Зоологический журнал, 2021, T. 100, № 4, стр. 374-384
New Faunistic Data on Oribatid Mites (Acari, Oribatida) Inhabiting the Soil and Termite Nests in the Franklin Game Reserve, Bloemfontein, South Africa
S. G. Ermilov a, *, E. A. Hugo-Coetzee b, c, **, A. A. Khaustov a, ***, V. A. Khaustov a, ****
a Tyumen State University
625003 Tyumen, Russia
b University of the Free State
9300 Bloemfontein, South Africa
c National Museum
9300 Bloemfontein, South Africa
* E-mail: ermilovacari@yandex.ru
** E-mail: lhugo@nasmus.co.za
*** E-mail: alkhaustov@mail.ru
**** E-mail: khaustov93@mail.ua
Поступила в редакцию 2.08.2019
После доработки 8.12.2019
Принята к публикации 4.02.2020
Аннотация
The present study is based on oribatid mite material collected from the soil and nests of the termite, Trinervitermes trinervoides (Isoptera, Termitidae) of the Franklin Game Reserve in South Africa. A list of identified taxa is presented and it includes 47 species belonging to 34 genera and 19 families. The species Phauloppia rauschenensis, the subgenus Lanceoppia (Baioppia) and the genus Phauloppia are recorded from the Ethiopian Region for the first time, while the genus Oribatula is new to southern Africa. A faunistic comparison of the mites from the soil and termite nests shows a lower abundance, but a higher species richness in the soil than in the nests. Soil samples contain dominant species (Zygoribatula contracta, Hypozetes andreii, Licnodamaeus sacculatus, Tectocepheus velatus sarekensis), which are absent from nest samples, while dominant species in the latter (Ausoribula bloemfonteinensis, Transoribates agricola, Bipassalozetes bidactylus, Coetzeella navalensis) are different from those in the soil samples. One species, Oribatula franklinensis sp. n. (Oribatulidae), is described. It differs from all species of the genus by the presence of very long notogastral porose areas Aa. An identification key to Oribatula species known from the Ethiopian Region is given.
Oribatid mites inhabiting termitaries have rarely been investigated worldwide (see Ermilov et al., 2017c for comprehensive list). Our study is part of a project to investigate mites in this habitat, and some data have already been published based on studies in the Franklin Game Reserve (Bayartogtokh et al., 2018; Ermilov et al., 2017–2017d), as well as in the Faan Meintjes Nature Reserve (Ermilov et al., 2019).
The primary goal of the paper is to present quantitative and qualitative data and to compare faunistic structure, abundance and occurrence of mites inhabiting the soil and nests.
During taxonomic identification, we found one new species, Oribatula franklinensis sp. n. (Oribatulidae). This genus Oribatula was described by Berlese (1896) with Notaspis tibialis Nicolet 1855 as type species. Currently, this genus comprises 35 species, which have a cosmopolitan distribution (except the Antarctic region). Additionally, we provide an identification key to known species of Oribatula in the Ethiopian region.
MATERIALS AND METHODS
Material. Mites were collected in the Franklin Game Reserve on Naval Hill (29°05′ S, 26°14′ E), which is located in the city of Bloemfontein (South Africa), in October 2018. The vegetation of the reserve is typical grassland with scattered bushes and trees. Samples were collected from soil and the termite nests (Isoptera, Termitidae: Trinervitermes trinervoides (Sjöstedt 1911)).
Methods. Two samples (125 cm3 each, data of both samples was combined for all analyses) from soil (20 samples consisting of soil, grass and leaf litter, mostly under bushes; five samples from four different places of the Reserve each) and from 20 termite nests (five nests from four different places of the Reserve each) were collected with special shovels (Fig. 1). However, two samples each of soil and nests were not extracted correctly, and therefore only 18 samples for each were used in faunistic comparison. Mites were extracted into 75% ethanol using Berlese’s funnels with electric lamp during five days in the laboratory.
Fig. 1.
Termite nests of Trinervitermes trinervoides in the Franklin Game Reserve, South Africa: a – sampling from a nest, b – active nest, c – abandoned nest.

Specimens were mounted in lactic acid on temporary cavity slides for measurement and illustration. Body length was measured in lateral view, from the tip of the rostrum to the posterior edge of the notogaster. Notogastral width refers to the maximum width of the notogaster in ventral view. Length of body setae was measured in lateral aspect. All body measurements are presented in micrometers. Formulas for leg setation are given in parentheses according to the sequence trochanter-femur-genu-tibia-tarsus (famulus included). Formulas for leg solenidia are given in square brackets according to the sequence genu–tibia–tarsus.
Drawings were made with a camera lucida using a Leica transmission light microscope “Leica DM 2500”.
General morphological terminology used in this paper mostly follows that of F. Grandjean: see Travé and Vachon (1975) for references, Norton (1977) for leg setal nomenclature, and Norton and Behan-Pelletier (2009) for overview.
The following abbreviations are used: lam = lamella; tlam = translamella; slam = sublamella; Al = sublamellar porose area; tu = tutorium; lc = lateral carina; ro, le, in, bs, ex = rostral, lamellar, interlamellar, bothridial and exobothridial setae, respectively; bo = bothridium; Ad = dorsosejugal porose area; D = dorsophragma; P = pleurophragma; len = lenticulus; c, da, la, dm, lm, dp, lp, h, p = notogastral setae; Aa, A1, A2, A3 = notogastral porose areas; im, ip, ih, ips = notogastral lyrifissures; gla = opisthonotal gland opening; a, m, h = subcapitular setae; or = adoral seta; v, l, d, cm, acm, ul, sul, vt, lt = palp setae; ω = palp and leg solenidion; cha, chb = cheliceral setae; Tg = Trägårdh’s organ; Pd I, Pd II = pedotecta I, II, respectively; 1a, 1b, 1c, 2a, 3a, 3b, 3c, 4a, 4b, 4c = epimeral setae; dis = discidium; cp = circumpedal carina; g, ag, an, ad = genital, aggenital, anal and adanal setae, respectively; iad = adanal lyrifissure; Ap = postanal porose area; p.o. = preanal organ; Tr, Fe, Ge, Ti, Ta = leg trochanter, femur, genu, tibia, tarsus, respectively; p.a. = = porose area; σ, φ = leg solenidia; ɛ = leg famulus; v, ev, bv, l, d, ft, tc, it, p, u, a, s, pv, pl = leg setae.
FAUNISTIC DATA
A total of 616 oribatid mite individuals belonging to 47 species (34 genera and 19 families) (Table 1) were extracted from 18 soil and 18 termite nest samples in the Franklin Game Reserve. One species Phauloppia rauschenensis (Sellnick 1908), representatives of the genus Phauloppia Berlese 1908 and subgenus Lanceoppia (Baioppia) Luxton 1985 are found for the first time in the Ethiopian region; the genus Oribatula Berlese 1896 was found for the first time in South Africa.
Table 1.
Oribatid mites recorded from soil and nests of the termite, Trinervitermes trinervoides in the Franklin Game Reserve
| Taxa | Soil | Termite nests | ||
|---|---|---|---|---|
| NI | NN | NI | NN | |
| Steganacaridae | ||||
| Notophthiracarus endroedyyoungai (Mahunka 1984) | 1 | 1 | 1 | 1 |
| Neoliodidae | ||||
| Neoliodes terrestris (Wallwork 1963) | 2 | 2 | – | – |
| Licnodamaeidae | ||||
| Licnodamaeus navalhillensis Bayartogtokh, Ermilov, Hugo-Coetzee et Khaustov 2018 | 3 | 1 | – | – |
| Licnodamaeus sacculatus Bayartogtokh, Ermilov, Hugo-Coetzee et Khaustov 2018 | 19 | 4 | – | – |
| Pedrocortesella africana Pletzen 1963 | 8 | 2 | – | – |
| Pedrocortesella parva Pletzen 1963 | 2 | 2 | – | – |
| Oppiidae | ||||
| Brachioppiella dawidi Hugo-Coetzee 2014 | 1 | 1 | – | – |
| Brachioppiella (Gressittoppia) moresonensis (Kok 1967) | – | – | 4 | 3 |
| Coetzeella navalensis Ermilov, Hugo-Coetzee et Khaustov 2017 | – | – | 36 | 5 |
| Kokoppia kaaimansensis Ermilov et Hugo-Coetzee 2019 | 4 | 2 | 2 | 1 |
| Kokoppia pectinata (Kok 1967) | 1 | 1 | – | – |
| Lanceoppia (Baioppia) sp. | 1 | 1 | – | – |
| Microppia minus (Paoli 1908) | – | – | 1 | 1 |
| Multioppia (Hammeroppia) wilsoni Aoki 1964 | 3 | 1 | – | – |
| Paroppia neethlingi Ermilov and Hugo-Coetzee 2019 | – | – | 1 | 1 |
| Ramusella filigera (Mahunka 1985) | 4 | 2 | – | – |
| Ramusella (Insculptoppia) tobiasi Hugo-Coetzee 2016 | 3 | 2 | 1 | 1 |
| Suctobelbidae | ||||
| Suctobelbella (Flagrosuctobelba) peracuta (Balogh et Mahunka 1980) | 3 | 2 | – | – |
| Suctobelbila sp. | – | – | 2 | 2 |
| Eremulidae | ||||
| Austroeremulus glabrus Mahunka 1985 | – | – | 1 | 1 |
| Eremulus flagellifer Berlese 1908 | 2 | 1 | – | – |
| Eremulus spindleformis Ermilov et Hugo-Coetzee 2012 | 1 | 1 | – | – |
| Tectocepheidae | ||||
| Tectocepheus velatus sarekensis Trägårdh 1910 | 13 | 7 | – | – |
| Tegeocranellidae | ||||
| Tegeocranellus sacchareus Kok 1968 | 2 | 2 | – | – |
| Cymbaeremaeidae | ||||
| Scapheremaeus rustenburgensis Engelbrecht 1975 | 2 | 2 | – | – |
| Scutoverticidae | ||||
| Ethiovertex sculperens (Kok 1968) | 5 | 2 | – | – |
| Passalozetidae | ||||
| Bipassalozetes bidactylus (Coggi 1900) | – | – | 49 | 5 |
| Bipassalozetes wolwekransensis (Engelbrecht 1974) | 1 | 1 | – | – |
| Oribatellidae | ||||
| Novoribatella transcripta (Mahunka 1985) | – | – | 2 | 2 |
| Haplozetidae | ||||
| Transoribates agricola (Nakamura et Aoki 1989) | – | – | 90 | 7 |
| Oribatulidae | ||||
| Ausoribula bloemfonteinensis Ermilov, Hugo-Coetzee et Khaustov 2017 | – | – | 205 | 11 |
| Ausoribula termitophila Ermilov, Hugo-Coetzee et Khaustov 2017 | – | – | 22 | 5 |
| Megatrichobates striatus Grobler 2000 | 7 | 1 | – | – |
| Phauloppia rauschenensis (Sellnick 1908) | 2 | 1 | – | – |
| Oribatula franklinensis sp. n. | 2 | 2 | 1 | 1 |
| Zygoribatula contracta Grobler 1994 | 41 | 9 | – | – |
| Zygoribatula setosa Evans 1953 | 3 | 2 | – | – |
| Scheloribatidae | ||||
| Scheloribates elsi Pletzen 1965 | 3 | 2 | – | – |
| Scheloribates fimbriatus Thor 1930 | 4 | 2 | 8 | 3 |
| Scheloribates sudafricanus Subías 2018 | – | – | 2 | 2 |
| Chamobatidae | ||||
| Hypozetesandreii Ermilov, Hugo-Coetzee, Khaustov et Kontschán 2019 | 19 | 5 | – | – |
| Humerobatidae | ||||
| Afroleius minor Mahunka 1984 | – | – | 2 | 2 |
| Punctoribatidae | ||||
| Antarctozetes translamellatus (Mahunka 1985) | 2 | 2 | – | – |
| Galumnidae | ||||
| Galumna curvifamulus Ermilov, Hugo-Coetzee, Khaustov et Theron 2017 | 2 | 1 | 5 | 1 |
| Galumna nuda Engelbrecht 1972 | – | – | 1 | 1 |
| Pilogalumna bloemfonteinensis Engelbrecht 1972 | 6 | 2 | – | – |
| Pilogalumna kimberleyensis Engelbrecht 1972 | 8 | 2 | – | – |
Soil. 180 individuals representing 33 species were recorded. Four species constituted 51% of all individuals sampled in the soil with high occurrence: Zygoribatula contracta Grobler 1994 (41 ind., in 9 samples), Hypozetes andreii Ermilov, Hugo-Coetzee, Khaustov et Kontschán 2019 (19 ind., 5 samples), Licnodamaeus sacculatus Bayartogtokh, Ermilov, Hugo-Coetzee et Khaustov 2018 (19 ind., 4 samples), Tectocepheus velatus sarekensis Trägårdh 1910 (13 ind., 7 samples). However, these species were completely absent from nests. Abundance and occurrence of other 29 species were low (1–8 ind. in 1–2 samples).
Termite nests. 436 individuals representing 20 species were recorded. Five species constituted 92% of all individuals sampled in nests with high occurrence: Ausoribula bloemfonteinensis Ermilov, Hugo-Coetzee et Khaustov 2017 (205 ind., 11 samples), Transoribates agricola (Nakamura et Aoki 1989) (90 ind., 7 samples), Bipassalozetes bidactylus (Coggi 1900) (49 ind., 5 samples), Coetzeella navalensis Ermilov, Hugo-Coetzee et Khaustov 2017 (36 ind., 5 samples), Ausoribula termitophila Ermilov, Hugo-Coetzee et Khaustov 2017 (22 ind., 5 samples). These species were found only in the termite nests. Abundance and occurrence of the other 15 species were low (1–10 ind., in 1–3 samples).
Remarks. Comparison of quantitative and qualitative data showed four important results:
(1) The abundance of oribatid mites was distinctly lower in soil than in nests (180 ind. versus 436 ind.), but species richness in soil was distinctly higher compared to nests (33 versus 20 spp.). The higher abundance of mites in the nests is probably due to variability and density of food resources compared to adjacent soils (see Ermilov et al. 2017d), while the higher species richness in soil can be ascribed to the high ecological niche of the soil i.e. litter of different grass species and leaf litter of different tree species.
(2) The oribatid mite communities are distinctly different between soil and nests. Only six species were found in both habitats: Notophthiracarus endroedyyoungai (Mahunka 1984), Kokoppia kaaimansensis Ermilov et Hugo-Coetzee 2019, Ramusella (Insculptoppia) tobiasi Hugo-Coetzee 2016, O. franklinensis sp. n., Scheloribates fimbriatus Thor 1930, Galumna curvifamulus Ermilov, Hugo-Coetzee, Khaustov et Theron 2017.
(3) Furthermore, species with highest abundance occur only in either habitat. However, some species confined to soil in this study (i.e. Z. contracta, L. sacculatus, T. velatus sarekensis) have been found in the nests of the same locality (Ermilov et al., 2017c).
(4) From this and previous studies (Ermilov et al., 2019), it became evident that the following species prefer termite nests: A. bloemfonteinensis, A. termitophila, T. agricola, B. bidactylus, C. navalensis, Saltarichus louiseae Ermilov, Hugo-Coetzee et Khaustov 2019.
DESCRIPTION OF NEW SPECIES
Oribatula franklinensis Ermilov, Hugo-Coetzee, A. Khaustov et V. Khaustov sp. n. (Figs 2–4)
Fig. 2.
Oribatula franklinensis sp. n., adult: a – dorsal view (legs omitted); b – posterior view; c – subcapitulum, ventral view; d – palp, left, antiaxial view; e – chelicera, right, antiaxial view. Scale bar (µm): a, b – 100; c–e – 20.
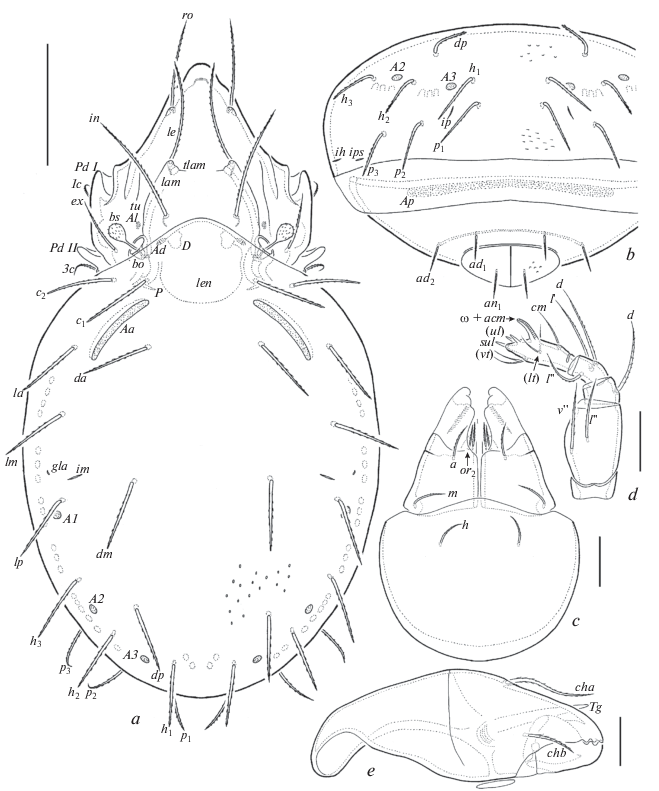
Fig. 3.
Oribatula franklinensis sp. n., adult: a – ventral view (legs omitted); b – anterior part of body, lateral view (legs omitted); c – posterior part of body, lateral view. Scale bar 100 μm.
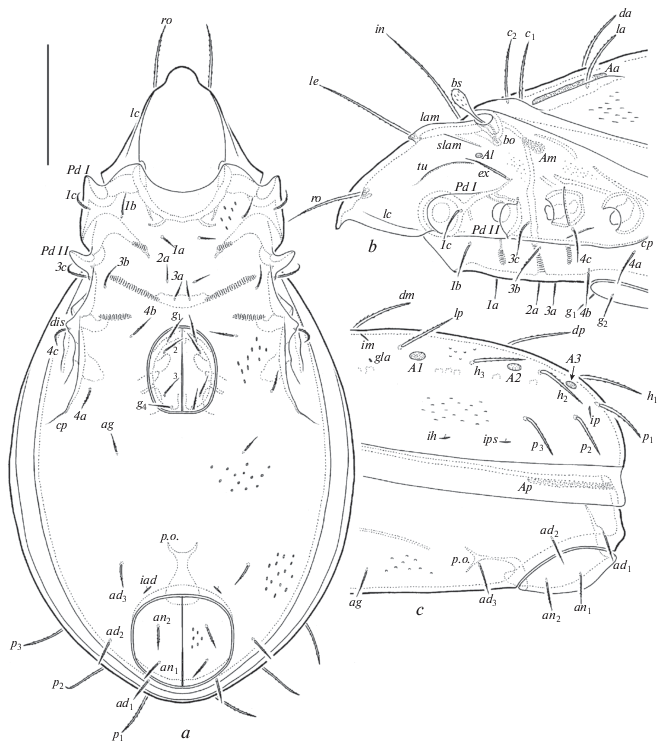
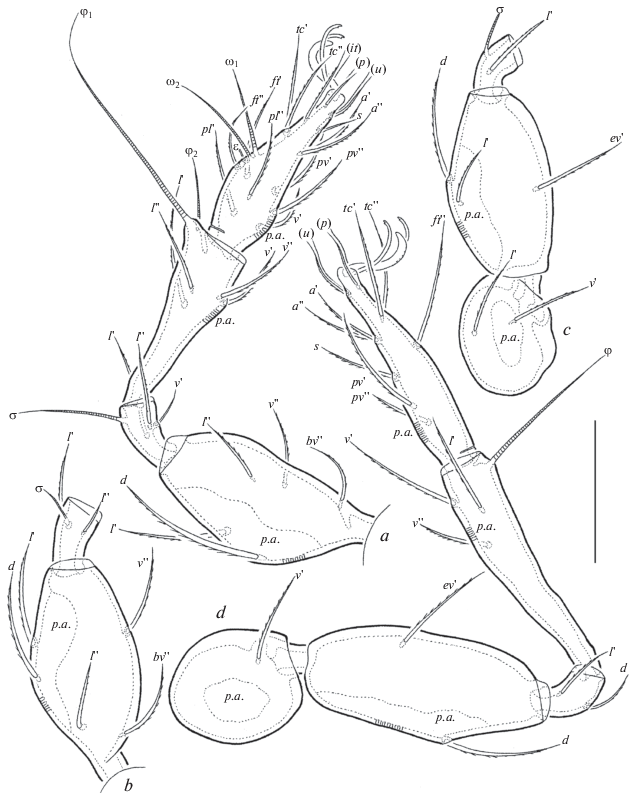
Fig. 4.
Oribatula franklinensis sp. n., adult: a – leg I, without trochanter, right, antiaxial view, b – femur and genu of leg II, right, antiaxial view, c – trochanter, femur and genu of leg III, left, antiaxial view, d – leg IV, right, paraxial view. Scale bar 50 μm.
Material. Holotype (♂) and 1 paratype (1♀): soil under bush vegetation; one paratype (♂): same data, but from a nest of termites.
The holotype is deposited in the collection of the National Museum Bloemfontein, South Africa; 2 paratypes are deposited in the collection of the Tyumen State University Museum of Zoology, Tyumen, Russia. All specimens are preserved in ethanol with a drop of glycerol.
Diagnosis. Body size: 498–531 × 282–315. Notogaster, epimeral and anogenital regions sparsely foveolate, lateral parts of notogaster and ventral plate lineolate. Rostrum protruding, rounded. Rostral, lamellar and interlamellar setae long, setiform, barbed; ro shortest, in longest. Bothridial setae short, clavate, barbed. Exobothridial setae of medium size, setiform, barbed. Lenticulus present. Fourteen pairs of notogastral setae of medium size, stiff, barbed; c1 and c2 located in transverse row. Notogastral porose areas, Aa very long, elongate oval, A1 oval, A2 and A3 small, rounded. Epimeral and anogenital setae setiform, barbed.
Description. Measurements. Body length: 514 (holotype: male), 498, 531 (two paratypes); notogaster width: 298 (holotype), 282, 315 (two paratypes).
Integument (Figs 2a, 2b; 3a–3c). Body color light brown to brown. Body surface punctate (visible under high magnification, ×1000). Notogaster, epimeral and anogenital regions sparsely foveolate (foveoles rounded or slightly elongate, their diameter or length up to 4). Lateral parts of prodorsum between bothridia and acetabula I–IV microgranulate. Lateral parts of notogaster and ventral plate lineolate, with fine, short (length up to 6) depressed lines.
Prodorsum (Figs 2a; 3b). Rostrum protruding, rounded. Lamellae well developed, located dorsally, shorter than half of prodorsum (measured in lateral view), with minute truncate cusps. Translamella represented by two short parts located close to lamellae. Sublamellae and tutoria lineate. Sublamellar porose areas (4–6 × 2–4) oval. Rostral (69–73), lamellar (86–90) and interlamellar (90–94) setae setiform, barbed; ro thinner than le and in. Bothridial setae (32–36) clavate, with short stalk and slightly longer, barbed head. Bothridia not covered by anterior margin of notogaster in dorsal view. Exobothridial setae (45–53) setiform, barbed. Dorsosejugal porose areas (6–8 × 4) oval, located posterolateral to interlamellar setae. Dorsophragmata comparatively short, slightly elongated.
Notogaster (Figs 2a, 2b; 3a–3c). Anterior notogastral margin distinctly convex medially. Lenticulus well visible. Fourteen pairs of notogastral setae (53–61) stiff, barbed; c1 and c2 located in transverse row. Four pairs of porose areas, Aa (65–73 × 10–16) very long, elongate oval, A1 (20–28 × 10–16) oval, A2 (8–14), A3 (8–14) round. All lyrifissures (except ia), opisthonotal gland openings, circumgastric scissure and circumgastric sigillar band distinct.
Gnathosoma (Figs 2c–2e). Subcapitulum longer than wide (106–110 × 82–90). Three pairs of subcapitular setae similar in length (20–24), setiform, barbed. Two pairs of adoral setae (12) setiform, heavily barbed. Palps (73–77) with setation 0-2-1-3-9(+ω). Postpalpal setae (6) thorn-like, smooth. Chelicerae (118–123) with two setiform, barbed setae, cha (36) longer than chb (20). Trägårdh’s organ of chelicerae elongate triangular.
Epimeral and lateral podosomal regions (Figs 3a, 3b). Epimeral setal formula: 3-1-3-3. Epimeral setae setiform, barbed; 1a, 2a and 3a (20–22) shorter than others (32–36). Pedotecta II rounded in ventral view. Discidia triangular. Circumpedal carinae of medium length, directed to pedotecta II.
Anogenital region (Figs 2b; 3a–3c). Four pairs of genital, one pair of aggenital, two pairs of anal and three pairs of adanal setae similar in length (20–22), setiform, barbed. Adanal lyrifissures located close and anterior to anal aperture. Adanal setae ad1 in posterior, ad2 in lateral, ad3 in anterior positions to anal plates. Postanal porose area (168–176 × 8–14) band-like. Preanal organ goblet-like.
Legs (Figs 4a–4d). All legs tridactylous. Median claw thick, lateral claws thin, with small tooth ventrodistally; all slightly barbed dorsally. Dorsoparaxial porose area on all femora and trochanters III, IV, ventroproximal porose area on all tarsi and ventrodistal porose area on all tibiae well visible. Formulas of leg setation and solenidia: I (1-5-3-4-19) [1-2-2], II (1-5-2-4-15) [1-1-2], III (2-3-1-3-15) [1-1-0], IV (1-2-2-3-12) [0-1-0]; homology of setae and solenidia indicated in Table 2. Famulus of tarsi I short, erect, truncate distally, located posterior to solenidion ω2. Dorsoanterior apophysis of tibiae I (bearing solenidion φ2) well developed.
Table 2.
Leg setation and solenidia of adult Oribatula franklinensis sp. n.
| Leg | Tr | Fe | Ge | Ti | Ta |
|---|---|---|---|---|---|
| I | v' | d, (l), bv'', v'' | (l), v', σ | (l), (v), φ1, φ2 | (ft), (tc), (it), (p), (u), (a), s, (pv), (pl), v', ɛ, ω1, ω2 |
| II | v' | d, (l), bv'', v'' | (l), σ | (l), (v), φ | (ft), (tc), (it), (p), (u), (a), s, (pv), ω1, ω2 |
| III | l ', v' | d, l ', ev' | l ', σ | l ', (v), φ | (ft), (tc), (it), (p), (u), (a), s, (pv) |
| IV | v ' | d, ev' | d, l ' | l ', (v), φ | ft'', (tc), (p), (u), (a), s, (pv) |
Remarks. Oribatula franklinensis sp. n. differs from all other species of the genus by the presence of very long notogastral porose areas Aa (versus rounded, oval or slightly elongated); in this trait, the new species is morphologically similar to some representatives of the related genus Zygoribatula, e.g., Z. diverseta (Bose, Mathur, Jain et Dogra 1996) from India (see Bose et al., 1996), Z. lineaporosa Grobler 1993 from South Africa (see Grobler, 1993), and Z. schauenbergi (Mahunka 1978) from the Ethiopian region (see Mahunka, 1978), but differs from these by the absence of complete translamella (versus present), the localization of notogastral setae c1 and c2 (in transverse row versus in longitudinal row), and the presence of rounded rostrum (versus triangular to pointed) and long notogastral setae (versus many setae comparatively short).
Etymology. The new species refers to the Franklin Game Reserve, where the type material was collected.
KEY TO SPECIES OF ORIBATULA IN THE ETHIOPIAN REGION
1. Notogastral porose areas Aa very long, elongate oval; notogastral setae c1 and c2 located in transverse row ………………. Oribatula franklinensis sp. n. Distribution: South Africa.
– Notogastral porose areas Aa rounded or oval; notogastral setae c1 and c2 located in longitudinal row ….............................................................. (2)
2. Rostrum rounded …………. Oribatula interrupta (Willmann 1939). Distribution: Palaearctic region, Ethiopia.
– Rostrum pointed ……………….....……………….…. (3)
4. Notogastral setae comparatively long, distinctly longer than diameter of notogastral porose areas ….............. ....................Oribatula acuminata Wallwork 1964. Distribution: Chad.
– Notogastral setae comparatively short, subequal to diameter of notogastral porose areas ……................ ................Oribatula incompleta Mahunka1987. Distribution: Nigeria.
Список литературы
Bayartogtokh B., Ermilov S.G., Hugo-Coetzee E.A., Khaustov A.A., 2018. Contribution to the knowledge of the oribatid mite genus Licnodamaeus Grandjean, 1931 and synonymy of Licnodamaeolus Covarrubias, 1998 (Acari, Oribatida, Licnodamaeidae) // Systematic and Applied Acarology. V. 23. № 1. P. 42–60. https://doi.org/10.11158/saa.23.1.4
Berlese A., 1896. Acari, Myriapoda et Scorpiones hucusque in Italia reperta. Ordo Cryptostigmata II (Oribatidae) // Portici, Padova. P. 1–98.
Bose A., Mathur R.B., Jain K.L., Dogra D., 1996. A new genus Rekharibatula (Acarina: Oribatulidae) from the soil of Eucalyptus plantation from India // Shashpa. V. 3. № 1. P. 25–26.
Ermilov S.G., Hugo-Coetzee E.A., Khaustov A.A., 2017. Coetzeella navalensis gen. nov., sp. nov. (Acari, Oribatida, Oppiidae) from South Africa // Systematic and Applied Acarology. V. 22. № 3. P. 403–409. https://doi.org/10.11158/saa.22.3.6
Ermilov S.G., Hugo-Coetzee E.A., Khaustov A.A., 2017a. Contribution to the knowledge of the oribatid mite genus Lamellarea (Acari, Oribatida, Lamellareidae) // Systematic and Applied Acarology. V. 22. № 11. P. 2008– 2022. https://doi.org/10.11158/saa.22.11.16
Ermilov S.G., Hugo-Coetzee E.A., Khaustov A.A., 2017b. Contribution to the knowledge of oribatid mites of the family Lohmanniidae (Acari, Oribatida) from South Africa // Systematic and Applied Acarology. V. 22. № 5. P. 666–682. https://doi.org/10.11158/saa.22.5.6
Ermilov S.G., Hugo-Coetzee E.A., Khaustov A.A., 2017c. Oribatid mites (Acari, Oribatida) inhabiting nests of the termite Trinervitermes trinervoides (Sjöstedt) in the Franklin Game Reserve (Bloemfontein, South Africa), with description of a new species of the genus Ceratobates (Tegoribatidae) // Systematic and Applied Acarology. V. 22. № 10. P. 1715–1732. https://doi.org/10.11158/saa.22.10.12
Ermilov S.G., Hugo-Coetzee E.A., Khaustov A.A., 2017d. Two new species of the genus Ausoribula (Acari, Oribatida, Oribatulidae) from termitaries of South Africa // Acarologia. V. 57. № 3. P. 643–650. https://doi.org/10.24349/acarologia/20174183
Ermilov S.G., Hugo-Coetzee E.A., Khaustov A.A., Theron P.D., 2019. Oribatid mites (Acari, Oribatida) inhabiting termite nests in the Faan Meintjes Nature Reserve (South Africa) // Systematic and Applied Acarology. V. 24. № 9. P. 1783–1798. https://doi.org/10.11158/saa.24.9.14
Grobler L., 1993. Species of the genus Zygoribatula Berlese, 1916 (Acari, Oribatida, Oribatulidae) from South Africa II // Navorsinge van die Nasionale Museum, Bloemfontein. V. 9. № 6. P. 181–212.
Mahunka S., 1978. Neue und interessante milben aus dem Genfer museum XXXIV. A compendium of the oribatid (Acari) fauna of Mauritius, Reunion and the Seychelles Is. II // Revue suisse de Zoologie. V. 85. № 2. P. 307–340.
Norton R.A., 1977. A review of F. Grandjean’s system of leg chaetotaxy in the Oribatei (Acari) and its application to the family Damaeidae // Dindal D.L., ed. Biology of oribatid mites. Syracuse, SUNY College of Environmental Science and Forestry. P. 33–61.
Norton R.A., Behan-Pelletier V.M., 2009. Oribatida // A Manual of Acarology (TX): Lubbock, Texas Tech University Press. P. 430–564.
Travé J., Vachon M., 1975. François Grandjean. 1882–1975 (Notice biographique et bibliographique) // Acarologia. V. 17. № 1. P. 1–19.
Дополнительные материалы отсутствуют.
Инструменты
Зоологический журнал


