Зоологический журнал, 2020, T. 99, № 10, стр. 1160-1186
A new genus of the family Crangonyctidae (Crustacea, Amphipoda) from the Palaearctic, with descriptions of two new species from the foothills of the Altai mountains
D. M. Palatov a, b, *, I. N. Marin b, **
a Lomonosov Moscow State University, Faculty of Biology
119992 Moscow, Leninskiye gory, 1, Russia
b Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences
119071 Moscow, Russia
* E-mail: triops@yandex.ru
** E-mail: coralliodecapoda@mail.ru
Поступила в редакцию 1.04.2020
После доработки 10.04.2020
Принята к публикации 17.04.2020
Аннотация
An overview of stygobiotic crangonyctid amphipods from the Altai Republic, Russia is presented, with the erection of the new genus Palearcticarellus Palatov et Marin gen. n. for three previously described Stygobromus-like species, as well as two new species: Palearcticarellus smirnovi Palatov et Marin sp. n. from several localities in the drainage basins of the Biya and Katun rivers, and Palearcticarellus sapozhnikovi Palatov et Marin sp. n. from the springs around Lake Kureevo. Three previously known species are transferred to the new genus: Palearcticarellus pusillus (Martynov 1930) comb. n., P. mikhaili (Sidorov, Holsinger et Takhteev 2010) comb. n., both endemic to the Altai Republic, and P. kazakhstanica (Kulkina 1992) comb. n., from the northern Tian Shan. The new genus is morphologically (but not phylogenetically) very similar to the North American Stygobromus in also demonstrating some troglomorphic features, including depigmented integuments and reduced eyes, but it can be separated by 1) the presence of coxal gills 7; 2) less than 6 plumose setae (bristles) on the inner plate of maxilla 1; and 3) the presence of 2–5 hooks in the retinacle of the pleopods. At the same time, a molecular study reveals that the new genus represents a well-isolated and well-supported sister clade to the Eurasian Synurella-related genera: the Caucasian Lyurella, the northeastern (Siberian) Synurella (= Eosynurella), and the northwestern (European) Synurella. However, it can clearly be separated from these genera by 1) free, non-fused uronites; 2) blunt posteroventral margins of epimeral plates 1–3; as well as 3) an unpigmented body; 4) mostly reduced eyes; 5) the presence of 2–5 hooks in the retinacle of the pleopods; and 6) the armature of gnathopods 1 and 2 (palmar margins of both gnathopods being armed with a double row of bifurcate robust setae, similar to those in most of North American species, but not Palaearctic ones). The new species can easily be distinguished from each other by 1) the number of hooks in the retinacle of the pleopods; 2) the shape of both gnathopods; 3) the shape and depth of a distal notch on the telson, and 4) the armature of uropod 3. The morphology and body size of smaller species of Palearcticarellus Palatov et Marin gen. n. (e.g., P. pusillus and P. mikhaili) are assumed to be more closely related to their neotenic origins rather than troglomorphy, the latter being more characteristic of larger species (e.g., P. smirnovi and P. sapozhnikovi).
The family Crangonyctidae Bousfield 1973 consists of predominantly fresh-water amphipods, mostly living in groundwater or epigean water reservoirs with hypogean connection or origination (e.g., Bousfield, 1973; Holsinger, 1978; Lowry, Myers, 2013, 2017; Zhang, Holsinger, 2003). Currently, there are 7 valid genera with more than 220 described species (according to WoRMS database): Bactrurus Hay 1902 (7 species), Crangonyx Spence Bate 1859 (49 species), Stygobromus Cope 1872 (142 species) and the monotypic Stygonyx Bousfield et Holsinger 1989 are characterized by Nearctic distribution (Holarctic for Crangonyx, see below), while Synurella Wrześniowski 1877 (20 species) is northern Palaearctic (from Europe to Yakutia), while Lyurella Derzhavin 1939 (2 species) and the monotypic Amurocrangonyx Sidorov et Holsinger 2007 are endemic for the Caucasus and the basin of the Amur River, respectively (Horton et al., 2020). The species richness of the family has recently been the highest throughout North America (e.g. Holsinger, 1974, 1978; Kornobis et al., 2012; Zhang, Holsinger, 2003; Horton et al., 2020), while the diversity in Eurasia is probably not less, but has been studied poorly and fragmentarily (e.g., Decu et al., 2019). The family Crangonyctidae is closely related to the East Asian Pseudocrangonyctidae Holsinger 1989 and the Icelandic endemic and monotypic Crymostygidae Kristjánsson et Svavarsson 2004, both being exclusively freshwater (Holsinger, 1994; Kornobis et al., 2011; Kristjánsson, Svavarsson 2004; Sidorov, Gontcharov, 2015).
At the moment, it is currently assumed that the family originated before the final break-up of Laurasia at the end of the Cretaceous (Copilaş-Ciocianu et al., 2019). Due to the specific ecology and life style, many species and groups within Crangonyctidae are characterized by a narrowly localized, endemic and even relict distribution (Zhang, Holsinger, 2003; Kornobis et al., 2012; Sidorov, Holsinger, 2007; Svavarsson, Kristjánsson, 2006; Copilaş-Ciocianu et al., 2019), and for this reason, the taxonomy of the family is still unclear and requires a comprehensive review. Recent morpho-molecular studies do not confirm the monophyly of the largest and widespread genera: Crangonyx, Stygobromus and Synurella (Kornobis et al., 2011; Copilaş-Ciocianu et al., 2019). Reconstructed phylogenetic relationships most often reflect the geographical proximity of species than their morphology (Copilaş-Ciocianu et al., 2019), and it is possible that the natural system of the family is most likely based mainly on geographic distribution, while morphological features can be considered as secondary.
Currently, eight species of the family Crangonyctidae belonging to 3 genera have been described from the North-Eastern Palearctic. Four species referring to the genus Stygobromus are reported from the mountain regions of the southern Siberia and Central Asia (see Fig. 1): S. pusillus (Martynov 1930) is known from the deep-water zone of Teletskoye Lake (Altai Republic, Russia) (Martynov, 1930; Holsinger, 1987; Sidorov et al., 2010); S. kazakhstanica Kulkina 1992 – from springs in the foothills of the western Tien Shan (Zhambyl region, Kazakhstan) (Kulkina, 1992); S. anastasiae Sidorov, Holsinger et Takhteev 2010 – from the springs near Baikal Lake (Irkutsk region, Russia) (Sidorov et al., 2010); and S. mikhaili Sidorov, Holsinger et Takhteev 2010 – from the springs in the valley of the Chuya river (Altai Republic, Russia) (Sidorov et al., 2010). All these species are characterized by troglomorphic features (troglomorphy), such as depigmentation of the integuments and an extremely small body size, not more than 3.8–5 mm, which is significantly smaller than in most species of North American Stygobromus. A larger, up to 10 mm, unidentified Stygobromus was recently discovered in the Kök-Tash Cave (Shebalinsky district, Altai Republic, Russia), the deepest known cave in Siberia (Turbanov et al., 2019).
Fig. 1.
The general map of distribution of known species of the genus Palearcticarellus Palatov et Marin gen. n. in the Altai Republic (upper) and distribution of other Palaearctic representatives of the family Crangonyctidae (lower) (chronologic). Upper map: yellow circle – Palearcticarellus sapozhnikovi Palatov et Marin gen. et sp. n., red triangles – Palearcticarellus smirnovi Palatov et Marin gen. et sp. n., purple triangles – Stygobromus sp. (after Turbanov et al., 2019), blue rhombus – Palearcticarellus mikhaili (Sidorov, Holsinger et Takhteev 2010) comb. n., green square – Palearcticarellus pusillus (Martynov 1930) comb. n. Lower map: 1 – Palearcticarellus pusillus (Martynov 1930) comb. n., Teletskoe Lake, Altai Republic; 2 – Synurella stadukhini Derzhavin 1930, springs and lakes in the Inya river basin on the northern coast of the Sea of Okhotsk (Okhotsk district, Magadan region) (Derzhavin 1930) and the spring Ozerniy (Magadan region) (Karaman, 1990); 3 – Synurella jakutana Martynov 1931, lakes in the vicinity of Tiksi (Bulunsky ulus, Yakutia (Sakha) Republic); 4 – Synurella jakutana elegans Martynov 1931, a small spring near the Zhigansk village (Zhigansky ulus, Yakutia (Sakha) Republic) (Martynov 1931), lately synonymized with S. jakutana (Karaman, 1974); 5 – Synurella levanidovae G.S. Karaman 1991, small spring in the valley of the Pucheveem River (Chaunsi District, Chukotka Autonomous Okrug); 6 – Palearcticarellus kazakstanica (Kulkina 1992) comb. n., a small spring near the Chernorechenskaya Cave (Kordaysky district, Jambyl Region, Kazakhstan); 7 – Palearcticarellus mikhaili (Sidorov, Holsinger et Takhteev 2010) comb. n., springs in valley of the Chuya River near the mouth of the Kyzyltash River (Kosh-Agach District, Altai Republic); 8 – Stygobromus anastasiae Sidorov, Holsinger et Takhteev 2010, a small spring in the city of Irkutsk and the Olkhinsky stream in the Shelekhovsky district (Irkutsk region); 9 – Palearcticarellus smirnovi Palatov et Marin gen. et sp. n. and Palearcticarellus sapozhnikovi Palatov et Marin gen. et sp. n.
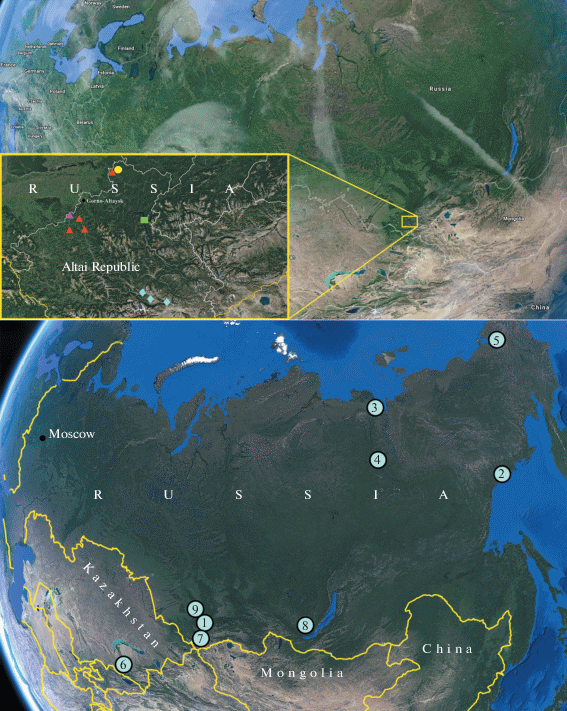
Fig. 2.
Palearcticarellus smirnovi Palatov et Marin gen. et sp. n., female: a – antenna 1; b – accessory flagellum of antenna 1; c – antenna 2; d – gnathopod 1; e – palmar margin of chela of Gn1; f – gnathopod 2; g – palmar margin of chela of Gn2.
Four species referring to the genus Synurella have been reported from epigean reservoirs, mainly tundra streams and lakes in the North-Eastern Palaearctic (see Fig. 1): S. stadukhini Derzhavin 1930 was described from in the Inya River basin on the northern coast of the Sea of Okhotsk (Magadan region, Russia) (Derzhavin, 1930; Karaman, 1990); S. jakutana Martynov 1931 – from tundra lakes of the Republic of Yakutia (Sakha) in the vicinity of Tiksi (Russia) (Martynov, 1931); S. jakutana elegans Martynov 1931 – from the central regions of the Republic of Yakutia (Sakha) (Russia) (Martynov, 1931; Karaman, 1974); and S. levanidovae G.S. Karaman 1991 – from the Pucheveem River of the Chukotka Autonomous Okrug (Russia) (Karaman, 1990). Moreover, S. johanseni Shoemaker 1920, described from Alaskan tundra ponds in the vicinity of Teller County (Alaska, USA) (Shoemaker, 1920; Martynov, 1931), is probably phylogenetically close to them according to the similarity of the main morphological features. Moreover, based on quite unique combination of morphological features in S. jakutana and S. johanseni, Martynov (1931) proposed a new subgenus (=genus) Eosynurella Martynov 1931, which obviously should also include S. stadukhini and S. levanidovae. Eosynurella Martynov 1931 is presently considered as a junior synonym of Synurella Wrześniowski 1877.
Finally, the stygobiotic Amurocrangonyx arsenjevi (Derzhavin 1927), known from the Khor River basin, a small tributary of the Ussuri River (Amur river basin) (see Fig. 1) (Derzhavin, 1927; Sidorov, Holsinger, 2007), is morphologically very similar to the North American Crangonyx, differing only in the structure of uropod 3, while phylogenetically it forms a well-recognized sister clade (Sidorov, Holsinger, 2007; Copilaş-Ciocianu et al., 2019).
At the same time, groundwater (stygobiotic) habitats of Siberia and, for example, the Altai Republic still hide various Crangonyctidae, which are probably much more diverse than it is currently known (Sidorov et al., 2010; Turbanov et al., 2019). Moreover, molecular studies have revealed that the Eurasian and North American species of the genus Stygobromus, as well as European and Siberian species of the genus Synurella belong to significantly distant clades, which suggests their independent evolution over a long time (Copilaş-Ciocianu et al., 2019). In this paper, we describe two new species from the groundwater of the foothills of Altai Mountains, belonging to a distinct and newly described crangonyctid genus.
MATERIALS AND METHODS
Amphipods were collected in different subterranean and epigean water resources of the Altai Republic (Russia) using a hand net in August and September of 2019. All animals were fixed and preserved in 90% solution of ethanol. The body length (bl., mm), the dorsal length from the distal margin of head to the posterior margin of telson, without the length of uropod 3 and antennas, is used as a standard measurement. Morphological photographs were made with a digital camera attached to light microscope Olympus ZX10 and Olympus CX21. Photographs of alive coloration of animals in situ were made using Canon G16 digital camera. SEM photographs were made in Electronic Microscopy Laboratory of the Biological Faculty of the Moscow State University using CamScan S2 microscope. The type material is deposited in the collection of Zoological Museum of Moscow State University, Moscow, Russia (ZMMU) and Laboratory of Ecology and Evolution of Marine Invertebrates of A.N. Severtsov Institute of Ecology and Evolution of Russian Academy of Sciences, Moscow, Russia (LEMMI).
The mitochondrial cytochrome c oxidase subunit I (COI mtDNA) gene marker was used as one of the most informative markers for genetic studies at population and species level to confirm the phylogenetic relationships of the studied species (Avise, 1993). Total genomic DNA was extracted from muscle tissue using the innuPREP DNA Micro Kit (AnalitikJena, Germany). The COI mtDNA gene marker was amplified with the help of the universal primers LCO1490 (5'–GGTCAACAAATCATAAAGATATTGG–3') and HC02198 (5'–TAAACTTCAGGGTGACCAAAAAATCA–3') (Folmer et al., 1994). PCR products were performed on amplificator T100 (Bio–Rad, USA) under the following conditions: initial denaturation at 96°C for 1.5 min followed by 42 cycles of 95°C for 2 min, 49°C for 35 sec, and 72°C for 1.5 min, followed by chain extension at 72°C for 7 min. The volume of 10uL of reaction mixture contained 1uL of total DNA, 2uL of 5xPCR mix (Dialat, Russia) and 1uL of each primer. The amplification products were separated by using gel electrophoresis of nucleic acids on a 1.5% agarose gel in 1xTBE, and then stained and visualized with 0.003% EtBr using imaging UV software. DNA nucleotide sequences were determined using Genetic Analyzer ABI 3500 (Applied Biosystems, USA) and BigDye 3.1 (Applied Biosystems, USA) with direct and reverse primers.
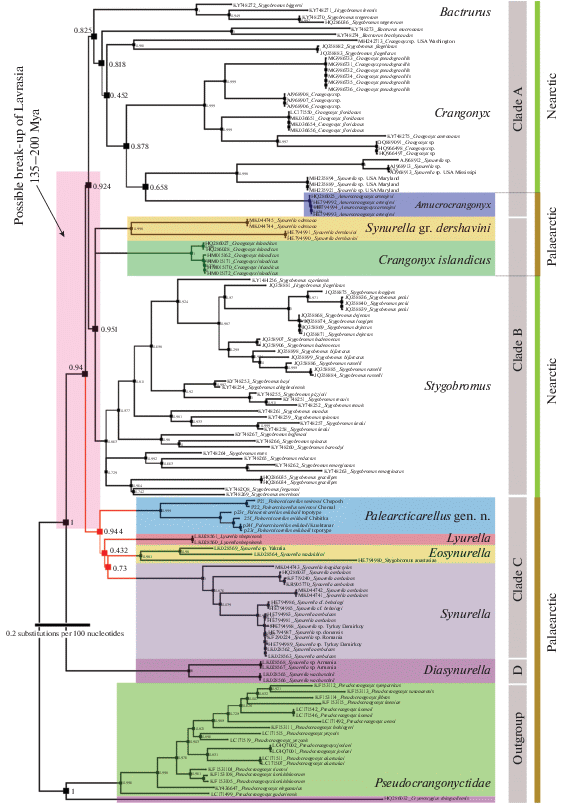
Dataset of aligned sequences of COI mtDNA gene markers (see Fig. 17) (taken from GenBank (NCBI)), about 658 base pairs, was used in the phylogenetic study. Consensus of complementary sequences was obtained with MEGA 7.0. The best evolutionary substitution model was determined using MEGA 7.0 and jModeltest2.1.141. A phylogenetic analysis was conducted using PhyML 3.0 (http://www.atgc-montpellier.fr/phyml/) using GTR+I+G model of evolution. The BIC analysis was conducted by sampling one tree every 1000 generations for 1 000 000 generations. Confidence values >50% are presented for ML analyses (bootstraps); pairwise sequence divergence (p-distances) were calculated using the Kimura-2-parameter (K2P) model in MEGA 7.0.
RESULTS
Genus Palearcticarellus Palatov et Marin gen. n.
Diagnosis (based on Holsinger, 1978). Size vary from relatively small (2.5 mm) to relatively large (10 mm). Body smooth, uronites always free, without dorsal spines; head without rostrum, lateral lobe rounded anteriorly; eye invisible, large yellow spots (“eyes”) present in large individuals. Antenna 1 longer than antenna 2; primary segments of flagellum with transparent aesthetascs; accessory flagellum 2-segmented. Antenna 2 of male without calceoli. Upper lip rounded apically, margin not incised. Mandible with well-developed incisor process and lacinia mobilis and underlying row of spines; molar process triturative; palp 3-segmented, with broad articles covered with setae. Maxilla 1: inner plate with 5–6 long plumose setae apically; outer plate with 7 mostly serrate spines apically; palp 2-segmented, with short setae and slender spines apically. Maxilla 2: inner plate mostly oval, broader than outer plate, with oblique row of long plumose setae along inner margin; both plates with numerous setae apically. Maxilliped: inner plate with several spines and stiff-like setae apically; outer plate with short stiff-like setae on apex and along inner margin; palp 4-segmented. Lower lip with well-developed outer lobes; inner lobes small, vestigial or absent; lateral processes rather short, usually narrowly rounded distally. Gnathopods 1 and 2 usually similar in size and shape, robust, with equal propodus (palm); palmar margin with a double row of typically distally notched spine teeth. Pereopods 3 and 4 subequal, with straight posterior margin. Pereopod 5 shorter than pereiopods 6 and 7. Coxal gills on pereopods 2–7 moderately small, pedicellate, oblong. Posterior corners of pleonal plates usually rounded, posterior margins usually with several short setae, ventral margins with small spines, sometimes reduced; ventral margin of pleonal segment 1 rarely with spines. Pleopods biramous, subequal in length, with 2–5 (usually 2) hooks (coupling curved spines) in retinacle on distal inner margin of peduncle. Uropods 1 and 2 biramous; rami and peduncles bearing spines; uropod 1 usually dimorphic in male (with small distal peduncular process). Uropod 3 uniramous, with short 2-segmented ramus. Telson quadrate, distal margin convex, with distinct distal notch. Only females are known.
Included species: Palearcticarellus smirnovi Palatov et Marin gen. et sp. n. (type species), Palearcticarellus sapozhnikovi Palatov et Marin gen. et sp. n., Palearcticarellus pusillus (Martynov 1930) comb. n., Palearcticarellus kazakhstanica (Kulkina 1992) comb. n.; Palearcticarellus mikhaili (Sidorov, Holsinger et Takhteev 2010) comb. n.
Etymology. The genus is named after its distribution in the Northern Palaearctic (or the Palearctic in Russian pronunciation).
Differential diagnosis. The new genus is morphological very similar to the Northern American genus Stygobromus with the type species Stygobromus vitreus Cope 1872 (after Holsinger, 1978)). Nevertheless, it can be separated by 1) the presence of coxal gills 7 (usually absent in Stygobromus); 2) the presence of less than 6 plumose setae on the inner plate of maxilla 1 (usually more than 6 setae in Stygobromus); and 3) the presence of 2–5 hooks in retinacle of pleopods (usually only 2 hooks in Stygobromus).
It is worth adding that it is very difficult to use morphological features to separate the genera within the family Crangonyctidae (Sidorov, Holsinger, 2007). Morphological differences within the genus often overlap the features of differential diagnoses of crangonyctid genera due to their long-time evolution (see Creaser, 1934; Sidorov, Holsinger, 2007; Svavarsson, Kristjánsson, 2006; Copilaş-Ciocianu et al., 2019), which is also characteristic for Palearcticarellus Palatov et Marin gen. nov. (see below). Therefore, the molecular research methods are best used to separate genera (see Fig. 17) (e.g., Copilaş-Ciocianu et al., 2019). According to our data (see Fig. 17), the new genus represents a well-isolated sister clade to Eurasian Synurella-related genera: Caucasian Lyurella, the northeastern (Siberian) Synurella (=Eosynurella) and northwestern (European) Synurella. Despite the morphological similarity, the new genus is phylogenetically strongly separated from North American Stygobromus (see Fig. 17; Copilaş-Ciocianu et al., 2019).
From the Caucasian genus Lyurella with the type species Lyurella hyrcana Derzhavin 1939 (after Derzhavin, 1939), the new genus can be separated by 1) not pigmented body and mostly reduced eyes (pigmented orange body and large well-developed black eyes in Lyurella); 2) free uronites (fused in Lyurella); 3) blunt posteroventral margins of epimeral plates 1–3 (sharply produced in Lyurella); 4) the absence of additional spines on dactyli of pereopods 3–7 (present in Lyurella); and 5) 2-segmented outer rami of uropod 3 (1-segmented in Lyurella). At the same time, Palearcticarellus sapozhnikovi Palatov et Marin gen. et sp. n. also has more than 2 hooks in retinacle of pleopods similar to the representatives of the genus Lyurella (3 in L. shepsiensis Sidorov 2015 (see Sidorov, 2015: Fig. 3g), and 4 in L. hyrcana (see Derzhavin, 1939: Fig. 10a) that separates them from other known Palaearctic crangonyctids, which have only 2 hooks in retinacle.
Fig. 3.
Palearcticarellus smirnovi Palatov et Marin gen. et sp. n., female: a – upper lip; b – lower lip; c, d – left mandible; e, f, g – right mandibles; d, e, g – incisor process and pars incisiva of mandibles; h – maxilla 1; i – distal margin of inner plate of maxilla 1; j – maxilla 2; k – maxilliped.

Fig. 4.
Palearcticarellus smirnovi Palatov et Marin gen. et sp. n., female: a – pereopod 3; b – dactylus of P3; c – pereopod 4; d – dactylus of P4; e – pereopod 5; f – dactylus of P5; g – pereopod 6; h – dactylus of P6; i – pereopod 7; j – dactylus of P7.
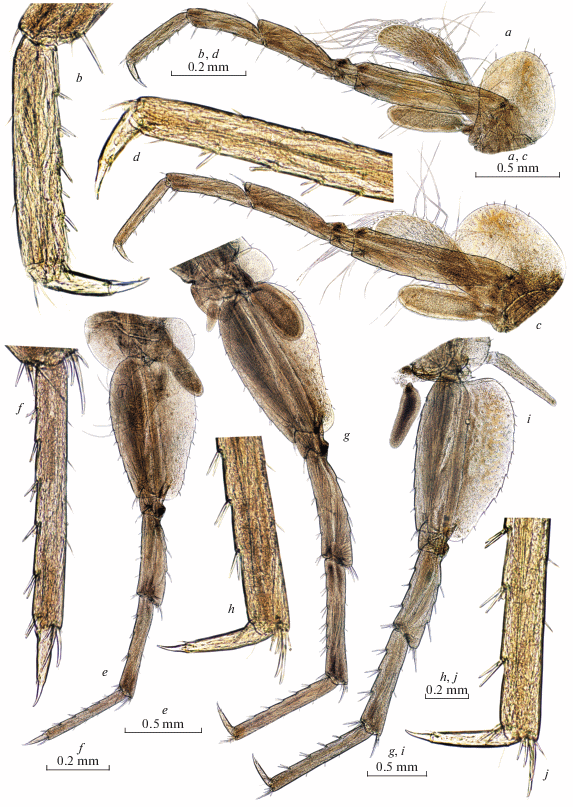
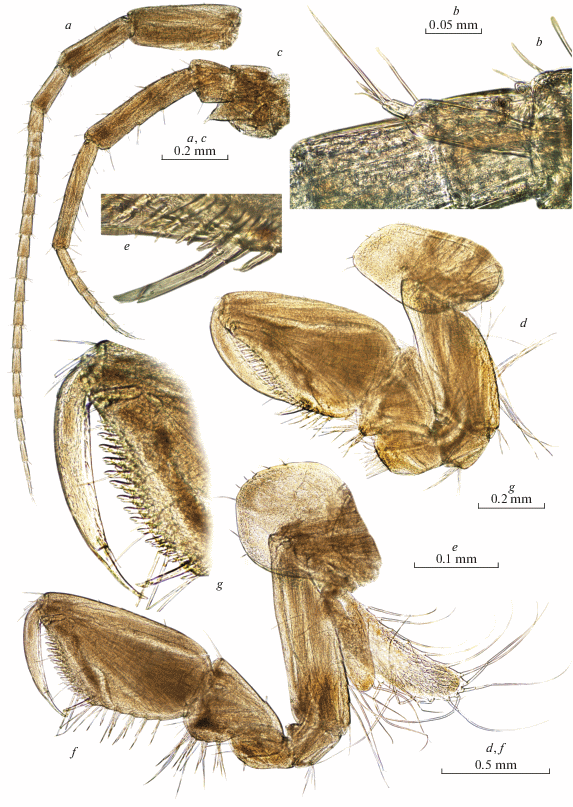
Fig. 5.
Palearcticarellus smirnovi Palatov et Marin gen. et sp. n., female: a–c – epimeral plates 1–3 ; d – coxal plate 7 ; e – pleopod 2; f – retinacle of pleopod 3; g, h – telson; i – uropod 1; j – uropod 2; k, l – uropods 3.
From the European genus Synurella with the type species Synurella ambulans (F. Müller 1846) (after Sidorov, Palatov, 2012), the new genus can be clearly separated by 1) not pigmented body and mostly reduced eyes (pigmented orange body and large well-developed black eyes in Synurella); 2) free uronites (fused in Synurella); 3) blunt posteroventral margins of epimeral plates 1–3 (sharply produced in Synurella); and 4) the presence of 2–5 hooks in retinacle of pleopods (usually only 2 hooks in Synurella).
From the northeastern (Siberian) Synurella (S. jakutana and S. jakutana elegans, placed in the genus Eosynurella (after Martynov, 1931)), as well as S. stadukhini and S. levanidovae, and probably related Alaskan S. johanseni (see above) (following as Eosynurella), the new species can be separated by 1) not pigmented body and mostly reduced eyes (pigmented orange body and large well-developed black eyes in Eosynurella); 2) longer antenna 1 and 2 and different shape of coxal gills (after Martynov, 1931); 3) free uronites (fused in Eosynurella); 4) blunt posteroventral margins of epimeral plates 1–3 (sharply produced in Eosynurella); 5) gnathopods 1 teardrop-shaped, with a strongly beveled palmar edge (gnathopods 1 rectangular or square with slightly oblique palmar margin in Eosynurella); and 6) the presence of 2–5 hooks in retinacle of pleopods (usually only 2 hooks in Eosynurella).
Distribution. All species are known from the Asian (Siberian) part of the Northern Palaearctic, namely, mountains of South Siberia and Central Asia (The Tian Shan).
Palearcticarellus smirnovi Palatov et Marin gen. et sp. n.
(Figs 2–5; 10a, 10c, 10e, 10g; 11a, 11b; 12a, 12b; 13a, 13b; 14a, 14b; 16a, 16c)
Material examined. Holotype, female (bl. 11 mm), ZMMU Mb-1156, springs (rheokren) on a shore of the Kureevo lake, 52°28.150'N 86°45.139'E, Biya River drainage basin, Turochaksky District, Altai Republic, Russia; coll. D. Palatov et I. Marin, 06 September 2019.
Paratypes, female (bl. 9 mm), ZMMU Mb-1157, same locality and data as holotype; female (bl. 8 mm), ZMMU Mb-1158, same location and collectors as holotype.
Additional material examined. 172 females, springs (rheokrens) on shores of the Kureevo Lake, 52°28.150′ N 86°45.139′ E, Biya River drainage basin, Turochaksky District, Altai Republic, Russia, coll. D. Palatov et I. Marin, 06 September 2019; 5 females, spring (rheokren) on the banks of the Katun River near Cheposh village, 51°35.443′ N 85°49.690′ E, Chemalsky District, Altai Republic, Russia, coll. D. Palatov, I. Marin et V. Maryinsky, 11 September 2019; 1 female, spring (rheokren) on banks of the Katun River near Chemal village, 51°23.437′ N, 86°00.297′ E, Chemalsky District, Altai Republic, Russia, coll. D. Palatov, I. Marin et V. Maryinsky, 11 September 2019; 13 females, spring (rheokren) on the banks of the Sema River near Cherga village, Katun River drainage basin, 51°33′34.7″ N 85°34′21.0″ E, Shebalinsky district, Altai Republic, Russia, coll. I. Marin, 19 September 2019.
Type locality. Springs (rheokrens) around the Kureevo Lake, 52°28.150′ N 86°45.139′ E, Biya River drainage basin, Turochaksky District, Altai Republic, Russia.
Diagnosis. Only females are known. Large sized (7.0–11.0 mm) stygobiotic species with well-developed gnathopods 1 and 2 armed with a double row of numerous robust setae along palmar margins; coxal gills present on coxal segments 2–7; pleopods 1–3 with 2 hooks in retinacle; uropod 3 with peduncle about twice longer than ramus, ramus with 4–5 apical spines, ramus 2-segmented; telson rectangular, about 1.2 times longer than broad; distal margin with shallow U-shaped distal notch, each lobe armed with 6–8 distal spines and some plumose submarginal setae.
Description. Female (Fig. 16a, 16c): 8.6 mm long. Eyes invisible, large individuals may show yellow pigment spots (“eyes”) (Fig. 13a, 13b). Inter-antennal lobe wide, bluntly rounded anteriorly (Fig. 11a).
Antenna 1 (Figs 2a, 13b) is about 40% of the body length, 2.0–2.1X longer than antenna 2; primary flagellum with 19 segments, with aesthetascs on distal segments, shorter than respective segments; accessory flagellum 2-segmented, overreaching the segment of the main flagellum in length (Fig. 2b).
Antenna 2 (Fig. 2c): gland clone distinct; peduncle 2.6X longer than flagellum, with robust setae tightly covering segments 3 and 4, peduncle of segment 4 subequal to ones of segment 5 in length; flagellum 7-segmented; calceoli absent on peduncle and flagellum.
Mandible (Fig. 3c–3g): left mandible incisor 5-dentate, lacinia mobilis 5-dentate, with 5–6 robust plumose accessory setae; molar process with 1 seta (Fig. 3c, 3d). Right mandible incisor 4-dentate, lacinia mobilis bifurcate, both lobes with numerous protuberances; underlying with a row of 5–6 robust plumose setae; molar process similar to left mandible (Fig. 3e–3g). Palp 3-segmented, segment 2 with 12–13 setae; segment 3 oval with straight posterior margin, with 14–15 separate D setae, 4 separate E setae and 2 separate B setae, lacking both A and C setae.
Upper lip (Fig. 3a): oval, elongated, apical margin of labrum with numerous fine setae.
Lower lip (Fig. 3b): inner lobes well developed.
Maxilla 1 (Fig. 3h, 3i): inner plate with 5 plumose marginal setae, outer plate with 7 apical comb-spines; palp 2-segmented, distal segment pubescent, apical margin of distal segment with 4 simple and 2 subdistal robust setae.
Maxilla 2 (Fig. 3j): inner, outer plates covered in pubescent setae; outer plate smaller than inner plate, weakly narrowing distally, with 12 apical setae; inner plate narrowing slightly distally, with 10–12 simple and 7 plumose facial apical setae.
Maxilliped (Fig. 3k): inner plate much shorter than outer plate, with 4 armed spines along apical margin, 1 plumose and 2 simple submarginal setae, surface of plate covered in fine pubescence; outer plate with 16–18 setae; palp 4-segmented, segment 1 with 1 seta, segment 2 with 32 marginal setae, segment 3 setaceous with numerous marginal/submarginal setae; dactylus with 2 outer and 1 inner setae.
Gnathopod 1 (Figs 2d, 2e; 10a, 10c; 12b): subequal in size to gnathopod 2; coxal plate weakly narrowing distally, with 3–5 apical setae; basis with numerous (19–20) long setae inserted along posterior margin and 5–6 long setae inserted along medial margin; ischium with 8 plumose setae; merus with 22–23 distal setae; carpus about 35% of the length of propodus, with 2 anterior and a group of posterior setae; propodus 1.6X longer than broad, with 3 groups (with 2–4 setae each) of medial and 7 inferior medial setae; palm oblique with double row of 8 inner and 16 outer bifurcate robust setae; palm groove (depression), where dactylus enters, with 5 inner robust, 1 large outer and 1 smaller robust setae (Figs 10c; 12b); dactylus with 1 outer and 7 inner setae.
Gnathopod 2 (Figs 2f, 2g; 10e, 10g): coxal plate rounded, with 8 apical setae; basis with numerous (16–17) long setae inserted along posterior margin; ischium with 3–4 long simple setae; merus with 10 distal setae; carpus about 40% of the length of propodus, with 5 anterior and 4 groups of plumose posterior setae; propodus 1.7X longer than broad, with 4 groups (3 setae each) of superior medial, 2 single, 4 groups (11 setae) of inferior medial and 5 groups of posterior setae; palm oblique with a double–row of 10 inner and 16 outer bifurcate robust setae; palm groove (depression), where dactylus enters, with 5 inner robust and 2 outer robust setae (Fig. 10g); dactylus with 1 outer and 6 inner setae.
Pereopod 3 (Fig. 4a, 4b): coxal plate with 8 apical setae; basis with numerous anterior (3 long and 3 short) and posterior (11–12) setae; merus 1.1X longer than carpus; propodus subequal to carpus in length; dactylus is about 40% of the length of propodus, with plumose seta on outer margin, stout seta on distal corner of inner margin followed by thin seta on distolateral margin.
Pereopod 4 (Fig. 4c, 4d): subequal to pereopod 3 in length; coxal plate slightly longer than broad, excavate behind, with 10 apical and numerous facial setae; merus 1.2X longer than carpus, carpus subequal to propodus in length; dactylus is approximately 30% of the length of propodus, setation similar to pereopod 3.
Pereopod 5 (Fig. 4e, 4f): coxal plate large, bilobate with distinct anterior, posterior lobes with 2 simple and sparse facial setae; basis with posterior margin weakly convex, armed with 16 shallow serrations, with convex distal corner, anterior margin with 9 split-tipped robust and 3 distal setae; carpus 1.1X longer that propodus in length, dactylus is approximately 30% of the length of propodus, setation similar to that of other pereopods.
Pereopod 6 (Fig. 4g, 4h): coxal plate bilobate, with distinct anterior, posterior lobe with 2 apical and sparse facial setae; basis with posterior margin armed with 15 shallow serrations, with convex distal corner, anterior margin with 10 split-tipped robust and 4 distal setae, with several facial setae near posterior margin; carpus 1.1X longer than propodus in length; dactylus is approximately 30% of the length of propodus, setation similar to that of other pereopods.
Pereopod 7 (Fig. 4i, 4 j): coxal plate small, with 1–3 posterior setae (Fig. 5d); basis with posterior margin convex, armed with 15 serrations, with weakly convex distal corner, anterior margin with 6 split–tipped robust and 3 distal setae; carpus 0.9X of propodus in length, dactylus is approximately 30% of the length of propodus, setation similar to that of other pereopods.
Gills, brood plates (Figs 2f; 4a, 4c, 4e, 4g, 4i): coxal gills on somites 2–7, somite 7 with lanceolate sternal gill. Slender, setaceous brood plates on somites 2–5, decreasing in size posteriorly.
Pleopods (Fig. 5e, 5f): pleopod 1 peduncle with 2 hooks and 1 distal seta in retinacle; outer and inner rami with 8 and 7 segments, respectively; basal segment of outer ramus lacking clothes–pin setae.
Pleopod 2 peduncle with 2 hooks and 1 distal seta in retinacle; outer and inner rami with 7 and 8 segments, respectively; basal segment of outer ramus lacking clothes-pin setae.
Pleopod 3 peduncle with 2 hooks in retinacle, without distal seta; outer and inner rami with 6 segments; basal segment of outer ramus lacking clothes-pin setae.
Epimera (Figs 5a–5c; 11b): epimeron 1 with unarmed ventral margin, posterior margin with 6 setae. Epimeron 2 with ventral margin armed with 4–5 spines, posterior margin with 5 setae. Epimeron 3 with ventral margin armed with 5 spines, posterior margin with 7 setae.
Urosome with free smooth segments, with sparse setae covering dorsal surface.
Uropod 1 (Fig. 5j): peduncle 1.4X length of rami, with outer and 11–12 inner robust spines; outer ramus subequal to inner ramus, with 4 outer, 3 inner robust and 5 apical robust spines; inner ramus with 6 outer robust and 4 apical robust spines, without inner robust spines.
Uropod 2 (Fig. 5i): peduncle subequal in length to inner ramus, with 5 outer robust and no inner robust spines; outer ramus is about 85% of the length of inner ramus, with 5 outer robust, 4 inner robust and 4 apical robust spines; inner ramus with 5 outer robust and 4 apical robust spines, without inner robust spines.
Uropod 3 (Fig. 5l, 5k): small, shorter than telson, uniramous; peduncle about twice longer than ramus in length, with 1–3 medial spines; ramus with 4–5 apical robust spines.
Telson (Fig. 5h) rectangular, 1.2X longer than broad; distal margin with shallow U-shaped distal notch, each lobe armed with 6–8 spines, with 2 additional submarginal plumose setae.
Coloration. The body and appendages yellowish transparent; with small pigmented spots on the anterior lobe of head (Figs 13a; 14a, 14b).
Body size. The largest collected female has bl. 11.0 mm.
GenBank accession numbers. MT231260, MT231261.
Taxonomic remarks. From all known Palaearctic Crangonyctidae, the new species can be well distinguished by 1) the larger size; 2) not pigmented (troglomorphic) body; 3) the reduced invisible eyes; 4) epimeral plates 1–3 with bluntly rounded posteroventral margins; and 5) the presence of shallow U‑shaped notch on distal margin of telson. For the morphological difference from other crangonyctid genera see the genus description, presented above.
From related Palearcticarellus pusillus (Martynov 1930) comb. n., Palearcticarellus kazakhstanica (Kulkina 1992) comb. n. and Palearcticarellus mikhaili (Sidorov, Holsinger et Takhteev 2010) comb. n., the new species can be clearly separated by 1) large size of mature individuals (up to 7–10 mm ); 2) longer antenna I; 3) the armature of oral appendages; 4) the armature of palmar margin of gnathopods 1 and 2 armed with a double row of bifurcate robust setae, similar to most of North American Stygobromus species (vs. these setae are absent or present only in the lower corner in a small number of known Palearctic species (for example, similar to Synurella anastasiae Sidorov, Holsinger et Takhteev 2010 (=Eosynurella))), but never cover the entire length of palmar margin).
For the differences from closely related Palearcticarellus sapozhnikovi Palatov et Marin gen. et sp. n., see below.
Distribution and Ecology. A relatively common species that lives in various spring reservoirs (rheokren/gelokren) in the drainage basins of Biya and Katun rivers in the foothills (up to 400 m above sea level) of the Altai Mountains (Altai Republic, Russia) (Fig. 14c–14e). The species was discovered together with such spring-dwelling crustaceans as Palearcticarellus sapozhnikovi Palatov et Marin gen. et sp. n. (see below), Gammarus angusticoxalis Martynov 1935 (Amphipoda, Gammaridae) and Asellus latifrons Birštein 1947 (Isopoda, Asellidae).
Etymology. The new species is named after Dr. Nikolay Nikolaevich Smirnov (07.01.1928–20.11.2019), one of the outstanding carcinologists of the twentieth century, the founder of the Russian Cladoceran scientific school and an honorary member of the Russian Crustacean Society.
Palearcticarellus sapozhnikovi Palatov et Marin gen. et sp. n.
(Figs 6–9; 10b, 10d, 10f, 10h; 11c, 11d; 12c, 12d; 16b)
Fig. 6.
Palearcticarellus sapozhnikovi Palatov et Marin gen. et sp. n., female: a – antenna 1; b – accessory flagellum of antenna 1; c – antenna 2; d, f, g – gnathopod 1; e – palmar margin of chela of Gn1; h – gnathopod 2; i – palmar margin of chela of Gn2.
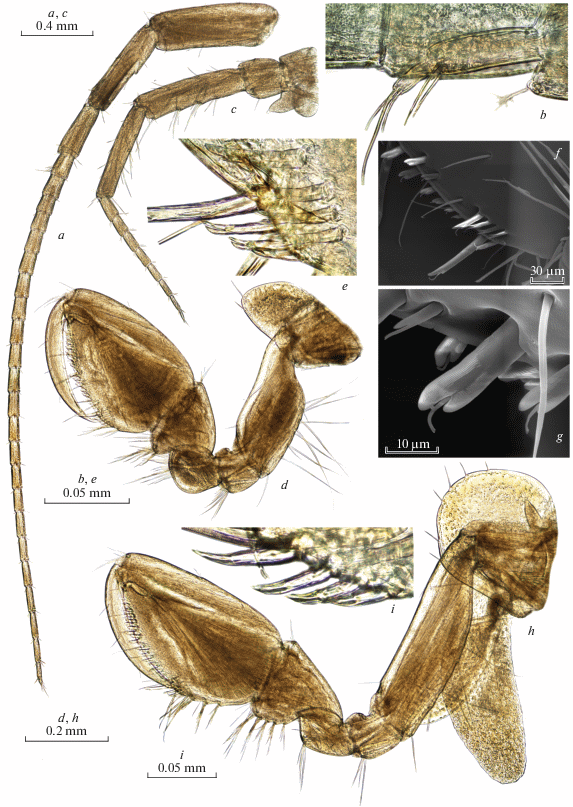
Fig. 7.
Palearcticarellus sapozhnikovi Palatov et Marin gen. et sp. n., female: a – upper lip; b – lower lip; c, d – left mandible; e, f – right mandible; d, f – incisor process and pars incisiva of mandibles; g – maxilla 1; h – distal margin of inner plate of maxilla 1; i – maxilla 2; j – maxilliped.
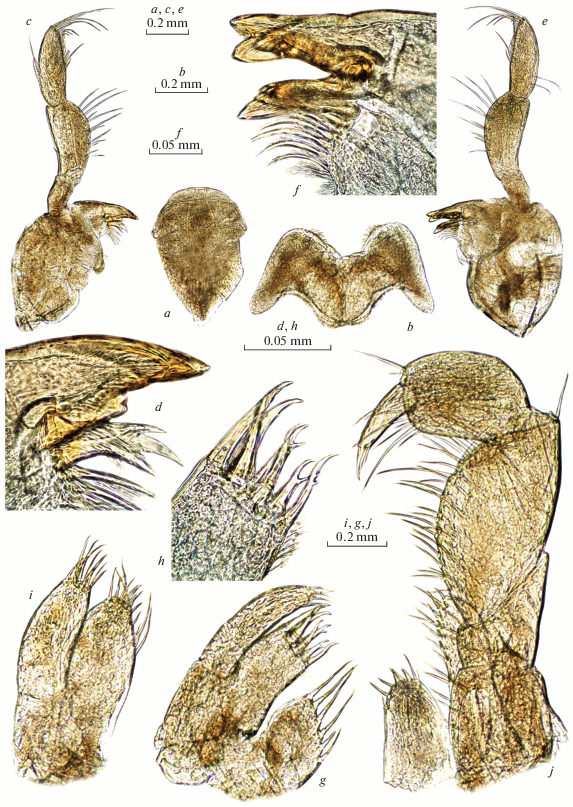
Fig. 8.
Palearcticarellus sapozhnikovi Palatov et Marin gen. et sp. n., female: a – pereopod 3; b – dactylus of P3; c – pereopod 4; d – dactylus of P4; e – pereopod 5; f – dactylus of P5; g – pereopod 6; h – dactylus of P6; i – pereopod 7; j – dactylus of P7.
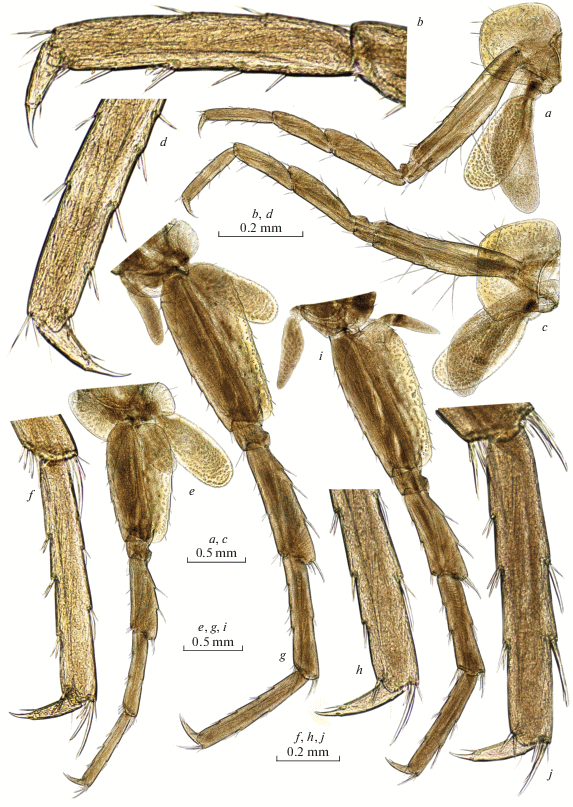
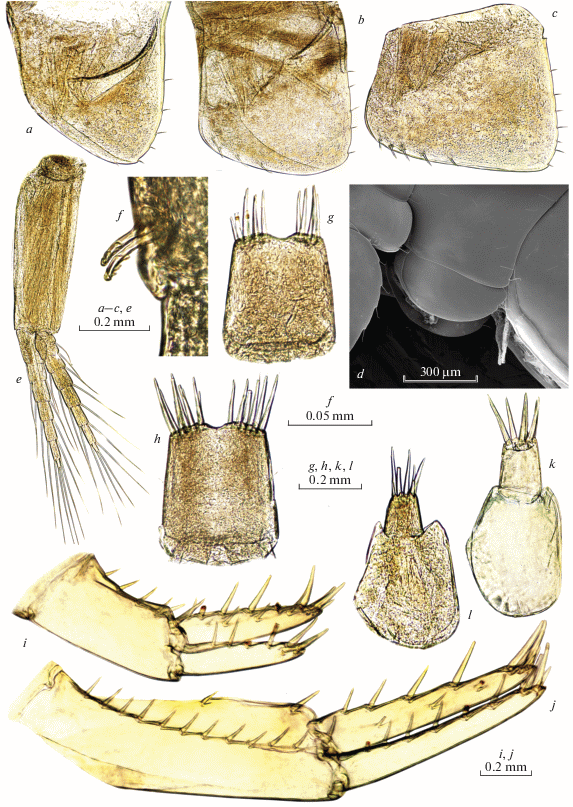
Fig. 9.
Palearcticarellus sapozhnikovi Palatov et Marin gen. et sp. n., female: a–c – epimeral plates 1–3 ; d – pleopod 3; e, f – retinacle of pleopod 3; g – telson; h – uropod 1; i – uropod 2; j – uropod 3; k – urosome.
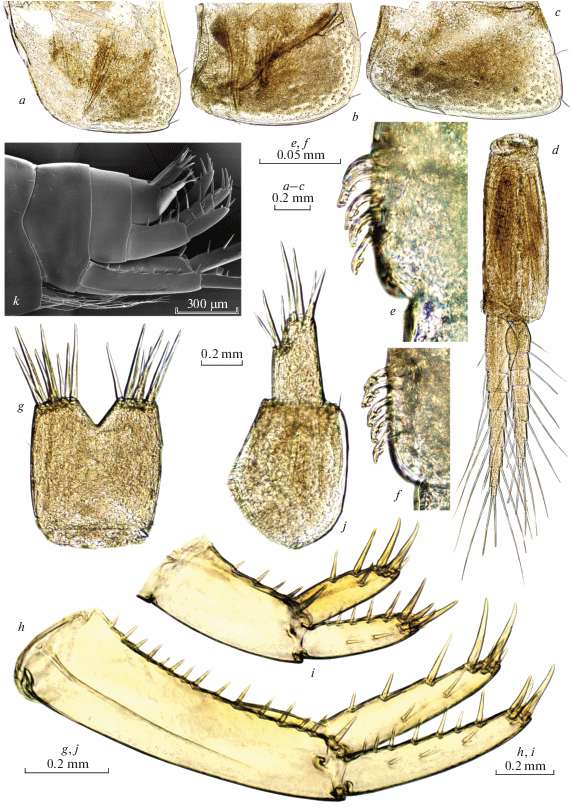
Fig. 10.
Palearcticarellus smirnovi Palatov et Marin gen. et sp. n. (a, c, e, g) and Palearcticarellus sapozhnikovi Palatov et Marin gen. et sp. n. (b, d, f, h), females: a, b – gnathopods 1; c, d – palmar margins of chela of Gn1; e, f – gnathopods 2; g, h – palmar margins of chela of Gn2.
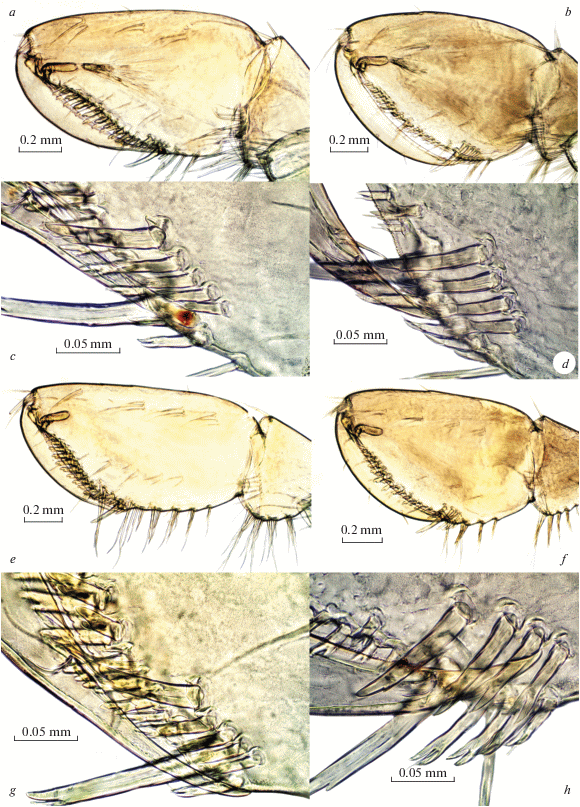
Fig. 11.
Palearcticarellus smirnovi Palatov et Marin gen. et sp. n. (a, b), Palearcticarellus sapozhnikovi Palatov et Marin gen. et sp. n. (c, d) and Palearcticarellus mikhaili (Sidorov, Holsinger et Takhteev 2010) comb. n. (e, f): a, c, e – head; b, d, f – epimeral plates 1–3 .
Material examined. Holotype, female (bl. 10 mm), ZMMU Mb-1159, springs (rheokrens) on a shore of the Kureevo Lake, 52°28.150′ N 86°45.139′ E, Biya River drainage basin, Turochaksky District, Altai Republic, Russia, coll. D. Palatov et I. Marin, 06 September 2019.
Paratype: 1 female (bl. 9 mm), LEMMI, same locality and data as holotype.
Type locality. Springs (rheokrens) on a shore of the Kureevo Lake, 52°28.150′ N 86°45.139′ E, Biya River drainage basin, Turochaksky District, Altai Republic, Russia.
Diagnosis. Only females are known. Large sized (about 10.0 mm) stygobiotic species, with large gnathopods 1 and 2, armed with a double row of numerous robust setae along palmar margins; coxal gills present on coxal segments 2–7; pleopods 1–3 with 5 hooks in retinacle; uropod 3 with peduncle 1.6X longer than ramus, ramus 2-segmented; telson mostly square, distal margin with distinct V-shaped distal notch, each lobe armed with 6–8 spines and 1 small submarginal plumose seta.
Description. Female (Fig. 16b): 8.6 mm long. Eyes invisible, yellow pigment spots (“eyes”) are expected in large individuals (see above for Palearcticarellus smirnovi Palatov et Marin gen. et sp. n. Inter-antennal lobe wide, bluntly rounded anteriorly.
Antenna 1 (Fig. 6a, 6b) about 40% of the body length, about 1.9–2.0X longer than antenna 2; flagellum with 24 segments, aesthetascs on distal segments, aesthetascs shorter than respective segments; accessory flagellum 2-segmented, similar or slightly overreaching the segment of the main flagellum in length (Fig. 6b).
Antenna 2 (Fig. 6c): gland clone distinct; peduncle 1.9–2.0X longer than flagellum, with robust setae broadly covering segments 3 and 4; segment 5 of peduncle about 1.1X longer than segment 4; flagellum 8-segmented; calceoli absent on peduncle and flagellum.
Mandibles (Fig. 7c–7f): left mandible incisor 5-dentate, lacinia mobilis 4-dentate, with 5 robust plumose accessory setae; molar process with 1 seta (Fig. 7f). Right mandible incisor process 4-dentate, lacinia mobilis with 3 teeth, covered with protuberance; accessory setae with a row of 5 robust plumose setae; molar process similar to left mandible (Fig. 7d). Palp 3-segmented, segment 2 with 11 setae; segment 3 narrowing distally, with 1 separate C setae, 5 separate E setae, 3 separate B seta, 15 simple separate D setae, without A setae.
Upper lip (Fig. 7a): oval, elongated, apical margin of labrum with numerous fine setae.
Lower lip (Fig. 7b): inner lobes well developed.
Maxilla 1 (Fig. 7g, 7h): inner plate with 6 plumose marginal setae; outer plate with 7 apical comb-spines; palp 2-segmented, distal segment pubescent; apical margin of distal segment with 5 simple and 2 marginal robust setae.
Maxilla 2 (Fig. 7i): inner, outer plates with plumose setae; outer plate smaller than inner, weakly narrowing distally, with 9 apical setae; inner plate narrowing slightly distally, with 10–12 apical and 4 plumose facial setae.
Maxilliped (Fig. 7j): inner plate much shorter than outer plate, with 5 spines along apical margin, 1 plumose and 1 simple submarginal setae, surface of plate covered with fine pubescence; outer plate with 15 setae; palp 4-segmented, segment 1 with 2 setae, segment 2 with 20–22 marginal and submarginal setae, segment 3 setaceous with numerous marginal/submarginal setae; dactylus with 1 outer and 2 inner setae.
Gnathopod 1 (Figs 6d–6g; 10b, 10d): subequal in size to gnathopod 2; coxal plate oval, with 2 apical setae; basis with numerous (up to 12) long setae inserted along posterior margin, 5 long setae inserted along medial margin and1 short seta on anterior margin; ischium with 8 plumose setae; merus with 17–18 distal setae; carpus is about 30% of the length of propodus, with 4 anterior and a group of posterior setae; propodus 1.4X longer than broad, with 3 groups (with 2–3 setae each) of medial and 3 groups (2 setae each) of inferior medial setae; palm oblique with a double row of 12 inner and 16 outer bifurcate robust setae; palm groove (depression), where dactylus enters, with 4 inner robust and 5 large outer robust setae (Figs 6e, 6f; 10d; 12d); dactylus with 3 outer and 7 inner setae.
Gnathopod 2 (Figs 6h, 6i; 10f, 10h): coxal plate rounded, with 6 apical setae; basis with numerous (up to 8) long setae inserted along posterior margin and 3 long setae inserted along anterior margin; ischium with 4 long simple setae; merus with 6 posterodistal setae; carpus 35% propodus length, with 2 anterior setae and 5 groups of plumose posterior setae; propodus 1,7X longer than broad with 4 groups of superior medial (2–3 setae each), 2 groups (5 setae) inferior medial and 3 groups of posterior setae; palm oblique with a double row of 12 inner and 16 outer, bifurcate robust setae; palm groove (depression), where dactylus enters, with 4 inner robust setae and 5 outer robust setae (Fig. 10h); dactylus with 3 outer and 4 inner setae.
Pereopod 3 (Fig. 8a, 8b): coxal plate with 5 apical setae; basis with numerous anterior (4 long and 4 short) and posterior (10–11) setae; merus 1.3X longer than carpus; propodus 1.1X longer than carpus; dactylus proximately is about 30% of the length of propodus, with plumose seta on outer margin, stout seta on distal corner of inner margin followed by thin seta on distolateral margin.
Pereopod 4 (Fig. 8c, 8d): subequal to pereopod 3 in length; coxal plate broader then long, with 5 apical and sparse facial setae; merus 1.2X longer than carpus, carpus subequal to propodus in length; dactylus is approximately 30% of the length of propodus, setation similar to pereopod 3.
Pereopod 5 (Fig. 8e, 8 f): coxal plate large, bilobate with distinct anterior, posterior lobes, with 2 setae on anterior and posterior lobe; basis posterior margin straight, with 10 shallow serrations, convex distal corner, anterior margin with 6 split-tipped robust and 3 distal setae; merus, carpus and propodus subequal in length, dactylus approximately 30% propodus length, setation similar to other pereopods.
Pereopod 6 (Fig. 8g, 8h): coxal plate bilobate, with distinct anterior, posterior lobes, posterior lobe with 2 apical setae, anterior lobe with distal seta; basis posterior margin straight, with 11 shallow serrations, convex distal corner, anterior margin with 7 split-tipped robust and 3 distal setae; merus 1.1X carpus in length; carpus subequal propodus in length; dactylus approximately 28% propodus length, setation similar to other pereopods.
Pereopod 7 (Fig. 8i, 8 j): coxal plate small, with 2 posterior setae; basis posterior margin straight with 10 serrations, weakly convex distal corner, anterior margin with 6 split–tipped robust and 2 distal setae; merus and carpus 0.9X propodus in length, dactylus approximately 30% propodus length, setation similar to other pereopods.
Gills and brood plates (Figs 6h; 8a, 8c, 8e, 8g, 8i): coxal gills on somites 2–7, somite 7 with lanceolate sternal gill. Slender, setaceous brood plates on somites 2–5, decreasing in size posteriorly.
Pleopods: pleopod 1 peduncle with 5 hooks in retinacle, without seta; outer, and inner rami with 10 and 11 segments, respectively; basal segment of outer ramus lacking clothes-pin setae.
Pleopod 2 peduncle with 5 hooks and 1 distal seta in retinacle; outer and inner rami with 8 and 11 segments, respectively; basal segment of outer ramus lacking clothes-pin setae.
Pleopod 3 (Fig. 9d–9f) peduncle with 5 hooks and 1 split-tipped robust seta in retinacle; outer and inner rami with 7 and 8 segments, respectively; basal segment of outer ramus lacking clothes-pin setae.
Epimera (Figs 9a–9c; 11d): epimeron 1 with unarmed ventral margin, posterior margin with 2 setae. Epimeron 2 with ventral margin armed with 2 spines, posterior margin with 2 setae. Epimeron 3 with ventral margin armed with 3 spines, posterior margin with 2 setae.
Urosome with free smooth segments, with sparse setae covering dorsal surface (Fig. 9k).
Uropod 1 (Fig. 9h): peduncle 1.4X of the length of rami, with outer and 14–15 inner robust spines; outer ramus subequal to inner ramus, with 3 outer, 2 inner robust and 4 apical robust spines; inner ramus with 7 outer robust, 3 inner robust and 4 apical robust spines.
Uropod 2 (Fig. 9i): peduncle 1.1X of the length of inner ramus, with 4–6 outer robust spines, without inner spines; outer ramus about 90% of the length of inner ramus, with 5 outer robust, 2 inner robust and 4 apical robust spines; inner ramus with 2 outer robust and 4 apical robust spines, without inner robust spines.
Uropod 3 (Fig. 9j): small, shorter than telson, uniramous; peduncle 1.6X of the length of ramus, with 1 apical spine; ramus with 4 apical and 3 subapical robust spines.
Telson (Fig. 9g) nearly square; distal margin with distinct U-shaped notch, each lobe armed with 6–8 spines and small plumose submarginal setae, with 2 additional submarginal plumose setae arise from outer margins.
Taxonomic remarks. From all known Palaearctic representatives of the family Crangonyctidae, the new species is well distinguished by 1) the large size; 2) not pigmented (troglomorphic) body; 3) the reduced invisible eyes; 4) epimeral plates 1–3 with bluntly rounded posteroventral margins; 5) the presence of 5 hooks in retinacle of pleopods; and 6) the presence of shallow U-shaped notch on distal margin of telson. Morphological differences from the closely related north-eastern Asian congeners are similar to Palearcticarellus smirnovi Palatov et Marin gen. et sp. n., discussed above. For the morphological difference from other crangonyctid genera see above description of the genus.
From the closely related Palearcticarellus smirnovi Palatov et Marin gen. et sp. n., described above, the new species differs by 1) shorter accessory flagellum of antenna 1, which just reaching and approximately equal to antennal segment 1 in length; 2) by the presence of 5 hooks in retinacle of pleopod 1–3; 3) the shape of both gnathopods and the presence of 5 large setae and basipodites of pereiopods 5–7; 4) the shape and depth U-shaped notch on distal margin of telson; and 5) the presence of subapical group of spines on proximal segment of outer ramus of uropod 3 (apical spines present only on distal segment of outer ramus, while proximal segment uropod 3 is unarmed in Palearcticarellus smirnovi Palatov et Marin gen. et sp. n.).
Distribution and ecology. The rare species that was found in spring reservoirs (rheokrens) around Kureevo Lake in the drainage basins of Biya River in the foothills (about 350 m above sea level) of the Altai Mountains (Altai Republic, Russia). The species was found together with other spring dwelling crustaceans, such as Palearcticarellus smirnovi Palatov et Marin gen. et sp. n., G. angusticoxalis and A. latifrons.
Etymology. The new species is named after Professor Vasily Vasilyevich Sapozhnikov (1861–1924), a famous Russian biologist, geographer and traveler, who explored the Russian and Mongolian Altai, the Sayan Mountains and other regions of the southern Siberia and Central Asia.
DISCUSSION
The discovery of two more unusual representatives of the family Crangonyctidae on a relatively small territory of the Altai Republic confirms that the crangonyctid diversity in the northern, and, especially, the north-eastern Palearctic is still far to be completely studied. The obtained molecular data and previously published studies (e.g., Copilaş-Ciocianu et al., 2019) showed that the described species, as well as previously known species, are relics of at least the late Paleogene period (about 65–20 Mya). The unique and relict nature of some species of freshwater crustaceans that live in the reservoirs of the Altai–Sayan Mountain system has already been shown for Cladocera (Zuykova et al., 2019). Unique microgastropod Sibirobythinella kuznetzkiana Johansen et Starobogatov 1982 also inhabit spring reservoirs in the region (see Johansen, Starobogatov, 1982), and relics are also noted among other groups of invertebrates living in the region (for example, Gryloblatella spp. (Notoptera, Grylloblattidae) (e.g., Storozhenko, Oliger, 1985; Bai et al., 2010). Thus, within the region, we are expecting new discoveries of stygomorphic/troglomorphic and crenobiotic animals, including amphipods. The presence of such fauna is often associated with a slight influence of the historical glaciation, to which the regions studied by us were not particularly exposed.
Moreover, our research revealed that not all crangonyctid species are endemic. During the study, we found Palearcticarellus mikhaili (Sidorov, Holsinger et Takhteev 2010) comb. n. (GenBank MT231256–MT231259) in other areas of the Kurai steppe in addition to the typical locality (50°13.764′ N 87°51.320′ E), specifically in small springs in the valley of the Chibitka River, near Chibit village (50°19′12.7″ N 87°31′26.0″ E) (see Fig. 16) and at one of springheads of the Kuektanar River (50°09.592′ N 88°18.785′ E) (see Fig. 1), which are quite far from the type locality. The distribution of Palearcticarellus smirnovi Palatov et Marin gen. et sp. n. described above also confirms this conclusion. We also assume that the rare records of crangonyctids are probably related to an extremely small size and cryptic lifestyle of these crustaceans. For example, specimens of Palearcticarellus mikhaili (Sidorov, Holsinger et Takhteev 2010) comb. n. were occasionally found among the swamp moss thickets (Fig. 16c–16e), where it was extremely difficult to discover them visually.
It is also worth noting that small-sized species of the new genus live in rather extreme habitats: Palearcticarellus mikhaili (Sidorov, Holsinger et Takhteev 2010) comb. n. occurs in cold-water springs and surrounding moss at an altitude of more than 1600 m above sea level in the Kurai steppe, while Palearcticarellus pusillus (Martynov 1930) comb. n. lives in a silt bottom at depths of more than 100 m in the Teletskoe Lake (Sidorov et al., 2010), where the water temperature rarely exceeds 5°C even on the surface. In our opinion, the morphology and body size of these species are related to their neotenic origin rather than to troglomorphy. The morphology of Palearcticarellus mikhaili (Sidorov, Holsinger et Takhteev 2010) comb. n. and Palearcticarellus pusillus (Martynov 1930) comb. n. confirm this assumption by 1) extremely small size with the total body length not exceeding 3–3.5 mm; 2) weakly expressed inter-antennal lobe of head (Fig. 11e); 3) weak armature of appendages (see Fig. 12e) and, especially, gnathopods (see Sidorov et al., 2010: Figs 18–21 ); and 4) relatively large setae and bristles (plumose setae) compared to the overall body size.
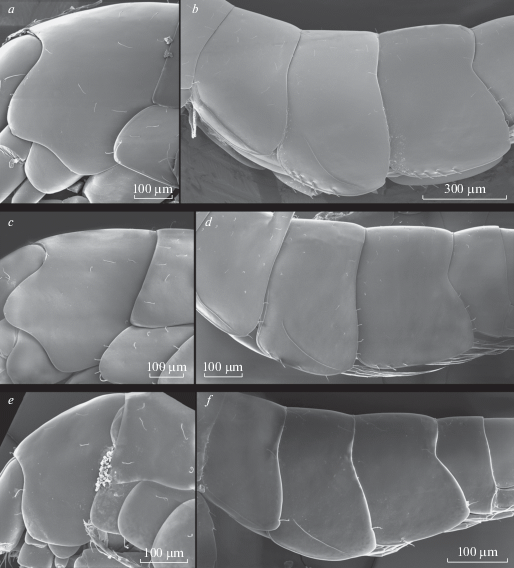
Fig. 12.
Palearcticarellus smirnovi Palatov et Marin gen. et sp. n. (a, b), Palearcticarellus sapozhnikovi Palatov et Marin gen. et sp. n. (c, d) Palearcticarellus mikhaili (Sidorov, Holsinger et Takhteev 2010) comb. n. (e, f): a, c, e – pleonal segments and uropods, lateral view; b, d – palmar margin of chela of Gn1; f – dactylus of P7.
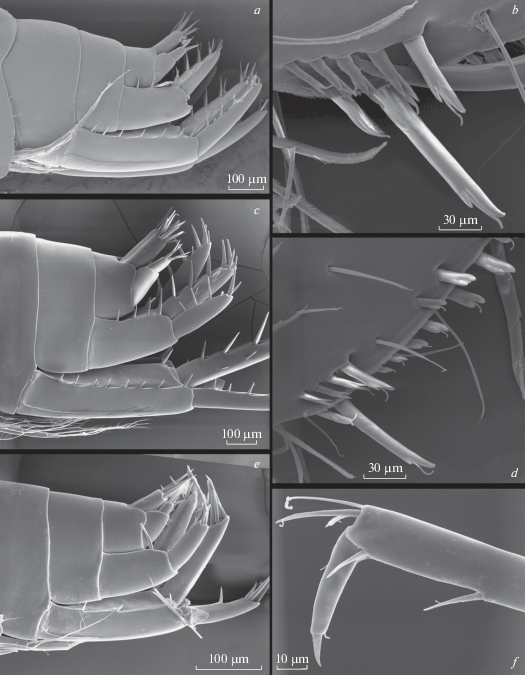
Fig. 13.
Alive coloration (a, b) and habitat (c–e) of Palearcticarellus smirnovi Palatov et Marin gen. et sp. n. in the Kureevo Lake in the drainage basin of the Biya River.

Fig. 14.
Alive coloration (a, b) and habitat (c–e) of Palearcticarellus smirnovi Palatov et Marin gen. et sp. n. in the Cheposh village in the valley of the Katun River.
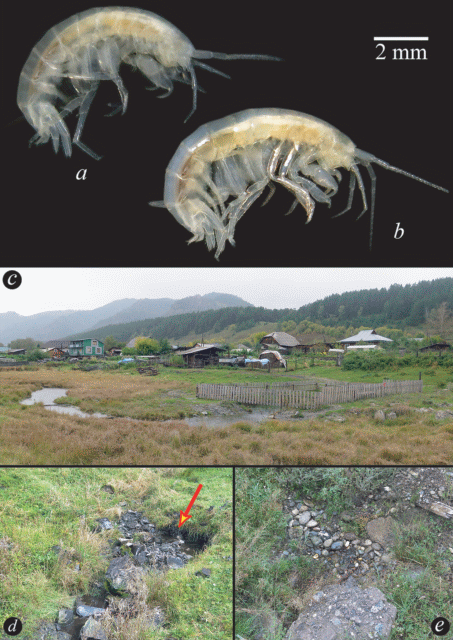
Fig. 15.
Habitat (a–c) and alive coloration (d, e) of Palearcticarellus mikhaili (Sidorov, Holsinger et Takhteev 2010) comb. n. in the Kurai highland valley (steppe) (near the Chibit village in the valley of the Chibitka River).

Fig. 16.
Size comparison of females of known crangonyctid amphipods from the Altai Republic: Palearcticarellus smirnovi Palatov et Marin gen. et sp. n. (a, c), Palearcticarellus sapozhnikovi Palatov et Marin gen. et sp. n. (b), Palearcticarellus mikhaili (Sidorov, Holsinger et Takhteev 2010) comb. n. (d, e) and damaged specimen of Palearcticarellus pusillus (Martynov 1930) comb. n. (f).

Fig. 17.
The reconstruction (tree) of the molecular phylogenetic (COI mtDNA gene marker) relations between the genus Palearcticarellus gen. n. and the representatives of the family Crangonyctidae (with the related Pseudocrangonyctidae and Crymostygidae as outgroups) (sequences from NCBI (GenBank)). Color indicates the sequences of the Palaearctic species/genera; nodes are indicated with the support based on ML algorithm. High-resolute version of the file is deposited on Google Disk: https://drive.google.com/file/d/1MWlSC8sOCL8MBQLaJHwxmWsnCcZ8gSLe/view?usp=sharing
Finally, we present the provisional key for the identification of the species of the genus Palearcticarellus Palatov et Marin gen. n.:
1. Total body length is more than 5 mm, up to 12 mm. Telson with the length more than width, with distal margin concave, sometimes with U-shaped notch .………………………………................................ 2
– Total body length less than 5 mm (usually smaller than 3.5 mm). Telson with the length equal to the width, with distal margin flat or convex ……………... 3
2. Pleopods 1–3 with 2 hooks in retinacle; gnathopods with 1 large setae on outer margin of dactylus; proximal segment of outer ramus of uropod 3 unarmed ……………Palearcticarellus smirnovi Palatov et Marin gen. et sp. n.
– Pleopods 1–3 with 5 hooks in retinacle; gnathopods with 5 large setae on outer margin of dactylus; proximal segment of outer ramus of uropod 3 armed with a group of simple setae ……… Palearcticarellus sapozhnikovi Palatov et Marin gen. et sp. n.
3. Total body length close to 5 mm. Telson with convex distal margin; inner lobe of maxilla 1 with 7 plumose setae; epimeral plates 2 and 3 with several (3–4) well-marked spines along ventral margin .... Palearcticarellus kazakhstanica (Kulkina 1992) comb. n.
– Total body length from 3 to 3.5 mm. Telson with flat distal margin; inner lobe of maxilla 1 with 2–4 plumose setae; only epimeral plates 3 with 1 well-marked spine along ventral margin ……………………. 4
4. Body densely covered by sensillae; inner plate of maxilla 2 with a row of 4 plumose setae, narrowing apically …………………………Palearcticarellus mikhaili (Sidorov, Holsinger et Takhteev 2010) comb. n.
– Body mostly smooth; inner plate of maxilla 2 rounded, not tapering, armed with fewer plumose setae ……………………............ Palearcticarellus pusillus (Martynov 1930) comb. n.
The presented phylogenetic analysis (Fig. 17), although it cannot be considered absolutely reliable, since it is based on only one gene marker (COI mtDNA), nevertheless it coincides with the results obtained earlier for other gene markers (Sidorov, Holsinger, 2007; Kornobis et al., 2011; Copilaş-Ciocianu et al., 2019). However, this analysis showed that the Palearctic forms are more basal, and it is highly likely that the family’s ancestors appeared around the area of modern Central Eurasia (Western Siberia or the Caucasus). Two predominantly Nearctic clades (Clades A and B) also contain Palearctic forms (several distantly living species, probably relic), while Clades C and D are completely Palearctic, as well as outgroups, which are considered the closest relatives of the family Crangonyctidae. It was additionally shown that different Synurella s. l. (“derzhavini”-gr. and “ambulans”-gr., belonging to different clades in our analysis) occur in unrelated geologic formations (platforms) with the territory of the northwestern Eurasia (Sidorov, Kovton, 2015). It is also clear from the analysis that the described species of the new genus Palearcticarellus Palatov et Marin gen. n., as well as the genus as a whole, are not phylogenetically related to the North American Stygobromus (even not a sister clade), and the external morphological similarity can be explained by a common family ancestor and genetic predispositions, similar lifestyle, and convergence. Thus, with the exception of the taxonomically unclear and phylogenetically related Crangonyx chlebnikovi Borutzky 1928 from the Ural caves and the European subterranean Crangonyx subterraneus Spence Bate 1859, the family currently include only the genus Crangonyx characterized by a Holarctic distribution, while the genus Stygobromus has become Nearctic only.
It is also obvious that, despite the relationship between the Palearctic genera from Clade C, which includes the new genus, they diverged very long time ago (about 65–100 Mya), close to the time of the division of the family into main modern clades (perhaps the time of the break-up of Laurasia, about 130–200 Mya (Copilaş-Ciocianu et al., 2019)), and due to their long genetic isolation and unique morphology, they deserve the status of separate genera. Here we resurrect their previously suggested generic names such as Eosynurella Martynov 1931 (see above) and Diasynurella Behning 1940 (for Diasynurella wachuschtii Behning 1940 and undescribed species from Armenia) (e.g., Behning, 1940) (see Fig. 17).
Список литературы
Avise J.C., 1993. Perspective: The evolutionary biology of aging, sexual reproduction, and DNA repair // Evolution. V. 47. № 5. P. 1293–1301. https://doi.org/10.1111/j.1558-5646.1993.tb02155.x
Bai M., Jarvis K., Wang S.-Y., Song K.-Q., Wang Y.-P., Wang Z.-L., Li W.-Z., Wang W., Yang X.-K., 2010. A second new species of ice crawlers from China (Insecta: Grylloblattodea), with thorax evolution and the prediction of potential distribution // PLoS ONE. V. 5. № 9. e12850. https://doi.org/10.1371/journal.pone.0012850
Behning A.L., 1940. Über einige Crustaceen der Umgebung von Bakuriani in Grusien // Trudy Biologicheskikh Stantsii Narkomprosa Gruzinskoi SSR. V. 1. P. 11–58.
Bousfield E.L., 1973. Shallow-water gammaridean Amphipoda of New England. Ithaca: Cornell University Press. 312 p.
Copilaș-Ciocianu D., Sidorov D.A., Gontcharov A., 2019. Adrift across tectonic plates: molecular phylogenetics supports the ancient Laurasian origin of old limnic crangonyctid amphipods // Organisms Diversity & Evolution. V. 19. P. 191–207. https://doi.org/10.1007/s13127-019-00401-7
Creaser E.P., 1934. A new genus and species of blind amphipod with notes on parallel evolution in certain amphipod genera // Occasional Papers of the University of Michigan Museum of Zoology. V. 282. P. 1–105.
Decu V., Juberthie C., Iepure S., Gheorghiu V., Nazareanu G., 2019. An overview on the subterranean fauna from Central Asia // Ecologica Montenegrina. V. 20. P. 163–188.
Derzhavin A.N., 1927. New Forms of Freshwater Gammarids from the Ussuri Region // Russkii Gidrobiologicheskii Zhurnal. V. 6. № 8/10. P. 176–179 [in Russian].
Derzhavin A.N., 1930. The freshwater Malacostraca of the Russian Far East // Russkii Gidrobiologicheskii Zhurnal. V. 9. P. 1–8 [in Russian with English abstract].
Derzhavin A.N., 1939. Presnovodnye perakaridyi Talysha // Trudy Zoologicheskogo Instituta Azerbaidjanskogo filiala AN, Baku. V. 10. P. 43–58 [in Russian].
Johansen B.G., Starobogatov Ya.I., 1982. A finding of freshwater mollusk of the family Triculidae (Gastropoda, Prosobranchia) in Siberia // Zoologicheskyi Zhurnal. V. 61. № 8. P. 1141–1147 [in Russian with English abstract].
Folmer O., Black M., Hoeh W., Lutz R., Vrijenhoek R., 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates // Molecular Marine Biology and Biotechnology. V. 3. P. 294–299.
Holsinger J.R., 1974. Systematics of the subterranean amphipod genus Stygobromus (Gammaridae). Part I: Species of the western United States // Smithsonian Contributions to Zoology. V. 160. P. 1–63. https://doi.org/10.5479/si.00810282.160
Holsinger J.R., 1978. Systematics of the subterranean amphipod genus Stygobromus (Gammaridae). Part II: Species of the eastern United States // Smithsonian Contributions to Zoology. V. 266. P. 1–144.
Holsinger J.R., 1994. Pattern and process in the biogeography of subterranean amphipods // Hydrobiologia. V. 287. № 1. P. 131–145. https://doi.org/10.1007/BF00006902
Holsinger J.R., 1987. Redescription of the stygobiont amphipod crustacean Stygobromus pusillus (Crangonyctidae) from the Soviet Union, with comments on taxonomic and zoogeographic relationships // Journal of Crustacean Biology. V. 7. P. 249–257. https://doi.org/10.1163/193724087X00207
Horton T., Lowry J., De Broyer C., Bellan-Santini D., Coleman C.O., Corbari L., Costello M.J., Daneliya M., Dauvin J.-C., Fišer C., Gasca R., Grabowski M., Guerra-García J.M., Hendrycks E., Hughes L., Jaume D., Jazdzewski K., Kim Y.-H., King R., Krapp-Schickel T., Le Croy S., Lörz A.-N., Mamos T., Senna A.R., Serejo C., Sket B., Souza-Filho J.F., Tandberg A.H., Thomas J.D., Thurston M., Vader W., Väinölä R., Vonk R., White K., Zeidler W., 2020. World Amphipoda Database. Crangonyctidae Bousfield, 1973 [Electronic Resource]. Accessed at: http://www.marinespecies.org/aphia.php?p=taxdetails&id=430454. Last update on 2020-03-01.
Karaman G.S., 1974. Genus Synurella Wrzes in Yugoslavia, with remarks on its all world known species, their synonymy, bibliography and distribution (Fam. Gammaridae) // Poljoprivreda i Sumarstvo. V. 20. № 2–3. P. 83–133.
Karaman G.S., 1990. New and interesting species of the genus Synurella Wrzes. 1877 (Fam. Crangonyctidae) from Soviet Union (U.S.S.R.). Contribution to the knowledge of the Amphipoda. 204 // Glasnik Republickog Zavoda za Zastitu Prirode. V. 23. P. 25–50 (published in 1991).
Kornobis E., Pálsson S., Sidorov D.A., Holsinger J.R., Kristjánsson B.K., 2011. Molecular taxonomy and phylogenetic affinities of two groundwater amphipods, Crangonyx islandicus and Crymostygius thingvallensis, endemic to Iceland // Molecular Phylogenetics and Evolution. V. 58. №. 3. P. 527–539. https://doi.org/10.1016/j.ympev.2010.12.010
Kornobis E., Pálsson S., Svavarsson J., 2012. Classification of Crangonyx islandicus (Amphipoda, Crangonyctidae) based on morphological characters and comparison with molecular phylogenies // Zootaxa. V. 66. P. 52–66. https://doi.org/10.1099/ijs.0.047324-0
Kristjánsson B.K., Svavarsson J., 2004. Crymostygidae, a new family of subterranean freshwater gammaridean amphipods (Crustacea) recorded from subarctic Europe // Journal of Natural History. V. 38. P. 1881–1894. https://doi.org/10.1080/00222930310001597295
Kulkina L.V., 1992. Stygobromus kazakhstanica sp. n. (Amphipoda, Crangonyctidae) from underground waters of Tien Shan // Zoologicheskii Zhurnal. V. 71. P. 40–45 [in Russian with English abstract].
Lowry J.K., Myers A.A., 2013. A phylogeny and classification of the Senticaudata subord. nov. (Crustacea: Amphipoda) // Zootaxa. V. 3610. P. 1–80. https://doi.org/10.11646/zootaxa.3610.1.1
Lowry J.K., Myers A.A., 2017. A phylogeny and classification of the Amphipoda with the establishment of the new order Ingolfiellida (Crustacea: Peracarida) // Zootaxa. V. 4265. P. 1–89. https://doi.org/10. 11646/zootaxa.4265.1.1
Martynov A.V., 1930. Amphipodous fauna of Teletszkoye Lake and its origin // Izvestiya Gosudarstvennogo Gidrologicheskogo Instituta. V. 29. P. 95–128 [in Russian with English abstract].
Martynov A.V., 1931. On Freshwater Amphipoda and Isopoda of North Yakutia // Ezhegodnik Zoologicheskogo Muzeya Akademii Nauk SSSR. V. 32. № 4. P. 523–540.
Shoemaker C.R., 1920. The amphipods of the Canadian Artic Expedition 1913-1918 // Report of the Canadian Arctic Expedition 1913–1918. V. 7E. P. 1–30.
Sidorov D., 2015. The spring-dwelling amphipod genus Lyurella (Peracarida, Amphipoda): systematics, distribution, and affinity, with description of the second representative from the Black Sea coast region // Crustaceana. V. 88. P. 27–50. https://doi.org/10.1163/15685403-00003392
Sidorov D.A., Holsinger J.R., 2007. Amurocrangonyx, a new genus of subterranean amphipod (Crangonyctidae) from the Russian Far East, with a redescription of the poorly known Crangonyx arsenjevi and comments on biogeographic relationships // Journal of Crustacean Biology. V. 27. P. 660–669. https://doi.org/10.1651/s-2817r.1
Sidorov D., Palatov D., 2012. Taxonomy of the spring dwelling amphipod Synurella ambulans (Crustacea: Crangonyctidae) in West Russia: with notes on its distribution and ecology // European Journal of Taxonomy. V. 23. P. 1–19. https://doi.org/10.5852/ejt.2012.23
Sidorov D.A., Gontcharov A.A., 2015. Preliminary analysis of phylogenetic relationships of the Asian-Pacific endemial subterranean amphipod genus Pseudocrangonyx among families and genera of crangonyctoidean amphipods inferred by partial LSU rDNA gene sequences // Zoological Science. V. 32. P. 178–182. https://doi.org/10. 2108/zs140129
Sidorov D.A., Kovtun O.A., 2015. Synurella odessana sp. n. (Crustacea, Amphipoda, Crangonyctidae), first report of a subterranean amphipod from the catacombs of Odessa and its zoogeographic importance // Subterranean Biology. V. 15. P. 11–27. https://doi.org/10.3897/subtbiol.15.8820
Sidorov D.A., Holsinger J.R., Takhteev V., 2010. Two new species of the subterranean amphipod genus Stygobromus (Amphipoda: Crangonyctidae) from Siberia, with new data on Stygobromus pusillus (Martynov) and remarks on morphology and biogeographic relationships // Zootaxa. V. 2478. P. 41–58. https://doi.org/10.11646/zootaxa.2478.1.2
Storozhenko S.Yu., Oliger A.I., 1985. A new species of Grylloblattida from northeastern Altai // Entomological Review. V. 63. № 4. P. 54–57.
Svavarsson J., Kristjánsson B.K., 2006. Crangonyx islandicus sp. nov., a subterranean freshwater amphipod (Crustacea, Amphipoda, Crangonyctidae) from springs in lava fields in Iceland // Zootaxa. V. 1365. P. 1–17. https://doi.org/10.11646/zootaxa.1365.1.1
Turbanov I.S., Schwartz D.B., Shelepin A.L., 2019. Kök-Tash Cave (Ecological). Atlas of caves of Russia // Russian Geographical Society, Russian Union of Speleologists. Moscow. P. 490–493.
Zhang J., Holsinger J.R., 2003. Systematics of the freshwater amphipod genus Crangonyx (Crangonyctidae) in North America // Virginia Museum of Natural History Memoir. V. 6. P. 1–274. https://doi.org/10.1163/156854008X354894
Zuykova E.I., Bochkarev N.A., Taylor D.J., Kotov A.A., 2019. Unexpected endemism in the Daphnia longispina complex (Crustacea: Cladocera) in Southern Siberia // PloS One. V. 14. № 9. e0221527. https://doi.org/10.1371/journal.pone.0221527
Дополнительные материалы отсутствуют.
Инструменты
Зоологический журнал


