Зоологический журнал, 2019, T. 98, № 11, стр. 1291-1303
Mountain Regions are the Putative Place of Origin of Many Arctic Animal and Plant Forms
A. A. Makhrov 1, *, I. N. Bolotov 2, 3, **, V. S. Artamonova 1, ***, E. A. Borovikova 4, ****
1 Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences
119071 Moscow, Russia
2 Northern (Arctic) Federal University
163002 Arkhangelsk, Russia
3 Laverov Federal Center for Integrated Arctic Research, Russian Academy of Sciences
163000 Arkhangelsk, Russia
4 Papanin Institute for Biology of Inland Waters, Russian Academy of Sciences
152742 Yaroslavl Oblast, Borok, Russia
* E-mail: makhrov12@mail.ru
** E-mail: inepras@yandex.ru
*** E-mail: valar99@mail.ru
**** E-mail: elena.ibiw@gmail.com
Поступила в редакцию 5.04.2019
После доработки 13.05.2019
Принята к публикации 24.05.2019
Аннотация
In this study, we tried to understand why the biota of northern regions is similar to that of southern mountain regions. Phylogeographic studies of several Arctic-alpine plants (Arabis alpina, Bistorta vivipara, Carex atrofusca, Gentiana sect. Cruciata, Koenigia islandica, Oxyria digyna, Ranunculus glacialis, Saxifraga oppositifolia, Sibbaldia procumbens, Trollius europaeus, Veronica alpina, Lagotis spp., and Pedicularis spp.), insects (Oeneis spp. and Arcynopteryx dichroa), and a mammal species (Ovibos moschatus) indicate that the respective groups emerged in the mountains of the temperate climatic zone and then migrated to the Arctic. As paleontological findings indicate, the mountains of the temperate and tropical zones provided habitats for the ancestors of several Salmonidae genera and at least some of the mammalian species common to the Eurasian mammoth steppe (Mammuthus primigenius, Coelodonta antiquitatis, Bos (Poëphagus) baikalensis, Alopex lagopus, and Panthera spelaea). A hypothesis is suggested to explain the crucial role of mountain regions in the evolution of northern forms. Additionally, colonization events by Arctic taxa in the mountains of temperate climatic zones have been demonstrated in a few studies.
The expansion of organisms from one temperature zone of Earth to another is a spectacular example of adaptive evolution (review: Hoffmann, Parsons, 1997). Adaptive changes occur in numerous modern populations as a response to global ecosystem and climate changes (reviewed in Pauls et al., 2013; Jaeschke et al., 2014; Holyoak, Heath, 2016; De Meester et al., 2018).
Examples of when living organisms colonized new climatic zones in the past are also known. The most intriguing example is provided by the formation of the terrestrial and freshwater flora and fauna in the Arctic. Their origin is a matter of long-standing interest (reviews: Tugarinov, 1935; Hultén, 1937; Kusnezov, 1938; Hulten, 1958; Tolmachev, 1960; Røen, 1994; Murray, 1995; Weider, Hobæk, 2000; Abbott, Brochmann, 2003; Brochmann et al., 2013), but it was difficult to choose among several hypotheses of how living organisms colonized the Arctic until a phylogeographic approach was developed. Additionally, research on the evolution of Arctic biota is a topic of great importance for the conservation of Arctic ecosystems, which have changed greatly under various anthropogenic impacts over the past few decades (Dmitrenko et al., 2008).
In the beginning of the 19th century, Alexander von Humboldt (von Humboldt, Bonpland, 1807) drew attention to the similarity among the floras of the northern regions and the mountains of the southern regions. The taxa inhabiting both the Arctic and southern mountain regions are known as Arctic-alpine taxa and are quite numerous. The most common current opinion is that mountain taxa could have originated from related Arctic taxa. Many studies have addressed the cold-adapted forms that inhabit the mountains of the temperate zone as relics of one of the glacial periods, when their ancestors migrated from the Arctic to the far south. Forbes (1846, p. 400) was the first to advance the hypothesis that “The alpine floras of Europe and Asia, so far as they are identical with the flora of the Arctic and sub-arctic zones of the Old World, are fragments of a flora which was diffused from the north…”.
Christ (1867) was the first to propose a different hypothesis in which mountains, i.e., Asian mountain ranges, are a prospective place of origin for Arctic flora.
Ample botanical evidence has accumulated to date to support both Forbes’s and Christ’s hypotheses (reviews: Murray, 1995; Abbott, Brochmann, 2003; Brochmann et al., 2013; Wen et al., 2014; Sun et al., 2017).
Kusnezov (1938) and Yakovlev (1964) assumed that the mountains of the temperate zone were a place of origin for several Arctic animal taxa. However, among zoologists, the predominant concept is that new taxa originate at equatorial or tropical latitudes, with subsequent spreading to high (polar) latitudes (Darlington, 1957). According to this hypothesis, southern ancestral lineages spread northwards during warm periods and then gradually adapt to the decrease in temperature during cold periods (Valentine, 1968). This phenomenon is referred to as the “equatorial pump” (Meyen, 1987).
Here, we review data demonstrating that a plethora of Arctic plant and animal taxa originated in mountain regions in temperate and even in tropical zones. We demonstrate that Forbes’s (1846) and Christ’s (1867) ideas are both true, and they supplement one another. We also propose a novel hypothesis that combines the assumptions that northern forms originate from mountains (Christ, 1867), with the concept of the “equatorial pump” (Valentine, 1968; Meyen, 1987).
METHODS
To review the body of available literature, we searched databases such as the Web of Science (https://apps.webofknowledge.com) and the Russian Scientific Electronic Library (https://elibrary.ru) using the following word combinations: “Arctic-alpine”, “Arctic evolution”, “Polar evolution”, “Arctic origin”, “Polar origin”, and “Glacial relict”. In summary, 66 appropriate references were analyzed.
Over the course of the literature analysis, we identified two groups of Arctic-alpine taxa, namely groups of mountain origin and groups of Arctic origin. The primary criterion for determining the center of origin using phylogeographic data was the presence of the most ancient haplotypes of mitochondrial and chloroplast DNA and variants in the sequences of different nuclear DNA fragments. For the paleontological studies, we combed the records of the most ancient representatives and/or their fossils for the groups under consideration. Studies in which the results preclude the migration paths of the respective taxa to be determined with sufficient accuracy were excluded from consideration.
An assessment of the putative time of origin for Arctic plant and animal species is beyond the scope of our study, as are the taxonomic ranks of related Arctic and mountain forms. The extent of divergence varies among pairs of Arctic and related mountain taxa, because the divergence of different Arctic-alpine taxa started during different periods and the evolutionary rate was not constant. Moreover, recent morphological and molecular data on various groups of animals indicate that many Arctic forms and those living in more southern mountain regions could be close to one another. In some cases, they have even been shown to be conspecific (Den Bakker et al., 2007; Varga, Schmitt, 2008; Sher et al., 2011; Tiberti, 2011; Barrio et al., 2013; Makarova, 2013; Artamonova et al., 2015; Kotov, 2016; Lindholm et al., 2016; Abeli et al., 2018; Makhrov et al., 2019a).
The term Central Asia, which is often used here, requires explanation. Von Humboldt (1843) coined the term in his well-known monograph, and then von Richthofen (1877, p. 7) was the first to demarcate this vast region as follows: “vom Hochland von Tibet im Süden zum Altai im Norden, und von der Wasserscheide am Pamir im Westen zu derjenigen der Riesenströme von China und dem Gebirge Khingan im Osten [from the highlands of Tibet in the south to the Altai in the north, and from the watershed on the Pamir in the west to those of the great rivers of China and the Khingan Range in the east]”.
The majority of researchers (including the authors of many studies referenced here) uses this definition. The region earlier known as Middle Asia has come to be named Central Asia in some recent studies. However, like any other change in terminology, this one often leads to misunderstandings and the term “Middle Asia” is recommended for use by geographers (Biske, Sevastyanov, 2003; Cowan, 2007). We use von Richthofen’s classical definition of Central Asia here. This region includes western China, Mongolia, and the southern mountains of Asian Russia, but it does not include Kazakhstan, Turkmenia, Kirgizia, Tadzhikistan, Uzbekistan and India.
RESULTS
Southern origin of certain Arctic-alpine forms: phylogeographic evidence. Because it is virtually impossible for diploids to originate from polyploids, records of diploid relatives of polyploid Arctic species in mountains of the temperate zone support the mountain origin of some Arctic-alpine species (review: Stebbins, 1984). However, polyploidy is most likely of adaptive significance, and the selection for polyploids against their diploid ancestors might have taken place in Arctic regions.
The results of phylogeographic studies provide evidence that many Arctic taxa originated in mountain regions (table 1). The Arctic-alpine taxa of mountain origin were even more numerous than the groups of Arctic origin. It is important that Central Asia is the primary center of origin for the Arctic-alpine species.
Table 1.
Taxa that have presumably migrated to the Arctic from temperate mountains based on molecular data
| Mountains inhabited by ancestral taxon | Taxon | Method | Reference |
|---|---|---|---|
| Qinghai-Tibetan Plateau | Plant, Koenigia islandica | Internal transcribed spacers (ITS) and chloroplast DNA haplotypes | Fan et al., 2013; Long et al., 2014 |
| Plant, Oxyria digyna | Three chloroplast DNA fragments (trnH-psbA, trnT-trnL and matK) and 11 nuclear loci | Wang et al., 2016 | |
| Plants, Gentiana sect. Cruciata | Four chloroplast DNA fragments | Zhang et al., 2009 | |
| Mountains of Central Asia | Plant, Carex atrofusca | AFLP (amplified fragment length polymorphism) and sequences of chloroplast DNA | Schönswetter et al., 2006 |
| Plants, Lagotis | Chloroplast genes and ITS of nuclear ribosomal DNA | Li et al., 2014 | |
| Plant, Saxifraga oppositifolia | Plastid sequence data set and AFLP data set | Winkler et al., 2012 | |
| Butterflies, Oeneis | Mitochondrial DNA haplotypes and alleles of three nuclear genes | Kleckova et al., 2015 | |
| Mountains of South and East Asia | Plant, Sibbaldia procumbens | Three plastid DNA non-coding regions (the atpI–atpH and trnL–trnF intergenic spacers and the trnL intron) | Allen et al., 2015 |
| Mammal, Ovibos moschatus | Complete mitochondrial genome | Hassanin et al., 2009; Yang et al., 2013 | |
| Anatolia | Plant, Arabis alpina | Sequences of the chloroplast DNA trnL-trnF region | Ansell et al., 2011 |
| Mountains of Asia | Plant, Bistorta vivipara | Two chloroplast DNA spacer regions, trnH–psbA and trnS–G | Marr et al., 2013 |
| Central European mountains | Plant, Ranunculus glacialis | AFLP, noncoding plastid DNA regions and nuclear ribosomal ITS | Schönswetter et al., 2003; Ronikier et al., 2012 |
| Eastern Carpathians | Plant, Trollius europaeus | AFLP markers | Despres et al., 2002 |
| Eastern Alps or Carpathians | Plant, Veronica alpina | Plastid DNA trnL-F sequences and AFLP fingerprints | Albach et al., 2006 |
| Europe (probably the Alps) | Plant, Saxifraga oppositifolia | Plastid sequence data set and AFLP data set | Winkler et al., 2012 |
| Central European highlands | Stonefly, Arcynopteryx dichroa | Mitochondrial sequence data and nuclear microsatellite data | Theissinger et al., 2013 |
| Eurasia and North America (independently evolved of 12–14 times) | Plants, Pedicularis |
Entire internal transcribed spacer region of the nuclear ribosomal DNA (ITS1-5.8S rRNA gene-ITS2) and chloroplast DNA matK–trnK region | Tkach et al., 2014 |
Analyzing the distribution of internal transcribed spacer (ITS) and chloroplast DNA haplotypes of the Arctic-alpine plant species Koenigia islandica L. has led to the substantiated conclusion that the Qinghai-Tibetan Plateau was its place of origin (Fan et al., 2013; Long et al., 2014). Arctic-alpine Gentiana sect. Cruciata plants also originated in this region (Zhang et al., 2009). Amplified fragment length polymorphism (AFLP) fingerprinting and a sequence analysis of chloroplast DNA showed that the Arctic-alpine plant Carex atrofusca Schkuhr most likely originated from the mountains of Central Asia (Schönswetter et al., 2006). The Arctic-alpine genus Lagotis also originated in this region (Li et al., 2014). The Arctic-alpine plant Sibbaldia procumbens L. “probably originated in the mountains of South and East Asia” (Allen et al., 2015). Bistorta vivipara (L.), another Arctic-alpine plant, also seems to originate from Asian mountains (Marr et al., 2013).
Mountain plants also colonized Arctic regions of Europe. Arctic populations of the glacier buttercup Ranunculus glacialis L. have been found to originate from central European mountain populations, based on the body of molecular researches (Schönswetter et al., 2003; Ronikier et al., 2012).
A broad-scale study of nuclear and chloroplast DNA diversity has shown that the Arctic taxa of the hemiparasitic plant genus Pedicularis have evolved independently 12–14 times, and they primarily originated in the lineages that otherwise occur in the high mountains of Eurasia and North America (Tkach et al., 2014).
The sequence data from the trnH-psbA and trnTtrnL fragments of chloroplast DNA spacer regions made it possible to assume that the mountain sorrel Oxyria digyna Hill, an Arctic-alpine plant, originated in the North American mountains (Allen et al., 2012). However, when additional samples were examined and a fragment of chloroplast DNA matK and sequences from 11 nuclear loci were included in the analysis, the results led to a conclusion that this plant species most likely originated on the Qinghai-Tibetan Plateau (Wang et al., 2016).
Chloroplast DNA diversity was studied in Arabis alpina L., another Arctic-alpine plant species. It was found that “All haplogroups occur within Anatolia, and all intermediate haplotypes linking the three haplogroups are endemic to central Anatolia and Levant, where haplotypic and nucleotide diversities exceeded all other regions.” Those findings provided a basis for believing that Anatolia is the cradle of origin for the global genetic diversification of the species, including its Arctic populations (Ansell et al., 2011).
A study on the Arctic-alpine plant Trollius europaeus L. using AFLP markers made it possible to assume that the Fennoscandian populations of this species originated from the eastern Carpathian refugium (Despres et al., 2002). The Scandinavian population of Veronica alpina L. probably originated from a refugium in the eastern Alps or Carpathians (Albach et al., 2006).
Phylogeographic analyses of a plastid sequence data set and an AFLP data set for the Arctic-alpine plant Saxifraga oppositifolia L. showed that Europe (probably the Alps) and Central Asia are most likely the ancestral areas of the two primary lineages (Winkler et al., 2012).
Examples known in animals are congruent with the examples in plants outlined above. According to the distributions of mitochondrial DNA haplotypes and the alleles of three nuclear genes, the Arctic-alpine butterfly genus Oeneis originated in the Central Asian mountains (Kleckova et al., 2015).
An analysis of complete mitochondrial genomes has shown that the closest relatives of the Arctic muskox, Ovibos moschatus (Zimmermann), are species belonging to the genera Capricornis (serow) and Naemorhedus (goral), from the East Asian mountains (Hassanin et al., 2009; Yang et al., 2013). With respect to the high levels of genetic and morphological diversity in both Capricornis and Naemorhedus, the Arctic muskox could have originated from a mountain ancestor.
As inferred from a diversity analysis of mitochondrial sequence and nuclear microsatellite data, the Arctic-alpine freshwater stonefly (Plecoptera) Arcynopteryx dichroa (McLachlan) colonized Fennoscandia from a refugium in the central European highlands (Theissinger et al., 2013).
Southern origin of certain Arctic-alpine forms: congruence of genetic and paleontological data. Salmonids are typical inhabitants of northern waters. Arctic charrs (Salvelinus), the name of which speaks for itself, appears to be the most ancient genus within this group based on phylogenetic reconstructions (Artamonova et al., 2018). The species of this genus live in Arctic and mountain water bodies and the cold, deep waters of large lakes. The most ancient salmonid fossils have been found in the mountain regions of North America (Wilson, Li, 1999) and Kamchatka (Sytchevskaya, 1986).
Several Salmonidae genera originated in temperate mountains with a subsequent expansion into Arctic water bodies, e.g., the genus Salmo originated in the Caucasus, according to molecular and paleontological data (Makhrov, Bolotov, 2019).
Freshwater pearl mussels (Margaritifera), the larvae (glochidia) of which parasitize fish gills, colonized the Arctic water bodies together with their primary hosts, salmonids. Paleontological records show that the earliest members of the family Margaritiferidae inhabited tropical and subtropical river systems in China (Fang et al., 2009), Europe (Delvene, Araujo, 2009), and northern Africa (Van Damme et al., 2015). Recent species are widespread in mountainous regions, e.g., European mountain ranges, the Cumberland Plateau in North America, the Atlas Mountains in Africa, and several mountain ranges in central Indochina (Bolotov et al., 2014; Lopes-Lima et al., 2018). The ranges of three recent species extend from the temperate highlands to the Arctic and Subarctic areas as follows: Margaritifera margaritifera (L.) (the northwestern edge of Europe, Newfoundland, and Labrador), M. falcata (Gould) (Alaska), and M. middendorffi Rosen (Kamchatka and North Kurile Islands) (Makhrov et al., 2014; Lopes-Lima et al., 2018).
The oldest remains of representatives from the freshwater fish genus Prosopium (Coregonidae) were found in the Pliocene sediments of Lake Idaho, in the Rocky Mountains (Smith, 1975). Currently, this genus is not only widespread throughout the northern part of North America, but it also inhabits water bodies in Siberia.
Paleontological data on mammals provide strong evidence to support the origin of several Arctic forms from mountain species. Deng et al. (2011) and Wang et al. (2014, 2016) described new Pliocene mammals from high-altitude areas of the western Himalaya region. The Tibetan woolly rhinoceros Coelodonta thibetana Deng et al. belongs to the genus, other species of which inhabited northern Eurasia. The Tibetan fox Vulpes qiuzhudingi Wang et al. is closely related to the Arctic fox Vulpes lagopus (L.). The Tibetan wild sheep Protovis himalayensis Wang et al. appears to be an ancestral lineage of Ovis (a member of the Ice Age megafauna). Deng et al. (2011) and Wang et al. (2014, 2016) concluded that at least several mammalian species common to the Eurasian mammoth steppe fauna originated in the rigorous climate of Tibet.
It should be noted that this conclusion was perceived with skepticism by some theriologists, including researchers with comprehensive experience in the Tibetan Plateau and other regions of Central Asia (Makhrov et al., 2019). In fact, the available paleontological data on fossil mammals from Tibet are still scarce, and this group deserves further research effort.
The woolly mammoth Mammuthus primigenius (Blumenbach) was the most charismatic member of the north Eurasian fauna during the Ice Age. Mammuthus subplanifrons Osborn, the most ancient species in the genus, lived during the late Miocene-early Pliocene. The earliest fossils of this species have been found in the Middle Awash Valley of modern Ethiopia, in Africa (Sanders, Haile-Selassie, 2012), and its fossil records seem to be confined to past mountain areas (WoldeGabriel et al., 2001). The earliest known Mammuthus trogontherii (Pohlig) fossils have been found in the Loess Plateau area of northern China (Lister, Sher, 2015).
Northern origin of certain Arctic-alpine forms. There is convincing evidence that several Arctic-alpine taxa colonized the southern (mountain) parts of their ranges during glacial periods, when Arctic forms spread far to the south. The taxa that have arisen in this manner are termed glacial relicts.
This mechanism of range formation was initially inferred from the broad distribution of recent Arctic-alpine species in deposits from the glacial age. For example, the terrestrial mollusk Vertigo genesii (Gredler), which is common in European deposits of the late Pleistocene age, is now widespread in Northern Europe and has recently been found in England and the Alpine highlands (Coles, Colville, 1980; Schenková, Horsák, 2013 and references therein). Mollusks and plants characteristic of the glacial-age deposits still inhabit the mountains of southern Siberia (Horsák et al., 2015) and North America (Miller, 1996). Similar examples are especially numerous in earlier publications (review: Birks, 2008). However, paleontological evidence for the Arctic origin of these taxa is usually absent since fossil remains had no chance of remaining in the glaciation zone under the ice sheet. Therefore, evidence for the Arctic origin of these Arctic-alpine taxa was only obtained using a molecular approach.
In several cases, molecular studies confirmed that recent mountain taxa originated from Arctic ancestors, the ranges of which expanded southward during one of the glacial periods. As shown in table 2, relict Arctic-alpine forms have been found in European mountains, the Himalayan-Hengduan Mountains, North America and Japan.
Table 2.
Taxa that have presumably migrated to temperate mountains from the Arctic based on molecular data
| Taxon | Mountains inhabited by relict forms | Method | Reference |
|---|---|---|---|
| Plant, Comastoma tenellum (Rottb.) Toyok. | Alps (Europe) | Amplified fragment length polymorphism (AFLP) | Schönswetter et al., 2004 |
| Plant, Ranunculus pygmaeus Wahlenb. | Alps (Europe) | AFLP and chloroplast DNA sequence | Schönswetter, et al., 2006a |
| Plant, Dryas octopetala L. | Tatra Mountains, Carpathians (Europe) | AFLP | Skrede et al., 2009 |
| Plants, Cassiope | Himalayan–Hengduan Mountains | Loci produced by restriction site associated DNA sequencing (RAD-seq) | Hou et al., 2016a |
| Plants, Diapensia | Himalayan–Hengduan Mountains | Sequences of four plastid DNA markers and the nuclear ribosomal internal transcribed spacer | Hou et al., 2016 |
| Plant, Phyllodoce nipponica Makino | Japan | Sequences of two plastid DNA markers and multiple nuclear loci | Ikeda et al., 2014 |
| Plants, Phyllodoce aleutica (Spreng.) A. Heller and P. glanduliflora (Hook.) Coville | East Asia and North America | Sequences of multiple nuclear loci | Ikeda, Setoguchi, 2017 |
| Plant, Kalmia procumbens (L.) Gift & Kron | Southern Europe, Japan | Sequences of multiple nuclear loci | Ikeda et al., 2017 |
| Arctic fairy shrimp, Branchinecta paludosa O.F. Müller | Tatra Mountains, Carpathians (Europe) | Sequences of mitochondrial cytochrome c oxidase I subunit | Lindholm et al., 2016 |
| Wolf spiders, Pardosa saltuaria (L. Koch) group | Central Alpine Region of Europe | Sequences of mitochondrial ND1 gene | Muster, Berendonk, 2006 |
| Arctic charr Salvelinus alpinus L. | Central Alpine Region of Europe | Sequences of mitochondrial control region | Brunner et al., 2001 |
It is important to note that the genetic differentiation of mountain and Arctic taxa is high in some cases, and that these mountain taxa can be considered relics of cold stages from a more distant past (review: Schmitt et al., 2010).
As indicated by molecular studies, several Arctic forms of mountain origin secondarily migrated to the mountains of another temperate region (the data are not included in table 2). The Arctic-alpine plants Saxifraga oppositifolia (Abbott et al., 2000), Bistorta vivipara (Marr et al., 2013), and Oxyria digyna (Wang et al., 2016) and the Arctic-alpine butterfly genus Oeneis (Kleckova et al., 2015) probably originated in Central Asia, from which they colonized the Arctic, and from there they migrated to the temperate mountains of Europe and North America. Two plant taxa, Gentiana sect. Cruciata (Zhang et al., 2009) and Carex atrofusca (Schönswetter et al., 2006), similarly originated in Central Asia, but they colonized only Arctic and European mountains.
Although the available data are still insufficient for statistical analysis, the Tibetan Plateau and adjacent regions seem to be the most important center of origin of Arctic-alpine taxa. Consequently, these taxa migrated mostly along the Tibetan sector of the Holarctic to spread northward. Both the colonization of temperate mountains by Arctic taxa and the oncoming migration of taxa of mountain origin to the Arctic occurred widely in other Holarctic sectors, such as Europe, Japan, and North America.
DISCUSSION
Origin of Arctic-alpine taxa. The several examples outlined above clearly demonstrate that a variety of Arctic animal and plant taxa originated from ancestral forms inhabiting the mountains of the temperate or even subtropical climatic zones (16 taxa and 27–29 cases of distribution, see table 1). Additionally, molecular studies have confirmed that several recent mountain taxa originated from Arctic ancestors (12 taxa and 12 cases of distribution, see table 2). At first glance, the known groups of Arctic origin are far less numerous than the groups of mountain origin among the Arctic-alpine taxa. A possible explanation is that the terrestrial Arctic ecosystems are relatively young, in that they originated only 2–3 million years ago (review: Brochmann et al., 2013), and only a few endemic species have originated in the Arctic so far.
In some cases, taxa originating in subtropical mountains colonized not only the Arctic, but also the temperate zone. Thus, the hypothesis of origin for many Palearctic taxa in Central Asia (Matthew, 1939) has received new support (review: Mosbrugger et al., 2018; Poplavskaya et al., 2018). Several forms that originated from the Tibetan Plateau currently share broader ranges (reviews: Wen et al., 2014; Favre et al., 2016).
Mountain taxa were apparently not the sole source of the terrestrial and freshwater fauna and flora of the Arctic. The Arctic flora includes not only taxa of mountainous origins, but also subarctic, boreal, mountain-steppe, and ancient Arctic taxa (Wulff, 1944). For example, the genus Saxifraga most likely originated in North America (Ebersbach et al., 2017). Aquatic taxa from plain water bodies in the temperate climatic zone and invaders from marine habitats colonized freshwater bodies of the European Arctic along with taxa of mountain origin according to molecular data (Makhrov, Bolotov, 2006). Mountain taxa still played a substantial role in the formation of northern biomes.
In particular, species of mountain origin account for approximately one-third of the mammoth faunal complex, which is a group of species that inhabited northern Eurasia during the late Pleistocene (reviews: Ukraintseva, 2013; Kahlke, 2014). Table 3 summarizes the primary mammalian components of this fauna in the Arctic zone of Eurasia. As shown, 6 out of 17 species (approximately 35%) originated from ancestors that inhabited the mountains of temperate or even subtropical climatic zones.
Table 3.
Origin of species of the mammoth faunal complex*
| Species (according to Ukraintseva, 2013) | Molecular data | Paleontological data | Mountain origin of the species |
|---|---|---|---|
| Woolly mammoth (Mammuthus primigenius) | N/A | Ancestral species inhabited African mountains (Sanders, Haile-Selassie, 2012) | Yes |
| Woolly rhinoceros (Coelodonta antiquitatis) | N/A | Ancestral species lived in Tibet (Deng et al., 2011) | Yes |
| Kamchatka marmot (Marmota camtschatica) | The closest relative inhabits the Himalayas and Tibet (Brandler et al., 2010) | N/A | Possible (it is unclear whether the northern or mountain species is ancestral) |
| Baikalian yak (Bos (Poëphagus) baikalensis Vereshchagin) | N/A | The species has not been found in plains (Malikov, 2015) | Yes |
| Muskox (Ovibos moschatus) | The closest relatives inhabit East Asian mountains (Hassanin et al., 2009; Yang et al., 2013) | N/A | Yes |
| Arctic fox (Alopex lagopus) | N/A | An ancestral species lived in Tibet (Wang et al., 2014) | Yes |
| Cave lion (Panthera spelaea (Goldfuss)) | N/A | An ancestor of the genus Panthera inhabited Tibet (Tseng et al., 2014) | Yes |
* Species in the mammoth faunal complex, the origin of which is unknown, are as follows: Pleistocene bison (Bison priscus occidentalis (Lucas)), Cherskiy horse (Equus lenensis Russanov), brown lemming (Lemmus sibiricus Kerr), reindeer (Rangifer tarandus L.), northern saiga (Saiga tatarica L.), wolf (Canis lupus L.), polar bear (Ursus maritimus Phipps), wolverine (Gulo gulo L.), cave bear (Ursus spelaeus Rosenmüller), Pleistocene arctic hare (Lepus arcticus Ross). N/A – not available.
The evolution of other species in the mammoth faunal complex is poorly understood, although their mountain origin cannot be excluded. For example, phylogenetic reconstructions from inter-SINE PCR in marmots (Marmota, Sciuridae, Rodentia) have shown that Marmota himalayana (Hodgson) from the Himalayas and Tibet is the closest relative of M. camtschatica (Pallas), which is widespread in Siberia (Brandler et al., 2010).
Great mountain chains as places for the adaptation of originally southern taxa to cold climatic conditions. A review presented above suggests that vast upland areas could have played a significant role in the development of high-latitude fauna and flora. A question remains as to what factors could facilitate their adaptation to the Arctic environment.
The gradual uplift of the Tibetan Plateau and other mountain systems is well known to geologists, and it might have contributed to the adaptation of the originally southern taxa to cold climatic conditions. In addition, climatic oscillations might act as “species pumps”, thus facilitating the colonization of mountain regions by species of lowland origin (reviewed in Hoorn et al., 2013, 2018; Favre et al., 2015; Pellissier et al., 2018; Deng et al., 2019; Muellner-Riehl, 2019).
However, climatic oscillations have not always led to speciation (Bennett, 2004). This variation is explained, among other factors, by the fact that peripheral populations decrease in size when exposed to adverse environmental changes. А random dispersal causes a gene flow from regions of high to low density and therefore hampers adaptation in the peripheral areas (Kirkpatrick, Barton, 1997; Cassel-Lundhagen, 2010).
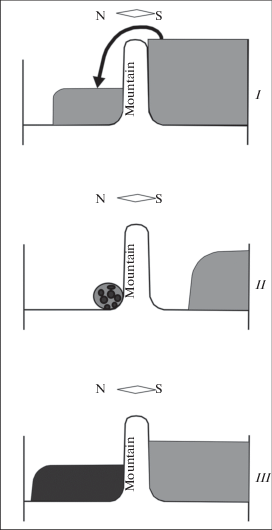
We believe that large mountain systems that extended in a latitudinal direction could similarly serve as “gates” for the equatorial pump. Southern species migrate to the regions north of the mountain chains during a warm climate period (Dynesius, Jansson, 2000; Hewitt, 2004). During the subsequent cold climate period, they are “pressed” against the northern slopes and forced to adapt to the cold environment. Fisher and Ford (1950, p. 118) noted that the “Sub-division into small isolated or semi-isolated populations is clearly favorable to evolutionary progress through the variety of environmental conditions to which the colonies are exposed”. Modern models (Garcia-Ramos, Kirkpatrick, 1997) and field data (e.g. Fu et al., 2016; Lagerholm et al., 2017) provide strong evidence in favor of Fisher and Ford’s opinion. Moreover, populations that were isolated in small northern refugia could be involved in hybridization with related species (Hassanin, 2015). Finally, during the next warm period, newly originated mountain species could expand their ranges to the north (fig. 1 ).
Fig. 1.
The “operation cycle” of a mountain “gate”. I and III – climate warming periods, II – a climate cooling period.
The characteristic pattern in the distribution of diploid and polyploid forms provides additional evidence supporting this hypothesis. Diploid forms of insects are common in the Alps, while polyploid forms mostly inhabit northern Europe (Suomalainen et al., 1976). Moreover, the distribution of the Arctic-alpine plant Potentilla crantzii (Crantz) Beck ex Fritsch indicates a ploidy-shaped Arctic-alpine disjunction, with tetraploids being limited to the central and southern European mountain chains and hexaploids restricted to the subarctic. In addition, hexaploids occur in the Alps and Carpathians (Paule et al., 2015). Upon analyzing data on the populations of Nepal and northern regions, Hedberg (1992, p. 390) concluded that “The ancestral population of Saxifraga hirculus L. in Central Asia must evidently have been diploid and polyploidization can only have occurred after the species reached North America via Beringia”. Apparently, increases in ploidy occurred in these insects and plants during the Ice Age, when small populations were confined to the areas immediately north of the Alps and Himalayas.
CONCLUSION
In general, the current molecular and paleontological findings fully support the hypothesis that several plant and animal taxa of the Arctic originate from ancestors that inhabited the mountains of more southern climatic zones. This finding explains in detail how, despite the relatively short existence of Arctic biota, deep adaptations by Arctic species arose in response to low temperatures. These adaptations emerged from the ancestors of Arctic species that inhabited the highlands. From this perspective, the comparison of Arctic ecosystems, including those that disappeared and the ecosystems of large highlands, primarily the Tibetan Plateau, is a topic of great significance. Furthermore, our findings are important for practical purposes, namely for the acclimatization of economically valuable species. Thus, it should be expected that invasions of Arctic ecosystems by species inhabiting the temperate mountains can be successful.
Список литературы
Abbott R.J., Brochmann C., 2003. History and evolution of the arctic flora: in the footsteps of Eric Hultén // Molecular Ecology. V. 12. P. 299–313.
Abbott R.L., Smith L.C., Milne R.I., Crawford R.M.M., Wolff K., Balfour J., 2000. Molecular Analysis of Plant Migration and Refugia in the Arctic // Science. V. 289. P. 1343–1346.
Abeli T., Vamosi J.C., Orsenigo S., 2018. The importance of marginal population hotspots of coldadapted species for research on climate change and conservation // Journal of Biogeography. V. 45. P. 977–985.
Albach D.C., Schönswetter P., Tribsch A., 2006. Comparative phylogeography of closely related species of the Veronica alpina complex in Europe and North America // Molecular Ecology. V. 15. P. 3269–3286.
Allen G.A., Marr K.L., McCormick L.J., Hebda R.J., 2012. The impact of Pleistocene climate change on an ancient arctic–alpine plant: multiple lineages of disparate history in Oxyria digyna // Ecology and Evolution. V. 2. P. 649–665.
Allen G.A., Marr K.L., McCormick L.J., Hebda R.J. 2015. Geographical origins, migration patterns and refugia of Sibbaldia procumbens, an arctic–alpine plant with a fragmented range // Journal of Biogeography. V. 42. P. 1665–1676.
Ansell S.W., Stenøien H.K., Grundmann M., Russell S.J., Koch M.A., Schneider H., Vogel J.C., 2011. The importance of Anatolian mountains as the cradle of global diversity in Arabis alpina, a key arctic–alpine species // Annals of Botany. V. 108. P. 241–252.
Artamonova V.S., Kolmakova O.V., Kirillova E.A., Makhrov A.A., 2018. Phylogeny of salmonoid fishes (Salmonoidei) based on mtDNA COI gene sequences (barcoding) // Contemporary Problems of Ecology. V. 11. P. 271–285.
Artamonova V.S., Kucheryavyy A.V., Makhrov A.A., 2015. Nucleotide sequence diversity of the mitochondrial cytochrome oxidase subunit I (COI) gene of the Arctic lamprey (Lethenteron camtschaticum) in the Eurasian part of the range // Hydrobiologia. V. 757. P. 197–208.
Barrio I.C., Schmidt B.C., Cannings S., Hik D.S., 2013. First records of the arctic moth Gynaephora groenlandica (Wocke) South of the Arctic Circle: A new alpine subspecies // Arctic. V. 66. P. 429–434.
Bennett K.D., 2004. Continuing the debate on the role of Quaternary environmental change for macroevolution // Philosophical Transactions of the Royal Society. London. B. V. 359. P. 295–303.
Birks H.H., 2008. The Late-Quaternary history of arctic and alpine plants // Plant Ecology & Diversity. V. 1. P. 135–146.
Biske Yu.S., Sevastyanov D.V., 2003. Why is “Middle Asia” disappering? // Herald of St. Petersburg University. Serie 7. P. 62–65.
Bolotov I., Vikhrev I., Bespalaya Ju., Artamonova V., Gofarov M., Kolosova Ju. et al., 2014. Ecology and conservation of endangered Indochinese freshwater pearl mussel, Margaritifera laosensis (Lea, 1863) in the Nam Pe and Nam Long rivers, Northern Laos // Tropical Conservation Science. V. 7. P. 706–719.
Brandler O.V., Lyapunova E.A., Bannikova A.A., Kramerov D.A., 2010. Phylogeny and systematics of marmots (Marmota, Sciuridae, Rodentia) inferred from Inter_SINE PCR Data // Russian Journal of Genetics. V. 46. P. 283–292.
Brochmann C., Edwards M.E., Alsos I.G., 2013. The dynamic past and future of arctic vascular plants: climate change, spatial variation and genetic diversity // The Balance of Nature and Human Impact. Ed. Rohde K. Cambridge etc.: Published by Cambridge University Press. P. 133–152.
Brunner P.C., Douglas M.R., Osinov A., Wilson C.C., Bernatchez L., 2001. Holarctic phylogeography of Arctic charr (Salvelinus alpinus L.) inferred from mitochondrial DNA sequences // Evolution. V. 55. P. 573–586.
Cassel-Lundhagen A., 2010. Peripheral Relict Populations of Widespread Species; Evolutionary Hotspots or Just More of the Same? // Relict Species. Phylogeography and Conservation Biology. Habel J.C., Assmann T. (Eds.). Heidelberg, Dordrecht, London, New York: Springer. P. 267–275.
Christ H., 1867. Ueber die Verbreitung der Pflanzen der alpinen Region der europäischen Alpenkette // Neue Denkschriften der allgemeinen Schweizerischen Gesellschaft fur die gesammten Naturwissenschaften. V. 22. P. 1–85.
Coles B., Colville B., 1980. A glacial relict mollusc // Nature. V. 286. P. 761.
Cowan P.J., 2007. Geographic usage of the terms Middle Asia and Central Asia // Journal of Arid Environments. V. 69. P. 359–363.
Darlington P.J., 1957. Zoogeography: The Geographical Distribution of Animals. London: Chapman and Hall. 657 p.
Delvene G., Araujo R., 2009. Early Cretaceous non-marine bivalves from the Cameros and Basque-Cantabrian basins of Spain // Journal of Iberian Geology. V. 35. P. 19–34.
Den Bakker H.C., Zuccarello G.C., Kuyper T.W., Noordeloos M.E., 2007. Phylogeographic patterns in Leccinum sect. Scabra and the status of the arctic-alpine species L. rotundifoliae // Mycological Research. V. 111. P. 663–672.
Deng T., Wang X., Fortelius M., Li Q., Wang Y., Tseng Z.J. et al., 2011. Out of Tibet: Pliocene Woolly Rhino Suggests High–Plateau Origin of Ice Age Megaherbivores // Science. V. 333. P. 1285–1288.
Deng T., Wang X., Wu F., Wang Y., Li Q., Wang S., Hou S., 2019. Review: Implications of vertebrate fossils for paleo-elevations of the Tibetan Plateau // Global and Planetary Change. V. 174. P. 58–69.
Despres L., Loriot S., Gaudeul M., 2002. Geographic pattern of genetic variation in the European globeflower Trollius europaeus L. (Ranunculaceae) inferred from amplified fragment length polymorphism markers // Molecular Ecology. V. 11. P. 2337–2347.
Dmitrenko I.A., Polyakov I.V., Kirillov S.A., Timokhov L.A., Frolov I.E., Sokolov V.T. et al., 2008. Toward a warmer Arctic Ocean: Spreading of the early 21st century Atlantic Water warm anomaly along the Eurasian Basin margins // Journal of Geophysical Research. V. 113. P. C05023.
Dynesius M., Jansson R., 2000. Evolutionary consequences of changes in species’ geographical distributions driven by Milankovitch climate oscillations // Proceedings of the National Academy of Sciences of USA. V. 97. P. 9115–9120.
Ebersbach J., Muellner-Riehl A.N., Michalak I., Tkach N., Hoffmann M.H., Röser M., et al., 2017. In and out of the Qinghai-Tibet Plateau: divergence time estimation and historical biogeography of the large arctic-alpine genus Saxifraga L. // Journal of Biogeography. V. 44. P. 900–910.
Fan D.-M., Chen J.-H., Meng Y., Wend J., Huang J.-L., Yang Y.-P., 2013. Molecular phylogeny of Koenigia L. (Polygonaceae: Persicarieae): Implications for classification, character evolution and biogeography // Molecular Phylogenetics and Evolution. V. 69. P. 1093–1100.
Fang Z.–J., Chen J., Chen C., Sha J., Lan X., Wen S., 2009. Supraspecific taxa of the Bivalvia first named, described, and published in China (1927–2007) // The University of Kansas Paleontological Contributions, New Series. V. 17. P. 1–157.
Favre A., Michalak I., Chen C.-H., Wang J.-C., Pringle J.S., Matuszak S. et al., 2016. Out-of-Tibet: the spatio-temporal evolution of Gentiana (Gentianaceae) // Journal of Biogeography. V. 43. P. 1967–1978.
Favre A., Päckert M., Pauls S.U., Jähnig S.C., Uhl D., Michalak I., Muellner-Riehlet A.N., 2015. The role of the uplift of the Qinghai-Tibetan Plateau for the evolution of Tibetan biotas // Biological Reviews. V. 90. P. 236–253.
Fisher R.A., Ford E.B., 1950. The “Sewall Wright effect” // Heredity. V. 4. P. 117–119.
Forbes E., 1846. On the connection between the distribution of the existing fauna and flora of the British Isles, and the geological changes which have affected their area, especially during the epoch of the Northern Drift // Memoirs of the Geological survey of Great Britain, and of the Museum of economic geology in London. V. 1. P. 336–432.
Fu P.-C., Gao Q.-B., Zhang F.-Q., Xing R., Khan G., Wang J.-L., et al., 2016. Responses of plants to changes in Qinghai-Tibetan Plateau and glaciations: Evidence from phylogeography of a Sibiraea (Rosaceae) complex // Biochemical Systematics and Ecology. V. 65. P. 72–82.
Garcia-Ramos G., Kirkpatrick M., 1997. Genetic models of adaptation and gene flow in peripheral population // Evolution. V. 51. P. 21–28.
Hassanin A., 2015. The role of Pleistocene glaciations in shaping the evolution of polar and brown bears. Evidence from a critical review of mitochondrial and nuclear genome analyses // Comptes Rendus Biologies. V. 338. P. 494–501.
Hassanin A., Ropiquet A., Couloux A., Cruaud C., 2009. Evolution of the Mitochondrial Genome in Mammals Living at High Altitude: New Insights from a Study of the Tribe Caprini (Bovidae, Antilopinae) // Journal of Molecular Evolution. V. 68. P. 293–310.
Hedberg K.O., 1992. Taxonomic differentiation in Saxifraga hirculus L. (Saxifragaceae) – a circumpolar Arctic-Boreal spedes of Central Asiatic origin // Botanical Journal of the Linnean Society. V. 109. P. 377–393.
Hewitt G.M., 2004. Genetic consequences of climatic oscillations in the Quaternary // Philosophical Transactions of the Royal Society. Series B. V. 359. P. 183–195.
Hoffmann A.A., Parsons P.A., 1997. Extreme Environmental Change and Evolution. Cambridge: Cambridge University Press. 259 p.
Holyoak M., Heath S.K., 2016. The integration of climate change, spatial dynamics, and habitat fragmentation: A conceptual overview // Integrative Zoology. V. 11. P. 40–59.
Hoorn C., Mosbrugger V., Mulch A., Antonelli A., 2013. Biodiversity from mountain building // Nature Geoscience. V. 6. P. 154.
Hoorn C., Perrigo A., Antonelli A., Eds. 2018. Mountains, Climate and Biodiversity. Oxford: John Wiley & Sons Ltd. 544 p.
Horsák M., Chytrý M., Hájková P., Hájek M., Danihelka J., Horsáková V., et al., 2015. European glacial relict snails and plants: environmental context of their modern refugial occurrence in southern Siberia // Boreas. V. 44. P. 638–657.
Hou Y., Bjorå C.S., Ikeda H., Brochmann C., Popp M., 2016. From the north into the Himalayan–Hengduan Mountains: fossil-calibrated phylogenetic and biogeographical inference in the arctic-alpine genus Diapensia (Diapensiaceae) // Journal of Biogeography. V. 43. P. 1502–1513.
Hou Y., Nowak M.D., Mirré V., Bjorå C.S., Brochmann C., Popp M., 2016a. RAD-seq data point to a northern origin of the arctic–alpine genus Cassiope (Ericaceae) // Molecular Phylogenetics and Evolution. V. 95. P. 152–160.
Hultén E., 1937. Outline of the history of Arctic and Boreal biota during the quaternary period. Stockholm: Bokförlags Aktiebolaget Thule. 168 p.
Hulten E., 1958. The amphi-atlantic plants and their phytogeographical connections. Stockholm: Almqvisk & Wiksell. 340 p.
de Humboldt A., 1843. Asia Centrale. Recherches sur les chaines de montagnes et la climatologie comparee. V. 1. Gide, Paris: Libraire-Editeur. 1172 p.
von Humboldt A., Bonpland A., 1807. Ideen zu einer Geographie der Pflanzen, nebst einem Naturgemälde der Tropenländer, auf Beobachtungen und Messungen gegründet, welche vom 10. Tübingen: F.G. Cotta. 182 p.
Ikeda H., Eidesen P.B., Yakubov V., Barkalov V., Brochmann C., Setoguchi H., 2017. Late Pleistocene origin of the entire circumarctic range of the arctic-alpine plant Kalmia procumbens // Molecular Ecology. V. 26. P. 5773–5783.
Ikeda H., Setoguchi H., 2017. Importance of Beringia for the divergence of two northern Pacific alpine plants, Phyllodoce aleutica and Phyllodoce glanduliflora (Ericaceae) // Biological Journal of the Linnean Society. V. 122. P. 249–257.
Ikeda H., Yakubov V., Barkalov V., Setoguchi H., 2014. Molecular evidence for ancient relicts of arctic-alpine plants in East Asia // New Phytologist. V. 203. P. 980–988.
Jaeschke A., Bittner T., Jentsch A., Beierkuhnlein C., 2014. The last decade in ecological climate change impact research: where are we now? // Naturwissenschaften. V. 101. P. 1–9.
Kahlke R.-D., 2014. The origin of Eurasian mammoth faunas (Mammuthus–Coelodonta faunal complex) // Quaternary Science Reviews. V. 96. P. 32–49.
Kirkpatrick M., Barton N.H., 1997. Evolution of a species' range // The American Naturalist. V. 150. P. 1–23.
Kleckova I., Cesanek M., Fric Z., Pellissier L., 2015. Diversification of the cold-adapted butterfly genus Oeneis related to Holarctic biogeography and climatic niche shifts // Molecular Phylogenetics and Evolution. V. 92. P. 255–265.
Kotov A.A., 2016. Faunistic Complexes of the Cladocera (Crustacea, Branchiopoda) of Eastern Siberia and the Far East of Russia // Zoologicheskiy Zhurnal. V. 95. P. 748–768.
Kusnezov N.J., 1938. The arctic fauna of Eurasia and its origin; A study based mainly on Lepidoptera // Travaux de l’Institut Zoologique de l’Académie des Sciences de l’URSS. V. 5. P. 1–85.
Lagerholm V.K., Noren K., Ehrich D., Ims R.A., Killengreen S.T., Abramson N.I., et al., 2017. Run to the hills: gene flow among mountain areas leads to low genetic differentiation in the Norwegian lemming // Biological Journal of the Linnean Society. V. 121. P. 1–14.
Li G.-D., Kim C., Zha H.-G., Zhou Z., Nie Z.-L., Sun H., 2014. Molecular phylogeny and biogeography of the arctic-alpine genus Lagotis (Plantaginaceae) // Taxon. V. 63. P. 103–115.
Lindholm M., Anglès d’Auriac M., Thaulow J., Hobæk A., 2016. Dancing around the pole: holarctic phylogeography of the Arctic fairy shrimp Branchinecta paludosa (Anostraca, Branchiopoda) // Hydrobiologia. V. 772. P. 189–205.
Lister A.M., Sher A.V., 2015. Evolution and dispersal of mammoths across the Northern Hemisphere // Science. V. 350. p. 805–809.
Long C., Min Y.J., Zhao X.X., Yany C.L., Sun H., Lü H.Y., et al., 2014. Origin area and migration route: Chloroplast DNA diversity in the arctic-alpine plant Koenigia islandica // Science China, Earth Sciences. V. 57. P. 1760–1770.
Lopes-Lima M., Bolotov I.N., Do V.T., Aldridge D.C., Fonseca M.M., et al., 2018. Expansion and systematics redefinition of the most threatened freshwater mussel family, the Margaritiferidae // Molecular Phylogenetics and Evolution. V. 127. P. 98–118.
Makarova O.L., 2013. Gamasid mites (Parasitiformes, Mesostigmata) of the European Arctic and their distribution patterns // Entomological Review. V. 93. P. 113–133.
Makhrov A.A, Artamonova V.S., Bobrov V.V., Koblik E.A., Lebedev V.S., Pavlova S.V., Sheftel B.I. 2019. From subtropics to taiga and “tundra-steppe”: a trip to the Qinling mountains and the eastern edge of Tibet (In Russian, summary in English) // Nature (Moscow). № 3. P. 70–83.
Makhrov A., Bespalaya Ju., Bolotov I., Vikhrev I., Gofarov M., Alekseeva Ya., Zotin A., 2014. Historical geography of pearl harvesting and current status of populations of freshwater pearl mussel Margaritifera margaritifera (L.) in the western part of Northern European Russia // Hydrobiologia. V. 735. P. 149–159.
Makhrov A.A., Bolotov I.N., 2006. Dispersal Routes and Species Identification of Freshwater Animals in Northern Europe: A Review of Molecular Evidence // Russian Journal of Genetics. V. 42. P. 1101–1115.
Makhrov A.A., Bolotov I.N., Spitsyn V.M., Gofarov M.Yu., Artamonova V.S. 2019a. Resident and Anadromous Forms of Arctic Charr (Salvelinus alpinus) from North-East Europe: An Example of High Ecological Variability without Speciation // Doklady Biochemistry and Biophysics. V. 485. P. 119–122.
Makhrov A.A., Bolotov I.N., 2019. Ecological causes of high morphological plasticity of members of a taxon inhabiting the center of its origin (Exemplified by the Noble Salmons, genus Salmo) // Biology Bulletin. V. 46. P. 38–46.
Malikov D., 2015. Zoogeographical features of Mammoth fauna of the south of Siberia (in Russian, Summary in English) // Tomsk State University Journal. V. 398. P. 233–242.
Marr K.L., Allen G.A., Hebda R.J., McCormick L.J., 2013. Phylogeographical patterns in the widespread arctic–alpine plant Bistorta vivipara (Polygonaceae) with emphasis on western North America // Journal of Biogeography. V. 40. P. 847–856.
Matthew W.D., 1939. Climate and evolution. Second edition, revised and enlarged. New York: New York Academy of Sciences. 223 p.
De Meester L., Stoks R., Brans K.I., 2018. Genetic adaptation as a biological buffer against climate change: Potential and limitations // Integrative Zoology. V. 13. P. 372–391.
Meyen S.V., 1987. Geography of macroevolution in higher plants // Journal of General Biology. V. 48. P. 291–309.
Miller N.G., 1996. On the distributional history of the arctic-alpine moss Cyrtomnium hymenophylloides (Mniaceae) in North America // The Bryologist. V. 99. P. 187–192.
Mosbrugger V., Favre A., Muellner-Riehl A.N., Packert M., Mulch A., 2018. Cenozoic Evolution of Geobiodiversity in the Tibeto-Himalayan Region // Mountains, Climate and Biodiversity. Hoorn C., Perrigo A., Antonelli A. (Eds). Oxford: John Wiley & Sons Ltd. P. 429–448.
Muellner-Riehl A.N., 2019. Mountains as Evolutionary Arenas: Patterns, Emerging Approaches, Paradigm Shifts, and Their Implications for Plant Phylogeographic Research in the Tibeto-Himalayan Region // Frontiers in Plant Science. V. 10. P. 195.
Murray D.F., 1995. Causes of Arctic plant diversity: origin and evolution // Arctic and Alpine Biodiversity: Patterns, causes and ecosystem consequences. Chapin III F.S., Korner C. (Eds). Berlin, Heidelberg: Springer-Verlag. P. 21–32.
Muster C., Berendonk T.U., 2006. Divergence and diversity: lessons from an arctic–alpine distribution (Pardosa saltuaria group, Lycosidae) // Molecular Ecology. V. 15. P. 2921–2933.
Pauls S.U., Nowak C., Balint M., Pfenninger M., 2013. The impact of global climate change on genetic diversity within populations and species // Molecular Ecology. V. 22. P. 925–946.
Paule Ju., Kolár F., Dobeš C., 2015. Arctic-alpine and serpentine differentiation in polyploidy Potentilla crantzii // Preslia. V. 87. P. 195–215.
Pellissier L., Heine C., Rosauer D.F., Albouy C., 2018. Are global hotspots of endemic richness shaped by plate tectonics? // Biological Journal of the Linnean Society. V. 123. P. 247–261.
Poplavskaya N.S., Bannikova A.A., Fang Y., Sheftel B.I., Ushakova M.V., Surov A.V., Lebedev V.S., 2018. Is the center of origin of long-tailed hamster Cricetulus longicaudatus Milne-Edwards 1867 (Rodentia, Cricetidae) located in Tibet? // Doklady Biological Sciences. V. 479. P. 70–73.
von Richthofen F.F., 1877. China. Ergebnisse eigener Reisen und darauf gegründeter Studien. Band 1. Berlin: Verlag von Dietrich Reimer. 758 p.
Ronikier M., Schneeweiss G.M., Schönswetter P., 2012. The extreme disjunction between Beringia and Europe in Ranunculus glacialis s. l. (Ranunculaceae) does not coincide with the deepest genetic split – a story of the importance of temperate mountain ranges in arctic–alpine phylogeography // Molecular Ecology. V. 21. P. 5561–5578.
Røen U., 1994. A theory for the origin of the Arctic freshwater fauna // Verhandlungen der Internationale Vereinigung fur Theoretische und Angewandte Limnologie. V. 25. P. 2409–2412.
Sanders W.J., Haile-Selassie Y., 2012. A new assemblage of Mid-Pliocene Proboscideans from the Woranso-Mille Area, Afar Region, Ethiopia: Taxonomic, evolutionary, and paleoecological considerations // Journal of Mammalian Evolution. V. 19. P. 105–128.
Schenková V., Horsák M., 2013. Refugial Populations of Vertigo lilljeborgi and V. genesii (Vertiginidae): New Isolated Occurrences in Central Europe, Ecology and Distribution // American Malacological Bulletin. V. 31. P. 323–329.
Schmitt T., Muster C., Schönswetter P., 2010. Are Disjunct Alpine and Arctic-Alpine Animal and Plant Species in the Western Palearctic Really “Relics of a Cold Past”? // Relict Species. Phylogeography and Conservation Biology. Habel J.C., Assmann T. (Eds). Heidelberg, Dordrecht, London, New York: Springer. P. 239–252.
Schönswetter P., Paun O., Tribsch A., Niklfeld H., 2003. Colonization of northern Europe by east Alpine populations of the glacier buttercup Ranunculus glacialis L. (Ranunculaceae) // Molecular Ecology. V. 12. P. 3373–3381.
Schönswetter P., Popp M., Brochmann C., 2006. Central Asian origin of and strong genetic differentiation among populations of the rare and disjunct Carex atrofusca (Cyperaceae) in the Alps // Journal of Biogeography. V. 33. P. 948–956.
Schönswetter P., Popp M., Brochmann C., 2006a. Rare arctic-alpine plants of the European Alps have different immigration histories: the snow bed species Minuartia biflora and Ranunculus pygmaeus // Molecular Ecology. V. 15. P. 709–720.
Schönswetter P., Tribsch A., Niklfeld H., 2004. Amplified fragment length polymorphism (AFLP) suggests old and recent immigration into the Alps by the arctic-alpine annual Comastoma tenellum (Gentianaceae) // Journal of Biogeography. V. 31. P. 1673–1681.
Sher A.V., Weinstock J., Baryshnikov G.F., Davydov S.P., Boeskorov G.G., Zazhigin V.S., Nikolskiy P.A., 2011. The first record of “spelaeoid” bears in Arctic Siberia // Quaternary Science Reviews. V. 30. P. 2238–2249.
Skrede I., Eidesen P.B., Portela R.P., Brochmann C., 2009. Refugia, differentiation and postglacial migration in arctic-alpine Eurasia, exemplified by the mountain avens (Dryas octopetala L.) // Molecular Ecology. V. 15. P. 1827–1840.
Smith G.R., 1975. Fishes of the Pliocene Glenns Ferry formation, Southwest Idaho // Papers on Paleontology, University of Michigan. V. 14. P. 1–68.
Stebbins G.L., 1984. Polyploidy and the distribution of the arctic-alpine flora: new evidence and a new approach // Botanica Helvetica. V. 94. P. 1–13.
Sun H., Zhang J., Deng T., Boufford D.E., 2017. Origins and evolution of plant diversity in the Hengduan Mountains, China // Plant Diversity. V. 39. P. 161–166.
Suomalainen E., Saura A., Lokki J., 1976. Evolution of partenogenetic insects // Evolutionary Biology. V. 9. Hecht M.K., Steere W.C., Wallace B. (Eds). New York, London: Plenum Press. P. 209–257.
Sytchevskaya E.K., 1986. Palaeogene freshwater fish fauna of the USSR and Mongolia (in Russian). Moscow: Nauka. 157 p.
Theissinger K., Bálint M., Feldheim K.A., Haase P., Johannesen J., Laube I., Pauls S.U., 2013. Glacial survival and post-glacial recolonization of an arctic–alpine freshwater insect (Arcynopteryx dichroa, Plecoptera, Perlodidae) in Europe // Journal of Biogeography. V. 40. P. 236–248.
Tiberti R., 2011. Morphology and ecology of Daphnia middendorffiana, Fisher 1851 (Crustacea, Daphniidae) from four new populations in the Alps // Journal of Limnology. V. 70. P. 239–247.
Tkach N., Ree R.H., Kuss P., Röser M., Hoffmann M.H., 2014. High mountain origin, phylogenetics, evolution, and niche conservatism of arctic lineages in the hemiparasitic genus Pedicularis (Orobanchaceae) // Molecular Phylogenetics and Evolution. V. 76. P. 75–92.
Tolmachev A.I., 1960. Der Autochthone Grundstock der arktischen Flora und ihre Beziehungen zu den Hochgebirgsfloren Nord- und Zentralasiens // Botanisk Tidsskrift. V. 55. P. 269–276.
Tseng Z.J., Wang X., Slater G.J., Takeuchi G.T., Li Q., Liu J., Xie G., 2014. Himalayan fossils of the oldest known pantherine establish ancient origin of big cats // Proceedings of the Royal Society. Series B. V. 281. e20132686
Tugarinov A.J., 1935. Essay on the history of the Arctic fauna of Eurasia // Transactions of the II International conference of the Association on the study of the Quaternary period in Europe. fasc. 5. Bykowski S.N. (Ed.). Leningrad, Moscow: Scientific technical geological and prospecting editorial office. P. 47–58.
Ukraintseva V.V., 2013. Mammoths and the environment. Cambridge: Cambridge University Press. 346 p.
Valentine J.W., 1968. Climatic regulation of species diversification and extinction // Geological Society of America, Bulletin. V. 79. P. 273–276.
Van Damme D., Bogan A.E., Dierick M., 2015. A revision of the Mesozoic naiads (Unionoida) of Africa and the biogeographic implications // Earth-Science Reviews. V. 147. P. 141–200.
Varga Z.S., Schmitt T., 2008. Types of oreal and oreotundral disjunctions in the western Palearctic // Biological Journal of the Linnean Society. V. 93. P. 415–430.
Wang X., Li Q., Takeuchi G.T., 2016. Out of Tibet: an early sheep from the Pliocene of Tibet, Protovis himalayensis, genus and species nov. (Bovidae, Caprini), and origin of Ice Age mountain sheep // Journal of Vertebrate Paleontology. V. 36. e1169190.
Wang Q., Liu J., Allen G.A., Ma Y., Yue W., Marr K.L., Abbott R.J., 2016. Arctic plant origins and early formation of circumarctic distributions: a case study of the mountain sorrel, Oxyria digyna // New Phytologist. V. 209. P. 343–353.
Wang X., Tseng Z.J., Li Q., Takeuchi G.T., Xie G., 2014. From ‘third pole’ to north pole: a Himalayan origin for the arctic fox // Proceedings of the Royal Society. Series B. V. 281. 20140893.
Weider L.J., Hobæk A., 2000. Phylogeography and arctic biodiversity: a review // Annales Zoologici Fennici. V. 37. P. 217–231.
Wen J., Zhang J.-Q., Nie Z.-L., Zhong Y., Sun H., 2014. Evolutionary diversifications of plants on the Qinghai-Tibetan Plateau // Frontiers in Genetics. V. 5. Article 4.
Wilson M.V.H., Li G.-Q., 1999. Osteology and systematic position of the Eocene salmonid †Eosalmo driftwoodensis Wilson from western North America // Zoological Journal of the Linnean Society. V. 125. P. 279–311.
Winkler M., Tribsch A., Schneeweiss G.M., Brodbeck S., Gugerli F., Holderegger R. et al., 2012. Tales of the unexpected: Phylogeography of the arctic-alpine model plant Saxifraga oppositifolia (Saxifragaceae) revisited // Molecular Ecology. V. 21. P. 4618–4630.
WoldeGabriel G., Haile-Selassie Y., Renne P.R., Hart W.K., Ambrosek S.H., Asfaw B. et al., 2001. Geology and palaeontology of the Late Miocene Middle Awash valley, Afar Rift, Ethiopia // Nature. V. 412. P. 175–178.
Wulff E.V., 1944. Historical geography of plants – History of the world’s flora. Moscow & Leningrad: Academy of Sciences of the U.S.S.R. 546 p.
Yakovlev V.N., 1964. History of formation of fish faunistic complexes // Voprosy Ichtyologii. V. 4. P. 10-22.
Yang C., Xiang C., Qi W., Xia S., Tu F., Zhang X. et al., 2013. Phylogenetic analyses and improved resolution of the family Bovidae based on complete mitochondrial genomes // Biochemical Systematics and Ecology. V. 48. P. 136–143.
Zhang X.-L., Wang Y.-J., Ge X.-J., Yuan Y.-M., Yang H.-L., Liu J.-Q., 2009. Molecular phylogeny and biogeography of Gentiana sect. Cruciata (Gentianaceae) based on four chloroplast DNA datasets // Taxon. V. 58. P. 862–870.
Дополнительные материалы отсутствуют.
Инструменты
Зоологический журнал


