Зоологический журнал, 2021, T. 100, № 2, стр. 159-169
Roncus ladestani sp. n. and Roncus pecmliniensis sp. n., two new Pseudoscorpions (Pseudoscorpiones, Neobisiidae) from Croatia and Bosnia and Herzegovina, respectively
B.P.M. Ćurčić a, T. Rađa b, *, R.N. Dimitrijević a, **, N.B. Ćurčić c, ***, S.B. Ćurčić a, ****
a Institute of Zoology, University of Belgrade – Faculty of Biology
11000 Belgrade, Serbia
b Špiljar Speleological Society
21000 Split, Croatia
c Geographical Institute “Jovan Cvijić”, Serbian Academy of Sciences and Arts
11000 Belgrade, Serbia
* E-mail: tonci.radja1@gmail.com
** E-mail: rajko@bio.bg.ac.rs
*** E-mail: n.curcic@gi.sanu.ac.rs
**** E-mail: srecko@bio.bg.ac.rs
Поступила в редакцию 25.11.2019
После доработки 15.07.2020
Принята к публикации 23.07.2020
Аннотация
Two new species of pseudoscorpions, Roncus ladestani sp. n. and R. pecmliniensis sp. n. (Pseudoscorpiones, Neobisiidae), from two underground sites [the Jama višje Zađa (= Jama za Zle Poje) Pit, island of Lastovo, Dalmatia, Croatia; and the Ravlića Pećina Cave, hamlet of Peć Mlini, village of Drinovci, near Grude, Herzegovina, Bosnia and Herzegovina], are described and diagnosed. All important morphological features of the new species are listed and illustrated. The taxa are compared with their close relatives inhabiting surrounding areas on the Balkan Peninsula. The new pseudoscorpions are endemic to the Dinaric mountain chain.
The richness of the pseudoscorpion fauna of the Balkan Peninsula varies from country to country, the highest numbers of taxa (species and subspecies) in the region having been recorded in Croatia (143, of which 121 are species and 22 are subspecies) (Ozimec, 2004; Ćurčić et al., 2012, 2012a, 2012b, 2012c, 2012d, 2012e, 2013, 2013a, 2013c, 2014, 2014a, 2015; Harvey, 2013; Dimitrijević, Rađa, 2016), Greece (142, of which 120 are species and 22 are subspecies) (Harvey, 2013) and Bosnia and Herzegovina (83, of which 59 are species and 24 are subspecies) (Harvey, 2013; Ćurčić et al., 2014, 2014b, 2014c; Dimitrijević, Rađa, 2017).
The genus Roncus L. Koch 1873 includes about 140 species and is endemic to Europe, North Africa, Southwest Asia and the Caucasus region (Harvey, 2013). Its taxa chiefly inhabit leaf litter and soil (Gabbutt, Vachon, 1967), as well as caves. Božidar Ćurčić and co-workers described about 58 species and subspecies of Roncus from the region of the Balkan Peninsula over the last 30 years, most of them being of epigean facies (Mahnert, Gardini, 2014). In total, 17 species of Roncus are currently known in Croatia, of which 11 are cave-dwelling (Ćurčić, 1988; Ćurčić et al., 2012c, 2012d, 2012e, 2014; Harvey, 2013). The territory of Bosnia and Herzegovina is inhabited by only six Roncus species, of which five are cavernicolous (Ćurčić, 1988; Ćurčić, Dimitrijević, 2007; Harvey, 2013; Ćurčić et al., 2014, 2014b). The cave-dwelling Roncus taxa are distributed in a wider Dinaric area and are mostly stenoendemics.
Well-expressed morphological variability, both interspecific and intraspecific, is observed in the diverse genus Roncus, especially among the Balkan taxa (Ćurčić, 1988; Zaragoza, Šťáhlavský, 2008; Šťáhlavský et al., 2013). Some Roncus species from the Balkan Peninsula are roncoid in form, i.e., epigean in appearance (Zaragoza et al., 2007), while others are parablothroid, cave-dwelling in appearance (Gardini, 1982).
Certain karstic regions remain still unexplored in both Croatia and Bosnia and Herzegovina, where findings of further new pseudoscorpion taxa can be expected in the future.
Several field trips in Dalmatia (southern Croatia) and Herzegovina (southwestern Bosnia and Herzegovina) conducted by the Špiljar Speleological Society (Split, Croatia) in 1997 and 2012 resulted in the discovery of two pseudoscorpion species new to science, descriptions and diagnostic characters of which are presented in the current study.
The presence of two new Roncus species from Dalmatia (Croatia) and Herzegovina (Bosnia and Herzegovina) confirms that Roncus populations in the Dinaric mountain chain show a high level of endemism.
METHODS
Specimens of pseudoscorpions were collected manually at two investigated cave localities in Croatia and Bosnia and Herzegovina. The samples were then studied in the laboratory at the Institute of Zoology, University of Belgrade – Faculty of Biology, Belgrade, Serbia. The collected individuals were dissected and mounted on microscope slides in glycerol, measured, illustrated and then fixed in a medium composed of Canada balsam and xylol. All important morphological features were analysed for comparison. A Carl Zeiss - Axioskop 40 microscope with a drawing attachment was used in this study.
Setal designations follow Beier (1963).
The following abbreviations are used: IZFB – collection of the Institute of Zoology, University of Belgrade – Faculty of Biology, Belgrade, Serbia; NHMS – collection of the Natural History Museum in Split R – range of total measurements performed.
SYSTEMATICS
Roncus ladestani Dimitrijević et B. Ćurčić sp. n. (Figs 1a–1h; Table 1)
Fig. 1.
Roncus ladestani sp. n., holotype male: a – pedipalpal chela, b – pedipalp, c – leg IV, d – chelicera, e – epistome, f – carapace, g – flagellum, h – abdominal sternites III and IV. Scale bar (mm): a–c, f – 0.25; d, e, g, h – 0.50.
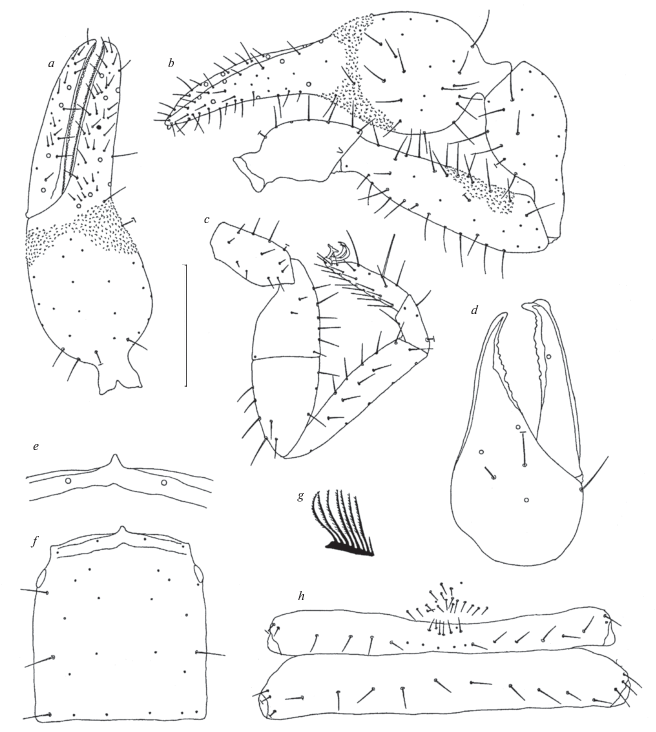
Table 1.
Linear measurements (in millimetres) and morphometric ratios in Roncus ladestani sp. n., R. trojanicus, R. diocletiani and R. almissae (modified after Ćurčić, 1988; Ćurčić et al., 2008, 2010, 2013b)
| Character | R. ladestani | R. trojanicus | R. diocletiani | R. almissae | |
|---|---|---|---|---|---|
| M | F | F | M | F | |
| Body | |||||
| Length (1) | 3.22 | 4.445 | 3.115 | 3.09 | 4.00 |
| Cephalothorax | |||||
| Length (2) | 0.805 | 1.10 | 0.815 | 0.88 | 0.97 |
| Breadth (2a) | 0.69 | 0.95 | 0.61 | 0.72 | 0.805 |
| Ratio 2/2a | 1.17 | 1.16 | 1.35 | 1.22 | 1.20 |
| Abdomen | |||||
| Length | 2.415 | 3.57 | 2.30 | 2.21 | 3.03 |
| Chelicerae | |||||
| Length (3) | 0.53 | 0.59 | 0.48 | 0.51 | 0.55 |
| Breadth (4) | 0.27 | 0.33 | 0.25 | 0.275 | 0.295 |
| Length of movable finger (5) | 0.38 | 0.40 | 0.34 | 0.36 | 0.40 |
| Ratio 3/5 | 1.39 | 1.475 | 1.41 | 1.42 | 1.375 |
| Ratio 3/4 | 1.96 | 1.79 | 1.92 | 1.85 | 1.86 |
| Pedipalps | |||||
| Length with coxa (6) | 4.29 | 6.895 | 3.98 | 4.30 | 4.825 |
| Ratio 6/1 | 1.33 | 1.48 | 1.28 | 1.39 | 1.21 |
| Length of coxa | 0.60 | 0.84 | 0.60 | 0.61 | 0.68 |
| Length of trochanter | 0.53 | 0.665 | 0.49 | 0.54 | 0.56 |
| Length of femur (7) | 0.92 | 1.26 | 0.835 | 0.815 | 1.00 |
| Breadth of femur (8) | 0.24 | 0.30 | 0.24 | 0.26 | 0.305 |
| Ratio 7/8 | 3.83 | 4.20 | 3.48 | 3.13 | 3.28 |
| Ratio 7/2 | 1.14 | 1.145 | 1.02 | 0.93 | 1.03 |
| Length of patella (tibia) (9) | 0.75 | 0.96 | 0.71 | 0.75 | 0.815 |
| Breadth of patella (tibia) (10) | 0.34 | 0.40 | 0.315 | 0.34 | 0.36 |
| Ratio 9/10 | 2.205 | 2.40 | 2.25 | 2.205 | 2.26 |
| Length of chela (11) | 1.49 | 1.88 | 1.345 | 1.585 | 1.77 |
| Breadth of chela (12) | 0.48 | 0.62 | 0.47 | 0.52 | 0.58 |
| Ratio 11/12 | 3.10 | 3.03 | 2.86 | 3.05 | 3.05 |
| Length of chelal palm (13) | 0.72 | 0.91 | 0.69 | 0.805 | 0.87 |
| Ratio 13/12 | 1.50 | 1.47 | 1.47 | 1.55 | 1.50 |
| Length of chelal finger (14) | 0.77 | 0.97 | 0.65 | 0.78 | 0.90 |
| Ratio 14/13 | 1.07 | 1.065 | 0.94 | 0.97 | 1.03 |
| Leg IV | |||||
| Total length | 3.11 | 3.93 | 2.71 | 2.975 | 3.265 |
| Length of coxa | 0.46 | 0.61 | 0.43 | 0.39 | 0.44 |
| Length of trochanter (15) | 0.38 | 0.47 | 0.34 | 0.36 | 0.42 |
| Breadth of trochanter (16) | 0.17 | 0.21 | 0.16 | 0.18 | 0.18 |
| Ratio 15/16 | 2.235 | 2.24 | 2.125 | 2.00 | 2.33 |
| Length of femur + patella (17) | 0.845 | – | 0.74 | 0.815 | 0.91 |
| Breadth of femur + patella (18) | 0.285 | – | 0.26 | 0.35 | 0.33 |
| Ratio 17/18 | 2.96 | – | 2.85 | 2.33 | 2.76 |
| Length of tibia (19) | 0.74 | 0.97 | 0.63 | 0.77 | 0.855 |
| Breadth of tibia (20) | 0.16 | 0.16 | 0.12 | 0.15 | 0.15 |
| Ratio 19/20 | 4.625 | 6.06 | 5.25 | 5.13 | 5.70 |
| Length of metatarsus (21) | 0.285 | 0.34 | 0.21 | 0.24 | 0.22 |
| Breadth of metatarsus (22) | 0.10 | 0.12 | 0.09 | 0.11 | 0.11 |
| Ratio 21/22 | 2.85 | 2.83 | 2.33 | 2.18 | 2.00 |
| Length of tarsus (23) | 0.40 | 0.48 | 0.36 | 0.40 | 0.42 |
| Breadth of tarsus (24) | 0.09 | 0.11 | 0.08 | 0.10 | 0.10 |
| Ratio 23/24 | 4.44 | 4.36 | 4.50 | 4.00 | 4.20 |
| TS ratio – tibia IV | 0.55 | 0.57 | 0.60 | 0.85 | 0.595 |
| TS ratio – metatarsus IV | 0.21 | 0.20 | 0.19 | 0.17 | 0.23 |
| TS ratio – tarsus IV | 0.38 | 0.385 | 0.31 | 0.38 | 0.39 |
Material. The holotype (♂), labelled as follows: “Croatia, Dalmatia, island of Lastovo, Jama višje Zađa (= Jama za Zle Poje) Pit, latitude 42°45′22.5″ N, longitude 16°52′32.5″ E, 12.VI.1997 (T. Rađa)” (white label, printed)/Holotypus Roncus ladestani sp. n. Dimitrijević et B. Ćurčić det. 2015 (red label, printed) (NHMS).
Diagnosis. Roncus ladestani sp. n. is phenetically and geographically close to the following species inhabiting Dalmatia (Croatia): R. trojanicus Ćurčić 1988, from the Baretina Špilja (= Grota) Cave, village of Okrug Gornji, near Trogir, island of Čiovo, Dalmatia; R. diocletiani Ćurčić, Dimitrijević et Rađa 2008, Omit “from Marasovića,” southern slope of the Marjan hill, Split, Dalmatia; and R. almissae B. Ćurčić, Rađa, S. Ćurčić et N. Ćurčić 2010, from the village of Podašpilje, northern slope of Mt. Omiška Dinara, near Omiš, Dalmatia (Fig. 2) (sharing the presence of eyes and of granulations on the pedipalpal articles). It differs from its close congeners in a number of characters: length of the carapace (0.805 mm in the male vs. 1.10 mm in the female of R. trojanicus vs. R 0.88–0.97 mm in both sexes of R. almissae), the ratio of carapace length to breadth (1.17 in the male vs. 1.35 in the female of R. diocletiani vs. R 1.20–1.22 in both sexes of R. almissae), cheliceral length (0.53 mm in the male vs. 0.59 mm in the female of R. trojanicus vs. 0.48 mm in the female of R. diocletiani), the ratio of cheliceral length to breadth (1.96 in the male vs. 1.79 in the female of R. trojanicus vs. R 1.85–1.86 in both sexes of R. almissae), pedipalpal length (4.29 mm in the male vs. 6.895 mm in the female of R. trojanicus vs. 3.98 mm in the female of R. diocletiani), the ratio of pedipalpal femur length to breadth (3.83 in the male vs. 4.20 in the female of R. trojanicus vs. 3.48 in the female of R. diocletiani vs. R 3.13–3.28 in both sexes of R. almissae), the ratio of pedipalpal femur length to carapace length (1.14 in the male vs. 1.02 in the female of R. diocletiani vs. R 0.93–1.03 in both sexes of R. almissae), the ratio of pedipalpal chela length to breadth (3.10 in the male vs. 3.03 in the female of R. trojanicus vs. 2.86 in the female of R. diocletiani vs. R 3.05 in both sexes of R. almissae), the presence/absence of microsetae close to trichobothrium eb on the pedipalpal chela (present in the male vs. absent from the female of R. trojanicus), leg IV length (3.11 mm in the male vs. 3.93 mm in the female of R. trojanicus vs. 2.71 mm in the female of R. diocletiani), the ratio of femur IV length to breadth (2.96 in the male vs. 2.85 in the female of R. diocletiani vs. R 2.33–2.76 in both sexes of R. almissae), the degree of granulation of the pedipalps (trochanter with no granulations, femur with some granulations on its interior side distally, chela with granulations both on its exterior and interior sides in the male vs. pedipalps mostly smooth, only a few inconspicuous tubercles borne on femur both anteriorly and laterally in the female of R. trojanicus vs. trochanter with granulations, femur with well-developed granulations, chela with granulations on its interior side in the female of R. diocletiani vs. trochanter with no granulations, femur with some granulations on its interior side medially, chela with some granulations on its interior side in both sexes of R. almissae) and many other morphometric ratios and linear measurements (Figs 1a–1h; Table 1) (Ćurčić, 1988; Ćurčić et al., 2008, 2010, 2013b).
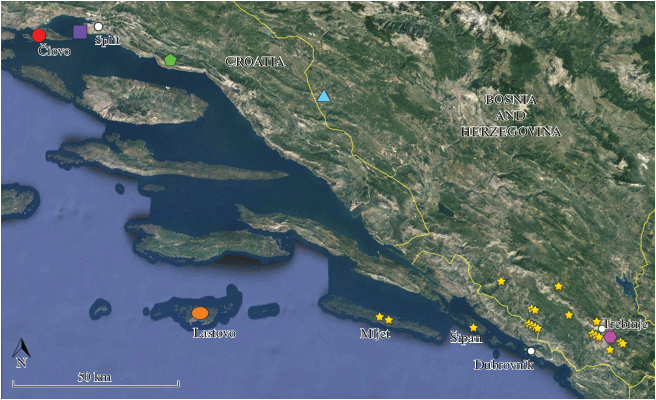
Fig. 2.
Distribution of new pseudoscorpion species from Croatia and Bosnia and Herzegovina and related species: red circle – R. trojanicus, violet square – R. diocletiani, green pentagon – R. almissae, blue triangle – R. pecmliniensis sp. n., orange ellipse – R. ladestani sp. n., yellow star – R. anophthalmus, pink hexagon – R. travuniensis.
Description. Body colour brownish. Carapace longer than broad, with a pair of eyes (Fig. 1f ). Epistome triangular, well-developed (Fig. 1e). Carapace with 26 setae in four rows (4 + 8 + 8 + 6). Preocular microsetae absent.
Setal formula of abdominal tergites I–X: 8–11–11–11–11–12–12–11–19–9. Abdominal sternite II carrying 26 setae. Sternite III with 22 setae (15 in posterior row) and two suprastigmal microsetae on each side (Fig. 1h). Sternite IV carrying 10 setae and three suprastigmal microsetae on each side. Sternites V–X with 16–15–15–13–11–11 posterior setae.
Cheliceral spinneret well-developed (Fig. 1d). Cheliceral palm with six setae, movable finger with one seta. Flagellum eight-bladed. All flagellar blades pinnate along anterior margins (Fig. 1g).
Pedipalpal coxa with four long setae. Fixed and movable pedipalpal fingers with eight and four trichobothria, respectively (Fig. 1a). Teeth of both fingers small, close-set, occupying almost whole length of fingers. Fixed pedipalpal finger with 62 teeth, movable pedipalpal finger bearing 58 teeth (Fig. 1a; Table 1).
Pedipalpal trochanter and tibia smooth. Some fine granulations on interior side of pedipalpal femur distally. Pedipalpal chela ovate, with granulations on both interior and exterior sides and a few microsetae close to trichobothrium eb (Figs 1a, 1b).
Certain morphological structures (pedipalpal lyrifissures, nodus ramosus, micropores on the patellar and chelal pedicel, more detailed structure of the coxa, genital apparatus) could not be observed due to the condition of the specimen.
Trichobothriotaxy. Trichobothria eb, esb, ib and isb at finger base; esb slightly distal to eb; it closer to eb than to ib; trichobothrium ist closer to isb than to est, medially located on pedipalpal chela. Trichobothria b and sb in proximal finger half, st and t in distal finger half. Trichobothrium b closer to st than to finger tip. Distance sb–st longer than b–sb; t–st shorter than b–sb or sb–st.
Metatarsus IV and tarsus IV each with a single tactile seta (Fig. 1c).
Measurements and morphometric ratios of different morphological structures as presented in Table 1.
Distribution. At the present time, the new species is known to inhabit only its type locality: Jama višje Zađa (= Jama za Zle Poje) Pit, island of Lastovo, Dalmatia, southern Croatia (Fig. 2).
Etymology. The name of the new species is derived from Ladestanos, the Greek name for the Adriatic island of Lastovo (Dalmatia, Croatia), its terra typica.
Roncus pecmliniensis B. Ćurčić et Rađa sp. n. (Figs 3a–3h; Table 2)
Fig. 3.
Roncus pecmliniensis sp. n., holotype female: a – pedipalpal chela, b – flagellum, c – pedipalp, d – chelicera, e – epistome, f – leg IV, g – carapace, h – genital area. Scale bar (mm): a, c, f, g – 0.25; b, d, e, h – 0.50.
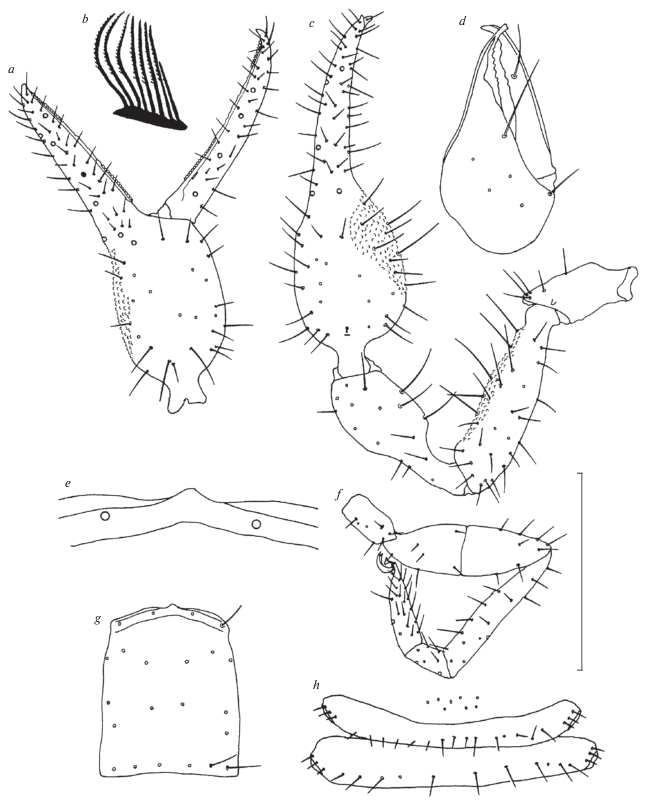
Table 2.
Linear measurements (in millimetres) and morphometric ratios in Roncus pecmliniensis sp. n., R. travuniensis and R. anophthalmus (modified after Ćurčić et al., 1995; Ćurčić, Dimitrijević, 2007)
| Character | R. pecmliniensis | R. travuniensis | R. anophthalmus |
|---|---|---|---|
| F | F | F | |
| Body | |||
| Length (1) | 2.855 | 2.845 | 3.09 |
| Cephalothorax | |||
| Length (2) | 0.855 | 0.92 | 1.03 |
| Breadth (2a) | 0.73 | 0.80 | 0.91 |
| Ratio 2/2a | 1.17 | 1.15 | 1.13 |
| Abdomen | |||
| Length | 2.00 | 1.925 | 2.06 |
| Chelicerae | |||
| Length (3) | 0.58 | 0.64 | 0.67 |
| Breadth (4) | 0.285 | 0.33 | 0.35 |
| Length of movable finger (5) | 0.40 | 0.44 | 0.49 |
| Ratio 3/5 | 1.45 | 1.45 | 1.37 |
| Ratio 3/4 | 2.035 | 1.94 | 1.91 |
| Pedipalps | |||
| Length with coxa (6) | 5.21 | 5.83 | 5.97 |
| Ratio 6/1 | 1.82 | 2.03 | 1.93 |
| Length of coxa | 0.72 | 0.75 | 0.82 |
| Length of trochanter | 0.62 | 0.67 | 0.70 |
| Length of femur (7) | 1.05 | 1.19 | 1.24 |
| Breadth of femur (8) | 0.275 | 0.305 | 0.33 |
| Ratio 7/8 | 3.82 | 3.90 | 3.76 |
| Ratio 7/2 | 1.23 | 1.29 | 1.20 |
| Length of patella (tibia) (9) | 0.89 | 1.02 | 1.02 |
| Breadth of patella (tibia) (10) | 0.36 | 0.41 | 0.41 |
| Ratio 9/10 | 2.47 | 2.49 | 2.49 |
| Length of chela (11) | 1.93 | 2.20 | 2.19 |
| Breadth of chela (12) | 0.57 | 0.63 | 0.68 |
| Ratio 11/12 | 3.385 | 3.49 | 3.22 |
| Length of chelal palm (13) | 0.89 | 1.11 | 1.14 |
| Ratio 13/12 | 1.56 | 1.76 | 1.68 |
| Length of chelal finger (14) | 1.04 | 1.09 | 1.05 |
| Ratio 14/13 | 1.17 | 0.98 | 0.92 |
| Leg IV | |||
| Total length | 3.23 | 3.58 | 3.335 |
| Length of coxa | 0.51 | 0.41 | 0.58 |
| Length of trochanter (15) | 0.36 | 0.45 | 0.445 |
| Breadth of trochanter (16) | 0.15 | 0.17 | 0.22 |
| Ratio 15/16 | 2.40 | 2.65 | 2.02 |
| Length of femur + patella (17) | 0.855 | 0.99 | – |
| Breadth of femur + patella (18) | 0.25 | 0.315 | – |
| Ratio 17/18 | 3.42 | 3.14 | – |
| Length of tibia (19) | 0.78 | 0.94 | 0.55 |
| Breadth of tibia (20) | 0.14 | 0.15 | 0.14 |
| Ratio 19/20 | 5.57 | 5.875 | 3.93 |
| Length of metatarsus (21) | 0.275 | 0.30 | 0.30 |
| Breadth of metatarsus (22) | 0.11 | 0.11 | 0.11 |
| Ratio 21/22 | 2.09 | 2.73 | 2.73 |
| Length of tarsus (23) | 0.45 | 0.49 | 0.48 |
| Breadth of tarsus (24) | 0.10 | 0.10 | 0.11 |
| Ratio 23/24 | 4.50 | 4.90 | 4.36 |
| TS ratio – tibia IV | 0.57 | 0.47 | 0.54 |
| TS ratio – metatarsus IV | 0.22 | 0.17 | 0.23 |
| TS ratio – tarsus IV | 0.36 | 0.35 | 0.36 |
Material. The holotype (♀), labelled as follows: “Bosnia and Herzegovina, Herzegovina, Grude, village of Drinovci, hamlet of Peć Mlini, Ravlića Pećina Cave, latitude 43°20′20.8″ N, longitude 17°19′24.0″ E, 26.VIII.2012 (T. Rađa)” (white label, printed)/Holotypus Roncus pecmliniensis sp. n. B. Ćurčić et Rađa det. 2015 (red label, printed) (IZFB).
Diagnosis. The two closest known congeners of the new species are Roncus travuniensis Ćurčić et Dimitrijević 2007 (from the Arenstorfova Pećina Cave, Petrina hill, near Trebinje, southern Herzegovina) and R. anophthalmus (Ellingsen 1910) (from a number of caves and pits in Herzegovina and on the islands of Mljet and Šipan, Dalmatia) (Fig. 2). Roncus pecmliniensis sp. n. shares with them the absence of eyes and has a similar ratio of pedipalpal length to breadth and a similar ratio of tibia of leg IV length to breadth, but differs clearly in pedipalpal length (5.21 mm in the female vs. 5.83 mm in the female of R. travuniensis vs. 5.97 mm in the female of R. anophthalmus), the ratio of pedipalpal chela length to breadth (3.385 in the female vs. 3.49 in the female of R. travuniensis vs. 3.22 in the female of R. anophthalmus), the ratio of pedipalpal chelal finger length to chelal palm length (1.17 in the female vs. 0.98 in the female of R. travuniensis vs. 0.92 in the female of R. anophthalmus), the presence/absence of microsetae close to trichobothrium eb on the pedipalpal chela (absent from the female vs. present in the female of R. travuniensis), the setal formula of abdominal tergites I–X (7–10–11–11–12–11–11–11–10–10 in the female vs. 6–8–9–10–10–10–9–9–9–8 in the female of R. travuniensis vs. 6–6–10–11–11–11–11–11–11–11 in the female of R. anophthalmus), the ratio of trochanter IV length to breadth (2.40 in the female vs. 2.65 in the female of R. travuniensis vs. 2.02 in the female of R. anophthalmus), the ratio of femur + patella IV length to breadth (3.42 in the female vs. 3.14 in the female of R. travuniensis), the ratio of metatarsus IV length to breadth (2.09 in the female vs. 2.73 in the female of R. travuniensis vs. 2.73 in the female of R. anophthalmus), the ratio of tarsus IV length to breadth (4.50 in the female vs. 4.90 in the female of R. travuniensis vs. 4.36 in the female of R. anophthalmus) and many other morphometric ratios and linear measurements (Figs 3a–3h; Table 2) (Beier, 1938, 1963; Ćurčić et al., 1995; Ćurčić, Dimitrijević, 2007). Specimens of R. anophthalmus from some Herzegovinian populations possess minute granulations in the middle part of the interolateral side of the pedipalpal femur, as in the case of R. pecmliniensis sp. n., while specimens of R. anophthalmus from Dalmatian populations and other Herzegovinian populations lack such granulations on the pedipalpal femur (Ćurčić et al., 1995). Granulation on the interior side of the pedipalpal chela in R. pecmliniensis sp. n. is more developed than that on the same podomere in R. travuniensis (Ćurčić, Dimitrijević, 2007).
Description. Body colour yellowish red. Carapace longer than broad (Fig. 3g; Table 2). Eyes not developed (Fig. 3g). Epistome small, apically rounded (Fig. 3e). Carapace with 22 setae in four rows (4 + 6 + + 6 + 6) (Fig. 3g). With no preocular microsetae. Carapace entirely reticulate.
Abdominal tergites and sternites uniseriate, entire, smooth. Setal formula of abdominal tergites I–X: 7–10–11–11–12–11–11–11–10–10. Abdominal sternite II with eight setae, sternite III with 15 posterior setae and three microsetae on each side, sternite IV with 11 posterior setae and three suprastigmal microsetae on each side (Fig. 3h). Sternites V–X with 13–14–13–13–13–12 setae. Abdominal segment XII with two pairs of small setae. Pleural membranes granulostriate.
Galea in the form of a slight elevation on movable cheliceral finger’s margin (Fig. 3d). Fixed cheliceral finger with six setae, movable cheliceral finger with one seta (Fig. 3d). Flagellum with eight blades, first seven of nearly equal size, pinnate along anterior margin, eighth blade smaller than others, not pinnate (Fig. 3b).
Apex of pedipalpal coxa with four long setae. Pedipalpal trochanter and tibia smooth, slender. Some fine granulations on interior side of pedipalpal femur medially. Pedipalpal chelal palm with some minute interior granulations (Figs 3a, 3c).
Fixed pedipalpal chelal finger with 56 teeth, movable pedipalpal chelal finger carrying 50 teeth (Fig. 3a). No microsetae close to trichobothrium eb on pedipalpal chela (Fig. 3a).
Certain morphological structures (pedipalpal lyrifissures, nodus ramosus, micropores on the patellar and chelal pedicel, more detailed structure of the coxa, genital apparatus) could not be observed due to the condition of the specimen.
Trichobothriotaxy. Trichobothria eb, esb, ib and isb at base of fixed finger. Esb slightly closer to ib than to eb. Trichobothrium it closer to est than to et; ist closer to isb than to est, medially located on pedipalpal chela; distance ist–ib shorter than ist–finger tip. Trichobothria b and sb in proximal finger half, st and t in distal finger half. Trichobothrium t closer to st than to finger tip. Distance sb–st somewhat longer than b–sb; t–st shorter than b–sb or sb–st (Figs 3a, 3c).
Tibia IV, metatarsus IV and tarsus IV each with a single tactile seta (Fig. 3f).
Measurements and morphometric ratios of different morphological structures as presented in Table 2.
Distribution. So far, the new species has been found only in the Ravlića Pećina Cave, village of Drinovci, Grude, Herzegovina, southwestern Bosnia and Herzegovina (Fig. 2).
Etymology. After the hamlet of Peć Mlini, village of Drinovci, where the Ravlića Pećina Cave, its terra typica, is located.
DISCUSSION
The type specimens of the new pseudoscorpion species were collected under stones in the inner, humid parts of the investigated underground sites, in total darkness (the distance of the collecting locality from the cave entrance and the locality’s depth were 25 and 8 m, respectively, for R. ladestani sp. n.; and 35 and 6 m, respectively, for R. pecmliniensis sp. n.). A few further efforts were made to collect additional individuals of the new pseudoscorpion species, but they were unsuccessful. Therefore, these taxa can be assumed to be rare. The presence of one pair of eyes in R. ladestani sp. n. indicates that this pseudoscorpion species seems to be epigeic in its lifestyle. The specimen most likely fell into the Jama višje Zađa (= Jama za Zle Poje) Pit through its entrance or through holes and fissures on its cap. The lack of eyes in R. pecmliniensis sp. n. suggests that this species is either cave-dwelling or endogean.
Due to morphological variability of the genus Roncus in the Balkan region, its systematics faces some difficulties. Traditional taxonomy is inadequate in some cases (e.g., in identification of closely related taxa), especially when the investigator uses pedipalp morphometry, with which only clearly different taxa can be separated (Zaragoza, Šťáhlavský, 2008). New taxonomy is more comprehensive and uses characteristics that were not previously considered (features of the legs, including the ratios and measurements of legs I and IV, form of the claws and subterminal setae, position of tactile setae on leg IV, structure of the genital apparatus, etc.) (Zaragoza, Šťáhlavský, 2008). For instance, the chelal microsetal pattern in Roncus, providing useful taxonomic characters, is of great help in distinguishing species and species groups (Gardini, 1983; Gardini, Rizzerio, 1985, 1986; Henderickx, Zaragoza, 2005).
The ratios of certain articles of appendages can be important as indicative of the way of life led by a given pseudoscorpion taxon. Contrary to epigean species, cave-dwelling pseudoscorpions have an enlarged body, elongated appendages and no eyes, while troglophilic forms are in an intermediate state. With respect to appendages, this pattern is particularly reflected in shape of the tibia and tarsus of legs I and IV, as well as in possession of enlarged pedipalps. The ratio of tibia IV length to breadth can be particularly useful in attempting to evaluate the degree of adaptation to cave life (Zaragoza, Šťáhlavský, 2008). Zaragoza and Šťáhlavský (2008) estimate that in Roncus an average ratio of tibia IV length to breadth of about 6.0 or higher suggests a troglophilic or troglobitic state of the species. The ratios in R. ladestani sp. n. (4.625) and R. pecmliniensis sp. n. (5.57) suggest that the former taxon exhibits epigean tendencies, while the latter one displays troglophilic affinities. The same authors state that a ratio of pedipalp femur length to width of about 4.0 points to cave-dwelling tendencies. To judge from the value of this ratio in it (3.54), R. ladestani sp. n. can again be treated as an epigean form, while the somewhat greater value of the ratio (3.82) in R. pecmliniensis sp. n. indicates its troglophilic condition.
These two new species probably belong to different evolutionary Roncus lineages. The relationships of numerous Balkan species of Roncus need to be further clarified (Mahnert, Gardini, 2014). As numerous Roncus species, including those described in the current study, are delimitated from each other on the basis of morphological and morphometric characters, there is a need for molecular taxonomic investigations in order to confirm the validity of these species. Special attention should be paid to this task in the future. A comprehensive approach, including several types of analysis, needs to be taken. This can be done by combining cytogenetic (karyological) analysis and DNA barcoding studies with comparison of morphological details (including structure of the genital apparatus, which is difficult to study properly, but which is becoming increasingly important in the systematics of Neobisiidae) in richer samples of individuals belonging to different taxa.
The colonization of Dinaric subterranean environments must have begun a long time ago and has passed through successive stages during different geological times, parallel with the development of karstic phenomena (Ćurčić, 1988). It is probable that the Dinaric area was colonized at the beginning of its existence by pseudoscorpions, which already inhabited Mediterranean forests. Cave-dwelling pseudoscorpions of the Dinarides represent the last vestiges of an old fauna, which found shelter in the underground domain of the Balkans (Ćurčić, 1988).
According to Guéorguiev (1977), different representatives of the genus Roncus originated or lived in regions and geological epochs with a humid and warm climate. With growing aridity and the creation of different niches underground, some taxa evolved as cave-dwelling and endogean inhabitants (Ćurčić, Dimitrijević, 2007), which is the case of R. pecmliniensis sp. n. Adaptation to life in caves, pits and deep soil represents an adjusting response of both epigean and humicolous species to survival in conditions of a typical or modified Mediterranean climate (Ćurčić, 1986, 1988).
The high diversity of pseudoscorpions in the Balkan region emphasizes the peninsula’s status as a glacial refugium (Schmitt, 2007), a zone of exchange and a home to old endemic forms (Murienne et al., 2010). A high rate of endemism is observed in several arachnid groups on the peninsula, such as spiders (Deltshev, 2004) and pseudoscorpions (Ćurčić et al., 2004). The great diversity of pseudoscorpions and related groups in the Balkans can be explained by the long and complex palaeogeographic history of the region (Parmakelis et al., 2006) and its high habitat heterogeneity, pronounced topographic diversity and great climatic variations (Murienne et al., 2010). A recently conducted phylogenetic analysis of a group of arachnids with a similar lifestyle (Opiliones: Cyphophthalmi) indicates that its diversification in the Balkans occurred 94.3 million years ago (105.7 million years after the group’s origin) (Murienne et al., 2010), which might imply early diversification of pseudoscorpions in this region too. On the other hand, in his recent analysis, Harms (2018) documented relatively faster morphological changes of an epigeic pseudoscorpion (Pseudotyrannochthonius giganteus Beier 1971) in caves. Molecular dating indicates that the age estimated for individual cave populations of P. giganteus coincides with the period of karst creation, suggesting that underground habitats were colonized as they emerged.
Список литературы
Beier M., 1938 . Vorläufige Mitteilung über neue Höhlenpseudoscorpione der Balkanhalbinsel // Studien aus dem Gebiete der allgemeinen Karstforschung, der wissenschaftlichen Höhlenkunde, der Eiszeitforschung und den Nachbargebieten, Biologische Serie. V. 3. № 8. P. 5–8.
Beier M., 1963. Ordnung Pseudoscorpionidea (Afterskorpione) // Bestimmungsbücher zur Bodenfauna Europas. V. 1. Berlin: Akademie-Verlag. 313 p.
Ćurčić B.P.M., 1986. On the origin and biogeography of some pseudoscorpions of the Balkan Peninsula // Biologia Gallo-Hellenica. V. 12. P. 85–92.
Ćurčić B.P.M., 1988. Cave-dwelling pseudoscorpions of the Dinaric Karst // Classis IV: Historia Naturalis, Opera 26, Institutum Biologicum Ioannis Hadži 8. Ljubljana: Academia Scientiarum et Artium Slovenica. 192 p.
Ćurčić B.P.M., Ćurčić S.B., Makarov S.E., 1995. On the identity of some cave representatives of Roncus L. Koch, 1873, from the Balkan Peninsula (Chelicerata: Pseudoscorpiones: Neobisiidae) // Annalen des Naturhistorischen Museums in Wien. V. 97B. P. 131–138.
Ćurčić B.P.M., Dimitrijević R.N., 2007. Roncus travuniensis sp. n. (Neobisiidae, Pseudoscorpiones), a troglobitic false scorpion from Bosnia-Herzegovina // Biologia, Bratislava. V. 62. № 1. P. 84–87.
Ćurčić B.P.M., Dimitrijević R.N., Legakis A., 2004. The pseudoscorpions of Serbia, Montenegro, and the Republic of Macedonia // Monographs. V. 8. Belgrade–Athens: Institute of Zoology, Faculty of Biology, University of Belgrade; Hellenic Zoological Society; Committee for Karst and Speleology, Serbian Academy of Sciences and Arts & Institute of Nature Conservation of the Republic of Serbia. 400 p.
Ćurčić B.P.M., Dimitrijević R.N., Rađa T., Makarov S.E., Ilić B.S., 2012. Archaeoroncus, a new genus of pseudoscorpions from Croatia (Pseudoscorpiones, Neobisiidae), with descriptions of two new species // Acta zoologica bulgarica. V. 64. № 4. P. 333–340.
Ćurčić B.P.M., Dimitrijević R.N., Rađa T., Milinčić M., 2012a. Chthonius (Chthonius) makirina (Chthoniidae, Pseudoscorpiones), a new species from Croatia // Archives of Biological Sciences, Belgrade. V. 64. № 2. P. 709–714.
Ćurčić B.P.M., Dimitrijević R.N., Rađa T., Rađa B., 2008. On two new species of pseudoscorpions from the Dinaric Karst // Archives of Biological Sciences, Belgrade. V. 60. № 2. P. 315–324.
Ćurčić B.P.M., Makarov S.E., Ćurčić S.B., Rađa T., Tomić V.T., Ilić B.S., 2012b. A new rare representative of Microchthonius Hadži (Chthoniidae, Pseudoscorpiones) from Dalmatia, Croatia // Acta zoologica bulgarica. V. 64. № 3. P. 229–234.
Ćurčić B.P.M., Makarov S.E., Rađa T., Ilić B.S., Antić D.Ž., 2012c. Roncus meledae n. sp. and Neobisium oculatum n. sp., from the Island of Mljet, Dalmatia (Neobisiidae, Pseudoscorpiones) // Archives of Biological Sciences, Belgrade. V. 64. № 4. P. 1567–1576.
Ćurčić B.P.M., Rađa T., Ćurčić S.B., Ćurčić N.B., 2010 . On Roncus almissae n. sp., R. krupanjensis n. sp., and R. radji n. sp., three new pseudoscorpions (Pseudoscorpiones, Neobisiidae) from Croatia and Serbia, respectively // Archives of Biological Sciences, Belgrade. V. 62. № 2. P. 503–513.
Ćurčić B.P.M., Rađa T., Ćurčić S.B., Ilić B.S., Tomić V.T., Makarov S.E., 2013. Microchthonius elegantissimus n. sp., a new troglobitic pseudoscorpion (Pseudoscorpiones, Chthoniidae) from Croatia // Archives of Biological Sciences, Belgrade. V. 65. № 1. P. 405–410.
Ćurčić B.P.M., Rađa T., Dimitrijević R.N., 2012d. On two new cave pseudoscorpions, Chthonius (Chthonius) pagus n. sp. (Chthoniidae) and Roncus navalia n. sp. (Neobisiidae), from the Island of Pag, Croatia // Archives of Biological Sciences, Belgrade. V. 64. № 4. P. 1555–1565.
Ćurčić B.P.M., Rađa T., Dimitrijević R.N., Ćurčić S.B., 2012e. On two new cave pseudoscorpions from Dalmatia (Croatia) (Chthoniidae and Neobisiidae, Pseudoscorpiones) // Archives of Biological Sciences, Belgrade. V. 64. № 3. P. 1099–1108.
Ćurčić B.P.M., Rađa T., Dimitrijević R.N., Ćurčić S.B., Ćurčić N.B., Makarov S.E., 2013a. A new cave pseudoscorpion from Dalmatia – Microchthonius tragurion n. sp. (Chthoniidae, Pseudoscorpiones) // Archives of Biological Sciences, Belgrade. V. 65. № 3. P. 1253–1259.
Ćurčić B.P.M., Rađa T., Dimitrijević R.N., Ćurčić S.B., Ćurčić N.B., Makarov S.E., 2014. On two new cave species of pseudoscorpions (Neobisiidae, Pseudoscorpiones) from Herzegovina and Dalmatia // Archives of Biological Sciences, Belgrade. V. 66. № 1. P. 377–384.
Ćurčić B.P.M., Rađa T., Dimitrijević R.N., Ćurčić S.B., Ćurčić N.B., Makarov S.E., Ilić B.S., 2014a. Microchthonius kasteli n. sp. (Chthoniidae, Pseudoscorpiones) – a new cave false scorpion from Croatia (Dalmatia) // Archives of Biological Sciences, Belgrade. V. 66. № 1. P. 437–443.
Ćurčić B.P.M., Rađa T., Dimitrijević R.N., Ćurčić S.B., Makarov S.E., Antić D.Ž., Ilić B.S., 2014b. A new pseudoscorpion from Bosnia: Roncus bosniensis n. sp. (Neobisiidae, Pseudoscorpiones) // Archives of Biological Sciences, Belgrade. V. 66. № 1. P. 363–368.
Ćurčić B., Rađa T., Dimitrijević R., Vesović N., Ćurčić S., 2015. On two new species of Microchthonius Hadži (Pseudoscorpiones, Chthoniidae) from Dalmatia, Croatia // Universiteti i Shkodrës „Luigj Gurakuqi“, Buletin Shkencor, Seria e Shkencave të Natyrës. V. 65. P. 80–93.
Ćurčić B.P.M., Rađa T., Makarov S.E., Dimitrijević R.N., Ćurčić S.B., Ilić B.S., 2013b. On the identity of types of Roncus diocletiani Ćurčić, Dimitrijević & Rađa and Archaeoroncus tenuis (Hadži) (Pseudoscorpiones, Neobisiidae) from Croatia // Archives of Biological Sciences, Belgrade. V. 65. № 2. P. 761–766.
Ćurčić B.P.M., Rađa T., Rađa B., Dimitrijević R.N., Ćurčić S.B., Ćurčić N.B., Ilić B.S., 2014c. Chthonius (Globochthonius) daorsoni n. sp. (Chthoniidae, Pseudoscorpiones) – a new cave false scorpion from Bosnia and Herzegovina // Archives of Biological Sciences, Belgrade. V. 66. № 2. P. 901–906.
Ćurčić B.P.M., Rađa T., Rađa B., Dimitrijević R.N., Tomić V.T., Ilić B.S., 2013c. A new cave pseudoscorpion from the Adriatic Isle of Šolta (Dalmatia): Microchthonius solentanus n. sp. (Pseudoscorpiones: Chthoniidae) // Acta zoologica bulgarica. V. 65. № 3. P. 283–288.
Deltshev C., 2004. A zoogeographical review of the spiders (Araneae) of the Balkan Peninsula // Griffiths H.I., Kryštufek B., Reed J.M., eds. Balkan biodiversity: pattern and process in the European hotspot. Dordrecht, Kluwer Academic Publishers. P. 193–200.
Dimitrijević R.N., Rađa T., 2016. On the biodiversity of pseudoscorpions in Croatia: Neobisium curcici (Pseudoscorpiones: Neobisiidae), a new cave-dwelling species from Dalmatia (Croatia) // Ecologica Montenegrina. V. 7. P. 259–265.
Dimitrijević R.N., Rađa T., 2017. Neobisium radjai n. sp. (Neobisiidae, Pseudoscorpiones), a new cave-dwelling pseudoscorpion from Bosnia and Herzegovina // Ecologica Montenegrina. V. 15. P. 10–16.
Gabbutt P.D., Vachon M., 1967. The external morphology and life history of the pseudoscorpion Roncus lubricus // Journal of Zoology. V. 153. № 4. P. 475–498.
Gardini G., 1982. Pseudoscorpioni cavernicoli Sardi // II. Neobisiidae e Chernetidae, con considerazioni sui Neobisiinae cavernicoli (Pseudoscorpioni d’Italia XII) // Fragmenta Entomologica. V. 16. № 2. P. 89–115.
Gardini G., 1983. Redescription of Roncus lubricus L. Koch, 1873, type-species of the genus Roncus L. Koch, 1873 (Pseudoscorpionida, Neobisiidae) // Bulletin of the British Arachnological Society. V. 6. № 2. P. 78–82.
Gardini G., Rizzerio R., 1985. Materiali per una revisione del genere Roncus L. Koch, 1873 // I. Ridescrizione dei tipi di alcune specie italiane non cavernicole (Pseudoscorpionida, Neobisiidae) // Fragmenta Entomologica. V. 18. № 1. P. 47–79.
Gardini G., Rizzerio R., 1986. Materiali per una revisione del genere Roncus L. Koch, 1873 // II. Ridescrizione dei tipi delle specie parablothroidi alpine e appenniniche // Fragmenta Entomologica. V. 19. № 1. P. 1–56.
Guéorguiev V.B., 1977. La faune troglobie terrestre de la péninsule Balkanique // Origine, formation et zoogéographie. Special edition. Sofia: Bulgarian Academy of Sciences. 182 p.
Harms D., 2018. The origins of diversity in ancient landscapes: deep phylogeographic structuring in a pseudoscorpion (Pseudotyrannochthoniidae: Pseudotyrannochthonius) reflects Plio-Pleistocene climate fluctuations // Zoologischer Anzeiger. V. 273. P. 112–123.
Harvey M.S., 2013. Pseudoscorpions of the world // Version 3.0 [E-resource]. Perth: Western Australian Museum. URL: http://www.museum.wa.gov.au/catalogues/ pseudoscorpions. Accessed on: 24.07.2019.
Henderickx H., Zaragoza J.A., 2005. Notes on Roncus (Pseudoscorpiones: Neobisiidae) from the Eastern Pyrenees: new synonymy and description of a new species // Revista Ibérica de Aracnología. V. 11. P. 47–59.
Mahnert V., Gardini G., 2014. Cave-inhabiting pseudoscorpion species of the genus Roncus (Pseudoscorpiones: Neobisiidae) from western Greece, including the Ionian Islands // Arachnologische Mitteilungen. V. 48. P. 28–37.
Murienne J., Karaman I., Giribet G., 2010. Explosive evolution of an ancient group of Cyphophthalmi (Arachnida: Opiliones) in the Balkan Peninsula // Journal of Biogeography. V. 37. № 1. P. 90–102.
Ozimec R., 2004. List of Croatian pseudoscorpion fauna (Arachnida, Pseudoscorpiones) // Natura Croatica. V. 13. № 4. P. 381–394.
Parmakelis A., Stathi I., Chatzaki M., Simaiakis S., Spanos L., Louis C., 2006. Evolution of Mesobuthus gibbosus (Brullé, 1832) (Scorpiones: Buthidae) in the northeastern Mediterranean region // Molecular Ecology. V. 15. № 10. P. 2883–2894.
Schmitt T., 2007. Molecular biogeography of Europe: Pleistocene cycles and postglacial trends // Frontiers in Zoology. V. 4. P. 11.
Šťáhlavský F., Christophoryová J., Henderickx H., 2013. A karyological study of four European species of Roncus (Pseudoscorpiones: Neobisiidae) // European Journal of Entomology. V. 110. № 3. P. 393–399.
Zaragoza J.A., De Mas E., Ribera C., 2007. Pseudoescorpiones del Parque Natural del Cadí-Moixeró (Pirineo Catalán): estudio ecológico, faunístico y taxonómico (Arachnida: Pseudoscorpiones) // Revista Ibérica de Aracnología. V. 14. P. 69–95.
Zaragoza J.A., Šťáhlavský F., 2008. A new Roncus species (Pseudoscorpiones: Neobisiidae) from Montseny Natural Park (Catalonia, Spain), with remarks on karyology // Zootaxa. V. 1693. № 1. P. 27–40.
Дополнительные материалы отсутствуют.
Инструменты
Зоологический журнал


