Зоологический журнал, 2019, T. 98, № 9, стр. 1072-1076
New Data on the Tongue Structure in the Springhare, Pedetes capensis (Rodentia, Pedetidae)
E. G. Potapova *
Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences
119071 Moscow, Russia
* E-mail: lena-potapova@yandex.ru
Поступила в редакцию 6.02.2019
После доработки 28.03.2019
Принята к публикации 28.03.2019
Аннотация
A highly specialised tongue structure in Pedetes capensis is documented in its proportions and in the distribution of different types of papillae on its dorsal side. The fixed part of the tongue is long, while its posterior part in the area of the lenticular papillae is greatly shortened. Neither a torus linguae nor any sulci are present on its dorsum. Circumvallate papillae are absent. The lateral organ is markedly enlarged. Numerous fungiform papillae are concentrated in two zones: at the tip of the tongue and on a low eminence at its base. The tongue of Pedetes is generally more similar to that of Anomalurus than to that of Idiurus. The similarity of tongue morphology in Pedetidae and Anomaluridae is based on features occurring not only in these families, but also appearing in other rodent taxa. Despite the specialised tongue structure in Pedetes, the data obtained do not contradict the hypothesis of a sister relationship between the above families, although this cannot be confirmed with certainty.
The morphology of the tongue is described in detail in the springhare, Pedetes capensis (Forster 1778), the only extant member of the rodent family Pedetidae. This family belongs to one of the basal branches of the rodent radiation and is characterized by a high level of morpho-functional specialization (Offermans, De Vree, 1989; Lopez-Antonanzas, 2016; Potapova, 2017; etc.). The scaly-tailed squirrel family Anomaluridae is considered a sister group to pedetids. These two families are grouped into one taxon, Anomalurimorpha (Montgelard et al., 2002), the position of which within the Rodentia is uncertain and has repeatedly been revised (Anderson, Jones, 1984; Luckett, Hartenberger, 1985; Simpson, 1945; Pavlinov, 2006). This group is currently believed to be phylogenetically close to myomorph rodents and is nested within the so-called “mouse-related clade” (Huchon et al., 2007; Blanga-Kanfi et al., 2009; etc.). Specifics of the morphological diversity of the tongue in rodents allow us to include data on its structure for purposes of the systematics and phylogenetics of the order (Tullberg, 1899; Sonntag, 1924; Potapova, 1976, 1979, 2018; Vorontsov, 1982; etc.).
The morphology of the tongue was recently described in five of the seven known modern species of anomalurids: Anomalurus beecrofti Fraser 1853, A. derbianus (Gray 1842), A. pusillus Thomas 1887, Idiurus macrotis Miller 1898, and I. zenkeri Matschie 1894 (Potapova, 2018). Comparable data on the springhare were not available as its tongue had never been studied using modern microscopy techniques. Therefore, illustrations of the tongue from Tullberg’s (1899) work were used for comparison.
After that article was released (Potapova, 2018), a specimen of young Pedetes transferred to the Zoological Museum of Moscow State University from the Moscow Zoo was obtained for study. The specimen was preserved in 10% formalin and then transferred to 70% alcohol. The study was conducted using stereomicroscopes Leica MZ7.5 (Leica, Czech Republic) and Stemi SV11 (Carl Zeiss, Germany) with an Axio Cam MRc digital camera (Carl Zeiss). As a result, important additional information on the tongue structure of Pedetes was acquired which made it possible to conduct a comprehensive comparison of its morphology in representatives of both Anomalurimorpha families.
RESULTS
The tongue in the springhare is rather short, broad, and thick. The fixed part of its body (corpus and radix linguae) is long. The anterior free part of the tongue (frp) and the caudal part of its root with the lenticular papillae (zpl) are short (Figs 1c, 1d; 2а, 2b).
Fig. 1.
Tongue of Pedetes capensis (a, c) and of Idiurus macrotis (b, d). Schematically: a, b – dorsal view; c, d – side view. b, d – after Potapova, 2018 (with changes). Abbreviations: al –apex of the tongue; cl – fixed part of the tongue; frp – free part of the tongue; pcv – circumvallate papillae; pfil – filiform papillae; pfol – foliate papillae (= lateral organ); pfun – fungiform papillae and zones of their concentration on the tip of the tongue (pfun1), on its body in front of the dorsum eminence (pfun2), and on this eminence (pfun3); pl – lenticular papillae; tl – the low intermolar eminence of the tongue dorsum, according to the position corresponding to torus linguae.
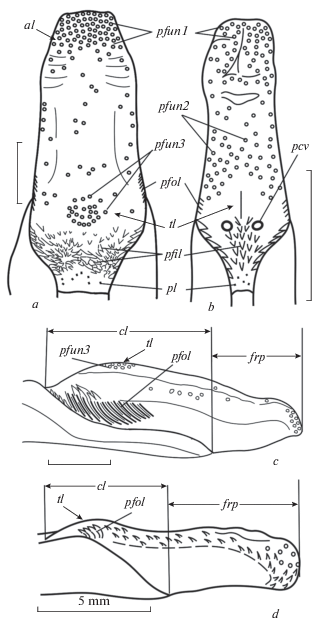
There are no folds on the tongue dorsum, neither the anterior median sulcus on the tip of the tongue, nor the semilunar sulcus that usually encircles the anterior border of the large median protrusion (the so called “tongue pad” – torus linguae). The torus linguae is not clearly expressed, the tongue dorsum is flat and only slightly convex at the posterior part. This low intermolar eminence (tl) corresponds to the torus linguae (Fig. 1a, 1c).
The dorsal surface of the tongue and its lateral margins are densely covered by numerous compact filiform papillae, which, as is known, do not contain taste buds and perform a mechanical function. At the tip of the tongue they extend onto its underside. In Pedetes, the filiform papillae are small, each with a single apex and slightly flattened at its base. At the tip of the tongue they extend onto its underside. They tightly overlap each other in a regular pattern, resembling scales (Fig. 2).
Fig. 2.
Details of the tongue structure in Pedetes capensis (a, c, e, f) and Anomalurus derbianus (b, d); a, b, e, f – dorsal view; c, d – side view. e – dorsum of the tongue in the zone of lenticular papillae (zpl), f – in the zone of the intermolar eminence (ztl). Other abbreviations as in Fig. 1. b, d – after Potapova, 2018 (with changes).

There is no gradual increase in size of the filiform papillae posteriorly; they are small both on the tip and on the body of the tongue. Only in the area of the dorsal eminence are they slightly enlarged (Fig. 2f), though less so than the equivalent in anomalurids (Fig. 2b, 2d). In some rodent taxa, the largest filiform papillae are concentrated exactly on this eminence. In Pedetes, the largest filiform papillae are located at the tongue root in front of the area of lenticular tubercles (Fig. 2a, 2e), where they are arranged in groups extended along the presumed paths of food transport (Fig. 2e).
In Pedetes, there are only two kinds of papillae that contain taste receptors: fungiform and foliate (=lateral organ). Both are rather numerous. The fungiform papillae are concentrated mainly in two zones: at the tip of the tongue and on the low intermolar eminence at its base. In the interval between these zones, papillae of this type are located singly or in small groups, one behind the other, mainly along the lateral edges of the dorsum of the tongue and on its lateral surfaces. At the tip of the tongue, the fungiform papillae are especially numerous and are much smaller than those on the dorsal eminence. The most prominent fungiform papillae arranged in a semicircle are located along the posterior edge of this protrusion, whereas in other rodents, this area is occupied by the circumvallate papillae (Fig. 2a, 2f). In its pattern of bizonal distribution of the fungiform papillae, concentrated on the tip of the tongue and on the dorsal eminence, Pedetes resembles Anomalurus, but differs from Idiurus.
The circumvallate papillae are absent. This is the only case of a reduction of these papillae reported in rodents.
The foliate papillae which form the so-called lateral organ are located on the lateral surface of the tongue (Fig. 2c). Their number is determined by the number of fissures into which pores of the taste buds open. The springhare specimen examined here had 15 long fissures on one side of the tongue and 16 on the other (Fig. 2с). The lateral organ in Pedetes is relatively much larger than that in anomalurids and in many other rodents. Probably the largest lateral organs among rodents occur in beavers (Castor – Zonntag, 1924).
Thus, besides the tip of the tongue, the root zone is also particularly rich in taste buds in Pedetes. The taste papillae form a “sensitive” semicircular area in the region of the dorsal eminence; the fungiform papillae are located at the top of the tongue, and the foliate papillae on the sides.
The lenticular papillae are shallow small pits at the base of the tongue root which include the lymphoid nodules and the salivary glands so, in fact, they are not true papillae. In Pedetes, they are not numerous and are poorly visible, and the zone of their distribution is quite limited (Fig. 2a, compare with 2b).
DISCUSSION
The tongue of the Pedetes specimen studied here looks very similar to that depicted by Tullberg (1899). This allows us to consider these features as species-specific rather than an individual variation in a particular specimen.
Our study revealed that tongue morphology in Pedetes capensis is noticeably different from that of other rodents. This applies to the proportions of the tongue (Fig. 1), as well as the number, size and location of different types of papillae on its surface. This specificity is expressed in a distinctive combination of the unique features with traits found in some other groups of rodents.
Some tongue features distinguish the springhare from scaly-tailed squirrels, whereas others are similar. The tongue of Pedetes is relatively shorter, wider and thicker than that of the anomalurids; its fixed part is long, whereas the root part in the region of the lenticular papillae is very short. The lateral organ is substantially enlarged. In addition, unlike anomalurids, the median sulcus is not expressed at the tip of the tongue in Pedetes, and there are no circumvallate papillae. The complete reduction of this kind of papillae is a unique feature of the springhare not found in other rodents.
Beside the differences listed above, there are similarities in the structure of the springhare tongue with either one or both of the studied anomalurid genera. The fungiform papillae in Pedetes are concentrated mainly in two zones: at the tip of the tongue and on the low intermolar eminence at its base. Similar bizonal distribution of fungiform papillae is characteristic of Anomalurus and representatives of certain other groups of rodents, such as beavers (Castoridae) or some squirrels (Sciuridae) (Zonntag, 1924). This pattern differs significantly from that observed in Idiurus, or, for example, in glirids (Potapova, 2018) in which the fungiform papillae are not represented on the dorsal eminence (Fig. 1b), but are always located only anteriorly, even being distributed in two zones.
Some other features of the tongue are common to the two families. These include the absence of the pronounced torus linguae, as well as the semilunar sulcus in front of it on the dorsum, and only a slight increase in the size of the filiform papillae posteriorly along the tongue dorsum, including the surface of the dorsal eminence (the papillae are significantly enlarged only in the posterior part of the tongue’s root).
However, the above similarities in tongue structure between Pedetidae and Anomaluridae are not specific only to these families. Individually, or in various combinations, they are found in other rodent taxa and therefore cannot be regarded as strong evidence of the sister relationship of these two families within the Rodentia. In general, the data obtained do not contradict the hypothesis of a close relationship between pedetids and anomalurids, although they do not provide reliable evidence in its support.
The tongue proportions in Pedetes could be due to the significant transformations of its skull, which led to changes in the size and form (shortening and deepening) of the oral cavity. It is possible that the “extreme” shortening of the rear part of the tongue behind the dorsal eminence could play a certain role in the reduction of the circumvallate papillae in this species.
Список литературы
Anderson S., Jones J.K. (eds), 1984. Orders and Families of Recent Mammals of the World. New York: John Wiley & Sons. 686 p.
Blanga-Kanfi S., Miranda H., Penn O., Pupko T., DeBry R.W., Huchon D., 2009. Rodent phylogeny revised: analysis of six nuclear genes from all major rodent clades // BMC Evolutionary Biology. V. 9. № 1. P. 71.
Huchon D., Chevret P., Jordan U., Kilpatrick C.W., Ranwez V., Jenkins P.D., Brosius J., Schmitz J., 2007. Multiple molecular evidences for a living mammalian fossils // Proceedings of the National Academy of Sciences of the USA. V. 104. P. 7495–7499.
Lopez-Antonanzas R., 2016. Family Pedetidae (springhare) // Wilson D.E., Lacher T.E. Jr, Mittereier R.A. (eds). Handbook of the Mammals of the World. V. 6. Lagomorphs and Rodents I. Barcelona: Lynx Editions. P. 280–287.
Luckett W.P., Hartenberger J.-L. (eds), 1985. Evolutionary Relationships among Rodents. A Multidisciplinary Analysis. New York: Plenum Press. 721 p.
Montgelard C., Bentz S., Tirard C., Verneau O., Catzeflis F.M., 2002. Molecular systematics of Sciurognathi (Rodentia): the mitochondrial cytochrome b and 12S rRNA genes support the Anomaluroidea (Pedetidae and Anomaluridae) // Molecular Phylogenetics and Evolution. V. 22. P. 220–233.
Offermans M., De Vree F., 1989. Morphology of the masticatory apparatus in the springhare, Pedetes capensis // Journal of Mammalogy. V. 70. № 4. P. 701–711. https://doi.org/10.2307/1381705
Pavlinov I.Ya., 2006. Systematics of Recent Mammals. 2nd ed. Moscow: Mosk. Univ. Publ. 297 p. (In Russian)
Potapova E.G., 1976. Structure of the tongue in some Dipodoidea // Zoologicheskii Zhurnal. V. 55. № 9. P. 1383–1389. (In Russian)
Potapova E.G., 1979. Features of the structure of the tongue of hamsters of the genus Mystromys Wagn. (Cricetidae, Rodentia) // Zoologicheskii Zhurnal. V. 58. № 2. P. 230–234. (In Russian)
Potapova E.G., 2017. Specificity and pathways on morpho-functional spezialization of the jaw apparatus in Anomaluridae (Rodentia, Mammalia) // Evolutionary and functional morphology of vertebrates. Moscow: KMK Scientific Press Ltd. P. 40–46. (In Russian)
Potapova E.G., 2018. Tongue structure in scaly-tailed scuirrels (Rodentia, Anomaluridae) // Biology Bulletin. V. 45. № 8. P. 865–871. [orig. Potapova E.G., 2018. Zoologicheskii Zhurnal. V. 97. № 2. P. 230–237. doi 10.7868/S0044513418020113 (In Russian)]https://doi.org/10.1134/S1062359018080137
Simpson G.G., 1945. The principles of classification and a classification of mammals // Bulletin of the American Museum of Natural History. V. 85. P. 1–350.
Sonntag C.F., 1924. The comparative anatomy of the tongues of the Mammalia. X. Rodentia // Proceedings of the Zoological Society of London. P. 725–741.
Tullberg T., 1899. Ṻber das System der Nagethiere: eine phylogenetische Studie // Nova Acta Regiae Societatis Scientiarum Upsaliensis. Bd. 18. Series 3. Uppsala: Akadem. Buchdruckerei. 514 S.
Vorontsov N.N., 1982. Lower Cricetidae of the World Fauna. Part 1. Morphology and Ecology. Fauna of the USSR. Mammals. V. 3. № 6. Leningrad: Nauka. 452 p. (In Russian)
Wilson D.E., Reeder D.M. (eds), 2005. Mammal Species of the World: a Taxonomic and Geographic Reference (3rd ed.). Baltimore: Johns Hopkins Univ. Press. 2142 p.
Дополнительные материалы отсутствуют.
Инструменты
Зоологический журнал


