Зоологический журнал, 2020, T. 99, № 4, стр. 405-412
A contribution to the Knowledge of Oribatid Mites (Acari, Oribatida) of New Caledonia
S. G. Ermilov a, *, N. J. Mary b, **, ***
a Tyumen State University
625003 Tyumen, Russia
b Etude des Hydrosystèmes Continentaux tropicaux (ETHYC’O)
B.P. 13 821, Nouméa Cedex 98 803, New Caledonia
* E-mail: ermilovacari@yandex.ru
** E-mail: nmary@free.fr
*** E-mail: ethyco2005@gmail.com
Поступила в редакцию 25.02.2019
После доработки 15.03.2019
Принята к публикации 15.05.2019
Аннотация
The present study is based on oribatid mite material collected in New Caledonia in 2001–2016. A list of 31 species/subspecies belonging to 20 genera and 16 families is provided. Of these, six species (Mochlozetes penetrabilis, Punctoribates (Minguezetes) insignis, Galumna acutirostrum, G. naturalisi, Pergalumna paraelongata, P. weberi) are being recorded from the Australian Region for the first time. A new species, Pergalumna caledonica sp. n., is described, which differs from Pergalumna remota (Hammer 1968) by the presence of two longitudinal prodorsal carinae. The new species differs also from Pergalumna minipora Ermilov, Chatterjee, Kumar Das, Bordoloi 2014 by its smaller body size, the presence of long interlamellar setae, and a rounded rostrum.
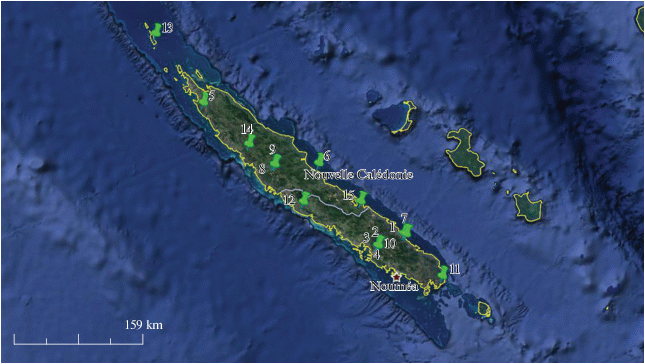
New Caledonia (land area of 18.576 km2) is an archipelago which includes the main island of Grande Terre, the Loyalty Islands, the Chesterfield Islands, the Belep Archipelago, the Isle of Pines, as well as a few remote islets. It is a part of the Melanesia subregion that is located to the south of Vanuatu, about 1.210 km east of Australia.
At present, oribatid mites (Acari, Oribatida) of New Caledonia are little known (e.g., Balogh, Balogh, 1983; Olszanowski, 1997; Niedbała, 2000; Niedbała, Penttinen, 2007; Ermilov et al., 2013). Our work is based on a random set of previously unstudied materials, which were collected by the second author with colleagues during several hydrobiological expeditions to New Caledonia conducted between 2001 and 2016. The primary goal of our paper is to provide the list of identified oribatid mite taxa.
During identification, we found one new species, belonging to the nominative subgenus of Pergalumna Grandjean 1936 (Galumnidae). The secondary goal of our paper is to describe and illustrate this new species. Pergalumna comprises two subgenera and about 160 species (Ermilov, Klimov, 2017; Subías, 2004, updated 2018) that have a cosmopolitan distribution. The generic and subgeneric diagnoses of Pergalumna were presented by Ermilov and Klimov (2017). Identification keys to species of Pergalumna from the Ethiopian, Neotropical, Oriental and Australian regions are provided by Ermilov et al. (2014, 2015), Ermilov and Starý (2018), and Ermilov and Friedrich (2019).
MATERIAL AND METHODS
Except for locality 13 (Bélep Island), oribatid mites were collected from the following places situated along various streams of Grande Terre Island (Fig. 1):
Locality 1, river Ni, site NI200, 166°30′36.9691″ E, 21°52′50.1503″ S, alt. 50 m, 11 September 2008 (collected by C. Flouhr, Hytec).
Locality 2, river Kouembélia, site AVAL AEROPORT, 166°12′21.4132″ E, 22°0′29.7065″ S, alt. 15 m, 04 October 2010 (collected by C. Flouhr, Hytec) and 08 July 2016 (collected by N. Mary).
Locality 3, river Kouembélia, site AMONT AEROPORT, 166°12′56.6381″ E, 22°0′35.9046″ S, alt. 15 m, 19 October 2009 (collected by C. Flouhr, Hytec).
Locality 4, river Kouembélia, site AMONT AEROPORT, 166°12′56.6381″ E, 22°0′35.9046″ S, alt. 15 m, 04 October 2010 (collected by C. Flouhr, Hytec).
Locality 5, river Creek à Paul, site BOSSU, 164°12′56.1971″ E, 20°29′8.9030″ S, alt. 110 m, 24 September 2010 (collected by N. Mary).
Locality 6, river Monéo, site NEEE100, 165°31′56.1216″ E, 21°8′11.0915″ S, alt. 10 m, 12 April 2011 (collected by C. Flouhr, Hytec).
Locality 7, river Ni, site AFF_NI400, 166°32′16.3273″ E, 21°53′19.5580″ S, alt. 25 m, 10 September 2008 (collected by C. Flouhr, Hytec).
Locality 8, river Papainda, site PAP100, 165°1′16.1836″ E, 21°8′53.1499″ S, alt. 165 m, 10 September 2010 (collected by C. Flouhr, Hytec).
Locality 9, river Pandanus, alt. site PND100, 165°1′46.8617″ E, 21°8′53.0041″ S, alt. 165 m, 11 September 2010 (collected by C. Flouhr, Hytec).
Locality 10, river Kouembélia, site AMONT AEROPORT, 166°12′56.6381″ E, 22°0′35.9046″ S, alt. 15 m, 04 October 2010 (collected by C. Flouhr, Hytec).
Locality 11, river Trou Bleu, site 3-C, 166°57′43.8278″ E, 22°20′14.2764″ S, alt. 10 m, 18 January 2013 (collected by C. Flouhr, Hytec).
Locality 12, river Deva, site DEVA050, 165°21′24.8544″ E, 21°32′50.3225″ S, alt. 55 m, 26 August 2009 (collected by C. Flouhr, Hytec).
Locality 13, river Gaawé la Madar, site AFF_GAA100, 163°40′22.1″ E, 19°44′14.4″ S, alt. 46 m, 06 December 2005 (collected by C. Flouhr, Hytec).
Locality 14, river Voh, site VOH-t-010, 164°44′5.4154″ E, 20°55′55.5838″ S, alt. 39 m, 06 September 2001 (collected by N. Mary).
Locality 15, river Xwê Nuo, site AVAL_CET 2007FW002, 166°0′43.6878″ E, 21°32′28.5673″ S, alt. 10 m, 11 December 2007 (collected by C. Flouhr, Hytec).
Specimens were mounted in lactic acid on temporary cavity slides for measurement and illustration. Body length was measured in lateral view, from the tip of the rostrum to the posterior edge of the notogaster. Notogastral width refers to the maximum width of the notogaster in dorsal view. Lengths of body setae were measured in lateral aspect. All body measurements are presented in micrometers. Formulas for leg setation are given in parentheses according to the sequence trochanter–femur–genu–tibia–tarsus (famulus included). Formulas for leg solenidia are given in square brackets according to the sequence genu–tibia–tarsus.
Drawings were made with a camera lucida using a Leica transmission light microscope “Leica DM 2500”.
Morphological terminology used in this paper follows that of F. Grandjean (see Ermilov and Klimov (2017) for review and application).
The following abbreviations are used: car – carina; L – lamellar line; S – sublamellar line; N – prodorsal leg niche; E, T – lateral ridges of prodorsum; ro, le, in, bs – rostral, lamellar, interlamellar and bothridial setae, respectively; Ad – sejugal porose area; D – dorsophragma; P – pleurophragma; c, la, lm, lp, h, p – notogastral setal alveoli or setae; Aa, A2, A3 – notogastral porose areas; mp – median pore; ia, im, ip, ih, ips – notogastral lyrifissures; gla – opisthonotal gland opening; h, m, a – subcapitular setae; or – adoral seta; ω – palp and leg solenidion; sac – axillary saccule; cha, chb – cheliceral setae; Tg – Trägårdh’s organ; Pd I, Pd II – pedotecta I, II, respectively; 1b, 3b, 3c, 4a, 4b, 4c – epimeral setae; dis – discidium; cp – circumpedal carina; g, ag, an, ad – genital, aggenital, anal and adanal setae, respectively; iad – adanal lyrifissure; p.o. – preanal organ; Tr, Fe, Ge, Ti, Ta – leg trochanter, femur, genu, tibia, tarsus, respectively; σ, φ – leg solenidia; ɛ – leg famulus.
LIST OF IDENTIFIED ORIBATID MITE TAXA FROM NEW CALEDONIA
Distribution: mostly from Subías (2004, updated 2018). All species (except holotype) are deposited in the collection of the Tyumen State University Museum of Zoology, Tyumen, Russia.
Trhypochthoniidae
(1) Allonothrus russeolus reticulatus Hammer 1972. Distribution: Australian and Oriental regions; New Caledonia: locality 3 (2 ex.).
(2) Archegozetes magnus (Sellnick 1925). Distribution: Tropical and Subtropical regions; New Caledonia: locality 2 (1 ex.).
(3) Trhypochthoniellus longisetus (Berlese 1904). Distribution: Cosmopolitan; New Caledonia: localities 5 (14 ex.), 9 (7 ex.).
Malaconothridae
(4) Malaconothrus dorsofoveolatus Hammer 1979. Distribution: Oriental and Australian regions; New Caledonia: localities 1 (11 ex.), 7 (17 ex.).
(5) Tyrphonothrus crassisetosus fijiensis (Hammer 1971). Distribution: Australian and Ethiopian regions; New Caledonia: localities 2 (1 ex.), 5 (1 ex.), 12 (2 ex.), 15 (15 ex.).
Neoliodidae
(6) Neoliodes bataviensis Sellnick 1925. Distribution: Java, Australian region, Japan; New Caledonia: locality 13 (2 ex.).
Plateremaeidae
(7) Plateremaeus novemsetosus J. et P. Balogh 1983. Distribution: Australia; New Caledonia: locality 15 (6 ex.).
Licnodamaeidae
(8) Licnodamaeidae sp. Distribution: New Caledonia: locality 12 (3 ex.).
Carabodidae
(9) Austrocarabodes falcatus Hammer 1973. Distribution: Australian, Neotropical and Oriental regions; New Caledonia: locality 11 (1 ex.).
Hydrozetidae
(10) Hydrozetes lemnae (Coggi 1897). Distribution: Semicosmopolitan; New Caledonia: localities 9 (8 ex.), 12 (29 ex.), 14 (1 ex.).
Scutoverticidae
(11) Scutovertex sp. Distribution: New Caledonia: locality 12 (1 ex.).
Caloppiidae
(12) Crassoribatula maculosa Hammer 1967. Distribution: New Zealand; New Caledonia: locality 11 (1 ex.).
Haplozetidae
(13) Peloribates magnisetosus Hammer 1967. Distribution: New Zealand; New Caledonia: locality 11 (1 ex.).
Scheloribatidae
(14) Scheloribates praeincisus (Berlese 1910). Distribution: Tropical and Subtropical regions; New Caledonia: localities 2 (1 ex.), 9 (1 ex.).
(15) Scheloribates praeincisus interruptus (Berlese 1916). Distribution: Tropical region; New Caledonia: locality 11 (1 ex.).
(16) Scheloribates tubuaiensis Sellnick 1959. Distribution: Australian region, Caucasus; New Caledonia: localities 2 (10 ex.), 3 (8 ex.), 4 (3 ex.), 9 (1 ex.), 10 (10 ex.), 14 (3 ex.).
Mochlozetidae
(17) Mochlozetes penetrabilis Grandjean 1930. Distribution: Neotropical region, Japan; New Caledonia: localities 6 (1 ex.), 8 (2 ex.), 9 (19 ex.), 15 (3 ex.). Thе species is recorded in the Australian region for the first time.
Ceratozetidae
(18) Ceratozetes hamobatoides Hammer 1967. Distribution: Australian region; New Caledonia: localities 3 (1 ex.), 15 (1 ex.).
Humerobatidae
(19) Humerobates rostrolamellatus Grandjean 1936. Distribution: Semicosmopolitan; New Caledonia: localities 2 (1 ex.), 14 (1 ex.), 15 (1 ex.).
Punctoribatidae
(20) Punctoribates (Minguezetes) insignis Berlese 1910. Distribution: Neotropical and Subtropical regions, Vietnam; New Caledonia: locality 15 (2 ex.). Thе species is recorded in the Australian region for the first time.
Galumnidae
(21) Galumna acutirostrum Ermilov et Anichkin 2010. Distribution: Vietnam; New Caledonia: locality 15 (17 ex.). Thе species is recorded in the Australian region for the first time.
(22) Galumna naturalisi Ermilov 2017. Distribution: Colombia; New Caledonia: locality 2 (3 ex.). Thе species is recorded in the Australian region for the first time.
(23) Galumna rugosa Hammer 1968. Distribution: New Zealand; New Caledonia: localities 2 (1 ex.), 3 (2 ex.).
(24) Galumna triquetra Aoki 1965. Distribution: Oriental and Australian regions; New Caledonia: localities 2 (5 ex.), 3 (1 ex.), 11 (2 ex.).
(25) Galumna valida Aoki 1994. Distribution: Australian region; New Caledonia: locality 8 (1 ex.).
(26) Pergalumna altera (Oudemans 1915). Distribution: Semicosmopolitan; New Caledonia: locality 2 (1 ex.).
(27) Pergalumna bimaculata Hammer 1973. Distribution: Australian region, Philippines; New Caledonia: locality 15 (1 ex.).
(28) Pergalumna caledonica sp. n. Distribution: New Caledonia: localities 2 (1 ex.), 4 (1 ex.), 10 (1 ex.), 15 (6 ex.).
(29) Pergalumna hawaiiensis (Jacot 1934). Distribution: Australian and Oriental regions; New Caledonia: locality 2 (1 ex.).
(30) Pergalumna paraelongata Ermilov et Anichkin 2012. Distribution: Vietnam; New Caledonia: locality 12 (3 ex.). Thе species is recorded in the Australian region for the first time.
(31) Pergalumna weberi (Jacot 1935). Distribution: USA (Florida); New Caledonia: localities 2 (1 ex.), 15 (7 ex.). Thе species is recorded in the Australian region for the first time.
Remarks. The list of the identified oribatid mites from New Caledonia includes 31 species from 20 genera and 16 families. Six species (Mochlozetes penetrabilis, Punctoribates (Minguezetes) insignis, Galumna acutirostrum, G. naturalisi, Pergalumna paraelongata, P. weberi) are recorded for the first time in the Australian region.
SYSTEMATICS
Pergalumna caledonica Ermilov et Mary sp. n. (Figs 2, 3)
Fig. 2.
Pergalumna caledonica sp. n., adult: a – dorsal view (legs not shown); b – ventral view (gnathosoma, legs and right pteromorph not shown); c – anterior part of prodorsum, dorsolateral view; d – subcapitulum, ventral view (left half not shown partially); e – right lip with adoral setae; f – palp, right, paraxial view (genu, tibia and tarsus turned by 180 degrees); g – chelicera, left, paraxial view (posterior part not shown). Scale bar (µm): a–c – 100; d–g – 20.
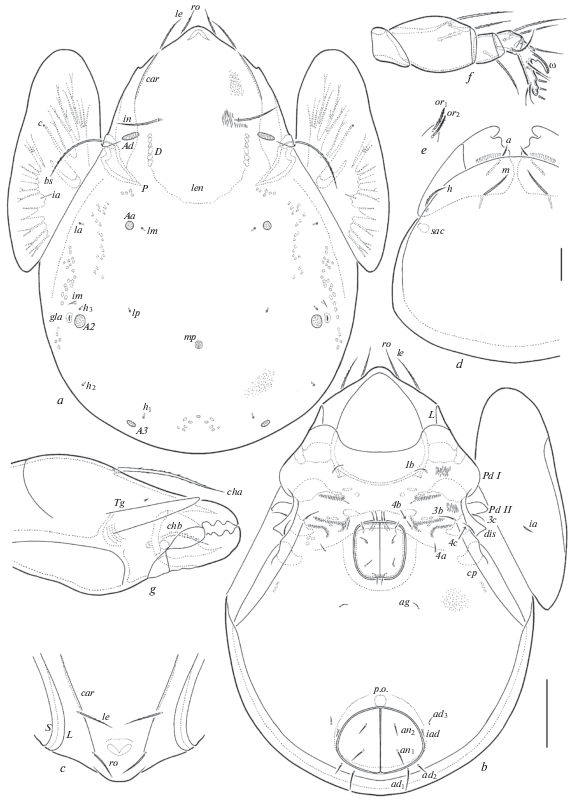
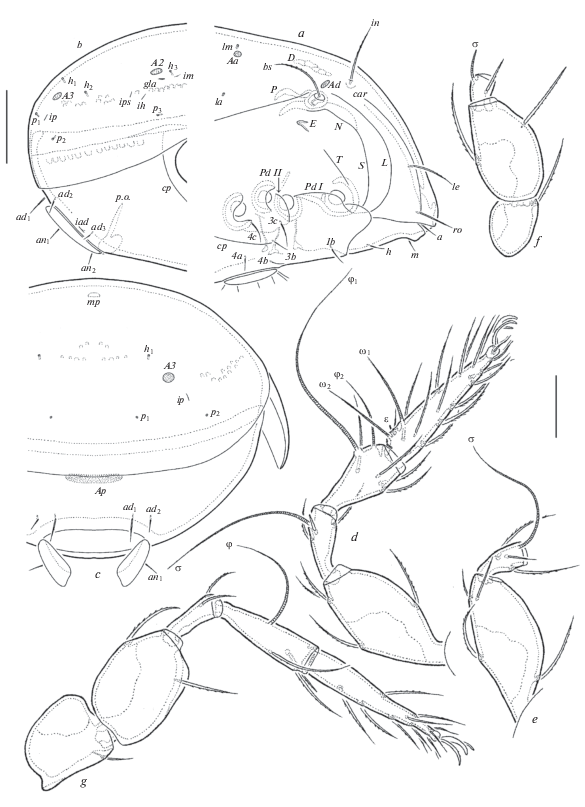
Fig. 3.
Pergalumna caledonica sp. n., adult: a – anterior part of body, lateral view (legs not shown); b – posterior part of body, lateral view; c – posterior view (left half not shown partially); d – leg I, without trochanter, right, antiaxial view; e – femur and genu of leg II, right, antiaxial view; f – trochanter, femur and genu of leg III, left, antiaxial view; g – leg IV, left, antiaxial view. Scale bar (µm): a–c – 100; d–g – 50.
Material. Holotype (♂) and 5 paratypes (3 ♀♀, 2 ♂♂): New Caledonia, Main island (Grande Terre), river Xwê Nuo, stream, site AVAL_CET 2007FW002, 166°0′43.6878″ E, 21°32′28.5673″ S, alt. 10 m, 11 December 2007 (collected by C. Flouhr, Hytec). Three specimens of this species from localities 2, 4 and 10 are not included in the type series.
The holotype (ethanol with drop of glycerol) is deposited in the collection of the Senckenberg Museum of Natural History, Görlitz, Germany; 5 paratypes (ethanol with drop of glycerol) are deposited in the collection of the Tyumen State University Museum of Zoology, Tyumen, Russia.
Diagnosis. Body size: 647–713 × 464–498. Body surface microgranulate; basal part of prodorsum and lateral parts of epimeres I, II striate. Rostrum rounded. Prodorsum with one pair of long, longitudinal carinae. Rostral, lamellar, interlamellar and bothridial setae long, setiform, barbed. Dorsosejugal suture absent. Notogaster with lenticulus and three pairs of rounded porose areas, A1 absent, Aa located between la and lm, close to lm. Median pore present. Epimeral setal formula: 1–0–2–3. Epimeral and anogenital setae setiform, slightly barbed. Adanal setae ad1 longer than ad2 and ad3. Circumpedal carinae directed to insertions of setae 3b. Postanal porose area elongate oval. Leg solenidion on tibiae IV inserted in the middle part of the segment.
Description. Measurements. Body length: 647 (holotype), 647–713 (paratypes); notogaster width: 464 (holotype), 464–498 (paratypes). No clear differences between females and males in body size, but females usually larger.
Integument. Body color brown. Body surface (including subcapitular mentum, genital and anal plates) densely microgranulate (diameter of granules less than 1), visible under high magnification. In addition, basal part of prodorsum and lateral parts of epimeres I, II with short, thin stria.
Prodorsum (Figs 1a, 1c, 2a). Rostrum slightly protruding, rounded. With one pair of long, longitudinal carinae. Lamellar and sublamellar lines thin, parallel, curving backwards at ventral ends. Prodorsal leg niches and lateral ridges of prodorsum well developed. Rostral (53–65), lamellar (61–73) and interlamellar (82–86) setae setiform, barbed. Insertions of rostral and lamellar setae located close to prodorsal carinae and clearly distanced from lamellar lines. Bothridial setae (123–143) setiform, barbed mediodistally. Exobothridial setae and their alveoli absent. Sejugal porose areas (24–28 × 8) elongate oval, transversely oriented, located posterolateral to interlamellar setae. Dorsophragmata long, longitudinally elongated.
Notogaster (Figs 1a, 2a–2c). Dorsosejugal suture absent. Lenticulus present, but poorly visible, without distinct borders. With 10 pairs of setal alveoli and three pairs of rounded porose areas (A1 absent), Aa (12–20) slightly larger than A2 and A3 (16–24). Areas Aa located between la and lm, close to lm and distanced from la. Median pore present, but poorly visible, large, located between A2. Opisthonotal gland openings and all lyrifissures distinct, im located close and anterolateral to A2, gla close and lateral to A2, ip between p2 and A3, ih and ips close to each other, medial to p3. Circumgastric sigillar slightly developed.
Gnathosoma (Figs 1d–1g). Subcapitulum size: 151–155 × 139–143. Subcapitular setae setifrom, similar in thickness, a (24–32) slightly longer than m and h (20–24). Adoral setae (16–20) setiform, heavily barbed. Length of palps: 114–123. Postpalpal setae (6) spiniform, smooth. Axillary saccules poorly visible. Length of chelicerae: 213–221. Cheliceral setae setiform, barbed, cha (61–69) longer than chb (41–45). Trägårdh’s organ of chelicerae long, elongate triangular.
Epimeral and lateral podosomal regions (Figs 1b, 2a). Anterior tectum of epimere I smooth. Pedotecta I narrowly rounded, pedotecta II narrowly rounded in ventral view. Discidia triangular. Epimeral setal formula: 1–0–2–3. Epimeral setae setiform, slightly barbed, 3c and 4c (36–41) longer than 1b and 3b (28–32) and 4a and 4b (12–16). Circumpedal carinae of medium size, thin, directed to insertions of setae 3b.
Anogenital region (Figs 1b, 2a–2c). Six pairs of genital (g1, g2, 18–20; others 12), one pair of aggenital (12), two pairs of anal (an1, 20–24; an2, 16–20) and three pairs of adanal (ad1, 32; ad2, 16–20; ad3, 12) setae setiform, slightly barbed. Anterior edge of genital plates with two setae, but third pair located also near to it. Aggenital setae located closer to genital plates than to anal plates. Adanal setae ad1 and ad2 posterior, ad3 lateral to anal aperture. Distance ad1–ad2 shorter than ad2–ad3. Adanal lyrifissures located close and parallel to anal plates. Postanal porose area present, but poorly visible, elongate oval (41–65 × 12–16).
Legs (Figs 2d–2g). Median claw distinctly thicker than laterals, all slightly barbed on dorsal side. Porose areas on all femora and on trochanters III, IV well visible. Formulas of leg setation and solenidia: I (1–4–3–4–20) [1–2–2], II (1–4–3–4–15) [1–1–2], III (1–2–1–3–15) [1–1–0], IV (1–2–2–3–12) [0–1–0]; homologies of setae and solenidia indicated in Table 1. Famulus on tarsi I inserted between seta ft '' and solenidion ω2. Solenidion on tibiae IV inserted in the middle part of the segment.
Table 1.
Leg setation and solenidia of adult Pergalumna caledonica sp. n.
| Leg | Tr | Fe | Ge | Ti | Ta |
|---|---|---|---|---|---|
| I | v' | d, (l), bv'' | (l), v', σ | (l), (v), φ1, φ2 | (ft), (tc), (it), (p), (u), (a), s, (pv), v ', (pl), l '', ɛ, ω1, ω2 |
| II | v' | d, (l), bv'' | (l), v', σ | (l), (v), φ | (ft), (tc), (it), (p), (u), (a), s, (pv), ω1, ω2 |
| III | v' | d, ev' | l ', σ | l ', (v), φ | (ft), (tc), (it), (p), (u), (a), s, (pv) |
| IV | v' | d, ev' | d, l ' | l ', (v), φ | ft '', (tc), (p), (u), (a), s, (pv) |
Remarks. Pergalumna caledonica sp. n. is morphologically most similar to Pergalumna remota (Hammer 1968) (see Hammer, 1968) from New Zealand and India in having: setiform bothridial setae; comparatively long rostral, lamellar and interlamellar setae; three pairs of rounded notogastral porose areas (Aa located close to setal alveoli lm); a large median pore; an elongate oval postanal porose area; and the absence of dorsosejugal suture. However, the new species differs from the latter by the presence of two longitudinal prodorsal carinae (versus absent in P. remota).
Also, Pergalumna caledonica sp. n. is similar to Pergalumna minipora Ermilov, Chatterjee, Kumar Das, Bordoloi 2014 (see Ermilov et al., 2014) from India in having two longitudinal prodorsal carinae. However, the new species differs from P. minipora by a smaller body size (647–713 × 464–498 versus 1162–1278 × × 898–1012); the presence of long interlamellar setae (versus absent); and a rounded (versus pointed) rostrum.
Etymology. The species name caledonica refers to the place of origin of the new species, New Caledonia.
Список литературы
Balogh J., Balogh P., 1983. New oribatids (Acari) from the Pacific region // Acta Zoologica Academiae Scientiarum Hungaricae. V. 29. № 4. P. 303–325.
Ermilov S.G., Friedrich S., 2019. To the knowledge of oribatid mites (Acari, Oribatida) of Samoa // Systematic and Applied Acarology. V. 24. № 1. P. 118–131.
Ermilov S.G., Klimov P.B., 2017. Generic revision of the large-winged mite superfamily Galumnoidea (Acari, Oribatida) of the world // Zootaxa. V. 4357. № 1. P. 1–72.
Ermilov S.G., Starý J., 2018. New taxa of oribatid mites from Korup National Park (Cameroon). The genus Pergalumna (Acari, Oribatida, Galumnidae), with description of three new species and a key to known species from the Ethiopian region // Zootaxa. V. 4425. № 2. P. 201–222.
Ermilov S.G., Alvarado-Rodríguez O., Retana-Salazar A.P., 2014. Two new species of Pergalumna (Acari, Oribatida, Galumnidae) from Costa Rica, including a key to all species of the genus from the Neotropical region // ZooKeys. № 435. P. 7–23.
Ermilov S.G., Chatterjee T., Das M.K., Bordoloi S., 2014. Three new species of oribatid mites of the genus Pergalumna (Acari: Oribatida: Galumnidae) from India // Biologia. V. 69. № 4. P. 489–497.
Ermilov S.G., Tolstikov A.V., Mary N., Schatz H., 2013. Oribatid mites (Acari, Oribatida) from riverine environments of some islands in Oceania // ZooKeys. № 318. P. 47–57.
Ermilov S.G., Sandmann D., Klarner B., Widyastuti R., Scheu S., 2015. Contributions to the knowledge of oribatid mites of Indonesia. 2. The genus Pergalumna (Galumnidae) with description of a new species and key to known species in the Oriental region (Acari, Oribatida) // ZooKeys. № 529. P. 87–103.
Hammer M., 1968. Investigations on the Oribatid fauna of New Zealand with a comparison between the oribatid fauna of New Zealand and that of the Andes Mountains, South America. Part III // Det Kongelige Danske Videnskabernes Selskab Biologiske Skrifter. V. 16. № 2. P. 1–96.
Niedbała W., 2000. The ptyctimous mites of the Oriental and Australian regions and their centers of its origin (Acari: Oribatida) // Genus. Supplement. P. 1–493.
Niedbała W., Pentinnen R., 2007. New species of ptyctimous mites (Acari, Oribatida, Steganacaridae) with some new records from Australasian region // Annales Zoologici. V. 57. № 3. P. 517–532.
Olszanowski Z., 1997. On the genus Holonothrus (Acari: Oribatida) in the Australian Region // Acarologia. V. 38. № 4. P. 385–402.
Subías L.S., 2004. Listado sistemático, sinonímico y biogeográfico de los ácaros oribátidos (Acariformes: Oribatida) del mundo (excepto fósiles) // Graellsia. V. 60 (número extraordinario). P. 3–305. Online version accessed in January 2018, 605 p.; http://bba.bioucm.es/cont/docs/RO_1.pdf
Дополнительные материалы отсутствуют.
Инструменты
Зоологический журнал