ПАРАЗИТОЛОГИЯ, 2021, том 55, № 1, с. 73-80.
УДК 576.895.121.5: 576.31
ULTRASTRUCTURE OF THE METACESTODE
APLOPARAKSIS SHIGINI BONDARENKO ET KONTRIMAVICHUS, 2006
(CESTODA: APLOPARAKSIDAE)
© 2021 N. A. Pospekhovaa,*, K. V. Regela
aInstitute of Biological Problems of the North,
Far Eastern Branch of the Russian Academy of Sciences,
Magadan, 685000 Russia
*e-mail: posna@ibpn.ru
Received 24.10.2020
Received in revised form 16.11.2020
Accepted 19.11.2020
The fine structure of the metacestode Aploparaksis shigini Bondarenko et Kontrimavichus, 2006
from the predatory leeches Erpobdella octoculata L. from the lakes of the Upper Kolyma River basin
was studied for the first time. The cysticercoid is similar to the floricercus, i.e. has an open cellular
exocyst and many processes at its base, however, it is distinguished by a long tail. The exocyst and
all its outgrowths (including the caudal process) are covered with long and thick microvilli. Excre-
tory canals of different diameter are noted in the exocyst, near the base of the caudal process. The
structure of the endocyst is typical of hymenolepidid metacestodes; the glycocalyx has a characteristic
reticular structure.
Keywords: metacestode, ultrastructure, floricercus, exocyst, excretory canals
DOI: 10.31857/S0031184721010063
Cysticercoids Aploparaksis shigini represent one of three species of metacestodes found
in the predatory pharyngeal leeches Erpobdella octoculata L. from the lakes of the Upper
Kolyma basin (Regel, 2016). Small aploparaksid metacestodes were freely located in the
fluid of lateral lacunae - the rudiments of the coelom, while the larger hymenolepidids from
the genus Kowalewskius Yamaguti, 1959 were found in the thickness of the botryoid tissue
of leeches (Regel, 2010). Following morphological features of K. formosus cysticercoids
were noted: closeness to the modification of cyclocercus and the presence of long, thick
microvilli on the tail appendage twining the cyst around (Regel, Pospekhova, 2019).
The structural features of the A. shigini metacestodes do not allow attributing them
to any of the known modifications. The presence of a bowl-shaped outgrowth of the tail
appendage, together with numerous lobes at the base of the endocyst, makes it similar to
the floricercus (Bondarenko, Krasnoshchekov, 1978; Bondarenko, Kontrimavichus, 2006).
However, the floricercus has a relatively short tail, whereas metacestodes from leech has
a long tail, forming a single conglomerate with the caudal processes of other metacestodes
(Regel, 2016). Similar structure of the caudal process was noted in metacestodes of the
73
“marine” species of aploparaksids, Wardium cirrosa (Krabbe, 1869) (Greben et al., 2019).
The authors could not attribute that metacestode to any of the known modifications as well.
The aim of our research is to identify the characteristic features of the fine morphology
of the cysticercoid A. shigini, which could be useful for specifying of the classification
system of metacestodes, as well as for establishing the generic relations with other repre-
sentatives of the family.
Material and methods
The original material was obtained by dissection of leeches Erpobdella octoculata L. from the
lakes of the Upper Kolyma basin. Metacestodes were fixed in a 2% glutaraldehyde solution in 0.1 M
phosphate buffer (pH 7.2) at a temperature of about 4 °C. After fixation the material was postfixed
in a 2 % OsO4 solution in a 0.2 M phosphate buffer (pH 7.2) for 12 hours, dehydrated, and embed-
ded in an EPON-araldite mixture. During dehydration, the specimens were stained with a saturated
uranyl acetate solution in 70 % ethanol for a night. Ultra-thin sections (90 nm), obtained on an LKB
ultratome (Sweden), were viewed in JEM-1011 (JEOL, Japan) operating at 80 kV and JEM-1400Plus
(JEOL, Japan) operating at 120 kV transmission electron microscopes.
Results
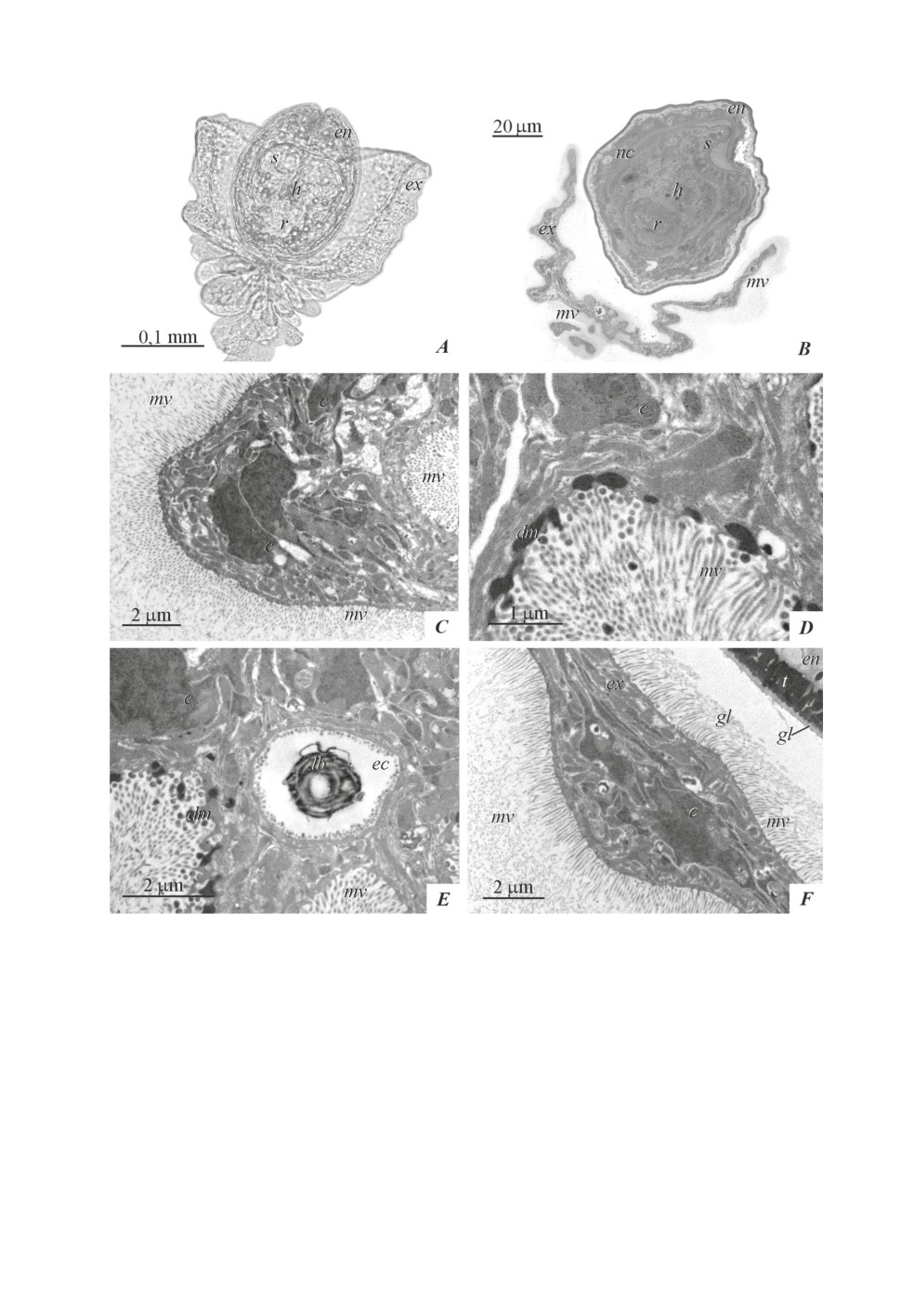
The location of the exocyst and endocyst on the sections is close to that observed in
living metacestodes, although preparation for electron microscopic examination leads to a
change of the envelopes’ form (fig. 1A, 1B). On the sections, the bowl-shaped profile of the
exocyst is located around the endocyst, and the long and dense microvilli of the exocyst
form a continuous zone around it (fig. 1B, 1C). The width of this zone sometimes exceeds
10 µm. At a distance of up to 2 μm from the tegument surface, the microvilli retain linear
outlines (fig. 1C), while the ends of the microvilli bend, forming numerous profiles on
the sections along the border periphery, which makes it difficult to measure the length of
separate microvilli. The transverse sections of the microvilli allow watching the fine fibers
that bind them into a single border.
Long (up to 100 μm) radial rays, discernible at the surface of the exocyst of some liv-
ing metacestodes, can be microvilli that shrivel up when processing the material and turn
into this border.
The exocyst tegument, containing microvilli, has a thin distal cytoplasm connected with
the underlying cytons. They are distinguished by dense cytoplasm and looser karyoplasm
with the large amount of heterochromatin (fig. 1C). The tegument of the inner surface of
the exocyst, at the base of the endocyst, secretes dense material. The latter is accumulated
in the surface cytoplasm, and rounded or oblong bodies are separated from it (fig. 1D, 1E).
Muscle cells with expanded channels of the granular endoplasmic reticulum were not
found, although muscle fibers of a small section were noted in the subtegument.
Excretory canals of different diameters are located in the area adjacent to the base of
the tail. It was not possible to trace the exact topography of the canals, however, larger
canals are located at the base of the bowl-shaped outgrowth of the exocyst; small canals
are located outside of large ones. The canal walls are formed by thin syncytium lined with
typical round microvilli. In the lumen of the canals, dense bodies and myelin figures are
occasionally observed (fig. 1E).
In the contact places of exocyst microvilli and the endocyst glycocalyx, the latter ad-
heres to the microvilli and separates from the endocyst, leaving only an inner homogeneous
glycocalyx layer on its surface (fig. 1F).
74
Figure 1. Metacestode of Aploparaksis shigini: exocyst.
A - living metacestode,
B - a thin section of the metacestode (tail process is outside the field of view),
C - microvilli of the exocyst tegument,
D - secretion of dense material from the inner surface of the exocyst,
E - excretory canal with a lamellar body,
F - adhesion of endocyst glycocalyx and exocyst microvilli. Abbreviations: c - cyton,
dm - dense material, ec - excretory canal, en - endocyst, ex - exocyst, gl - glycocalyx,
h - rostellar hook, lb - lamellar body, mv - microvilli, nc - neck, r - rostellum, s - sucker,
t - distal cytoplasm of tegument.
75
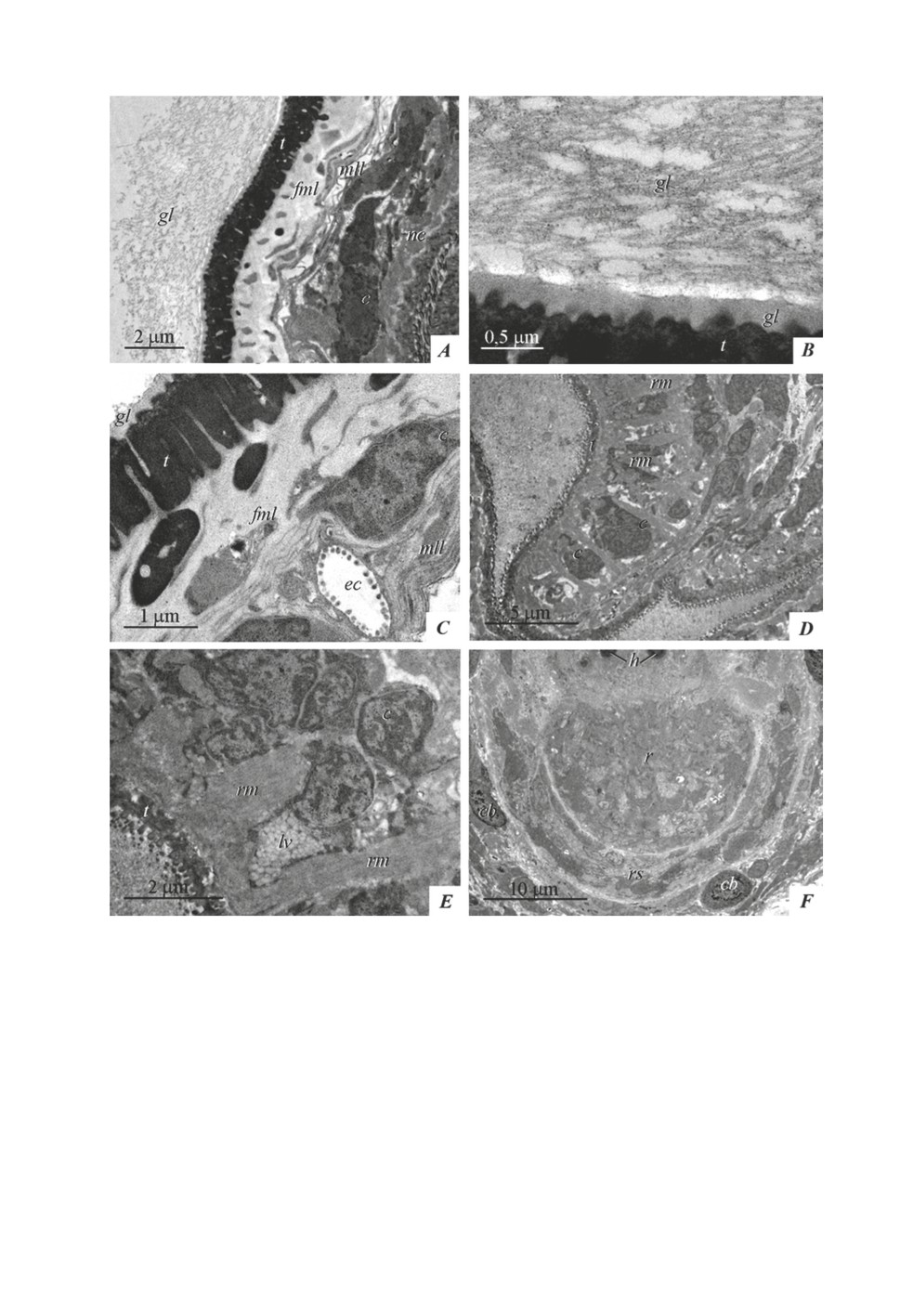
The endocyst wall structure of A. shigini is shown in figs. 2A-2C. The endocyst tegu-
ment is covered with a tubular-fibrous glycocalyx up to 6-7 µm thick; its inner layer consists
of a homogeneous material of about 200 nm thick (fig. 2B). The tubules form a reticular
structure, interspersed with fine fibers and vesicles; there is usually a narrow light zone in
the area of attachment of the outer part of the glycocalyx to the inner homogeneous layer
(fig. 2A, 2B). The distal cytoplasm of the endocyst, about 1.5 µm thick, is filled with dense
material with small cavities (fig. 2A-2C). Fibrous-muscular layers are located under the basal
plate: external circular one (with the inclusion of muscle fibers of the same orientation)
and internal longitudinal one with longitudinal muscle fibers. The deeper layer of cellular
elements (muscle cells, excretory canals and cells whose processes form the pseudomyelin
layer at the endocyst border) usually constitutes about ¼ of the endocyst wall, in rare cases -
up to half of it (fig. 2C).
Large oval calcareous bodies, up to 8-9 µm in diameter, lie in the parietal part of the
neck and at the base of the scolex, under the suckers and rostellar sac (figs1B, 2F). The
neck and scolex are covered with microtriches, which are longer in the area of the suckers.
In the tegument of the suckers, non-cilia sensory endings can be distinguished; among the
muscles of the suckers, there are cells containing numerous light vesicles, about 100 nm
in diameter. The endocyst cavity is filled with flaky material and vesicles. The retracted
pyriform rostellum with hooks is surrounded behind by the rostellar sac with cords of cells
without visible synthetic activity.
Discussion
For the first time, conglomerates of metacestodes with intertwining caudal processes
(“larvophores”) were noted in the caudate diplocysts Wardium fryei Mayhew, 1925; the
author suggested the possibility of asexual proliferation of metacestodes from bud-like
outgrowths on the tail appendage (Bondarenko, 1997). The presence of long intertwined
caudal processes with rounded thickenings was noted in A. shigini by Regel (2016), and
subsequently in W. cirrosa by Greben et al. (2019), and in the latter case, the authors also
suggested the possibility of the development of several cysticeroids from one oncosphere.
No evidence of asexual reproduction of A. shigini by budding was found in our mate-
rial, although we do not exclude this possibility. The cellular exocyst of A. shigini, together
with all its processes, is a modified tail appendage (cercomer according to Freeman, 1973;
Gulyaev, 1989; Chervy, 2002; Greben et al., 2019 and other authors). In some cyclophyllids,
it is the homologues of the tail appendage that contain poorly differentiated cells capable of
forming new individuals, as noted, for example, in the multicercus Mircia shigini (Gulyaev
et Konyaev, 2006) (Gulyaev, 1989; Regel, Pospekhova, 2012; Pospekhova, Regel, 2015).
The presence of a thick border of long microvilli is characteristic of the tail appendage
and its homoloque in two examined (by electron microscopy) metacestodes from leeches
E. octoculata (K. formosus and A. shigini), although they have different localization (tissue
and cavity), belong to different families (Hymenolepididae and Aploparaksidae ), are close
to different morphological modifications (cyclocercus and floricercus) and parasitize in
different definitive hosts (Laridae and Anatidae) (Regel, 2016). The only common feature
of these metacestodes is the same intermediate host - the pharyngeal leech E. octoculata.
Supposedly, long dense microvilli, which are a kind of frame for the glycocalyx of the
tail appendage tegument, is a general adaptation of various modifications of cyclophyllids
metacestodes in the body of pharyngeal leeches.
76
Figure 2. Metacestode of Aploparaksis shigini: endocyst and definitive part.
A - endocyst and parietal part of the neck,
B - endocyst glycocalyx,
C - endocyst wall,
D - sucker,
E - a cell with light vesicles in a sucker,
F - rostellum and rostellar sac.
Abbreviations: c - cyton, cb - calcereous body, ec - excretory canal, en - endocyst,
fml - fibrous-muscular layer, gl - glycocalyx, h - rostellar hook, lb - lamellar body,
lv - light vesicles, mll - myelin-like layer, nc - neck, r - rostellum, rm - radial muscles,
rs - rostellar sac, s - sucker, t - distal cytoplasm of tegument.
77
A possible protective role of exocyst long microvilli has been noted for a typical diplocyst
Aploparaksis bulbocirrus Deblock et Rausch, 1968: in the area of the exocyst outlet, the
microvilli are denser and longer, comparing to those, on the lateral surface. They intertwine
and form a “plug” (Nikishin, 2009). It is possible that this “plug” prevents the penetration
of host immune cells into the exocyst cavity.
Studying the postembryonic development of aploparaksids at the light-microscopic level,
the presence of excretory canals and cyrtocytes in the cellular exocyst of A. birulai floric-
ercus, typical diplocysts A. scolopacis, and caudate diplocysts W. friey was noted (Bonda-
renko, Krasnoshchekov, 1978; Bondarenko, 1993, 1997). Our finding of excretory canals in
the exocyst of A. shigini and the absence of similar data in ultrastructural studies of other
cysticercoids of the Aploparaksidae family (Nikishin, Krasnoshchekov, 1979; Nikishin,
2009) may indicate only local functioning period the of the exocyst excretory system in
postembryonic development, or be associated with specific structural features of a particular
type of cestodes in the intermediate host.
The endocyst walls of the studied species have the same morphological features that
are noted for the endocysts of hymenolepidids: the distal cytoplasm of the tegument is
filled with dense material, the muscle fibers of the subtegumental muscles are embedded
into fibrous layers of the same orientation, the wall of the endocyst is separated from the
parietal part of the neck by the so-called pseudomyelin (myelin-like) layer (Caley, 1974).
However, the endocyst glycocalyx of A. shigini is quite different from those previously
described (see review by Nikishin, 2017). It also differs from the glycocalyx of another
species of metacestodes from leeches, K. formosus (Regel, Pospekhova, 2019). In the latter
case, the endocyst glycocalyx has a fibrous structure typical of hymenolepidids, which is
denser near the tegument surface.
Analysis of the available literature and our observations show that the main role in
the formation of metacestodes morphological polymorphism belongs to temporary larval
structures, which undergo significant changes during the postembryonic development. This
is especially evident in metacestodes of the Aploparaksidae family. Such features, as the
degree of immersion of the endocyst into the exocyst, the shape of the exocyst, and the
length of the tail process can vary depending on the stage of development, the intensity of
invasion, and many other factors, which are still unknown.
An even more significant (in our opinion) difference in the postembryonic development
of some aploparaksids is the presence of two invaginations (most cyclophyllids have only
one), as well as the displacement of the primary lacuna into the tail appendage primordium
(Gulyaev, 1977; Bondarenko, 1978; Bondarenko, Kontrimavichus, 2006; Nikishin, 2009;
Pospekhova, 2017).
Considering these circumstances, as well as a significant prevalence of molecular genetic
research methods in modern helminthology, the study of life cycles (Blasco-Costa, Poulin,
2017) and the morphological characteristics of various modifications of metacestodes is of
particular importance, since it is obvious that to obtain a complete picture “… it is necessary
to study all existing modifications of cestodes’ larvae” (Mrazek, 1927).
Acknowledgements
The research was carried out in the course of fulfilling the state assignment on the project
“Taxonomic, morphological and ecological diversity of helminths of vertebrates in North
Asia” No. AAA-A17-117012710031-6. The authors are grateful to the respected reviewers
for constructive and friendly reviews.
78
References
Blasco-Costa I., Poulin R. 2017. Parasite life-cycle studies: a plea to resurrect an old parasitological tradition.
Bondarenko S.K. 1978. Postembryonal development of cestodes of the genus Aploparaksis Clerc, 1903 (Hyme-
nolepididae) with a cysticercoid of the diplocyst type. Parazitologiya 12 (4): 345-348 [In Russian]. https://
Bondarenko S.K. 1993. Aploparaksis scolopacis and some questions of its ecology. Parazitologiya 27 (3): 251-259.
Bondarenko S.K. 1997. The life cycle of the Wardium fryei (Cestoda: Hymenolepididae). Parazitologiya 31 (2):
pdf
Bondarenko S.K., Kontrimavichus V.L. 1976. Polymorphism of the larvae of the genus Aploparaksis Clerc, 1903
Bondarenko S.K., Kontrimavichus V.L. 2006. Aplaparaksidae of wild and domesticated birds. Fundamental of
Cestodology. T.14. Moscow, Nauka, 443 p. [in Russian].
Bondarenko S.K., Krasnoshchekov G.P. 1978. Postembryonic development of the cestode Aploparaksis birulai
(Hymenolepididae). Zoologichesky Zhurnal 57 (4): 485-494. [in Russian].
Caley J. 1974. The functional significance of scolex retraction and subsequent cyst formation in the cysticercoid
Chervy L. 2002. The terminology of larval cestodes or metacestodes. Systematic Parasitology 52 (1): 1-33. https://
doi.org/10.1023/A:1015086301717
Freeman R.S. 1973. Ontogeny of cestodes and its bearing on their phylogeny and systematics. Advances in Para-
Greben O., Kudlai O., Kornyushin V.V. 2019. The intermediate hosts of Wardium cirrosa (Krabbe, 1869) Spassky,
1961 (Cestoda, Cyclophyllidea, Aploparaksidae) in Ukraine. Parasitology Research 118: 3129-3137. https://
doi.org/10.1007/s00436-019-06453-0
Gulyaev V.D. 1977. Larvogenesis of diplocyst of Aploparaksis furcigera (Rud., 1819) Fuhrmann, 1926 (Cestoda,
content/1977/prz_1977_1_3_Guljaev.pdf
Gulyaev V.D. 1989. New morpho-ecological types of the cycticercoids of cestodes of subfamily Schistotaeniinae
Johry, 1959. Ekologiya gelmintov pozvonochnykh Sibiri. Novosibirsk, Nauka, Sibirskoe Otdelenie, 199-213
[in Russian].
Mrázek A. 1927. Organisace a ontogenie larvy druhu Tatria acanthorhyncha (Wedl.). Věstník Kralovske Českĕ
Společnosti Nauk 7: 1-12 [in Czech].
Nikishin V.P. 2009. Structure and differentiation of the tissues of cysticercoids. 2. Differentiation of the exocyst
in typical diplocyst Aploparaksis bulbocirrus (Cestoda, Hymenolepididae). Invertebrate Zoology 6 (2):
Nikishin.pdf
Nikishin V.P. 2017. Morphofunctional diversity of glycocalyx in tapeworms. Biology Bulletin Reviews 7 (2):
Nikishin V.P., Krasnoshchekov G.P. 1979. Ultrastructural organization of the cercomere of Aploparaxis polystictae
Schiller, 1955 and Aploparaksis furcigera (Rudolphi, 1819) diplocysts. In: Sonin M.D. (ed.). Oecology and
morphology of the vertebrates’ helminthes in Chukotka. Moskwa, Nauka, 133-138 [In Russian].
Pospekhova N.A. 2017. Location of the primary lacuna in the postembryonic development of selected Cyclophyl-
lidea (Cestoda): a review. Invertebrate Zoology 14 (2): 167-173. doi: 10.15298/invertzool.14.2.11
Pospekhova N.A., Regel K.V. 2015. Morphology and ultrastructure of two schistotaeniid cysticercoids (Cestoda:
Cyclophyllidea) from the haemocoele of the dragonfly larvae. Parazitologiya 49 (5): 339-351. https://www.
zin.ru/journals/parazitologiya/content/2015/prz_2015_5_2_Pospekhova.pdf
Regel K.V. 2010. Leeches Erpobdella octoculata L. - intermediate hosts of Kowalewskius parvula (Kowalewski,
1904) and Kowalewskius formosa (Dubinina, 1953) comb. nov. in the Kolyma basin. International Symposium
“Parasites of the Holarctic Region”, Petrozavodsk, 4-8 October 2010, 70-73 [in Russian].
Regel К.V. 2016. On taxonomic position of aploparaksid’ metacestodes found in leeches Erpobdella octoculata
in the Upper Kolyma river basin. Transactions of Center of Severtsov Institute of Ecology and Evolution
RAS 49: 121-123 [In Russian].
Regel K.V., Pospekhova N.A. 2012. Biodiversity and morphological features of metacestodes - dragonflies larvae’
parasites of the North-East Asia. Bulletin of the NESC FEB RAS. 4: 75-83 [in Russian].
Regel K.V., Pospekhova N.A. 2019. On the morphology of metacestodes of the genus Kowalewskius - parasites of
the leeches Erpobdella octoculata from the Kolyma river basin. Parazitologiya 53 (2): 91-104 [in Russian]
DOI: 10.1134/S0031184719020017
79
Ультраструктура метацестоды
AploparaKSis shigini Bondarenko et Kontrimavichus, 2006
(Cestoda: Aploparaksidae)
Н. А. Поспехова, К. В. Регель
Ключевые слова: метацестода, ультраструктура, экзоциста, флорицерк
РЕЗЮМЕ
Впервые изучено тонкое строение метацестоды Aploparaksis shigini Bondarenko
et Kontrimavichus, 2006 от глоточных пиявок Erpobdella octoculata L. из озёр бассейна Верхней
Колымы. Цистицеркоид схож с флорицерком, т.е. имеет незамкнутую клеточную экзоцисту и
множество отростков у её основания, однако отличается длинным хвостом. Экзоциста и все
её выросты (включая хвостовой отросток) покрыты длинными, густыми микроворсинками.
Экскреторные каналы разного диаметра отмечены в экзоцисте, вблизи основания хвостового
отростка. Структура эндоцисты типична для гименолепидидных метацестод, гликокаликс имеет
характерное сетчатое строение.
80