Зоологический журнал, 2019, T. 98, № 9, стр. 988-1002
A Survey of the Belgrandiella-Like Gastropods of the Northern Black Sea Region (Mollusca, Gastropoda, Hydrobiidae s. l.): Morphological Variability and Morphospecies
M. V. Vinarski a, b, *, **, D. M. Palatov c, d, ***
a Saint-Petersburg State University
199034 Saint-Petersburg, Russia
b Omsk State Pedagogical University
644099 Omsk, Russia
c Severtzov Institute of Ecology and Evolution, Russian Academy of Sciences
119071 Moscow, Russia
d Lomonosov Moscow State University
119991 Moscow, Russia
* E-mail: radix.vinarski@gmail.com
** E-mail: m.vinarsky@spbu.ru
*** E-mail: triops@yandex.ru
Поступила в редакцию 16.12.2018
После доработки 18.02.2019
Принята к публикации 3.03.2019
Аннотация
A taxonomic revision of the snails from the northern Black Sea coastal region previously assigned to the genus Belgrandiella A.J. Wagner 1928 (Hydrobiidae) is presented. As shown anatomically, these mollusks can be classified neither in Belgrandiella nor any other conchologically similar genera of hydrobiids (Pontobelgrandiella Radoman 1978; Agrafia Szarowska et Falniowski 2011). In consequence, a new genus, Tschernomorica gen. n., is established. It encompassed five species: T. kimmeria sp. n. (the type species), T. adsharica (Lindholm 1913), T. caucasica (Starobogatov 1962), T. inconspicua sp. n., and T. lindholmi sp. n. The range of the new genus covers the Crimean Peninsula and the Caucasian part of the Black Sea coast. Brief accounts of all five species of Tschernomorica gen. n. are provided, with a key given for their identification on the basis of anatomical and conchological traits.
The members of the hydrobiid genus Belgrandiella A.J. Wagner 1928 are minute snails, with ovate-conical shells, which inhabit springs and underground waters of southern Europe (Wagner, 1928; Radoman, 1983; Arconada, Ramos, 2003; Grego et al., 2017; Osikowski et al., 2018). The type species of this genus, Belgrandia kusceri A.J. Wagner 1914, was described from Slovenia. Anatomically, the genus is characterized by the presence of a prominent hook-shaped lateral outgrowth of a penis (Radoman, 1983). The generic name Belgrandiella was among the earliest ones proposed for the European Hydrobiidae, and since 1928 a number of nominal species, found in various countries, have been assigned to this genus. Consequently, the range of Belgrandiella was assumed to be huge, stretching from the Iberian Peninsula in the west (Arconada, Ramos, 2003) eastward to the Caucasus (Starobogatov, 1962; Vinarski, Kantor, 2016) and the Near East (Schütt, Şeşen, 1993; Haase, 1994), southward to Tunisia (Haase, 1994). At least three nominal species of this genus were recorded from the former USSR territory. These are: Belgrandiella abchasica Starobogatov 1962 (type locality – Abkhasia, Nizhne-Shakuranskaya Cave), B. caucasica Starobogatov 1962 (type locality – Russia, Krasnodar Region, Sochi district, Krasnoaleksandrovskaya Cave), and B. nemethi Schütt et Şeşen 1993 (type locality – Ukraine [sic!], Sochi Area, Hosta [= Khosta], valley near the village Rosswet [sic!], spring of a brook). All the three species were delineated on the basis of shell characters only, and none of them has been studied anatomically. In addition, Schütt and Şeşen (1993) included into the genus Belgrandiella the species Bythinella adzharica (Lindholm 1913), described from the vicinities of Batum City, Georgia. Its internal morphology also remains unknown.
The most recent studies on diversity and taxonomy of the European hydrobiids are based chiefly on molecular data. As an outcome of these works, the range of the genus Belgrandiella has been drastically reduced. It turned out that many species, earlier assigned to this genus, should be re-classified as representatives of other genera. For example, all species of the Bulgarian malacofauna, once placed into Belgrandiella, proved to be members of another genus, Pontobelgrandiella Radoman 1978 (Rysiewska et al., 2016). According to Boeters et al. (2017), the actual range of Belgrandiella does not extend far to eastern Europe; the easternmost reliable findings of snails of this genus are located in Kosovo, Balkans. It raises a question about the generic position and phylogenetic affinities of the Belgrandiella-like snails of the former USSR territory. As it was stated above, none of these species has hitherto been characterized anatomically, and the molecular data on Caucasian and Transcaucasian hydrobiid snails remain scarce (Glöer et al., 2015; Anistratenko et al., 2017; Sitnikova et al., 2018). Recently, Grego et al. (2017), having studied genetically a rather limited sample of hydrobiids from Georgia, determined them as belonging to the genus Agrafia Szarowska et Falniowski 2011, previously known from Northern Greece (Szarowska, Falniowski, 2011). Though the authors lacked any data about the anatomy of Belgrandiella caucasica, they hypothesized it is a member of Agrafia (Grego et al., 2017). As it will be discussed below, it is hardly acceptable, though one cannot deny that hydrobiid genera other than Belgrandiella may live in Caucasus and adjacent areas.
The aim of our study is to outline the shell and (partially) genital morphology of Belgrandiella-like stygo- and crenobiont molluscs occurring within a north and east part of the Black Sea coastal area of the former USSR (Caucasus, Transcaucasia, and Crimea). We discuss all nominal taxa of Belgrandiella recorded from this area, establish a new genus and describe three new species of hydrobiid snails. Also, morphological variability of shell and penis of the Caucasian Belgrandiella-like molluscs is provided here for the first time as well as original data on their geographic distribution. Prior to this study, the data on this subject was very scant. For example, Starobogatov (1962) used a single empty shell as the material for description of B. abchasica, whereas the type series of B. caucasica consists of only two specimens. Since 1962, nobody found these two species, and their original descriptions and type specimens have remained the sole source of information on these snails.
This situation is common for the stygo- and crenobiont Hydrobiidae; many nominal species of this family, described from Europe, are still known from very limited samples and from a single or few localities. Their intraspecific variability and anatomical traits remains poorly known.
MATERIALS AND METHODS
The snails for this study were collected in 2009–2016 by Mikhail Chertoprud and Dmitry Palatov in various regions of Caucasus, Transcaucasia, and the Crimea Peninsula. The type localities of all nominal species of Belgrandiella of the ex-USSR fauna have been visited to collect the topotypic specimens. The snails were gathered during surveys of caves and springs, by hands or by means of a sieve.
We examined the type series of Belgrandiella abchasica and B. caucasica kept in the Zoological Institute of the Russian Academy of Sciences, Saint-Petersburg (ZIN hereafter). Sitnikova et al. (2017) illustrated the types of both species and commented on their present state. A key proposed by Starobogatov (1962) was used as a means for primary identification of these snails.
The measurements of shells were carried out following an original scheme (Fig. 1). In addition to absolute measurements, the whorl number (WN) was counted. To assess the extent of intraspecific variation in shell traits of Belgrandiella we applied the standard statistical procedures, including multivariate analysis. We measured several dozens of shells of two nominal species of Belgrandiella described by Starobogatov (1962) collected from several localities, including two samples of topotypic specimens (Table 1). To make a comparison, the data of shell measurements of an undescribed species of Belgrandiella-like snails from the Crimea Peninsula were added to the analyses.
Fig. 1.
A scheme of measurement of a hydrobiid shell applied in this paper. Abbreviations: SH – shell height, SW – shell width, SpH – spire height, BWH – body whorl height, AH – aperture height, AW – aperture width, BWHap – body whorl height measured above aperture, PWH – penultimate whorl height, PWW – penultimate whorl width.

Table 1.
The list of shell samples of Belgrandiella used for statistical analyses*
| Species | Sampling dates | Sampling site | n |
|---|---|---|---|
| Belgrandiella abchasica | 13.04.2012 | Abkhazia, Nizhnyaya Shakuranskaya Cave (topotypes) | 10 |
| Belgrandiella abchasica | 23.02.2009 24.02.2009 |
Russia, Krasnodar Region, Kudepsta River valley, in springs | 38 |
| Belgrandiella abchasica | 02.01.2010 | Abkhazia, Novyi Afon Town, a spring near Psyrtskha Lake | 40 |
| Belgrandiella caucasica | 04.02.2009 24.02.2009 |
Russia, Krasnodar Region, Kudepsta River valley, in springs | 35 |
| Belgrandiella caucasica | 01.05.2015 | Russia, Krasnodar Region, vicinity of Sochi, a spring in Kalezh village | 22 |
| Belgrandiella caucasica | 01.05.2015 | Russia, Krasnodar Region, vicinity of Sochi, Krasnoaleksandrovskaya Cave (topotypes) | 22 |
| Belgrandiella sp. n. | 06.11.2010 | Russia, Crimea Peninsula, springs of the Zuya River valley | 24 |
The studied material is deposited mainly in the collection of the Laboratory of Macroecology and Biogeography of Invertebrates (LMBI), Saint-Petersburg State University. Some voucher specimens, including the holotypes and paratypes (partly) of all species described here as new, were donated to ZIN collection. Some paratypes were deposited in the Senckenberg Natural History Museum (SMF), Frankfurt am Main, Germany, and in the Zoological Museum of the Moscow State University (ZMMU), Moscow.
RESULTS
An assessment of the intraspecific variation in Belgrandiella-like snails and its taxonomic implications
The two previously known Caucasian species of Belgrandiella (B. abchasica, B. caucasica) have been found by us in several localities, including some sites where they live syntopically (see Table 1). It allowed us to conduct a comparative study of their conchological variation based on representative samples.
The absolute shell sizes of the two species as well as their shell proportions proved very similar, and the mean values of many quantitative conchological traits are close, if not identical (Table 2). The results of the discriminant analysis confirm this observation: there are no clear differences between the populations of B. abchasica and B. caucasica (Table 3). It seems that the two species are indistinguishable by means of either the standard shell measurements or the shell proportions. In particular, it is true for the syntopic samples of B. abchasica and B. caucasica collected in the Kudepsta River valley (see Table 2). The general shell shape, as well as the proportions and sculpture of protoconch are almost identical in the two Caucasian species of Belgrandiella (Fig. 2), those slight differences in shell appearances, which are seen in Fig. 3, may be explained by interpopulation variation.
Table 2.
Morphometric characteristics of shells of Belgrandiella-like gastropods of the Caucasus and Crimea. Above lines – limits of variation (min–max), below lines – mean value ± standard deviation (σ)
| Character/index | Species/locality* | ||||||
|---|---|---|---|---|---|---|---|
| Belgrandiella abchasica | Belgrandiella caucasica | Belgrandiella sp. | |||||
| Novyi Afon | Kudepsta | Topotypes | topotypes | Kudepsta | Kalezh | Crimea | |
| Whorl number | $\frac{{3.50{\kern 1pt} --{\kern 1pt} 4.25}}{{3.85 \pm 0.16}}$ | $\frac{{3.25{\kern 1pt} --{\kern 1pt} 4.25}}{{3.75 \pm 0.23}}$ | $\frac{{3.50{\kern 1pt} --{\kern 1pt} 4.25}}{{3.89 \pm 0.06}}$ | $\frac{{3.37{\kern 1pt} --{\kern 1pt} 3.87}}{{3.63 \pm 0.14}}$ | $\frac{{3.25{\kern 1pt} --{\kern 1pt} 4.00}}{{3.60 \pm 0.19}}$ | $\frac{{3.50{\kern 1pt} --{\kern 1pt} 4.00}}{{3.70 \pm 0.13}}$ | $\frac{{3.12{\kern 1pt} --{\kern 1pt} 3.62}}{{3.39 \pm 0.14}}$ |
| Shell height (SH) | $\frac{{1.58{\kern 1pt} --{\kern 1pt} 2.00}}{{1.82 \pm 0.10}}$ | $\frac{{1.50{\kern 1pt} --{\kern 1pt} 2.05}}{{1.67 \pm 0.10}}$ | $\frac{{1.63{\kern 1pt} --{\kern 1pt} 1.90}}{{1.78 \pm 0.09}}$ | $\frac{{1.60{\kern 1pt} --{\kern 1pt} 1.75}}{{1.66 \pm 0.04}}$ | $\frac{{1.65{\kern 1pt} --{\kern 1pt} 1.90}}{{1.75 \pm 0.06}}$ | $\frac{{1.60{\kern 1pt} --{\kern 1pt} 1.78}}{{1.68 \pm 0.04}}$ | $\frac{{1.30{\kern 1pt} --{\kern 1pt} 1.63}}{{1.46 \pm 0.11}}$ |
| Shell width (SW) | $\frac{{0.93{\kern 1pt} --{\kern 1pt} 1.25}}{{1.11 \pm 0.08}}$ | $\frac{{0.88{\kern 1pt} --{\kern 1pt} 1.18}}{{0.95 \pm 0.07}}$ | $\frac{{0.90{\kern 1pt} --{\kern 1pt} 1.00}}{{0.96 \pm 0.04}}$ | $\frac{{0.90{\kern 1pt} --{\kern 1pt} 1.03}}{{0.98 \pm 0.04}}$ | $\frac{{0.85{\kern 1pt} --{\kern 1pt} 1.13}}{{0.99 \pm 0.06}}$ | $\frac{{0.95{\kern 1pt} --{\kern 1pt} 1.05}}{{1.00 \pm 0.02}}$ | $\frac{{0.85{\kern 1pt} --{\kern 1pt} 1.05}}{{0.94 \pm 0.07}}$ |
| Spire height (SpH) | $\frac{{0.93{\kern 1pt} --{\kern 1pt} 1.20}}{{1.08 \pm 0.07}}$ | $\frac{{0.88{\kern 1pt} --{\kern 1pt} 1.25}}{{1.02 \pm 0.07}}$ | $\frac{{0.98{\kern 1pt} --{\kern 1pt} 1.18}}{{1.10 \pm 0.07}}$ | $\frac{{0.90{\kern 1pt} --{\kern 1pt} 1.08}}{{0.97 \pm 0.04}}$ | $\frac{{0.98{\kern 1pt} --{\kern 1pt} 1.16}}{{1.07 \pm 0.05}}$ | $\frac{{0.90{\kern 1pt} --{\kern 1pt} 1.08}}{{0.98 \pm 0.05}}$ | $\frac{{0.68{\kern 1pt} --{\kern 1pt} 0.98}}{{0.83 \pm 0.09}}$ |
| Body whorl height (BWH) | $\frac{{1.10{\kern 1pt} --{\kern 1pt} 1.40}}{{1.23 \pm 0.07}}$ | $\frac{{1.00{\kern 1pt} --{\kern 1pt} 1.38}}{{1.15 \pm 0.08}}$ | $\frac{{1.08{\kern 1pt} --{\kern 1pt} 1.35}}{{1.22 \pm 0.08}}$ | $\frac{{1.08{\kern 1pt} --{\kern 1pt} 1.25}}{{1.17 \pm 0.04}}$ | $\frac{{1.11{\kern 1pt} --{\kern 1pt} 1.30}}{{1.21 \pm 0.05}}$ | $\frac{{1.10{\kern 1pt} --{\kern 1pt} 1.25}}{{1.18 \pm 0.04}}$ | $\frac{{0.93{\kern 1pt} --{\kern 1pt} 1.13}}{{1.05 \pm 0.06}}$ |
| Aperture height (AH) | $\frac{{0.67{\kern 1pt} --{\kern 1pt} 0.90}}{{0.79 \pm 0.05}}$ | $\frac{{0.65{\kern 1pt} --{\kern 1pt} 0.85}}{{0.72 \pm 0.05}}$ | $\frac{{0.70{\kern 1pt} --{\kern 1pt} 0.83}}{{0.75 \pm 0.04}}$ | $\frac{{0.68{\kern 1pt} --{\kern 1pt} 0.80}}{{0.76 \pm 0.03}}$ | $\frac{{0.71{\kern 1pt} --{\kern 1pt} 0.85}}{{0.76 \pm 0.03}}$ | $\frac{{0.71{\kern 1pt} --{\kern 1pt} 0.80}}{{0.77 \pm 0.02}}$ | $\frac{{0.60{\kern 1pt} --{\kern 1pt} 0.73}}{{0.68 \pm 0.04}}$ |
| Aperture width | $\frac{{0.55{\kern 1pt} --{\kern 1pt} 0.72}}{{0.65 \pm 0.04}}$ | $\frac{{0.50{\kern 1pt} --{\kern 1pt} 0.75}}{{0.60 \pm 0.06}}$ | $\frac{{0.50{\kern 1pt} --{\kern 1pt} 0.63}}{{0.58 \pm 0.04}}$ | $\frac{{0.55{\kern 1pt} --{\kern 1pt} 0.68}}{{0.61 \pm 0.03}}$ | $\frac{{0.53{\kern 1pt} --{\kern 1pt} 0.68}}{{0.62 \pm 0.03}}$ | $\frac{{0.55{\kern 1pt} --{\kern 1pt} 0.65}}{{0.61 \pm 0.03}}$ | $\frac{{0.50{\kern 1pt} --{\kern 1pt} 0.85}}{{0.57 \pm 0.07}}$ |
| Body whorl height above aperture | $\frac{{0.85{\kern 1pt} --{\kern 1pt} 1.08}}{{0.98 \pm 0.05}}$ | $\frac{{0.80{\kern 1pt} --{\kern 1pt} 1.00}}{{0.86 \pm 0.04}}$ | $\frac{{0.85{\kern 1pt} --{\kern 1pt} 0.98}}{{0.90 \pm 0.04}}$ | $\frac{{0.83{\kern 1pt} --{\kern 1pt} 0.95}}{{0.90 \pm 0.03}}$ | $\frac{{0.83{\kern 1pt} --{\kern 1pt} 0.96}}{{0.90 \pm 0.04}}$ | $\frac{{0.83{\kern 1pt} --{\kern 1pt} 0.95}}{{0.90 \pm 0.04}}$ | $\frac{{0.75{\kern 1pt} --{\kern 1pt} 0.88}}{{0.81 \pm 0.04}}$ |
| Penultimate whorl height (PWH) | $\frac{{0.28{\kern 1pt} --{\kern 1pt} 0.41}}{{0.36 \pm 0.03}}$ | $\frac{{0.28{\kern 1pt} --{\kern 1pt} 0.48}}{{0.33 \pm 0.04}}$ | $\frac{{0.30{\kern 1pt} --{\kern 1pt} 0.40}}{{0.35 \pm 0.03}}$ | $\frac{{0.28{\kern 1pt} --{\kern 1pt} 0.35}}{{0.30 \pm 0.02}}$ | $\frac{{0.29{\kern 1pt} --{\kern 1pt} 0.38}}{{0.38 \pm 0.02}}$ | $\frac{{0.28{\kern 1pt} --{\kern 1pt} 0.35}}{{0.31 \pm 0.02}}$ | $\frac{{0.20{\kern 1pt} --{\kern 1pt} 0.35}}{{0.27 \pm 0.04}}$ |
| Penultimate whorl width | $\frac{{0.68{\kern 1pt} --{\kern 1pt} 0.88}}{{0.79 \pm 0.05}}$ | $\frac{{0.61{\kern 1pt} --{\kern 1pt} 0.75}}{{0.69 \pm 0.03}}$ | $\frac{{0.63{\kern 1pt} --{\kern 1pt} 0.80}}{{0.75 \pm 0.05}}$ | $\frac{{0.65{\kern 1pt} --{\kern 1pt} 0.75}}{{0.70 \pm 0.03}}$ | $\frac{{0.66{\kern 1pt} --{\kern 1pt} 0.81}}{{0.72 \pm 0.03}}$ | $\frac{{0.65{\kern 1pt} --{\kern 1pt} 0.75}}{{0.71 \pm 0.03}}$ | $\frac{{0.55{\kern 1pt} --{\kern 1pt} 0.75}}{{0.65 \pm 0.06}}$ |
| SW/SH | $\frac{{0.55{\kern 1pt} --{\kern 1pt} 0.65}}{{0.61 \pm 0.03}}$ | $\frac{{0.52{\kern 1pt} --{\kern 1pt} 0.64}}{{0.57 \pm 0.03}}$ | $\frac{{0.52{\kern 1pt} --{\kern 1pt} 0.59}}{{0.54 \pm 0.02}}$ | $\frac{{0.55{\kern 1pt} --{\kern 1pt} 0.61}}{{0.59 \pm 0.02}}$ | $\frac{{0.51{\kern 1pt} --{\kern 1pt} 0.62}}{{0.57 \pm 0.03}}$ | $\frac{{0.56{\kern 1pt} --{\kern 1pt} 0.63}}{{0.60 \pm 0.02}}$ | $\frac{{0.61{\kern 1pt} --{\kern 1pt} 0.73}}{{0.65 \pm 0.03}}$ |
| SpH/SH | $\frac{{0.57{\kern 1pt} --{\kern 1pt} 0.63}}{{0.59 \pm 0.02}}$ | $\frac{{0.56{\kern 1pt} --{\kern 1pt} 0.64}}{{0.61 \pm 0.02}}$ | $\frac{{0.57{\kern 1pt} --{\kern 1pt} 0.66}}{{0.62 \pm 0.03}}$ | $\frac{{0.55{\kern 1pt} --{\kern 1pt} 0.62}}{{0.58 \pm 0.02}}$ | $\frac{{0.57{\kern 1pt} --{\kern 1pt} 0.64}}{{0.61 \pm 0.02}}$ | $\frac{{0.54{\kern 1pt} --{\kern 1pt} 0.61}}{{0.58 \pm 0.02}}$ | $\frac{{0.52{\kern 1pt} --{\kern 1pt} 0.61}}{{0.57 \pm 0.03}}$ |
| BWH/SH | $\frac{{0.63{\kern 1pt} --{\kern 1pt} 0.72}}{{0.67 \pm 0.02}}$ | $\frac{{0.65{\kern 1pt} --{\kern 1pt} 0.75}}{{0.69 \pm 0.03}}$ | $\frac{{0.64{\kern 1pt} --{\kern 1pt} 0.73}}{{0.69 \pm 0.03}}$ | $\frac{{0.66{\kern 1pt} --{\kern 1pt} 0.75}}{{0.71 \pm 0.02}}$ | $\frac{{0.66{\kern 1pt} --{\kern 1pt} 0.74}}{{0.69 \pm 0.02}}$ | $\frac{{0.66{\kern 1pt} --{\kern 1pt} 0.74}}{{0.70 \pm 0.02}}$ | $\frac{{0.66{\kern 1pt} --{\kern 1pt} 0.76}}{{0.72 \pm 0.02}}$ |
| PWH/SH | $\frac{{0.40{\kern 1pt} --{\kern 1pt} 0.49}}{{0.44 \pm 0.02}}$ | $\frac{{0.39{\kern 1pt} --{\kern 1pt} 0.48}}{{0.43 \pm 0.02}}$ | $\frac{{0.40{\kern 1pt} --{\kern 1pt} 0.45}}{{0.42 \pm 0.02}}$ | $\frac{{0.42{\kern 1pt} --{\kern 1pt} 0.48}}{{0.46 \pm 0.02}}$ | $\frac{{0.41{\kern 1pt} --{\kern 1pt} 0.48}}{{0.43 \pm 0.02}}$ | $\frac{{0.42{\kern 1pt} --{\kern 1pt} 0.48}}{{0.46 \pm 0.01}}$ | $\frac{{0.41{\kern 1pt} --{\kern 1pt} 0.52}}{{0.46 \pm 0.03}}$ |
| PWH/BWH | $\frac{{0.25{\kern 1pt} --{\kern 1pt} 0.33}}{{0.29 \pm 0.02}}$ | $\frac{{0.16{\kern 1pt} --{\kern 1pt} 0.26}}{{0.19 \pm 0.04}}$ | $\frac{{0.18{\kern 1pt} --{\kern 1pt} 0.21}}{{0.20 \pm 0.01}}$ | $\frac{{0.23{\kern 1pt} --{\kern 1pt} 0.31}}{{0.26 \pm 0.02}}$ | $\frac{{0.17{\kern 1pt} --{\kern 1pt} 0.21}}{{0.19 \pm 0.01}}$ | $\frac{{0.17{\kern 1pt} --{\kern 1pt} 0.21}}{{0.19 \pm 0.01}}$ | $\frac{{0.19{\kern 1pt} --{\kern 1pt} 0.33}}{{0.25 \pm 0.03}}$ |
* For more information on samples studied see Table 1.
Table 3.
Classification of populations of ‘Belgrandiella’ by discriminant analysis, based on 10 conchological characters
| Group (code) | % correct | Predicted grouping | ||||||
|---|---|---|---|---|---|---|---|---|
| ct | ck | ckd | an | akd | at | nsp | ||
| caucasica topotypes (ct) | 36.4 | 8 | 7 | 1 | 1 | 5 | 0 | 0 |
| caucasica Kalezh (ck) | 45.5 | 7 | 10 | 2 | 2 | 1 | 0 | 0 |
| caucasica Kudepsta (ckd) | 68.6 | 1 | 0 | 24 | 3 | 4 | 3 | 0 |
| abchasica Novy Afon (an) | 85.0 | 1 | 1 | 3 | 34 | 1 | 0 | 0 |
| abchasica Kudepsta (akd) | 57.7 | 2 | 0 | 5 | 1 | 15 | 0 | 3 |
| abchasica topotypes (at) | 80.0 | 0 | 0 | 1 | 0 | 1 | 8 | 0 |
| n. sp. Crimea (nsp) | 100.0 | 0 | 0 | 0 | 0 | 0 | 0 | 23 |
| Total | 68.5 | 19 | 18 | 36 | 41 | 27 | 11 | 26 |
Fig. 2.
Shells (a–e, i–m) and protoconchs (f–h, n, o) of Belgrandiella spp. SEM-photos. a–c, f, g – B. caucasica, a spring in Kalezh village; d, e, h, i–m – B. caucasica, Russia, vicinity of Sochi, a brook in the Malaya Khosta River valley; k, n – B. abchasica, Novoafonskaya cave; l, o – B. abchasica, Nizhnyaya Shakuranskaya Cave; m – B. abchasica, Abkhazia, a cave near Gumrysh village. Scale bars – 30 μm (f), 100 μm (g, h, l, m), 300 μm (shells, except of j), 500 μm (j).
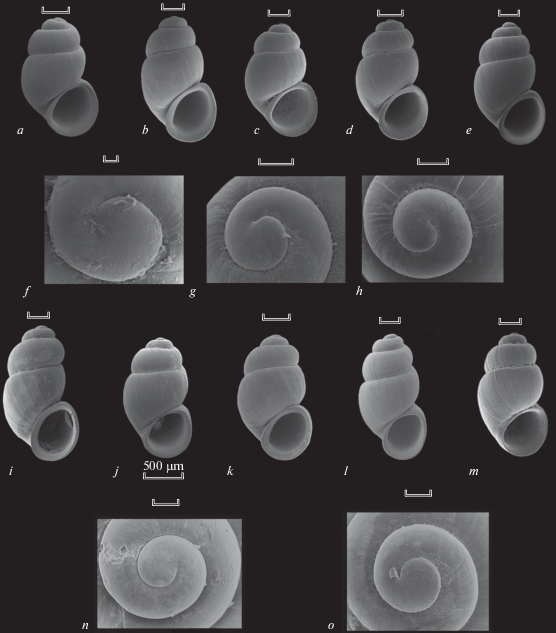
Fig. 3.
Results of a canonical analysis of conchological variation in three species of Belgrandiella.
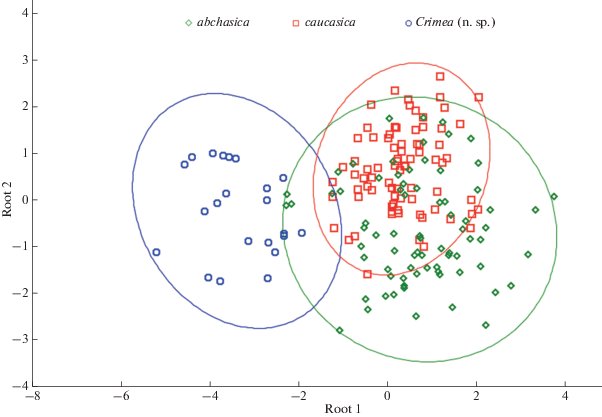
On the other hand, a sample of the Belgrandiella-like snails collected in Crimea demonstrate a clear difference from the two Caucasian taxa. In addition to smaller absolute size, the Crimean molluscs are characterized by relatively wider shells (higher values of the SW:SH ratio), with relatively lower spire (lower values of the SpH:SH ratio). Though there is no a ‘full’ morphological hiatus between the Caucasian and Crimean snails, the latter form a more or less separate ‘cloud’ of points in the multivariate space, whereas the ‘clouds’ corresponding to the two Caucasian taxa demonstrate an almost full overlap (Fig. 3). The discriminate analysis was able to distinguish between the Crimean and Caucasian species of ‘Belgrandiella’ with 100% accuracy (Table 4).
Table 4.
Classification of supposed species of ‘Belgrandiella’ by discriminant analysis, based on 10 conchological characters
| Group | % correct | Predicted grouping | ||
|---|---|---|---|---|
| abchasica | caucasica | n. sp. Crimea | ||
| abchasica | 68.4 | 52 | 21 | 3 |
| caucasica | 77.2 | 18 | 61 | 0 |
| n. sp. Crimea | 100.0 | 0 | 0 | 23 |
| Total | 76.4 | 70 | 82 | 26 |
Because of the absence of representative samples, Starobogatov (1962) used some qualitative characters to distinguish between B. abchasica and B. caucasica, including the aperture shape. According to him, B. caucasica has a round aperture, whereas in another species the aperture shape is roundish-quadrangular. Having examined more than 100 specimens of the Caucasian Belgrandiella, we found that this feature is not useful since the aperture shape varies greatly and all series of intergradations from almost round to roundish-quadrangular aperture may be found (see Fig. 2). It is not surprising since Starobogatov (1962) based his observations on an extremely small amount of shells (a single specimen of B. abchasica and two shells of B. caucasica).
To illustrate the variation in penial anatomy in ‘Belgrandiella’, we studied two series of dissections of snails collected in Abkhazia (Fig. 4). It was found that the main diagnostic feature of Belgrandiella, i.e. the presence of a hook-shaped lateral outgrowth (or a lobe) of a penis (Pezzoli, Giusti, 1980; Radoman, 1983; Arconada, Ramos, 2003) is lacking in all studied populations. Though some individuals possessed a penial outgrowth, its shape can hardly be described as a ‘hook-like’. If it exists, its relative size is smaller than in Belgrandiella kusceri, the type species of the genus (see Fig. 4c), or in B. saxatilis (Reyniés, 1844), another species of this group, whose anatomical variation was studied (Pezzoli, Giusti, 1980). Moreover, in most specimens, dissected during this work, this penial outgrowth is more or less reduced and not rarely it is completely absent. A portion of individuals with well-developed outgrowth is rather small. The reduced state of this structure seems to be typical for the Caucasian ‘Belgrandiella’, whereas all genuine Belgrandiella from Italy and the Balkan waterbodies demonstrate well-developed outgrowths (see Pezzoli, Giusti, 1980, pl. 14; Osikowski et al., 2018, fig. 5).
Fig. 4.
Shells and copulative organs of Belgrandiella abchasica from Novyi Afon (a) and Belgrandiella sp. from the Pitsunda Cape, Abkhazia (b). Images of the penises of B. kusceri (c) and Agrafia wiktori (d) are given for comparison. Each penis is shown from two projections. Arrows indicate the position of the lateral outgrowth of the penis. Abbreviations: bv – bottom view, lv – lateral view. a, b – original, c – after Radoman (1983), d – after Szarowska, Falniowski (2011), modified. Scale bars – 300 μm (shells), 0.5 mm (d), 1 mm (all penises in a and b).
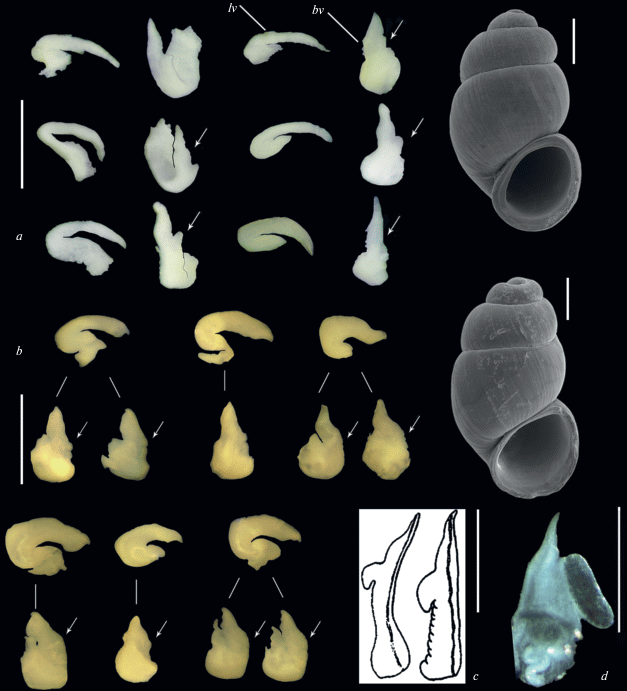
Fig. 5.
Copulative organs of Tschernomorica snails. a – Tschernomorica kimmeria; b – T. caucasica comb. n. (= Belgrandiella abchasica, a topotype); c – T. caucasica comb. n. (= B. caucasica sensu Starobogatov, 1962), a brook in the Malaya Khosta River valley; d – T. adsharica comb. n., Georgia, Adjara; e – T. inconspicua sp. n., Abkhazia, Pitsunda Cape; f – T. lindholmi sp. n., type locality. g – T. lindholmi sp. n., Abrskila Cave. Scale bars – 0.5 mm. Arrows indicate the positions of the two lateral outgrowths in the penis of P. lindholmi.

On the other hand, the shape and proportions of the distal end of the penis are much more stable within a population. It was found in both studied samples, where the relative length of the distal end, as well as its general appearance are almost the same among specimens (see Fig. 4). Moreover, these two samples differ from each other by the distal end of penis proportions. In snails from Novyi Afon (see Fig. 4a) it is relatively long, narrow and tapered, while in the Pitsunda Cape population it is short, thick, and blunt (see Fig. 4b). The relative stability in the shape and length of the distal end of the penis was found in another Belgrandiella-like snail, B. saxatilis (Pezzola, Giusti, 1980).
It is important to stress here, that no of specimens dissected by us demonstrated the penial structure characteristic to the genus Agrafia, the type species of which, Agrafia wiktori Szarowska et Falniowski 2011, possesses a penis of a peculiar type, with well developed tubular gland (see Fig. 4d). The distal end of the penis in A. wiktori is short and acute, much shorter than in ‘Belgrandiella’ of the Black Sea region. Thus, it is improbable to place Belgrandiella caucasica to this genus, at is was proposed by Grego et al. (2017). On the other hand, we had no samples from Georgia, from where the genus Agrafia has recently been recorded (Grego et al., 2017), and there is a possibility that some representatives of this genus may live in this country.
Some conclusions can be made on the basis of the results presented above.
1. ‘Belgrandiella’-like snails of the Black Sea region are conchologically very similar to the species of Belgrandiella and Agrafia from south Europe, but anatomically cannot be classified as belonging to either of these two genera. In addition, the lack of reliable findings of Belgrandiella in countries lying eastward from Kosovo, for example in Bulgaria, gives another evidence that no true Belgrandiella are living in the Crimea and Caucasus. The snails previously placed into Belgrandiella by Russian authors (Starobogatov, 1962; Vinarski, Kantor, 2016) should be separated in a special genus, which is described below.
2. ‘Belgrandiella’ abchasica and B. caucasica cannot be distinguished on the basis of their shell size and proportions; the qualitative differences between the two species listed by Starobogatov (1962) do not work as well. The structure of their copulative organs (Figs 5b–5d) is virtually the same. We regard therefore these two species as synonyms.
3. The most stable structure of the penial anatomy in the studied molluscs is the distal end of the penis. Its shape, size, and proportions may be species-specific and thus useful for delineation of (morpho-)species in this group, especially if these characters are in parallel with differences in shell habitus.
The study of conchological and anatomical variation in the ‘Belgrandiella’-like snails of the Black Sea region allows us to present below a systematic survey of all morphologically defined species of this group, with description of some new taxa of the generic and species rank. The absence of molecular support does not preclude from such description since even today it is legitimate to ground new hydrobiid species on purely morphological data (see, for example, Boeters et al., 2017; Grego et al., 2017а). In addition, we think that the formal taxonomic description of these snails is desirable from the conservation point of view, since these taxa may need a legislative protection. It is known that the underground and spring water ecosystems are especially vulnerable to human impact, their fauna is very prone to extinction (Culver et al., 2000; Hutchins, 2018), and many species of hydrobiid snails in Europe are under protection now (Arconada, Ramos, 2003; Cuttelod et al., 2011).
Systematic part
genus Tschernomorica Vinarski et Palatov gen. n.
Type species: Tschernomorica kimmeria Vinarski et Palatov sp. n.
Etymology: the generic name is derived from “Chernoye more” (Black Sea in Russian).
Diagnosis. Shell shortly-turreted to almost ovoid, with relatively high spire and rounded whorls. Eyes present. Penis simple or, sometimes, with a single lateral outgrowth, which typically is very small and poorly visible (occasionally, two weakly developed outgrowths are present). This trait differs Tschernomorica from two other conchologically similar genera – Belgrandiella and Pontobelgrandiella. From all other hydrobiid genera of the Caucasian region Tschernomorica differs by the presence of well-developed eyes.
The genus includes five species occurring in the Caucasian part of the Black Sea Coast and in Crimea. Three of these species are described below as new.
Tschernomorica kimmeria Vinarski et Palatov sp. n. (Figs 5a, 6a, 7)
Fig. 6.
Shells of Tschernomorica snails. a – Tschernomorica kimmeria (the holotype); b – T. adsharica comb. n., Georgia, Adjara; c – T. inconspicua sp. n., Abkhazia, Pitsunda Cape (the holotype); d – T. lindholmi sp. n. (the holotype). e – T. lindholmi sp. n. (a paratype). Scale bars 1 mm.

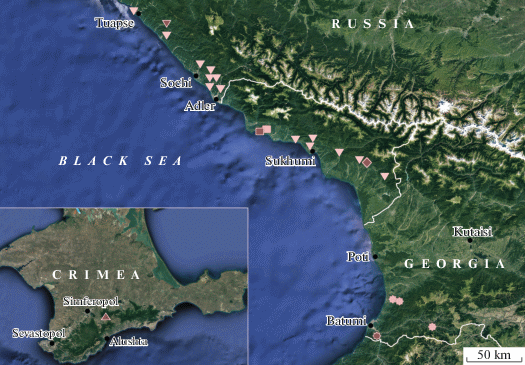
Fig. 7.
A map of localities of hydrobiid species discussed in this paper (red signs designate the species’ type localities): T. kimmeria (triangle), T. caucasica (inverted triangles), T. adharica (stars), T. inconspicua (squares), T. lindholmi (rhomb). Red signs denote the type localities.
Type series. The type series includes the holotype (see Fig. 6a) and 34 paratypes. The holotype and 23 paratypes are in ZIN, the rest of the paratypes in ZMMU (accession numbers Lc-40559 and Lc-40562). The accession number of the holotype is ZIN 503-2019-1.
Type locality. Russia, Crimea Peninsula, Belgorodsky district, a spring of the Zuya River valley (44°55′12.6″ N, 34°22′10.9″ E). 11.11.2010. leg. M.V. Chertoprud.
Etymology. Named after Kimmeria (Κι μμέρια) – an ancient Greek name for the Crimean Peninsula and adjacent areas of the northern Black Sea coast.
Shell dimensions of the holotype (in mm). WN = 3.50; SH = 1.55; SW = 1.03; SpH = 0.88; BWH = 1.10; AH = 0.70; AW = 0.60; BWHap = 0.85; PWH = 0.28; PWW = 0.70. For morphometric characteristics of the entire type series see Table 2.
Description. Shell small (SH ≤ 1.65 mm), shortly cylindrical or barrel-shaped to almost ovoid, dark-white or yellowish. Whorl number up to 3.75. Whorls rounded and visibly convex, separated by deep suture. Shell relatively wide, SW/SH ratio is not less than 0.60. Spire high, its height constitutes 0.50–0.60 of SH. Body whorl is high and very weakly inflated, its width slightly exceeds the width of the penultimate one. Tangent-line is strongly convex. Aperture occasionally with a small angle in its upper part. Operculum of light-orange color.
Penis simple, without lateral outgrowth. The distal end of the penis relatively narrow, not acute; it is visibly swollen in its last third (see Fig. 5a).
Distribution. Endemic to Crimea. At the moment, T. kimmeria sp. n. is known from several springs of the Zuya River valley (11.11.2010, leg. M.V. Chertoprud).
Tschernomorica caucasica (Starobogatov 1962) comb. n. (Figs 2, 4a, 5b–5c, 7)
– Belgrandiella caucasica Starobogatov 1962: 48, fig. 1 (Д); Vinarski, Kantor, 2016: 215; Sitnikova et al., 2017: 277, fig. 9, I ; Palatov, Sokolova, 2019: 3, fig. 2, A, C, D, E.
– Belgrandiella abchasica Starobogatov 1962: 48, fig. 1 (E); Vinarski, Kantor, 2016: 215; Sitnikova et al., 2017: 277, fig. 9, G, H .
– Belgrandiella nemethi Schütt, Şeşen 1993: 167, fig. 18 .
Type series. ZIN (Nos. 1 and 2 in the systematic catalogue). The type series includes the holotype (see Fig. 6a) and a single paratype (see Sitnikova et al., 2017 for details).
Type locality. Russia, Krasnodar Region, Sochi district, Krasnoaleksandrovskaya Cave (44°00′55.3″ N 39°21′49.8″ E).
Description. Shell relatively large for the genus (SH reaches 2.05 mm), shortly cylindrical or barrel-shaped, dark-white or yellowish. Whorl number up to 4.25. Whorls rounded and weakly convex, separated by shallow suture. Shell moderately wide, SW/SH ratio is between 0.50 and 0.63. Spire is a bit higher than in previous species, its height constitutes 0.55–0.66 of SH. Body whorl is relatively high and very weakly inflated, its width slightly exceeds the width of the penultimate one. Tangent-line is convex. Aperture shape varies from almost round to roundish-quadrangular, sometimes it has a small angle in its upper part.
Penis usually with a single lateral outgrowth, whose size demonstrates all degrees of reduction, sometimes this outgrowth is almost absent. The distal end of the penis relatively narrow and acute; it is not swollen in its last third (see Figs 5a–5b).
Distribution. Western Caucasus. Findings of this species are known from Russia (Krasnodar Region, alongside the Black Sea coast, Tuapse to Adler) and Abkhazia (eastwards to the Tkuarchyl District).
Ecology. Inhabits springs (rheocrenes, helocrenes), cave brooks (in caves Dolgaya, Nizhnyaya Shakuranskaya, Verkhnyaya Shakuranskaya, Adzaba, Golova Otapa). Found in lowland rivers (for example, in the Bolshaya Khosta River), on small pebbles. All localities of this species are situated below 500 m a.s.l.
Remark. T. caucasica comb. n. demonstrates a high level of conchological variation. The populations of this species from vicinities of Sochi were described under the name Belgrandiella nemethi Schütt in Schütt et Şeşen 1993. Having examined a large number of specimens collected from the type locality of B. nemethi, we could not find any differences from T. caucasica and, thus, we considered B. nemethi as a junior synonym of the latter (see also Palatov, Vinarski, 2015; Palatov, Sokolova, 2019).
Tschernomorica adsharica (Lindholm 1913) comb. n. (Figs 5d, 6c, 7)
– Bythinella adsharica Lindholm, 1913: 67; Vinarski, Kantor, 2016: 182.
– Belgrandiella adsharica Schütt, Şeşen, 1993: 167.
Type series. Not traced; absent in ZIN.
Type locality. Georgia, Adzharia, vicinities of Batum Town, a spring near river Adsharis-Tskhali at its entrance to Tschoroch.
Morphology. Conchologically, this species is hardly distinguishable from T. caucasica comb. n., except of being smaller and having a slightly narrower shell (compare Tables 2 and 5). The main anatomical difference between the two taxa lies in the penial anatomy. In T. adsharica, the distal end of the penis is relatively longer than in T. caucasica comb. n.; it is not acute, and its last third is slightly swollen (see Fig. 5d). A gap in the geographical distribution of these snails (see Fig. 7) is another evidence of their specific distinctness. Possibly, they form a pair of vicariant species (or a pair of subspecies?), inhabiting the western (T. caucasica) and the eastern (T. adsharica) part of the Caucasian Region.
Table 5.
Morphometric characteristics of shells of Tschernomorica adsharica comb. n., T. inconspicua sp. n., and T. lindholmi sp. n.
| Character/index | Species | ||
|---|---|---|---|
| T. adsharica* | T. inconspicua** | T. lindholmi*** | |
| Whorl number | $\frac{{3.25{\kern 1pt} --{\kern 1pt} 4.00}}{{3.69 \pm 0.24}}$ | $\frac{{3.50{\kern 1pt} --{\kern 1pt} 4.50}}{{3.78 \pm 0.16}}$ | $\frac{{3.50{\kern 1pt} --{\kern 1pt} 4.00}}{{3.71 \pm 0.13}}$ |
| Shell height (SH) | $\frac{{1.40{\kern 1pt} --{\kern 1pt} 1.70}}{{1.55 \pm 0.10}}$ | $\frac{{1.45{\kern 1pt} --{\kern 1pt} 1.75}}{{1.61 \pm 0.09}}$ | $\frac{{1.65{\kern 1pt} --{\kern 1pt} 2.00}}{{1.82 \pm 0.09}}$ |
| Shell width (SW) | $\frac{{0.75{\kern 1pt} --{\kern 1pt} 0.90}}{{0.83 \pm 0.04}}$ | $\frac{{0.85{\kern 1pt} --{\kern 1pt} 1.05}}{{0.94 \pm 0.06}}$ | $\frac{{1.08{\kern 1pt} --{\kern 1pt} 1.18}}{{1.13 \pm 0.04}}$ |
| Spire height (SpH) | $\frac{{0.83{\kern 1pt} --{\kern 1pt} 1.03}}{{0.91 \pm 0.07}}$ | $\frac{{0.85{\kern 1pt} --{\kern 1pt} 1.08}}{{0.96 \pm 0.06}}$ | $\frac{{0.90{\kern 1pt} --{\kern 1pt} 1.22}}{{1.07 \pm 0.08}}$ |
| Body whorl height (BWH) | $\frac{{0.93{\kern 1pt} --{\kern 1pt} 1.13}}{{1.04 \pm 0.06}}$ | $\frac{{1.00{\kern 1pt} --{\kern 1pt} 1.25}}{{1.12 \pm 0.06}}$ | $\frac{{1.25{\kern 1pt} --{\kern 1pt} 1.45}}{{1.38 \pm 0.05}}$ |
| Aperture height (AH) | $\frac{{0.60{\kern 1pt} --{\kern 1pt} 0.78}}{{0.67 \pm 0.05}}$ | $\frac{{0.63{\kern 1pt} --{\kern 1pt} 0.80}}{{0.71 \pm 0.04}}$ | $\frac{{0.80{\kern 1pt} --{\kern 1pt} 0.93}}{{0.88 \pm 0.04}}$ |
| Aperture width | $\frac{{0.45{\kern 1pt} --{\kern 1pt} 0.73}}{{0.55 \pm 0.08}}$ | $\frac{{0.48{\kern 1pt} --{\kern 1pt} 0.68}}{{0.57 \pm 0.05}}$ | $\frac{{0.58{\kern 1pt} --{\kern 1pt} 0.77}}{{0.68 \pm 0.04}}$ |
| Body whorl height above aperture | $\frac{{0.68{\kern 1pt} --{\kern 1pt} 0.83}}{{0.76 \pm 0.05}}$ | $\frac{{0.80{\kern 1pt} --{\kern 1pt} 0.95}}{{0.86 \pm 0.04}}$ | $\frac{{1.00{\kern 1pt} --{\kern 1pt} 1.13}}{{1.07 \pm 0.03}}$ |
| Penultimate whorl height (PWH) | $\frac{{0.25{\kern 1pt} --{\kern 1pt} 0.35}}{{0.30 \pm 0.03}}$ | $\frac{{0.28{\kern 1pt} --{\kern 1pt} 0.38}}{{0.32 \pm 0.03}}$ | $\frac{{0.23{\kern 1pt} --{\kern 1pt} 0.38}}{{0.29 \pm 0.04}}$ |
| Penultimate whorl width | $\frac{{0.58{\kern 1pt} --{\kern 1pt} 0.70}}{{0.64 \pm 0.03}}$ | $\frac{{0.65{\kern 1pt} --{\kern 1pt} 0.75}}{{0.71 \pm 0.04}}$ | $\frac{{0.70{\kern 1pt} --{\kern 1pt} 0.88}}{{0.78 \pm 0.05}}$ |
| SW/SH | $\frac{{0.48{\kern 1pt} --{\kern 1pt} 0.57}}{{0.54 \pm 0.02}}$ | $\frac{{0.56{\kern 1pt} --{\kern 1pt} 0.61}}{{0.58 \pm 0.02}}$ | $\frac{{0.58{\kern 1pt} --{\kern 1pt} 0.67}}{{0.62 \pm 0.03}}$ |
| SpH/SH | $\frac{{0.51{\kern 1pt} --{\kern 1pt} 0.63}}{{0.59 \pm 0.04}}$ | $\frac{{0.56{\kern 1pt} --{\kern 1pt} 0.64}}{{0.60 \pm 0.02}}$ | $\frac{{0.54{\kern 1pt} --{\kern 1pt} 0.63}}{{0.59 \pm 0.02}}$ |
| BWH/SH | $\frac{{0.63{\kern 1pt} --{\kern 1pt} 0.72}}{{0.67 \pm 0.02}}$ | $\frac{{0.64{\kern 1pt} --{\kern 1pt} 0.74}}{{0.69 \pm 0.03}}$ | $\frac{{0.70{\kern 1pt} --{\kern 1pt} 0.80}}{{0.76 \pm 0.03}}$ |
| PWH/SH | $\frac{{0.38{\kern 1pt} --{\kern 1pt} 0.46}}{{0.43 \pm 0.02}}$ | $\frac{{0.41{\kern 1pt} --{\kern 1pt} 0.46}}{{0.44 \pm 0.01}}$ | $\frac{{0.45{\kern 1pt} --{\kern 1pt} 0.55}}{{0.48 \pm 0.02}}$ |
| PWH/BWH | $\frac{{0.17{\kern 1pt} --{\kern 1pt} 0.22}}{{0.19 \pm 0.01}}$ | $\frac{{0.18{\kern 1pt} --{\kern 1pt} 0.23}}{{0.20 \pm 0.01}}$ | $\frac{{0.12{\kern 1pt} --{\kern 1pt} 0.19}}{{0.16 \pm 0.02}}$ |
* N = 17 (05.02.2013. Georgia, Adzharia, a spring in the Kintrishi River valley); ** N = 22 (the holotype and 21 paratypes of ZIN collection); *** N = 21 (the holotype and 20 paratypes of ZIN collection). Above lines – limits of variation (min–max), below lines – mean value ± standard deviation (σ).
Distribution. Georgia (Adzharia) and Asia Minor (Schütt, Şeşen, 1993; Vinarski, Kantor, 2016).
Ecology. Inhabits springs (rheocrenes, helocrenes), lives mainly on submerged leaves.
Remark. The topotypic specimens of this species differ anatomically from the type species of both Agrafia and Belgrandiella (see above); the structure of their penises corresponds to that of other species of Tschernomorica gen. n., therefore we place B. adsharica into this new genus. The original placement of this species into the genus Bythinella Moquin-Tandon 1856 (Lindholm, 1913) is untenable as the topotypic specimens lack the flagellum, which is characteristic for the representatives of Bythinella (Glöer, Georgiev, 2009; Glöer, Pešič, 2010).
Tschernomorica inconspicua Vinarski et Palatov sp. n. (Figs 5e, 6c, 7)
– Belgrandiella sp. Palatov, Sokolova, 2019: 5, fig. 2, F, G.
Type series. The type series includes the holotype (see Fig. 6c) and 70 paratypes. The holotype and 21 paratypes are in ZIN, 20 paratypes in LMBI collection, 20 paratypes in ZMMU (accession number Lc-40 560), 9 paratypes in SMF. The accession number of the holotype is ZIN 502-2019-1.
Type locality. Abkhazia, Gudauta district, Pitsunda Cape, a spring in the Myussera River valley (43°11′52.30″ N, 40°27′0.03″ E). 19.02.2010. leg. D.M. Palatov.
Etymology. The name is derived from the Latin “inconspicuus” – unremarkable.
Shell dimensions of the holotype (in mm). WN = 3.75; SH = 1.75; SW = 1.05; SpH = 1.05; BWH = 1.15; AH = 0.80; AW = 0.60; BWHap = 0.95; PWH = 0.35; PWW = 0.75. For morphometric characteristics of the paratypes see Table 5.
Description. Shell small (SH ≤ 1.75 mm), shortly cylindrical or barrel-shaped, dark-white or yellowish. Whorl number up to 4.50. Whorls are evenly rounded and weakly convex, separated by shallow suture. Shell moderately slender, SW/SH ratio is usually less than 0.60. Spire high, its height constitutes 0.56–0.64 of SH. Body whorl is high and very weakly inflated, its width slightly exceeds the width of the penultimate one. Tangent-line is slightly convex. Aperture rounded, with a small angle in its upper part.
Penis simple, without lateral outgrowth. The distal end of the penis is thick, blunt, and usually bears a large bulbous inflation in its last third (see Fig. 5e).
Distribution. Registered from springs of the Myussera River basin (in the Pitsundo-Myussersky Nature Reserve).
Ecology. Inhabits springs (mainly helocrenes).
Remark. Conchologically, this new species is very similar to both Tschernomorica caucasica comb. n. and T. adsharica, but the peculiar structure of the penis allows to distinguish it easily from all congenerics. Besides, T. inconspicua differs from T. caucasica by its smaller shell size: the maximal shell heights registered in these species are 1.75 and 2.05 mm, respectively.
Tschernomorica lindholmi Vinarski et Palatov sp. n. (Figs 5f–5g, 6d–6e, 7)
Type series. Includes the holotype (see Fig. 6d) and 63 paratypes. The holotype and 20 paratypes are in ZIN,14 paratypes in LMBI collection, 20 paratypes in ZMMU (accession number Lc-40561), 9 paratypes in SMF. The accession number of the holotype is ZIN 504-2019-1.
Type locality. Abkhazia, Ochamchira district, Otap village, a brook outflowing from the Abrskila Cave (42°55′13.81″ N, 41°33′16.56″ E). 03.05.2015. leg. D.M. Palatov.
Etymology. Named in memory of Wassily A. Lindholm (1874–1935), the Russian malacologist, who greatly contributed to the knowledge of land and aquatic continental gastropods of Caucasus.
Shell dimensions of the holotype (in mm). WN = 3.75; SH = 1.75; SW = 1.12; SpH = 1.00; BWH = 1.35; AH = 0.88; AW = 0.73; BWHap = 1.10; PWH = 0.30; PWW = 0.70. For morphometric characteristics of the patarypes see Table 5.
Description. Shell relatively large (SH reaches 2.0 mm), its shape varies from shortly cylindrical to almost perfectly ovoid. Shell surface is dark-white or yellowish. Whorl number up to 4.00. Whorls rounded and weakly convex, separated by shallow suture. Shell relatively wide, SW/SH ratio is about 0.60. Spire high, its height constitutes 0.54–0.63 of SH. Body whorl is high and moderately inflated, its width visibly exceeds width of the penultimate one. Tangent-line is convex. Aperture rounded, sometimes with a very small angle in its upper part.
Penis bears a single or, occasionally, two weakly developed lateral outgrowths on its left side; the distal end of the penis is relatively narrow and pointed in its last third.
Distribution. In addition to the type locality, this species was registered in the Abrskila Cave.
Ecology. The type locality of T. lindholmi is a large brook (about one meter width), outflowing from the Abrskila Cave; in addition, specimens of this species were found in small tributaries (helocrenes) of this brook. In the Abrskila Cave, snails were not found beyond its lighted zone (30 meters from the entrance). Mollusks were collected from submerged stones and pebble, at water velocity 0.1–0.3 m/s.
Remark. Externally, this new species resembles Tschernomorica kimmeria sp. n., but the pointed distal end of the penis distinguishes T. lindholmi from the latter species. The presence of two lateral outgrowths on the left side of the penis (see Fig. 5g) may mean that this species should be included into the genus Pontobelgrandiella, which is characterized by this feature (Radoman, 1983; Georgiev, 2013).
Key to identification of species of the genus Tschernomorica
1(6) Penis simple, without outgrowth(s). Its distal end blunt and visibly swollen in the apical part.
2(3) Shell shape close to ovoid. Inhabits Crimea ………………………............. T. kimmeria sp. n.
3(4) Shell shape shortly cylindrical or barrel-shaped. Inhabits Caucasus.
4(5) Distal end of the penis thick, its apical part greatly inflated……...................... T. inconspicua sp. n.
5(4) Distal end of the penis narrow, its apical part weakly inflated…….....T. adsharica (Lindholm 1913) comb. n.
6(1) Penis with a single (rarely two) weakly developed outgrowth, which sometimes may be almost invisible. Its distal end pointed, not swollen in the apical part.
7(8) Shell ovoid.…………………… T. lindholmi sp. n.
8(7) Shell shortly cylindrical…………………. T. caucasica (Starobogatov 1962) comb. n.
DISCUSSION
The five species of Belgrandiella-like snails united here under a new generic name Tschernomorica are characterized by a rather broad diversity of their penial anatomy, whereas their conchological differences are much less obvious, and at least in a single pair of species (T. adsharica – T. caucasica) the shell characters are virtually useless for species identification.
In general, all species of Tschernomorica may be divided into two groups – the first one unites species with the pointed distal end of the penis (T. caucasica, T. lindholmi), whereas the three other species (T. kimmeria, T. inconspicua, T. adsharica), possessing the blunt penial apex, form the second division. However, lack of the molecular data does not allow to decide if these groups are phylogenetically distinct [independent] units. In the meantime a spatial distribution of these groups correlates with some physical-geographical peculiarities of the studied region. The species of the first group are restricted to the karst massifs; neither of these species has been found in the plain part of the Caucasian region. The localities of species of the second group are situated beyond the karst zone; these species have never been recorded from caves. Perhaps, this fact gives an indirect evidence of a rather deep phylogenetic split between the two divisions of Tschernomorica, whose divergence might have been driven by adaptation to different types of localities. Molecular phylogenetic and/or more in-depth anatomical studies are required to test this hypothesis.
Список литературы
Anistratenko V., Peretolchina T., Sitnikova T., Palatov D., Sherbakov D., 2017. A taxonomic position of Armenian endemic freshwater snails of the genus Shadinia Akramowski, 1976 (Caenogastropoda: Hydrobiidae): combining morphological and molecular evidence // Molluscan Research. V. 37. P. 212–221.
Arconada B., Ramos M.A., 2003. The Iberico-Balearic region: one of the areas of highest Hydrobiidae (Gastropoda, Prosobrancha, Rissooidea) diversity in Europe // Graellsia. V. 59. P. 91-104.
Boeters H., Reischütz P., Reischütz A., 2017. The first record of Pontobelgrandiella Radoman, 1978 from Greece (Caenogastropoda: Truncatelloidea: Hydrobiinae) with the description of a new species // Folia Malacologica. V. 25. P. 249–255.
Culver D.C., Master L.L., Christman M.C., Hobbs H.H., 2000. Obligate cave fauna of the 48 contiguous United States // Conservation Biology. V. 14. P. 386–401.
Cuttelod A., Seddon M., Neubert E., 2011. European Red List of Non-marine Molluscs. Luxembourg: Publications Office of the European Union. 97 p.
Georgiev D., 2013. Catalogue of the stygobiotic and troglophilous freshwater snails (Gastropoda: Rissooidea: Hydrobiidae) of Bulgaria with descriptions of five new species // Ruthenica: Russian Malacological Journal. V. 23. P. 59–67.
Glöer P., Bößneck U., Walther F., Neiber M.T., 2015. New taxa of freshwater molluscs from Armenia (Caenogastropoda: Truncatelloidea: Hydrobiidae) // Folia Malacologica. V. 24. P. 3–8.
Glöer P., Georgiev D., 2009. New Rissooidea from Bulgaria (Gastropoda: Rissooidea) // Mollusca (Dresden). V. 27. P. 123–136.
Glöer P., Pešič V., 2010. The freshwater snails of the genus Bythinella Moquin-Tandon (Gastropoda: Rissooidea: Hydrobiidae) from Montenegro // Archives of Biological Sciences. V. 62. P. 441–447.
Grego J., Hofman S., Mumladze L., Falniowski A., 2017. Agrafia Szarowska et Falniowski, 2011 (Caenogastropoda: Hydrobiidae) in the Caucasus // Folia Malacologica. V. 25. P. 237–247.
Grego J., Glöer P., Eröss Z.P., Fehér Z., 2017a. Six new subterranean freshwater gastropod species from northern Albania and some new records from Albania and Kosovo (Mollusca, Gastropoda, Moitessieriidae and Hydrobiidae) // Subterranean Biology. V. 23. P. 85–107.
Haase M., 1994. Differentiation of selected species of Belgrandiella and the redefined genus Graziana (Gastropoda: Hydrobiidae) // Zoological Journal of the Linnean Society. V. 111. P. 219–246.
Hutchins B.T., 2018. The conservation status of Texas groundwater invertebrates // Biodiversity & Conservation. V. 27. P. 475–501.
Lindholm W.A., 1913. Beschreibung neuer Arten und Formen aus dem Kaukasus-Gebiete // Nachrichtsblatt der Deutschen Malakozoologischen Gesellschaft. B. 45. S. 62–69.
Osikowski A., Hofman S., Rysiewska A., Sket B., Prevorčnik S., Falniowski A., 2018. A case of biodiversity overestimation in the Balkan Belgrandiella A. J. Wagner, 1927 (Caenogastropoda: Hydrobiidae): molecular divergence not paralleled by high morphological variation // Journal of Natural History. V. 52. P. 323–344.
Palatov D.M., Sokolova A.M., 2019. Stygobiotic faunal elements in spring assemblages of West Transcaucasia // Ecosystem Transformation. V. 2. № 1. P. 1–9. https://doi.org/10.23859/estr-180128
Palatov D.M., Vinarski M.V., 2015. Ecology and distribution of gastropods of the subfamily Belgrandiellinae (Mollusca: Gastropoda: Hydrobiidae s. lato) in the Western Transcaucasia // Biospeleologiya Kavkaza i drugikh rayonov Rossii. Materialy Vserossiyskoy molodezhnoy konferentsii (Moskva, 3-4 dekabrya 2015). Kostroma: Kostromskoy pechatnyi dom. P. 49–54. [In Russian].
Pezzoli E., Giusti F., 1981. Primo contributo alla revisione del genere Belgrandiella in Italia (Prosobranchia, Hydrobioidea) // Atti del IV° Congresso della Società Malacologica Italiana (Siena, 6-9 ottobre 1978). Siena. P. 319–351.
Radoman P., 1983. Hydrobioidea – a superfamily of Prosobranchia (Gastropoda). I. Systematics // Monographs of the Serbian Academy of Sciences and Arts. V. 547. P. 1–256.
Rysiewska A., Georgiev D., Osikowski A., Hofman S., Falniowski A., 2016. Pontobelgrandiella Radoman, 1973 (Caenogastropoda: Hydrobiidae): A recent invader of subterranean waters? // Journal of Conchology. V. 42. P. 1–11.
Schütt H., Şeşen R., 1993. Pseudamnicola species and other freshwater gastropods (Mollusca, Gastropoda) from East Anatolia (Turkey), the Ukraine and the Lebanon // Basteria. V. 57. P. 161–171.
Sitnikova T.Ya., Sysoev A.V., Kijashko P.V., 2017. Species of freshwater gastropods described by Ya. L. Starobogatov: Pulmonata (Acroloxidae), Heterobranchia (Valvatidae) and Caenogastropoda (Viviparoidea, Truncatelloidea and Cerithioidea) // Trudy Zoologicheskogo Instituta RAN. V. 321. P. 247–299.
Sitnikova T.Ya., Peretolchina T.E., Anistratenko V.V., Anistratenko O.Yu., Palatov D.M., 2018. Variability and taxonomy of South Caucasian freshwater snails of the genus Shadinia Akramowski, 1976 (Caenogastropoda: Hydrobiidae) based on new morphological and molecular data // Archiv für Molluskenkunde. V. 147. P. 63–76.
Starobogatov Ya.I., 1962. Contribution to knowledge of mollusks from subterranean waters of the Caucasus // Byulleten’ Moskovskogo Obshchestva Ispytateley Pripody, otdel biologicheskiy. V. 67. P. 42–54. [In Russian].
Szarowska M., Falniowski A., 2011. An unusual, flagellum-bearing hydrobiid snail (Gastropoda: Rissooidea: Hydrobiidae) from Greece, with descriptions of a new genus and a new species // Journal of Natural History. V. 45. P. 2231–2246.
Vinarski M.V., Kantor Yu.I., 2016. Analytical catalogue of fresh and brackish water molluscs of Russia and adjacent countries. Moscow: A.N. Severtsov Institute of Ecology and Evolution of RAS. 542 p.
Wagner A.J., 1928. Studien zur Molluskenfauna der Balkanhalbinsel mit besonderer Berücksichtigung Bulgariens und Thraziens, nebst monographischer Bearbeitung einzelner Gruppen // Annales Zoologici Musei Polonici Historiae Naturalis. V. 6. P. 263–399.
Дополнительные материалы отсутствуют.
Инструменты
Зоологический журнал


