЛС экспрессия хемокинов CXCL12 и CX3CL1 оказывает на ДК такое же влияние, что и CCL21 в ЛК [15]. Попадая непосредственно в сегменты лимфатических коллекторов ДК, вероятно, избегают медленной активной миграции в капиллярах и быстрее достигают ЛУ, чем основная масса ДК, проникших через капилляры. Полагают, что этот эволюционно сформированный механизм позволяет значительно ускорить доставку ДК из очага воспаления в дренирующие ЛУ, что способствует ускорению развития иммунных реакций.
Вполне очевидно, что более быстрая доставка ДК в ЛУ и индукция адаптивного иммунитета может быть выгодным в отношении инфекций, вызываемых быстро размножающимися патогенами. Кроме того, все больше данных свидетельствует о том, что ЛЭК ЛК выполняют иммуномодулирующие функции и экспрессируют молекулы, которые могут подавлять созревание ДК [32]. Таким образом, проникновение ДК в коллекторы позволяет не только избежать задержки в ЛК, но и уклоняться от иммуносупрессивного влияния ЛЭК ЛК. Этот путь миграции используется только частью ДК, в более поздние сроки основную массу ДК составляют клетки, проникшие через ЛК. Представляется интересным, что и некоторые регуляторные Т-клетки проникают в лимфатическую систему, используя этот, зависимый от VCAM-1, путь миграции [25].
Собирательные ЛС экспрессируют цитокиновые и толл-подобные рецепторы [43] и реагируют на воспалительные стимулы не только изменениями пролиферации и проницаемости [10]. ЛЭК периферических ЛС также могут влиять на миграцию лейкоцитов, модулируя доступность хемокинов посредством экспрессии атипичного хемокинового рецептора D6 (“поглотитель хемокинов”) [70]. Показано, что ЛЭК в ЛС кожи и кишечника человека экспрессируют значительное количество D6. Возрастание экспрессии D6 при воспалении, возможно, нейтрализует воспалительные β-хемокины и предотвращает индуцированную хемокинами прочную адгезию лейкоцитов к ЛЭК. Подобный антиадгезионный механизм улучшает перенос в ЛУ лейкоцитов, попавших в ЛС.
Одним из важнейших открытий последних лет в лимфологии и иммунологии является установление факта поглощения и архивирования антигенов ЛЭК ЛК и ЛС. Tamburini et al. показали, что поглощенные ЛЭК антигены длительное время сохраняются в ЛЭК с целью последующей передачи обратно в ДК или другим антигенпрезентирующим клеткам [129]. В стационарных условиях LEC могут презентировать собственные антигены, чтобы вызвать толерантность Т-клеток либо посредством экспрессии антигенов периферических тканей, либо путем приобретения внеклеточных антигенов посредством фагоцитоза, а также посредством поглощения предварительно загруженных молекул MHC II из ДК [102]. Молекулярные механизмы, посредством которых ЛЭК захватывают и удерживают антиген, пока неясны. Однако известно, что в физиологических условиях LEC экспрессируют молекулы MHC класса I и MHC класса II [97]. Какими бы ни были механизмы переноса антигена, понятно, что некоторые ДК, переносимые лимфой из периферических тканей, захватывают антиген из ЛЭК в процессе миграции по ЛК и ЛС и переносят его в ЛУ, где представляют лимфоцитам. Авторы исследования полагают, что лимфодренаж в физиологических условиях способствует периферической толерантности, доставляя собственные АГ к лимфоцитам, находящимся в ЛУ и обеспечивая постоянную экспозицию периферических антигенов ЛЭК, которые поддерживают толерогенную перекрестную презентацию таких антигенов [55]. Интересно, что подобный механизм используется не только для хранения собственных антигенов, но и для архивирования вирусных антигенов. Впоследствии эти антигены переносятся в ДК для стимуляции Т-клеток памяти и усиления защитного иммунитета [114].
ЛИМФАТИЧЕСКИЕ УЗЛЫ
Лимфа, перемещаемая по афферентным ЛС за счет ритмических сокращений лимфангионов, обязательно проходит через один или несколько ЛУ. У человека, по данным разных авторов, насчитывается от 500 до 800 ЛУ. В большинстве случаев ЛУ имеют округлую, несколько уплощенную форму, диаметром от нескольких мм до нескольких см. Снаружи ЛУ покрыт соединительнотканной капсулой, в которую включены пучки ГМК. Соединительная ткань из капсулы продолжается в трабекулы – внутренние перегородки ЛУ. Капсула и трабекулы в ЛУ выполняют функцию каркаса. В ЛУ представлена сложная сеть лимфатических синусов, окружающих и пронизывающих высокоорганизованную паренхиму, структурной основой которой являются стромальные клетки, создающие оптимальную среду для активации и протекания иммунных ответов [7, 80]. Ряд авторов рассматривают лимфатические синусы ЛУ как продолжение периферической лимфатической сети, хотя они сильно отличаются как от ЛК, так и от собирательных ЛС уникальными структурными и молекулярными особенностями. Распределенные по всему телу ЛУ являются вторичными лимфоидными органами, фильтрующими лимфу и играющими ключевую роль в адаптивном иммунитете. Они представляют собой важный перекресток, где резидентные и мигрирующие иммунные клетки взаимодействуют, чтобы инициировать антиген-специфические иммунные ответы [88].
В ЛУ принято выделять три зоны: кора, паракортикальная зона и мозговое вещество. Кора включает лимфоидную ткань, образующую лимфатические узелки (В-зависимые зоны) и межузелковые скопления клеток. Здесь имеются зародышевые центры, где В-клетки пролиферируют в плазматические клетки, секретирующие антитела. В этих областях также присутствуют макрофаги вместе с ДК. Макрофаги и ДК улавливают антигены и представляют их на своей поверхности В-клеткам.
Под корковым слоем находится паракортекс. Основными отличительными чертами паракортикальной зоны являются отсутствие лимфоидных узелков и большое количество Т-лимфоцитов (CD4+ и CD8+). Здесь происходит их антиген-зависимая пролиферация и дифференцировка с формированием различных субпопуляций. В паракортикальной зоне рядом с лабиринтами располагаются венулы с высоким эндотелием (HEV), через которые циркулирующие лимфоциты попадают в паренхиму узла. HEV экспрессируют аквапорин-1 и участвуют в поглощении воды из лимфы, поступающей по афферентным ЛС.
Мозговое вещество образовано ветвящимися и анастомозируюшими тяжами лимфоидной ткани, между которыми располагаются мозговые синусы. В мозговой ткани представлено большое количество плазматических клеток, а также В-лимфоциты и макрофаги (в меньшем количестве). Плазматические клетки могут длительно находиться в мозговой ткани и активно секретировать антитела, часть этих клеток с лимфой поступает в кровоток.
Синусы ЛУ. Под капсулой ЛУ располагается субкапсулярный синус (СКС) – узкое пространство, сформированное ЛЭК ЛУ и пронизанное отростками ЛЭК и тонкими ретикулярными волокнами. В коре и паракортексе расположены трабекулярные синусы, переходящие в мозговом веществе в большой мозговой синус (рис. 4). Все синусы имеют выстилку из ЛЭК, отростки ЛЭК и ретикулярных клеток формируют в просвете синусов сложную трехмерную сеть, поры которой имеют размеры, не превышающие размеры ДК и лимфоцитов, так что эти клетки, протискиваясь через поры сети, контактируют с большим количеством ЛЭК и макрофагов. Жидкость и мигрирующие клетки, прибывающие в ЛУ, проходят через ЛУ преимущественно в следующем порядке: СКС, трабекулярные синусы, мозговой синус. Часть лимфы и мигрирующих клеток также попадают в кору через отверстия в дне СКС.
Рис. 4.
Строение лимфатического узла: А – лимфатический узел в целом. Стрелками показаны потоки лимфы, поступающей в субкапсулярный синус лимфатического узла: коричневая – основной поток лимфы по субкапсулярному синусу, желтая – поток по трабекулярному синусу, синяя – поток низкомолекулярных частиц в фолликулы лимфатического узла. Б – увеличенный фрагмент лимфатического узла, субкапсулярный синус с ЛЭК, расположенными на капсуле (потолок) и ЛЭК, расположенными на паренхиме ЛУ (пол). Показаны В- и Т-лимфоциты и макрофаги субкапсулярного синуса (М), погруженные в паренхиму и выступающие в просвет субкапсулярного синуса.
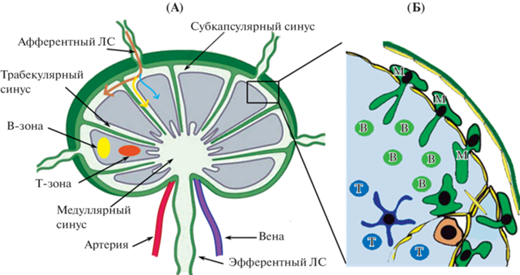
Дно СКС выстлано слоем уплощенных ЛЭК. Синусоидальные ЛЭК имеют многочисленные поверхностные инвагинации, цитоплазматические везикулы, транспортные канальцы и мультивезикулярные тельца, которые указывают на активные эндоцитозные процессы [59]. Проникновение частиц через ЛЭК дна СКС происходит очень быстро, у мышей введенные меченые молекулы массой около 150 кДа специфически окрашивают свои клетки-мишени в паренхиме дренирующих ЛУ через несколько секунд после инъекции. Было установлено, что перенос антител через ЛЭК пола СКС является независимым от макрофагов [66].
Между ЛЭК СКС располагаются многочисленные макрофаги. Макрофаги СКС имеют “головку”, которая выступает в просвет СКС, и длинные “хвостовые” отростки, которые простираются в нижележащий В-фолликул, при этом “шейка” плотно прилегает к ЛЭК дна СКС ЛУ (рис. 4Б). Дно СКС заселяют не только макрофаги СКС, но и некоторое количество ДК. Макрофаги СКС и ДК могут непосредственно захватывать патоген или частицы из лимфы, проходящей по СКС. Многофотонная прижизненная микроскопия показала, что расположенные на дне макрофаги СКС функционируют как “липучка” для захвата частиц вируса [64]. Функция “липучки” макрофагов дна СКС также проявляется и по отношению к бактериям, передающимся через лимфу [20, 64]. Макрофаги СКС захватывают поступающие с лимфой вирусы и иммунные комплексы своими “головками”, а затем перемещают эти частицы в область “хвостов” для контакта с фолликулярными В-клетками. Фолликулярные В-клетки мигрируют между хвостовыми отростками макрофагов СКС, совершая “случайные блуждания” по фолликулу, и часто вступают в тесный контакт с макрофагами. Имеются данные, что В-клетки получают антиген непосредственно от макрофагов через их В-клеточный рецептор [64].
Макрофаги СКС, как и макрофаги других тканей, являются фагоцитирующими клетками, которые захватывают и разрушают частицы и патогены и высвобождают медиаторы, информирующие адаптивную иммунную систему об опасности [53]. Эти макрофаги захватывают свободно плавающие крупные и средние антигены в афферентной лимфе в течение нескольких минут после их появления в СКС, в то время как мелкие антигены могут проникать в кондуиты ЛУ, где они захватываются резидентными ДК ЛУ [75]. Установлено, что первая волна активации иммунных реакций в ЛУ происходит за несколько часов до того, как ДК тканевого происхождения, несущие антиген, попадают в ЛУ. Фактически, даже в отсутствие мигрирующих в тканях ДК резидентные ДК ЛУ способны генерировать защитный иммунный ответ против вторжения патогенов [45]. Таким образом, резидентные макрофаги ЛУ функционируют как передняя линия иммунной защиты от патогенов.
Макрофаги СКС имеют относительно низкую фагоцитарную активность и не могут уничтожать микробы напрямую [41], но обладают способностью продуцировать значительное количество цитокинов [86]. После активации макрофаги СКС функционируют, связываясь с другими резидентными клетками ЛУ или рекрутируя другие клетки в СКС, чтобы обеспечить быстрый и сильный антимикробный ответ на переносимые лимфой антигены. В этом случае натуральные киллеры становятся подвижными, мигрируют в СКС и задерживаются в нем на протяжении нескольких часов. Их число в течение первых трех суток после заражения возрастает в 10–15 раз [35, 54].
Макрофаги СКС в каждом конкретном случае продуцируют различные цитокины с целью запустить наиболее эффективные типы иммунных реакций, что приводит к рекрутированию различных иммунных клеток против разных патогенов, поступающих с лимфой. В ответ на переносимые лимфой вирусные возбудители макрофаги СКС продуцируют α-интерферон, в итоге они дополнительно рекрутируют плазмацитоидные ДК в СКС для инициации противовирусного иммунитета. В случае бактериальной инфекции значительно возрастает продукция γ-интерферона. Дефицит макрофагов СКС сопровождается значительным снижением продукции цитокинов и ограничивает привлечение важных врожденных иммунных клеток [67]. Это также негативно влияет на инициацию адаптивного иммунитета.
Кортикальные синусы плотно заполнены выходящими лимфоцитами и, в отличие от СКС и медуллярного синуса, содержат мало макрофагов. Поток лимфоцитов в разветвленной сети корковых синусов начинается в области слепых концов синусов. Многие корковые синусы расположены рядом с HEV и лимфоциты в физиологических условиях поступают в синусы в течение нескольких минут после попадания в ЛУ. Однако при воспалении лимфоциты быстро активируют CD69, что ограничивает их доступ к корковым синусам и вынуждает задерживаться в ЛУ, это ключевое событие для успешного иммунного надзора [64].
Медуллярные синусы (МС) представляют собой многочисленные заполненные жидкостью пространства неправильной формы, выстланные ЛЭК и пересекаемые тонкими ретикулярными тяжами. Они содержат многочисленные макрофаги МС, которые прикрепляются к стенкам синусов и ретикулярным волокнам в просвете МС [81]. Медуллярные макрофаги ЛУ известны своей способностью захватывать, фагоцитировать и уничтожать патогены, поступающие из очагов инфекции, что помогает предотвратить их системное распространение [41]. В первые часы после подкожной инъекции меченого антигена основным местом накопления метки в ЛУ является медуллярная область, и меченый антиген выявляется в макрофагах МС. На основании морфологических особенностей (большие лизосомы и гетерогенные скопления везикул) и количества интернализированного меченого антигена эти клетки классифицируются как высокоактивные фагоциты [50].
МС также содержат лимфоциты, находящиеся в процессе выхода из ЛУ, и небольшое количество плазматических клеток. Мозговое вещество в состоянии покоя представляет собой небольшую часть ЛУ, но значительно увеличивается на пике реакции плазматических клеток. Полагают, что мозговое вещество ЛУ выполняет, по меньшей мере, три важнейших функции: 1) фагоцитоз и удаление патогенов и частиц антигенов из лимфы; 2) поддержание выживания короткоживущих плазматических клеток и 3) обеспечивает транспортную магистраль для выхода клеток и антител в эфферентную лимфу.
Стромальные клетки ЛУ. ЛУ имеют сложную высокоорганизованную архитектуру, сформированную стромальными клетками ЛУ и сформированную в субанатомически разделенные области, создающие оптимальные условия для индукции и протекания иммунных ответов [49]. Стромальные клетки формируют трехмерную сеть, которая является основой для миграции иммунных клеток. Имеются убедительные доказательства того, что стромальные клетки ЛУ обладают иммунологическими свойствами, которые имеют решающее значение для выживания и регуляции иммунных клеток, а также для поддержки структуры ЛУ. Лимфоциты, находящиеся в ЛУ, интенсивно взаимодействуют со стромальными клетками на протяжении всего времени их пребывания в ЛУ. Помимо обеспечения структурной поддержки, стромальные клетки также обеспечивают сигналы выживания, питательные вещества, растворимые факторы и антигены, которые в совокупности необходимы для иммунного надзора, а также для генерации и контроля адаптивных иммунных ответов [30].
Стромальные клетки ЛУ представляют собой две основные популяции: это эндотелиальные клетки (кровеносных сосудов и лимфатические) и фибробластные ретикулярные клетки (ФРК). В ЛУ выявлено несколько подмножеств эндотелиальных клеток кровеносных сосудов: это артериальные ЭК, пять подвидов капиллярных ЭК, два подвида венозных ЭК и медуллярные ЭК (209). Каждая разновидность ЭК выполняет свои специфические функции. Особо необходимо отметить эндотелий венул HEV. Эти клетки секретируют CCL21 и создают градиент для привлечения в ЛУ из крови Т-лимфоцитов и ДК, а также экспрессируют аквапорин-1, что позволяет обеспечить переход значительного количества воды из лимфы в кровь [76].
Лимфатические эндотелиальные клетки (ЛЭК) в ЛУ довольно гетерогенны, в ЛУ человека выявлено шесть подвидов ЛЭК. В СКС ЛУ были идентифицированы две разновидности ЛЭК: потолочные ЛЭК (слой ЛЭК, обращенный к капсуле) и напольные LEC (покрывают паренхиму), все они участвуют в трансмиграции лимфоцитов в ЛУ [127]. Отростки ЛЭК пересекают СКС от потолка ЛУ до пола, образуя трехмерное сито для фильтрации поступающих клеток и крупных частиц. На дне синуса ЛЭК экспрессируют TNFRSF9, CCL20 и CXCL5 и привлекают зрелые В-клетки, Т-клетки и ДК. ЛЭК потолка СКС выполняют важнейшую функцию нейтрализации CCL19, CCL21 и CCL25, они поглощают и инактивируют эти хемокины, способствуя тем самым миграции лимфоцитов в паренхиму ЛУ посредством трансцитоза [119]. ЛЭК кортикальных и мозгового синусов экспрессируют CCL21 и SPHK1 (фермент, необходимый для производства сфингозин-1-фосфата (S1P), тем самым регулируя задержку или выход CCR7+ клеток из ЛУ [48]. CCL21 не только привлекает Т-лимфоциты, но и удерживает Т-клетки в ЛУ. В кортикальном синусе выход лимфоцитов регулируется с помощью LEC, экспрессирующих S1P, который связывается с S1P1R на Т-клетках. После клональной экспансии активированные Т-клетки подавляют CCR7 и активируют S1PR1, способствуя тем самым миграции в корковые синусы и в дальнейшем в МС и эфферентные ЛС [106]. В МС ЛЭК в сотрудничестве с макрофагами привлекают нейтрофилы во время развития инфекционного процесса [28]. ЛЭК МС также регулируют адгезию нейтрофилов в мозговом веществе ЛУ, что предотвращает распространение патогенов через ЛУ.
Как уже упоминалось ранее, не все антигены поглощаются ДК, часть их поступает в ЛУ в свободном виде. Свободные антигены, поступающие в СКС, имеют возможность перемещаться дальше как минимум четырьмя путями: 1) трансцитоз через ЛЭК пола СКС, 2) попадание в кондуиты ЛУ 3) поглощение макрофагами СКС и ДК, 4) немедленная доставка в МС через трабекулярные синусы, без проникновения в паренхиму ЛУ. ЛЭК СКС играют центральную роль при сортировке антигенов, содержащихся в лимфе.
ЛЭК ЛУ экспрессируют эндоцитарные рецепторы и могут поглощать различные материалы, иногда в больших количествах. Например, наночастицы оксида железа, покрытые кремнеземом, преимущественно поглощались ЛЭК, выстилающими СКС, а не макрофагами, а вирусные частицы были обнаружены преимущественно в ЛЭК, выстилающих медуллярный синус [64]. Вирусные и вакцинные антигены обнаруживаются в ЛЭК ЛУ в течение как минимум 5 нед. после воздействия, т.е. ЛЭК ЛУ могут выполнять функцию резервуара для антигенов (архивирование антигена), облегчая индукцию защитных иммунных ответов при вакцинации и вирусной инфекции [122]. Хранение антигена может сопровождаться его медленным внутриузловым высвобождением даже после того, как острое воздействие периферического антигена прекратится. Подобный процесс представляет собой механизм, который может иметь особое значение для формирования долговременных иммунологических ответов. Предполагается, что ЛЭК ЛУ представляют антигены напрямую, но также возможно, что они индуцируют толерантность косвенно, перенося антигены в ДК или получая антигенные комплексы от ДК [38]. ЛЭК ЛУ также действуют как непрофессиональные антигенпрезентирующие клетки, экспрессируя MHC класса I и II, но не экспрессируя костимулирующие молекулы [78].
Недавно было показано, что LEC ЛУ служат также петлей отрицательной обратной связи иммунной системы для противодействия чрезмерной активации Т-клеток. В частности, в воспаленных ЛУ ЛЭК ингибируют пролиферацию Т-клеток посредством секреции NO [241], тем самым контролируя пул активированных Т-клеток в ЛУ. Кроме этого ЛЭК могут ингибировать деление Т-клеток посредством экспрессии индоламин-2,3-диоксигеназы (IDO) [129].
Вторую группу стромальных клеток ЛУ составляют фибробластные ретикулярные клетки (ФРК). ФРК являются иммунологически специализированными миофибробластами, они составляют в ЛУ до 50% всех клеток негематопоэтического происхождения [39]. Они формируют в ЛУ трехмерную сеть с многочисленными межклеточными контактами, по которой мигрируют лейкоциты. ФРК продуцируют ретикулярные волокна и создают сеть проводников (кондуитов), по которой растворимые антигены и сигнальные молекулы быстро транспортируются глубоко в паренхиму ЛУ [96]. В ЛУ описано не менее 5 разновидностей ФРК, отличающихся по их расположению и экспрессии функциональных маркеров. ФРК обеспечивают прочность ЛУ и способность к быстрому изменению размеров, а также формируют компартментализацию B- и T-клеток, направляя движение лейкоцитов с помощью секреции хемокинов. Т-клетки и ДК находятся в постоянном контакте с ФРК, мигрируя по сети и сканируя друг друга на предмет антиген-специфического сродства.
ФРК паракортикальной зоны секретируют CCL19 и CCL21, привлекая тем самым CCR7+ наивные Т-клетки и ДК, которые перемещаются по сети ФРК в поисках антиген-специфических взаимодействий, оставаясь при этом в основном в паракортексе. ФРК также секретируют IL-7, который способствует выживанию Т-клеток [73]. ФРК обеспечивают поддержку ДК и их миграцию. Перемещение ДК является активным процессом, требующим амебоидного движения и наличия структуры, по которой можно ползать. ФРК создают такую структуру, а продуцируемый ими подопланин регулирует образование мембранных выпячиваний и подвижность ДК [9].
В настоящее время общепризнано, что лимфоидный компартмент ЛУ представляет собой структуру, экранированную от лимфы и крови, при этом клетки могут проходить через слой ЛЭК в кору ЛУ. Уникальная организация лимфоидного компартмента ЛУ создает среду с минимальным количеством жидкости, в которой плотно упакованные лимфоциты, перемещаясь по сети ФРК, могут взаимодействовать с ДК.
Единственное движение жидкости в этом отделе происходит в системе кондуитов, которая транспортирует часть поступающей лимфы из СКС непосредственно в кровеносную систему (HEV). Эта проводящая система состоит из сети трубчатых коллагеновых волокон, покрытых отростками ФРК, начинается между ЛЭК дна СКС, проходит по всему паракортексу и достигает фолликулов В-клеток. Волокна синтезируются ФРК, которые соединены друг с другом отростками, образуя трехмерную сеть, а лимфоциты заполняют пространства этой сети. Ретикулярные волокна спускаются от слоя ЛЭК СКС в направлении HEV, где иммунные клетки крови попадают в паренхиму ЛУ. Система канальцев выполняет функцию молекулярного сита, ограничивая размеры транспортируемых молекул (менее 70 кДа), вместе с тем она обеспечивает их быстрый транспорт из СКС к HEV, где заканчивается большинство кондуитов [11]. Таким образом, система кондуитов обеспечивает перекрестное взаимодействие между компонентами лимфы и кровью [99].
Существует мнение, что кондуиты могут действовать как система быстрой доставки информации, обеспечивая транспорт сигнальных молекул, таких как хемокины и цитокины, из ткани, дренируемой конкретным ЛУ к порту входа лимфоцитов – HEV. Хемокины, продуцируемые в месте воспаления, достигают ЛУ с афферентной лимфой и быстро транспортируются в HEV через систему кондуитов [11]. Эта модель, известная, как функция дистанционного управления, предполагает, что иммунная система может ощущать начало воспаления в отдаленной области (дренируемой ЛУ), и способна направлять соответствующие типы клеток в конкретный ЛУ, где должен быть инициирован иммунный ответ. ФРК не только транспортируют хемокины через свою проводниковую систему, но также активно продуцируют гомеостатические хемокины CCL19 и CCL21 для привлечения наивных Т-клеток [103]. Эти хемокины поддерживают Т-клетки в активном миграционном состоянии, благодаря чему они непрерывно перемещаются вдоль ФРК и ассоциированных ДК.
Что касается возможного проникновения в кондуиты антигенов, то известно, что в систему каналов лимфоидного компартмента проникают только антигены с молекулярной массой менее 70 кДа. Т-клетки в Т-клеточной зоне не имеют доступа к антигену, поскольку он экранирован от них внутри кондуита. Только резидентные ДК, связанные с кондуитом, могут получить доступ к содержимому кондуита, пересекая базальную мембрану своими отростками. Они могут контактировать с антигенами в кондуите и представлять их находящимся рядом Т-клеткам. Быстрое обнаружение антигенов и хемокинов, поступающих из дренируемой области периферической ткани через кондуит приводит к немедленной активации иммунных реакций в ЛУ, что может способствовать более раннему развитию иммунного ответа. Позднее, через нескольких часов, в ЛУ прибывают ДК из тканей и развивается полноценный иммунный ответ [60].
Высокоорганизованная система фильтрации афферентной лимфы на уровне кондуитов эффективно предотвращает попадание патогенов, таких как бактерии и вирусы, в кровоток. Любая крупная частица, поступающая в составе лимфы в СКС, обязательно захватывается одним из многих макрофагов в СКС и МС. Кроме того, ДК, расположенные на дне синуса, активно поглощают и обрабатывают крупные антигены.
Отличительной чертой инициации адаптивного иммунного ответа является быстрое ремоделирование (увеличение) ЛУ, необходимое для размещения привлекаемых и подвергающихся клональной экспансии Т- и В-лимфоцитов. Сеть ФРК способна к значительной пролиферации в ответ на инфекцию или воспаление ЛУ. Время, необходимое для пролиферации, по-видимому, зависит от используемого стимула и варьируется от 24 ч до 12 дней. При некоторых видах воспаления объем ЛУ может увеличиваться в 15–20 раз с сохранением оригинальной структуры ЛУ [33]. Разрешение воспаления сопровождается выходом и сокращением популяций лимфоцитов и возвращением размеров ЛУ к их гомеостатическому значению.
ЗАКЛЮЧЕНИЕ
Лимфатическая система играет определяющую роль в иммунитете, выходящую далеко за рамки простого формирования каналов для перемещения лейкоцитов и антигенов из очага воспаления в ЛУ. ЛЭК в различных отделах лимфатической системы отличаются, и высоко специализированы для выполнения строго определенных ролей в иммунитете.
ЛК непрерывно собирают иммунологическую информацию (антигены, иммунные клетки и растворимые медиаторы) о текущем состоянии периферических тканей. ЛЭК ЛК имеют особые межклеточные соединения для эффективного поглощения интерстициальной жидкости и макромолекул. Они экспрессируют хемокин CCL21, выделяют его в интерстициальное пространство и формируют в нем гаптотактический градиент хемокина CCL21 с максимальной концентрацией вблизи стенки ЛК, благодаря которому мигрирующие CCR7+ иммунные клетки (ДК, Т-лимфоциты и моноциты) находят путь к порталам ЛК. Высокая концентрация CCL21 на люминальной поверхности ЛК обеспечивает возможность трансмиграции иммунных клеток в просвет ЛК. Оказавшись в просвете ЛК, ДК и Т-клетки активно ползают по люминальной поверхности ЛЭК и взаимодействуют друг с другом, обмениваясь информацией. ЛЭК ЛК являются платформой для взаимодействия ДК и Т-клеток и способны “архивировать” антиген. При воспалении или инфекции ЛЭК ЛК продуцируют растворимые факторы и оказывают тем самым “дистанционное управление” на ЛУ, модулируя миграцию лейкоцитов через венулы с высоким эндотелием из крови в паренхиму дренирующих ЛУ.
ЛС опосредуют транспорт антигена и иммунных клеток к дренирующим ЛУ, служа тем самым иммунологическими коммуникационными магистралями между периферическими тканями и ЛУ. ЛС активно транспортируют лимфу и под действием различных сигнальных молекул способны значительно изменять силу и частоту сокращений, модулируя скорость доставки с периферии в ЛУ антигенов, иммунных клеток и различных цитокинов. В гомеостатических условиях стенка ЛС непроницаема для макромолекул и клеток, однако при воспалении под действием медиаторов воспаления и высвобождаемых из клеток ферментов проницаемость стенки ЛС возрастает и ДК могут поступать из воспаленной ткани в просвет ЛС, минуя ЛК, что значительно ускоряет доставку антигена в ЛУ.
ЛУ являются высокоорганизованными структурами, расположенными в критических местах для исследования антигенов из периферических тканей, создавая оптимальные условия для встречи антигенпрезентирующих клеток и лимфоцитов. Внутри ЛУ стромальные клетки (ЛЭК и ФРК) формируют изолированные компартменты, благодаря которым иммунные клетки располагаются в строго определенных местах, что позволяет им эффективно реагировать на информацию, доставляемую лимфой, и либо способствовать иммунному гомеостазу, либо обеспечивать защитные иммунные ответы. Эти ответы включают активацию и функциональное сотрудничество нескольких различных типов клеток и адаптированы к конкретным воспалительным состояниям. ЛЭК и ФРК ЛУ создают маршруты для клеток и молекул в различные компартменты ЛУ. ЛЭК ЛУ совместно с ДК принимают участие в презентации антигенов, а ЛЭК кортикальных и медуллярного синусов активно регулируют вход и выход лимфоцитов из ЛУ. Еще одной ключевой функцией ЛЭК в ЛУ является продукция разнообразных хемокинов, что позволяет направлять ДК и Т-лимфоциты в строго определенные области ЛУ. Кроме того, они продуцируют факторы, способствующие выживанию поступающих иммунных клеток.
ФРК, активно реагируя на различные сигнальные молекулы в процессе воспаления, способствуют ремоделированию ЛУ, что сопровождается его гипертрофией с сохранением строго организованной структуры.
Макрофаги СКС ЛУ непосредственно подвергаются воздействию антигенов афферентной лимфы, фагоцитируют антигены и прямо или косвенно стимулируют другие клетки врожденного иммунитета ЛУ для борьбы с патогенами, а также активируют Т-клетки и В-клетки для развития адаптивного иммунитета.
Работа выполнена при поддержке Госпрограммы 47 ГП “Научно-технологическое развитие Российской Федерации” (2019–2030), тема 0134-2019-0001.
Список литературы
Абдрешов С.Н., Балхыбекова А.О., Демченко Г.А., Лобов Г.И. Лимфодинамика и адренергическая иннервация почки и почечных лимфатических узлов при токсическом гепатите // Регионарное кровообращение и микроциркуляция. 2020. № 19(3). С. 73–79.https://doi.org/10.24884/1682-6655-2020-19-3-73-79
Борисов А.В. Функциональная анатомия лимфангиона // Морфология. 2005. Т. 128. № 6. С. 18–27.
Лобов Г.И. Лимфатическая система в норме и при патологии // Успехи физиологических наук. 2022. Т. 53. № 2. С. 15–38. https://doi.org/10.31857/S0301179822020060
Лобов Г.И. Электрофизиологические свойства мембраны гладкомышечных клеток лимфатических сосудов //Доклады Академии наук СССР. 1984. Т. 277. № 1. С. 244–247.
Лобов Г.И., Орлов Р.С. Саморегуляция насосной функции лимфангиона // Физиол. журн. СССР им. И.М. Сеченова. 1988. Т. 74. № 7. С. 977–988.
Лобов Г.И., Унт Д.В. Дексаметазон предотвращает сепсис-индуцированное угнетение сократительной функции лимфатических сосудов и узлов посредством ингибирования индуцибельной NO-синтазы и циклооксигеназы-2 // Рос. физиол. журн. им. И.М. Сеченова. 2019. Т. 105. № 1. С. 76–88. https://doi.org/10.1134/S0869813919010059
Сапин М.Р., Никитюк Д.Б. Лимфатическая система и ее роль в иммунных процессах. М.: Медицинская книга, 2014. 40 с.
Abadie V., Badell E., Douillard P., Ensergueix D. et al. Neutrophils rapidly migrate via lymphatics after Mycobacterium bovis BCG intradermal vaccination and shuttle live bacilli to the draining lymph nodes // Blood. 2005. V. 106. P. 1843–1850. https://doi.org/10.1182/blood-2005-03-1281
Acton S.E., Astarita J.L., Malhotra D. et al. Podoplanin-rich stromal networks induce dendritic cell motility via activation of the C-type lectin receptor CLEC-2 // Immunity. 2012. V. 37(2). P. 276–289. https://doi.org/10.1016/j.immuni.2012.05.022
Aebischer D., Iolyeva M., Halin C. The inflammatory response of lymphatic endothelium // Angiogenesis. 2014. V. 17(2). P. 383–393. https://doi.org/10.1007/s10456-013-9404-3
Ager A. High endothelial venules and other blood vessels: critical regulators of lymphoid organ development and function // Front. Immunol. 2017. 8. 45. https://doi.org/10.3389/fimmu.2017.00045
Akl T.J., Nagai T., Cote G.L., Gashev A.A. Mesenteric lymph flow in adult and aged rats // Am J. Physiol. Heart Circ. Physiol. 2011. V. 301(5). P. H1828–H1840. https://doi.org/10.1152/ajpheart.00538.2011
Aldrich M.B., Sevick-Muraca E.M. Cytokines are systemic effectors of lymphatic function in acute inflammation // Cytokine. 2013. V. 64(1). P. 362–369. https://doi.org/10.1016/j.cyto.2013.05.015
Alrumaihi F. The Multi-Functional Roles of CCR7 in Human Immunology and as a Promising Therapeutic Target for Cancer Therapeutics // Front Mol. Biosci. 2022. V. 9. 834149. https://doi.org/10.3389/fmolb.2022.834149
Arasa J., Collado-Diaz V., Kritikos I. et al. Upregulation of VCAM-1 in lymphatic collectors supports dendritic cell entry and rapid migration to lymph nodes in inflammation // J. Exp. Med. 2021. V. 218:e20201413. https://doi.org/10.1084/jem.20201413
Arasa J., Collado-Diaz V., Halin C. Structure and Immune Function of Afferent Lymphatics and Their Mechanistic Contribution to Dendritic Cell and T Cell Trafficking // Cells. 2021. V. 10(5). 1269. https://doi.org/10.3390/cells10051269
Arokiasamy S., Zakian C., Dilliway J. et al. Endogenous TNFα orchestrates the trafficking of neutrophils into and within lymphatic vessels during acute inflammation // Sci. Rep. 2017 V. 7:44189. https://doi.org/10.1038/srep44189
Aukland K., Reed R.K. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume // Physiol. Rev. 1993. V. 73(1). P. 1–78. https://doi.org/10.1152/physrev.1993.73.1.1
Baluk P., Fuxe J., Hashizume H. et al. Functionally specialized junctions between endothelial cells of lymphatic vessels // J. Exp. Med. 2007. V. 204(10). P. 2349–2362. https://doi.org/10.1084/jem.20062596
Barral P., Polzella P., Bruckbauer A. et al. CD169(+) macrophages present lipid antigens to mediate early activation of iNKT cells in lymph nodes // Nat. Immunol. 2010. V. 11. P. 303–312. https://doi.org/10.1038/ni.1853
Beauvillain C., Cunin P., Doni A. et al. CCR7 is involved in the migration of neutrophils to lymph nodes // Blood. 2011. V. 117. P. 1196–1204. https://doi.org/10.1182/blood-2009-11-254490
Billaud M., Lohman A.W., Johnstone S.R. et al. Regulation of Cellular Communication by Signaling Microdomains in the Blood Vessel Wall // Pharmacol. Rev. 2014. V. 66(2). P. 513–569. https://doi.org/10.1124/pr.112.007351
Bouta E.M., Wood R.W., Brown E.B. et al. In vivo quantification of lymph viscosity and pressure in lymphatic vessels and draining lymph nodes of arthritic joints in mice // J. Physiol. 2014. V. 592. P. 1213–1223. https://doi.org/10.1113/jphysiol.2013.266700
Breslin J.W. ROCK and cAMP promote lymphatic endothelial cell barrier integrity and modulate histamine and thrombin-induced barrier dysfunction // Lymphat. Res. Biol. 2011/ V. 9. P. 3–11. https://doi.org/10.1089/lrb.2010.0016
Brinkman C.C., Iwami D., Hritzo M.K. et al. Treg engage lymphotoxin beta receptor for afferent lymphatic transendothelial migration // Nat. Commun. 2016. V. 7. 12021. https://doi.org/10.1038/ncomms12021
Brown M.N., Fintushel S.R., Lee M.H. et al. Chemoattractant receptors and lymphocyte egress from extralymphoid tissue: changing requirements during the course of inflammation // J. Immunol. 2010. V. 185:4873–82. https://doi.org/10.4049/jimmunol.1000676
Brulois K., Rajaraman A., Szade A. et al. A molecular map of murine lymph node blood vascular endothelium at single cell resolution // Nat. Commun. 2020. V. 11. 3798. https://doi.org/10.1038/s41467-020-17291-5
Camara A., Cordeiro O.G., Alloush F. et al. Lymph node mesenchymal and endothelial stromal cells cooperate via the RANK–RANKL cytokine axis to shape the sinusoidal macrophage niche // Immunity. 2019. V. 50. P. 1467–1481 https://doi.org/10.1016/j.immuni.2019.05.008
Card C.M., Yu S.S., Swartz M.A. Emerging roles of lymphatic endothelium in regulating adaptive immunity // J. Clin. Invest. 2014. V. 124. P. 943–952. https://doi.org/10.1172/JCI73316
Chang J.E., Turley S.J. Stromal infrastructure of the lymph node and coordination of immunity // Trends Immunol. 2015. V. 36(1). P. 30–39. https://doi.org/10.1016/j.it.2014.11.003
Chen H., Ye F., Guo G. Revolutionizing immunology with single-cell RNA sequencing // Cell Mol. Immunol. 2019. V. 16(3). P. 242–249. https://doi.org/10.1038/s41423-019-0214-4
Christiansen A.J., Dieterich L.C., Ohs I. et al. Lymphatic endothelial cells attenuate inflammation via suppression of dendritic cell maturation // Oncotarget. 2016. V. 7. P. 39421–39435. https://doi.org/10.18632/oncotarget.9820
Collin M., Bigley V. Human dendritic cell subsets: an update // Immunology. 2018. V. 154(1). P. 3–20. https://doi.org/10.1111/imm.12888
Debes G.F., Arnold C.N., Young A.J. et al. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues // Nat. Immunol. 2005. V. 6. P. 889–894. https://doi.org/10.1038/ni1238
Detienne S., Welsby I., Collignon C. et al. Central role of CD169+ lymph node resident macrophages in the adjuvanticity of the QS-21 component of AS01 // Sci. Rep. 2016. V. 6. 39475. https://doi.org/10.1038/srep39475
Dixon J.B., Raghunathan S., Swartz M.A. A tissue-engineered model of the intestinal lacteal for evaluating lipid transport by lymphatics // Biotechnol. Bioeng. 2009. V. 103. P. 1224–1235. https://doi.org/10.1002/bit.22337
Dixon J.B., Zawieja D.C., Gashev A.A., Coté G.L. Measuring microlymphatic flow using fast video microscopy // Biomed. Opt. 2005. V. 10(6). 064016. https://doi.org/10.1117/1.2135791
Dubrot J., Duraes F.V., Potin L. et al. Lymph node stromal cells acquire peptide-MHCII complexes from dendritic cells and induce antigen-specific CD4(+) T cell tolerance // J. Exp. Med. 2014. V. 211. 1153–1166. https://doi.org/10.1084/jem.20132000
Fletcher A.L., Malhotra D., Acton SE. et al. Reproducible isolation of lymph node stromal cells reveals site-dependent differences in fibroblastic reticular cells // Front. Immunol. 2011. V. 2. 35. https://doi.org/10.3389/fimmu.2011.00035
Forster R., Davalos-Misslitz A.C., Rot A. CCR7 and its ligands: balancing immunity and tolerance // Nat. Rev. Immunol. 2008. V. 8. P. 362–71. https://doi.org/10.1038/nri2297
Fossum S. The architecture of rat lymph nodes. IV. Distribution of ferritin and colloidal carbon in the draining lymph nodes after foot-pad injection // Scand. J. Immunol. 1980. V. 12. P. 433–441. https://doi.org/10.1111/j.1365-3083.1980.tb00087.x
Garnier L., Gkountidi A.O., Hugues S. Tumor-Associated Lymphatic Vessel Features and Immunomodulatory Functions // Front. Immunol. 2019. V. 10. 720. https://doi.org/10.3389/fimmu.2019.00720
Garrafa E., Imberti L., Tiberio G. et al. Heterogeneous expression of toll-like receptors in lymphatic endothelial cells derived from different tissues // Immunol. Cell Biol. 2011. V. 89. P. 475–481. https://doi.org/10.1038/icb.2010.111
Gascoigne N.R.J., Rybakin V., Acuto O., Brzostek J. TCR signal strength and T cell development // Annu. Rev. Cell Dev. Biol. 2016. V. 32. P. 327–348. https://doi.org/10.1146/annurev-cellbio-111315-125324
Gerner M.Y., Torabi-Parizi P., Germain R.N. Strategically localized dendritic cells promote rapid T cell responses to lymph-borne particulate antigens // Immunity. 2015. V. 42. P. 172–185. https://doi.org/10.1016/j.immuni.2014.12.024
Ghani S., Feuerer M., Doebis C. et al. T cells as pioneers: antigen-specific T cells condition inflamed sites for high-rate antigen-non-specific effector cell recruitment // Immunology. 2009. V. 128. e870–e880. https://doi.org/10.1111/j.1365-2567.2009.03096.x
Ginhoux F., Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis // Nat. Rev. Immunol. 2014. V. 14. P. 392–404. https://doi.org/10.1038/nri3671
Gómez D., Diehl M.C., Crosby E.J. et al. Effector T cell egress via afferent lymph modulates local tissue inflammation // J. Immunol. 2015. V. 195. P. 3531–3536. https://doi.org/10.4049/jimmunol.1500626
Grasso C., Pierie C., Mebius R.E., van Baarsen L.G.M. Lymph node stromal cells: subsets and functions in health and disease // Trends Immunol. 2021. V. 42(10). P. 920–936. https://doi.org/10.1016/j.it.2021.08.009
Gray E.E., Jason G., Cyster J.G. Lymph Node Macrophages // J. Innate. Immun. 2012. V. 4(5–6). P. 424–436. https://doi.org/10.1159/000337007
Guyton A.C., Taylor A.E., Brace R.A. A synthesis of interstitial fluid regulation and lymph formation // Fed. Proc. 1976. V. 35(8). P. 1881–1885.
Hampton H.R., Chtanova T. Lymphatic Migration of Immune Cells // Front. Immunol. 2019. V. 10. 1168. https://doi.org/10.3389/fimmu.2019.01168
Hashimoto D., Miller J., Merad M. Dendritic cell and macrophage heterogeneity in vivo // Immunity. 2011. V. 35. P. 323–335. https://doi.org/10.1016/j.immuni.2011.09.007
Heesters B.A., van der Poel C.E., Das A., Carroll M.C. Antigen presentation to B cells // Trends Immunol. 2016. V. 37. P. 844–854. https://doi.org/10.1016/j.it.2016.10.003
Hirosue S., Vokali E., Raghavan V.R. et al. Steady-state antigen snging, cross-presentation, and CD8+ T cell priming: a new role for lymphatic endothelial cells // J. Immunol. 2014. V. 192. P. 5002–5011. https://doi.org/10.4049/jimmunol.1302492
Hunter M.C., Teijeira A., Montecchi R. et al. Dendritic Cells and T Cells Interact Within Murine Afferent Lymphatic Capillaries // Front. Immunol. 2019. V. 10. 520. https://doi.org/10.3389/fimmu.2019.00520
Jackson D.G. Leucocyte Trafficking via the Lymphatic Vasculature- Mechanisms and Consequences // Front. Immunol. 2019. V. 10. 471. https://doi.org/10.3389/fimmu.2019.00471
Jakubzick C., Gautier E.L., Gibbings S.L. et al. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes // Immunity. 2013. V. 39. P. 599–610. https://doi.org/10.1016/j.immuni.2013.08.007
Jalkanen S., Salmi M. Lymphatic endothelial cells of the lymph node // Nat. Rev. Immunol. 2020. V. 20(9). 566–578. https://doi.org/10.1038/s41577-020-0281-x
Johnson L.A. In Sickness and in Health: The Immunological Roles of the Lymphatic System // Int. J. Mol. Sci. 2021. V. 22(9). P. 4458. https://doi.org/10.3390/ijms2209445
Johnson L.A, Jackson D.G. Inflammation-induced secretion of CCL21 in lymphatic endothelium is a key regulator of integrin-mediated dendritic cell transmigration // Int. Immunol. 2010. V. 22(10). P. 839–49. https://doi.org/10.1093/intimm/dxq435
Johnson L.A., Clasper S., Holt A.P. et al. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium // J. Exp. Med. 2006. V. 203(12). P. 2763–2777. https://doi.org/10.1084/jem.20051759
Johnson L.A., Banerji S., Lagerholm B.C., Jackson D.G. Dendritic cell entry to lymphatic capillaries is orchestrated by CD44 and the hyaluronan glycocalyx // Life Sci. Alliance. 2021. V. 4(5). e202000908. https://doi.org/10.26508/lsa.202000908
Junt T., Moseman E.A., Iannacone M. et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells // Nature. 2007. V. 450. P. 110–114. https://doi.org/10.1038/nature06287
Kabashima K., Shiraishi N., Sugita K. et al. CXCL12-CXCR4 engagement is required for migration of cutaneous dendritic cells // Am. J. Pathol. 2007. V. 171. P. 1249–1257. https://doi.org/10.2353/ajpath.2007.070225
Kähäri L., Fair-Mäkelä R., Auvinen K. et al. Transcytosis route mediates rapid delivery of intact antibodies to draining lymph nodes // J. Clin. Invest. 2019. V. 129. P. 3086–3102. https://doi.org/10.1172/JCI125740
Kastenmüller W., Torabi-Parizi P., Subramanian N. et al. A spatially-organized multicellular innate immune response in lymph nodes limits systemic pathogen spread // Cell. 2012. V. 150. P. 1235–1248. https://doi.org/10.1016/j.cell.2012.07.021
Kim H., Kataru R.P., Koh G.Y. Regulation and implications of inflammatory lymphangiogenesis // Trends Immunol. 2012. V. 33(7). P. 350–356. https://doi.org/10.1016/j.it.2012.03.006
Lammermann T., Bader B.L., Monkley S.J. et al. Rapid leukocyte migration by integrin-independent flowing and squeezing // Nature. 2008. V. 453. P. 51–55. https://doi.org/10.1038/nature06887
Lee K.M., McKimmie C.S., Gilchrist D.S. et al. D6 facilitates cellular migration and fluid flow to lymph nodes by suppressing lymphatic congestion // Blood. 2011. V. 118. P. 6220–6229. https://doi.org/10.1182/blood-2011-03-344044
Leirião P., del Fresno C., Ardavín C. Monocytes as effector cells: activated Ly-6C(high) mouse monocytes migrate to the lymph nodes through the lymph and cross-present antigens to CD8+ T cells // Eur. J. Immunol. 2012. V. 42. P. 2042–2051. https://doi.org/10.1002/eji.201142166
Levick J.R., Michel C.C. Microvascular fluid exchange and the revised Starling principle // Cardiovasc. Res. 2010. V. 87. P. 198–210. https://doi.org/10.1093/cvr/cvq062
Link A., Vogt T.K., Favre S. et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells // Nat. Immunol. 2007. V. 8. P. 1255–1265. https://doi.org/10.1038/ni1513
Lobov G.I. Location and properties of the pacemaker cells of the lymphangion // Doklady Biological Sciences. 1987. V. 294(2). P. 503–506.
Louie D.A.P., Liao S. Lymph Node Subcapsular Sinus Macrophages as the Frontline of Lymphatic Immune Defense // Front. Immunol. 2019. V. 28(10). 347. https://doi.org/10.3389/fimmu.2019.00347
Low S., Hirakawa J., Hoshino H. et al. Role of MAdCAM-1-expressing high endothelial venule-like vessels in colitis induced in mice lacking sulfotransferases catalyzing l-selectin ligand biosynthesis // J. Histochem. Cytochem. 2018. V. 66. P. 415–425. https://doi.org/10.1369/0022155417753363
Lukacs-Kornek V., Malhotra D., Fletcher A.L. et al. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes // Nat. Immunol. 2011. V. 12. P. 1096–1104. https://doi.org/10.1038/ni.2112
Ma Q., Dieterich L.C., Detmar M. Multiple roles of lymphatic vessels in tumor progression // Curr. Opin. Immunol. 2018. V. 53. P. 7–12. https://doi.org/10.1016/j.coi.2018.03.018
Maddaluno L., Verbrugge S.E., Martinoli C. et al. The adhesion molecule L1 regulates transendothelial migration and trafficking of dendritic cells // J. Exp. Med. 2009. V. 206. P. 623–635. https://doi.org/10.1084/jem.20081211
Malhotra D., Fletcher A.L., Turley S.J. Stromal and hematopoietic cells in secondary lymphoid organs: partners in immunity // Immunol. Rev. 2013. V. 251. P. 160–176. https://doi.org/10.1111/imr.12023
Martens J.H., Kzhyshkowska J., Falkowski-Hansen M. et al. Differential expression of a gene signature for scavenger/lectin receptors by endothelial cells and macrophages in human lymph node sinuses, the primary sites of regional metastasis // J. Pathol. 2006. V. 208. P. 574–589. https://doi.org/10.1002/path.1921
Mazzone M., Bergers G. Regulation of blood and lymphatic vessels by immune cells in tumors and metastasis // Ann. Rev. Physiol. 2019. V. 81. P. 535–560. https://doi.org/10.1146/annurev-physiol-020518-114721
Michel C.C., Nanjee M.N., Olszewski W.L., Miller N.E. LDL and HDL transfer rates across peripheral microvascular endothelium agree with those predicted for passive ultrafiltration in humans // J. Lipid Res. 2015. V. 56. P. 122–128. https://doi.org/10.1194/jlr.M055053
Miteva D.O., Rutkowski J.M., Dixon J.B. et al. Transmural flow modulates cell and fluid transport functions of lymphatic endothelium // Circ. Res. 2010. V. 106. P. 920–931. https://doi.org/10.1161/CIRCRESAHA.109.207274
Mehta D., Malik A.B. Signaling mechanisms regulating endothelial permeability // Physiol. Rev. 2006. V. 86(1). P. 279–367. https://doi.org/10.1152/physrev.00012.2005
Moseman E.A., Iannacone M., Bosurgi L. et al. B cell maintenance of subcapsular sinus macrophages protects against a fatal viral infection independent of adaptive immunity // Immunity. 2012. V. 36. P. 415–426. https://doi.org/10.1016/j.immuni.2012.01.013
Nitschké M., Aebischer D., Abadier M. et al. Differential requirement for ROCK in dendritic cell migration within lymphatic capillaries in steady-state and inflammation // Blood. 2012. V. 120(11). P. 2249–2258. https://doi.org/10.1182/blood-2012-03-417923
Ohtani O., Ohtani Y. Structure and function of rat lymph nodes // Arch. Histol. Cytol. 2008. V. 71(2). P. 69–76. https://doi.org/10.1679/aohc.71.6
Palframan R.T., Jung S., Cheng G. et al. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues // J. Exp. Med. 2001. V. 194 P. 1361–1373. https://doi.org/10.1084/jem.194.9.1361
Permanyer M., Bošnjak B., Förster R. Dendritic cells, T cells and lymphatics: dialogues in migration and beyond // Curr. Opin. Immunol. 2018. V. 53. P. 173–179. https://doi.org/10.1016/j.coi.2018.05.004
Pflicke H., Sixt M. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels // J. Exp. Med. 2009. V. 206. P. 2925–2935. https://doi.org/10.1084/jem.20091739
Poirot J., Medvedovic J., Trichot C., Soumelis V. Compartmentalized multicellular crosstalk in lymph nodes coordinates the generation of potent cellular and humoral immune responses // Eur. J. Immunol. 2021. V. 51(12). P. 3146–3160. https://doi.org/10.1002/eji.202048977
Quast T., Zölzer K., Guu D. et al. A Stable Chemokine Gradient Controls Directional Persistence of Migrating Dendritic Cells // Front. Cell Dev. Biol. 2022. V. 10. 943041. https://doi.org/10.3389/fcell.2022.943041
Randolph G.J., Bala S., Rahier J.F. et al. Lymphoid aggregates remodel lymphatic collecting vessels that serve mesenteric lymph nodes in Crohn disease // Am. J. Pathol. 2016. V. 186(12). P. 3066–3073. https://doi.org/10.1016/j.ajpath.2016.07.026
Reed R.K., Rubin K. Transcapillary exchange: role and importance of the interstitial fluid pressure and the extracellular matrix // Cardiovasc. Res. 2010. V. 87(2). P. 211–217. https://doi.org/10.1093/cvr/cvq143
Roozendaal R., Mempel T.R., Pitcher L.A. et al. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles // Immunity. 2009. V. 30. P. 264–276. https://doi.org/10.1016/j.immuni.2008.12.014
Rouhani S.J., Eccles J.D., Riccardi P. et al. Roles of lymphatic endothelial cells expressing peripheral tissue antigens in CD4 T-cell tolerance induction // Nat. Commun. 2015. V. 6. 6771. https://doi.org/10.1038/ncomms7771
Russo E., Nitschké M., Halin C. Dendritic cell interactions with lymphatic endothelium // Lymphat. Res. Biol. 2013. V. 11(3). P. 172–82. https://doi.org/10.1089/lrb.2013.0008
Russo E., Teijeira A., Vaahtomeri K. et al. Intralymphatic CCL21 promotes tissue egress of dendritic cells through afferent lymphatic vessels // Cell Rep. 2016. V. 14. P. 1723–1734. https://doi.org/10.1016/j.celrep.2016.01.048
Sagris M., Theofilis P., Antonopoulos A.S. et al. Inflammation in Coronary Microvascular Dysfunction // Int. J. Mol. Sci. 2021. V. 22(24). 13471. https://doi.org/10.3390/ijms222413471
Santambrogio L. The Lymphatic Fluid // Int. Rev. Cell Mol. Biol. 2018. V. 337. P. 111–133. https://doi.org/10.1016/bs.ircmb.2017.12.002
Santambrogio L., Berendam S.J., Engelhard V.H. The Antigen Processing and Presentation Machinery in Lymphatic Endothelial Cells // Front. Immunol. 2019. V. 10. 1033. https://doi.org/10.3389/fimmu.2019.01033
Saxena V., Li L., Paluskievicz C., Kasinath V. et al. Role of lymph node stroma and microenvironment in T cell tolerance // Immunol. Rev. 2019. V. 292(1). P. 9–23. https://doi.org/10.1111/imr.12799
Schineis P., Runge P., Halin C. Cellular traffic through afferent lymphatic vessels // Vascul. Pharmacol. 2019. V. 112. P. 31–41. https://doi.org/10.1016/j.vph.2018.08.001
Schmid-Schönbein G.W. Microlymphatics and lymph flow // Physiol. Rev. 1990. V. 70(4). P. 987–1028. https://doi.org/10.1152/physrev.1990.70.4.987
Schwab S.R., Cyster J.G. Finding a way out: lymphocyte egress from lymphoid organs // Nat. Immunol. 2007. V. 8(12). P. 1295–1301. https://doi.org/10.1038/ni1545
Schwager S., Detmar M. Inflammation and Lymphatic Function //Front. Immunol. 2019. V. 10. 308. https://doi.org/10.3389/fimmu.2019.00308
Shields J.D., Fleury M.E., Yong C. et al. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling // Cancer Cell. 2007. V. 11. P. 526–538. https://doi.org/10.1016/j.ccr.2007.04.020
Stewart R.H. A Modern View of the Interstitial Space in Health and Disease // Front. Vet. Sci. 2020. V. 7. 609 583. https://doi.org/10.3389/fvets.2020.609583
Sura R., Colombel J.F., Van Kruiningen H.J. Lymphatics, tertiary lymphoid organs and the granulomas of Crohn’s disease: an immunohistochemical study // Aliment. Pharmacol. Ther. 2011. V. 33(8). P. 930–939. https://doi.org/10.1111/j.1365-2036.2011.04605.x
Swartz M.A., Fleury M.E. Interstitial Flow and Its Effects in Soft Tissues // Annu. Rev. Biomed. Eng. 2007. V. 9. P. 229–256. https://doi.org/10.1146/annurev.bioeng.9.060906.151850
Ta O., Lim H.Y., Gurevich I. et al. DC mobilization from the skin requires docking to immobilized CCL21 on lymphatic endothelium and intralymphatic crawling // J. Exp.Med. 2011. V. 208. P. 2141–2153. https://doi.org/10.1084/jem.20102392
Talsma D.T., Katta K., Boersema M. et al. Increased migration of antigen presenting cells to newly-formed lymphatic vessels in transplanted kidneys by glycol-split heparin // PLoS One. 2017. V. 12(6). e0180206. https://doi.org/10.1371/journal.pone.0180206
Tamburini B.A., Burchill M.A., Kedl R.M. Antigen capture and archiving by lymphatic endothelial cells following vaccination or viral infection // Nat. Commun. 2014. V. 5. 3989. https://doi.org/10.1038/ncomms4989
Tecchio C., Micheletti A., Cassatella M.A. Neutrophil-derived cytokines: facts beyond expression // Front. Immunol. 2014. V. 5. 508. https://doi.org/10.3389/fimmu.2014.00508
Teijeira A., Palazon A., Garasa S. et al. CD137 on inflamed lymphatic endothelial cells enhances CCL21-guided migration of dendritic cells // FASEB J. 2012. V. 26. P. 3380–3392. https://doi.org/10.1096/fj.11-201061
Teijeira A., Hunter M.C., Russo E. et al. T cell migration from inflamed skin to draining lymph nodes requires intralymphatic crawling supported by ICAM-1/LFA-1 interactions // Cell Rep. 2017. V. 18. P. 857–865. https://doi.org/10.1016/j.celrep.2016.12.078
Theocharis A.D., Manou D., Karamanos N.K. The extracellular matrix as a multitasking player in disease // FEBS J. 2019. V. 286(15). P. 2830–2869. https://doi.org/10.1111/febs.14818
Thomson C.A., van de Pavert S.A., Stakenborg M. et al. Expression of the atypical chemokine receptor ACKR4 identifies a novel population of intestinal submucosal fibroblasts that preferentially expresses endothelial cell regulators // J. Immunol. 2018. V. 201. P. 215–229. https://doi.org/10.4049/jimmunol.1700967
Tomura M., Honda T., Tanizaki H. et al. Activated regulatory T cells are the major T cell type emigrating from the skin during a cutaneous immune response in mice // J. Clin. Invest. 2010. V. 120. P. 883–93. https://doi.org/10.1172/JCI40926
Triacca V., Guc E., Kilarski W.W., Pisano M., Swartz M.A. Transcellular pathways in lymphatic endothelial cells regulate changes in solute transport by fluid stress // Circ. Res. 2017. V. 120. P. 1440–1452. https://doi.org/10.1161/CIRCRESAHA.116.309828
Ueno H., Klechevsky E., Morita R. et al. Dendritic cell subsets in health and disease // Immunol Rev. 2007. V. 219. P. 118–142. https://doi.org/10.1111/j.1600-065X.2007.00551.x
Ulvmar M.H., Mäkinen T. Heterogeneity in the lymphatic vascular system and its origin // Cardiovasc. Res. 2016. V. 111(4). P. 310–321. https://doi.org/10.1093/cvr/cvw175
Vigl B., Aebischer D., Nitschke M., Iolyeva M. et al. Tissue inflammation modulates gene expression of lymphatic endothelial cells and dendritic cell migration in a stimulus-dependent manner // Blood. 2011. V. 118. P. 205–215. https://doi.org/10.1182/blood-2010-12-326447
Weber M., Hauschild R., Schwarz J. et al. Interstitial dendritic cell guidance by haptotactic chemokine gradients // Science. 2013. V. 339(6117). P. 328–332. https://doi.org/10.1126/science.1228456
Wiig H., Swartz M.A. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer // Physiol. Rev. 2012. V. 92(3). P. 1005–1060. https://doi.org/10.1152/physrev.00037.201
Xiang M., Grosso R.A, Takeda A. et al. A single-cell transcriptional roadmap of the mouse and human lymph node lymphatic vasculature // Front. Cardiovasc. Med. 2020. V. 7. 52. https://doi.org/10.3389/fcvm.2020.00052
Xu H., Guan H., Zu G., Bullard D. et al. The role of ICAM-1 molecule in the migration of Langerhans cells in the skin and regional lymph node // Eur. J. Immunol. 2001. V. 31. P. 3085–3093. https://doi.org/10.1002/1521-4141(2001010)31:10<3085::aid-immu3085>3.0.co;2-b
Yan Y., Zhang G.X., Gran B., Fallarino F. et al. IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis // J. Immunol. 2010. V. 185(10). P. 5953–5961. https://doi.org/10.4049/jimmunol.1001628
Yanagawa Y., Onoe K. CCR7 ligands induce rapid endocytosis in mature dendritic cells with concomitant up-regulation of Cdc42 and Rac activities // Blood. 2003. V. 101. P. 4923–4929. https://doi.org/10.1182/blood-2002-11-3474
Yawalkar N., Hunger R.E., Pichler W.J. et al. Human afferent lymph from normal skin contains an increased number of mainly memory / effector CD4(+) T cells expressing activation, adhesion and co-stimulatory molecules // Eur. J. Immunol. 2000. V. 30. P. 491–497. https://doi.org/10.1002/1521-4141(200002)30:2<491::AID-IMMU491>3.0.CO;2-H
Zawieja D.C., Thangaswamy S., Wang W. et al. Lymphatic Cannulation for Lymph Sampling and Molecular Delivery // J. Immunol. 2019. V. 203(8). P. 2339–2350. https://doi.org/10.4049/jimmunol.1900375
Zhu J., Yamane H., Paul W.E. Differentiation of effector CD4 T cell populations // Annu. Rev. Immunol. 2010. V. 28. P. 445–489. https://doi.org/10.1146/annurev-immunol-030409-101212
Дополнительные материалы отсутствуют.
Инструменты
Успехи физиологических наук


