Зоологический журнал, 2020, T. 99, № 3, стр. 261-274
Morphology and Phylogenetic Position of Two Microphalloid Trematode Species, Parasites of the Vesper Bat Pipistrellus kuhlii in the Lower Volga Region of Russia
S. G. Sokolov a, *, S. V. Shchenkov b, A. P. Kalmykov c, A. D. Smirnova b
a Severtsov Institute of Ecology and Evolution, Russian Academy of Science
Moscow 119071, Russia
b Saint-Petersburg State University
St. Petersburg 199034, Russia
c Astrakhan Nature Reserve
Astrakhan’ 414021, Russia
* E-mail: sokolovsg@mail.ru
Поступила в редакцию 9.04.2019
После доработки 6.07.2019
Принята к публикации 10.11.2019
Аннотация
Two species of microphalloid trematodes, Parabascus oppositus and Lecithodendrium сf. skrjabini, were found in the Vesper bat, Pipistrellus kuhlii, caught in the Astrakhan Oblast’, Lower Volga region, Russia. A morphological revision of a paratype of P. oppositus, as well as a study of the specimens collected during this survey indicate this species to be devoid of a cirrus-sac. Instead, the species has a convoluted seminal vesicle, a tubular pars prostatica surrounded by an extensive field of prostatic cells, and a short ejaculatory duct. These structures lie freely in the parenchyma. The genital pore is submedian and is situated at the level of the ventral sucker. The body length of Lecithodendrium cf. skjabini differs from that of L. skjabini sensu stricto. A phylogenetic analysis using 28S rDNA unites P. oppositus together with G. amphoraeformis and Gyrabascus sp. in the Gyrabascus spp. clade (Pleurogenidae). On this basis, P. oppositus has been included into the genus Gyrabascus, thus correcting its previous diagnosis. Lecithodendrium cf. skjabini is clustered with other members of the genus Lecithodendrium. The monophyly of the Lecithoodendriidae is confirmed. Our results support the integration of this family together with Microphallidae, Phaneropsolidae, and Stomylotrematidae into a large clade. In the present study, we show the paraphyly of the family Pleurogenidae and the monophyly of the Pleurogenidae + Collyriclidae group.
Bats are an extraordinary group of mammals. Their prominent feature is the ability to fly. Bats are also unique among mammals as they possess a diverse assemblage of host-specific helminths (Khotenovsky, 1972).
Fourteen species of bats are now recorded in the Lower Volga region of Russia in southeastern Europe (Zavialov, 2009; Smirnov et al., 2018). Some of these species have recently appeared in the region as a result of habitat expansion to the north. Kuhl’s pipistrelle Pipistrellus kuhlii (Kuhl 1817) appeared in this region in the 20th century, as recent as the 1960s (Strelkov et al., 1985), apparently originating from the southeastern Transcaucasus (Strelkov, Ilyin, 1990). Limited data exists on the helminth fauna of bats inhabiting the Lower Volga region (Dubinin, 1952; Kurochkin, Kurochkina, 1962; Ivanov et al., 2012), and no information is available on helminths infecting Kuhl’s pipistrelle.
During the parasitological investigation of Kuhl’s pipistrelle in this region two trematode species were observed, Parabascus oppositus Zdzitowiecki 1969 and Lecithodendrium cf. skrjabini Mazaberidse 1963. Parabascus oppositus was originally described from the serotine bat, Eptesicus serotinus (Schreder 1774), captured near Warsaw (Zdzitowiecki, 1969). According to Zdzitowiecki (1969), the first specimens of this species were found in the common bent-wing bat Miniopterus schreibersi Kuhl 1817 from the former Czhechoslovakia by Mituch (1965), who mistakenly identified them as P. semisquamosum Braun 1900. Otherwise, no other records exist for this species. Khotenovsky (1985) synonymized P. oppositus with Parabascus lepidotus Looss 1907, but in his work there are no appropriate explanations for this synonymization. Sharpilo et al. (1989) did not include P. oppositus in the list of P. lepidotus synonyms. Niewiadomska and Pojmańska (2018) considered P. oppositus and P. lepidotus as a separate species. Morphological and molecular phylogenetic investigation of the P. oppositus specimens collected for this study, as well as the re-examination of the paratype, facilitates the revision the genus affiliation of this species, resulting in the transfer to the genus Gyrabascus Macy 1935.
Lecithodendrium skrjabini was described from Nathusius’ pipistrelle, Pipistrellus nathusii (Keyserling et Blasius 1839), captured in the Caucasius in western Georgia (Matsaberidze, 1963). Two more records exist for this species, in addition to the first description (Matsaberidze, Khotenovsky, 1967; Kirillov et al., 2012). The specimens collected during this investigation are assigned to L. skrjabini, however some doubts remain and are discussed below.
This paper provides the description, drawings, and results of molecular phylogenetic analysis of Gyrabascus oppositus comb. n. and Lecithodendrium cf. skrjabini.
MATERIALS AND METHODS
Specimen collection
Trematodes were collected from six Kuhl’s pipistrelles specimens; five captured on the 18th September, 2014 and one on the 29th May, 2018 in the Kalinino village (46°20′ N, 48°52′ E), Astrakhan Oblast, Russia (Lower Volga region). Adult worms were collected for morphological study and relaxed in freshwater at room temperature, fixed in 70% ethanol, and subsequently stained with acetocarmine. All measurements were made in micrometers unless otherwise indicated. Mean values are provided in parentheses. Specimens for genetic analysis were fixed in 96% ethanol and stored at +4°C.
Paratype examination
Zdzitowiecki (1969) has described P. oppositus based on holotype and several paratypes. Holotype deposit location remains unknown (according to personal communication by Dr. R. Salamatin). A set of horizontal optical sections of body region between the intestinal bifurcation and the testes of P. oppositus paratype were studied from photographs. The paratype is deposited in the Natural History Museum, London (Parasites & Vectors Division, Department of Life Sciences, cat. # 1992.12.3.25) (Natural History Museum, 2014). Photos were kindly provided by Dr. Rodney Bray.
DNA extraction, amplification, sequencing, and phylogenetic analysis
To obtain LSU sequences, DNA was isolated from one G. oppositus specimen and one Lecithodendrium cf. skrjabini specimen using Chelex-100 and Proteinase K. The D1-D3 regions of 28S rDNA (up to 1,200 bp) were amplified with primer pairs 28Sy/28Sz (Hillis, Dixon, 1991) for Lecithodendrium cf. skrjabini and LSU5/1500R (Tkach et al., 2003) for G. oppositus. Amplicons were purified using a Cleanup mini Purification Kit (Eurogene) and sequenced on the ABI-Prism 3500xl using the same primers.
Newly generated sequences were deposited in GenBank (www.ncbi.nlm.nih.gov) as follows: G. oppositus – MK575195; Lecithodendrium cf. skrjabini – MK575196. The sequences were mounted in general alignment with 61 species (Tkach et al., 2000, 2001, 2003; Olson et al., 2003; Al-Kandari et al., 2011; Galaktionov et al., 2012; Lord et al., 2012; Heneberg, Literák, 2013; Boyce et al., 2014; Presswell et al., 2014; Kanarek et al., 2014, 2015, 2017; Kudlai et al., 2015, 2016; Hernandez-Orts et al., 2016; Bell et al., 2018; Galaktionov, Blasco-Costa, 2018) (Table 1). First, sequences were automatically aligned using the Muscle algorithm (Edgar, 2004) as implemented in SeaView 4.0 (Gouy et al., 2010), and then the alignment was adjusted manually. The final length of alignment was 1267 bp. The phylogenetic analysis was performed using the Maximum Likelihood method as implemented in the PhyML program (Guindon, Gascuel, 2003), with the GTR + G + I model, as suggested by the Modeltest program (Posada, Crandall, 1998). The stability of clades was assessed using a non-parametric bootstrap with 1,000 pseudoreplicates. Bayesian analysis was performed using MrBayes 3.2.6 at the CIPRES Science Gateway (Miller et al., 2010). Trees were run as two separate chains (default heating parameters) for 15 million generations, by which time they had ceased converging: the final average standard deviation of the split frequencies was less than 0.01. The quality of chains was estimated using built-in MrBayes tools and using the Tracer 1.6 package (Rambaut et al., 2014). Based on the estimates by Tracer, the first 7,000 generations were discarded for burn-in.
Table 1.
List of species, incorporated into molecular analysis
| Species | Host species | Geographical region | GenBank accession number | Reference |
|---|---|---|---|---|
| Collyriclidae | ||||
| Collyriclum faba (Bremser in Schmalz 1831) | Saxicola rubetra (Linnaeus 1758) (Aves; Muscicapidae) | OrlickéZáhoří, Chech Republic | JQ231122 | Heneberg, Literák, 2013 |
| Lecithodendriidae | ||||
| Lecithodendrium linstowi Dollfus 1931 | Nyctalus noctula (Schreber 1774) (Mammalia; Vespertilionidae) | Sumy Region, Ukraine | AF151919 | Tkach et al., 2000 |
| Lecithodendrium sp. | Bithynia tentaculata (Linnaeus 1758) (Gastropoda; Bithyniidae) | Curonian lagoon, Lithuania | KJ126726 | Kudlai et al., 2015 |
| Lecithodendrium spathulatum (Ozaki 1929) | Pipistrellus pipistrellus (Schreber 1774) (Mammalia; Vespertilionidae) | England | JF784193 | Lord et al., 2012 |
| Ophiosacculus mehelyi (Mödlinger 1930) | Eptesicus serotinus (Schreber 1774) (Mammalia; Vespertilionidae) | Ukraine | AF480167 | Tkach V.V., direct submission |
| Paralecithodendrium chilostomum Mehlis 1931 | N. noctula | Sumy Region, Ukraine | AF151920 | Tkach et al., 2000 |
| Paralecithodendrium hurkovaae Dubois 1960 | Myotis daubentoni (Kuhl 1817) (Mammalia; Vespertilionidae) | Kiev Region, Ukraine | AF151922 | Tkach et al., 2000 |
| Paralecithodendrium longiforme (Bhalerao 1926) | M. daubentoni | Kiev Region, Ukraine | AF151921 | Tkach et al., 2000 |
| Paralecithodendrium parvouterus (Bhalerao 1926) | Miniopterus schreibersi (Kuhl 1817) (Mammalia; Miniopteridae) | Rubielos de Mora, Spain | AY220617 | Tkach et al., 2003 |
| Paralecithodendrium sp. | P. pipistrellus | England | JF784196 | Lord et al., 2012 |
| Pycnoporus heteroporus (Dujardin 1845) | Pipistrellus kuhli (Kuhl 1817) (Mammalia; Vespertilionidae) | Kherson Region, Ukraine | AF151918 | Tkach et al., 2000 |
| Pycnoporus megacotyle (Ogata 1939) | P. kuhli | Kherson Region, Ukraine | AF151917 | Tkach et al., 2000 |
| Microphallidae | ||||
| Floridatrema heardi Kinsella et Deblock 1994 | Oryzomys palustris (Harlan 1837) (Mammalia; Cricetidae) | Florida, USA | AY220632 | Tkach et al., 2003 |
| Levinseniella sp. | Somateria mollissima (Linnaeus 1758) (Aves; Anatidae) | Sea of Okhotsk, Skipper Creek, Russia | MG783585 | Galaktionov, Blasco-Costa, 2018 |
| Longiductotrema tethepae Kudlai, Cribb et Cutmore 2016 | Gymnothorax pseudothyrsoideus (Bleeker 1852) (Actinopterygii; Muraenidae) | Lizard Island, Queensland, Australia | KX712085 | Kudlai et al., 2016 |
| Maritrema arenaria Hadley et Castle 1940 | Barnacle | United Kingdom | AY220629 | Tkach et al., 2003 |
| Maritrema brevisacciferum Shimazu et Pearson 1991 | Posticobia brazieri (Smith 1882) (Gastropoda; Hydrobiidae) | Moggil Creek, Australia | KT355819 | Kudlai et al., 2015 |
| Maritrema corai Hernández-Orts, Pinacho-Pinacho, García-Varela et Kostadinova 2016 | Eudocimus albus (Linnaeus 1758) (Aves; Threskiornithidae) | Mexico | KT880222 | Hernandez-Orts et al., 2016 |
| Maritrema deblocki Presswell, Blasco-Costa et Kostadinova 2014 | Anas platyrhynchos Linnaeus 1758 (Aves; Anatidae) | New Zealand | KJ144173 | Presswell et al., 2014 |
| Maritrema eroliae Yamaguti 1939 | Clypeomorus bifasciatus (Sowerby II 1855) (Gastropoda; Cerithiidae) | Kuwait | JF826247 | Al-Kandari et al., 2011 [as Maritrema cf. eroliae] |
| Maritrema novaezealandense Martorelli, Fredensborg, Mouritsen et Poulin 2004 | Zeacumantus subcarinatus (Sowerby II 1855) (Gastropoda; Batillariidae) | New Zealand | KJ144178 | Presswell et al., 2014 |
| Maritrema oocysta Lebour 1907 | Peringia ulvae (Pennant 1777) (=Hydrobia ulvae (Pennant 1777) (Gastropoda; Hydrobiidae) | United Kingdom | AY220630 | Tkach et al., 2003 |
| Maritrema poulini Presswell, Blasco-Costa et Kostadinova 2014 | Paracorophium excavatum (Thomson 1884) (Malacostraca; Corophiidae) | New Zealand | KJ144177 | Presswell et al., 2014 |
| Maritrema prosthometra Deblock et Heard 1969 | O. palustris | USA | AY220631 | Tkach et al., 2003 |
| Maritrema subdolum Jägerskiöld 1909 | P. ulvae | Kandalaksha Bay, White Sea, Russia | HM584135 | Galaktionov et al., 2012 |
| Microphallus abortivus Deblock 1974 | P. ulvae | United Kingdom | AY220626 | Tkach et al., 2003 |
| Microphallus basodactylophallus (Bridgman 1969) | O. palustris | USA | AY220628 | Tkach et al., 2003 |
| Microphallus calidris Belopolskaia et Ryjikov 1963 | Littorina sitkana Philippi 1846 (Gastropoda; Littorinidae) | Kunashir, KurilIslands, Russia | HM584125 | Galaktionov et al., 2012 |
| Microphallus fusiformis Reimer 1963 | P. ulvae | United Kingdom | AY220633 | Tkach et al., 2003 [as Microphallidae gen. sp.] |
| Microphallus minutus Johnston 1948 | P. brazieri | Queensland, Churchbank Weir, Australia | KT355823 | Kudlai et al., 2015 |
| Microphallus ochotensis Galaktionov et Blasco-Costa 2018 | Falsicingula kurilensis (Pilsbry 1905) (Gastropoda; Falsicingulidae) | Sea of Okhotsk, Skipper Creek, Russia | MG783589 | Galaktionov, Blasco-Costa, 2018 |
| Microphallus piriformes Galaktionov 1983 | Littorina saxatilis (Olivi 1792) (Gastropoda; Littorinidae) | Vaygach Island, SE Barents Sea, Russia | HM584123 | Galaktionov et al., 2012 |
| Microphallus pseudopygmaeus Galaktionov 2009 | L. saxatilis | Kandalaksha Bay, White Sea, Russia | HM584127 | Galaktionov et al., 2012 |
| Microphallus pygmaeus (Levinsen 1881) | L. saxatilis | Kandalaksha Bay, White Sea, Russia | HM584134 | Galaktionov et al., 2012 |
| Microphallus similis (Jägerskiöld 1900) | Larus schistisagus Stejneger 1884 (Aves; Laridae) | Impoveem, Sea of Okhotsk, Russia | HM584138 | Galaktionov et al., 2012 |
| Microphallus triangulatus Galaktionov 1984 | Somateria mollissima v-nigrum Bonaparte 1855 (Aves; Anatidae) | Yamskaya Bay, Sea of Okhotsk, Russia | HM584139 | Galaktionov et al., 2012 |
| Microphallus sp. | Littorina natica Reid 1996 (Gastropoda; Littorinidae) | Bering Sea, Chukotka, Russia | HM584129 | Galaktionov et al., 2012 |
| Microtrema barusi Sitko 2013 | Prunella modularis (Linnaeus 1758) (Aves; Prunellidae) | Czech Republic | KJ700421 | Kanarek et al., 2014 |
| Pachypsolidae | ||||
| Pachypsolus irroratus (Rudolphi 1819) | Lepidochelyso livacea (Eschscholtz 1829) (Reptilia; Cheloniidae) | Oaxaca, Mexico | AY222274 | Olson et al., 2003 |
| Phaneropsolidae | ||||
| Phaneropsolus praomidis Baer 1971 | Rhabdomys sp. (Mammalia; Muridae) | Malawi | KJ700422 | Kanarek et al., 2015 |
| Phaneropsolus sp. | Terpsiphone paradise (Linnaeus 1758) (Aves; Monarchidae) | Guangxi Province, China | KY982862 | Kanarek et al., 2017 |
| Pleurogenidae | ||||
| Brandesia turgida (Brandes 1888) | Pelophylax lessonae (Camerano 1882) (=Rana lessonae Camerano 1882) (Amphibia; Ranidae) | Near Lesniki, Kiev Region, Ukraine | AY220622 | Tkach et al., 2003 |
| Candidotrema loossi (Africa 1930) | Pelophylax ridibundus (Pallas 1771) (=Rana ridibunda Pallas 1771) (Amphibia; Ranidae) | Vilkovo, Kiliya, Ukraine | AY220621 | Tkach et al., 2003 |
| Cortrema magnicaudata (Bykhovskaya-Pavlovskaya 1950) | Hirundo rustica Linnaeus 1758 (Aves; Hirundinidae) | Near Záhlinice, Czech Republic | KJ700420 | Kanarek et al., 2014 |
| Gyrabascus amphoraephormis (Mödlinger 1930) | M. daubentoni | Kiev, Ukraine | AY220620 | Tkach et al., (2003) [as Allassogonoporus amphoraeformis (Mödlinger 1930)] |
| Gyrabascus sp. | Dromiciops bozinovici D'Elía, Hurtado et D’Anatro 2016 (Mammalia; Microbiotheriidae) | Chile | KY921598 | Bell et al., 2018 |
| Langeronia macrocirra Caballero et Bravo 1949 | Lithobates berlandieri (Baird 1859) (=Rana berlandieri Baird1859) (Amphibia; Ranidae) | Guatemala | AY220624 | Tkach et al., 2003 [as Loxogenes macrocirra (Caballero et Bravo 1949)] |
| Leyogonimus polyoon (Linstow 1887) | Fulica atra Linnaeus 1758 (Aves; Rallidae) | Poland | KY752116 | Kanarek et al., 2017 |
| Macyella massanae (Vaucher 1968) | Erithacus rubecula (Linnaeus 1758) (Aves; Muscicapidae) | Near Zahlinice, Czech Republic | KP682451 | Kanarek et al., 2015 [as Collyricloides massanae Vaucher 1968] |
| Macyella postgonoporus Neiland 1951 | Dendrocopus major (Linnaeus 1758) (Aves; Picidae) | Czech Republic | KY752115 | Kanarek et al., 2017 |
| Parabascus duboisi Hurková 1961 | M. daubentoni | Kiev, Ukraine | AY220618 | Tkach et al., 2003 |
| Parabascus joannae (Zdzitowiecki 1967) | M. daubentoni | Kiev, Ukraine | AY220619 | Tkach et al., 2003 |
| Parabascus semisquamosus (Braun 1900) | P. kuhli | Kherson Region, Ukraine | AF151923 | Tkach et al., 2000 |
| Pleurogenes claviger (Rudolphi 1819) | Rana temporaria Linnaeus 1758 (Amphibia; Ranidae) | Kiev Region, Ukraine | AF151925 | Tkach et al., 2000 |
| Pleurogenoides medians (Olsson 1876) | P. lessonae | Ukraine | AF433670 | Tkach et al., 2001 |
| Prosotocus confusus (Looss 1894) | P. lessonae | Kiev Region, Ukraine | AY220623 | Tkach et al., 2003 |
| Prosthogonimidae | ||||
| Prosthogonimus cuneatus (Rudolphi 1809) | Sturnus vulgaris Linnaeus 1758 (Aves; Sturnidae) | Nezhin, Chernigiv Region, Ukraine | AY220634 | Tkach et al., 2003 |
| Prosthogonimus ovatus (Rudolphi 1803) | Pica pica (Linnaeus 1758) (Aves; Corvidae) | Chernigiv region, Ukraine | AF151928 | Tkach et al., 2000 |
| Prosthogonimus rarus Braun 1901 | Anas querquedula (Linnaeus 1758) (Aves; Anatidae) | Golopristansky district, Kherson Region, Ukraine | AY116869 | Tkach et al., 2003 [as Schistogonimus rarus (Braun 1901)] |
| Stomylotrematidae | ||||
| Stomylotrema vicarium Braun 1901 | Sclerurus mexicanus Sclater 1857 (Aves; Furnariidae) | Tocache, Peru | KY982863 | Kanarek et al., 2017 |
| Outgroup | ||||
| Plagiorchioidea | ||||
| Plagiorchiidae | ||||
| Plagiorchis elegans (Rudolphi 1802) | Apodemus sylvaticus (Linnaeus 1758) (Mammalia; Muridae) | United Kingdom | JX522535 | Boyce et al., 2014 |
RESULTS
Systematics
Family Pleurogenidae Looss 1899
Genus Gyrabascus Macy 1935
Gyrabascus oppositus (Zdzitowiecki 1969) comb. n. (fig. 1)
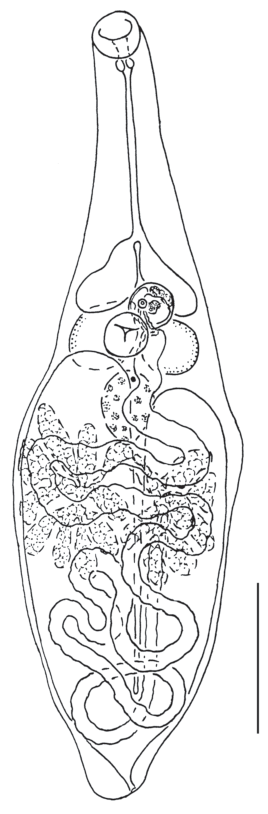
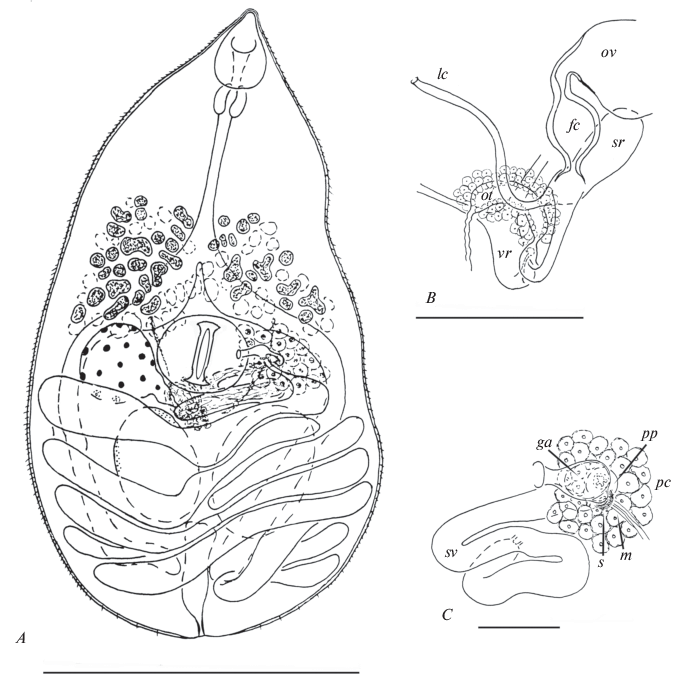
Fig. 1.
Gyrabascus oppositus: A – whole specimen, ventral view; B – ovarian complex, dorsal view; C – terminal genitalia, ventral view; fc – fertilization chamber, ga – common genital atrium, lc – Laurer’s, canal, m – metraterm, ot – ootype with Mehlis’ gland, ov – ovary, pc – prostatic cells, pp – pars prostatica, s – sphincter, sr – seminal receptacle, sv – seminal vesicle, vr – vitelline reservoir. Scale bars (mm): A – 0.4; B, C – 0.05.
Syns: Parabascus oppositus Zdzitowiecki 1969, Parabascus semisquamosum (Braun 1900) sensu Mituch 1965, Parabascus lepidotus Looss 1907 sensu Khotenovsky 1985 pro parte.
Host: Pipistrellus kuhlii (Kuhl 1817) (Chiroptera: Vespertilionidae).
Locality: Kalinino village, Astrakhan Oblast of Russia (46°20′ N, 48°52′ E).
Date of collection: 18 September, 2014 and 29 May, 2018.
Site of infection: intestine.
Prevalence and intensity: in 5 of 6, 5–10 ind.
Specimens deposited: mounts № 14277, 14278 in the Museum of Helminthological Collections at the Centre of Parasitology of the A.N. Severtsov Institute of Ecology and Evolution (IPEE RAS) in Moscow, Russia.
Description (based on 15 adult specimens). Body pyriform with conical anterior extremity and rounded posterior end; length 0.62–1.01 (0.80) mm, maximum width 0.33–0.44 (0.40) mm at level of testes, rare of ventral sucker. Body length to width ratio 1.44–2.57 : 1 (2.02 : 1); body width to length ratio 1 : 0.39–0.69 (1 : 0.51). Forebody 290–491 (390) which represents 38.9–69.4 (50.7)% of body length. Tegument spinose. Oral sucker drop-shaped with elongated antero-dorsal lobe, 89–108 (97) × 57–70 (64). Mouth opening subterminal. Prepharynx usually not observed. Pharynx 38–51 (43) × 38–51 (45). Oral sucker to pharynx width ratio 1.25–1.67 : 1 (1.44 : 1). Oesophagus 120–215 (163); intestinal bifurcation in posterior third of forebody. Intestinal branches ending blindly far behind testes. Ventral sucker transverse-oval and recessed into body, 82–108 (95) × 108–133 (123). Oral sucker to ventral sucker width ratio 0.50–0.55 : 1 (0.53 : 1). Two testes, round to oval, entire, opposite, in anterior half of hindbody; sinistral 95–152 (122) × 76–138 (122), dextral 90–139 (125) × × 76–138 (110). Cirrus sac absent. Seminal vesicle tubular, convoluted, free in parenchyma. Pars prostatica tubular, surrounded by extensive field of prostatic cells. Ejaculatory duct short, opening into tubular common genital atrium. Genital pore submedian, at level of ventral sucker, on opposite side to ovary. Ovary round to oval, entire, sinistral or dextral, at level of ventral sucker; 82–114 (98) × 95–152 (115). Proximal part of oviduct forms vesicular fertilization chamber. Seminal receptacle canalicular. Laurer’s canal opens on dorsal side of body in ventral sucker region. Ootype with Mehlis’ gland and vitelline reservoir median, between ventral sucker and testes. Vitellarium follicular in posterior half of forebody but overlaps with ventral sucker to some extent; follicles in two sublateral fields confluent dorsally. Uterus with transversal loops fills almost all space in hindbody. Metraterm with somewhat thickened walls, terminating with sphincter, and opening into common genital atrium ventral to male pore. Eggs numerous, oval, operculate, 24–26 (26) × × 13–16 (13). Excretory pore terminal; vesicle I-shaped and usually reaches posterior edge of testes.
Remarks. Our specimens are consistent with the prominent morphological features of P. oppositus, as described by Zdzitowiecki (1969), specifically the body size and shape, configuration of the inner organs, ratio of the suckers, and eggs size. In Zdzitowiecki’s (1969) paper, no data exists on morphology of the male terminal genitalia, however drawing of P. oppositus made by Mituch (1965) (named as P. semisquamosum by this author) does not indicate that the seminal vesicle is enclosed into the cirrus-sac.
Only the tubular pars prostatica with a field of prostate cells were visible on photos of the paratype terminal genitalia, while the wall of the cirris-sac separating this part of the male duct from the parenchyma is absent. Thus, the paratype of P. opposites obviously does not have a cirrus-sac.
Family Lecithodendriidae Looss 1902
Genus Lecithodendrium Lühe 1896
Lecithodendrium cf. skjabini Mazaberidse 1963 (fig. 2)
Host: Pipistrellus kuhlii (Kuhl 1817) (Chiroptera: Vespertilionidae).
Locality: Kalinino village, Astrakhan Oblast of Russia (46°20′ N, 48°52′ E).
Date of collection: 29 May, 2018.
Site of infection: intestine.
Prevalence and intensity: in 1 of 6, 22 ind.
Specimens deposited: mounts № 14 279, 14 280 in the Museum of Helminthological Collections at the Centre of Parasitology of the A.N. Severtsov Institute of Ecology and Evolution (IPEE RAS), in Moscow, Russia.
Description (based on 11 adult specimens). Body fusiform; length 1.04–1.33 (1.18) mm, maximum width 0.30–0.40 (0.38) mm at mid-level of body. Body length to width ratio 2.56–3.61 : 1 (3.17 : 1); body width to length ratio 1 : 0.28–0.39 (1 : 0.32). Forebody 390–516 (440), which represents 32.3–40.2.4 (37.4)% of body length. Tegument unarmed. Oral sucker rounded, 60–76 (67) × 57–79 ( 68). Mouth opening subterminal. Prepharynx absent, pharynx 19–28 (23) × 25–32 (27). Oral sucker to pharynx width ratio 2.00–3.00 : 1 (2.60 : 1). Oesophagus 222–294 (251), intestinal bifurcation in forebody. Intestinal branches end near anterior edge of testes. Ventral sucker spherical to subsperical, 63–82 (75) × 63–82 (70). Oral sucker to ventral sucker width ratio 0.77–1.10 : 1 (0.98 : 1). Testes two, entire, round to oval, opposite, at level of ventral sucker; sinistral 82–127 (114) × 82–120 (104), dextral 95–133 (124) × 95–133 (110). Pseudocirrus-sac elongate-oval, 125 × 62–76 (70), with convoluted seminal vesicle, lies transversely to ventral surface of body and communicates with short thick-walled common genital atrium. Genital pore median or slightly sinistro-submedian, near anterior edge of ventral sucker. Ovary elongate-oval, entire, median, or slightly sinistro-median or dextro-submedian; post-testicular; contiguous with testes or separated, 95–133 (113) × 76–120 (97). Seminal receptacle canalicular. Laurer’s canal opens on dorsal side of body, at level of posterior edge of ovary or distinctly posterior to ovary. Ootype with Mehlis’ gland and vitelline reservoir near postero-sinistral margin of ovary or at level of posterior half ovary. Vitellarium follicular; elongated follicles in two dorsal clusters, 7–9 in each. Clusters postovarian but partly overlapped by ovarian area. Uterus fills almost all space in hindbody. Metraterm of similar length to pseudocirrus-sac, running over posterior surface of pseudocirrus-sac, and opening into common genital atrium. Eggs numerous, oval, operculate, 19–22 (20) × 10–13 (10). Excretory pore terminal; vesicle V-shaped and reaches to testes.
Remarks. Previous descriptions of L. skrjabini were deficient. Matsaberidze (1963) and Matsaberidze and Khotenovsky (1967) presented a description based on deformed specimens with compressed forebodies (Matsaberidze, 1963; Matsaberidze, Khotenovsky 1967). Kirillov et al. (2012) described specimens with a stretched forebody, as indicted by the equatorial position of the ventral sucker. Also, in the paper of Matsaberidze and Khotenovsky (1967) there is confusion with the drawing of L. skrjabini, which is replaced with the drawing of L. linstowi Dollfus 1931. The actual drawing of L. skrjabini is depicted in figure 1.
Comparison of the descriptions published by Matsaberidze (1963), Matsaberidze and Khotenovsky (1967) and Kirillov et al. (2012) enable the reconstruction of the body shape of relaxed L. skrjabini adults: it is fusiform, while the ventral sucker occupies the pre-equatorial position. The samples within this study are similar to L. skrjabini in terms of body shape, internal organ position, sucker size and ratio, the vitellarium morphology, and egg size. However, these trematodes differ from L. skrjabini in terms of body length and forebody and oesophagus lenght. This study presents specimens lengths of 1.04–1.33 mm, and the maximum length of L. skrjabini is 0.89 mm (Matsaberidze, Khotenovsky 1967). However, the publications of Matsaberidze (1963), and Matsaberidze and Khotenovsky (1967) take measurements from worms with compressed forebodies. At the same time, the adults in this study are evidently larger than the individuals collected by Kirillov et al. (2012). It is put forward that this difference is due to the phenomenon of host-induced morphological variability.
Molecular phylogenetic analysis
In both the ML and BI analysis, G. oppositus appears as a member of the well-supported Gyrabascus spp. clade that is also includes G. amphoraeformis (Mödlinger 1930) and Gyrabascus sp. (fig. 3). However, the phylogenetic relationships among representatives within the Gyrabascus spp. clade are poorly resolved. In turn, the Gyrabascus spp. clade is situated within the major Pleurogenidae + Collyriclidae clade. The Pleurogenidae + Collyriclidae clade appears to be monophyletic, with statistically significant support. Within the Pleurogenidae + Collyriclidae clade, the Gyrabascus spp. appears a sister clade to the group, containing representatives of the genera Cortrema Tang 1951, Leyogonimus Ginetsinskaya 1948 and Macyella Neiland 1951, but support of this sister connection is weak.
Fig. 3.
Phylogenetic position of Gyrabascus oppositus and Lecithodendrium cf skrjanbini based on the analysis of 28S rDNA partial sequences using ML and BI algorithms. Nodal support: ML/BI. The clade which is absent in ML, but present in BI noted with asterisk. Newly obtained sequences are shown in bold. Plagiorchis elegans is designated as outgroup taxa.
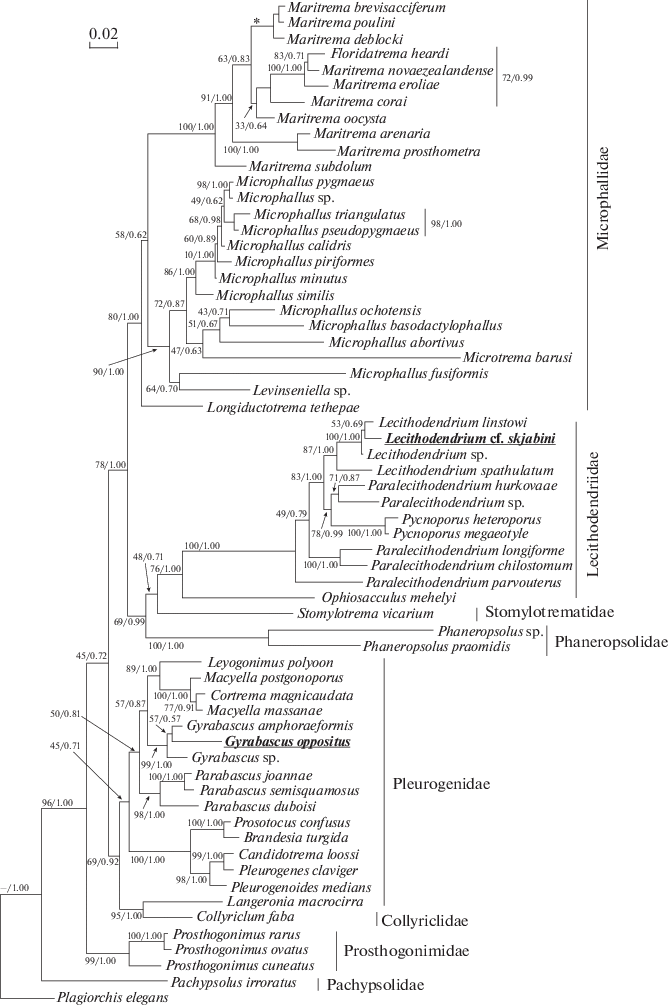
Lecithodendrium cf. skrjabini together with congeneric species form the Lecithodendrium spp. clade, which is strongly supported by BI analysis and moderately supported by ML analysis. Inside this clade, Lecithodendrium cf. skrjabini appears as a weakly supported sister taxon to L. linstowi. The Lecithodendrium cf. skrjabini + L. linstowi group is combined with the Lecithodendrium sp. into a well-supported monophyletic clade. In turn, the Lecithodendrium cf. skrjabini + + L. linstowi + Lecithodendrium sp. clade is strongly supported by BI analysis and moderately supported by ML analysis sister group to Lecithodendrium spathulatum (Ozaki 1929). The Lecithodendrium spp. clade appears as a member of the family Lecithodendriidae (fig. 3). The family Lecithodendriidae is closely related to Stomylotrematidae, however, this is weakly supported in both ML and BI analyses. The Lecithodendriidae + + Stomylotrematidae group is clustered with the Phaneropsolidae, strongly supported by BI analysis and moderately supported by ML analysis. The Lecithodendriidae + Stomylotrematidae + Phaneropsolidae clade is a sister group to the family Microphallidae, this is also strongly supported by BI analysis and moderately supported by ML analysis.
Meanwhile, the major clade of trematodes containing the Lecithodendriidae, Stomylotrematidae, Phaneropsolidae and Microphallidae appears as a low-supported sister group to the Pleurogenidae + + Collyriclidae clade (fig. 3). In turn, the [(Lecithodendriidae +Stomylotrematidae + Phaneropsolidae) + + Microphallidae] + (Pleurogenidae + Collyriclidae) clade is nested within the well-supported monophyletic group of microphalloids, that also includes the Prosthogonimidae. The Pachypsolidae occupies a basal position to all of the aforementioned taxa.
DISCUSSION
The genus Parabascus Looss 1907 combines pleurogenids with a well-developed cirrus sac, a submedian genital pore which lies behind the anterior edge of the ventral sucker, a V-shaped excretory vesicle, and several other morphological characters (Khotenovsky, 1985; Lotz, Font, 2008a; Kanarek et al., 2014). However, P. oppositus does not have consistent morphological features characteristic to this genus. The morphology of the male terminal genitalia and other features of P. oppositus, excluding the genital pore position, correspond to those for the genus Gyrabascus (= Allassogonoporus Olivier 1938) (compare with Olivier, 1938; Macy, 1940; Podvyaznaya, 1996; Lotz, Font, 2008). Six well-known representatives of this genus have a marginally located genital pore (Olivier, 1938; Macy, 1940; Dubois, 1956; Khotenovsky, 1974; Tkach, Bray, 2001; Brugni, Flores, 2007; Lotz, Font, 2008; Bell et al., 2018). However, for some specimens of G. amphoraeformis (=Allassogonoporus amphoraeformis), the sinistro-lateral position of genital pore was described to be located at the midpoint between the edge of the ventral sucker and the sinistral margin of the body (Sharpilo, Iskova, 1989 – fig. 67, g ). Molecular genetic data supports the belonging of G. oppositus to the genus Gyrabascus, which requires an amendment of the diagnosis for this genus.
Genus Gyrabascus Macy 1935 emend
Diagnosis (based on Lotz, Font (2008) with changes). Body broadly oval, nearly as wide as long. Oral sucker terminal to subterminal. Ventral sucker equatorial or preequatorial. Prepharynx very short. Testes in middle third of body. Cirrus-sac absent. Seminal vesicle lies transversely or irregularly coiled. Genital pore marginal or submedian, sinistral or dextral, locates in region of ventral sucker, opposite to ovary, not anterior to vitellarium. Ovary entire to lobed, extends anterior to testes. Uterus comprised of overlapping coils that fill posterior half of body. Eggs small, numerous. Vitellarium follicular, in forebody, sometimes reaches into ventral sucker region. In bats and rodents; North America and Eurasia. Type species G. brevigastus Macy 1935.
Integration of the Gyrabascus spp. into the family Pleurogenidae (fig. 3) can be verified through phylogenetic reconstructions from other authors (Tkach et al., 2002, 2003; Kanarek et al., 2014, 2015, 2017; Bell et al., 2018). However, in phylogram, presented in this article, the family appears to be paraphyletic, because Langeronia macrocirra Caballero et Bravo 1949 (Pleurogenidae) forms a highly supported sister relationship with Collyriclum faba (Bremser in Schmalz 1831) (Collyriclidae). Earlier, the sister relationship between C. faba and L. macrocirra was demonstrated by Heneberg and Literák (2013). The combination of C. faba with pleurogenid trematodes is supported by the morphological data on cercariae. Cercaria of C. faba, has four pairs of penetration glands and a bilobed virgula in the posterior half of the oral sucker, similar to the larvae of other pleurogenids (Heneberg et al., 2015).
The results of Kanarek et al. (2014, 2015, 2017) and Bell et al. (2018) indicate a close relationship between the genera Gyrabascus and Cortrema. According to our own data, there is no direct phylogenetic relationship between these taxa, as the only species of the genus Cortrema that has been sequenced, Cortrema magnicaudata (Bykhovskaya-Pavlovskaya 1950), is clustered with two members of the genus Macyella: Macyella massanae (Vaucher 1968) and Macyella postgonoporus Neiland 1951. Furthermore, M. massanae was closer to C. magnicaudata than to congeneric species (fig. 3). It should be noted that previous to reassigning M. massanae to the genus Macyella, based on the phylogenetic data of Kanarek et al. (2017), it was considered to be member of the genus Collyricloides Vaucher 1968. Consequently, a sister group to the Gyrabascus spp. clade cannot be clearly supported based on our own phylogenetic data.
The presented molecular phylogenetic data clearly demonstrates the monophyly of the genus Lecithodendrium and the family Lecithodendriidae as a whole. The data also clearly indicate the close relationships Lecithodendriidae with the families Phaneropsolidae and Stomylotrematidae, which supports the results of a recent study by Bell et al. (2018). In the study by Bell et al. (2018), the relationship of the Phaneropsolidae + + Stomylotrematidae + Lecithodendriidae clade with the Pleurogenidae, Prosthogonimidae, and Microphallidae was unclear. According to the results of our phylogenetic analysis, the Phaneropsolidae + Stomylotrematidae + Lecithodendriidae clade has a common ancestor with microphallid trematodes. However, the relationships between the Prostogonimidae and the Pleurogenidae + Collyriclidae and Microphallidae + + (Phaneropsolidae + Stomylotrematidae + Lecithodendriidae) clades were poorly resolved.
Список литературы
Al-Kandari W.Y., Al-Bustan S.A., Alnaqeeb M., 2011. Ribosomal DNA sequence characterization of Maritrema cf. eroliae Yamaguti, 1939 (Digenea: Microphallidae) and its life cycle // Journal of Parasitology. V. 97. P. 1067–1074. https://doi.org/10.1645/GE-2869.1
Bell J.A., González-Acuña D., Tkach V.V., 2018. First record of Gyrabascus (Digenea, Pleurogenidae) from Dromiciops bozinovici D’Elia et al., 2016 (Marsupialia: Microbiotheriidae) in Chile and its phylogenetic relationships // Comparative Parasitology. V. 85. P. 58–65. https://doi.org/10.1654/1525-2647-85.1.58
Boyce K., Hide G., Craig P.S., Reynolds C., Hussain M., et al., 2014. A molecular and ecological analysis of the trematode Plagiorchis elegans in the wood mouse Apodemus sylvaticus from a periaquatic ecosystem in the UK // Journal of Helminthology. V. 88. P. 310–320. https://doi.org/10.1017/S0022149X13000199
Brugni N., Flores V.R., 2007. Allassogonoporus dromiciops n. sp. (Digenea: Allassogonoporidae) from Dromiciops gliroides (Marsupialia: Microbiotheriidae) in Patagonia, Argentina // Systematic Parasitology. V. 68. P. 45–48. https://doi.org/10.1007/s11230-006-9083-1
Dubois G., 1956. Contribution à l’étude des Trématodes de Chiroptéres // Revue Suisse de Zoologie. V. 63. P. 683–695 [in French].
Dubinin V.B., 1952. Fauna of larvae of parasitic worms of vertebrates in the delta of the Volga River // Parazitologicheskii Sbornik. V. 14. P. 213–265 [in Russian].
Edgar R.C., 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput // Nucleic Acids Research. V. 32. P. 1792–1797. https://doi.org/10.1093/nar/gkh340
Galaktionov K.V., Blasco-Costa I., 2018. Microphallus ochotensis sp. nov. (Digenea, Microphallidae) and relative merits of two-host microphallid life cycles // Parasitology Research. V. 117. P. 1051–1068. https://doi.org/10.1007/s00436-018-5782-1
Galaktionov K.V., Blasco-Costa I., Olson P.D., 2012. Life cycles, molecular phylogeny and historical biogeography of the ‘pygmaeus’ microphallids (Digenea: Microphallidae): widespread parasites of marine and coastal birds in the Holarctic // Parasitology. V. 139. P. 1346– 1360. https://doi.org/10.1017/S0031182012000583
Gouy M., Guindon S., Gascuel O., 2010. SeaView Version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building // Molecular Biology and Evolution. V. 27. P. 221–224. https://doi.org/10.1093/molbev/msp259
Guindon S., Gascuel O., 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by Maximum Likelihood // Systematic Biology. V. 52. P. 696–704. https://doi.org/10.1080/10635150390235520
Heneberg P., Faltýnková A., Bizos J., Malá M., Žiak J., Literák I., 2015. Intermediate hosts of the trematode Collyriclum faba (Plagiochiida: Collyriclidae) identified by an integrated morphological and genetic approach // Parasites & Vectors. V. 8. P. 85. https://doi.org/10.1186/s13071-015-0646-3
Heneberg P., Literák I., 2013. Molecular phylogenetic characterization of Collyriclum faba with reference to its three host-specific ecotypes // Parasitology International. V. 62. P. 262–267. https://doi.org/10.1016/j.parint.2013.01.002
Hernandez-Orts J.S., Pinacho-Pinacho C.D., Garcia-Varela M., Kostadinova A., 2016. Maritrema corai n. sp. (Digenea: Microphallidae) from the white ibis Eudocimus albus (Linnaeus) (Aves: Threskiornithidae) in Mexico // Parasitology Research. V. 115. P. 547–559. https://doi.org/10.1007/s00436-015-4771-x
Hillis D.M., Dixon M.T., 1991. Ribosomal DNA: molecular evolution and phylogenetic inference // The Quarterly review of biology. V. 66. P. 411–453. https://doi.org/10.1086/417338
Ivanov V.M., Semenova N.N., Kalmykov A.P., 2012. Helminths in ecosystem of the delta of the Volga River. Astrakhan’: GP AO. 255 p. [in Russian].
Kanarek G., Zaleśny G., Czujkowska A., Sitko J., Harris P.D., 2015. On the systematic position of Collyricloides massanae Vaucher, 1969 (Platyhelminthes: Digenea) with notes on distribution of this trematode species // Parasitology Research. V. 114. P. 1495–1601. https://doi.org/10.1007/s00436-015-4333-2
Kanarek G., Zalesny G., Sitko J., Tkach V.V., 2014. Phylogenetic relationships and systematic position of the families Cortrematidae and Phaneropsolidae (Platyhelminthes: Digenea) // Folia Parasitologica. V. 61.P. 523–528. https://doi.org/10.14411/fp.2014.057
Kanarek G., Zalesny G., Sitko J., Tkach V.V., 2017. The systematic position and structure of the genus Leyogonimus Ginetsinskaya, 1948 (Platyhelminthes: Digenea) with comments on the taxonomy of the superfamily Microphalloidea Ward, 1901 // Acta Parasitologica. V. 62. P. 617–624. https://doi.org/10.1515/ap-2017-0075
Khotenovsky I.A., 1972. On the evolution of trematodes from bats // Parazitologiya. V. 6 P. 79–82 [in Russian].
Khotenovsky I.A., 1974. Family Allassogonoporidae Skarbilovich, 1947 // Skryabin K.I. (ed), Osnovy Trematodologii. V. 25. Moscow: Nauka. P. 245–258 [in Russian].
Khotenovsky I.A., 1985. Trematodes of the genus Parabascus (Trematoda, Pleurogenidae) from the bats of the Holarctic // Parazitologicheskii Sbornik. V. 33. P. 125–133 [in Russian].
Kirillov A.A., Kirillova N.Yu., Vekhnik V.P., 2012. Trematodes (Trematoda) of bats (Chiroptera) from the Middle Volga Region // Parazitologiya. V. 46. P. 384–413 [in Russian].
Kudlai O., Cribb T.H., Cutmore S.C., 2016. A new species of microphallid (Trematoda: Digenea) infecting a novel host family, the Muraenidae, on the northern Great Barrier Reef, Australia // Systematic Parasitology. V. 93. P. 863–876. https://doi.org/10.1007/s11230-016-9670-8
Kudlai O., Stunžėnas V., Tkach V., 2015. The taxonomic identity and phylogenetic relationships of Cercaria pugnax and C. helvetica XII (Digenea: Lecithodendriidae) based on morphological and molecular data // Folia Parasitologica. V. 62. 003. https://doi.org/10.14411/fp.2015.003
Kurochkin Yu.V., Kurochkina Z.A., 1962. On the helminth fauna of bats of the Astrakhan reserve // Trudy Astrakhanskogo zapovednika V. 6. P. 127–134 [in Russian].
Lord J.S., Parker S., Parker F., Brooks D.R., 2012. Gastrointestinal helminths of pipistrelle bats (Pipistrellus pipistrellus / Pipistrellus pygmaeus) (Chiroptera: Vespertilionidae) of England // Parasitology. V. 139. P. 366–374. https://doi.org/10.1017/S0031182011002046
Lotz J.M., Font W.F., 2008. Family Gyrabascidae Macy, 1935 // Bray R.A., Gibson D.J., Jones A. (eds). Keys to Trematoda. V. 3. London, U.K.: CAB International and Natural History Museum. P. 523–525.
Lotz J.M., Font W.F., 2008a. Family Phaneropsolidae Mehra, 1935 // Bray R.A., Gibson D.J., Jones A. (eds). Keys to Trematoda. V. 3. London, U.K.: CAB International and Natural History Museum, London. P. 545–562.
Macy R.W., 1940. A new species of trematode, Allassogonoporus vespertilionis (Lecithodendriidae), from an Oregon bat, Myotys californicuscaurinus Miller // Transactions of the American Microscopical Society. V. 59. P. 48–51. https://doi.org/10.2307/3222815
Matsaberidze G.V., 1963. Lecithodendrium skrjabini, a new trematode from bat // Bulletin of the Academy of Sciences of the Georgian SSR. V. 31. P. 695–698 [in Russian].
Matsaberidze G.V., Khotenovsky I.A., 1967. On trematode fauna of bats in Georgia // Gel’mintofauna zhivotnykh i rasteniy Gruzii. Tbilisi: Mutsniereba. P. 83–94 [in Russian].
Miller M.A., Pfeiffer W., Schwartz T., 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees // Proceedings of the Gateway Computing Environments Workshop (GCE). Piscataway: IEEE Press New Orleans. P. 1–8. https://doi.org/10.1109/GCE.2010.5676129
Mituch J., 1965. Beitrag zur Ercenntnis der Helminthenfauna von Miniopterus schreibersi (Kuhl, 1819) in der Slovakei (ČSSR) // Helminthologia. V. 6. P. 109–119.
Natural History Museum, 2014. Dataset: Collection specimens. Resource: Specimens. Natural History Museum Data Portal (data.nhm.ac.uk). Avaliable from: https://doi.org/10.5519/0002965 (accessed February 2019)
Niewiadomska K., Pojmańska T., 2018. Przywry (Trematoda): część systematyczna – Digenea: Plagiorchiida. Łódz: Wydawnictwo Uniwersytetu Łódzkiego. 387 p. [in Polish].
Olivier L., 1938. A new trematode, Allassogonoporus marginalis, from the muskrat // Journal of Parasitology. V. 24. P. 155–160. https://doi.org/10.2307/3272494
Olson P.D., Cribb T.H., Tkach V.V., Bray R.A., Littlewood D.T.J., 2003. Phylogeny and classification of Digenea (Platyhelminthes: Trematoda) // International Journal for Parasitology. V. 33. P. 733–755. https://doi.org/10.1016/S0020-7519(03)00049-3
Podvyaznaya I.M., 1996. Fine structure of the male reproductive system and genital atrium of the parasite of bats, Allassogonoporus amphoraeformis (Trematoda: Allassogonoporidae) // Parazitologiya. V. 30. P. 229–235 [in Russian].
Posada D., Crandall K.A., 1998. Modeltest: testing the model of DNA substitution // Bioinformatics. V. 14. P. 817–818. https://doi.org/10.1093/bioinformatics/14.9.817
Presswell B., Blasco-Costa I., Kostadinova A., 2014. Two new species of Maritrema Nicoll, 1907 (Digenea: Microphallidae) from New Zealand: morphological and molecular characterization // Parasitology Research. V. 113. P. 1641–1656. https://doi.org/10.1007/s00436-014-3809-9
Rambaut A., Suchard M.A., Xie D., Drummond A.J., 2014. Tracer v1.6. Avaliable from: http://beast.bio.ed.ac.uk/ Tracer (accessed February 2019)
Sharpilo V.P., Iskova N.I., 1989. The Fauna of the Ukraine. Trematoda: Plagiorchiata. Kiev: Naukova Dumka. 279 p. [in Russian].
Sharpilo V.P., Iskova N.I., Tkach V.V., 1989. Genus Parabascus Looss, 1907 // Sharpilo V.P., Iskova N.I. The Fauna of the Ukraine. Trematoda: Plagiorchiata. Kiev: Naukova Dumka. P. 192–199 [in Russian].
Smirnov D.G., Vekhnik V.P., Sokolova I.V., Lukyanenko A.M., 2018. Materials to the bat fauna (Chiroptera) of the south of Astrakhan region // Plecotus et al. V. 21. P. 22–34 [in Russian].
Strelkov P.P., Il’in V.J., 1990. Bats (Chiroptera, Vespertilionidae) in the South of the Middle and Lower Volga Regions // Trudy Zoologicheskogo Instituta Akademii nauk SSSR. V. 225. P. 42–167 [in Russian].
Strelkov P.P., Unkurova V.J., Medvedeva G.A., 1985. New data on Pipistrellus kuhlii and dynamics of its range in the USSR // Zoologicheskii zhurnal. 64. P. 87–97 [in Russian].
Tkach V.V., Bray R.A., 2001. Allassogonoporus callosciuri n. sp. (Digenea: Allassogonoporidae) from the plantain squirrel Callosciurus notatus (Boddaert, 1785) (Rodentia: Sciuridae) on the Island of Borneo, Malaysia // Systematic Parasitology. V. 48. P. 37–40. https://doi.org/10.1023/A:1026581409478
Tkach V.V., Snyder S.D., Swiderski Z., 2001. On the phylogenetic relationships of some members of Macroderoididae and Ochetosomatidae (Digenea, Plagiorchioidea) // Acta Parasitologica. V. 46. P. 267–275.
Tkach V., Lotz J.M., Swiderski Z., Esteban J.G., 2002. On the systematic position of Ophiosacculus Macy, 1935 (Digenea: Lecithodendriidae), with the erection of the Ophiosacculinae n. subfam. // Systematic Parasitology. V. 53. P. 159–167. https://doi.org/10.1023/A:1021199108887
Tkach V.V., Littlewood D.T.J., Olson P.D., Kinsella J.M., Swiderski Z., 2003. Molecular phylogenetic analysis of the Microphalloidea Ward, 1901 (Trematoda: Digenea) // Systematic Parasitology. V. 56. P. 1–15. https://doi.org/10.1023/A:1025546001611
Tkach V.V., Pawlowski J., Mariaux J., 2000. Phylogenetic analysis of the suborder Plagiorchiata (Platyhelminthes, Digenea) based on partial lsrDNA sequences // International Journal for Parasitology. V. 30. P. 83–93. https://doi.org/10.1016/S0020-7519(99)00163-0
Zavialov E.V. (ed.), 2009. Mammals of the Northern Lower-Volga region, I. Saratov: Saratov University Press. 248 p. [in Russian].
Zdzitowiecki K., 1969. Helminths of bats in Poland. III. Trematodes of the subfamily Lecithodendriidae, exept for Lecithodendriinae // Acta Parasitologica Polonica. V. 16. P. 227–237.
Дополнительные материалы отсутствуют.
Инструменты
Зоологический журнал