Зоологический журнал, 2019, T. 98, № 2, стр. 130-148
New species of the genus Anurida (Collembola, Neanuridae) from the Far East of Russia
A. Б. Бабенко a, *, Ю. Б. Швеенкова b, **, Н. А. Кузнецова c, ***
a Институт экологии и эволюции им. А.Н. Северцова РАН
119071 Москва, Россия
b Государственный природный заповедник “Приволжская лесостепь”
440031 Пенза, Россия
c Московский государственный педагогический университет
129278 Москва, Россия
* E-mail: lsdc@mail.ru
** E-mail: jushv@mail.ru
*** E-mail: mpnk@yandex.ru
Поступила в редакцию 19.05.2018
После доработки 23.08.2018
Принята к публикации 17.07.2018
Аннотация
A review of the Anurida fauna of the Far East of Russia was performed, based on fresh material from forest ecosystems of Primorsky Krai. This taxon generally seems to be highly diverse and rather peculiar in the region under consideration. Seven new species of the genus are described and illustrated. Four of them, A. pallens sp. n., A. armifera sp. n., A. multisensillata sp. n. and A. olae sp. n., belong to the weberi-group widespread in Siberia and North America. They can be distinguished by colouration, the number of ocelli, fine differences in the structure of the mouthparts and in the set of sensilla on the antennae. Anurida elegans sp. n. is a typical representative of the Beringian hammerae-group most similar to A. luciae Fjellberg 1985. Anurida longilamellata sp. n. resembles two Asiatic blind species, viz. A. nadezhdae Babenko 1998, described from central Siberia, and A. kazukoae Tamura 1997, known from China. The other described blind species, i.e. A. aspera sp. n., can be compared to a number of congeners, most of which are also known only from adjacent regions of the East Palaearctic. A key to the known non-cave Asiatic species of the genus is provided.
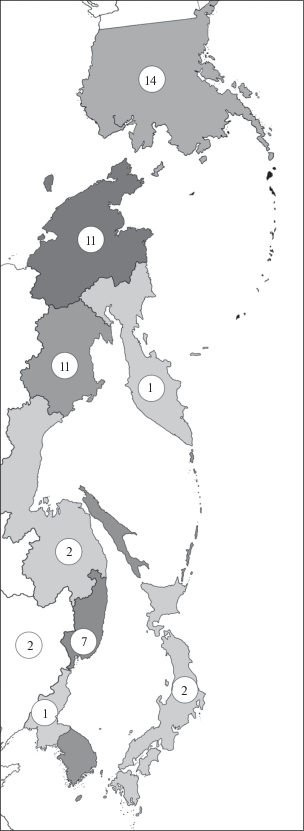
Arne Fjellberg, a famous Norwegian collembologist, was the first to draw the attention to an increased diversity of the Anurida-complex on both coasts of the Northern Pacific. He revealed as many as 14 species of the genus in Alaska (Fjellberg, 1985), described several additional species from NE Siberia and the northern Cordillera (Fjellberg 1985a), and emphasized that whether this richness in species has its centre in Beringia or not, is early to say (Fjellberg 1985, p. 122). The Anurida fauna of northeastern Russia also appears to be rather rich. Thus, eight species of the genus (Babenko, 2017) have been found at one locality on the eastern coast of the Chukchi Peninsula, and no less than 16 species east of the Kolyma basin, i.e. in Chukchi Peninsula and Magadan Province taking together (Babenko, 1997, 1998; Babenko, Fjellberg, 2006; Potapov et al., 2018). Against the background of a very poor non-cavernicolous fauna of the group in the more southern parts of eastern Asia, these estimates look quite impressive (Fig. 1). For example, only the widespread A. tullbergi Schött 1891 has been found on the Korean Peninsula (Weiner, Najt, 1985). Three terrestrial forms, A. trioculata Kinoshita 1916, A. papillosoides (Hammer 1953) and A. abashiriensis Babenko et Nakamori 2018, have been recorded in Japan, although the genus includes many cave forms in both Japan and Korea (Yosii, 1956, 1972, 1977, Lee, Park, 2016). The genus is equally impoverished across the vast territories of China, with only two species involved: A. kazukoae Tamura 1997 and A. tamurae Yue et Yin 1999. In the adjoining regions of the Russian Far East, the genus has only recently become studied. In our ecological research devoted to the spatial distribution of springtails in the multi-species communities of the Far East, we have encountered not only a rather rich regional fauna of Anurida and allied genera, but also serious problems in species identification. The present paper solves some of existing taxonomic problems.
Fig. 1.
Map of Russian Far East and surrounding countries with the numbers of recorded terrestrial Anurida species in different regions (from top to bottom: Alaska, Chukchi Peninsula, Magadan Province, Kamchatka, Khabarovsk Krai, Primorsky Krai, Northeast China, Japan, North Korea).
SPECIES DESCRIPTIONS
Anurida pallens Babenko, Shveenkova et Kuznetsova sp. n. Figs 2, 1; 2, 4; 3, 1–5
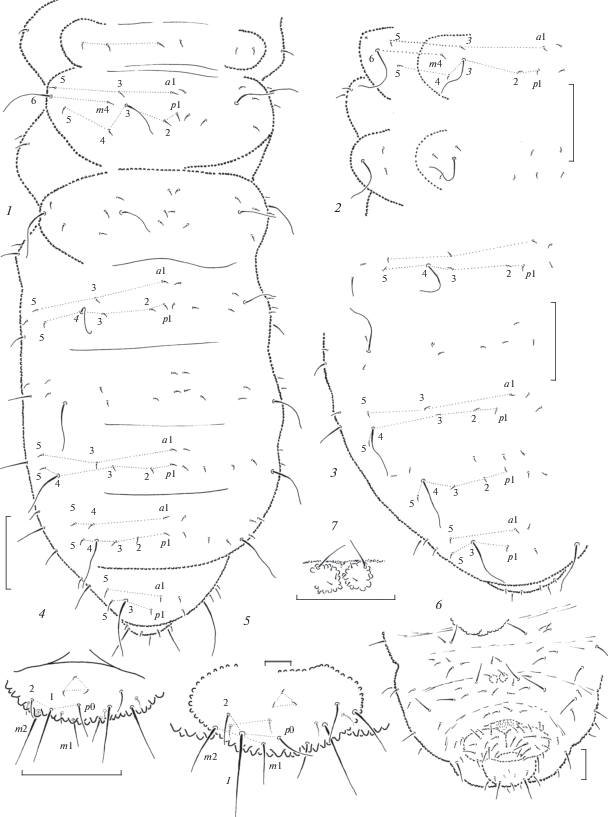
Fig. 2.
Anurida pallens sp. n. (1, 4) and A. armifera sp. n. (2–3, 5–7): 1 – dorsal chaetotaxy; 2 – dorsal chaetotaxy of Th.2–3; 3 – dorsal chaetotaxy of abdomen; 4–5 – ventral side of Abd.6; 6 – ventral chaetotaxy of abdomen; 7 – furcal remnant. Scales (mm): 1–4, 6–7 – 0.1; 5 – 0.01.
Diagnosis. A whitish species of the weberi-group characterized by the presence of 2 + 2(3) indistinct ocelli, six usual blunt sensilla on Ant.4, oval PAO with 15–18 lobes, the mandibles with 4 + 2 teeth set in a line, and serrate maxillary L.1.
Type material. Holotype, preadult male, Far-East of Russia, Northern Primorye, Sikhote-Alin Nature Reserve, Kabaniyy station [N 45.1384°, E 135.8870°], coniferous wood with Rhododendron fauriei, litter, 932 m alt., 8.08.2017, leg. N. Kuznetsova, A. Geraskina and A. Kuprin. Paratypes, 4 males and 3 females, Sikhote-Alin Nature Reserve, Blagodatny station [N 44.9817°, E 136.5341°], 95 m alt., oak wood with hazel, litter, 7.08.2017, leg. N. Kuznetsova, A. Geraskina and A. Kuprin. The types are kept in MSPU.
Additional material. About 30 specimens from the same region.
Description. Length without antennae 1.0–1.2 mm. General colour on available slides whitish including ocelli and ocular area of head22. Body shape typical of weberi-group, rather stout and somewhat flattened, Abd.6 rounded, not truncate, partly hidden under Abd.5. Integument granulation coarse and almost uniform.
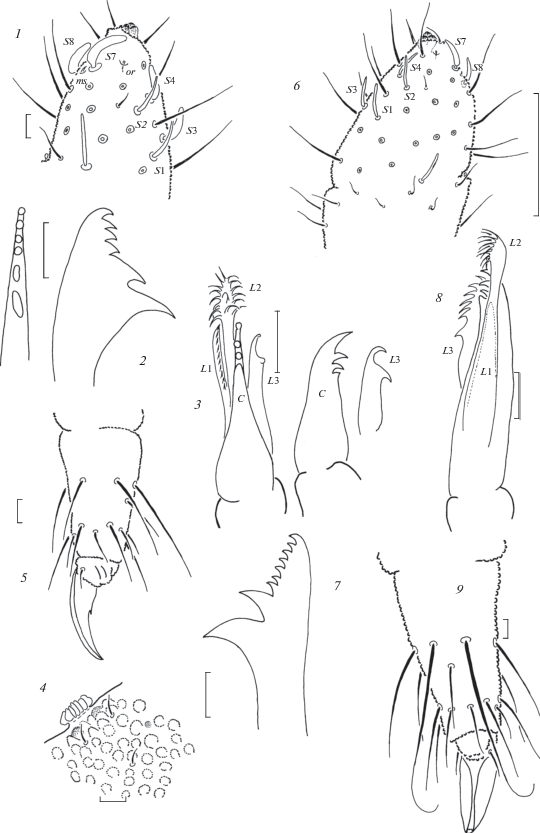
Ocelli present, 2 + 2(3), small, about as large as skin granules, usually uncolored and hard to detect. PAO roughly circular or slightly elongate, with 15–18 lobes (Fig. 3, 4). Antennae clearly shorter than head, of typical generic shape. Ant.4 with 3-lobed apical bulb, usual curved sensilla, S1 and S2 thinner than S3 and S4, in lateral group S7 clearly longer and thicker than S8, subapical ms and organite present (Fig. 3, 1). AO on Ant.3 typical, outer sensilla widely separated, small ms present ventrally. Ant.1–2 with 7 and (12)13 setae, respectively. Labral formula as 4/2-3-5-2. Labium with 11 setae on each side including three setae on its apical part also armed with two small sensillar papillae. Mandibles (Fig. 3, 2) with 4 apical teeth set in line and 2 larger basal ones. Maxillary capitulum with 3 apical teeth on capitulum followed by straight cutting edge, clearly serrate L.1, rather long L.2 reaching well beyond tip of capitulum, and L.3 with 2 strong hooks (Fig. 3, 3).
Fig. 3.
Anurida pallens sp. n. (1–5) and A. armifera sp. n. (6–9): 1 – antennal tip; 2 – mandible; 3 – maxilla; 4 – PAO and ocelli; 5 – distal part of leg 3; 6 – antennal tip; 7 – mandible; 8 – maxilla; 9 – distal part of leg 3. Scales (mm): 6 – 0.1; 1–5, 7–9 – 0.01.
Common dorsal setae fine and short, differentiated into macro and microsetae only on Abd.6 (and also on abdominal sterna), sensilla thin and long, 5–6 times longer than ordinary setae, lateral microsensilla (ms) present on Th.2. Dorsal chaetotaxy typical of weberi-group (Fig. 2, 1). Main characteristics: tergum of Th.1 with 3 + 3 setae. Th.2–3 with seta p2 slightly posteriorly to p1, sensilla (p3) clearly moved forward and set practically at level with setae m4, only three setae (a1, a3, and a5) of a-row present. Abd.1–4 with 3 + 3 p-setae between sensilla (p4) and three widely separated setae in a-row (a1, a3, and a5). Abd.5 with only 2 + 2 setae (a1 and p1) between sensilla (p3). Differentiation of setae on Abd.6 as on Fig. 2, 4. Thoracic sterna without setae. Ventral tube usually with 4 + 4 setae. Furca remnant as two small warts, each with one seta and few setulae, in anterior part of Abd.4 sternum.
Legs 1–3 chaetotaxy as following: upper subcoxae – 1, 2, 2; lower subcoxae – 0, 2–3, 2–3; coxae – 2–3, 5, 5–7; trochanters – 6, 6, 5–6 (one posterior seta on trochanter of leg 3 clearly thinner and shorter than others); femora – 12–13, 11–12, 11; tibiotarsi – 19, 19, 18 setae, respectively. Tibiotarsal setae of moderate length, longest inner setae of B-whorl about as long as 1.3–1.4 of inner unguis edge (Fig. 3, 5). Unguis with clear inner tooth in basal half of inner edge.
Etymology. The name reflects the pale coloration of the new species, this being not very typical of the weberi-group: from the Latin pallens, meaning pale, dim, faded.
Affinities. Anurida pallens sp. n. is a representative of the weberi-group of the genus as defined by Babenko (2002) in sharing the same type of maxillae and mandibles, a typical arrangement of dorsal setae, and a broadened and somewhat flattened body with a small roundish Abd.6 partly hidden under Abd.5. Among the known species of the group, it seems to be most similar to A. interior Fjellberg 1985, described from the inner parts of Alaska. Both species are whitish and have a reduced number of ocelli (3 or less), this being not too common in the group. In addition, both are characterized by the almost identical differentiation of sensilla on Ant.4, with S1 and S2 being clearly thinner than others. Nevertheless, A. pallens sp. n. can be reliably distinguished from A. interior through the presence of an additional tooth in the apical group on the mandibles (4 + 2 vs 3 + 2 in A. interior), the serrate maxillary L.1 (vs smooth in A. interior), and the significantly longer sensilla on the terga as compared to ordinary setae.
There is also one Palaearctic species of the weberi-group, which is even more similar to A. pallens sp. n., i.e. A. parapapillosa Tshelnokov 1988. It is also white (with dark ocelli), characterized by the mandibles and maxillae identical to those in A. pallens sp. n., but shows 4 + 4 clear ocelli and no differentiated sensilla on Ant.4. The original description of A. parapapillosa was based on a single specimen from the inner Chukotka. A later revision of the holotype (Babenko, 1997) revealed that it was an early juvenile, this explaining the absence of antennal sensilla. Additional uncertainty arose due to the discovery from the same locality of another, similar juvenile with 8 (3 dorsal and 5 lateral) antennal sensilla. Thus, the status of A. parapapillosa remains unclear and it can be distinguished from A. pallens sp. n. only by the number of ocelli (4 + 4 coloured ocelli in A. parapapillosa vs 2 or 3 barely visible ones in A. pallens sp. n.).
There is also one more pale [Farbe reingelb bis braungelb im Leben und fast reinweiss im Alkohol (Yosii, 1954, p. 137)] species with 3 + 3 ocelli in the neighbouring regions, namely, A. trioculata Kinoshita 1916, from Japan. It appears to be a member of the unrelated hammerae-group in having strongly differentiated setae, even though the sole available illustration of its chaetotaxy (Yosii, 1956, fig. 6) is too schematic for a definite conclusion. In any case, A. trioculata is larger (up to 3 mm) and has more lobes in PAO (25–32 vs 15–18 in A. pallens sp. n.), 5 teeth on the mandibles (6 in A. pallens sp. n.), and all maxillary lamellae are clearly serrate.
Yosii (1972), when recording A. papillosoides (Hammer 1953) from Hokkaido (North Cirque, Mt. Poroshiri), noted that one example at hand coincides well with Hammer’s description, but the body colour is totally white (ibid., p. 80). Thus, this form cannot be excluded to be the same as or closely related to A. pallens sp. n. or some other whitish species of the weberi-group described in the present paper.
Distribution. Known from two different sites (coniferous and oak woods) within a territory of Sikhote-Alin State Nature Reserve.
Anurida armifera Babenko, Shveenkova et Kuznetsova sp. n. Figs 2, 2–3; 2, 5–7; 3, 6–9
Diagnosis. A dark coloured species of the genus Anurida with 4 + 4(3) ocelli, six usual blunt sensilla on Ant.4, roundish PAO with 12–16 lobes, the mandibles with 7(6) + 2 teeth set in a line, and maxillary L.3 bearing several strong hooks.
Type material. Holotype, female on slide, Far-East of Russia, South Primorye, Ussuriisky Nature Reserve, Komarovskoye Forest District, Turova Nipple, Khripunovsky Pass [N 43.6481°, E 132.3515°], 207 m alt., Pinus sibirica forest on slope, litter, 22.07.2016, leg. N. Kuznetsova and M. Potapov. Paratypes, 2 females and 3 juveniles, same site and date; one female, same State Reserve, Suvorovskoye Forest District, Anikinsky Station, valley of Anikin River [N 43.6684° E 132.4985°], 156 m alt., broadleaf wood with Pinus sibirica, litter, 13.08.2017, leg. N. Kuznetsova, A. Geraskina and A. Kuprin. The types are kept in the collection of MSPU.
Additional material. About 25 juvenile specimens from the same sites.
Description. Length without antennae 1.8–2.0 mm. Colour probably dark blue but only partly cleared specimens available. Body shape as in species of weberi-group, wide and flattened, Abd.6 rounded, practically hidden under Abd.5. Integument granulation coarse and almost uniform.
Ocelli present, 4 + 4(3), anterior pair clearly larger than skin granules and easily detectable, posterior ocelli smaller and hard to detect, especially median one. PAO roughly circular or slightly elongate, with 10–16 lobes. Antennae clearly shorter than head, of typical generic shape. Ant.4 with 3-lobed apical bulb, 6 usual curved sensilla, those in lateral group (S7 and S8) usually thicker than dorsal sensilla (S1–S4), subapical ms and organite present (Fig. 3, 6). AO on Ant.3 typical, outer sensilla widely separated, small ms present ventrally. Ant.1–2 with 8 and 13 setae, respectively, dorsal setae on Ant. 1–3 significantly shorter than ventral ones. Labral formula as usual (4/2-3-5-2). Labium with 11 setae on each side including three setae on its apical part also armed with two small sensillar papillae. Mandibles (Fig. 3, 7) with 6–8 small apical teeth set in line and 2 larger basal ones. Maxillary capitulum with 3 apical teeth on main part followed by straight cutting edge, fine, hardly detectable L.1 (not clearly seen), rather long L.2 reaching well beyond tip of capitulum, and L.3 with several strong hooks (Fig. 3, 8).
Common dorsal setae fine and short, differentiated into macro and microsetae only on Abd.6 (and also on abdominal sterna), sensilla thin and long, 6–7 times longer than ordinary setae, lateral microsensilla (ms) present on Th.2. Dorsal chaetotaxy as typical of weberi-group (Figs 2, 2–3), but abnormalities and asymmetry in seta position rather frequent. Main characteristics: tergum of Th.1 with 3 + 3 setae. Th.2–3 with seta p2 slightly posteriorly to p1, sensilla (p3) clearly moved forward and set practically at level with setae m4, only three setae (a1, a3, and a5) of a-row present (Fig. 2, 2). Abd.1–4 with 3 + 3 p-setae between sensilla (p4) and three widely separated setae in a-row (a1, a3, and a5), sensilla in anterior position well in front of p3–p5 line. Abd.5 with only 2 + 2 setae (a1 and p1) between sensilla (p3). Differentiation of setae on Abd.6 as on Fig. 2, 5. Thoracic sterna without setae. Ventral tube usually with 4 + 4 setae. Furca remnant as two small warts, each with one seta and few setulae, in anterior part of Abd.4 sternum (Fig. 2, 6–7).
Legs 1–3 chaetotaxy as following: upper subcoxae – 1, 2, 2; lower subcoxae – 0, 2, 2; coxae – 3, 6(7), 6(7); trochanters – 6, 6, 6 (one posterior seta on trochanter of Leg 3 clearly thinner and shorter than others); femora – 13, 12, 11; tibiotarsi – 19, 19, 18 setae, respectively. Tibiotarsal setae rather long, especially inner ones of B-whorl (Fig. 3, 9). Unguis usually with small tooth in middle of inner edge, sometimes hardly visible.
Etymology. Named after the numerous mandibular teeth, from the Latin armifer, meaning armed.
Affinities. Taking into account an arrangement of the dorsal setae and the general body shape of A. armifera sp. n. with small roundish Abd.6 hidden under Abd.5, A. armifera could be placed in the weberi-group, which is common in the region. Nonetheless, it differs from all other species of the group in having several strong hooks on maxillary L.3 vs two typical of the group. It is this trait that has been considered as the main diagnostic character of the weberi-group by Babenko (2002) and therefore the diagnosis of the group needs revision. The number of hooks on L.3 in A. armifera sp. n. appears to be size/age dependent (smaller/younger specimens have less serrations on L.3) and it can well be derived from typical two-hooked lamella of the weberi-group.
Another characteristic feature of A. armifera sp. n. is the shape of the mandibles with an increased number of teeth, set in a line. A similar type of the mandibles is known only in two Nearctic species: A. amorita Folsom 1902 and A. dentata Christiansen et Bellinger 1980. The former has also been recorded from the eastern Palearctic by Axelson (1903), but this record seems to be dubius. It differs from A. armifera sp. n. in having more ocelli (5 + 5 vs 4 + 4), more lobes in PAO (30–40 vs 10–16), all three maxillary lamellae clearly serrate, fine integument granulations and a larger size (up to 4 mm); its chaetotaxy is unknown. Anurida dentata also has 5 + 5 ocelli and it is characterized by a pronounced differentiation of dorsal setae on the abdominal tip (see Christiansen, Bellinger, 1980, fig. 245A ); its maxillary head with long serrate lamellae also differs from that in A. armifera sp. n.
Apart from three species mentioned above, several congeners with multidentate mandibles are known from Japanese caves, for instance, A. speobia Yosii 1954, A. assimilis Yosii 1956 or A. persimilis Yosii 1956, but all of them are blind.
It is also noteworthy that, although such a type of mandibles is not common among the known Palaearctic congeners of the weberi-group, it has been seen in two undescribed forms from the adjacent region (Kedrovaya Pad Nature Reserve). One of them differs from A. armifera sp. n. in having a rather thin maxillary capitulum with two small apical teeth and a blunt tip, L.2 being barely drawn behind the tip of the capitulum, and L.3 with several irregular hooks. The other one possesses two-hooked maxillary L.3, which is typical of the group, and 5 + 2 teeth on the mandible. Unfortunately, only small juveniles of both these forms are present in the available material, rendering their formal description impossible.
Distribution. Known only from the Ussuriisky State Nature Reserve.
Anurida multisensillata Babenko, Shveenkova et Kuznetsova sp. n. Figs 4, 6–7; 5, 1–3
Fig. 4.
Anurida olae sp. n. (1–4), A. weberi (5), and A. multisensillata sp. n. (6–7): 1 – antennal tip; 2–3 – maxilla; 4 – mandible; 5–6 – antennal tip; 7 – labrum and labium. Scales (mm): 1 – 0.1; 2–7 – 0.01.
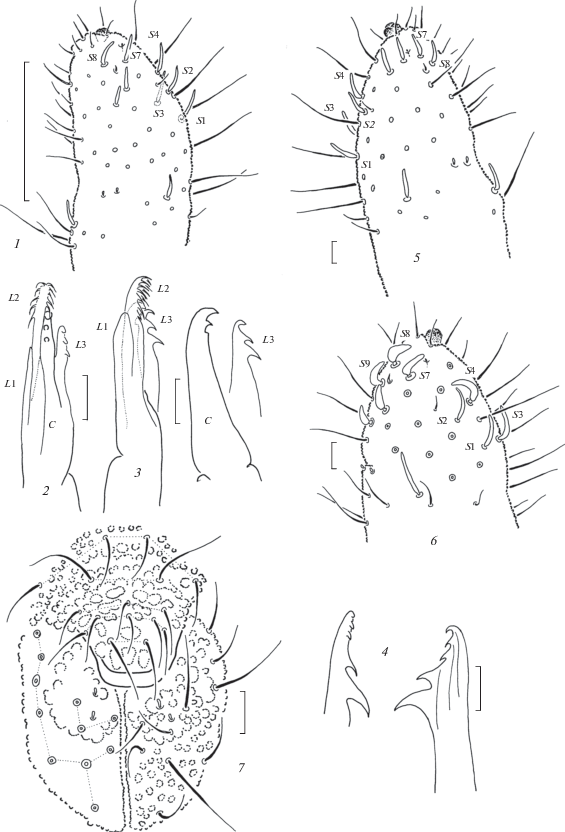
Diagnosis. A white species of the weberi-group of Anurida characterized by the presence of 4 + 4 ocelli, three additional lateral sensilla on Ant.4 (totally 9), roundish PAO with 15–20 lobes, and the mandibles and maxillae of the most typical type.
Type material. Holotype, female on slide, Far-East of Russia, South Primorye, Kedrovaya Pad Nature Reserve [N 43.1010° E 131.5613°], 149 m alt., oak wood, litter, 28.06.2016, leg. N. Kuznetsova and M. Potapov. Paratypes, preadult male, same site, rotten wood; one juvenile, same State Reserve [N 43.1159° E 131.5070°], mixed forest with Abies holophylla and Pinus sibirica, litter, 29.07.2016, leg. N. Kuznetsova and M. Potapov; preadult male and juvenile, Ussuriisky Nature Reserve, Komarovskoye Forest District, Grabovaya Nipple [N 43.6367°, E 132.3499°], 383 m alt., mixed forest on slope, litter, 24.07.2016, leg. N. Kuznetsova and M. Potapov; one male, same State Reserve, Komarovskoye Forest District, Turova Nipple, Khripunovsky Pass [N 43.6481°, E 132.3515°], 207 m alt., Pinus sibirica forest on slope, litter, 22.07.2016, leg. N. Kuznetsova and M. Potapov. The types are kept in the collection of MSPU.
Additional material. 12 juveniles from the Ussuriisky State Nature Reserve.
Description. Length without antennae 1.0–1.1 mm. Colour white excluding ocular area of head. Body shape typical of weberi-group, broadened and flattened, Abd.6 almost completely hidden under Abd.5. Integument granulation coarse and uniform.
Ocelli present, 4 + 4(3), anterior pair distinct, about as large as skin granules, usually colored, posterior ocelli smaller, hardly visible. PAO roughly circular or slightly elongate, with 15–20 lobes (Fig. 5, 3). Antennae shorter than head, of typical generic shape. Ant.4 with 3-lobed apical bulb, subapical ms and organite present; sensillar set enriched: 4 usual sensilla (S1–S4) present in dorsal group (S1 and S2 thinner than S3 and S4) and 5 sensilla laterally (S7–S9 and two additional sensilla below) (Fig. 4, 6). AO on Ant.3 typical, outer sensilla widely separated, small ms present ventrally. Ant.1–2 with 7 and (12)13 setae, respectively. Labral formula as usual 4/2-3-5-2 (Fig. 4, 7). Labium also typical with 11 setae on each side including three setae on its apical part also armed with two sensillar papillae (Fig. 4, 7). Mandibles with 3 small apical teeth set in line and 2 larger basal ones. Maxillary capitulum with 3 apical teeth on main part followed by straight cutting edge, fine (smooth ?) L.1 (not clearly seen), rather long L.2 reaching well beyond tip of capitulum, and L.3 with 2 strong hooks as on Fig. 3, 3.
Fig. 5.
A. multisensillata sp. n. (1–3) and Anurida olae sp. n. (4–6): 1 – dorsal chaetotaxy of Th.1–3 (lateral view); 2 – dorsal chaetotaxy of abdomen (lateral view); 3 – PAO and ocelli; maxilla; 4 – dorsal chaetotaxy of Th.2 (lateral view); 5 – dorsal chaetotaxy of Abd.4–6 (lateral view); 6 – PAO and ocelli. Scales (mm): 1–2, 4–6 – 0.1; 3 – 0.01.
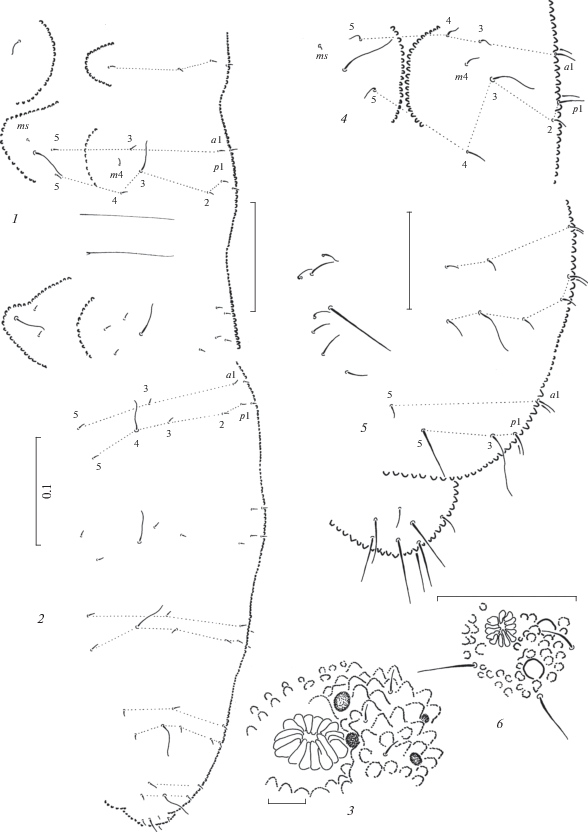
Fig. 6.
Anurida elegans sp. n.: 1 – dorsal chaetotaxy of head and Th.1–2; 2 – dorsal chaetotaxy of Abd.3–6; 3 – antennal tip; 4 – PAO and ocelli; 5–6 – tip of mandible; 7 – maxillary head. Scales (mm): 1–2 – 0.1; 3–7 – 0.01.
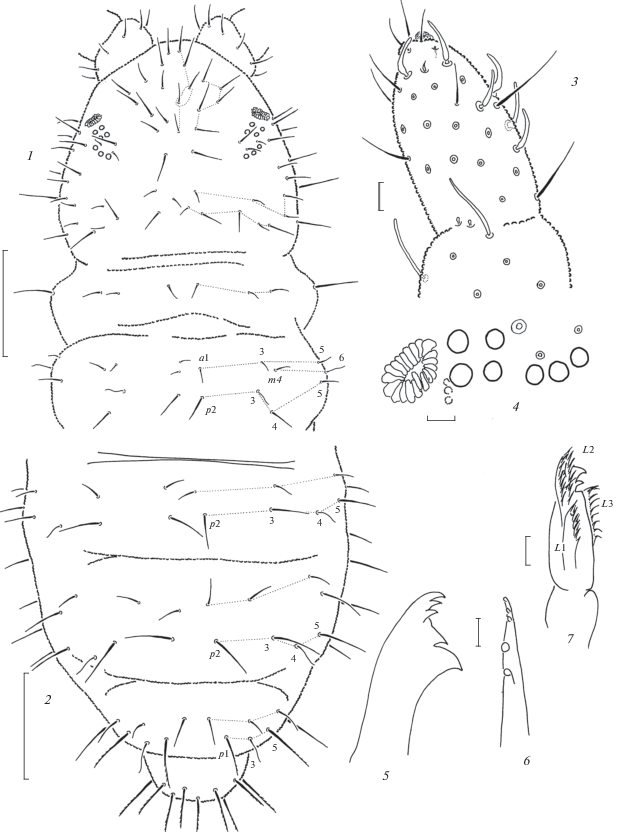
Common dorsal setae fine and short, differentiated into macro and microsetae only on Abd.6 (and also on abdominal sterna), sensilla thin and long, 4–5 times longer than ordinary setae, lateral microsensilla (ms) present on Th.2. Dorsal chaetotaxy typical of the weberi-group (Figs 5, 1–2). Thoracic sterna without setae. Ventral tube usually with 4+4 setae. Furca remnant as two small warts, each with one seta and few setulae, in anterior part of Abd.4 sternum.
Chaetotaxy of legs 1–3 as following: upper subcoxae – 1, 2, 2; lower subcoxae – 0, 2, 2; coxae – 3, 6, 7; trochanters – 6, 6, 6 (one posterior seta on trochanter of Leg.3 clearly thinner and shorter than others); femora –13, 12, 11; tibiotarsi – 19, 19, 18 setae, respectively. Tibiotarsal setae of rather short, longest inner setae of B-whorl about as long as inner unguis edge (1.0–1.1 : 1). Unguis without inner or lateral tooth.
Etymology. The name reflects the increased number of antennal sensilla, the main diagnostic trait of the new species.
Affinities. Anurida multisensillata sp. n. is quite typical of the weberi-group in having the most common type of maxillae and mandibles, a typical arrangement of the dorsal setae, and a broadened and somewhat flattened body with a small roundish Abd.6 partly hidden under Abd.5 as in the majority of congeners in the group. Nevertheless, A. multisensillata sp. n. occupies an isolated position in this complex due to the almost complete absence of colouration and therefore it can be compared to only two known Palaearctic species: A. parapapillosa Tshelnokov 198833 and A. pallens sp. n. The presence of additional antennal sensilla further aligns A. multisensillata sp. n. with A. parapapillosa (more correctly, with A. cf. parapapillosa sensu Babenko, 1997). These two latter species differ from A. multisensillata sp. n. by showing 6 mandibular teeth (vs 5 teeth in A. multisensillata sp. n.) and serrate maxillary L.1 (apparently smooth in A. multisensillata sp. n.).
Distribution. Coniferous-broadleaved forests of Ussuriisky Nature Reserve and Kedrovaya Pad Nature Reserve.
Anurida olae Babenko, Shveenkova et Kuznetsova sp. n. Figs 4, 1–4; 5, 4–6
Diagnosis. A dark coloured species of the weberi-group of Anurida characterized by the presence of 4 + 4 ocelli, (1)2 additional lateral sensilla on Ant.4, roundish PAO with 13–18 lobes, the mandibles with 3 + 2 teeth set in a line, and three hooks on dorsal maxillary lamella (L.3).
Type material. Holotype, female on slide, North-East of Russia, Magadan Province, about 120 km N of Magadan, upper reaches of Ola River [N 60.65° E 151.27°], larch forest, 830 m alt., 11.08.2011, leg. A. Babenko. Paratypes, two males and three females, same data as holotype. The types are kept in the collection of MSPU.
Description. Length without antennae 1.6–2.3 mm. Colour dark blue, paler ventrally. Body shape typical of weberi-group, widened and flattened, Abd.6 partly hidden under Abd.5. Integument granulation coarse and uniform.
Ocelli present, 4 + 4, anterior pair distinctly larger than skin granules, posterior ocelli slightly smaller, but usually detectable. PAO roughly circular with 15–20 lobes (Fig. 5, 6). Antennae shorter than head, of typical generic shape. Ant.4 with 3-lobed apical bulb, subapical ms and organite present; sensillar set enriched: 4 usual sensilla (S1–S4) present in dorsal group, two lateral (S7–S8) and 1–2(3) additional sensilla below (Fig. 4, 1). AO on Ant.3 typical, outer sensilla widely separated, small ms present ventrally. Ant.1–2 with 7 and 13–14 setae, respectively. Labral formula as usual 4/2-3-5-2, two basal labral setae clearly longer. Labium with 11 setae on each side, three setae of which set on its apical part, two sensillar papillae present. Mandibles with 3 small apical teeth set in line and 2 larger basal ones (Fig. 4, 4). Maxillary capitulum with 2 apical teeth on main part followed by straight cutting edge, smooth L.1, rather long L.2 reaching well beyond tip of capitulum, and L.3 with 3 clear hooks as on Figs 4, 2–3.
Common dorsal setae fine and short, clearly differentiated into macro and microsetae laterally and ventrally, lateral microsensilla (ms) present on Th.2. Dorsal chaetotaxy typical of weberi-group (Figs 5, 4–5). Th.2–3 with three setae (a3, a4 and m4) in dorso-lateral group above sensilla p3. Thoracic sterna without setae. Ventral tube with 5–6(8) setae on each side. Furca remnant as two small warts, each with one seta and few setulae, in anterior part of Abd.4 sternum.
Chaetotaxy of legs 1–3 as following: upper subcoxae – 2, 3, 3–5; lower subcoxae – 0, 2, 2; coxae – 3, 6, 7; trochanters – 6, 6, 6; femora –13, 13, 11; tibiotarsi – 19, 19, 18 setae, respectively. Tibiotarsal setae rather long, longest inner setae of B-whorl clearly longer than inner edge of unguis (1.5–1.9 : 1). Unguis usually with clear inner tooth.
Etymology. Named after the type-locality – upper reaches of Ola River.
Affinities. Anurida olae sp. n. is very similar to A. weberi Christiansen et Bellinger 1980 as defined by Fjellberg (1985). Both species have 1–2 additional antennal sensilla, 4 + 4 ocelli, a circular PAO with about 15 lobes, the identical dorsal chaetotaxy, the slightly increased number of setae on the subcoxae and VT, the long tibiotarsal setae, and the inner tooth on the unguis. The only notable difference between these species is the shape of dorsal maxillary lamella (L.3): with two usual hooks in A. weberi, vs three such hooks in A. olae sp. n. Apart from this, the position of additional antennal sensilla in these two species appears to be slightly different: below S7 and S8 in A. olae sp. n., vs between the lateral and dorsal groups in A. weberi (cf. Fig. 4, 1 and Fig. 4, 5). The stability of the latter difference needs confirmation.
Anurida olae sp. n. can also be compared to A. kolymensis Tshelnokov 1988, a similar species of the same group, characterized by the presence of numerous (up to six) additional antennal sensilla and of the maxillae with serrate L.1 and two-hooked L.3.
Three species of the weberi-group described above, A. pallens sp. n., A. armifera sp. n., and A. multisensillata sp. n., can easily be distinguished from A. olae sp. n. by the presence of only two setae (a3 and m4) in front of sensilla p3 on Th.2–3, whereas in A. olae sp. n. (as well as in A. weberi and A. kolymensis) this setal group additionally includes seta a4 (Fig. 5, 4).
Distribution. Known only from the type locality.
Anurida elegans Babenko, Shveenkova et Kuznetsova sp. n. Figs 6, 1–7
Diagnosis. A species of the hammerae-group characterized by the presence of 7 + 7 ocelli, six usual blunt sensilla on Ant.4, an oval PAO with about 20 lobes, the mandibles with 3 + 2 teeth, the maxillae with three serrate lamellae, and by the absence of axial setae p1 on all terga of Th.2–Abd.4, as well as of setae p2 on Abd.5.
Type material. Holotype, male on slide, Far-East of Russia, Terneyski District, nearby Sikhote-Alin Reserve, Brusnichnaya River (tributory of Kema) [N 45.6482°, E137.0097°], 228 m alt., valley mixed forest, litter, 6.08.2017, leg. N. Kuznetsova, A. Geraskina and A. Kuprin. Paratypes, immature female, Far-East of Russia, Sikhote-Alin Reserve, Kavalerovski District [N 44.3844°, E 135.3639°], 378 m alt., larch-wood, litter, 9.08.2017; 2 immature males, Far-East of Russia, Sikhote-Alin Reserve, Kabaniy station [N 45.1384°, E 135.8870°], 932 m alt., coniferous wood with Rhododendrom fauriei, litter, 8.08.2017, all leg. N. Kuznetsova, A. Geraskina and A. Kuprin. The types are kept in the collection of MSPU.
Description. Length of holotype without antennae 1.6 mm. Largest specimen (holotype) with traces of blue pigment all over body, ocelli dark; other available material whitish, usually without dark pigment even on ocular area. Body shape typical of hammerae-group, rather slender and elongate, but Abd.6 rounded and not constricted at base. Integument granulation fine and uniform.
Ocelli present, 7 + 7, anterior group with 4 ocelli (Fig. 6, 4). PAO elongate, with 18–24 lobes (Fig. 6, 4). Antennae clearly shorter than head, of typical generic shape. Ant.4 with large 3-lobed apical bulb, 6 usual curved sensilla, subapical ms and organite present (Fig. 6, 3). AO on Ant.3 typical, outer sensilla long and widely separated, small ms present ventrally. Ant.1–2 with 7 and 13 setae, long erect setae on basal segments of antennae only slightly longer (1.2–1.4 : 1) than those on apical ones. Labrum with usual seta set, distributed as 4/2-3-5-2. Apical part of labium with three setae and two small sensillar papillae; its basal part with 8 usual setae. Mandibles (Fig. 6, 5–6) with 3 small apical teeth set in oblique line and 2 larger basal ones. Maxillary capitulum with 2 apical teeth on main part followed by straight cutting edge and three serrate lamellae, L.2 reaching beyond tip of capitulum (Fig. 6, 7).
Common dorsal setae straight, rather strong and slightly serrate; their differentiation into macro and microsetae clear only on abdominal tip, sensilla subequal (on thorax) or shorter (on abdomen) than other setae of p-rows, lateral microsensilla (ms) present on Th.2. Dorsal chaetotaxy as on Figs 6, 1–2. Main characteristics: tergum of Th.1 with 3 + 3 setae. Th.2–3 without setae p1, sensilla (p3) in anterior position in front of p4, only three setae (a1, a3, and a5) of a-row and seta m4 present (Fig. 6, 1). Abd.1–4 with 2 + 2 p-setae between sensilla (p4), i.e. setae p1 absent on all abdominal terga (Fig 6, 2). Abd.5 with only 2 + 2 setae (a1 and p2) between sensilla (p3). Thoracic sterna without setae. Ventral tube with 4 + 4 setae. Furcal field with few tiny setulae in anterior part of Abd.4 sternum.
Chaetotaxy of legs 1–3 as following: upper subcoxae – 1, 2, 2; lower subcoxae – 0, 2, 2; coxae – 3, 8, 8; trochanters – 6, 6, 5; femora – 13, 12, 11; tibiotarsi – 19, 19, 18 setae, respectively. Tibiotarsal setae of moderate length and slightly thickened, longest inner setae of B-whorl shorter than inner unguis edge (0.8 : 1). Unguis without inner or lateral teeth.
Etymology. The name derives from the Latin elegans, meaning elegant, graceful.
Affinities. Fjellberg (1985a) described four definite stages in the reduction of axial chaetotaxy in species of the hammerae-group. Anurida elegans sp. n. belongs to the most advanced stage 4 with the loss of p1-seta on all terga of Th.2–Abd.4. The same type of reduction is known only for A. reducta Fjellberg 1985 and A. narli Fjellberg 1985, the species with 0 + 0 and 3 + 3 ocelli, respectively. Another species, A. luciae Fjellberg 1985, described from the upper Kolyma River, Chukotka, has been characterized as having a “weak” stage 4 with p1 sometimes present on Th.2–3. Anurida elegans sp. n. shares with the latter species an almost complete set of ocelli (7 + 7) and an identical maxillary structure. Nevertheless, A. luciae is clearly larger (up to 2.0 mm) and has more strongly differentiated dorsal setae. Thus, the ratio of macroseta/microsetae in the posteromedial group on the head varies from 4.0 to 4.2 : 1 in A. luciae being less than 2.0 : 1 in A. elegans sp. n. Apart from this, A. elegans sp. n. shows only 3 microsetae on each side of Th.1 (vs 2 macrosetae and 3–4 microsetae in A. luciae), 1 + 1 macrosetae between sensilla p3 on Abd.5 (additional p2 macrosetae are present in A. luciae), PAO with less than 25 lobes (vs 30–40 in A. luciae), the mandible with 2 + 3 teeth (vs 3 + 3 in A. luciae), the ventral tube with 4 + 4 distal setae (vs 5 + 5 in A. luciae), the longest tibiotarsal setae of B-whorl shorter than the inner edge of the unguis (vs 1.5–1.6 U3 in A. luciae), and no inner tooth on the unguis.
A weak reduction of the number of ocelli as in A. elegans sp. n. is not typical of the genus. Among 76 congeners listed on www.collembola.org (Bellinger et al., 1996–2018), only A. luciae and A. vontoernei Nosek 1962 have more than 5 + 5 ocelli. Unfortunately, the available description of the latter European species which has a full number of ocelli is deficient and not fully trustworthy. Nonetheless, A. vontoernei seems to be hardly too close to A. elegans sp. n. in being smaller (0.6–0.7 mm), intensely coloured and having different maxillae with 4 teeth in the main part, the PAO with about ten lobes and only one seta on VT.
Distribution. Sikhote-Alin Reserve and its surroundings.
Anurida longilamellata Babenko, Shveenkova et Kuznetsova sp. n. Figs 7, 1–3; 8, 1–2
Fig. 7.
Anurida longilamellata sp. n. (1–3) and A. aspera sp. n. (4–5): 1 – dorsal chaetotaxy; 2 – antennal tip; 3 – Abd.6 (ventral side); 4 – dorsal chaetotaxy of Th.1–2; 5 – dorsal chaetotaxy of Abd.3–6. Scales (mm): 1, 4–5 – 0.1; 2–3 – 0.01.
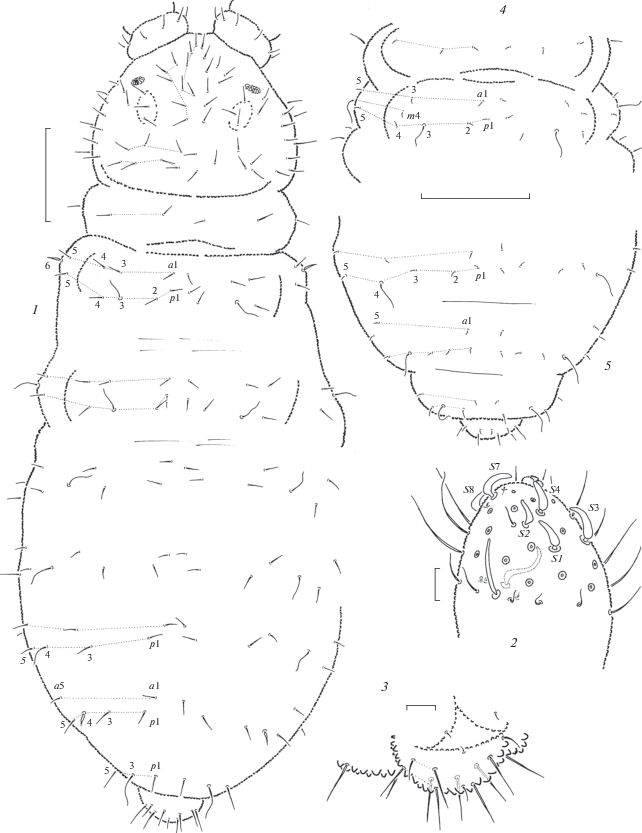
Diagnosis. A white, blind species of the genus Anurida with the common type of mandibles with 3 + 2 teeth and prolonged maxillary L.2. It is also characterized by a peculiar chaetotaxy with flame-like sensilla m6 on Th.2 and p4 on Abd.4, while setae p2 on Abd.1–4 and all setae of a-row on Abd.5 are absent.
Type material. Holotype, female on slide, Far-East of Russia, Kedrovaya Pad Nature Reserve, Central shelter [N 43.1147°, E 131.4872°], 119 m alt., northern slope, mixed forest with Pinus sibirica and Abies holophylla, litter, 27.07.2016, leg. N. Kuznetsova and M. Potapov. Paratypes, one juvenile, same data as holotype; two specimen (sex unknown), same State Reserve, river valley [N 43.1159°, E 131.5070°], 101 m alt., mixed forest with A. holophylla and P. sibirica, litter, 29.07.2016, leg. N. Kuznetsova and M. Potapov. The types are kept in the collection of MSPU.
Description. Length of holotype without antennae 0.9 mm. Colour white. Integument granulation rather coarse and uniform.
Ocelli absent. PAO roughly circular, with 9–11 lobes. Antennae shorter than head, of typical generic shape. Ant.4 with 3-lobed apical bulb (in holotype, in juveniles simple), subapical ms and organite present; six usual subequal sensilla (S1–S4, S7 and S8) present (Fig. 7, 2). AO on Ant.3 with typical sensorial set, ventral outer sensillum S-shaped. Ant.1–2 with 7 and 12 setae, respectively. Number of prelabral and labral setae: 4/12. Chaetotaxy of labium also typical: three setae and two sensillar papillae (organite) on apical part of labium and eight setae on its basal part. Mandibles rather fine with three small apical teeth set in line and two larger basal ones (Fig. 8, 2). Maxillary capitulum with two apical teeth on main part, probably smooth L.1 (not clearly seen), thin and long L.2, more than twice as long as capitulum, covered by several long filaments, and delicate L.3 with 2 hooks (Fig. 8, 1).
Fig. 8.
Anurida longilamellata sp. n. (1–2) and A. aspera sp. n. (3–7): 1 – maxillary head; 2 – tip of mandible; 3 – maxillary head; 4 – tip of mandible; 5 – PAO; 6 – antennal tip; 7 – distal part of leg. Scales 0.01 mm.
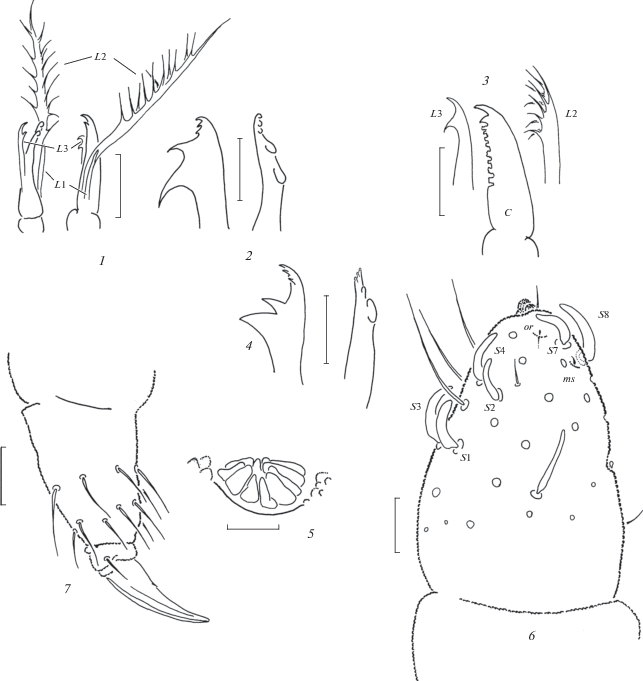
Common dorsal setae straight, smooth and rather strong, without visible differentiation, sensilla usually thinner and only slightly longer than ordinary setae, those on Th.2 (m6) and on Abd. 4 (p4) clearly shortened and widened, flame-shaped, lateral microsensilla (ms) present on Th.2. Dorsal chaetotaxy rather special (Fig. 7, 1). Main characteristic: Th.1 with 2 + 2 setae. Th.2–3 with 3 + 3 axial setae (a1, p1 and p2), both p2 and sensilla p3 in backward position, at level with p2–p4 line, four setae present in a-row (a1, a3, a4, a5), m4 absent. Abd.1–4 with 2 + 2 setae between sensilla p4 (setae p2 absent). Abd.5 without p2 as well as all setae of a-row. Differentiation of setae on Abd.6 as on Fig. 7, 3. Thoracic sterna without setae. Ventral tube with 4 + 4 setae. Furca remnant as two small warts in anterior part of Abd.4 sternum, each with one seta.
Legs 1–3 chaetotaxy as following: upper subcoxae – 1, 2, 2; lower subcoxae – 0, 2, 2; coxae – 3, 6, 7; trochanters – 6, 6, 6 (one posterior seta on trochanter of Leg.3 clearly thinner and shorter than others); femora –12(13), 12, 11; tibiotarsi – 19, 19, 18 setae, respectively. Tibiotarsal setae rather short, longest ones of B-whorl as long as or slightly shorter than those of A-whorl and about as long as inner edge of unguis. Unguis without inner or lateral tooth.
Etymology. The species is named after the long and unusual ventral maxillary lamella (L.2).
Affinities. Among the known species of the genus, A. longilamellata sp. n. is obviously most similar to A. nadezhdae Babenko 1998, described from the Putorana Plateau, Yenisei River basin, Siberia. Both these species share such unusual features as an S-shaped ventral sensillum of AO (more common for Micranurida) and the maxillae with long and thin L.2, as well as two hooks on L.3 (rare in blind congeners). The main characteristics of the dorsal chaetotaxy of these species are also almost identical. Thus, both species have Th.1 with 2 + 2 setae, a backward position of p2 and p3 on Th.2–3, broadened, flame-shaped sensilla m6 on Th.2 and p4 on Abd.4, and no p2 setae on Abd.1–4. The new species differs from A. nadezhdae in having longer antennal sensilla (vs short, hammer-like in A. nadezhdae), labial sensorial papillae (not seen in A. nadezhdae), a complete set of tibiotarsal setae (seta M is absent from A. nadezhdae, 18-18-17 tibiotarsal setae totally), and no seta of a-row on Abd.5 (vs a1 and a5 present in A. nadezhdae).
The Chinese species A. kazukoae Tamura 1997 with 2 + 2 setae on Th.1 and broadened sensilla m6 on Th.2 and m4 on Abd.4 most probably belongs to the same group. Nevertheless, its maxilla is quite different and there are 3 + 3 p-setae between sensilla p4 on Abd.1–4.
An S-shaped ventral sensillum in AO, 2 + 2 setae on Th.1, 2 + 2 p-setae between sensilla p4 on Abd. 1–4, and the absence of a1-setae on Abd.5 are also characteristic of the European A. balatovae Rusek 1970. Both maxilla and mandible of the latter species appear to differ from those in A. longilamellata sp. n.
The maxillary head, surprisingly similar to that of A. longilamellata sp. n. and A. nadezhdae, is also known for two species from the West-European genus Rusekella Deharveng 1982, namely for R. flagellata (Arbea et Jordana 1985) and R. gamae Arbea et Jordana 1991. Nevertheless, this similarity hardly reflects the close relationship of these species, since Rusekella, having a papillate seta L on the labium, is believed to belong to another phylogenetic line of Neanuridae (Deharveng, 1982).
Distribution. Coniferous-broadleaved forests of Kedrovaya Pad State Nature Reserve.
Anurida aspera Babenko, Shveenkova et Kuznetsova sp. n. Figs 7, 4–5; 8, 3–7
Diagnosis. A small, white and blind species of the genus Anurida characterized by the usual set of six subequal antennal sensilla, the mandibles with five teeth, of which three apical ones are positioned in an oblique line, the maxillae with a multidentate capitulum and two-hooked L.3, and by the presence of 3 + 3 p-setae between sensilla p4 on all terga of Abd.1–4.
Type material. Holotype, male on slide, Far-East of Russia, Ussuriisky Nature Reserve, Komarovskoye Forest District, Grabovaya Nipple [N 43.6367°, E132.3499°], 383 m alt., mixed forest on slope, litter, 23.07.2016, leg. N. Kuznetsova and M. Potapov. Paratypes, one male, same data as holotype; two males, same State Reserve, Komarovskoye Forest District, Turova Nipple, Khripunovsky Pass [N 43.6481°, E 132.3515°], 207 m alt., Pinus sibirica forest on slope, litter, 22.07.2016, leg. N. Kuznetsova and M. Potapov. The types are kept in the collection of MSPU.
Description. Length without antennae 0.6–0.8 mm. Colour white. Integument granulation coarse and uniform.
Ocelli absent. PAO roughly circular, with 9–11 lobes (Fig. 8, 5). Antennae shorter than head, of typical generic shape. Ant.4 with 3-lobed apical bulb, subapical ms, organite, and six usual subequal sensilla (S1–S4, S7 and S8) present (Fig. 8, 6). AO on Ant.3 with typical sensorial set. Ant.1–2 with 7 and 12 setae, respectively. Number of prelabral and labral setae as usual: 4/12, in single specimen reduced to 4/10. Chaetotaxy of labium also typical: three setae and two sensillar papillae (organite) on apical part of labium and eight setae on its basal part. Mandibles rather fine with three small apical teeth set in oblique line and two larger basal ones (Fig. 8, 4). Maxillary capitulum with two strong apical teeth on main part, followed by disrupt cutting edge forming several irregular teeth, L.1 not clearly seen (smooth ?), L.2 with 2–3 irregular rows of long filaments, projecting beyond tip of capitulum, and delicate L.3 with 2 hooks (Fig. 8, 3).
Common dorsal setae fine and short, without visible differentiation, only sensilla clearly longer, lateral microsensilla (ms) present on Th.2. Main characteristics of dorsal chaetotaxy (Figs 7, 4–5): Th.1 with usual 3 + 3 setae. Th.2–3 with both p2 and sensilla p3 in backward position, at level with p4, three setae present in a-row (a1, a3, a5), and one in m-row (m4). Abd.1–3 with complete set of seta: a1, a3, a5 and p1–p5. Abd.4 similar but without a3 setae. Ventral tube with 4 + 4 setae. Furca remnant as two small warts in anterior part of Abd.4 sternum, each with one seta and 2–3 tiny setulae.
Legs 1–3 with basic set of setae: upper subcoxae – 1, 2, 2; lower subcoxae – 0, 2, 2; coxae – 3, 6, 7; trochanters – 6, 6, 6; femora –13, 12, 11; tibiotarsi – 19, 19, 18 setae, respectively. Tibiotarsal setae rather short, longest ones about as long as inner edge of unguis. Inner tooth on unguis usually present (Fig. 8, 7), sometimes invisible.
Etymology. From the Latin word “asper” which means grainy.
Affinities. One of the most peculiar characters of A. aspera sp. n. is the shape of dorsal maxillary L.3 with two strong hooks. Such a character is very typical of the weberi-group, but among blind congeners A. aspera sp. n. shares it with only three described species: A. sakhensis Babenko 1997, A nadezhdae Babenko 1998, and A. longilamellata sp. n. However, the chaetotaxy of the two latter species (2+2 setae between sensilla p4 on Abd.1–4 and flame-like sensilla m6 on Th.2 and p4 on Abd.4) is so very special that they can hardly be too close to A. aspera sp. n.
As regards A. sakhensis, it appears to be very similar to A. aspera sp. n. in having the identical mandibles with three apical teeth set in an oblique line (a not very common feature). These two species can be distinguished by a number of fine chaetotic peculiarities. Thus, contrary to A. sakhensis, in A. aspera sp. n. all dorsal setae are practically identical, not differentiated into macro- and microsetae, setae a4 on Th.2–3 are absent, and Abd.4 with p2 setae present, but setae a3 absent. Apart from this, A. sakhensis is larger (1.4 mm vs <1.0 mm) and the maxillae appear to be slightly different; ventral L.1 is probably smooth in A. aspera sp. n. vs serrate in A. sakhensis.
There are also a number of other blind species of the genus occurring in the eastern Palaearctic, which can be compared to A. aspera sp. n. For instance, A. annatshagi Tshelnokov 1988, described from the Magadan Area, also shows mandibular teeth arranged in two different planes, but a different maxillary shape and a more strongly reduced chaetotaxy without setae p2 on Abd.1–4. Anurida subarctica Fjellberg 1985, another similar blind species widespread in the northern parts of the eastern Palaearctic, is identical to A. aspera sp. n. in thoracic chaetotaxy, but that of Abd.4 is quite different (only 1 + 1 p-setae present between sensilla p4).
Distribution. Coniferous-broadleaved forests of Ussuriisky Nature Reserve.
A key to non-cave Asiatic species of the genus Anurida
1. Th.2–3 with complete set of p-setae (p1–p5 present). With or without dark pigment and ocelli....3
– Setae p4 on Th.2–3 absent. White species with uncoloured ocelli................................................... 2
2. Ocelli 3 + 3. Th.2–3 with two setae above sensilla p3. Abd.1–4 with 2 + 2 p-setae between sensilla p4. Maxillary L.3 serrate..................... alpina Agrell 1939
= tundricola Tshelnokov 1988
Transpalaearctic
– Ocelli 2 + 2. Th.2–3 with three setae above sensilla p3, i.e. a3–a4 and m4 present. Abd.1–4 with 3 + 3 p-setae between sensilla. Maxillary L.3 with three strong hooks........................... tatianae Babenko 1997
East Palaearctic
3. Sensilla p3 on Th.2–3 moved forward and set in front of setae p4. Ocelli present................................ 4
– Sensilla p3 on Th.2–3 set at a level with or behind setae p4. Ocelli usually absent, rarely present......... 26
4. Head with 7 + 7(8) ocelli................................ 5
– At most 5 + 5 ocelli......................................... 6
5. Th.1 with 2 + 2 macrosetae and few microsetae. Abd.5 with p2 present, i.e. 2 + 2 p-setae between sensilla p3. PAO with 30–40 lobes. Mandible with 3 + 3 teeth. Inner tooth on unguis present.......... luciae Fjellberg 1985
= macrochaetosa Tshelnokov 1988
Magadan Province
– Th.1 with common 3 + 3 microsetae. Abd.5 without p2, i.e. only 1 + 1 p-setae present between sensilla p3. PAO with less than 25 lobes. Mandible with 3 small apical and 2 larger basal teeth. Inner tooth on unguis absent.................................................... elegans sp.n.
North Primorye
6. Th.2–3 with 2 + 2 p-setae in the axial group.... 7
– Th.2–3 with 1 + 1 p-setae in the axial group. Ocelli 3 + 3................................. narli Fjellberg 1985
= tshuktshorum Tshelnokov 1988
Nearctic – East Palaearctic
7. Th.2–3 with p2 in front of p1. Ocelli 5 + 5(4)... 8
– Th.2–3 with p2 at a level with or behind of p1. Ocelli 5 + 5 or lesser................................................ 9
8. More than one row of setae on Th.1. Th.2–3 with seta m5. Abd.1–3 with 3 + 3 p-setae between sensilla p4 (microsetae p1 present). Abd.4–5 with both p1 and p2 present...........................hammerae Christiansen 1951
=mandibulata Tshelnokov 1988; =polychaetosa Tshelnokov 1988
Nearctic – East Palaearctic
– Th.1 with a common set of setae (3 + 3). Th.2–3 without seta m5. Abd.1–4 with 2 + 2 p-setae between sensilla p4, i.e. microsetae p1 absent. Abd.5 without seta p2..........................................beringi Fjellberg 1985
= neriensis Tshelnokov 1988
Nearctic – East Palaearctic
9. Dark coloured species................................... 10
– Paler species, diffuse dark pigment on body and ocular plate present or absent................................ 22
10. Body with distinct dorsal cuticular tubercles. Th.2–Abd.5 with both a1 and a2 setae (2 + 2 a-setae in the axial group)........... neanuriformis Babenko 2002
Khakassia
– Body without cuticular tubercles. Th.2–Abd.5 with only 1 + 1 a-setae in the axial group............... 11
11. Common setae fine, short and undifferentiated, only sensilla distinct.............................................. 12
– Dorsal setae strongly differentiated into macro- and microsetae. Abd.1–4 with 2 + 2 p-setae between sensilla (p3 absent). Mandible with 7 teeth. Maxillary L.3 serrate...................................tullbergi Schött 1891
Transpalaearctic
12. Dorsal chaetotaxy clearly plurichaetotic, especially pronounced on lateral parts of a body. Each upper subcoxae of legs 1–3 with more than 10 setae.............................. asiatica Babenko 2002
East Palaearctic
– Dorsal chaetom without additional setae. No more than 5 setae on each subcoxae....................... 13
13. Th.2–3 with only one seta (a3) in front of p3 sensilla. Abd. 1–4 with 2+2 p-setae between sensilla p4 (p3 absent). Mandibles with 4–5 small apical and 2 larger basal teeth. Maxillary L.3 with few serrations.......................... decemoculata Hammer 1953
Nearctic – East Palaearctic
– Th.2–3 with two setae (a3 and a4 or m4) in front of p3 sensilla. Abd. 1–4 with complete set of p-setae (3 + 3) between sensilla p4.................................... 14
14. Ant.4 with lateral sensilla S7 and S8 fused forming arch. Abd.5 with 1(2) p2-setae.... papillosa (Axelson 1902)
East Palaearctic – [?] Nearctic
– Lateral sensilla S7 and S8 on Ant.4 free. Abd.5 without setae p2..................................................... 15
15. Ant.4 with some additional sensilla in lateral group.................................................................... 16
– Ant.4 with basic set of six sensilla (S1–S4, S7–S8)........................................................ 18
16. Ant.4 with 4–6 additional sensilla. Maxillary L.1 clearly serrate. Ventral tube with 6–7 setae. Upper subcoxae of legs 1–3 with 2–3, 2–4, 4–5 setae....................... kolymensis Tshelnokov 1988
East Palaearctic
– Ant.4 with 1–2 additional sensilla. Maxillary lam.1 smooth. Subcoxae with 1–2, 2–3, 2–5 setae.....17
17. Maxillary L.3 with two strong hooks........ weberi Christiansen et Bellinger 1980
Nearctic – East Palaearctic
– Maxillary L.3 with three strong hooks..... olae sp. n.
Magadan Province
18. Mandible with (6)7 apical teeth followed by two larger basal ones. Maxillary L.3 with several strong hooks.................................................. armifera sp. n.
South Primorye
– Mandible with 3 + 2 teeth. Maxillary L.3 with two hooks.............................................................. 19
19. Setae of m-row on Abd.6 almost as long as macrosetae p1.............................................................. 20
– Setae of m-row on Abd.6 as microsetae, clearly shorter than macrosetae p1.................................... 21
20. Dorsal maxillary L.2 passing well beyond tip of capitulum. Intensity of pigmentation on ventral and dorsal sides of a body almost equal......... palustris Babenko 1997
East Palaearctic
– Dorsal maxillary L.2 short, not passing beyond tip of capitulum. Ventral side of a body clearly lighter............................... zanokhae Babenko 1998
Mid Siberia
21. Maxilla prolonged, almost styliform, its capitulum with 2 tiny teeth, lamellae short and indistinct.................................... confinis Babenko 1998
East Palaearctic
– Maxilla typical of the genus, its capitulum with 4 teeth, lamellae distinct......dynkengda Babenko 1998
Mid Siberia
22. Ant.4 with additional lateral sensilla............. 23
– Ant. 4 with basic set of six sensilla (S1–S4, S7–S8)........................................................... 24
23. Mandible with 4 small apical teeth and two larger basal ones..........parapapillosa Tshelnokov 1988
Chukotka
– Mandible with 3 + 2 teeth.... multisensillata sp. n.
South Primorye
24. Th.2–3 usually with three setae (a3, a4, m4) above sensilla p3. Setae p1 on Abd.5 clearly longer than a1................................................................. 25
– Th.2–3 with two setae (a3, m4) above sensilla p3. Setae p1 on Abd.5 subequal to a1............. pallens sp.n.
North Primorye
25. All teeth on mandible set in a line. Common dorsal setae practically undifferentiated, setae p1 clearly longer than a1 only on Abd.5. Maxillary lam.3 with two hooks...........................azurea Babenko 1997
East Palaearctic
– Apical teeth on mandible set in oblique row. Dorsal setae clearly differentiated, on all terga setae p1 longer than a1. All three maxillary lamella serrate......................... bondarenkoae Tshelnokov 1988
= jakutica Tshelnokov 1988
East Palaearctic
26. Ocelli (4 + 4) and dark pigment on body present............................. papillosoides (Hammer 1953)
= arctica Tshelnokov 1988
Nearctic and East Palaearctic
– Ocelli and pigment absent............................. 27
27. Th.2–3 with seta p2 anterior to p1 seta......... 28
– Th.2–3 with seta p2 posterior to p1 seta.......... 30
28. Ant.4 with usual lateral sensilla (S7 and S8).......................... martynovae Fjellberg 1985
Nearctic – East Palaearctic
– Ant.4 with one additional lateral sensillum (S9)... 29
29. Th.2–3 without seta m5. Maxillary lam.3 with two strong hooks followed by 1–2 minute teeth................................... similis Fjellberg 1985
Nearctic – East Palaearctic
– Th.2–3 with seta m5 present in lateral group. All maxillary lamellae clearly serrate.... kyshyensis Babenko et Nakamori 2018
Chukotka
30. Ant.4 with additional sensillum (S9) in the lateral group................................. polaris Hammer 1953
= frigida Fjellberg 1973
= wrangelensis Tshelnokov 1988
Circumpolar
– Ant.4 with basic set of sensilla (S1–S4, S7–S8)... 31
31. Th.1 with 2 + 2 setae. Lateral sensilla (m6) on Th.2 and dorsal one (p4) on Abd.4 flame-like......... 32
– Th.1 with 3 + 3 setae. All lateral and dorsal sensilla long, seta-like................................................. 34
– Th.1 with at least 4 + 4 setae. Mandibles very peculiar with strong apical and basal teeth and 3 intermediate oblique, transverse rows of long thin filaments. All three maxillary lamella with long filaments passing well above tip of capitulum......abashiriensis Babenko et Nakamori 2018
Japan
32. Abd.1–4 with 3 + 3 p-setae between sensilla p4..................................... kazukoae Tamura 1997
China
– Abd.1–4 with 2 + 2 p-setae between sensilla p4... 33
33. Abd.5 without a-row of setae... longilamellata sp. n.
South Primorye
– Setae a1 and a5 on Abd.5 present...... nadezhdae Babenko 1998
Mid Siberia
34. Th.2–3 with two setae above p3–p4 line, i.e. a4 or m4 absent.......................................................... 35
– Th.2–3 with three setae (a3–a4 and m4) above p3–p4 line............................................................. 36
35. Abd.4 with 1 + 1 or 2 + 2 p-setae between sensilla p4. Mandible with 3 + 2 teeth set in a line..................................subarctica Fjellberg 1985
=tajmyrica Tshelnokov 1988
Nearctic – East Palaearctic
– Abd.4 with 3 + 3 p-setae between sensilla p4. Mandible with 3 + 2 teeth, three apical ones set in oblique row............................................ aspera sp. n.
South Primorye
36. All antennal sensilla subequal. Abd.1–3 usually with p2 setae. Maxillary L.3 with two strong hooks................................ sakhensis Babenko 1997
East Palaearctic
– One of antennal sensilla (S3) clearly thinner. Abd.1–3 without setae p2. Maxillary L.3 serrate..............................annatshagi Tshelnokov 1988
= balagannakhi Tshelnokov 1988
East Palaearctic
Two further insufficiently described Asiatic species are not included in the above key:
Anurida trioculata Kinoshita 1916 (Japan): yellow in life and almost white in alcohol, Ant.4 with 6 long sensilla, 3 + 3 ocelli, PAO with 25–32 lobes, mandibles with 5 teeth not set in a line, maxillary head with three strong apical teeth and 2 [?] serrate lamellae, unguis with a clear inner tooth. Dorsal chaetotaxy, according to Yosii (1956): setae strongly differentiated in size, all terga from Th.2 to Abd. 5 with 3 + 3 axial setae, middle ones (m1 in Yosii [=p2 ?]) much longer. This appears to be a member of the hammerae-group despite a posterior position of sensilla on Th.2 in the single available illustration (Yosii, 1956, fig. 6).
Anurida tamurae Yue et Yin 1999 (China): white and blind, PAO elliptical with 19–21 lobes, sensilla p3 on thorax in anterior position, according to fig. 16 in Yue and Yin (1999), abdominal chaetotaxy complete (ibid., fig. 12 ).
CONCLUSION
Altogether, 78 species of the genus Anurida are listed on www.collembola.org (Bellinger et al., 1996–2018). This number includes two nomina nuda: A. orientalis Martynova in Stebaeva, 1976 and A. borealis Martynova in Stebaeva, 1976, as well as three junior synonyms: A. frigida Fjellberg 1973, A. wrangelensis Tshelnokov 1988, and A. mandibulata Tshelnokov 1988. On the other hand, two Korean cave congeners, A. decipiens Yosii 1966 and A. troglodyta Lee et Park 2016, are omitted from the list. As a result, altogether with the seven species described in the present paper, the known global diversity of the genus amounts to 82 species, including 13 true troglobionts. A significant part of the remaining 69 non-cave congeners (38 included in the above key and 2 additional ones) are known to occur in Asia, mainly in its fairly boreal parts. This interesting observation obviously needs an explanation and clearly requires further efforts to study the fauna of the region.
Список литературы
Axelson W.M., 1903. Beitrage zur Kenntniss der Collembolen-Fauna Sibiriens // Öfversigt af Finska Vetenskaps-Societetens Förhandlingar. V. 45. № 20. P. 1–13.
Babenko A.B., 1997. The taxonomy and distribution of the genus Anurida (Collembola, Neanuridae) in the northern Palaearctic // European Journal of Entomology. V. 94. P. 511–536.
Babenko A.B., 1998. New Palaearctic species of the genus Anurida (Collembola, Neanuridae) // Zoologicheskii Zhurnal. V. 77. № 6. P. 648–661 [in Russian].
Babenko A.B., 2002. Two new species of the genus Anurida (Collembola, Neanuridae) from mountain regions of southern Siberia // Entomological Review. V. 82. № 3. P. 369–375 [original Russian text: Zoologicheskii Zhurnal. V. 81. № 8. P. 945–951].
Babenko A.B., 2017. Springtails (Collembola) of eastern Chukotka: characteristics of the fauna and species assemblages // Entomological Review. V. 97. № 7. P. 870–892. http://dx.doi.org/10.1134/S0013873817070041 [original Russian text: Zoologicheskii Zhurnal. V. 96. № 10. P. 1141–1164].
Babenko A.B., Fjellberg A., 2006. Collembola septentrionale: A catalogue of springtails of the Arctic regions. M.: KMK Press. 190 p.
Bellinger P.F., Christiansen K.A., Janssens F., 1996–2018. Checklist of the Collembola of the World. http:// www.collembola.org. Date of access: 19.07.2018
Christiansen K.A., Bellinger P.F., 1980 (1981). The Collembola of North America, North of the Rio Grande (1st edition). Grinnell, Iowa: Grinnell College. 1321 p.
Deharveng L., 1982. Contribution a la connaissance taxonomique et phylogénétique des Neanuridae. 1. Le genre Rusekella n. g. et ses implications phylogénétiques // Bulletin de la Société d’Histoire Naturelle de Toulouse. V. 118. P. 235–251.
Fjellberg A., 1985. Arctic Collembola I. Alaskan Collembola of the families Poduridae, Hypogastruridae, Odontellidae, Brachystomellidae and Neanuridae // Entomologica Scandinavica, Suppl. V. 21. 126 p.
Fjellberg A., 1985a. Elements of dorsal chaetotaxy in Neanuridae with descriptions of two new species of Anurida (Collembola) // Entomologica Scandinavica. V. 15. P. 349–362.
Lee I., Park K.-H., 2016. Cave species of the genus Anurida (Collembola: Neanuridae) from Korea, with the description of new species // Zootaxa. V. 4184. № 3. P. 589–599. http://doi.org/10.11646/zootaxa.4184.3.12
Potapov M., Kuprin A., Kuznetsova N., Shveenkova Yu., 2018. Checklist of Collembola of the Far East of Russia // Zootaxa (in press)
Stebaeva S.K., 1976. A state of knowledge of Collembola fauna of Siberia from a zonal standpoint // Trudy Biologicheskogo Instituta Sibirskogo otdeleniya AN SSR. V. 18. P. 85–133 [in Russian].
Weiner W.M., Najt J. 1985. Collemboles de Coree du Nord. IV. Brachystomellinae, Frieseinae et Pseudachorutinae ad partem // Acta Zoologica Cracoviensa. V. 28. № 6. P. 245–276.
Yosii R., 1954. Die Kulturpflanzenschädigenden Collembolen Japans // Ōyō-Kontyū. V. 10. № 2. P. 137–141.
Yosii R., 1956. Monographie zur Hölencollembolen Japan // Contribution from the Biological Laboratory, Kyoto University. V. 3. P. 1–131.
Yosii R., 1972. Collembola from the alpine region of Mt. Poroshiri in the Hidaka Mountains, Hokkaido // Memoirs of the National Science Museum, Tokyo. № 5. P. 75–99.
Yosii R., 1977. Critical check list of the Japanese species of Collembola // Contribution from the Biological Laboratory, Kyoto University. V. 25. № 2. P. 141–170.
Yue Q., Yin W., 1999. Two new species of Collembola (Arthropleona: Neanuridae, Pseudachorutidae) from Shanghai, China // Entomologica Sinica. V. 6. № 3. P. 222–226.
Дополнительные материалы отсутствуют.
Инструменты
Зоологический журнал


