Зоологический журнал, 2020, T. 99, № 11, стр. 1203-1222
Stygobiotic atyid shrimps (Crustacea, Decapoda, Atyidae) from the Amtkel karst system, Western Abkhazia, Caucasus, with a redescription of Xiphocaridinella osterloffi and the description of two new co-occurring species
A.N. Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences
119071 Moscow, Russia
* E-mail: coralliodecapoda@mail.ru
** E-mail: vanomarin@yahoo.com
Поступила в редакцию 1.03.2020
После доработки 16.03.2020
Принята к публикации 17.03.2020
Аннотация
A morphogenetic revision of Caucasian stygobiotic shrimps from the Amtkel karst system in western Abkhazia is presented. The neotype of Xiphocaridinella osterloffi (Juzbaš’jan 1941) comb. n. is designated and carefully re-described based on material from the type locality, the Lower Shakuran (= Nizhnya-Shakuranskya) Cave, Gulripshi District. Two new Xiphocaridinella species are described from several different caves, showing a connection between them within the Amtkel karst system. Xiphocaridinella falcirostris Marin sp. n. is described from underground streams of both Lower Shakuran and Besletka caves, Sukhumi District, and Xiphocaridinella smirnovi Marin sp. n. from the Besletka Cave. These records also represent additional cases of stygobiotic shrimp co-occurrence in the western Caucasus, simultaneously revealing the historical fragmentation of a formerly single underground karst habitat and speciation in the ancestral population. Both new species can be separated from congeners by morphological features, mainly the shape and armature of the rostrum, as well as genetically. Thus, the diversity of Caucasian Xiphocaridinella known from the Kolkhida coastal lowland (Colchis) of the eastern Black Sea region is increased to twelve species.
Knowledge about Caucasian stygobiotic shrimp genus Xiphocaridinella Sadowsky 1930 (Sadowsky, 1930), living in underground (subterranean) habitats of the Kolkhida coastal lowland plain (Colchis) of the Eastern Black Sea region, is still incomplete (Zakšek et al., 2007; Sket, Zakšek, 2009) and new species were discovered and described so far (Marin, 2017, 2017a; 2018, 2018a; 2019, 2019a; Marin, Sinelnikov, 2017).
The Amtkel karst system, located in the southwestern part of the plain in the valley of the Kodori river, is represented by numerous deep and branched karst caves, which are relatively well studied by speleologists and geologists (e.g., Amelichev et al., 2007). Stygobiotic shrimps from the Amtkel karst system were first recorded by A.E. Osterloff, a famous collector of Caucasian fauna, in the Lower Shakuran Cave (=Nizhnya-Shakuranskya) and briefly described by Juzbaš’jan (1940), who noted that they can be distinguished from Troglocaris schmidti kutaissiana (=Xiphocaridinella kutaissiana) with a short unarmed rostrum, “similar to Troglocaris schmidti inermis and other Abkhazian species”, and rarely reaching 2/3 of the length of the basal antennal segment. The study of numerous specimens allowed to generalize the measurement of the length of the rostrum for this species as “a short unarmed rostrum about 1/3–2/3 of the length of the basal antennular segment”, with the exception of one large female (tbl. 22 mm), having a rostrum reaching the distal margin of the basal antennular segment and armed with a single dorsal spine. At the same time, the described shrimp species was not named by Juzbaš’jan (1940) in the primary description. However, his next publication (Juzbaš’jan, 1941) includes a comparative table for Caucasian stygobiotic shrimps, which he studied with the introduction of a new scientific name – Troglocaris kutaissiana osterloffi (see Juzbaš’jan, 1941: 933), for the species from the Lower Shakuran Cave, named by him “in honor of the indefatigable cave explorer of Georgia A. E. Osterloff” (Juzbaš’jan, 1941: 935). Thus, the scientific name Troglocaris kutaissiana osterloffi (=Xiphocaridinella osterloffi) was proposed by Juzbaš’jan in 1941, not in 1940, as mentioned in the world databases, for example, WoRMS (2019), and scientific literature (e.g., Marin, Sokolova, 2014; Barjadze et al., 2015; Marin, 2017a). The current taxonomic name of the species should be changed for Xiphocaridinella osterloffi (Juzbaš’jan 1941) comb. n.
At the same time, the molecular-genetic analysis of Xiphocaridinella shrimps from the Lower Shakuran Cave revealed the presence of two different genetic lineages. After a thorough morphological study, Xiphocaridinella osterloffi, described by Juzbaš’jan (1940, 1941), was identified among samples from the Lower Shakuran Cave, as well as the second species in the same cave, previously mentioned by Juzbaš’jan (1940) as “a large female specimen (22 mm) with rostrum, reaching the distal margin of the basal segment of the antenna and dorsally armed with 1 dorsal spine”, was also distinguished. The study of the collections of the Russian zoological museums, in which specimens of Troglocaris kutaissiana osterloffi (=Xiphocaridinella osterloffi), studied by S.M. Juzbaš’jan, could be deposited, showed the absence of the holotype specimen. Thus, the neotype of Xiphocaridinella osterloffi (Juzbaš’jan 1941) comb. n. is designated herewith.
A large amount of precipitation in Abkhazia in May 2017 caused heavy flooding in the area, including in underground habitats and caves. Several specimens of stygobiotic shrimps were collected at the entrance of the Besletka Cave (43°01′48.1″ N 41°04′36.0″ E) using a large net placed at the entrance of the cave during the flooding. Analysis of the collected specimens also revealed the presence of several distinct genetic lineages, one of which was identical to the previously observed in the Lower Shakuran Cave of the Amtkel karst system, indicating a connection between these caves. The co-occurrence of sibling stygobiotic Troglocaris-like shrimp species within the same cave system was previously recorded in karst regions of NE Italy, Slovenia, Croatia, Hercegovina (Cobolli Sbordoni et al., 1991; Sket, Zakšek, 2009; Jugovic et al., 2012) and the Western Caucasus (Marin, 2018), as well as has been known for other subterranean animals (e.g., a review in Cobolli Sbordoni et al., 1991; Sket, 1994; Guzik et al., 2011). Stygobiotic shrimps have already been recorded from the Sukhumi region of Abkhazia as “Troglocaris schmidti” from the Tskaro (=Tsqaro) Cave (Birštein, Lopashov, 1940; Barjadze et al., 2015). The described cave is located about 6 km from the center of Sukhumi in the valley of the Basla River near the Besleti Bridge, also known as the Queen Tamar Bridge, and is probably the Besletka Cave (modern name). However, Troglocaris schmidti Dormitzer 1853 was described from SE Slovenia and is currently considered as a junior synonym of Troglocaris anophthalmus (Kollar 1848) (after Jugovic et al., 2012).
MATERIALS AND METHODS
Specimens of stygobiotic shrimps were collected in an subterranean stream flowing from the Lower Shakuran (=Nizhnya-Shakuranskya) Cave (43°01′47.8″ N 41°20′02.0″ E) in January and April of 2012 and the Besletka Cave (43°01′48.1″ N 41°04′36.0″ E) in May 2017. The water temperature in both caves was about 12–14°C. The shrimps were collected using a hand net and large net fixed in a subterranean stream formed as a result of flooding caused by torrential rains in May 2017. All collected specimens were fixed and preserved in 90% solution of ethanol. Postorbital carapace length (pcl., in mm), the length from the posterior orbit to the posterodorsal margin of carapace, and total body length (tbl., in mm), dorsal length from the tip of rostrum till the distal margin of telson, are used as standard measurements. The type material is deposited in the collection of Zoological Museum of Moscow State University, Moscow, Russia (ZMMU) and the Laboratory of Ecology and Evolution of Marine Invertebrates of A.N. Severtzov Institute of Ecology and Evolution of Russian Academy of Sciences, Moscow, Russia (LEMMI).
Local endemism of closely related (cryptic) species in separate cave systems is quite common and even characteristic for the genus Troglocaris s. l. (e.g., Sket, Zakšek, 2009). Thus, it is very convenient to use the mitochondrial cytochrome c oxidase subunit I (COI mtDNA) gene marker, one of the most informative markers for genetic studies at the population and species level (Avise, 1993), for marking species within the genus Xiphocaridinella. Total genomic DNA was extracted from muscle tissue using the innuPREP DNA Micro Kit (AnalitikJena, Germany). The COI mtDNA gene marker was amplified with the help of the universal primers LCO1490 (5'–GGTCAACAAATCATAAAGATATTGG–3') and HC02198 (5'–TAAACTTCAGGGTGACCAAAAAATCA–3') (Folmer et al., 1994). PCR products were performed on amplificator T100 (Bio-Rad, USA) under the following conditions: initial denaturation at 96°C for 1.5 min followed by 42 cycles of 95°C for 2 min, 49°C for 35 seconds, and 72°C for 1.5 min, followed by chain extension at 72°C for 7 min. The volume of 10uL of reaction mixture contained 1uL of total DNA, 2uL of 5xPCR mix (Dialat, Russia) and 1uL of each primer. The amplification products were separated by using gel electrophoresis of nucleic acids on a 1.5% agarose gel in 1xTBE, and then stained and visualized with 0.003% EtBr using imaging UV software. DNA nucleotide sequences were determined using Genetic Analyzer ABI 3500 (Applied Biosystems, USA) and BigDye 3.1 (Applied Biosystems, USA) with direct and reverse primers.
Dataset of aligned sequences of COI mtDNA gene markers, about 658 base pairs in length used in the study were taken from GenBank (NCBI) and author data (see Appendix 1; Marin 2018a b). The consensus of complementary sequences was obtained with MEGA 7.0. The best evolutionary substitution model was determined using MEGA 7.0 and jModeltest2.1.141. Phylogenetic analyses were conducted using MrBayes v.3.2.6 for Bayesian analysis (BA) using NKY+I+G model of evolution and MEGA 7.0 for Maximum-Likelihood (ML) and Neighbour-Joining (NJ) analyses using the Kimura-2-parameter (K2P) model. Bayesian analysis was conducted by sampling one tree every 1.000 generations for 1.000.000 generations. Confidence values >50% are presented for ML, NJ and BA analyses (bootstraps); pairwise sequence divergence (p-distances) were calculated using the Kimura-2-parameter (K2P) model in MEGA 7.0.
RESULTS
Molecular Identification of the species of the genus Xiphocaridinella
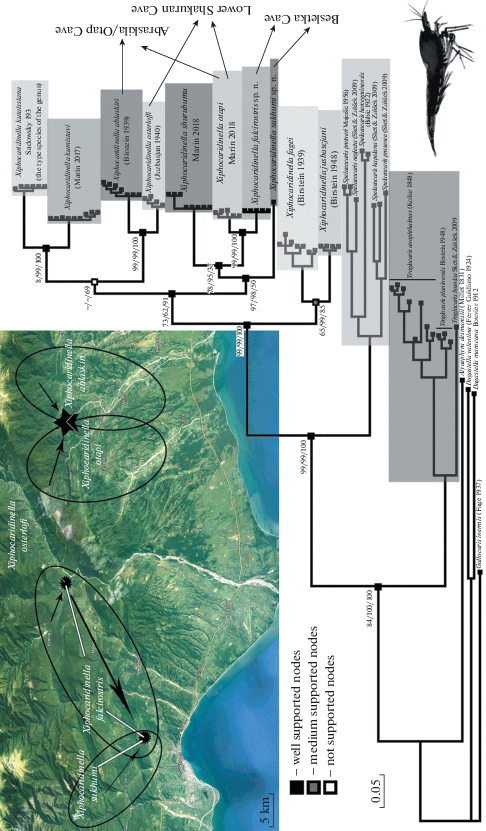
Interspecific pairwise genetic distances (p-distances) within the studied population of Xiphocaridinella osterloffi (Juzbaš’jan 1941) comb. n. from the Lower Shakuran Cave is 0.003 ± 0.002 substitutions per 100 nucleotides (about 0.3%) (n = 5), while p-distances between known species of the genus vary from 0.047 to 0.107 substitutions per 100 nucleotide positions (about 4–11%). The genetically close (phylogenetically relative) Xiphocaridinella ablaskiri (Birštein 1939) is known from Abrskila Cave, while the most genetically distant are Xiphocaridinella jusbaschjani (Birštein 1948) from Agura River, Sochi, Russia (39°48′40.54″ E 43°32′57.26″ N) and Xiphocaridinella fagei (Birštein 1939) from New Athos Cave, Abkhazia (43°5′44″ N 40°48′53″ E), which, oddly enough, are very similar morphologically, also having short unarmed rostrum (see below) (Birštein, 1939, 1948). The results of phylogenetic reconstruction (trees), obtained using the Maximum–Likelihood algorithm with high bootstrap support, also suggests the division of Xiphocaridinella osterloffi (Juzbaš’jan 1941) comb. n. as a separate species (Fig. 9) (after Knowlton et al., 1993; Knowlton, Weigt, 1998; Hebert et al., 2003; Sites, Marshall, 2004; Zakšek et al., 2007, 2019; Lefébure et al., 2006, 2007; Marin, 2017а).
Interspecific pairwise genetic distances within the studied population of Xiphocaridinella falcirostris Marin sp. n. from the Lower Shakuran Cave is 0.002 ± ± 0.001 substitutions per 100 nucleotide positions (about 0.2%) (n = 7). A specimen from the Besletka Cave (MT066038) genetically differs from the studied population from the Lower Shakuran Cave by 0.004 substitutions per 100 nucleotide positions (about 0.4%) (n = 1). At the same time, intraspecific pairwise genetic distances between known species of the genus (Table 1) varies from 0.04 to 0.121 substitutions per 100 nucleotide positions (about 4–12%) that genetically separate the new species from the congeners (Table 1; Fig. 9).
Table 1.
Uncorrected pairwise genetic (COI mtDNA) distances (p-distances) between Xiphocaridinella osterloffi (Juzbaš’jan 1941) comb. n. (n = 5), Xiphocaridinella falcirostris sp. n. (n = 7), Xiphocaridinella sukhumi sp. n. (n = 2), known Caucasian Xiphocaridinella species (COI mtDNA data after Marin, 2018) and relative taxa
| Species | X. osterloffi | X. falcirostris sp. n. | X. sukhumi sp. n. |
|---|---|---|---|
| X. otapi (n = 5) | 0.095 ± 0.013 | 0.040 ± 0.007 | 0.081 ± 0.012 |
| X. kumistavi (n = 9) | 0.091 ± 0.013 | 0.109 ± 0.014 | 0.091 ± 0.013 |
| X. shurubumu (n = 6) | 0.092 ± 0.013 | 0.109 ± 0.014 | 0.092 ± 0.013 |
| X. kutaissiana (n = 6) | 0.102 ± 0.015 | 0.121 ± 0.015 | 0.109 ± 0.014 |
| X. jusbaschjani (n = 5) | 0.104 ± 0.014 | 0.109 ± 0.014 | 0.107 ± 0.014 |
| X. fagei (n = 7) | 0.107 ± 0.014 | 0.102 ± 0.013 | 0.112 ± 0.014 |
| X. ablaskiri (n = 8) | 0.047 ± 0.009 | 0.105 ± 0.014 | 0.114 ± 0.014 |
| X. osterloffi (n = 5) | – | 0.112 ± 0.014 | 0.115 ± 0.014 |
| Spelaeocaris pretneri (n = 2) | 0.162 ± 0.017 | ||
| Troglocaris anophthalmus (n = 3) | 0.231 ± 0.023 | ||
For Xiphocaridinella smirnovi Marin sp. n., the most genetically related species is Xiphocaridinella otapi Marin 2018, which differ from the new species for more than 0.08 substitutions per 100 nucleotide positions (about 8%) (Table 1; Fig. 9).
Genus Xiphocaridinella Sadowsky 1930
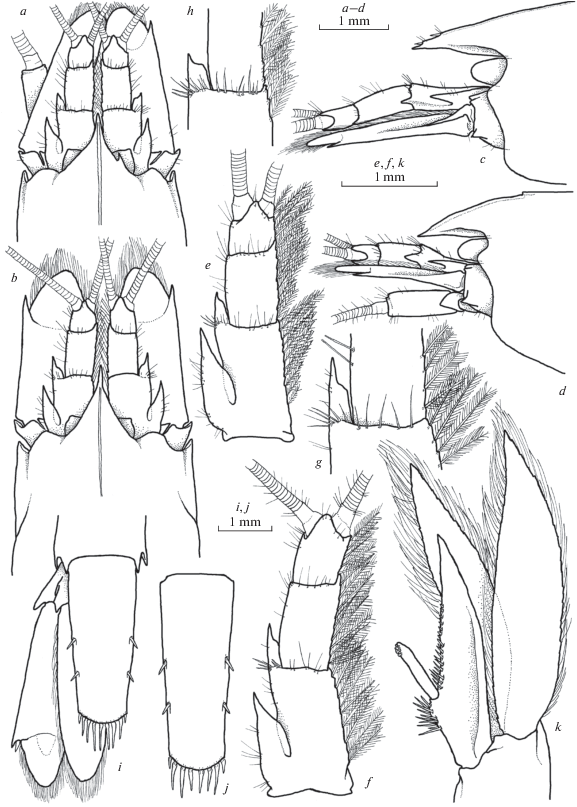
Xiphocaridinella osterloffi (Juzbaš’jan 1941) comb. n. (Figs 1c, 2, 3)
Fig. 1.
General view and proportions of Xiphocaridinella falcirostris Marin sp. n. (a – male, b – female) and Xiphocaridinella osterloffi (Juzbaš’jan 1941) comb. n. (c – female) from the Lower Shakuran Cave, Western Caucasus.
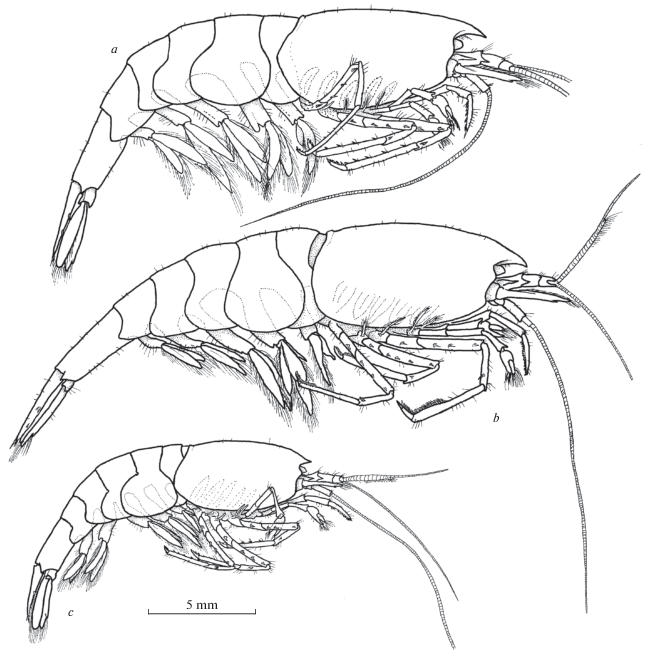
Fig. 2.
Xiphocaridinella osterloffi (Juzbaš’jan 1941) comb. n. from the Lower Shakuran Cave, Western Caucasus, male (a, b, e, g, h–j), female (c, d, f): a–g – front of carapace, h – antennula, i – distal margin of the basal antennular segment, j – telson.
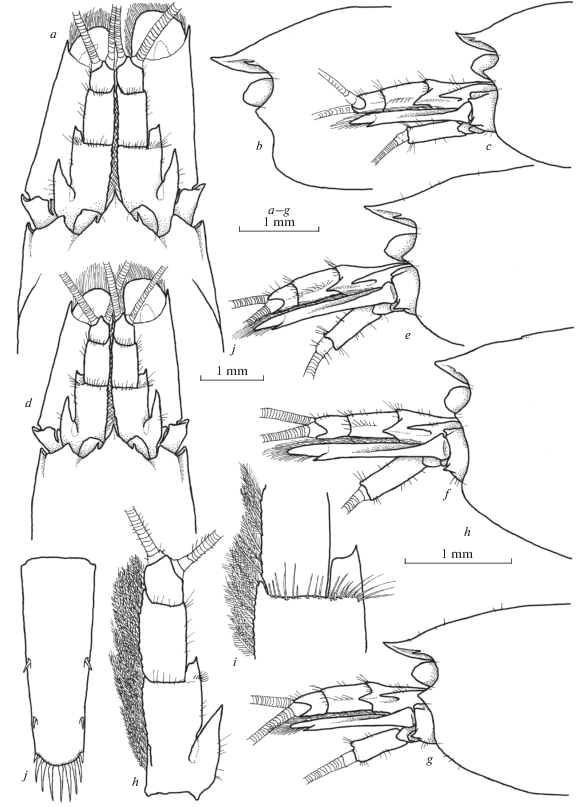
Fig. 3.
Xiphocaridinella osterloffi (Juzbaš’jan 1941) comb. n. from the Lower Shakuran Cave, Western Caucasus, female: a – pereiopod I, b – pereiopod II, c – pereiopod III, d – dactylus of pereiopod III, e – pereiopod IV, f – pereiopod V, g – dactylus of pereiopod V.
Troglocaris kutaissiana osterloffi Juzbaš’jan 1941: 933 (type locality – the Lower Shakuran Cave, Abkhazia, Western Caucasus)
Xiphocaridinella osterloffi. – Marin, 2017a: 325.
Material examined. Neotype, ovigerous female, pcl. 5.2 mm, tbl. 16.0 mm (ZMMU Ma 6008), Western Caucasus, Abkhazia, Sukhumi region, Amtkel karst system, Lower Shakuran Cave (=Nizhnya-Shakuranskya), 43°01′47.8″ N 41°20′02.0″ E, about 233–235 m above sea level, in subterranean stream, coll. D. M. Palatov, 30 Jan. 2012; 12 non-ovigerous females and 7 males, (LEMMI), same locality and date as neotype.
Description. Small-sized shrimp with swollen, smooth, subcylindrical body (Fig. 1). Carapace swollen, smooth, without dorsal carina in frontal part (Fig. 2a, 2d). Rostrum (Fig. 2a–2g) short, not reaching the midlength of basal antennular segment, triangular in shape, dorsally and ventrally unarmed, broad proximally, with developed lateral lamina sharply pointed distally, with tip turned forward.
Abdominal somites (Fig. 1) smooth, unarmed; tergites non-carinate, without dorsal lobes, not posteriorly produced; pleura of pleomeres I–IV posteroventrally and ventrally rounded; pleomere V small, with feebly developed pleura, rounded posterodistally; distal and distoventral margins of pleomere VI rounded posterodorsally. Telson (Fig. 2j) relatively slender, smooth, about 3 times as long as wide proximally, expanded distally, with 2 pairs of slender dorsal spines, situated at about 0.5 and 0.8 telson length respectively; distal margin convex, with 4 pairs of distal spines, including a pair of short lateral spines, a pair of long intermediate spines and 2–3 pairs of slender medial marginal spines.
Eyes (Fig. 2a–2g) partly reduced, subcylindrical, partly concealed by carapace; cornea reduced and feebly marked, without pigment; eyestalk stout, swollen, cylindrical, about as long as wide.
Antennula (Fig. 2h) with stout unarmed articles, basal peduncular segment robust, about twice longer than proximal width, without ventromedial tooth, distolateral angle with broadly produced sharp projection (Fig. 2i); stylocerite stout, sharpening distally, overreaching the midlength of basal segment; second and third segments stout, unarmed; second segment about 1.5 times as long as wide and about 1.5–2 times as long as distal segment; distal segment about as long as wide, unarmed.
Antenna with basicerite stout, about 1.5 times as wide as long, with distolateral margin unarmed; carpocerite robust, about 1.5 times as long as wide, not reaching midlength of scaphocerite; scaphocerite (Fig. 2a, 2d) well developed, broad, with small but well-marked distolateral tooth, lamella bluntly rounded distally.
Maxilliped III with slender segments; epipodite stout, bluntly rounded; arthrobranch reduced; exopodite slender, overreaching the distal margin of antepenultimate segment; antepenultimate segment slender, about 6–7 times as long as wide; penultimate segment about 6–7 times as long as wide, with straight lateral margins; ultimate (distal) segment slender, equal to penultimate segment, about 6–7 times as long as wide, tapering distally, with tufts of short simple stick-like setae along ventral and lateral margins, with several tufts of long setae dorsally.
Pereiopods I (Fig. 3a) equal in size and similar in shape, and similar in females and males, with smooth unarmed segments; coxa with well-developed slender epipodite and tuft of long simple setobranchs; basis about as long as wide, with well-developed exopodite overreaching carpo-meral articulation; ischium about 1.5 times longer than wide, with straight margins; merus slender, about 2 times as long as wide, slightly longer than ischium, with straight margins; carpus stout, significantly widening distally, equal to merus, about as 1.5 times as long as maximal width; palm relatively stout, about as long as wide, subcylindrical in cross-section, smooth; fingers relatively slender, subcylindrical, smooth, with blunt distal margins, about 2.5–3 times as long as proximal width, armed with a row of stout strong plumose setae distally.
Pereiopods II (Fig. 3b) equal in size and similar in shape, similar in males and females and almost similar to pereiopod I; coxa with well-developed slender epipodite and tuft of setobranchs; basis about as long as wide, with well-developed exopodite overreaching carpo-meral articulation; ischium about 2 times as long as wide, with straight margins, unarmed; merus slender, about 3 times as long as wide, usually longer than ischium, with straight margins; carpus slender, about 4–5 times as long as maximal width, widening distally, longer than merus; palm stout, similar to palm of pereiopod I, about as long as wide, subcylindrical in cross-section, smooth; fingers relatively slender, subcylindrical, smooth tapering distally, about 2–2.5 times as long as proximal width, with simple and straight cutting edges, with broad blunt distal margin, armed with a row of stout strong plumose setae distally.
Pereiopod III in females (Fig. 3c) with rectangular coxa, about as long as wide, with tuft of long simple setobranchs, with small epipodite; basis about as long as wide, with well-marked exopodite almost reaching the midlength of merus; ischium about 1.5 times as long as wide, with well-marked distoventral spine; merus about 5 times as long as wide, with straight margins, with 3–4 well marked spines along ventral margin; carpus relatively slender, about 4 times as long as wide, slightly widening distally, about 2 times shorter than merus and propodus, with bluntly projecting distodorsal margin overlapping carpo-propodal articulation, with 1 subdistal spine; propodus about 8 times as long as wide, with straight margins, armed with 5–6 spines along proximal half of ventral margin; dactylus (Fig. 3d) about 2–2.5 times longer than wide, biunguiculate, ventral margin armed with 4–5 small spines, main unguis smooth, curved and sharp; accessory unguis triangular, sharp, larger than ventral teeth, about twice shorter than main unguis. Pereiopod III in males with relatively slender segments; coxa rectangular, about as long as wide, with tuft of long simple setobranchs, with epipodite; basis about as long as wide, with well-marked exopodite overreaching the distal margin of ischium; ischium about 1.5 times as long as wide, with well-marked distoventral spine; merus about 5 times as long as wide, with straight margins, with 3 or 4 well-marked spines along ventral margin; carpus relatively slender, about 4 times as long as wide, slightly widening distally, about 2 times shorter than merus and propodus, with bluntly projecting distodorsal margin slightly overlapping carpo-propodal articulation, with subdistal spine or without it sometimes; propodus about 8–9 times as long as wide, with straight margins, distal third of propodus widening and armed with a series of small spines along its ventral margin; dactylus about 2.5–3 times as long as wide, with single unguis, ventral margin armed with small relatively stout sharp teeth, main unguis smooth, curved and sharp.
Pereiopod IV (Fig. 3e) generally similar to pereiopod III, similar in males and females; merus armed with 2–3 spines, carpus with subdistal spine or without it; exopodite smaller than in pereiopod III, not reaching the midlength of ischium. Pereiopod V (Fig. 4f) generally similar to pereiopods III and IV, similar in male and females, without armature on basal segments and exopodite on basis; segments covered with simple setae dorsally and ventrally; propodus about 8–9 times as long as wide, with straight margins, armed with 7–8 long spines along ventral margin and pair of long slender spines at distoventral angle; ventral margin of dactylus (Fig. 3f) armed with a dense “brush” consisting of small simple sharp setae; without accessory unguis, main unguis curved, triangular, sharp distally.
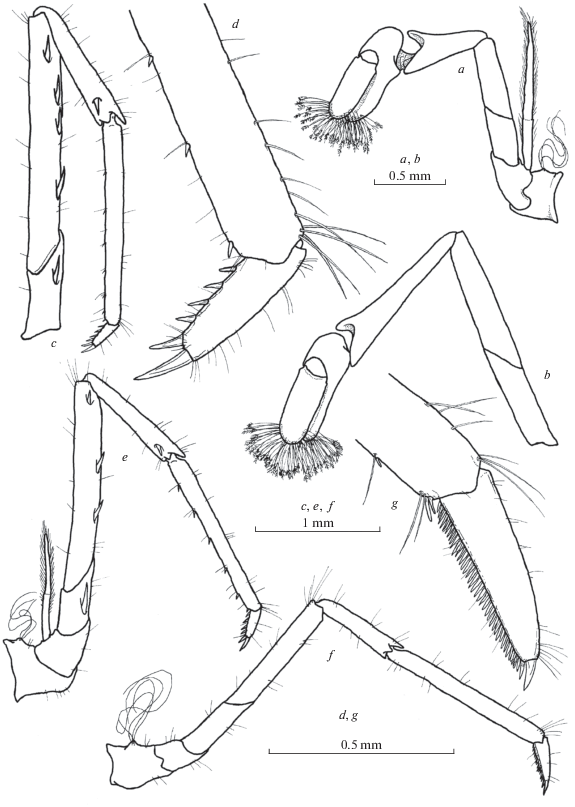
Fig. 4.
Rostrum and front of carapace of Xiphocaridinella falcirostris Marin sp. n. from the Lower Shakuran Cave, Western Caucasus: a–c – female, d–f – male.
Pleopods I and II in females normal, characteristic for the genus without specific differentiating features. Pleopod I in males with endopod bearing well marked appendix interna. Pleopod II in males characteristic for the genus, with well-developed appendix interna and appendix masculina; appendix interna with small cincinnuli distally; appendix masculina covered with numerous small sharp stout simple spines.
Uropods relatively stout, slightly exceeding telson; lateral margin of uropodal exopodite straight, with sharp triangular distolateral angle and large curved distolateral spine; dieresis simple, without spines.
Coloration. The body and appendages of shrimps are transparent whitish and yellowish; the cornea of eyes is albescent; the internal organs (gonads and hepatopancreas) are whitish or yellowish; numerous small transparent fat granules are visible through the carapace.
Body size. The largest collected female has pcl. 6.5 mm, tbl. 20.0 mm; the largest collected male has pcl. 6.0 mm, tbl. 19.0 mm.
GenBank accession numbers. MT065905–MT065909.
Taxonomic remarks. Xiphocaridinella osterloffi (Juzbaš’jan 1941) comb. n. is mostly (morphologically and phylogenetically) related to Xiphocaridinella ablaskiri (Table 1; Fig. 9); both species have short unarmed rostrum with a blunt tip. At the same time, the species can be clearly separated by much shorter rostrum, not reaching the midlength of the basal antennular segment (Fig. 2a–2g), while rostrum is almost reaching the distal margin of the basal antennular segment in Xiphocaridinella ablaskiri (see Marin, 2018: Fig. 4). Morphologically similar congeners with an unarmed rostrum are Xiphocaridinella jusbaschjani, which also have a blunt rostrum not reaching slightly the midlength of the basal antennular segment (Marin, Sokolova, 2014: Fig. 2a–2c), and Xiphocaridinella fagei, which have a long and distally thinning rostrum reaching the distal margin of the basal antennular segment (Marin, Sokolova, 2014: Fig. 14 ), while these species can be clearly separated by rostral shape, slenderer ambulatory pereiopods, as well as genetically (Table 1; Fig. 9) and by known geographical areas (see above).
Distribution. The species is known exclusively from an underground stream of the Lower Shakuran (=Nizhnya-Shakuranskya) Cave (43°01′47.8″ N 41°20′02.0″ E), Amtkel karst system of the southwestern Caucasus.
Xiphocaridinella falcirostris Marin sp. n. (Figs 1a, 1b; 4–6)
urn:lsid:zoobank.org:act:BE0CCAA9-DA8C-40C5-BB39-D75F915968BF
Material examined. Holotype, male, pcl. 7.5 mm, tbl. 24.0 mm (ZMMU Ma 6009), Western, Caucasus, Abkhazia, Sukhumi region, Amtkel karst system, Lower Shakuran Cave (=Nizhnya-Shakuranskya), 43°01′47.8″ N 41°20′02.0″ E, about 233–235 m above sea level, in subterranean stream, coll. Palatov, 30 Jan. 2012; 5 non-ovigerous females and 5 males (LEMMI), same locality and date as neotype; 5 non-ovigerous females, 5 males, (LEMMI), same locality as neotype, coll. Palatov, 30 Apr. 2012; 1 damaged specimen (LEMMI), Abkhazia, Sukhumi region, Besletka Cave, 43°01′48.1″ N 41°04′36.0″ E, about 68–70 m above sea level, collected with net in a stream flowing from the cave entrance in the period of high water, coll. Marin and Sinelnikov, 25 May 2017.
Description. Medium-sized shrimp with swollen, smooth, subcylindrical body (Fig. 1a, 1b). Carapace swollen, smooth, with well-marked dorsal carina in its frontal part (Fig. 4a, 4b). Rostrum (Fig. 4; 5a–5d) curved, robust proximally and slender distally, triangular in lateral and dorsal views, reaching the distal margin of basal antennular segment, dorsally unarmed in females and armed with 0–2 small spines in males, ventrally unarmed, sharply pointed distally, with tip turned downward, with relatively developed lateral lamina (Fig. 5a, 5b).
Fig. 5.
Xiphocaridinella falcirostris Marin sp. n. from the Lower Shakuran Cave, Western Caucasus, female (a, e, h, i), male (b–g, j, k): a–d – front of carapace, e, f – antennula, g, h – same, distal segments, i, j – telson, k – pleopod II.
Abdominal somites smooth, unarmed (Fig. 1a, 1b); tergites non-carinate, without dorsal lobes, not posteriorly produced; pleura of pleomeres I–IV posteroventrally and ventrally rounded; pleomere V small, with feebly developed pleura, bluntly rounded posteriorly; distal and distoventral margins of pleomere VI bluntly produced posterodorsally. Telson (Fig. 5i, 5j) stout, smooth, about 2.5–3 times as long as wide proximally, expanded distally, with 2 pairs of slender dorsal spines, each about 0.08 of telson length, situated at about 0.45 and 0.75 telson length respectively; distal margin convex, with 4 pairs of distal spines, including a pair of short lateral spines, a pair of long intermediate spines and 2–3 pairs of slender medial marginal spines.
Eyes (Fig. 5a, 5b) partly reduced, subcylindrical, partly covered by carapace; cornea rounded, reduced and feebly marked, without pigment; eyestalk stout, swollen, cylindrical, about as long as wide.
Antennula (Fig. 5e–5h) with stout unarmed articles, basal peduncular segment robust, about twice longer than proximal width, without ventromedial tooth, distolateral angle with sharply produced projection (Fig. 5h, 5g); stylocerite stout, sharpening distally, overreaching the midlength of basal segment; second and third segments stout, unarmed; second segment about 1.5 times as long as wide and about 1.5 times as long as distal segment; distal segment about as long as wide.
Antenna with basicerite stout, about as long as wide, with distolateral margin unarmed; carpocerite robust, about 1.5 times as long as wide, not reaching midlength of scaphocerite; scaphocerite well developed, broad, with small but well-marked distolateral tooth, lamella bluntly rounded distally.
Maxilliped III with slender segments; epipodite stout, bluntly rounded; arthrobranch reduced; exopodite slender, overreaching the distal margin of antepenultimate segment; antepenultimate segment slender, about 6–7 times as long as wide; penultimate segment about 6–7 times as long as wide, with straight lateral margins; ultimate (distal) segment slender, equal to penultimate segment, about 6–7 times as long as wide, tapering distally, with tufts of short simple stick-like setae along ventral and lateral margins, with several tufts of long setae dorsally.
Pereiopods I (Fig. 6b) equal in size and similar in shape, and similar in females and males, with stout, smooth and unarmed segments; coxa with well-developed slender epipodite and tuft of long simple setobranchs; basis about as long as wide, with well-developed exopodite overreaching carpo-meral articulation; ischium about 1.5 times longer than wide, with straight margins; merus slender, about 1.5–2 times as long as wide, equal to ischium and carpus, with straight margins; carpus tout, significantly widening distally, equal to merus, about as long as maximal width; palm relatively stout, about as long as wide, subcylindrical in cross-section, smooth; fingers relatively slender, subcylindrical, smooth, with blunt distal margins, about 2 times as long as proximal width, armed with a row of stout strong plumose setae distally.
Fig. 6.
Xiphocaridinella falcirostris Marin sp. n. from the Lower Shakuran Cave, Western Caucasus, male (a, f–h), female (b, c, d, e): a – pleopod I, b – pereiopod I, c – pereiopod II, d, f – pereiopod III, e, g, h – dactylus of pereiopod III.
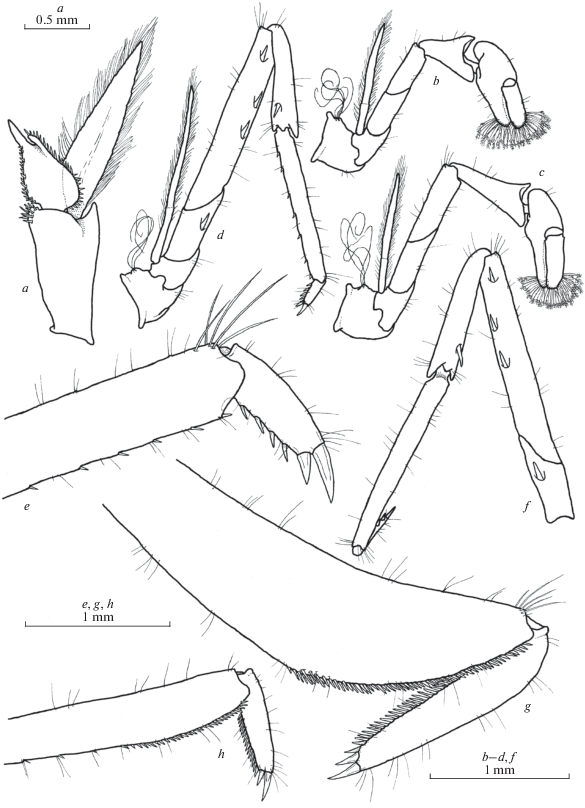
Pereiopods II (Fig. 6c) equal in size and similar in shape, similar in males and females and almost similar to pereiopod I, with stout, smooth and unarmed basal segments; coxa with well-developed slender epipodite and tuft of setobranchs; basis about as long as wide, with well-developed exopodite reaching carpo-meral articulation; ischium about 2 times as long as wide, with straight margins, unarmed; merus slender, about 2–2.5 times as long as wide, usually equal to ischium or lightly longer, with straight margins; carpus slender, about 2 times as long as maximal width, widening distally; palm stout, similar to palm of pereiopod I, about as long as wide, subcylindrical in cross-section, smooth; fingers relatively slender, subcylindrical, smooth tapering distally, about 2.5–3 times as long as proximal width, with simple and straight cutting edges, with broad blunt distal margin, armed with a row of stout strong plumose setae distally.
Pereiopod III in females (Figs. 6d) with rectangular coxa, about as long as wide, with tuft of long simple setobranchs, with small epipodite; basis about as long as wide, with well-marked exopodite almost reaching the midlength of merus; ischium about 1.5 times as long as wide, with well-marked distoventral spine; merus about 5 times as long as wide, with straight margins, with 2–3 well marked spines along ventral margin; carpus relatively slender, about 3 times as long as wide, slightly widening distally, about 2 times shorter than merus propodus, with bluntly projecting distodorsal margin overlapping carpo-propodal articulation, with 1 subdistal tooth or without it sometimes; propodus about 8 times as long as wide, with straight margins, armed with 5–7 small spines along proximal half of ventral margin; dactylus (Fig. 6e) about 2.5 times longer than wide, biunguiculate, ventral margin armed with 4–5 small spines, main unguis smooth, curved and sharp; accessory unguis triangular, sharp, larger than ventral teeth, about twice shorter than main unguis. Pereiopod III in males (Fig. 6f) with relatively slender segments; coxa rectangular, about as long as wide, with tuft of long simple setobranchs, with epipodite; basis about as long as wide, with well-marked exopodite overreaching the distal margin of ischium; ischium about 1.5 times as long as wide, with well-marked distoventral spine; merus about 6 times as long as wide, with straight margins, with 2–3 well marked spines along ventral margin; carpus relatively slender, about 4 times as long as wide, slightly widening distally, about twice shorter than merus and 1.5 times shorter than propodus, with bluntly projecting distodorsal margin slightly overlapping carpo-propodal articulation, with subdistal spine or without it sometimes; propodus about 7–8 times as long as wide, distal third of propodus widening and armed with a series of small spines along its ventral margin; dactylus (Fig. 6g) about 4 times as long as wide, with single unguis, ventral margin armed with small relatively stout sharp teeth, main unguis smooth, curved and sharp.
Pereiopod IV generally similar to pereiopod III; merus armed with 2 spines, carpus with or without subdistal spine; exopodite smaller than in pereiopod III. Pereiopod V generally similar to pereiopods III and IV, but without armature on basal segments and exopodite on basis; segments covered with simple setae dorsally and ventrally; propodus about 7–8 times as long as wide, with straight margins, armed with a series of small spines at distoventral angle (Fig. 6h); dactylus (Fig. 6h) about 3 times as long as wide, with ventral margin armed with a dense “brush” consisting of small simple sharp setae; without accessory unguis, main unguis curved, triangular, sharp distally.
Pleopods I and II in females normal, characteristic for the genus without specific differentiating features. Pleopod I in males with endopod bearing well marked appendix interna. Pleopod II in males (Fig. 6a) with well-developed appendix interna and appendix masculina (Fig. 5j); appendix interna with small cincinnuli distally; appendix masculina covered with numerous small sharp stout simple spines.
Uropods relatively stout, slightly exceeding telson; lateral margin of uropodal exopodite straight, with sharp triangular distolateral angle and large curved distolateral spine; dieresis simple, without spines.
Coloration. The body and appendages are transparent whitish and yellowish; the cornea of eyes albescent; the internal organs (gonads and hepatopancreas) are whitish or yellowish; numerous small transparent fat granules are visible through the carapace.
Body size. The largest collected female has pcl. 8.5 mm, tbl. 24.0 mm; the largest collected male has pcl. 7.0 mm, tbl. 20.0 mm.
GenBank accession numbers. MT066033–MT066038.
Taxonomic remarks. Xiphocaridinella falcirostris Marin sp. n. morphologically differs from Caucasian congeners by a specific dorsally armed and distally curved rostrum (Figs. 4; 5a–5d), which is a relatively constant morphological feature that allows distinguishing the new species from the congeners. The shape of rostrum is one of the most useful morphological features for distinguishing species within the Caucasian Xiphocaridinella (see Marin, 2017, 2017a; 2018). The closely related Caucasian Xiphocaridinella otapi, known from the Otap Cave (42°55′19.3″ N 41°32′19.6″ E), is recorded about 22 km south-east from the Lower Shakuran Cave (43°01′47.8″ N 41°20′02.0″ E), the type locality of Xiphocaridinella falcirostris Marin sp. n. (see Fig. 9). The COI mtDNA genetic marker demonstrates well-supported genetic divergence with high (99%) bootstrap node support (see Fig. 9). Moreover, Xiphocaridinella otapi can be clearly separated from the new species by a longer subulate rostrum, wide in its proximal part and thinning distally, exceeding the distal margin of the basal antennular segment (see Marin, 2018a: Fig. 9), which allow to separate this species even without genetic analysis. The possibility of morphological identification and allopatric distribution are the main criteria within the phenetic species concept (e.g., Ridley, 1993; Singh, 2012; Aldhebiani, 2018). Besides, genetic p-distances (4 ± 0.7% by COI mtDNA) and the current species distribution (see Fig. 9) suggest that the reason of the species divergence was the deepening of the Kodor riverbed, which dissected the karst habitat of the ancestral species (see Fig. 9) about 150.000–300.000 years ago (according to “molecular clocks” suggested by Knowlton et al. (1993), Knowlton, Weigt (1998), Jugovic et al. (2010, 2011, 2012) and Zakšek et al. (2019)).
Distribution. The species is known from an underground stream of the Lower Shakuran Cave (43°01′47.8″ N 41°20′02.0″ E) (the type locality) in the Amtkel karst system of the southwestern Caucasus, and the Besletka Cave (43°01′48.1″ N 41°04′36.0″ E), located in the Sukhumi region of Abkhazia. It is likely that these caves are connected and have a common watercourse.
Etymology. The new species is named after the unusual rostrum, which is crescent-shaped and distally curved downward (see Fig. 4); from Latin “falx, falcem” – crescent, sickle (Eng.)
Xiphocaridinella smirnovi Marin sp. n. (Figs 7, 8)
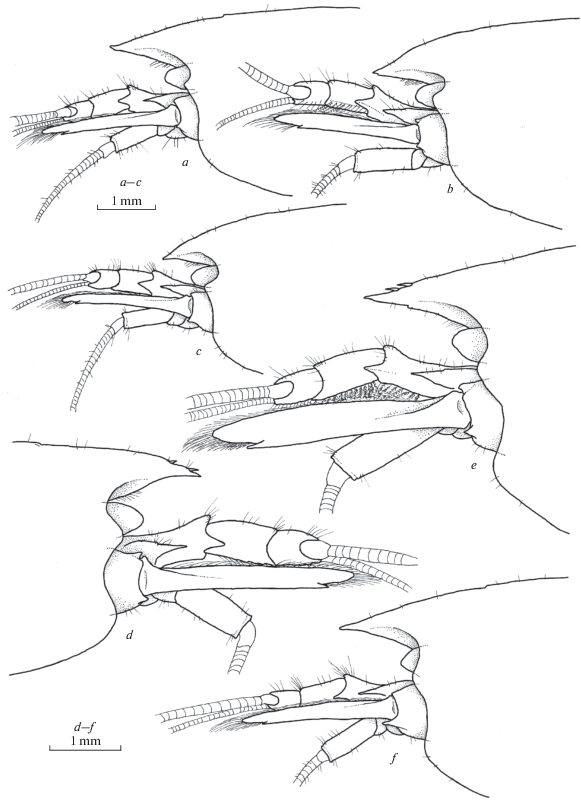
Fig. 7.
Xiphocaridinella smirnovi Marin sp. n., Besletka Cave, Abkhazia, Western Caucasus, female (a, b, e–g), male (c): a–c – front of carapace, d – antennula, e – antenna, f – telson, g – maxilliped III.
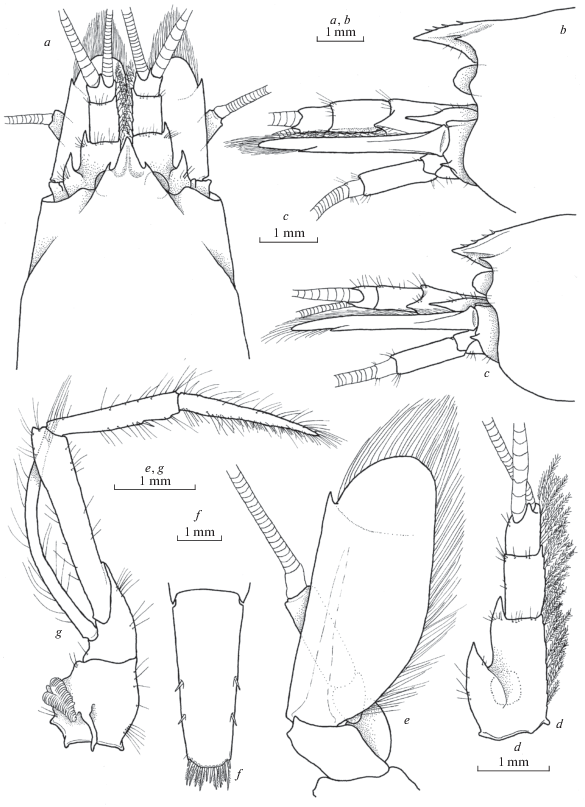
Fig. 8.
Xiphocaridinella smirnovi Marin sp. n., Besletka Cave, Abkhazia, Western Caucasus, female (a, b, e, f), male (c, d, g, h): a, c – pereiopod I; b, d – pereiopod II; e, g – pereiopod III; f, h – dactylus of pereiopod III.
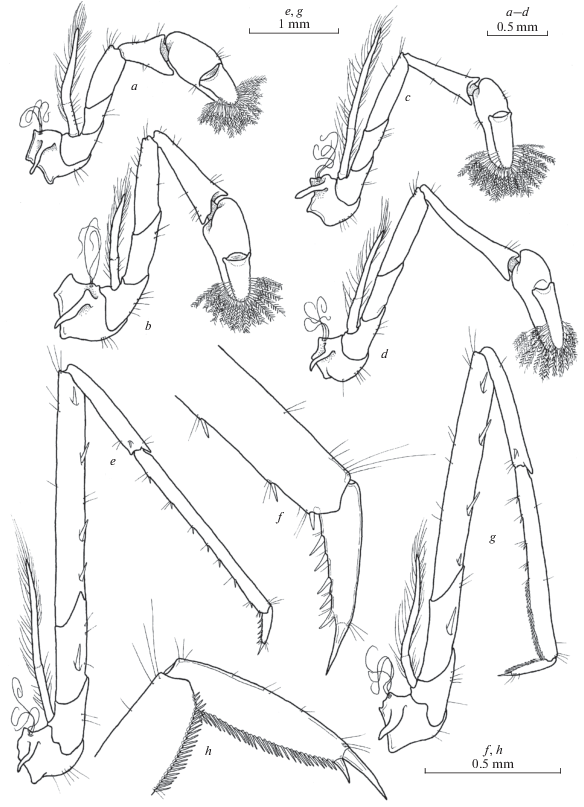
Fig. 9.
Phylogenetic reconstruction (based on COI mtDNA gene marker) using Bayesian (BA), Maximum–Likelihood (ML) and Neighbor-joining (NJ) analyses. The color insert indicates geographical map of distribution of Xiphocaridinella falcirostris Marin sp. n., Xiphocaridinella smirnovi Marin sp. n. and related taxa; black arrows indicate the direction of hypothetic subterranean water streams spreading shrimps.
urn:lsid:zoobank.org:act:2E12B46F-2A70-45EC-85D1-1A14D7EF8A4A
Material examined. Holotype, male, pcl. 6.5 mm, tbl. 20.0 mm (ZMMU Ma 6010), Abkhazia, Sukhumi region, Besletka Cave, 43°01′48.1″ N 41°04′36.0″ E, about 68–70 m above sea level, collected with net in a stream flowing from the cave entrance in the period of high water, coll. Marin and Sinelnikov, 25 May 2017; damage female, pcl. 4.5 mm, tbl. 16.0 mm (LEMMI), same locality and data as holotype.
Description. Small-sized shrimp with swollen, smooth, subcylindrical body. Carapace swollen, smooth, with small dorsal rostral carina in its frontal part (Fig. 7a–7c), with well-marked supraorbital tooth. Rostrum (Fig. 7a–7c) short, reaching the distal margin of basal antennular segment, triangular in shape, armed with 4 small spines, unarmed ventrally, broad proximally.
Abdominal somites smooth, unarmed; tergites non-carinate, without dorsal lobes, not posteriorly produced; pleura of pleomeres I–IV posteroventrally and ventrally rounded; pleomere V small, with feebly developed pleura, rounded posterodistally; distal and distoventral margins of pleomere VI rounded posterodorsally. Telson (Fig. 7j) relatively slender, smooth, about 2.5 times as long as wide proximally, expanded distally, with 2 pairs of slender dorsal spines, situated at about 0.5 and 0.7 of telson length, respectively; distal margin convex, with 4 or 5 pairs of distal spines, including a pair of short lateral spines, a pair of long intermediate spines and 2–3 pairs of slender medial marginal spines.
Eyes (Fig. 7a–7c) partly reduced, subcylindrical, partly concealed by carapace; cornea reduced and feebly marked, without pigment; eyestalk stout, swollen, cylindrical, about as long as wide.
Antennula (Fig. 7d) with robust basal segment, about twice longer than proximal width, without ventromedial tooth, distolateral angle with broadly produced sharp projection; stylocerite stout, sharpening distally, overreaching the midlength of basal segment; second and third segments stout, unarmed; second segment about 1.5 times as long as wide and about 1.5 times as long as distal segment; distal segment about as long as wide, unarmed.
Antenna (Fig. 7e) with basicerite stout, about as long as wide, with distolateral margin unarmed; carpocerite robust, about 3 times as long as wide, reaching the midlength of scaphocerite; scaphocerite well developed, broad, with well-marked distolateral tooth, lamella bluntly rounded distally.
Maxilliped III (Fig. 7g) with slender segments; epipodite stout, bluntly rounded; arthrobranch small, reduced; exopodite slender, slightly overreaching the distal margin of antepenultimate segment; antepenultimate segment slender, about 5–6 times as long as wide; penultimate segment about 5–6 times as long as wide, with straight lateral margins; ultimate (distal) segment slender, equal to penultimate segment, about 6–7 times as long as wide, tapering distally, with tufts of short simple stick-like setae along ventral and lateral margins, with several tufts of long setae dorsally.
Pereiopods I (Fig. 8a, 8c) equal in size and similar in shape, but differ in collected specimens by proportions (such difference was also described for Xiphocaridinella jusbaschjani (see Marin, Sokolova, 2013); coxa with well-developed slender epipodite and tuft of long simple setobranchs; basis about as long as wide, with well-developed exopodite overreaching carpo-meral articulation; ischium about 1–1.5 times longer than wide, with straight margins; merus slender, about 2–3 times as long as wide, equal to ischium; carpus about as 1–2.5 times as long as maximal width, significantly widening distally, equal to merus,; palm relatively stout, about as long as wide, subcylindrical in cross-section, smooth; fingers relatively slender, subcylindrical, smooth, with blunt distal margins, about 1.5–2.5 times as long as proximal width, armed with a row of stout strong plumose setae distally.
Pereiopods II (Fig. 8b, 8d) equal in size and similar in shape, similar in males and females and almost similar to pereiopod I and also slightly differ in collected specimens by proportions; coxa with well-developed slender epipodite and tuft of setobranchs; basis about as long as wide, with well-developed exopodite overreaching carpo-meral articulation; ischium about 1.5 times as long as wide, with straight margins, unarmed; merus slender, about 3 times as long as wide, longer than ischium but shorter than carpus, with straight margins; carpus slender, about 2.5–5 times as long as maximal width, widening distally, longer than merus; palm stout, similar to palm of pereiopod I, about as long as wide, subcylindrical in cross-section, smooth; fingers relatively slender, subcylindrical, smooth tapering distally, about 1.5–2.5 times as long as proximal width, with simple and straight cutting edges, with broad blunt distal margin, armed with a row of stout strong plumose setae distally.
Pereiopod III in females (Fig. 8e) with rectangular coxa, about as long as wide, with tuft of long simple setobranchs, with small epipodite; basis about as long as wide, with well-marked exopodite overreaching the ischium-merus articulation; ischium about 1.5 times as long as wide, with well-marked distoventral spine; merus about 5 times as long as wide, with straight margins, with 4 well marked spines along ventral margin; carpus relatively slender, about 4–5 times as long as wide, slightly widening distally, about 2 times shorter than merus and propodus, with bluntly projecting distodorsal margin protruding carpo-propodal articulation, with 1 subdistal spine; propodus about 8–10 times as long as wide, with straight margins, armed with 7–8 spines along ventral margin; dactylus (Fig. 8f) about 2–2.5 times longer than wide, biunguiculate, ventral margin armed with 4–5 spines, main unguis smooth, curved and sharp; accessory unguis small, triangular, sharp, equal or slightly larger than ventral teeth, about twice shorter than main unguis. Pereiopod III in males (Fig. 8g) with relatively slender segments; coxa rectangular, about as long as wide, with tuft of long simple setobranchs, with epipodite; basis about as long as wide, with well-marked exopodite overreaching the ischium-merus articulation; ischium about 1.5 times as long as wide, with well-marked distoventral spine; merus about 5–6 times as long as wide, with straight margins, with 4 well marked spines along ventral margin; carpus relatively slender, about 4 times as long as wide, slightly widening distally, about 2 times shorter than merus and propodus, with bluntly projecting distodorsal margin slightly protruding carpo-propodal articulation, with subdistal spine; propodus about 8–9 times as long as wide, with straight margins, distal third of propodus widening and armed with a series of small spines along its ventral margin; dactylus (Fig. 8h) about 2.5–3 times as long as wide, with single unguis, ventral margin armed with small relatively stout sharp teeth, main unguis smooth, curved and sharp.
Pereiopod IV generally similar to pereiopod III, similar in males and females; merus armed with 2–3 spines, carpus with subdistal spine or without it; exopodite smaller than in pereiopod III, not reaching the midlength of ischium. Pereiopod V generally similar to pereiopods III and IV, similar in male and females, without armature on basal segments and exopodite on basis; segments covered with simple setae dorsally and ventrally; propodus about 8–9 times as long as wide, with straight margins, armed with 7–8 long spines along ventral margin and pair of long slender spines at distoventral angle; ventral margin of dactylus armed with a dense “brush” consisting of small simple sharp setae; without accessory unguis, main unguis curved, triangular, sharp distally.
Pleopods I and II in females normal, characteristic for the genus without specific differentiating features. Pleopod I in males with endopod bearing well marked appendix interna. Pleopod II in males characteristic for the genus, with well-developed appendix interna and appendix masculina; appendix interna with small cincinnuli distally; appendix masculina covered with numerous small sharp stout simple spines.
Uropods relatively stout, slightly exceeding telson; lateral margin of uropodal exopodite straight, with sharp triangular distolateral angle and large curved distolateral spine; dieresis simple, without spines.
Coloration. Body and appendages of shrimps are transparent whitish and yellowish; cornea of eyes albescent; internal organs (gonads and hepatopancreas) are whitish or yellowish; numerous small transparent fat granules can be seen through carapace.
GenBank accession numbers. MT066032.
Taxonomic remarks. The new species is mostly phylogenetically close to Xiphocaridinella otapi and Xiphocaridinella shurubumu (see Fig. 9), also having a relatively short rostrum that slightly extends beyond the distal margin of the basal antennular segment (see Marin, 2018, 2018a). At the same time, the new species can be clearly separated from these species by the presence of several dorsal rostral spines compared to the mostly unarmed rostrum in Xiphocaridinella otapi and Xiphocaridinella shurubumu. The dorsal spines on telson in Xiphocaridinella smirnovi Marin sp. n. are situated at about 0.5 and 0.7 of telson length vs about 0.4 and 0.6 in Xiphocaridinella otapi and 0.25 and 0.5 of telson length in Xiphocaridinella shurubumu.
Distribution. The species is known only from an underground stream of the Besletka Cave (43°01′48.1″ N 41°04′36.0″ E), located in the Sukhumi region of Abkhazia, Western Caucasus.
Etymology. The new species is named in honor of Dr. Nikolay Nikolaevich Smirnov (07.01.1928– 20.11.2019), one of the greatest carcinologists of the XX century, the founder of the Russian school of Cladoceran studies, which currently occupies a leading position in world science.
Список литературы
Aldhebiani A.Y., 2018. Species concept and speciation // Saudi Journal of Biological Sciences. V. 25. № 3. P. 437–440. https://doi.org/10.1016/j.sjbs.2017.04.013
Amelichev G.N., Vakhrushev B.A., Dublyansky V.N., 2007. Hydrodynamics and evolution of speleomorphogenesis of the Amtkel karst system (Western Abkhazia) // Geopolitica i Ecogeodynamika Regionov. V. 2. P. 52–60 [in Russian].
Avise J.C., 1993. Molecular markers. Natural History and Evolution. New York: Chapman & Hall. 511p.
Barjadze S., Murvanidze M., Arabuli T., Mumladze L., Pkhakadze V., Djanashvili R., Salakaia M., 2015. Annotated list of invertebrates of the Georgian karst caves. Tbilisi: Georgian Academic Book. 119 p.
Birštein Y.A., 1939. About cave shrimps of Abkhazia // Zoologichesky Zhurnal. V. 18. P. 960–974 [in Russian].
Birštein Y.A., 1948. [The occurrence of the cave shrimp Troglocaris in underground water of Mazesta and related problems] // Byulleten’ Moskovskogo Obshchestva Ispytatelei Prirody. Otdel Biologicheskii. Moscow. V. 53. P. 3–10 [in Russian].
Birštein Y.A., Lopashov G.V., 1940. Biospeologica Sovietica 1. Investigation of the cave fauna in USSR in 1935–1939 // Bulletin de la Société impériale des naturalistes de Moscou, Section Biologique. V. 49. № 3/4. P. 29–38 [in Russian].
Cobolli Sbordoni M., Mattoccia M., LaRossa G., DeMatthaeis E., Sbordoni V., 1991. Secondary sympatric occurrence of sibling species of subterranean shrimps in the Karst // International Journal of Speleology. V. 19. P. 9–27. https://doi.org/10.5038/1827-806X.19.1.2
Folmer O., Black M., Hoeh W., Lutz R., Vrijenhoek R., 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates // Molecular Marine Biology & Biotechnology. V. 3. P. 294–299.
Guzik M.T., Cooper S.J.B., Humphreys W.F., Ong S., Kawakami T., Austin A.D., 2011. Evidence for population fragmentation within a subterranean aquatic habitat in the Western Australian desert // Heredity. V. 107. № 3. P. 215–230. https://doi.org/10.1038/hdy.2011.6
Hebert P.D.N., Cywinska A., Ball S.L., De Waard J.R., 2003. Biological identifications through DNA barcodes // Proceedings of the Royal Society of London B. V. 270. P. 313–322. https://doi.org/10.1098/rspb.2002.2218
Jugovic J., Jalžić B., Prevorčnik S., Sket B., 2012. Cave shrimps Troglocaris s. str. (Dormitzer, 1853), taxonomic revision and description of new taxa after phylogenetic and morphometric studies // Zootaxa. V. 3421. P. 1–31. https://doi.org/10.11646/zootaxa.3421.1.1
Jugovic J., Prevorčnik S., Aljančič G., Sket B., 2010. The atyid shrimp (Crustacea: Decapoda: Atyidae) rostrum: phylogeny versus adaptation, taxonomy versus trophic ecology // Journal of Natural History. V. 44. P. 2509–2533. https://doi.org/10.1080/00222933.2010.502258
Jugovic J., Prevorčnik S., Blejec A., Sket B., 2011. Morphological differentiation in the cave shrimps Troglocaris (Crustacea: Decapoda: Atyidae) of the Dinaric karst – a consequence of geographical isolation or adaptation? // Journal of Zoological Systematic and Evolutionary Research V. 49. № 3. P. 185–195. https://doi.org/10.1111/j.1439-0469.2010.00611.x
Juzbaš’jan S.M., 1940. [On a cave shrimp from Shakuran] // Trudy Biologiceskoj Stancii Narkomprosa Gruzinskoy SSR. V. 1. P. 73–86 [in Russian].
Juzbaš’jan S.M., 1941. [On the subspecies and intraspecific differentiation in Troglocaris kutaissiana Sadowsky. Communication I] // Soobshcheniya Akademii Nauk Gruzinskoy SSR. V. 2. № 10. P. 929–935 [in Russian].
Knowlton N., Weigt L.A., 1998. New dates and new rates for divergence across the Isthmus of Panama // Proceedings of the Royal Society of London, Series B. V. 265. P. 2257–2263. https://doi.org/10.1098/rspb.1998.0568
Knowlton N., Weight L.A., Solorzano L.A., Mills D.K., Bermingham E., 1993. Divergence in proteins, mitochondrial DNA and reproductive compatibility across the Isthmus of Panama // Science. V. 260. P. 1629–1632. https://doi.org/10.1126/science.8503007
Lefébure T., Douady C.J., Gouy M., Trontelj P., Briolay J., Gibert J., 2006. Phylogeography of a subterranean amphipod reveals cryptic diversity and dynamic evolution in extreme environments // Molecular Ecology. V. 15. P. 1797–806. https://doi.org/10.1111/j.1365-294X.2006.02888.x
Lefébure T., Douady C.J., Malard F., Gibert J., 2007. Testing dispersal and cryptic diversity in a widely distributed groundwater amphipod (Niphargus rhenorhodanensis) // Molecular Phylogenetics and Evolution. V. 42. P. 676–686. https://doi.org/10.1016/j.ympev.2006.08.020
Marin I., 2017. Troglocaris (Xiphocaridinella) kumistavi sp. nov., a new species of stygobiotic atyid shrimp (Crustacea: Decapoda: Atyidae) from Kumistavi Cave, Imereti, Western Georgia, Caucasus // Zootaxa. V. 4311. P. 576–588. https://doi.org/10.11646/zootaxa.4311.4.9
Marin I.N., 2017a. COXI based phylogenetic analysis of Caucasian clade of European Troglocaris s. l. (Crustacea: Decapoda: Atyidae) with the suggestion of a new taxonomic group structure // Biosystems Diversity. V. 25. P. 323–327. https://doi.org/10.15421/011749
Marin I., 2018. Cryptic diversity of stygobiotic shrimp genus Xiphocaridinella Sadowsky, 1930 (Crustacea: Decapoda: Atyidae): the first case of species co-occurrence in the same cave system in the Western Caucasus // Zootaxa. V. 4441. P. 201–224. https://doi.org/10.11646/zootaxa.4441.2.1
Marin I., 2018a. Xiphocaridinella shurubumu Marin sp. n. (Crustacea: Decapoda: Atyidae) – a new stygobiotic atyid shrimp species from Shurubumu and Mukhuri caves, Chkhorotsku, Western Georgia, Caucasus // Zoologichesky Zhurnal. V. 97. № 10. P. 1238–1256. https://doi.org/10.1134/s0044513418100082
Marin I.N., 2019. Crustacean “cave fishes” from the Arabika karst massif (Abkhazia, Western Caucasus): new species of stygobiotic crustacean genera Xiphocaridinella and Niphargus from the Gegskaya Cave and adjacent area // Arthropoda Selecta. V. 28. № 2. P. 225–245. https://doi.org/10.15298/arthsel.28.2.05
Marin I.N., 2019a. A new stygobiotic Xiphocaridinella (Crustacea: Decapoda: Atyidae) from the Motena Cave, Samegrelo-Zemo Svaneti region of Georgia, Caucasus // Zootaxa. V. 4648. P. 592–600. https://doi.org/10.11646/zootaxa.4648.3.12
Marin I.N., Sinelnikov S.Y., 2017. Preliminary data on larval development of Caucasian cave-dwelling shrimp Troglocaris (Xiphocaridinella) kumistavi Marin, 2017 (Crustacea: Decapoda: Atyidae) // Arthropoda Selecta. V. 26. № 4. P. 297–302. https://doi.org/10.15298/arthsel.26.4.03
Marin I., Sokolova A., 2014. Redescription of the stygobiotic shrimp Troglocaris (Xiphocaridinella) jusbaschjani Birštein, 1948 (Decapoda: Caridea: Atyidae) from Agura River, Sochi, Russia, with remarks on other representatives of the genus from Caucasus // Zootaxa. V. 3754. P. 277–298. https://doi.org/10.11646/zootaxa.3754.3.3
Ridley M., 1993. Evolution // Journal of Evolutional Biology. V. 6. P. 615–617.
Sadowsky A.A., 1930. [Xiphocaridinella kutaissiana nov. gen. et sp. (Fam. Atyidae) aus einer unterirdischen Höhle bei Kutais] // Zakavkazskij kraevedstenny sbornik naučnoissledovatel’nogo kraevedstvenogo kabineta Universiteta Tiflis. V. 1. P. 93–104 [in Russian].
Singh B.N., 2012. Concepts of species and modes of speciation // Current Science. V. 103. P. 784–790. https://doi.org/www.jstor.org/stable/24088835
Sites J.W., Marshall J.C., 2004. Operational criteria for delimiting species // Annual Review of Ecology, Evolution and Systematics. V. 35. P. 199–227. https://doi.org/10.1146/annurev.ecolsys.35.112202.130128
Sket B., 1994. Distribution patterns of some subterranean Crustacea in the territory of the former Yugoslavia // Hydrobiologia. V. 287. P. 65–75. https://doi.org/10.1007/BF00006897
Sket B., Zakšek V., 2009. European cave shrimp species (Decapoda: Caridea: Atyidae), redefined after a phylogenetic study; redefinition of some taxa, a new genus and four new Troglocaris species // Zoological Journal of the Linnaean Society. V. 155. P. 786–818. https://doi.org/10.1111/j.1096-3642.2008.00473.x
WoRMS, 2019. Xiphocaridinella osterloffi Juzbasjan, 1940. [Electronic resource]. Accessed at: http://www.marinespecies.org/aphia.php?p=taxdetails&id=1261947. Updated: 2018-06-29.
Zakšek V., Sket B., Trontelj P., 2007. Phylogeny of the cave shrimp Troglocaris: evidence of a young connection between Balkans and Caucasus // Molecular Phylogenetics and Evolution. V. 42. P. 223–235. https://doi.org/10.1016/j.ympev.2006.07.009
Zakšek V., Delić T., Fišer C., Jalžić B., Trontelj P., 2019. Emergence of sympatry in a radiation of subterranean amphipods // Journal of Biogeography. V. 1. P. 1–13. https://doi.org/10.1111/jbi.13514
Дополнительные материалы отсутствуют.
Инструменты
Зоологический журнал


