ПАРАЗИТОЛОГИЯ, 2022, том 56, № 3, с. 188-196.
УДК 616:576.8
EFFICACY OF IVERMECTIN AGAINST
GASTROINTESTINAL NEMATODES OF GOATS
IN THE SUB-HUMID SAVANNA ZONE OF NIGERIA
© 2022 I. K. Idikaa, S. U. Asogwaa, C. F. Obia*, C. O. Nwosua
aDepartment of Veterinary Parasitology & Entomology, Faculty of Veterinary Medicine,
University of Nigeria,
Nsukka, Enugu State, Nigeria
*e-mail: chukwunonso.obi@unn.edu.ng
Received 25.01.2022
Received in revised form 03.04.2021
Accepted 08.04.2021
Faecal egg count reduction test (FECRT) was used to determine the efficacy of ivermectin in eight
purposively selected goat farms with history of no anthelmintic treatment for a 2-month period. Faecal
egg counts (FEC) were conducted on faecal samples collected per rectum from individual goats prior
to treatment with ivermectin, and the egg per gram (EPG) of faeces recorded as the Pre-treatment
FEC (PRFEC) for each animal. A second FEC was carried out on the same animals on day 14 post
anthelmintic treatment and the EPG recorded as post treatment FEC (PTFEC). Ivermectin resistance
was confirmed where the fecal egg count reduction (FECR) percentage was less than 95% and the
lower 95% confidence limit (LCL) was less than 90% but if only one of the two criteria was met,
resistance was suspected. Faecal samples were also pooled and cultured for larval identification and
count. FECRT results showed that ivermectin treatment produced >95% reduction of the PRFEC
in 5 farms and <95% reduction in 3 farms. Ivermectin resistance was suspected in two goat farms
but was confirmed in one goat farm on the basis of 95% LCL and FECRT. Larval identification
showed the occurrence of Haemonchus contortus (79%), Trichostrongylus colubriformis (17.5%) and
Oesophagostomum (3.5%). The study revealed efficacy of ivermectin against GI nematode parasites
of small ruminants in the study area at the level FECRT, resistance, however, was suspected and
confirmed in 2 and 1 farms, respectively.
Keywords: resistance, GI nematodes, Nigeria, ivermectin, small ruminants
DOI: 10.31857/S0031184722030024, EDN: FFQLYS
Small ruminants are very important in poverty alleviation, especially in developing
economies (Elzaki et al., 2019), contributing majorly to food security, in addition to their
188
other cultural and religious values. Economically, small ruminant production compares
favourably with other livestock, as they require little initial capital to set up. However,
threats by diseases, particularly, gastrointestinal nematode (GIN) parasitism remain a major
constraint to profitable small ruminant enterprise, especially in the developing countries
with serious economic consequences (Biffa et al., 2006; Idika et al., 2012). Nevertheless,
prophylactic and more so, therapeutic use of broad spectrum anthelmintics help in
maximizing productivity of grazing livestock, including small ruminants as they diminish
the effects of these parasites in these animals. However, overtime usage of anthelmintics
could lead to emergence of worm strains that resist and survive exposure to therapeutic
doses of the drugs. Resistance to anthelmintic drugs is a growing concern and threat to the
beneficial and effective use of anthelmintics, as it militates against continued efficacy and
sustainable use of the drug, and by implication, profitable livestock farming.
Anthelmintic resistance has been reported all over the world, under various climatic
conditions, but more in areas with high annual number of circles of infection, requiring
several anthelmintic treatments per year (Han et al., 2017; Arsenopoulos et al., 2021).
Nevertheless, resistance to anthelmintics could be acquired by nematode parasites after
only a few treatments (Ihler, 2010). The rapid rates of nucleotide sequence evolution seen
in some parasitic nematodes of veterinary importance and their extremely large effective
population sizes that give the worms an exceptionally high level of genetic diversity are
genetic features that favour development of anthelmintic resistance in these parasites
(Jimenez Castro et al., 2019). Anthelmintic resistance affects all classes of anthelmintics
including macrocyclic lactones, of which ivermectin is the most popular.
A number of drugs are available to control gastrointestinal nematode infestation in
small ruminants in Nigeria, however, ivermectins are among the most commonly used.
Ivermectins are preferred owing to their effectiveness against a wide range of nematodes
and ectoparasites such as lice, mites, and ticks (Campbell, 1989) as well as their wide
safety margin (González Canga et al., 2008).
The present study was therefore designed to determine the ivermectin resistance status
of GIN of goats in Nsukka Local Government Area (LGA) of Enugu State, Nigeria
using the Faecal Egg Count Reduction Test (FECRT). The study has become necessary
considering the economic importance of GINs in small ruminants and the widespread use
of ivermectin both in treatment and prophylaxis in small ruminant production in the study
area and Nigeria in general.
MATERIALS AND METHODS
Study area
The study was conducted in Nsukka Local Government Area, a sub-humid derived savannah
zone in Enugu State, lying approximately between longitude 6ᵒ52՛-7ᵒ53՛ E and latitude 6ᵒ38՛-7ᵒ8՛ N.
Small ruminant farming, especially goats is very common in the area, where they are reared mainly
as a subsidiary rural agricultural activity.
189
Study design
The study was conducted using 80 West African Dwarf (WAD) goats from eight purposively
selected farms. Ten goats were randomly selected in each of the farms designated 1-8. Farms with
faecal egg counts above 100 eggs per gram of faeces (epg), and whose goats did not receive any
anthelmintic treatment for at least two months prior to the study were included in the study. Pregnant
and nursing animals, as well as suckling kids were excluded. Ivermectin susceptibility or resistance
was based on the faecal egg count reduction (FECR) percentage and the confidence limits.
Anthelmintic susceptibility test
The individual body weights (kg) of the randomly selected goats per farm were determined
using a weigh band (we-bo®). Egg counts were conducted on faecal samples collected per rectum
from individual goats in each farm prior to treatment with ivermectin, and the egg per gram (EPG)
of faeces recorded as the Pre-treatment FEC (PRFEC) for each animal. Thereafter, the goats
were given 1% ivermectin (Ivermectine®) at the dose of 0.02 mg/Kg as recommended by the
manufacturer. A second FEC was carried out on the same animals on day 14 post anthelmintic
treatment and the EPG recorded as post treatment FEC (PTFEC). All faecal egg counts were
carried out by the modified McMaster counting technique (MAFF, 1997). Left over faecal samples
for the pre- and post-treatment FECs were pooled and routinely cultured for larval identification
and count (Hansen, Perry, 1994). The FECR (%) was thereafter calculated using the formula 100
(1-[T2/T1]) (Mckenna, 2006), where T1 and T2 represent the pre- and post-treatment FEC respectively.
The confidence limits were calculated as described by Dobson et al. (2012). Ivermectin resistance
was confirmed where the FECR percentage was less than 95 % and the lower 95 % confidence limit
was less than 90 % but if only one of the two criteria was met, resistance was suspected.
Ethical Approval
The University of Nigeria, national and international guidelines for the ethical care and use of
laboratory animals were fully adhered to. Also, appropriate ethical clearance/approval was gotten
from the Ethics Committee for Medical and Scientific Research of the University of Nigeria, Nsukka
(UNN/FVM/VPE/2592). The informed consent of the farmers were duly sought for and obtained.
RESULTS
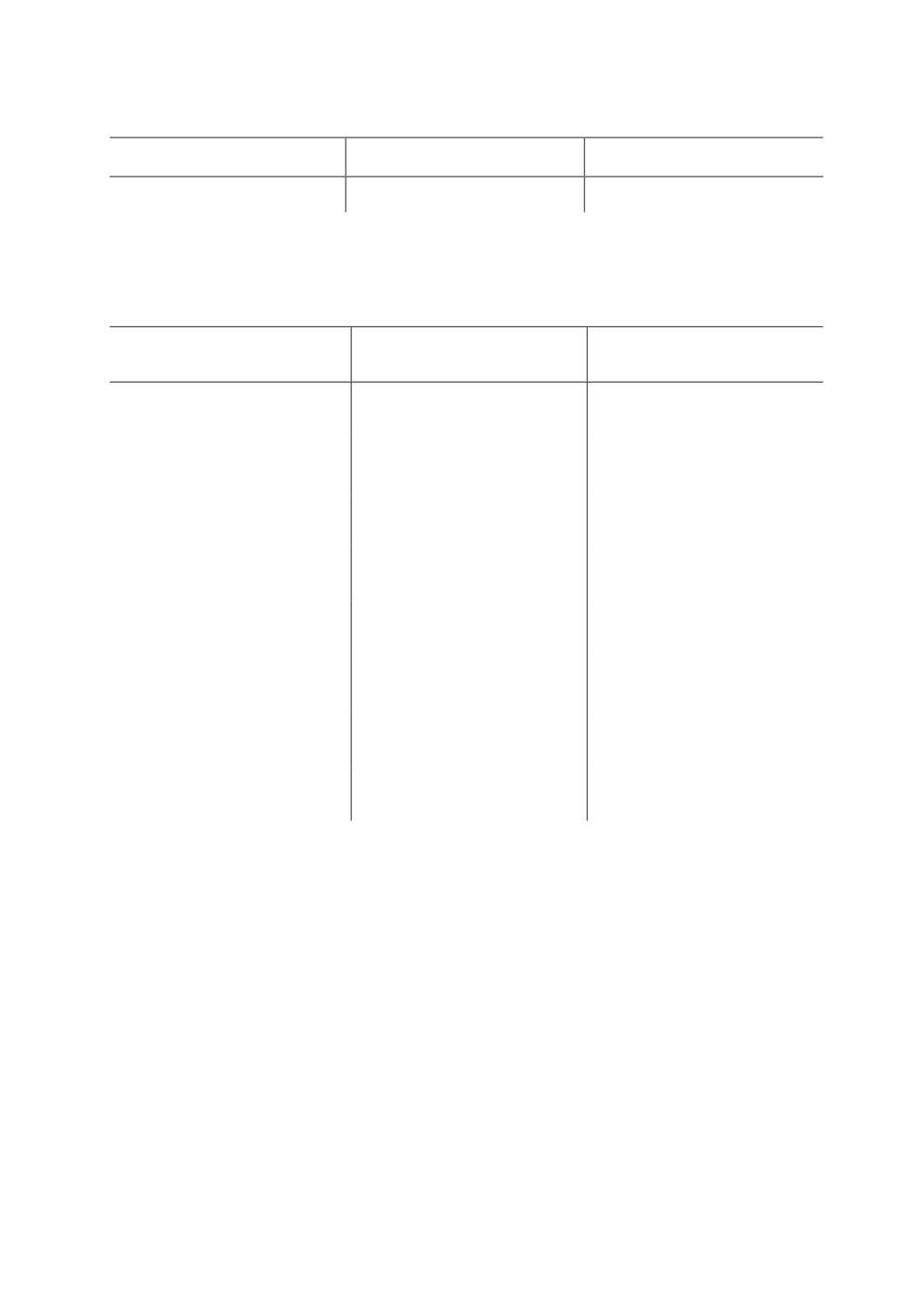
An overall GIN prevalence of 67.5% among the 8 selected goat farms has been
demonstrated (Tab. 1). Culture and larval identification of pooled pre-treatment faecal
samples (Tab. 2) indicate that 79.0% of the recovered worm eggs were those of
Haemonchus contortus (Rud.) 1915 with a mean larval count of 700 ± 49.01 L3/ml. The
proportion of the recovered eggs that belonged to Trichostrongylus colubriformis (Giles,
1892) and Oesophagostomum spp. were 17.49 and 3.45 % respectively with mean counts
of 155 ± 19.0 and 31.0 ± 4.3 L3/ml, respectively. The culture and larval identification of
post treatment faecal samples showed that 100 % of recovered strongyle eggs were those
of H. contortus (Tab. 2).
190
Table 1. Prevalence of GI trichostrongyloid nematodes in 8 selected goat farms
in Sub-humid Savanna Zone of Nigeria
No. Examined
No. Infected
Prevalence
80
54
67.5%
Table 2. Species of nematodes determined by culture of pooled faeces obtained
from selected goat farms in Sub-humid Savanna Zone of Nigeria
Larval count
Proportion of recovery
Nematode species
(Mean ± SEM) (x 102)
(Mean ± SEM), %
Pre-treatment faecal samples
Haemonchus contortus
7.00 ± 0.49
79.00
Trichostrongylus colubriformis
1.55 ± 0.19
17.49
Oesophagostomum spp.
0.31 ± 0.04
3.50
Post treatment faecal samples
Haemonchus contortus
0.40 ± 0.09
100
Trichostrongylus colubriformis
Nil
Oesophagostomum spp
Nil
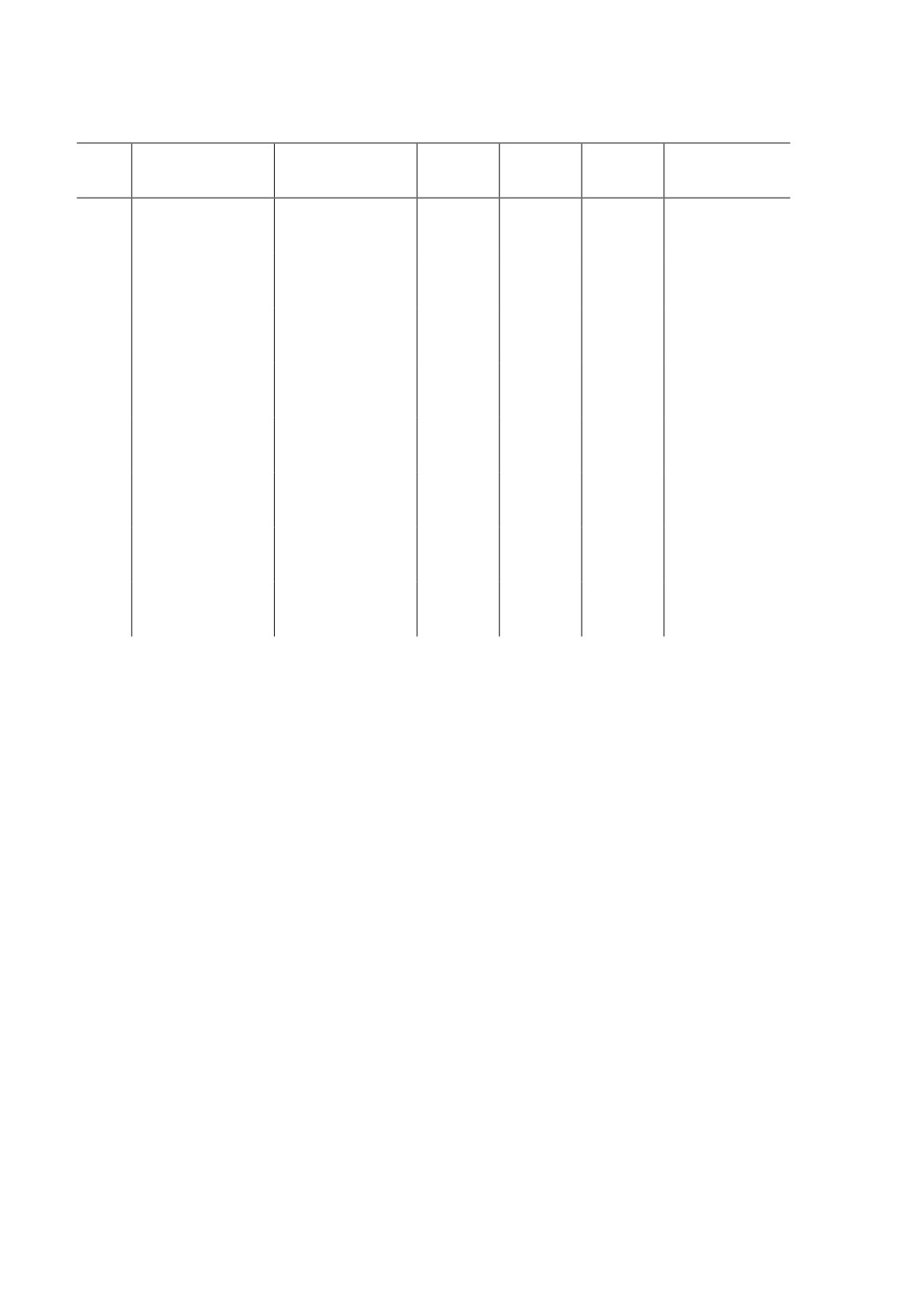
Table 3 shows the mean pre- and post- treatment FEC of the goats in different farms.
The animals possesed mean pre-treatment FEC that ranged between 179.00 ± 69.29 and
490.00 ± 165.03 epg and post-treatment FEC of 0.67 ± 0.67 and 37.67 ± 37.17.25%.
The results of the FECRT showed that a single dose treatment with ivermectin produced
> 95% reduction of the PRFEC in 5 farms, namely, farms 1, 3, 4, 5, and 7 with their
95% LCL above 90 %. Consequently, the ivermectin produced < 95% reduction in 3 farms,
namely, farms 2, 6, and 8. However, the 95% LCL was less than 90% in farm 6 and above
90% in farms 2 and 8. Therefore, resistance was suspected in farms 2 and 8 but present
in farm 6 on the basis of the FECRT. Consequently, 62.5% of the farms sampled were
susceptible to ivermectin, 12.5% were resistant while 25% had GIN parasites that suspected
of resistance to ivermectin.
191
Table 3. Mean faecal strongyle-egg count reduction percentage in WAD ruminants treated
with ivermectin 0.02 mg/Kg in Sub-humid Savanna Zone of Nigeria
Resistance
Farms
PREFEC
PTFEC
FECR
LCL
UCL
status
1
335.00 ± 70.30
4.00 ± 2.12
98.81
96.30
99.68
Susceptible
2
490.00 ± 165.03
27.33 ± 27.33
94.42
91.21
96.60
Suspected
3
186.67 ± 107.29
1.00 ± 1.00
99.46
96.11
99.94
Susceptible
4
393.33 ± 158.36
0.67 ± 0.67
99.83
98.30
99.99
Susceptible
5
316.67 ± 192.73
3.00 ± 3.00
99.05
96.59
99.79
Susceptible
6
433.33 ± 197.01
37.67 ± 37.17
91.31
87.26
94.26
Resistant
7
179.00 ± 69.29
2.33 ± 1.20
98.70
94.64
99.75
Susceptible
8
400.00 ± 165.03
24.33 ± 24.33
93.92
90.18
96.40
Suspected
DISCUSSION
The present study showed that goats in Nsukka LGA of Enugu State, Nigeria are
commonly infested with GI trichostrongyloid nematodes with 67.5 % prevalence rate. Larval
identification revealed the predominance of Haemonchus contortus above other GI nematode
parasites in goats in the study area. These findings collaborate with prevalence rates of
70-90 % of GI nematode infections of small ruminants, been reported by previous studies
(Chiejina, 1986; Idika et al., 2012) in the study area, of which Haemonchus contortus
has consistently been predominant. Nsukka is situated within the derived Savannah zone
of Nigeria, with annual temperature range of 16 to 30°С (rarely below 12 and above
32°С) and 9.3 months (mid February to late November) of rainfall that support survival
and development of pre-parasitic stages of nematodes almost all year round. Hence, grazing
livestock in the area is usually associated with high annual number of circles of infection
per year, with several anthelmintic treatments per year. The implication of such frequent
treatment especially with a particular type of anthelmintics resulted in the unwanted
emergence of nematode populations with heritable ability to resist anthelmintic doses.
In the study area, ivermectin is the most commonly used anthelmintic for treatment of small
192
ruminants. Campbell (1989) reports that ivermectin is a drug of choice in small ruminants,
due to its wide margin of action, been effective against endo- and ecto-parasites, as well
as its wide safety margin.
It was a core objective of the present study to provide information on the state of
ivermectin resistance by GI trichostrongyloid nematodes of goats in the study area, using
the Faecal egg count reduction test (FECRT). The study therefore, identified varying degrees
of efficacy and possible presence of ivermectin resistance. The results of the present study
showed that at the recommended therapeutic dose of 0.02 mg/kg, ivermectin produced
over 95% reduction of the pre-treatment FECs in five farms. Therefore, ivermectin was
efficacious in these farms and their gastrointestinal nematode (GIN) parasite population
judged susceptible to ivermectin. On the other hand, the GIN population in farm 6 was
assumed resistant to ivermectin on the basis of a less than 95% FECR produced by the
ivermectin with a 95% LCL of < 90%. However, ivermectin resistance was suspected in
farms 2 and 8 given that the FECR percentage in these farms were less than 95% (94.42
and 93.92% respectively) but the 95% LCLs were >90% (96.6 and 96.4% respectively).
Frequent use and misuse of antiparasiticides, especially diminazene, ivermectin, and
albendazole by farmers and animal attendants in the study area was reported following
a study by Obi et al. (in press). The ivermectin resistance noted in this study was attributed
to frequent and over usage of the drug, given that ivermectin serves dual purposes been
effective against nematode- and ecto-parasistes. Farmers and veterinarians often use
ivermectins against ectoparasites in animals including without any iota of regard to their
nematode parasites in these animals. Ectoparasitism, particularly mite infestation is very
common among small ruminants in the study area, requiring frequent treatment with
acaricides of which Ivermectin is widely used due it its efficacy, wide safety margin,
cost effectiveness and ease of administration. This invariably imposes continuous selection
pressure on nematode strains in favour of resistant genes. Such frequent and often
unnecessary uses of anthelmintics with respect to nematode parasites are known risk factors
for the development of anthelmintic resistance (Shalaby, 2013).
Culture of pooled post treatment faecal samples suggests that the observed ivermectin
resistance was demonstrated by H. contortus. This worm is known to have a high propensity
to develop resistance (Redman et al., 2012). Haemonchus contortus is the most important
GI nematode of goats in the study area, with very high daily egg output, rapid build-up
of infective L3 on pasture under suitable climatic conditions, high establishment rate and
very short latent period (Chiejina, 1986; Mghomga et al., 2012). Therefore, the result
of the present study calls for great caution with the use of ivermectins in goats. Coles
et al. (2006) report that early stages of anthelmintic resistance usually go unnoticed as the
anthelmintics may still be effective, however, complete efficacy is lost when resistance
193
reaches higher levels either in individual host or proportion of affected worm population.
It is also important to note that resistance to an anthelmintic by GI nematodes could lead to
resistance to other compounds with similar mode of action, irrespective of whether or not,
the nematode has been exposed to that particular anthelmintic. Resistance to ivermectin in
small ruminants has been reported in so many countries under different farm management.
Adediran, Uwalaka (2015) reported a low level of resistance against ivermectin in West
African Dwarf goats. Likewise, Dey et al. (2020) reported resistance to ivermectin as well
as to levamisole and albendazole in sheep and goats in Bangladesh.
CONCLUSION
This study confirmed and suspected ivermectin resistance in 1 and 2 farms respectively,
out of the 8 farms studied at the level of FECRT. The importance of this finding in
the study area cannot be over-emphasized given that many veterinarians and animal
handlers in the area rely heavily on ivermectin for treatment against GI nematode- and
ectoparasites in small ruminants. The FECRT, though highly recommended for detecting
anthelmintic resistance in farm animals, (Coles et al., 1992), lack sensitivity as it only
detects anthelmintic resistance in populations were more than 25 % of worms are resistant
(Domke et al., 2012). This, therefore, calls for great caution on the use of ivermectin in
the study area to limit the pressure imposed on the selection for resistant genes. Molecular
detection technique is however required for further confirmation of the ivermectin resistant
status of small ruminants in the area.
References
Adediran O.A., Uwalaka C.E. 2015. Effectiveness evaluation of levamisole, albendazole, ivermectin, and
Vernonia amygdalina in West African Dwarf Goats. Journal of Parasitology Research 5 p. https://doi.
org/10.1155/2015/706824
Arsenopoulos K.V., Fthenakis G.C., Fthenakis E.I., Papadopoulos E. 2021. Haemonchosis: a challenging parasitic
Biffa D., Jobre Y., Chakka H. 2006. Ovine helminthosis, a major health constraint to productivity of sheep in
Campbell W.C. 1989. Ivermectin and Abamectin. New York, Springer-Verlag, 363 pp.
Chiejina S.N. 1986. The epizootiology and control of parasitic gastroenteritis of domesticated ruminants in Nigeria.
Helminthological Abstracts 55: 413-429.
Coles G.C., Borgsteede F.H., Geerts S., Klei T.R., Taylor M.A., Walle P.J. 1992. World Association for the
Advancement of Veterinary Parasitology (W.A.A.V.P) methods for the detection of anthelmintic resistance
4017(92)90141-U
Coles G.C., Jackson F., Pomroy W.E., Prichard R.K., von Samson-Himmelstjerna G., Silvestre A., Taylor M.A.,
Vercruysse J. 2006. The detection of anthelmintic resistance in nematodes of veterinary importance. Vete-
194
Dey A.R., Begum N., Anisuzzaman Alim A., Alam M. 2020. Multiple anthelmintic resistance in gastrointestinal
nematodes of small ruminants in Bangladesh. Parasitology International 77 : 102105. https://doi.
org/10.1016/j.parint.2020.102105
Dobson, R.J., Hosking, B.C., Jacobson, C.L., Cotter, J.L., Besier, R.B., Stein, P.A., Reid, S.A. 2012. Preserving
new anthelmintics: A simple method for estimating faecal egg count reduction test (FECRT) confidence
limits when efficacy and/or nematode aggregation is high. Vet. Parasitol. 186, 79-93. https://doi.
org/10.1016/j.vetpar.2011.11.049
Domke A.V.M., Chartier C., Gjerde B. et al. 2012. Prevalence of anthelmintic resistance in gastrointestinal
nematodes of sheep and goats in Norway. Journal Parasitology Research 111: 185-193. https://doi.
org/10.1007/s00436-012-2817-x
Elzaki R., Abdalla S., Al-Mahish M. 2019. Small ruminants as a pathway to reduce urban poverty: An empirical
González Canga A., Sahagún Prieto A.M., Diez Liébana M.J., Fernández Martínez N., Sierra Vega M., García
Vieitez J.J. 2008. The pharmacokinetics and interactions of ivermectin in humans--a mini-review. The
Han T., Wang M., Zhang G., Han D., Li X., Liu G., Li X., Wang Z. 2017. Gastrointestinal nematodes infections
and anthelmintic resistance in grazing sheep in the Eastern Inner Mongolia in China. Acta Parasitologica
Hansen, J., Perry, B. 1994. The epidemiology, diagnosis and control of helminth parasites of ruminants a hand
book. International Library of Research in Animal Diseases, Nairobi, Kenya, 62-72.
Idika I.K., Iheagwam C.N., Ezemonye C.N., Nwosu C.O. 2012. Gastrointestinal nematodes and body condition
scores of goats slaughtered in Nsukka, Nigeria. Nigeria Veterinary Journal 33 (1): 440-447.
Ihler C.F. 2010. Anthelmintic resistance. An overview of the situation in the Nordic countries. Acta Veterinaria
Jimenez Castro P.D., Howell S.B., Schaefer J.J. et al. 2019. Multiple drug resistance in the canine hookworm
019-3828-6
MAFF 1977. Manual of veterinary laboratory diagnostic techniques. Bulletin Number 18. Ministry of Agriculture
Fisheries and Food. London, HMSO, 5-50.
McKenna P.B. 2006. Further comparism of fecal egg count reduction test procedure: sensitivity and specificity.
Mhomga L.I., Nnadi P.A., Chiejina S.N., Idika I.K., Ngongeh L.A. 2012. Effects of dietary protein supplementation
on the performance of West African dwarf (WAD) goats infected with Haemonchus contortus and
Trichostrongylus colubriformis. Turkish Journal of Veterinary and Animal Science 36 (6): 668-675. https://
doi.org/10.3906/vet-1106-21
Redman E., Sargison N., Whitelaw F., Jackson F., Morrison A., Bartley D.J., Gileard S.J. 2012. Introgression
of ivermectin resistance genes into a susceptible Haemonchus contortus strain by multiple backcrossing.
Shalaby H.A. 2013. Anthelmintics resistance; how to overcome it? Iranian journal of parasitology 8 (1): 18-32.
195
ЭФФЕКТИВНОСТЬ ИВЕРМЕКТИНА
ПРОТИВ НЕМАТОД ЖЕЛУДОЧНО-КИШЕЧНОГО ТРАКТА У КОЗ
В СУБГУМИДНОЙ ЗОНЕ САВАННЫ В НИГЕРИИ
И. К. Идика, С. У. Асогва, Ц. Ф. Оби, Ц. О. Нвосу
РЕЗЮМЕ
Тест на уменьшение подсчитанных яиц в фекалиях (FECRT) был использован для определе-
ния эффективности применения ивермектина в восьми специально отобранных козьих фермах,
в которых антигельминтные мероприятия не проводились, по крайней мере, в течение 2 меся-
цев. Подсчеты яиц в фекалиях (FEC) проводили в образцах, собранных per rectum от отдельных
особей коз, и обозначенных как предшествовавшие опытам (PRFEC) для каждого животного
отдельно. Повторный тест (FEC) был выполнен на тех же самых животных на 14-й день после
использования антигельминтного препарата и обозначен как PTFEC. Резистентность к ивер-
мектину считалась подтвержденной, когда коэффициент FECRT был меньше 95%, а нижний
доверительный предел (LCL) составлял менее 90%, но если был выявлен только один из крите-
риев, резистентность только подозревалась. Образцы, полученные из фекалий, были объединены
и культивированы для определения личинок и их подсчета. Результаты теста FECRT показали,
что обработка ивермектином привела к более чем 95% уменьшению PRFEC на пяти козьих фер-
мах и менее чем 95% уменьшению на трех фермах. Резистентность к ивермектину находилась
под подозрением на двух козьих фермах, но была подтверждена на единственной ферме, на
основании 95% LCL и FECRT. Определение личинок показало, что среди гельминтов присут-
ствовали Haemonchus contortus (79%), Trichostrongylus colubriformis (17.5%) и Oesophagostomum
(3.5%). Исследование показало эффективность использования ивермектина против желудочно-
кишечных нематод, паразитов мелкого рогатого скота на исследованной территории на уровне
FECRT, при этом, однако, резистентность к ивермектину была обнаружена у животных с одной
фермы, и предположительно подозреваема у животных с двух ферм.
196