Зоологический журнал, 2019, T. 98, № 10, стр. 1186-1202
Identification of subfossil mammal fur from ancient eskimo settlements of Chukotka
O. F. Chernova a, *, D. D. Vasyukov a, **, A. B. Savinetsky a, ***
a Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences
119071 Moscow, Russia
* E-mail: chernova@sevin.ru
** E-mail: tipa2128506@gmail.com
*** E-mail: arkadybs@rambler.ru
Поступила в редакцию 20.02.2019
После доработки 12.03.2019
Принята к публикации 3.04.2019
Аннотация
We analyzed 19 samples of mammal hair from two ancient Eskimo settlements of the Chukotka Peninsula (Russia) using light microscopy and SEM. They belonged at least to five well-identified mammal species typical of the Chukotka fauna: Lepus timidus, Pusa hispida, Rangifer tarandus, Ursus maritimus, and Canis lupus familiaris. Hairs of L. timidus and C. l. familiaris were the most abundant. We demonstrate here that mammalian hairs recovered from archaeological sites are identifiable and can be integrated into zooarchaeological studies. This study included a comparison of ancient dog hairs with those of modern Chukchi dogs and wolves. The architectonics of both ancient and modern dogs reflects their use as sled dogs and the adaptation to the harsh environment.
Subfossil mammal fur is of considerable archaeological interest, as it can answer numerous questions about the habitat and economic activities of different peoples in antiquity (Appleyard, Wildman, 1969; Rowe, 2010). Due to its keratin nature, the hairs are well preserved in tombs, solid layers of soil, and permafrost. In addition, the architectonics (fine structure) of fur is species specific. Thus it is possible to identify subfossil hairs using scanning electron microscopy (SEM).
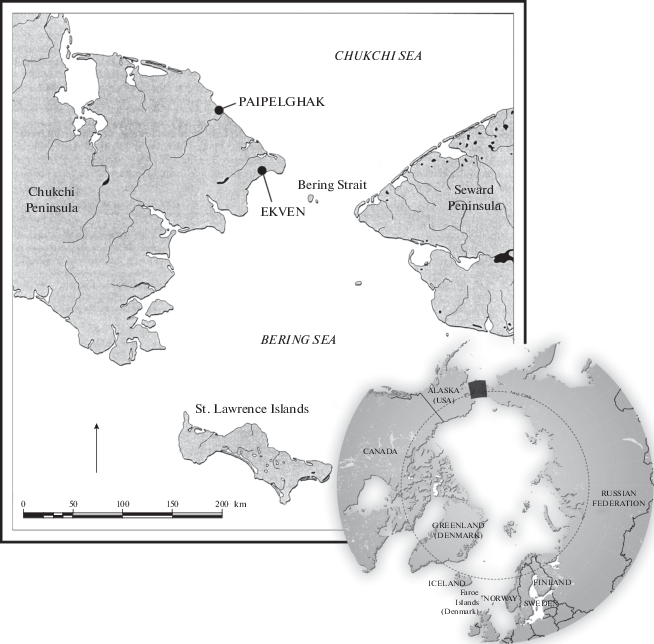
The samples of mammal hairs in this study come from two ancient Eskimo settlements, located on the northeast coast of the Chukotka Peninsula–Ekven and Paipelghak (fig. 1). For more than one thousand years, people lived in both settlements (Dinesman et al., 1999). According to the zooarchaeological analyses, they had domestic dogs for transportation and food and hunted sea mammals (seals and whales), but also hunted terrestrial species, like hare, reindeer and foxes.
Identification of mammalian hairs, in particular Arctic species, have been the subject of numerous investigations; thus the sizes, colors, and microstructures of the hairs are known for most of the species, see for example Alaska fur ID project 2009, among others (Scheffer, 1964; Keller, 1980, 1981, 1984; Kennedy, 1982; Wallis, 1993; Wolf, Long, 1997). Most of these studies used light microscopy or histology on numerous undamaged hairs of modern animals, although there are a few studies that used scanning electron microscopy (Verhoeven, 1972; Kisin, Golovin, 1992). Archaeological samples of hairs are very rare, damaged, and fragmented; it is difficult to measure and identify them, much less understand their inner structure on the light optical level, which is why we use scanning electron microscopy (SEM) to analyze the subfossil hairs.
MATERIALS AND METHODS
One sample comes from House-18 of Ekven site (table 1) that dates to cal AD 390–860 (Dneprovsky, 2001; Savinetsky, 2002). Another 18 samples come from Paipelghak settlement, which is radiocarbon dated cal 100 BC–AD 1600 (Vasyukov, 2011). However, not all samples were associated with dated layers.
Table 1.
Mammal species identified by the fossil hairs found in ancient Eskimo settlements in Chukotka
| Sample no | Site | Feature | Provenience | Species identified |
|---|---|---|---|---|
| 200 | Ekven | House-18 | Square 100/98 | Canis lupus familiaris |
| 201 | Paipelghak | House-1 | On the edge of A and V squares | C. l. familiaris |
| 202 | Paipelghak | House-1 | Square Z; Level 2 | Ursus maritimus |
| 203 | Paipelghak | House-1 | Square Z; Level 2 | Lepus timidus |
| 204 | Paipelghak | House-1 | Square Z; Level 4 | C. l. familiaris |
| 205 | Paipelghak | House-1 | Square A (under the 2nd floor level of Room 4) | L. timidus (hairs from a foot) |
| 206 | Paipelghak | House-1 | Square ZH; Level 13 | L. timidus + C. l. familiaris |
| 207 | Paipelghak | House-1 | Square A (under the 2nd floor level of Room 4) | L. timidus + C. l. familiaris |
| 208 | Paipelghak | House-1 | Square ZH; Level 11 (meat pit) | L. timidus + Rangifer tarandus |
| 209 | Paipelghak | House-1 | Square Z; Level 5 | C. l. familiaris |
| 210 | Paipelghak | House-1 | Square ZH; Levels 12-13 | L. timidus |
| 211 | Paipelghak | House-1 | Square D.east; Level 18 | C. l. familiaris + Pusa hispida (?) |
| 212 | Paipelghak | House-1 | Square A (under the 3rd floor level of Room 4) | L. timidus |
| 213 | Paipelghak | House-1 | Square A; Level 6 | L. timidus + R. tarandus + + C. l. familiaris |
| 214 | Paipelghak | House-1 | Square A (outside the Room 4) | L. timidus |
| 215 | Paipelghak | House-1 | Square Z; Level 1 | L. timidus |
| 216 | Paipelghak | House-1 | Square A; Level 3 | L. timidus |
| 217 | Paipelghak | House-1 | Square K; Level 3; Accumulation 2 | P. hispida (?) |
| 218 | Paipelghak | House-1 | Square ZH; Levels 9-10, burial | C. l. familiaris |
We used the hairs of modern species for comparison: modern dogs from Chukotka, wolves from Chukotka, and several seal species, collected in Kamchatka (table 2). The samples of fur from different dogs were collected in summer; they were taken from the withers of adult sled dogs–three male Chukotka Sled Dogs and five male crossbreeds, all collected in Chukotka. Chukotka Sled Dog is a local breed used for sledding; all the information about sampled dogs comes from their masters and no specific analyses were carried out to identify the breed affiliation. To compare modern and ancient dog fur, we examined the winter fur of the Tundra Wolf (Canis lupus albus Kerr 1792, hereinafter Wolf): four adult females and two adult males from Chukotka near Innymney mtn. (66.17° N, 172.92° W). The autumn seal fur samples were collected in western Kamchatka on the Ptichiy Island (57.17° N, 156.54° E) and in the Russian Far East on the Chkalov Island (53.40° N, 141.26° E) and consisted of Bearded Seal (Erignathus barbatus Erxleben 1777), Harbour Seal (Phoca vitulina L. 1758), and Ringed Seal (Pusa hispida Schreber 1775) adult males (table 2). We also used the database of the mammal hair architectonics that we previously developed (Chernova, Tselikova, 2004; Chernova et al., 2011).
Table 2.
Modern hair samples of mammal species of interest, chosen for the comparison
| Sample no | Species | Locality | Sampling date | Sex | Age | Collector |
|---|---|---|---|---|---|---|
| 1 | Chukchi Sled Dog (Canis l. familiaris) | Anadyr, Chukotka, Russia | 3 Jul. 2010 | Male | 1 yr. | Vasyukov D.D. |
| 2 | Crossbreed dog (Canis l. familiaris) |
Anadyr, Chukotka, Russia | 3 Jul. 2010 | Male | 1.5 yr. | Vasyukov D.D. |
| 3 | Crossbreed dog (Canis l. familiaris) |
Anadyr, Chukotka, Russia | 3 Jul. 2010 | Male | 1 yr. | Vasyukov D.D. |
| 14 | Chukchi Sled Dog (Canis l. familiaris) | Anadyr, Chukotka, Russia | 3 Jul. 2010 | Male | 11 yrs. | Vasyukov D.D. |
| 17 | Chukchi Sled Dog (Canis l. familiaris) | Anadyr, Chukotka, Russia | 3 Jul. 2010 | Male | 1 yr. | Vasyukov D.D. |
| 29 | Crossbreed dog (Canis l. familiaris) |
Lavrentiya, Chukotka, Russia | 5 Jul. 2010 | Male | 2 yrs. | Vasyukov D.D. |
| 41 | Crossbreed dog (Canis l. familiaris) |
Lavrentiya, Chukotka, Russia | 5 Jul. 2010 | Male | 2–7 yrs. | Vasyukov D.D. |
| 42 | Crossbreed dog (Canis l. familiaris) |
Lavrentiya, Chukotka, Russia | 5 Jul. 2010 | Male | 2–7 yrs. | Vasyukov D.D. |
| 303 | Wolf (Canis l. albus) | Chukotka, Russia | NA | Male | ad | Eyneucheyvun V.D. |
| 304 | Wolf (Canis l. albus) | Innymney mtn., Chukotka, Russia | Feb. 2014 | female | 1 yr. | Eyneucheyvun V.D. |
| 305 | Wolf (Canis l. albus) | Innymney mtn., Chukotka, Russia | Winter 2014 | Male | ad | Eyneucheyvun V.D. |
| 306 | Wolf (Canis l. albus) | Innymney mtn., Chukotka, Russia | Winter 2014 | female | 1.5 yrs | Eyneucheyvun V.D. |
| 307 | Wolf (Canis l. albus) | Innymney mtn., Chukotka, Russia | Dec. 2014 | female | 2 yrs. | Eyneucheyvun V.D. |
| 308 | Wolf (Canis l. albus) | Innymney mtn., Chukotka, Russia | Dec. 2014 | female | 2 yrs. | Eyneucheyvun V.D. |
| 309 | Ringed seal (Pusa hispida) |
West Kamchatka, the Ptichiy Island and the Chkalov Island | Autumn | Male | ad | NA* |
| 310 | Harbor seal (Phoca vitulina) |
West Kamchatka, the Ptichiy Island and the Chkalov Island | Autumn | Male | ad | NA |
| 311 | Bearded seal (Erignathus barbatus) | West Kamchatka, the Ptichiy Island and the Chkalov Island | Autumn | Male | ad | NA |
The hairs of each sample were separated into categories and orders under a binocular microscope – overhairs (lead), guard hairs of several orders, and downy (wool, underhairs) hair. This distinction was made by the thickness and configuration of the hair shafts. The hair and medullar thickness were measured under an “Amplival” light microscope (VEB Carl Zeiss, Jena) and Leica DMLS microscope with a digital video camera (Germany) using a ×10 eyepiece and ×10, ×40, and ×63 lenses, and a motorize microscope Keyence Biorevo BZ-9000 (Japan). The biggest of the lead and guard hairs were studied under JSM 840A (Japan) and TESCAN (Czech Republic) scanning electron microscopes. For the SEM, the hairs were washed and degreased in shampoo, then washed in distilled water, and dehydrated in alcohol of increasing concentrations. Longitudinal and transversal (cross) sections were cut using a sharp razor blade and fixed on stubs with clear nail polish. The samples were coated in an Edwards S-150 A (UK) gold sputter, then viewed and photographed in the accelerating voltage of 15 kV. Electronic graphs made from longitudinal and cross sections of the base and the mid-length of the shaft, and from the cuticle surface along the shaft from the base to the mid-length of the shaft and to the top.
The study was conducted using equipment of the Joint Usage Centre “Instrumental methods in ecology” at the IPEE RAS. The measurement scheme is shown on fig. 2. Some absolute measurements are obtained in the program ‘TESCAN ATLAS’. All statistics and plotting were carried out in R v. 3.5.2 (R Core Team, 2018). Data for PCA analysis were scaled before the analysis.
Fig. 2.
Scheme of the main hair measurements: Dmax –maximum diameter of the hair; Dmin – minimal diameter of the hair; С – maximum diameter of medullar cavity; D – diameter of a shaft base; H – maximum height of a cuticular scale; Me – maximum diameter of a medullar canal in a hair granna.
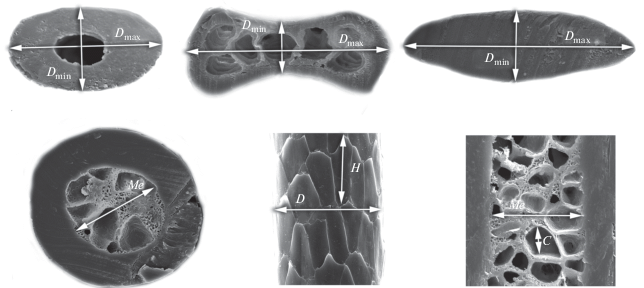
We chose the following patterns and indexes to measure hair structures:
(i) Shaft configuration at the widest part (hereafter ‘granna’), based on the form of the cross sections of the shaft – its maximum diameter relative to the minimal one, multiplied by 10;
(ii) Index of cuticle in a shaft base and in a granna – ratio of the maximum height of the cuticle scale along a shaft to the thickness of the shaft at the same location, multiplied by 100.
(iii) Index of medulla in a granna – ratio of the maximum width of medulla to the width of the shaft at the same location, multiplied by 100.
(iiii) Configuration of a shaft along the hair – ratio of a granna width to the width of a hair base, multiplied by 10.
RESULTS AND DISCUSSION
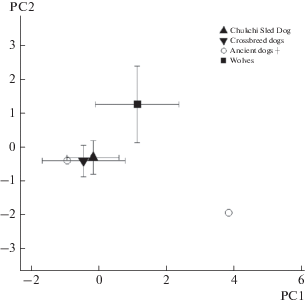
Twenty five individuals, identified in 19 archaeological samples, belonged to five mammal species (table 1). Using hair architectonics (thin structure of cuticle and medulla), we identified subfossil hairs typical of modern Chukotka fauna, such as the mountain hare, ringed seal, polar bear, reindeer, and domestic dog, the latter was part of our prior research (Chernova et al., 2016). Hairs of mountain hare and domestic dog were most numerous; polar bear, reindeer, and seals were represented by single hairs. Result of PCA analysis shows good differentiation of the species in the space of first and second principal components (fig. 3).
Fig. 3.
Ancient and modern hair samples SEM measurements (table 4) in the space of two first principal components. Points are placed in the mean PC values, bars show standard deviations.
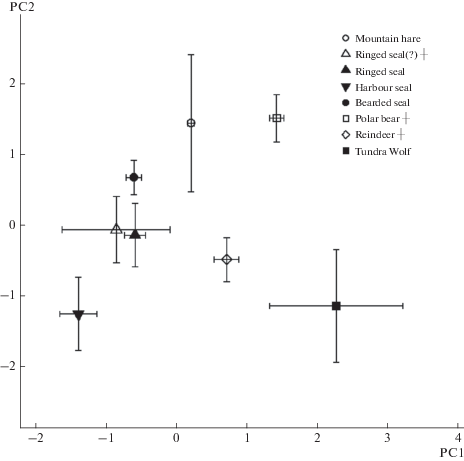
Table 3.
The averaged light microscopy and SEM metric data of the guard hairs of studied modern dogs, fossil dogs and wolves of Chukotka (MED ± MAD)*
| Species or breed | N/n | Dmax [μm] | Me [μm] | C [μm] | Me × 100/Dmax | C × 100/Me | H × 100/Dmax |
|---|---|---|---|---|---|---|---|
| Chukchi Sled Dog | 3/30 | 110.0 ± 11.1 | 52.9 ± 11.5 | 15.1 ± 4.8 | 50.0 ± 7.4 | 28.5 ± 6.7 | 13.0 ± 5.2 |
| Crossbreed dogs | 7/70 | 98.0 ± 17.8 | 44.0 ± 14.8 | 19.1 ± 9.8 | 50.0 ± 8.9 | 44.5 ± 18.5 | 12.5 ± 3.7 |
| Ancient dogs (№№ 213, 218) |
2/20 | 96.5 ± 39.3; 70 ± 14.8 | 42.4 ± 11.0; NA | NA; NA | 38 ± 11.9; NA | NA; NA | 14 ± 7.2; 41 ± 17.1 |
| Tundra Wolves | 6/30 | 101 ± 20.1 | 56 ± 14.5 | 23 ± 8.5 | 57.0 ± 12.5 | 50.0 ± 13.8 | 51.0 ± 19.4 |
* Median ± Median Absolute Deviation; N – the number of specimens; n – the number of tested hairs; Dmax – maximum diameter of a hair (in μm); Me – maximum diameter of medulla canal in the hair grana (in μm); С – maximum diameter of medullar cavity (in μm); H – maximum height of cuticular scale. Find the scheme of the main measurements in fig. 2, and the sample details – in tables 1, 2.
Table 4.
The relative averaged SEM data of mammal hairs identified in ancient Eskimo settlements in Chukotka in comparison to the modern representatives of the species (MED ± MAD)*
| Species | N/n | Dmax × 10/Dmin | H × 100/d | H × 100/Dmax | Me/Dmax × 100 | Dmax × 10/d |
|---|---|---|---|---|---|---|
| Lepus timidus (№ 203) † | 1/3 | 35.0 ± 5.9 | 47.0 ± 7.4 | 11.0 ± 2.9 | 77 ± NA | 28.0 ± 7.4 |
| Pusa hispida (?) (№№ 211, 217) † |
2/9 | 32.0 ± 10.4 | 14.0 ± 3.0 | 6.0 ± NA | NA | 22.0 ± 5.9 |
| Pusa hispida | 1/4 | 36.5 ± 3.0 | 19.0 ±3.0 | 7.5 ± 0.7 | NA | 28.0 ± 6.7 |
| Phoca vitulina | 1/3 | 38 ± NA | 15.0 ± 1.5 | 6.0 ± 1.5 | NA | 42.0 ± 3.0 |
| Erignathus barbatus | 1/3 | 44.0 ± 1.5 | 20.0 ± 4.5 | 6.0 ± 1.5 | NA | 15.0 ± 1.5 |
| Ursus maritimus (№ 202) † | 1/3 | 16.0 ± NA | 40.0 ± 3.0 | 13.0 ± 1.5 | 33.0 ± NA | 17.0 ± 3.0 |
| Rangifer tarandus (№ 208) † | 1/3 | 12.0 ± NA | 20.0 ± NA | 12.0 ± NA | 80.0 ± NA | 26.0 ± 1.5 |
| C. lupus albus | 1/4 | 14.0 ± 3.7 | 17.0 ± 3.7 | 40.5 ± 16.3 | 59.0 ± 10.4 | 17.5 ± 1.5 |
* Median ± Median Absolute Deviation; † – archaeological samples; N – the number of specimens; n – the number of tested hairs; Dmax – maximum diameter of the hair; Dmin – minimal diameter of the hair; d – diameter of a shaft base; Me – maximum diameter of medulla canal in the hair grana; H – maximum height of cuticular scale. Find the scheme of the main measurements in fig. 2, and the sample details – in tables 1, 2.
Mountain Hare – Lepus timidus L. 1758. Identification of this species is not difficult, because big white guard hairs (up to 90 μm) of hares have a characteristic dumb-bell configuration in cross section (figs 4a; 5a). The medium part of the shaft is very wide and flattened, with a deep groove along its ventral and dorsal sides, creating the dumb-bell configuration. The medulla is well developed and contains large rectangular cavities with rounded corners. The cavities are aligned in several rows along the shaft, forming multi-serial ladder or columnar medulla that is typical for hares (fig. 4c). The cuticular pattern significantly changes along the shaft (fig. 4a). A chevron pattern occurs above the hair base, which is formed by elongated longitudinal scales. This pattern is typical for hares (fig. 4a, first image from left). The cuticle becomes flattened semicircular when higher on the shaft and the scale does not envelope the shaft (fig. 4a).
Fig. 4.
Cuticular pattern along the shaft from base to granna (look from left to right) (a, b) and medullar architectonics (c) of the ancient guard hairs of the Polar Hare, Lepus timidus: (a) – sample № 214 (see table 1), Guard I of the trunk, the longitudinal groove is shown by arrow; (b) – sample № 205, Guard II of a foot sole; a restriction and a rotate of the shaft, and also a longitudinal groove are shown by arrows; (c) – sample № 214, the multi-serial ladder (columnar) medulla of Guard I of the trunk; medullar cavities are shown by arrows. SEM. Scale bar 10 μm.
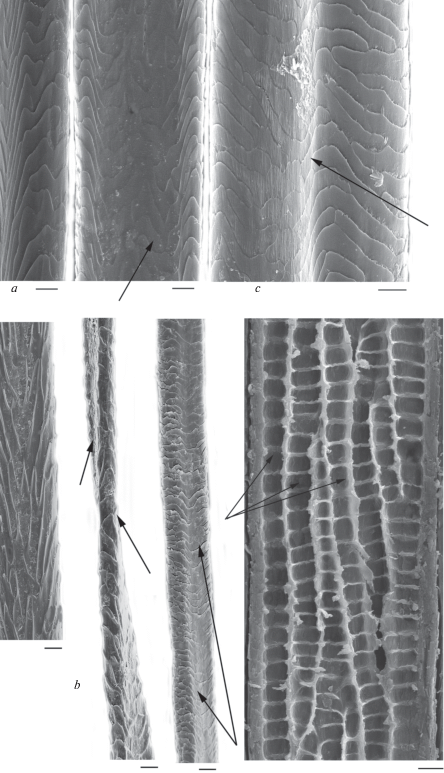
Some samples contained the remains of hairy foot soles with pads and nails of hares. The numerous big white hairs of mountain hare foot soles (and possibly other leporine species) have a specific structure, which differs from trunk hairs (figs 4b; 5b). The shaft of this hair also has the typical dumb-bell configuration on cross section, but the grooves are wider and deeper compared to trunk hairs and its lateral edges have protrusions that look like hooks. Probably, these hooks serve as a clutch for neighbor hairs, forming a dense fur layer that protects the hare’s pads and fingers from snow and ice. These hairs do not contain medulla and consist of dense cortex that significantly improves their mechanical properties. The cuticle of the biggest guard hairs (Guard I) is similar to trunk hairs including the chevron pattern (fig. 4b, first photo from the left). Smaller guard hairs (Guard II) have the characteristic restrictions and rotations of a shaft before its granna and the beginning of a groove (fig. 4b, second photo from left).
True Seals – Phocidae Gray 1821. Hairs of seals have a specific configuration and microstructure that is easily differentiated from the hairs of other species (Scheffer, 1964; Chernova, Tselikova, 2004). Their guard hairs are short, thick, flattened, and look like a javelin or lancet. Ventral and dorsal sides of the shaft are flattened and lateral edges are rounded creating cross sections with an elongated ellipsoid configuration, typical of semi-aquatic and underground mammals. An absence of medulla is a distinctive feature of the true seals (Phocidae) hairs and is used to differentiate them from the hairs of the eared seals (Otariidae) hairs. A cuticle with hastate pattern (or ‘pineal’ or ‘diamond petal’ or ‘cuticle of carnivores’) occurs above the base of the shaft in hairs of both families.
Fig. 5.
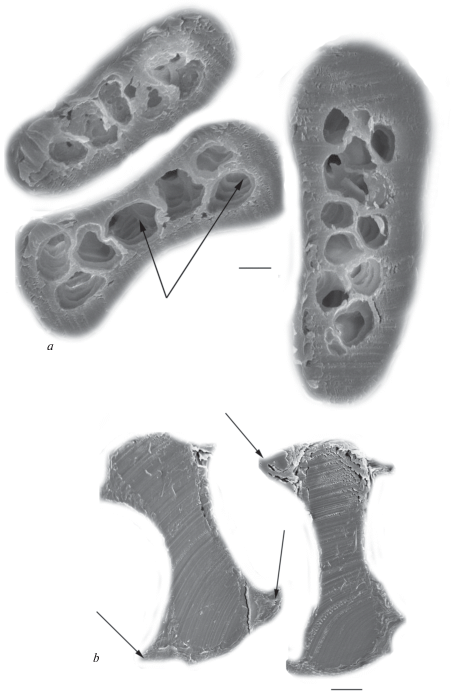
Cross sections of ancient Guard I of the trunk (a) and the sole of a foot (b) of the Polar Hare, Lepus timidus: (a) – sample № 214 (see table 1); medullar cavities are shown by arrows; (b) – sample № 205; the bent ends are shown by arrows. SEM. Scale bar 10 μm.
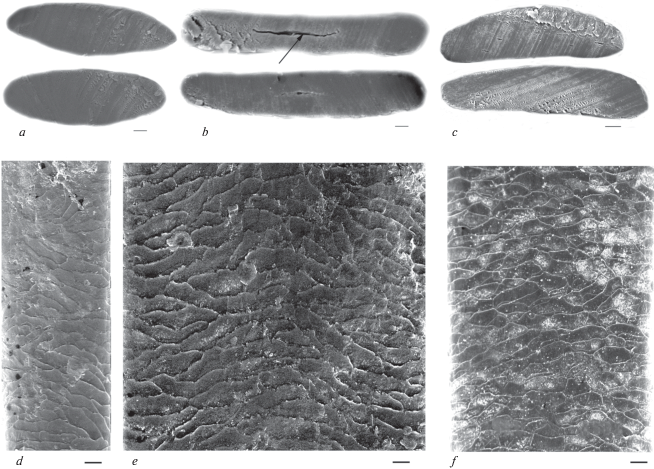
In the two tested samples (№№ 211, 217, see table 1), we found two hairs with characteristic lancet configuration. Their length is not more than 13 mm and width is 113 ± 11 and 226 ± 6 μm, respectively. The shaft configuration as well as the absent medulla indicate they are hairs of Phocidae. The cuticle is semi-circular and flattened; it forms a wavy pattern and consists of narrow and flattened scales with weakly wavy apical edges, which are not serrated like the cuticle of terrestrial mammals (figs 6d; 6e). In both specimens, the cross section configuration and absence of the specific hastate cuticle indicate the hairs belonged to a ringed seal, which is also proved by PCA analysis (fig. 3). Differences in the shaft configuration (compare fig. 6a and fig. 6b) of the two samples may be explained due to topographic differences; presence of the medullar slit (fig. 6b), probably, is the result of mechanical damage.
Fig. 6.
Cross sections of a granna (a–c) and cuticular pattern (d–f) of ancient guard hairs of the Ringed Seal, Pusa hispida: (a) – sample № 211 (see table 1); (b, d, e) – the same of sample № 217; (c, f) – a sample of the recent Ringed Seal; (a) – a base of the shaft; (e, f) – a granna. A central slit is shown by arrow. SEM. Scale bar 10 μm.
Polar Bear – Ursus maritimus Phipps 1774. Only sample № 202 (table 1) contained hairs of polar bear. Polar bear hairs were thick (up to 135 ± 11 μm) and thin (50–113 μm), with a length not longer than 44 mm. Big white hairs have long and thin base-stem, which gradually becomes thicker and slightly flattened. The hair contains weakly developed medulla (up to 20% of the shaft width); in the upper part, it has thick granna also with medulla (up to 40%) with large cavities (fig. 7a). The fine structure of medulla was not preserved. The cuticle of base and granna differs from each other: the scales become elongated above granna and cuticle pattern resembles that of the diamond petal ornamentation (fig. 7b).
Fig. 7.
Cross sections of the shaft (a) and cuticular pattern (b) (from a base to a middle of the shaft, look from left to right) of the ancient guard hair of the Polar Bear, Ursus maritimus: (a) – sample № 202 (see table 1); (b) – damaged medulla is shown by arrows. SEM. Scale bar 10 μm.
Reindeer – Rangifer tarandus L. 1758. Two samples (№№ 208, 213, table 1) contained small fragments of reindeer hairs (their thicknesses were 68, 56, 34 and 17 μm). They were identified using both light microscopy and SEM according to the following features: the shafts are cylindrical (round cross-sections), with a narrow base without any medulla; the base gradually becomes thickened granna with thick medulla, which occupies up to 86% of the shaft and contains large cavities (fig. 8). The cuticle of the hairs was destroyed.
Fig. 8.
Cross sections of the shaft (from a base to a granna along the shaft, look from left to right) of the ancient Guard hair of the Reindeer, Rangifer tarandus, sample № 208 (see table 1); destroyed medulla is shown by arrows. SEM. Scale bar 10 μm.
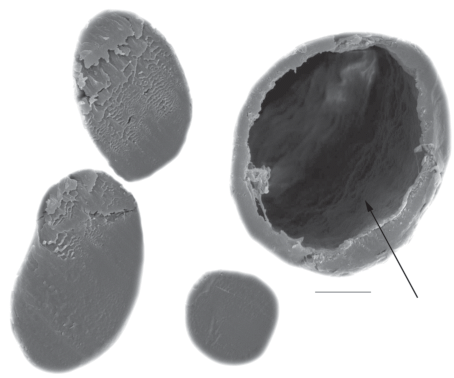
Domestic Dog – Canis lupus familiaris L. 1758. Dog and wolf fur have two main layers tiers consisting of lead (overhair) hairs – straight, long or short, resilient and brilliant guard hairs (hereinafter GHs) and the soft and dense underfur – wavy downy hairs (DHs). Dogs, besides fully dark and light colored hairs, have guard hairs of bicolor coloring (dark and light) or alternation of the brown (or black) and white bends on the shaft. Wolves have white hairs, although there are pigment granules in the medulla of big hairs. The fur is well differentiated in the main categories: lead hairs, guard hairs, and downy hairs (wool) of several orders. The shaft of lead and GHs have no standard widths, but gradually expand from the base to the middle (conditionally – granna) or slightly wavy, sometimes with a well-developed base stem. The thickness of GHs is limited in dogs from 50 to 150 μm (fig. 9). In general, the thickness of GHs I does not usually exceed 100 μm and the length can be up to 60 mm. GHs II have slightly wavy shaft with a diameter up to 79 μm. GHs III (up to 56 μm) and GHs IV (up to 36 μm) are thinner. The wavy DHs are short (up to 40 mm), thick (11–23 μm), and may contain the medulla. The winter hairs of wolves reach a maximum thickness of 136 μm and the medulla may occupy up to 70% of the shaft.
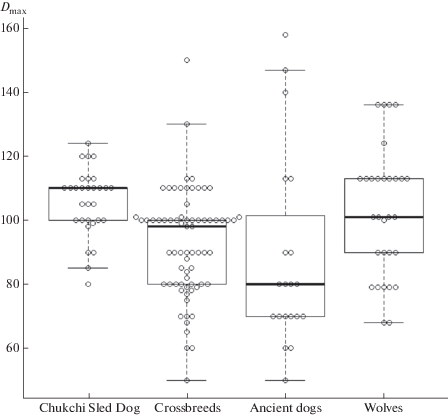
Fig. 9.
Maximum diameter of the Guard hairs of studied modern dogs, ancient dogs and wolves of Chukotka. Bold lines show the median values, boxes show standard deviation.
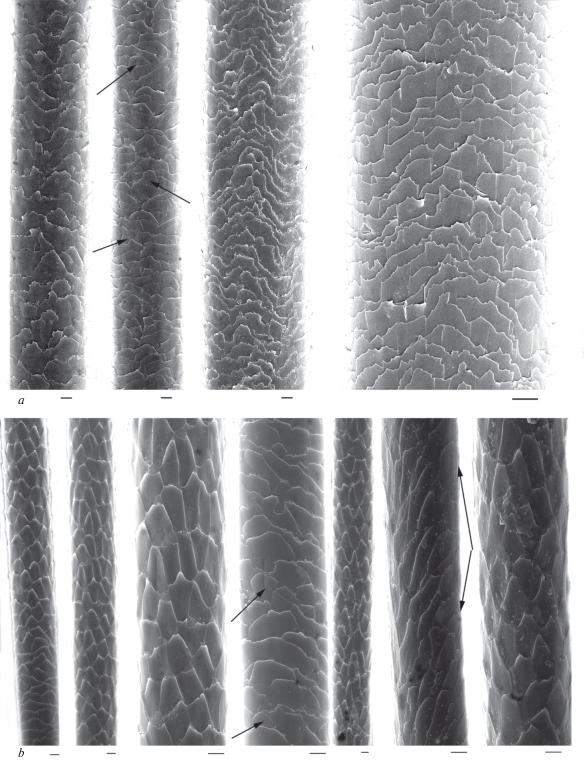
In modern and ancient dog hairs, the cuticle of the lead and most of GHs usually has semicircular structure, typical for canines. Its pattern changes little along the shaft from the base to the top (fig. 10a). The cuticle consists of big scales (their maximum is up to 10–15 μm). It is known that wild canines and some breeds of domestic dogs have the hastate cuticle, characteristic for hairs of different categories including DHs. In an intermediate (usually narrow) zone, which lies between the base and granna, cuticular scales become hastate and elongated longitudinally along the shaft. Such hastate (‘diamond petal’) cuticle found in the hairs of minks, polecats, red and arctic foxes, and in guard and downy hairs of wolves adult wolves and their pups, jackals, pariah dogs and some dog breeds (Sokolov et al., 1988; Chernova, Tselikova, 2004; Chernova et al., 2011). This specific cuticle did not occur in most of the tested hairs of modern and ancient dogs, with the exception of Chukotka Sled Dog, some ancient dog hairs (figs 10b; 11a), and the wolves. In the wolf, the specific cuticle covers some parts of the shaft above the base up to the lower third of the slightly crimped GHs I–IV and downy hairs. The absolute and relative height of hastate scales is several times bigger than the usual flattened cuticle scales. For example, in the Chukotka Sled Dog, it is twice as big as the flattened scales (respectively 23.87 ± 3.79 μm and 10.33 ± 2.2 μm, n = 10). The same in the ancient dog is 29.30 ± 3.48 μm and 15.65 ± 3.99 μm, n = 10 (figs 10b; 11a). The presence of specific hastate cuticles combined with numerous long and thick lead hairs in the Chukotka Sled Dog and in the wolf shows that the hair of these canines increasingly conserved features of wild type hair, compared with crossbreed Chukchi dogs. In addition, the dorsal surface of hair waves is covered by multi-layered overlapping specific thickenings, oriented at an angle to the long axis of the shaft cuticle (fig. 10b), which improves the mechanical properties of the hair.
Fig. 10.
Cuticular pattern of Lead (a) and Guard I (b) hairs from a shaft base to a granna along the shaft (look from left to right) of the Chukotka Sled Dog; intercalary scales and specialized cuticle covering the thickened dorsal surface of a twisted hair are shown by arrows. SEM. Scale bar 10 μm.
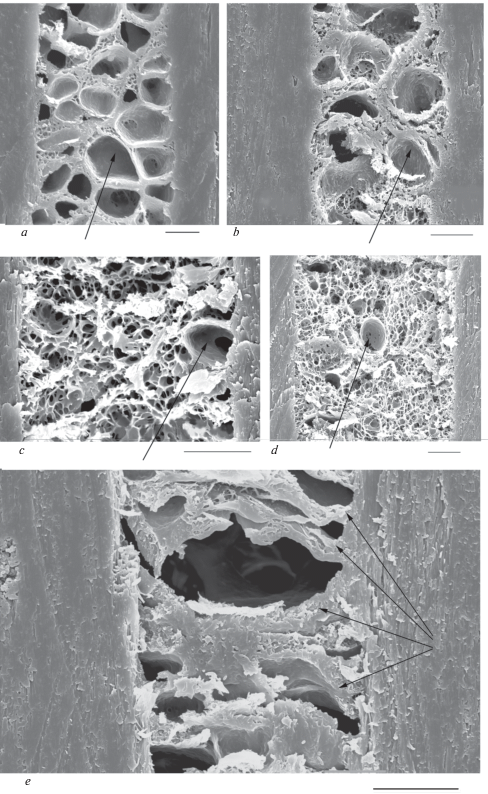
Medulla of tested dog hairs is not very thick and its median relative thickness is around 50% in modern dogs (table 3). In big GHs, the medulla stretches along a medium part of the hair (fig. 12). In wavy hairs (especially in DHs), in places of greatest curvature of the shaft, the medulla is shifted to the ventral side of the hair, typical for the hairs of this configuration. In medulla, the walls of cavities are covered by small (diameter is less than 1 μm) (fig. 12e) and large (diameter is more than 2 μm) (fig. 13a) perforations, and also contain rod-shaped pigment granules with a length less than 1 μm. The medullar canal is cylindrical in the lower part of the shaft, but it becomes slightly flattened in the upper part of the shaft. Medulla of hairs of the Chukotka Sled Dog consists of large round cavities (figs 11a; 13a; 13e), reminiscent of the wolf and may serve as evidence of the similarity of their living conditions. The other type of medulla consists of small polymorphous cavities separated by thick wavy walls covered by processes (fig. 13c). This medulla is found in some crossbreed Chukchi dogs (fig. 13d). It is also characteristic for GHs of wolves and jackals and in light lead hairs of German shepherds and the West Siberian Laika (Chernova, Tselikova, 2004).
Fig. 11.
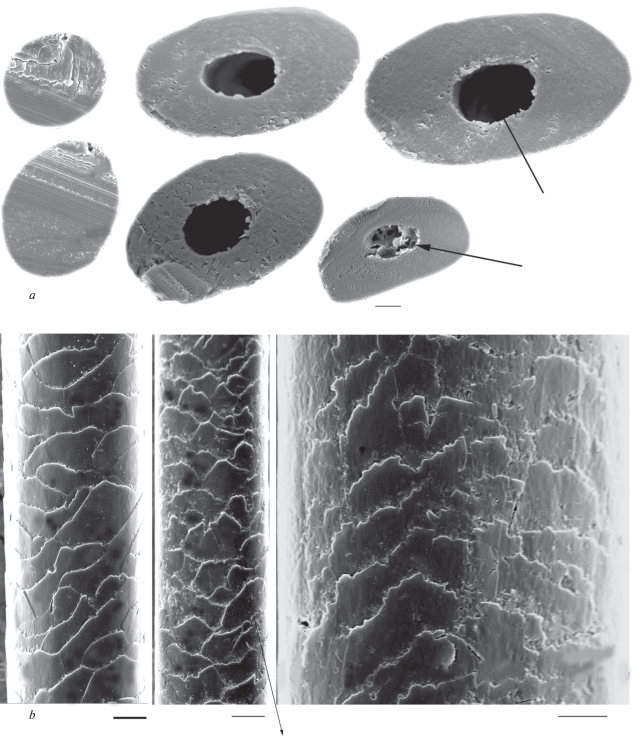
Cuticular pattern (a) and cross sections (b) along the shaft from a base to a granna (look from left to right) of the Guard hairs of ancient dogs (sample № 207, see table 1). (a) – the places of the shaft covered by the diamond petal cuticle are shown by arrows; (b) – deformed destroyed medulla is shown by arrow, on the left photo; medulla wall with large perforations is shown by arrow on the right photo. SEM. Scale bar 10 μm.
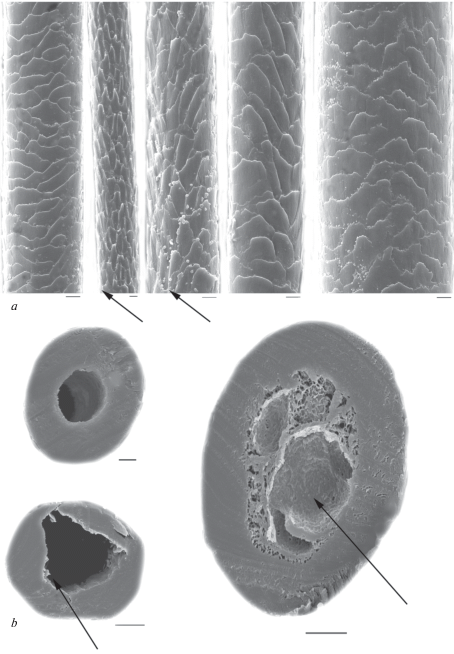
Fig. 12.
Cross sections of a base and a granna of the shaft (look from left to right) of the Guard hairs of the different recent dogs of Chukotka. (a) – the Chukotka Sled Dog; medulla with large cavities is shown by arrows; b – the same; deformed medullar is indicated by arrow; (c, d, e) – different crossbreed dogs; medulla overgrown by cortex is shown by arrow; medulla with small cavities is shown by arrow. SEM. Scale bar 50 μm.
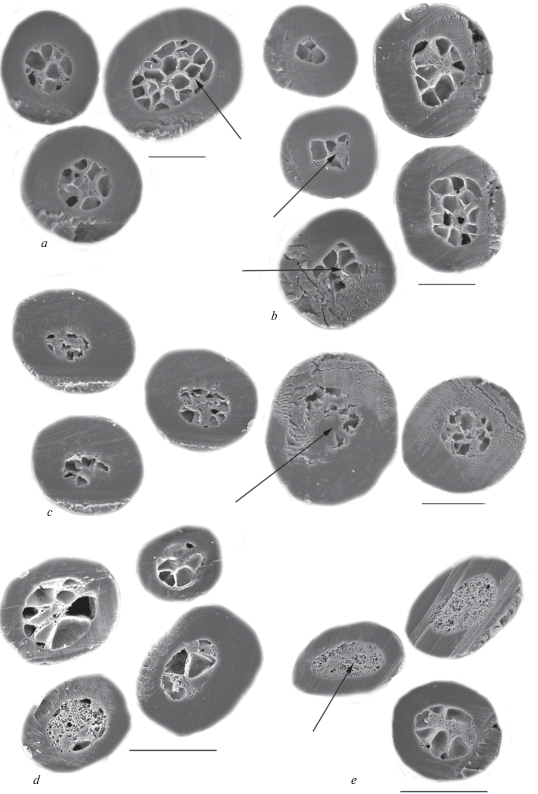
Fig. 13.
Medulla architectonics of the Guard hairs of modern and ancient dogs of Chukotka. (a) – Chukotka Sled Dog; (b, c, d) – crossbreed dogs; the large medullar cavities are shown by arrows; (e) – ancient dog (sample № 218, see table 1); the thickened walls between cavities are shown by arrows. SEM. Scale bar 10 μm.
Shafts of some hairs of modern Chukchi dogs are bent, irregularly thickened and deformed as the medulla strand, which develops a polyhedron configuration (fig. 12b) that does not occur in wild species (Chernova, Tselikova, 2004; Chernova et al., 2011). As a response for constant mechanical treatment during and after periods of growth, the hairs of the nape of sled dogs have the following structural modifications, increasing their mechanical strength:
(i) The walls between medullar cavities become thicker, tight, and have numerous attachments to the cortex (fig. 13e).
(ii) The dorsal side of hairs has a thickened cortex due to a shift in the medulla towards the ventral side of shaft (fig. 12c).
(iii) The surface of hairs mosaic covered by specialized solid cuticle (figs 10b; 11a).
In one case we found deformed medulla in the ancient dog hair (fig. 11b), which may be due to the pressure of the harness or to mechanical pressure of the burial ground. The latter assumption is not supported by the absence of similar deformation of medulla in hairs of some other wild mammals found in the same burials.
Principal component analysis showed no difference between crossbreed dogs and Chukchi Sled Dog guard hairs; however, wolves significantly differ from dogs (fig. 14). Two samples of ancient Eskimo dogs occupy different places in the space of two principal components: one sample is consistent with modern Chukchi dogs, while the other one is separated from both wolves and Chukchi dogs (fig. 14).
CONCLUSION
Using SEM, we identified ancient hairs of the typical representatives of the Chukotka fauna, such as the mountain hare, ringed seals, polar bear, reindeer, and domestic dog in ancient Holocene Eskimo settlements.
Even if the preservation factors allow recovering the fur remains, they are rarely collected from archaeological sites due to typical archaeological excavation methodology; however, the high degree of utilization of the mammal skins (e.g. by ancient Eskimos) could also play a significant role. The most common hairs were of hares and domestic dogs, and the rarest were hairs of commercial species such as seals and polar bears, which could be explained by the place where those animals were killed and/or skinned.
The structure of hairs of modern and ancient sled dogs reflects their economic use as a sled dog, as modified under the influence of a constant mechanical pressure of collar and harness, especially during hair formation and growing.
Список литературы
Alaska fur ID project, 2009. Retrieved February 14, 2019, from https://alaskafurid.wordpress.com/
Appleyard H.M., Wildman A.B., 1969. Fibers of archaeological interest: Their examination and identification // Science in Archaeology. 2nd edition. Brothwell, D., Higgs (Eds). Bristol: Thames and Hudson. P. 213–220.
Chernova O.F., Tselikova T.N., 2004. Atlas volos mlekopitayuschikh (Atlas of Mammal Hairs). Moscow: Tovarishchestvo Nauchnykh Izdaniy KMK. 429 p. [in Russian]
Chernova O.F., Perfilova T.V., Kiladze A.B., Zhukova F.A., Novikova V.M., Marakova T.I., 2011. Atlas volos mlekopitayuschikh — ob’ektov biologicheskoi expertizy (Atlas of mammal hairs — objectives of biological expertize). Moscow: Russian Federal Center for Forensic Expertize. 262 p. [in Russian]
Chernova O.F., Vasyukov D.D., Savinetsky A.B., 2016. Architectonics of the Hair of Sled Dogs of Chukotka // Doklady Biological Sciences. V. 467. P. 75–81.
Dneprovsky K.A., 2001. Dinamika drevneeskimosskoi kultury Chukotki v epokhu Birnirka i rannego Punuka (Dynamics of the ancient Eskimo culture of Chukotka in Birnirk and early Punuk epochs). Ph.D. Thesis. Moscow: Institute of Archaeology of RAS. 27 p. [in Russian]
Dinesman L.G., Kiseleva N.K., Savinetsky A.B., Khassanov B.F., 1999. Secular dynamics of coastal zone ecosystems of the north-eastern Chukchi peninsula (Chukotka: cultural layers and natural depositions from the last millennia). Tubingen: Mo Vince Verlag. 131 p.
Keller A., 1980. Dètermination des mammifères de la Suisse par leur pelage: II. Diagnose des families. III. Lagomorpha, Rodentia (partim) // Revue Suisse de Zoologie. V. 87. № 3. P. 781–796.
Keller A., 1981. Dètermination des mammifères de la Suisse par leur pelage: V. Carnivora, VI. Artiodactyla // Revue Suisse de Zoologie. V. 88. № 3. P. 803–820.
Keller A., 1984. Etude de la structure fine des jarres dorsaux de quelques Canidès sauvages et domestiques du genre Canis (Mammalia: Canidae) // Revue Suisse de Zoologie. V. 91. № 4. P. 973–992.
Kennedy A.J., 1982. Distinguishing characteristics of the hairs of wild and domestic canids from Alberta // Canadian Journal of Zoology. V. 60. P. 536–541.
Kisin M.V., Golovin A.V., 1992. The use of the SEM for the investigation of hairs having similar structure // Journal of Microscope. V. 40. P. 259–264.
R Core Team, 2018. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org
Rowe W.F., 2010. Forensic hair and fiber examination in archaeology: Analyses of materials from gravesity at the home of Samuel Washington // Technical Briefs in Historical Archaeology. V. 5. P. 43–51.
Savinetsky A., 2002. Mammals and birds harvested by early Eskimos of Bering Strait // University of Oregon Anthropological Papers. V. 59. P. 275–305.
Scheffer V.B., 1964. Hair patterns in seals (Pinnipedia) // Journal of Morphology. V. 115. P. 291–303
Sokolov V.E., Skurat L.N., Stepanova L.V., Sumina E.B., Shabadash S.A., 1988. Rukovodstvo po izucheniyu kozhnogo pokrova mlekopitayuschikh (A Manual for the Study of Mammal Skin). Moscow: Nauka. 280 p. [in Russian]
Vasyukov D.D., 2011. Sobaki (Canis lupus familiaris) iz drevnikh poselenij severo-vostochnogo poberezhya Chukotki (Dogs (Canis lupus familiaris) from the ancient coastal settlements of the Northeastern Chukotka). Ms.Sci. Thesis. Moscow: Moscow State University. 126 p. [in Russian]
Verhoeven L.E., 1972. The advantages of the scanning electron microscopy in the investigative studies of hair // The Journal of Criminal Law, Criminology, and Police Science. V. 63. № 1. P. 125–128
Wallis R.L., 1993. A key for the identification of guard hair of some Ontario mammals // Canadian Journal of Zoology. V. 71. P. 587–591.
Wolfe A., Long A.M., 1997. Distinguishing between the hair fibres of the rabbit and the mountain hare in scats of the red fox // Journal of Zoology. V. 242. P. 370–375.
Дополнительные материалы отсутствуют.
Инструменты
Зоологический журнал