УДК 578.4(470.22)
ARTHROPOD-BORNE AND ARTHROPOD-RELATED VIRUSES
IN IRAN AND NEIGHBORING COUNTRIES
© 2023 S. Azari-Hamidiana,b*, R. E. Harbachc
aResearch Center of Health and Environment, School of Health,
Guilan University of Medical Sciences, Rasht, Iran
bDepartment of Medical Parasitology, Mycology and Entomology, School of Medicine,
Guilan University of Medical Sciences, Rasht, Iran
cDepartment of Life Sciences, Natural History Museum, London, UK
Correspondence: Prof. Dr. Shahyad Azari-Hamidian, Research Center of Health
and Environment, School of Health, Guilan University of Medical Sciences,
Rasht, Iran, P.O. Box: 3391, Rasht, Iran, Tel./Fax: 0098 13 33822877
The present article is dedicated to my wife Elaheh and my son Arvin who have patiently
supported me during my professional currier, especially providing this article
*e-mail: azari@gums.ac.ir
Received May 07, 2023
Revised August 30, 2023
Accepted September 20, 2023
Arthropods are very significant for human and veterinary medicine and health because of the
burden of diseases caused by the pathogens they transmit. Databases, including the Web of Science,
PubMed, Scopus, Google Scholar, CABI, Scientific Information Database, IranMedex and Magiran
were searched to the end of December 2022 for publications concerning infections in Iran caused
by arboviruses. Pertinent information was extracted and analyzed. Thirty-three viral infections occur
in Iran, which are biologically or mechanically known or assumed to be transmitted by arthropods.
Information about agents (viruses), distribution (in 31 Iranian provinces), hosts (human and animals)
and known vectors in Iran was obtained for each disease. Also, a list of arboviruses was provided for
the countries neighboring Iran, including Afghanistan, Armenia, Azerbaijan, Bahrain, Iraq, Kuwait,
Oman, Pakistan, Qatar, Saudi Arabia, Turkey, Turkmenistan and the United Arab Emirates, as well
as Djibouti, Somalia, Sudan, Syria and Yemen, which do not neighbor Iran but, like Iran, occur in
the World Health Organization Eastern Mediterranean Region. This list includes 40 viruses which
are not formally recorded in Iran. The viruses are members of 19 genera representing 14 families
in which three, four, 20 and 29 viruses are sandfly-borne, biting midge-borne, mosquito-borne and
tick-borne, respectively.
Keywords: arboviruses, biological transmission, mechanical transmission, mobovirus, reservoirs,
vectors, zoonoses
356
DOI: 10.31857/S0031184723050010; EDN: PTKYLO
About 17% of the global burden of infectious and parasitic diseases is caused by
vector-borne pathogens. After lower respiratory infections, diarrhoeal diseases, HIV/AIDS
and tuberculosis, malaria displays the fifth highest burden among infectious and parasitic
diseases (World Health Organization, 2008). Traditionally, malaria and leishmaniasis are
major diseases in the World Health Organization (WHO) Eastern Mediterranean Region,
caused by vector-borne malarial protozoa (mosquito-borne) and trypanosomes (sandfly-
borne), respectively. Many other arthropod-borne viral (arboviral) infections, such as
Crimean-Congo hemorrhagic fever, dengue fever, Japanese encephalitis, Rift Valley fever,
sandfly fever, West Nile fever and yellow fever, are of lesser or more local importance
(World Health Organization, 2004). While, the burden of malaria has decreased and the
burden of leishmaniasis has not changed during recent years in the region (World Health
Organization, 2004, 2008, 2017), some arboviral infections, such as Crimean-Congo
hemorrhagic fever, Chikungunya fever, dengue fever, Rift Valley fever and West Nile
fever, which are classified as neglected, emerging or reemerging infectious diseases (EIDs
or RIDs), have been introduced into the region or Iran (World Health Organization, 2010;
Parhizgari et al., 2017; Pouriayevali et al., 2019). It has been estimated that the majority of
EIDs are zoonotic (60.3%) and 71.8% of these are caused by pathogens that originated from
wildlife, such as Ebola virus, Nipah virus and severe acute respiraotory syndrome (SARS)
virus. While 25.4% of EIDs are caused by viral and prion pathogens, 22.8% of EIDs
are vector-borne (Jones et al., 2008). Some arthropod-borne viruses (arboviruses) are not
pathogenic for humans but they are for domesticated animals; thus, they are very important
in view of food production and/or have economical importance because of loss of eggs,
milk or meat production, unhealthy offspring and loss of herds or fowl populations due to
diseases such as African horse sickness (Dennis et al., 2019), African swine fever (Dixon et
al., 2019), bluetongue (Maclachlan et al., 2015), bovine ephemeral fever (Walker, Klement,
2015), fowl pox (Della-Porta, 2001), rinderpest (Roeder et al., 2013) and Schmallenberg
virus infection (Collins et al., 2019). Also, the possible use of some arthropods infected with
certain arboviruses as weapons or bioterrorism is mentioned in published literature, such
as mosquitoes infected with dengue, Rift Valley fever and yellow fever viruses and ticks
infected with Colorado fever and Crimean-Congo hemorrhagic fever viruses (Lockwood,
2012). There are more than 600 known arboviruses (Conway et al., 2014) and about 100
of these infect humans and some 40 infect livestock (Hart, 2001). Fifty arboviruses are
known to cause disease in homeotherm (endotherm) wild and domestic mammals and birds
(Hubálek et al., 2014a).
Iran is located in the Middle East and southwestern Asia where the Afrotropical,
Oriental and Palaearctic Regions converge. Iran is connected with Central Asia through
Turkmenistan in the northeast, with southern Asia and the Oriental Region through Pakistan
in the southeast and with the Afrotropical Region through the Arabian Peninsula in the
south. For this reason, the region is interesting in view of biodiversity while at the same
357
time complicating interventions for vector control and integrated vector management (IVM)
aimed at reducing the transmission of vector-borne pathogens and parasites and the burden
of diseases. Iran also resides in the WHO Eastern Mediterranean Region along with 21
other countries: Afghanistan, Bahrain, Djibouti, Egypt, Iraq, Jordan, Kuwait, Lebanon,
Lybia, Morocco, Oman, Pakistan, Palestine, Qatar, Saudi Arabia, Somalia, Sudan, Syria,
Tunisia, the United Arab Emirates and Yemen (World Health Organization, 2004).
There are some recent and useful reviews of different arboviruses that occur in some
of the aforementioned countries, such as Failloux et al. (2017) who reviewed arboviruses
in the Mediterranean and Black Sea Regions, Atkinson and Hewson (2018) who reviewed
arboviruses in Central Asia and Braack et al. (2018) who reviewed mosquito-borne viruses
(moboviruses) in Africa. Also, there are some useful reviews on specific infections that
occur in the region, such as African horse sickness (Dennis et al., 2019), African swine
fever (Dixon et al., 2019), Akabane virus infection (Kirkland, 2015), Bhanja virus infection
(Hubálek, 1987), bluetongue virus infection (Maclachlan et al., 2015), bovine ephemeral
fever (Walker, Klement, 2015), bovine herpes (Chatterjee et al., 2016), Chikungunya virus
infection (Silva et al., 2018), Crimean-Congo hemorrhagic fever (Nasirian, 2019), Hantaan
virus infection (Bi et al., 2008), Rift Valley fever (Kenawy et al., 2018), rinderpest (Roeder
et al., 2013), sandfly fever (Depaquit et al., 2010), Schmallenberg virus infection (Collins
et al., 2019), West Nile fever (Eybpoosh et al., 2019) and Zika virus infection (Epelboin
et al., 2017), as well as reviews for specific countries, such as Afghanistan (Wallace et al.,
2002), Pakistan (Hayes, Burney, 1981), Sudan (Ahmed et al., 2020) and Turkey (Ergunay
et al., 2011; Inci et al., 2013, 2016, 2018; Düzlü et al., 2020).
Recently, Azari-Hamidian et al. (2019) reviewed 14 mosquito-borne pathogens and
parasites in Iran, including six viral infections (avian or fowl pox, bovine ephemeral
fever, dengue, Rift Valley fever, Sindbis and West Nile fever), two bacterial infections
(anthrax and tularemia), four helminthoses (Deraiophoronema evansi infection, dirofilariasis,
lymphatic filariasis and setariasis) and two protozoal infections (avian and human malarias)
and updated the checklist of Iranian mosquitoes. Also, Parhizgari et al. (2021) reviewed
some selected diseases in Iran caused by vector-borne pathogens. They reviewed, for
example, four arboviruses: Crimean-Congo hemorrhagic fever, dengue fever, sandfly fever
and West Nile fever.
In the present article, we provide a comprehensive review of infections caused by
arboviruses in Iran. We also provide a list of arboviruses in the countries neighboring
Iran, as well as Djibouti, Somalia, Sudan, Syria and Yemen, which, like Iran, located in
the WHO Eastern Mediterranean Region, and have not received much attention in recent
reviews of arboviruses (Failloux et al., 2017; Braack et al., 2018). Thus, the present review
includes 19 countries. Additionally, it includes some viruses that are not (true) arboviruses
or are arthropod-related viruses which arthropods may mechanically transmit to humans
and domestic animals, and were not included in the aforementioned reviews of arboviruses.
358
METHODS
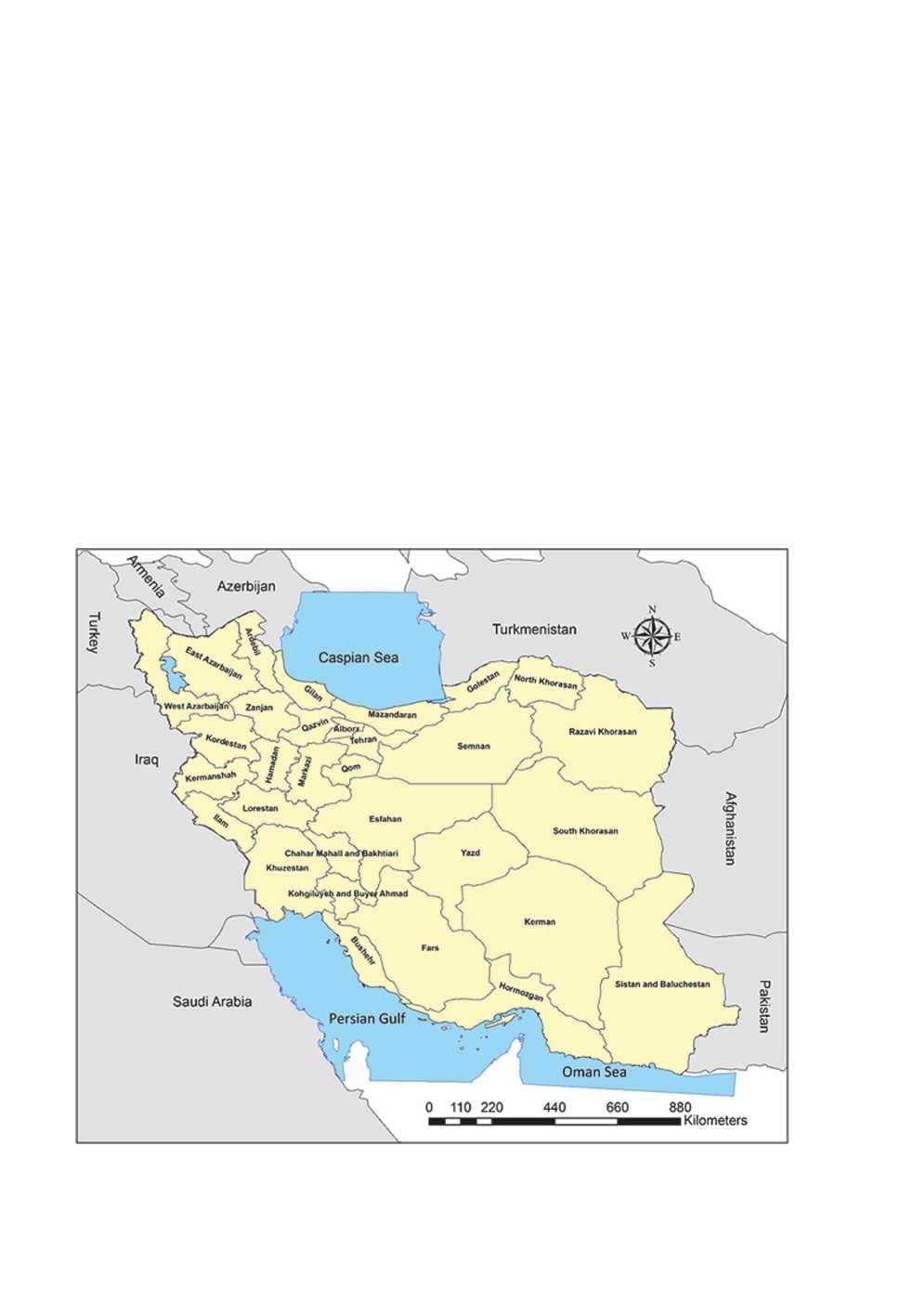
Iran, with an area of approximately 1,648,195 km2, is located between 25-40o N latitude
and 44-63o E longitude and formally includes 31 provinces (Fig. 1). Iran is bordered by
Armenia, Azerbaijan and Turkmenistan in the north, Afghanistan and Pakistan in the east,
Iraq and Turkey in the west and the Persian Gulf and Oman Sea in the south, across
which lie the countries of Bahrain, Kuwait, Oman, Qatar, Saudi Arabia and the United
Arab Emirates. Hereafter, Iran, the aforementioned countries and five countries of the
WHO Eastern Mediterranean Region, including Djibouti, Somalia, Sudan, Syria and Yemen,
are referred to as “the region”. Most parts of Iran and many countries in “the region”
have arid climate based on different climate classifications. This investigation is based on
publications listed in the Web of Science (Clarivate), PubMed, Scopus, Google Scholar,
CABI, Scientific Information Database (SID), IranMedex and Magiran databases prior
to December 2022. Firstly, principal textbooks on medical and veterinary entomology
(for example Harwood, James, 1979; Lane, Crosskey, 1993; Mullen, Durden, 2019) were
reviewed for information on diseases caused by arboviruses. Secondly, we searched the
aforementioned databases using terms such as “arthropod-borne diseases”, “arboviruses”,
“mosquito-borne viruses” and “moboviruses” to identify the names of viral infections
associated with arthropods. Afterwards, the databases were searched to obtain literature
reporting the occurrence of those diseases in animals and humans in Iran, Central Asia,
the Middle East, southwestern Asia and the WHO Eastern Mediterranean Region (Harbach,
1988; World Health Organization, 2004). Finally, the searches were conducted using the
keywords “extracted arthropod-borne viral disease names, Iran, Iranian” and “extracted
arthropod-borne virus names, Iran, Iranian”. Also, the searches were conducted with the
names of the countries neighboring Iran and the five additional countries of the WHO
Eastern Mediterranean Region. The names of diseases or infections comprised more than
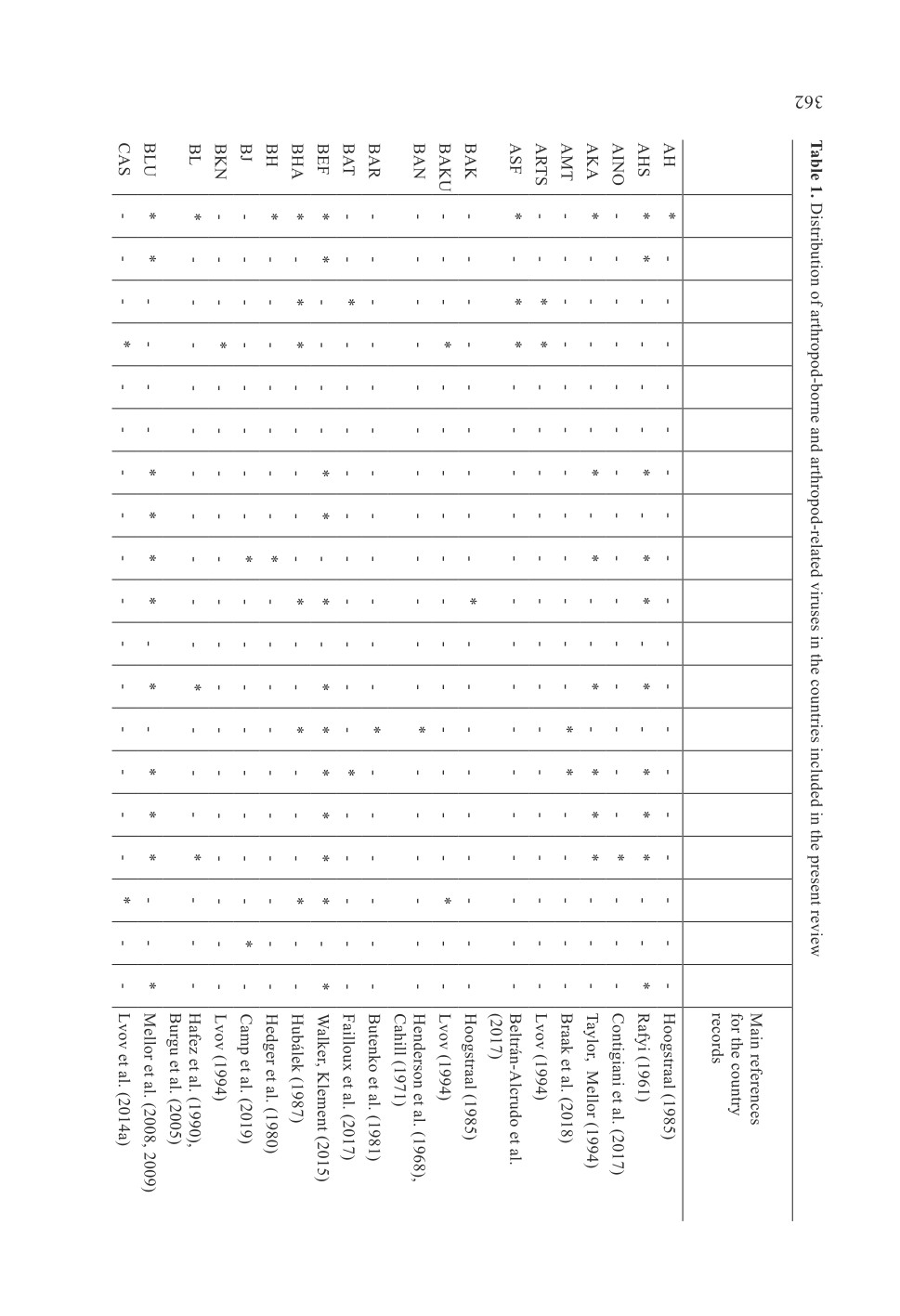
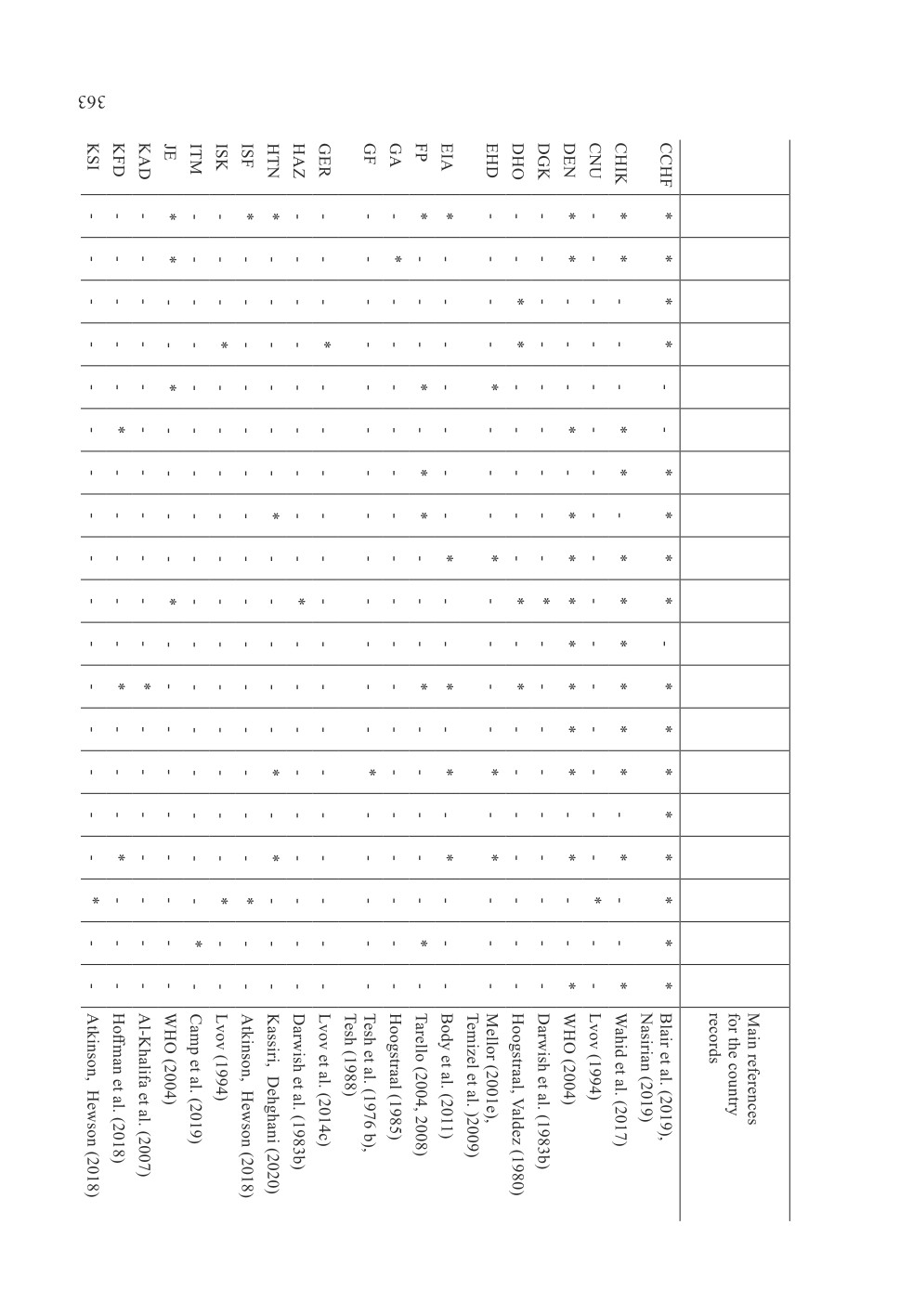
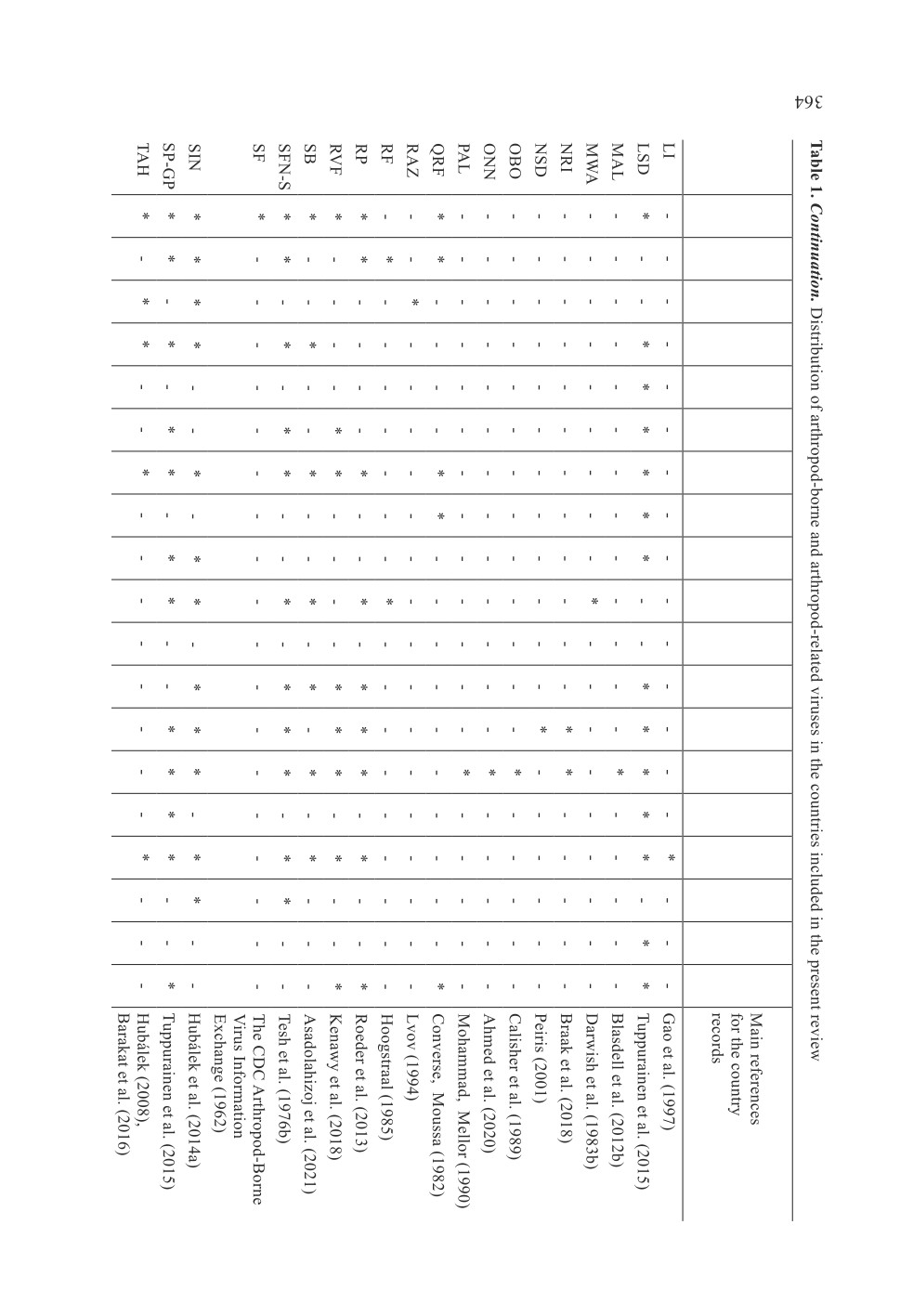
73 keywords (names or terms) which were mentioned in the search results (Table 1,
Fig. 2). It should be mentioned that there were more than one name or term for some
infections or diseases. The generic names of arthropod-borne and arthropod-related viruses
included Alphavirus, Asfavirus, Avipoxvirus, Bandavirus, Capripoxvirus, Deltaretrovirus,
Ephemerovirus, Flavivirus, Lentivirus, Morbillivirus, Orbivirus, Orthobunyavirus,
Orthohantavirus, Orthonairovirus, Phlebovirus, Thogotovirus, Varicellovirus, Vesiculovirus
and Zamolirhabdovirus. Additionally, references cited in the retrieved publications were
also reviewed to increase the coverage of the literature. Likewise, unpublished documents
such as the Centers for Disease Control and Prevention (CDC) Arthropod-Borne Virus
coverage. With few exceptions, only information obtained from books and peer-reviewed
articles was used to prepare this review. Information about infectious agents (viruses),
distribution (in 31 Iranian provinces) (Fig. 1), reservoirs or hosts (human and animals) and
known vectors in Iran was obtained for each infection. Six mosquito-borne viral infections,
which were recently reviewed by Azari-Hamidian et al. (2019), were mentioned only for
359
distributional records in the region or possible new data in Iran. Maes et al. (2018) was
consulted for the latest classification of arboviruses of the order Bunyavirales. The capital
letter abbreviations used for the names of viruses are based on the “International catalog
of arboviruses including certain other viruses of vertebrates” (available at https://wwwn.
cdc.gov/arbocat/VirusBrowser.aspx). There is one exception: all sandfly-borne phleboviruses
were mentioned in one keyword “Sandfly fever”. Those are abbreviated SFN-SV because
the most common viruses among them are Naples (SFNV) and Sicilian (SFSV) viruses,
and also to distinguish them from Semliki Forest virus (SFV). Also, sheep pox virus (SPV)
and goat pox virus (GPV) were mentioned in one search result. Though they are different
viruses, their clinical diseases are similar. The abbreviations of mosquito and sandfly genera
and subgenera follow Reinert (2009) and Galati et al. (2017), respectively. For the valid
species names of different arthropod taxa, the following references and webpages were
consulted: biting midges (Borkent, Dominiak, 2020), horseflies (Moucha, 1976), mosquitoes
(Azari-Hamidian et al., 2019, 2020; Harbach, 2023), sandflies (Secombe et al., 1993) and
ticks (Gugliemone et al., 2010, 2014; Hosseni-Chegeni et al., 2019).
Figure 1. Map of Iran and its 31 provinces.
360
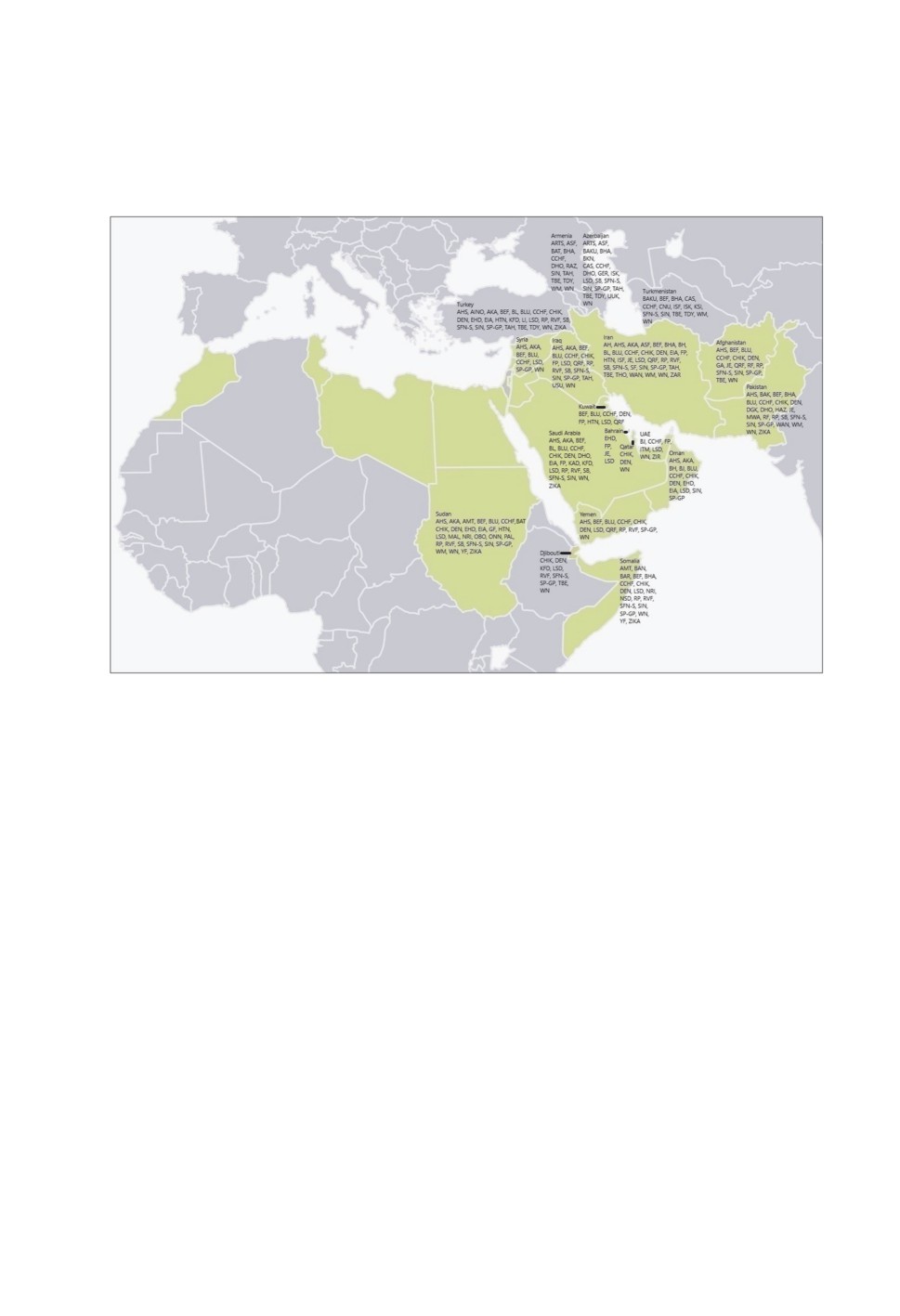
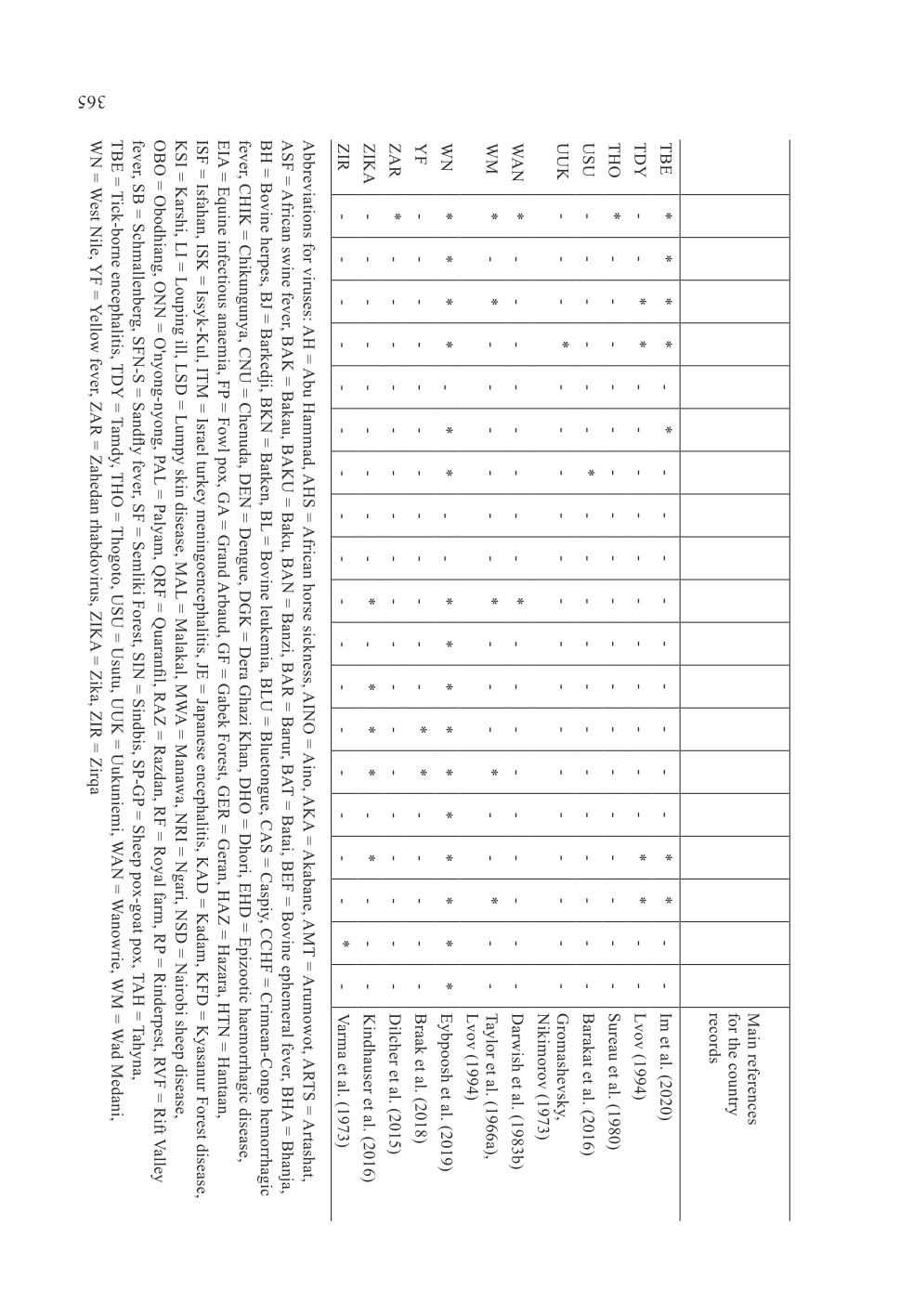
Figure 2. Map showing the arthropod-borne and arthropod-related viruses in the countries
included in the present review and the highlighted countries of the World Health Organization
Eastern Mediterranean Region. Abbreviations for viruses: AH = Abu Hammad, AHS = African
horse sickness, AINO = Aino, AKA = Akabane, AMT = Arumowot, ARTS = Artashat,
ASF = African swine fever, BAK = Bakau, BAKU = Baku, BAN = Banzi, BAR = Barur,
BAT = Batai, BEF = Bovine ephemeral fever, BHA = Bhanja, BH = Bovine herpes,
BJ = Barkedji, BKN = Batken, BL = Bovine leukemia, BLU = Bluetongue, CAS = Caspiy,
CCHF = Crimean-Congo hemorrhagic fever, CHIK = Chikungunya, CNU = Chenuda,
DEN = Dengue, DGK = Dera Ghazi Khan, DHO = Dhori, EHD = Epizootic haemorrhagic
disease, EIA = Equine infectious anaemia, FP = Fowl pox, GA = Grand Arbaud, GF = Gabek
Forest, GER = Geran, HAZ = Hazara, HTN = Hantaan, ISF = Isfahan, ISK = Issyk-Kul,
ITM = Israel turkey meningoencephalitis, JE = Japanese encephalitis, KAD = Kadam,
KFD = Kyasanur Forest disease, KSI = Karshi, LI = Louping ill, LSD = Lumpy skin disease,
MAL = Malakal, MWA = Manawa, NRI = Ngari, NSD = Nairobi sheep disease,
OBO = Obodhiang, ONN = O'nyong-nyong, PAL = Palyam, QRF = Quaranfil, RAZ = Razdan,
RF = Royal farm, RP = Rinderpest, RVF = Rift Valley fever, SB = Schmallenberg,
SFN-S = Sandfly fever, SF = Semliki Forest, SIN = Sindbis, SP-GP = Sheep pox-goat pox,
TAH = Tahyna, TBE = Tick-borne encephalitis, TDY = Tamdy, THO = Thogoto, USU = Usutu,
UUK = Uukuniemi, WAN = Wanowrie, WM = Wad Medani, WN = West Nile, YF = Yellow
fever, ZAR = Zahedan rhabdovirus, ZIKA = Zika, ZIR = Zirqa.
361
Viruses
Iran
Afghanistan
Armenia
Azerbaijan
Bahrain
Djibouti
Iraq
Kuwait
Oman
Pakistan
Qatar
Saudi Arabia
Somalia
Sudan
Syria
Turkey
Turkmenistan
UAE
Yemen
Viruses
Iran
Afghanistan
Armenia
Azerbaijan
Bahrain
Djibouti
Iraq
Kuwait
Oman
Pakistan
Qatar
Saudi Arabia
Somalia
Sudan
Syria
Turkey
Turkmenistan
UAE
Yemen
Viruses
Iran
Afghanistan
Armenia
Azerbaijan
Bahrain
Djibouti
Iraq
Kuwait
Oman
Pakistan
Qatar
Saudi Arabia
Somalia
Sudan
Syria
Turkey
Turkmenistan
UAE
Yemen
Viruses
Iran
Afghanistan
Armenia
Azerbaijan
Bahrain
Djibouti
Iraq
Kuwait
Oman
Pakistan
Qatar
Saudi Arabia
Somalia
Sudan
Syria
Turkey
Turkmenistan
UAE
Yemen
Infections in Iran caused by arthropod-borne viruses or the viruses
which may be mechanically transmitted by arthropods
Asfaviridae
African swine fever
African swine fever is caused by the African swine fever virus (ASFV) (Asfaviridae:
Asfavirus), the only DNA arbovirus that is pathogenic for animals. There are four antigenic
types and 22 genotypes of ASFV. The disease occurs in Africa, America, Asia and Europe.
Infections occur in Armenia and Azerbaijan. The virus infects all members of the pig
family (Suidae). The disease is transmitted via direct route and also by the bite of soft ticks
of the genus Ornithodoros (Parasitiformes: Argasidae) (Gibbs, 2001; Labuda, Nuttall,
2008; Hubálek, Rudolf, 2012; Vlasova et al., 2012; Hubálek et al., 2014a; Beltrán-Alcrudo
et al., 2017; Dixon et al., 2019). The principal vectors are O. erraticus (Lucas), species
of the O. moubata (Murray) complex (such as O. moubata and O. porcinus Walton),
O. savignyi (Audouin) and O. sonrai Sautet et Witkowski in Africa, O. erraticus in Europe
and O. coriaceus Koch, O. puertoricensis Fox and O. turicata (Dugès) in the Americas.
Transovarial, transstadial and sexual (venereal) transmission of the virus occur throughout
the life of the ticks (Plowright et al., 1970; Hoogstraal, 1985; Hess et al., 1987; Gibbs, 2001;
Boinas et al., 2004, 2011; de la Fuente et al., 2008; Ravaomanana et al., 2010; Gallardo
et al., 2011; Hubálek et al., 2014a; Beltrán-Alcrudo et al., 2017). There is some evidence
that the stable fly Stomoxys calcitrans (Linnaeus) (Diptera: Muscidae) may be involved
in mechanical transmission while feeding, or infection is due to ingestion of an infected
fly by the host (Mellor et al., 1987; Baldacchino et al., 2013; Olsen et al., 2018a, b).
The virus has been found in wild boars in East and West Azerbaijan Provinces of Iran
(Rahimi et al., 2010; Beltrán-Alcrudo et al., 2017). At least 11 species of soft ticks,
including four species of Ornithodoros, one being O. erraticus, occur in Iran (Hosseni-
Chegeni et al., 2019; Hosseini-Chegeni, Tavakoli, 2020), however there is no information
about the vector(s) of the virus in the country.
Flaviviridae
Dengue fever
Dengue fever, caused by the dengue fever virus (DENV) (Flaviviridae: Flavivirus),
was reviewed by Azari-Hamidian et al. (2019). Some published documents which might
be added to the Iranian literature are Baniasadi et al. (2016), Salehi-Vaziri et al. (2016),
Heydari et al. (2018), Tavakoli et al. (2020) and Firooziyan et al. (2022). The virus has
also been found in Afghanistan (Arsenʼeva, 1982; Wallace et al., 2002; Elyan et al., 2014),
Djibouti (World Health Organization, 2004; Andayi et al., 2014; Braak et al., 2018), Kuwait
(Mustafa et al., 2001; Pacsa et al., 2003), Oman (Al-Abri et al., 2015), Pakistan (Hayes,
Burney, 1981; World Health Organization, 2004; Afzal et al., 2015; Khan et al., 2016;
Yaqub et al., 2017; Ahmad et al., 2020), Qatar (Humphrey et al., 2019), Saudi Arabia
366
(World Health Organization, 2004; Khan et al., 2008; Zaki et al., 2008; Memish et al., 2011;
Shibl et al., 2012; Ahmed, 2015), Somalia (Oldfield et al., 1993; World Health Organization,
2004; Braak et al., 2018), Sudan (Watts et al., 1994; World Health Organization, 2004;
Farnon et al., 2010; Braak et al., 2018; Ahmed et al., 2020), Turkey (Ergunay et al., 2011)
and Yemen (World Health Organization, 2004; Shibl et al., 2012; Ciccozzi et al., 2014;
Rezza et al., 2014; Alghazali et al., 2019; Al-Samadi, Ali, 2020; Abdul-Ghani et al., 2021).
There are no recent reports of Aedes aegypti (Linnaeus) [Stegomyia aegypti] (Diptera:
Culicidae), the main vector, in Iran (Azari-Hamidian et al., 2019). The other important
vector, Ae. albopictus Skuse [Stegomyia albopicta], was recorded just one time in Iran
based on five larvae and six adults found in Sistan and Baluchistan Province (Doosti
et al., 2016). The species has not been recorded since and there is no evidence for
indigenous transmission of DENV in the country.
Japanese encephalitis
Japanese encephalitis, caused by the Japanese encephalitis virus (JEV) (Flaviviridae:
Flavivirus), is known from Asia and Australia. The virus has been isolated from different
domesticated and wild mammals, such as bats, cattle, dogs, donkeys, monkeys, horses,
pigs, rodents and water buffaloes, and also birds, including chickens, ducks, egrets, herons
and water hens; however, important amplifying hosts in the epidemiology of the disease
seem to be pigs and aquatic birds. The virus has been identified in different mosquito
species of the genera Aedes, Anopheles, Armigeres, Culex and Mansonia, however the
most important vector is Culex tritaeniorhynchus Giles, the rice field mosquito. Vertical
(transovarial) transmission and sexual (venereal) transmission are also known for mosquito
vectors (Barrett, 2001; Hubálek et al., 2014a; Gould et al., 2017). The virus has also
been isolated from the biting midge Forcipomyia (Lasiohelea) taiwana Shiraki (Diptera:
Ceratopogonidae) (Linley et al., 1983) and the hard ticks Dermacentor marginatus (Sulzer)
(Parasitiformes: Ixodidae) and Ixodes ricinus (Linnaeus), as reported by Anastos (1957).
Also, Hoogstraal (1966) listed a number of hard tick species of the genera Dermacentor,
Ixodes, Haemaphysalis, Hyalomma and Rhipicephalus which might serve as JEV hosts
in nature. The virus is known in Pakistan and is suspected to be present in Afghanistan
(Arsen'eva, 1982; Darwish et al., 1983a; Igarashi et al., 1994; Wallace et al., 2002; World
Health Organization, 2004; Khan et al., 2016). Also, one febrile patient who entered
China from Bahrain was positive for JEV-specific IgM antibody (Shi et al., 2016).
Japanese encephalitis virus has been isolated from a number of mosquitoes, including
Aedes albopictus, Ae. curtipes (Edwards) [Cancraedes curtipes], Ae. vexans (Meigen)
[Aedimorphus vexans], Anopheles barbirostris van der Wulp, An . sinensis Wiedemann,
An. subpictus Grassi s. l., An. vagus Dönitz, Armigeres obturbans (Walker), Ar. subalbatus
(Coquillett), Culex annulus Theobald, Cx. annulirostris Skuse, Cx. bitaeniorhynchus Giles,
Cx. epidesmus Theobald, Cx. fuscocephala Theobald, Cx. gelidus Theobald, Cx. modestus
Ficalbi, Cx. pipiens Linnaeus, Cx. pseudovishnui Colless, Cx. quinquefasciatus Say,
367
Cx. sitiens Wiedemann, Cx. theileri Theobald, Cx. vishnui Theobald, Cx. whitmorei (Giles),
Mansonia annulifera (Theobald), Ma. bonneae Edwards, Ma. dives (Schiner), Ma. indiana
Edwards and Ma. uniformis (Theobald), according to Simpson et al. (1970, 1974), Peiris
et al. (1994), Reuben et al. (1994), Dhanda et al. (1997), Barrett (2001) and Wang et al.
(2007). According to unpublished data in Iran, the antibodies for the virus have been found
in humans (3.4%) using the neutralization test (the CDC Arthropod-Borne Virus Information
published documentation about the occurrence of the virus in the country. The main vector,
Culex tritaeniorhynchus, has been found in at least 17 Iranian provinces (Zaim, 1987;
Sofizadeh et al., 2018). The species is very abundant in three northern provinces, Golestan,
Guilan and Mazandaran, with vast rice fields (Azari-Hamidian et al., 2018; Nikookar
et al., 2018; Sofizadeh et al., 2018). Other mosquito species in Iran from which the virus
has been isolated elsewhere include Aedes albopictus, Culex bitaeniorhynchus, Cx. pipiens,
Cx. pseudovishnui, Cx. quinquefasciatus, Cx. sitiens, Cx. theileri and Mansonia uniformis
(Reuben et al., 1994; Barrett, 2001; Wang et al., 2007; Azari-Hamidian et al., 2019, 2020).
Also, the aforementioned hard ticks occur in Iran (Hosseni-Chegeni et al., 2019).
Tick-borne encephalitis
Tick-borne encephalitis, caused by tick-borne encephalitis virus (TBEV) (Flaviviridae:
Flavivirus), has been found in Asia and Europe. TBEV is the most important tick-borne
pathogenic flavivirus in humans. The virus consists of three subtypes, also called clusters,
including the western European subtype (formerly central European encephalitis virus -
CEEV), the Siberian subtype (formerly West Siberian encephalitis virus - WSEV) and
the far-eastern subtype (formerly Russian spring-summer encephalitis virus - RSSEV).
The main reservoirs of the virus are small mammals, such as rodents and insectivores,
and some wild carnivores, such as foxes, however the virus has also been isolated from
chamois (Rupicapra rupicapra), dogs, horses and sheep. The main route of transmission
is the bite of hard ticks; however, some local epidemics have been caused by consumption
of unpasteurized milk or milk products. Ixodes ricinus is the main vector in Europe
(the western European subtype) and I. persulcatus Schulze is the main vector in Asia
(the Siberian and the far-eastern subtypes). Transovarial and transstadial transmission have
been observed in both main vectors (Heinz, Holzmann, 2001; de la Fuente et al., 2008;
Labuda, Nuttall, 2008; Wójcik-Fatla et al., 2011; Hubálek, Rudolf, 2012; Valarcher et al.,
2015). According to Anastos (1957), TBEV [as (Russian) spring-summer encephalitis virus]
has been isolated, in addition to Ixodes ricinus and I. persulcatus, from the following ticks
(in the former USSR): Dermacentor marginatus, D. nuttalli Olenev, D. silvarum Olenev,
Haemaphysalis concinna Koch, H. japonica Warburton, Hyalomma dromedarii Koch,
H. excavatum Koch and Ixodes trianguliceps Birula. Additionally, the virus has been isolated
from Dermacentor reticulatus (Fabricius) in Germany (Chitimia-Dobler et al., 2019),
368
Poland (Wójcik-Fatla et al., 2011) and Russia (Kislenko et al., 1987), Ixodes hexagonus
Leach in the Czech Republic (Krivanec et al., 1988) and Croatia (Jemeršić et al., 2014),
Haemaphysalis punctata Canstrini et Fanzago in the Czech Republic (Hubálek et al., 1989),
Ixodes ovatus Neumann in Japan (Takeda et al., 1998), Haemaphysalis flava Neumann,
H. japonica, H. longicornis Neumann and I. nipponensis Kitaoka et Saito in South Korea
(Kim et al., 2009; Yun et al., 2012), Hyalomma marginatum Koch in Crimea (Hubálek,
Rudolf, 2012), Ixodes gibbosus Nuttall in the Mediterranean (Hubálek, Rudolf, 2012),
Dermacentor silvarum, Ixodes pavlovskyi Pomerantzev and I. lividus Koch (as I. plumbeus
Leach) in Russia (Mikryukova et al., 2014; Pukhovskaya et al., 2018). Also, TBEV has
been shown experimentally to be vectored by Dermacentor marginatus, Haemaphysalis
inermis Birula and Ixodes arboricola Schulze et Schlottke (Lichard, Kozuch, 1967; Nosek
et al., 1972; Nosek, Kožuch, 1985). The virus has also been isolated from fleas, including
Ceratophyllus indages (Rothschild) (synonym: Ceratophyllus tamias Wagner) (Siphonaptera:
Ceratophyllidae), Palaeopsylla soricis (Dale) (Siphonaptera: Hystrichopsyllidae), gamasid
mites (Federov et al., 1959; Sotnikova, Soldatov, 1964; Naumov, Gutova, 1984),
the horsefly Hybomitra lundbecki Lynborg (Diptera: Tabanidae) (Krinsky, 1976), the poultry
red mite Dermanyssus gallinae (De Geer) (Mesostigmata: Dermanyssidae) (Sparagano et al.,
2014) and the mosquito Aedes vexans (Pukhovskaya et al., 2018). The virus has been found
in Afghanistan, Armenia, Azerbaijan, Djibouti, Turkey and Turkmenistan (Gromashevsky,
Nikimorov, 1973; Heinz, Holzmann, 2001; de la Fuente et al., 2008; Ergunay et al., 2011;
Inci et al., 2013, 2016; Elyan et al., 2014; Failloux et al., 2017; Atkinson, Hewson, 2018;
Im et al., 2020). The disease was recently recorded in Mazandaran Province of northern
Iran using ELISA. Among 448 serum samples, 3.6% were positive (Salehi-Vaziri et al.,
2020). There is no information about the vector(s) in Iran, however Ixodes ricinus,
the main vector, is a prevalent hard tick in northern areas of the country, especially
the Caspian Sea littoral. Dermacentor marginatus, Haemaphysalis concinna, H. inermis,
H. punctata, Hyalomma dromedarii, H. excavatum and H. marginatum also occur in Iran
(Rahbari et al., 2007; Hosseni-Chegeni et al., 2019).
West Nile fever
West Nile fever, caused by West Nile fever virus (WNV) (Flaviviridae: Flavivirus)
(synonyms or subtypes: Kunjin and Rabensburg viruses), was reviewed for Iran by Azari-
Hamidian et al. (2019) and for the WHO Eastern Mediterranean Region by Eybpoosh
et al. (2019). Information in the following publications might be added to those reviews:
Shamsizadeh et al. (2015), Shahhosseini, Chinikar (2016), Ziyaeyan et al. (2018), Adham
et al. (2019), Amini et al. (2019), Amini et al. (2020), Dehghani et al. (2020), Shahhosseini
et al. (2020), Bakhshi et al. (2021) and Staji et al. (2021). The virus has also been found in
Afghanistan (Arsenʼeva, 1982; Wallace et al., 2002; Elyan et al., 2014), Armenia (Failloux
et al., 2017), Azerbaijan (Gromashevsky, Nikimorov, 1973; Mirzoeva et al., 1974), Djibouti
369
(Andayi et al., 2014), Iraq (Barakat et al., 2016), Pakistan (Hayes, Burney, 1981; Hayes
et al., 1982; Reisen et al., 1982; Darwish et al., 1983a; Sugamata, 1988; Sugamata et al.,
1988; Igarashi et al., 1994; Bryan et al., 1996; Zohaib et al., 2015; Khan et al., 2016;
Niazi et al., 2017; Yaqub et al., 2017), Qatar (DeCarlo et al., 2017; Dargham et al.,
2021), Saudi Arabia (Al-Ghamdi, 2014; Hemida et al., 2019; Alqahtani, 2020), Somalia
(Henderson et al., 1968; Cahill, 1971; Oldfield et al., 1993), Sudan (Salim, Porterfield,
1973; Watts et al., 1994; McCarthy et al., 1996; Depoortere et al., 2004; Farnon et al.,
2010; Yousof et al., 2018; Ahmed et al., 2020), Syria (Azmi et al., 2017), Turkey (Inci et
al., 2013; Failloux et al., 2017; Düzlü et al., 2020; Yildirim et al., 2021), Turkmenistan
(Atkinson, Hewson, 2018), the United Arab Emirates (Wernery et al., 2007; Alfaresi,
Elkoush, 2008) and Yemen (Qassem, Jaawal, 2014). Three mosquito species are known
vectors of WNV in Iran: Aedes caspius (Pallas) s. l. [Ochlerotatus caspius s. l.] (Bagheri
et al., 2015), Culex pipiens (Shahhosseini et al., 2017) and Cx. theileri (Shahhosseini
et al., 2020). Other species which are known principal vectors in other countries that also
occur in Iran include Aedes albopictus, Coquillettidia richiardii (Ficalbi), Cx. modestus,
Cx. perexiguus Theobald, Cx. pipiens, Cx. quinquesfasciatus, Cx. tritaeniorhynchus and
Mansonia uniformis (see Hubálek et al., 2014a; Azari-Hamidian et al., 2019, 2020).
Hantaviridae
Hantaan infection
Hantaviruses (Hantaviridae: Orthohantavirus), which cause haemorrhagic fever with
renal syndrome (HFRS) in the Old World and hantavirus pulmonary syndrome (HPS) or
hantavirus cardiopulmonary syndrome (HCPS) in the New World, are distributed worldwide.
The main reservoirs of rural epidemiological pattern are the rodents of the genera Apodemus
and Clethrionomys in Asia and Europe and Microtus and Peromyscus in the Americas,
whereas in urban pattern domestic rodents (Rattus species) are reservoirs and in animal
houses (vivaria) colonized experimental rats are reservoirs. The viruses that cause HFRS in
the Old World include Dobrava (DOBV), Hantaan (HTNV), Puumala (PUUV), Saaremaa
(SAAV) and Seoul (SEOV). Dobrava virus is found primarily in the Balkans. Haantan virus
is widely distributed in eastern Asia, particularly in China, Russia and Korea. Puumala virus
is found in Scandinavia, western Europe and western Russia. Saaremaa is found in central
Europe and Scandinavia. Seoul virus is found worldwide. The virus is usually transmitted
by contamination of wounds by the saliva, urine or faeces of rodents or by rodent bite
(Xu, 2001; Bi et al., 2008; Zowghi et al., 2008; Kassiri, Dehghani, 2020). The virus has
been found in Kuwait (Pacsa et al., 2002, 2003), Sudan (Ibrahim et al., 2017) and Turkey
(Oncul et al., 2011; Gozalan et al., 2013). There is some evidence for transmission of the
virus by the tropical rat mite Ornithonyssus bacoti (Hirst) (Mesostigmata: Macronyssidae),
trombiculid mites (Prostigmata: Trombiculidae), such as Eutrombicula splendens (Ewing),
Leptotrombidium scutellare (Nagayo, Miyagawa, Mitamura, Tamiya et Tenjin), L. subpalpale
Vercammen-Grandjean et Langston, an unidentified ixodid tick (Houck et al., 2001; Xu,
370
2001) and the gamasid mites Haemolaelaps glasgowi (Ewing) and Eulaelaps stabularis
(Koch) (Mesostigmata: Haemogamasidae) (Li, 1986). The first verified record of Hantaan
virus in Iran was among street cleaners (4.5%) in Isfahan Province using ELISA and
molecular tests as an EID (Chinikar et al., 2014). Later, positive sera were detected in
10 provinces of the country, East Azerbaijan, Fars, Ilam, Isfahan, Kerman, Mazandaran,
Razavi Khorasan, South Khorasan, Tehran and Yazd, based on the results of ELISA (Salehi-
Vaziri et al., 2019, 2021). Parhizgari et al. (2017) recognized the disease in Iran as an
EID, and it was reviewed by Kassiri and Dehghani (2020). However, the aforementioned
references mentioned that IgG to hantaviruses has been identified in Iran, which are genus-
specific due to the close antigenic relationship of the Old World hantaviruses causing HFRS.
In fact, HFRS is caused by various hantaviruses, not necessarily by Hantaan. The Hantaan
virus itself circulates in the Far East and is unlikely to be detected in Iran. Ornithonyssus
bacoti has been found on various rodents in different areas of Iran (Kamali et al., 2001)
and at least 85 species of the mite family Trombiculidae are known to be present in the
country (Stekolnikov et al., 2019), however there is no information about the possible role
of mites in the transmission of Hantaan virus in Iran.
Herpesviridae
Bovine herpes
Bovine herpes, caused by the bovine herpes virus (BHV) (Herpesviridae: Varicellovirus),
has a worldwide distribution. The virus has been found in different wild and domesticated
ruminants, especially camels, cattle, goats and sheep. The disease generally displays two
clinical syndromes, respiratory and genital. The disease causes significant financial losses
because of a drop in milk production, abortion and deaths in cattle. The virus is mostly
transmitted through respiratory infection and less importantly via genital tract infection
(Wentink et al., 1993; Chatterjee et al., 2016). However, there is some evidence that the
stable fly Stomoxys calcitrans, the face fly Musca autumnalis De Geer (Diptera: Muscidae)
and the soft tick Ornithodoros coriaceus may be involved in mechanical transmission
(Gibbs et al., 1972, 1973a, b; Taylor et al., 1982; Johnson et al., 1991; Baldacchino et al.,
2013). Bovine herpes virus has been found in Oman (Hedger et al., 1980). The virus has
been detected in bufalloes, camels, cattle, dogs, horses, humans, Indian gazelles and pigs,
using serological and molecular tests, in the following provinces of Iran: Chaharmahal
and Bakhtiari, Fars, Guilan, Hamedan, Isfahan, Kerman, Khorasan, Khuzistan, Kurdistan,
Qazvin, Semnan, Tehran and Zanjan (Afshar, Tadjbakhsh, 1970; Hazrati et al., 1981; Kargar
Moakhar et al., 2001, 2003; Sakhaee et al., 2009; Raoofi et al., 2012a; Sadri, 2012b;
Shirvani et al., 2012; Bahari et al., 2013; Ezzi et al., 2013; Safarpoor Dehkordi et al.,
2013; Nikbakht et al., 2015; Sharifzadeh et al., 2015; Hemmatzadeh et al., 2016; Seyfi
Abad Shapouri et al., 2016; Adeli et al., 2017; Kaveh et al., 2017; Erfani et al., 2019;
Noaman, Nabinejad, 2020; Hashemi et al., 2022). There is no information about possible
transmission of the virus by arthropods in the country.
371
Nairoviridae
Abu Hammad virus
Abu Hammad virus (AHV) (Nairoviridae: Orthonairovirus) was first found in the soft
tick Argas hermanni Audouin (Parasitiformes: Argasidae) in Egypt (Converse et al., 1974;
Darwish et al., 1976; Casals, Tignor, 1980; Hoogstraal, 1985; Labuda, Nuttall, 2008; Kuhn
et al., 2016). It has also been isolated from A. hermanni in Dormian Village of Isfahan
Province in central Iran (Tesh, 1976, personal communication, cited by the CDC Arthropod-
Argas hermanni is not mentioned in the most recent checklist of the soft ticks (10
species) in Iran (Hosseni-Chegeni et al., 2019). Although Hosseni-Chegeni and Tavakoli
(2020) recently recorded the species in Lorestan Province in western Iran, there is no new
information about AHV in Iran.
Crimean-Congo hemorrhagic fever
Crimean-Congo hemorrhagic fever (CCHF), caused by CCHF virus (CCHFV)
(Nairoviridae: Orthonairovirus), occurs in Africa, Asia and Europe and is the most widely
distributed medically important arboviral disease after dengue fever. The disease is the
most significant tick-borne viral disease in humans. The virus has been found in different
wild and domestic animals (birds and mammals). It has been isolated from more than
30 species of hard and soft ticks, however the main vector is Hyalomma marginatum.
CCHFV is biologically transmitted to humans by the bite of an infected tick (horizontal
transmission) or by direct contact with infected blood, body fluid, tissues and, possibly,
crushed ticks (direct transmission). The virus can be transmitted via different routes in
certain ticks, including transovarial, trans-stadial and sexual (venereal) transmission
(Hoogstraal, 1979, 1981, 1985; Nuttall, 2001; Labuda, Nuttall, 2008; Chinikar et al., 2010b;
Hubálek, Rudolf, 2012; Bente et al., 2013; Spengler et al., 2016; Al-Abri et al., 2017;
Contigiani et al., 2017; Blair et al., 2019; Saleem et al., 2020). Also, as reported by
Hoogstraal (1979) and Nuttall (2001), the virus has been isolated from species of Culicoides
(Diptera: Ceratopogonidae). CCHFV occurs in Afghanistan (Hoogstraal, 1979; World Health
Organization, 2004), Armenia (Karapetyan et al., 1974; Hoogstraal, 1979; Lvov, 1994;
Failloux et al., 2017; Gevorgyan et al., 2019), Azerbaijan (Gromashevsky, Nikimorov, 1973;
Semashko et al., 1974; Hoogstraal, 1979; Lvov, 1994), Iraq (Al-Tikriti et al., 1981; World
Health Organization, 2004; Abul-Eis et al., 2012), Kuwait (Al-Nakib et al., 1984), Oman
(Scrimgeour et al., 1996, 1999; Williams et al., 2000; Al-Zadjali et al., 2013; Body et al.,
2016; Al-Abri et al., 2019), Pakistan (Begum et al., 1970a, d; Hoogstraal, 1979; Hayes,
Burney, 1981; Darwish et al., 1983b; World Health Organization, 2004; Kasi et al., 2020),
Saudi Arabia (El-Azazy, Scrimgeour, 1997; Hassanein et al., 1997; Memish et al., 2011),
Somalia (Spengler et al., 2016), Sudan (Watts et al., 1994; Aradaib et al., 2011; Elata
et al., 2011; Osman et al., 2013; Ibrahim et al., 2015; Spengler et al., 2016; Suliman et al.,
2017; Ahmed et al., 2020), Syria (Blair et al., 2019), Turkey (Inci et al., 2013, 2016; Düzlü
372
et al., 2020), Tukmenistan (Aristova et al., 1973; Hoogstraal, 1979; Lvov, 1994; Atkinson,
Hewson, 2018), the United Arab Emirates (Suleiman et al., 1980; Baskerville et al., 1981;
Khan et al., 1997; Rodriguez et al., 1997; Schwarz et al., 1997; Al-Dabal et al., 2016;
Aijazi et al., 2020; Camp et al., 2020; Khalafalla et al., 2021) and Yemen (Cecaro et al.,
2013). Historically, Jorjani (Gorgani) (1042-1136 AD), in his monumental book “Treasure
of the Khwarazm Shah” (Zakhireye Kharazmshahi), a Persian medical encyclopedia,
described a hemorrhagic and arthropod caused disease about one thousand years ago that
seemed to be CCHF (Hoogstraal, 1979; Jorjani, 2001). The first scientific reports of clinical
signs of CCHF in humans in Iran date back to the 1960s (Aminol-Achrafi, Noraniyan,
1966a, b). The first records of antibodies to the virus in different domesticated and wild
animals were identified in the early 1970s using the agar gel diffusion precipitation (AGDP)
test (Chumakov et al., 1970; Chumakov, Smirnova, 1972; Saidi et al., 1975). Also, the first
records of CCHFV antibodies in humans were identified in 4% of individuals tested in the
Caspian Sea littoral provinces of Golestan, Guilan and Mazandaran using the
hemagglutination inhibition (HI) test (Saidi, 1974) and 13% of people tested in six
provinces of the country using the AGDP test (Saidi et al., 1975). Phylogenetic analysis
showed that CCHFV in Iran includes at least five genomic variants (Senegalese, Pakistani,
Iraqi, Afghani and Russian) and seven genotypes of six clades or lineages: clades I (Africa
3), III (Africa 1), IV (Asia 1 and 2), V (Europe 1), VI (Europe 2) and a new clade VII
(Iran) (Chinikar et al., 2004, 2013b, 2016a, b; Morovvati et al., 2012; Kayedi et al., 2015;
Al-Abri et al., 2017; Nasirian, 2020). Saidi et al. (1975), using the AGDP test, found
positive antibodies in different domesticated and wild mammals in six provinces, East
Azerbaijan, Golestan, Guilan, Razavi Khorasan, Isfahan and Tehran as follow: 38% of
sheep, 36% of gaots, 18% of cattle and 3% of small mammals such as Myotis blyti,
Nyctalus noctula, Allactaga williamsoni, Mus musculus and Meriones crassus. CCHFV
was also serologically detected in a number of mammals and birds using ELISA, including
goats (46%) and sheep (77.5%) in North Khorasan, Razavi Khorasan and South Khorasan
Provinces (Bokaie et al., 2008; Chinikar et al., 2012b); in cattle (30%), goats (33.3%) and
sheep (41.9%) in Ardebil Province (Telmadarraiy et al., 2010); in cattle (5.9%) in five
Iranian provinces (Chaharmal and Bakhtiari, Razavi Khorasan, Semnan, Sistan and
Baluchistan and South Khorasan) (Lotfollahzadeh et al., 2011); in sheep (15.5%) in East
Azerbaijan Province (Rezazadeh et al., 2012, 2013); in cattle (25%), goats (24.8%) and
sheep (58.7%) in differenet areas of Iran (Mostafavi et al., 2013a); in ostriches (20%) and
sheep (54.2%) in Isfahan Province (Izadi et al., 2007; Mostafavi et al., 2013b); in sheep
(0.8%) in Kohgiluyeh and Boyerahmad Province (Ghasemian et al., 2021); in sheep (3.7%)
(Mostafavi et al., 2012) and (38.7%) (Faghihi et al., 2015) in Mazandaran Province; in
cattle (9.6%) in Razavi Khorasan and South Khorasan Provinces (Lotfollahzade et al.,
2009); and in camels (5.29%) in North Khorasan, Razavi Khorasan and South Khorasan
Provinces (Champour et al., 2014). In general, infections of CCHFV in humans have been
reported in at least 25 Iranian provinces (out of 31), with the highest rates in Sistan and
373
Baluchistan, Isfahan and Fars Provinces (Chinikar, 2003; Chinikar et al., 2002, 2005, 2008,
2009, 2010a, c; Mostafavi et al., 2013a). Specific documents have been published about
human cases of the disease in different provinces, including Ardebil (Asefi, 1974, 1977;
Adham et al., 2021; Habibzadeh et al., 2021; Abazari et al., 2022), Chaharmahal and
Bakhtiari (Mahzounieh et al., 2012), East Azerbaijan (Aminol-Achrafi, Noraniyan, 1966a,
b; Asefi, 1974; Saidi et al., 1975; Ardoin, Karimi, 1982; Ardalan et al., 2006), Fars (Raoofi
et al., 2012b; Rezaei et al., 2012), Golestan (Saidi, 1974; Saidi et al., 1975; Abbasi, Moradi,
2005); Guilan (Saidi, 1974; Saidi et al., 1975; Asefi, 1977), Hormozgan (Fazlalipour et al.,
2019), Isfahan (Saidi et al., 1975; Chinikar et al., 2012a), Khuzistan (Sharififard et al.,
2016), Kohgiluyeh and Boyerahmad (Hadinia et al., 2012), Kurdistan (Firouzmanesh et al.,
2017; Shahbazi et al., 2019), Mazandaran (Saidi, 1974; Sadeghi et al., 2013), North
Khorasan, Razavi Khorasan and South Khorasan (Saidi et al., 1975; Bokaie et al., 2008;
Ebadiazar et al., 2011; Ziyaei et al., 2011; Chinikar et al., 2013a; Heydari, Movahed
Danesh, 2013; Naderi et al., 2013; Shahhosseini et al., 2018); also co-infections of
brucellosis and CCHF (Hashemian, Ebrahimi, 2010), Qazvin (Nikoonejad, Bijani, 2016),
Qom (Saghafipour et al., 2012a, b; Farzinnia et al., 2013), Semnan (Arab-Ameree,
Mirshafee, 2006), Sistan and Baluchistan (Izadi et al., 2003, 2004, 2006; Alavi-Naini
et al., 2006; Sharifi Mod, Metanat, 2006; Owaysee Oskooei et al., 2008; Sharifi-Mood
et al., 2014; Mostafavi et al., 2017; Nili et al., 2020); also co-infections of malaria and
CCHF (Sharifi-Mood et al., 2011) and Tehran (Saidi et al., 1975). Some 53 to 154 human
cases of CCHF were found in at least 24 provinces of Iran during 2006-2011, with highest
number of cases in Sistan and Baluchistan, Isfahan, Razavi Khorasan, Khuzistan and Fars
Provinces (Ramezankhani, Kaveh, 2014). Blair et al. (2019) reported that the total number
of confirmed human cases of the virus in the country was 1256, with total deaths being
177 during 1999-2017 and cases per year ranging from 18 and 150. At least 47 species
of ticks (11 species of soft ticks and 36 species of hard ticks) occur in Iran (Hosseni-
Chegeni et al., 2019; Hosseini-Chegeni, Tavakoli, 2020). CCHFV has been isolated from
different tick species using RT-PCR in various provinces, including Ardebil (28% of tested
ticks were positive) (Telmadarraiy et al., 2010); East Azerbaijan (5.0%) (Shafei et al.,
2016); Fars (4.5%) (Farhadpour et al., 2015); Golestan (5.3%) (Sedaghat et al., 2017);
Hamedan (16.4%) (Telmadarraiy et al., 2008) (19.3%) (Tahmasebi et al., 2010); Ilam (6.6%)
(Sharifinia et al., 2015); Kermanshah (3.8%) (Mohammadian et al., 2016); Kurdistan (5.6%)
(Fakoorziba et al., 2012); Lorestan (6.7%) (Kayedi et al., 2015); Mazandaran (9.52%)
(Faghihi et al., 2015); Qom (7.9%) (Telmadarraiy et al., 2012); Razavi Khorasan (3.8%)
(Fakoorziba et al., 2015), (5%) (Maghsood et al., 2020); Semnan (4.3%) (Faghihi et al.,
2018); Sistan and Baluchistan (4.3%) (Mehravaran et al., 2013); South Khorasan (15.9%)
(Jafari et al., 2020); West Azerbaijan (8.33%) (Morovvati et al., 2012) and Yazd (5.71%)
(Salim Abadi et al., 2011). The virus was isolated from the following species of ticks:
Alveonasus lahorensis (Neumann), Dermacentor marginatus, Haemaphysalis inermis,
H. punctata, Hyalomma anatolicum Koch, H. asiaticum Schulze et Schlottke, H. scupense
374
Schulze (synonym: H. detritum Schulze), H. dromedarii, H. marginatum, H. schulzei
Olenev, Rhipicephalus bursa Canstrini et Fanzago, Rh. sanguineus (Latreille) and
Rh. turanicus Pomerantsev, Matikashvili et Lotosky (Sureau, Klein, 1980; Sureau et al.,
1980; Fakoorziba et al., 2015; Telmadarraiy et al., 2015). Fakoorziba et al. (2015) reported
finding CCHFV in Rhipicephalus appendiculatus Neumann collected in Razavi Khorasan
Province, however the record of this Afrotropical species in Iran is doubtful and the species
is not mentioned in the checklist of Iranian ticks (Hosseni-Chegeni et al., 2019). Among
the ticks from which the virus has been isolated in other countries, the following species
occur in Iran: Argas persicus (Oken), Dermacentor niveus Neumann, Ixodes ricinus,
Rhipicephalus annulatus (Say) and Rh. rossicus Yakimov et Kol-Yakimova (Hoogstraal,
1979; Hoogstraal, Valdez, 1980; Hosseni-Chegeni et al., 2019). A number of reviews of
CCHF in Iran (Emadi-Kouchak et al., 2003; Chinikar et al., 2010b; Keshtkar-Jahromi et
al., 2013; Keshtkar Jahromi, 2014; Mostafavi et al., 2014; Kouhpayeh, 2019; Mardani,
2019; Kassiri et al., 2020c), including two mata-analyses (Nasirian, 2019, 2020), have been
published.
Orthomyxoviridae
Quaranfil virus
Quaranfil virus (QRFV) (Orthomyxoviridae: Thogotovirus) was first isolated from
humans, the soft ticks Argas arboreus Kaiser, Hoogstraal et Kohls and A. hermanni and
pigeon squabs in Egypt (Taylor et al., 1966b; Mourya et al., 2019). The virus has been
found in several African and Asian countries. It has been isolated from the soft ticks
Argas arboreus, A. reflexus (Fabricius), A. hermanni, A. vulgaris Filippova and the hard
tick Hyalomma dromedarii (Hoogstraal, 1966, 1981, 1985; Taylor et al., 1966b; Williams
et al., 1970; Converse, Moussa, 1982; Labuda, Nuttall, 2008; Presti et al., 2009). The virus
has been found in Afghanistan, Iraq, Kuwait and Yemen (Williams et al., 1970; Converse,
Moussa, 1982). One isolation of Quaranfil virus was obtained from Argas vulgaris collected
near pigeon and sparrow nests in Razavi Khorasan Province of Iran (Klein et al., 1979;
Sureau, Klein, 1980). Eleven species of soft ticks, including A. hermanni and 36 species
of hard ticks, as well as Hyalomma dromedarii, are listed in the most recent checklist
of Iranian ticks (Hosseni-Chegeni et al., 2019; Hosseini-Chegeni, Tavakoli, 2020), however
there is no recent verification of Argas vulgaris in the country and it is not listed in
the checklist. Nothing more is known about the virus in Iran.
Thogoto virus
Thogoto virus (THOV) (Orthomyxoviridae: Thogotovirus) is known to occur in Africa,
Europe (Italy and Portugal) and Asia (Iran and Japan). The virus infects livestock (camels,
cattle, goats and sheep), migratory birds and occasionally humans. The virus is transmitted
by a number of species of hard ticks of the genera Amblyomma, Haemaphysalis, Hyalomma
and Rhipicephalus and can cause abortion in sheep (Haig et al., 1965; Albanese et al.,
1972; Williams et al., 1973; Filipe, Calisher, 1984; Woodall, 2001c; Labuda, Nuttall, 2008;
375
Hubálek, Rudolf, 2012; Hubálek et al., 2014a; Yoshii et al., 2015). The virus has been
isolated from Amblyomma variegatum (Fabricius), Haemaphysalis longicornis, Hyalomma
anatolicum, H. truncatum Koch, Rhipicephalus annulatus, Rh. appendiculatus, Rh. bursa,
Rh. decoloratus Koch, Rh. evertsi Neumann and Rh. sanguineus (see Haig et al., 1965;
Albanese et al., 1972; Williams et al., 1973; Johnson et al., 1980; Filipe, Calisher, 1984;
Jones et al., 1989; Woodall, 2001c; Hubálek et al., 2014a; Yoshii et al., 2015). There is just
one record of Thogoto virus in Iran, which was found in Hyalomma anatolicum collected
from cattle in Razavi Khorasan Province (Sureau, Klein, 1980; Sureau et al., 1980). Among
other ticks from which the virus has been isolated, Rhipicephalus annulatus, Rh. bursa and
Rh. sanguineus occur in Iran (Hosseni-Chegeni et al., 2019).
Paramyxoviridae
Rinderpest (cattle plague)
Rinderpest (cattle plague), caused by the rinderpest virus (RPV) (Paramyxoviridae:
Morbillivirus), has sometimes been found in Africa, Asia, Australia, Europe and South
America. The virus affected various mammals, including humans, but especially ruminants,
primilarily buffaloes and cattle. The disease was economically very important. In the 1990s,
Afghanistan, Iran, Iraq, Pakistan, Saudi Arabia, Somalia, Sudan, Turkey, Yemen and some
other African and Asian countries were identified as the last active foci of rinderpest.
Finally, after about 65 years and a global eradication program involving vaccinations and
zoosanitory procedures, the disease was officially declared eradicated in 2011. Rinderpest is
only the second disease to be eradicated and the greatest veterinary achievement of our time
(Njeumi et al., 2012; Roeder et al., 2013). This disease is mentioned here as a historical
example of successful international collaboration and acheivment, and the importance of
a One Health approach. Rinderpest was not considered to be an arbovirus and was mainly
transmitted via direct route, however there was some evidence, natural and experimental,
for its mechanical transmission by horseflies, for example Tabanus orientis Walker (Krinsky,
1976; Foil, 1989). There is no historical information with regard to what the vector of
virus may have been in Iran.
Peribunyaviridae
Akabane virus
Akabane virus (AKAV) (Peribunyaviridae: Orthobunyavirus) has been found in Africa,
Asia and Australia. The virus infects various wild and domesticated mammals, including
buffaloes, camels, cattle, elephants, giraffes, goats, horses, pigs and sheep. Infections in
pregnant cattle, goats or sheep causes a variety of abnormalities in the fetus, principally
arthrogryposis and hydranencephaly. Epizootics may cause a significant economical loss.
Infections in adult animals are entirely subclinical. Certain species of Culicoides are the
biological vectors of AKAV. The virus has also been isolated from a number of mosquitoes,
for example Aedes vexans, Anopheles funestus Giles, An. vagus, Culex tritaeniorhynchus
376
and Cx. vishnui; however, they do not biologically transmit the virus and are of lesser
importance as vectors (Oya et al., 1961; Wirth, Hubert, 1989; Mellor et al., 2000; Mellor,
2001c; Bryant et al., 2005; Hubálek et al., 2014a; Kirkland, 2015; Contigiani et al., 2017).
The virus has been found in Iraq (Alsaad et al., 2017; Al-Salihi, Al-Dabhawi, 2019), Oman
(Al-Busaidy, Mellor, 1991b), Saudi Arabia (Abu Elzein et al., 1998b), Sudan (Mohamed
et al., 1996), Syria (Taylor, Mellor, 1994) and Turkey (Taylor, Mellor, 1994; Dagalp
et al., 2021). The main vectors are Culicoides brevitarsis Kieffer and C. wadai Kitaoka
in Australia, C. oxystoma Kieffer in Japan, C. imicola Kieffer and C. milnei Austen in
Africa and C. imicola in Oman (St George et al., 1978; Kurogi et al., 1987; Al-Busaidy,
Mellor, 1991b; Mellor, 2001c; Hubálek et al., 2014a). Also, C. nubeculosus (Meigen) and
C. variipennis (Coquillett) have been shown to be capable of experimentally transmitting
the virus (Jennings, Mellor, 1989). Serological tests, such as hemagglutination inhibition
(HI) and enzyme-linked immunosorbent assay (ELISA), have been used to identify the
virus in Iran: in Charmahal and Bakhtiari Province (15% in goats and 5.88% in sheep)
(Kojouri et al., 2015), Golestan Province (10% in sheep, 80% in cattle) (Ahourai et al.,
1992), Khuzistan Province (39.72% in sheep, 85.87% in cattle) (Ahi et al., 2015; Karami
Boldaji et al., 2016), Semnan Province (23.3% in cattle) (Mohajer et al., 2019) and Tehran
Province (56.52% in cattle) (Dehghan Rahimabadi et al., 2020). There are at least four
genera of biting midges (Ceratopogonidae), Atrichopogon (three species), Culicoides
(43 species), Dasyhelea (four species) and Forcipomyia (one species), with at least
51 species in Iran (Navai, 1974; Dominiak, Alwin, 2013; Pilvari et al., 2016), however
there is no information about the vectors of the virus in the country. Among known possible
vectors, Culicoides nubeculosus occurs in Iran (Jennings, Mellor, 1989; Abdigoudarzi,
2016). Two mosquito species, Aedes vexans and Culex tritaeniorhynchus, from which
the virus was first isolated in Japan, also occur in Iran (Oya et al., 1961; Azari-Hamidian
et al., 2019).
Schmallenberg virus
Schmallenberg virus (SBV) (Peribunyaviridae: Orthobunyavirus) occurs in Africa, Asia
and Europe. SBV, as a newly emerging virus, was first detected in Germany (Schmallenberg
City) and the Netherlands in 2011 (Gibbens, 2012; Hoffmann et al., 2012). The virus
RNA or antibodies have been identified in a wide range of wild and domestic ruminants,
including cattle, goats, sheep, buffaloes, camels, chamois, deer, llamas, moufflons and
reindeer, and also non-ruminant species such as dogs, elephants, horses, pigs, wild boars
and zebras. SBV infection is economically very important. Infections in adult cattle,
goats and sheep are mild or subclinical or with clinical signs such as fever, drop in milk
production and diarrhea; however, infections in pregnant cattle, goats or sheep may cause
abortions or serious congenital malformation in offspring, such as arthrogryposis and
hydranencephaly. Infection is not considered a zoonosis. The virus is biologically vectored
by certain species of Culicoides biting midges (Gibbens, 2012; Hoffmann et al., 2012;
377
Nekoei et al., 2015b; Collins et al., 2019; Asadolahizoj et al., 2021). Infections have been
found in Azerbaijan (Zeynalova et al., 2019), Iraq (Al-Barawary, 2018; Al-Baroodi, 2021),
Pakistan (Wernery et al., 2013), Saudi Arabia (Taha et al., 2015), Sudan (Wernery et al.,
2013) and Turkey (Azkur et al., 2013; Yilmaz et al., 2014; Tonbak et al., 2016). SBV
has been isolated from C. chiopterus (Meigen), C. dewulfi Goetghebuer and C. obsoletus
(Meigen) in Belgium (De Regge et al., 2012), from C. chiopterus, C. obsoletus and
C. scoticus Downes et Kettle in the Netherlands (Elbers et al., 2013), from C. obsoletus and
C. punctatus (Meigen) in Poland (Larska et al., 2013), from C. imicola (experimentally)
and C. obsoletus in Spain (Pages et al., 2018) and from C. chiopterus, C. deltus Edwards
(synonym: C. lupicaris Downes et Kettle), C. dewulfi, C. imicola, C. newsteadi Austen,
C. nubeculosus, C. obsoletus, C. pulicaris (Linnaeus) and C. scoticus in France (Segard
et al., 2018). Additionally, the Nearctic C. sonorensis Wirth et Jones has experimentally
been shown to be an efficient vector (Veronesi et al., 2013). Transovarial transmission
is also known for Culicoides vectors (Larska et al., 2013). Rasekh et al. (2018) detected
SBV-specific antibodies in 5% of samples from horses using ELISA in North Khorasan
and Razavi Khorasan Provinces. This was the first time that antibodies against SBV were
detected in horses. However, the results should be verified using virus neutralization tests,
PCR and SBV RNA isolation (Collins et al., 2019). Also, Rasekh et al. (2022) detected
SBV-specific antibodies in 12.45% of samples from cattle using ELISA in Razavi Khorasan,
South Khorasan and Sistan, Baluchistan Provinces. Among known vectors of the virus,
C. nubeculosus, C. pulicaris and C. punctatus occur in Iran (Navai, 1974; Larska
et al., 2013; Abdigoudarzi, 2016; Segard et al., 2018). Although at least 43 species
of Culicoides are found in Iran (Navai, 1974), there is no information about the vector(s)
of Schmallenberg virus in the country.
Tahyna virus
Tahyna virus (TAHV) (Valtice fever) (Peribunyaviridae: Orthobunyavirus) (synonyms:
Lumbo, Trojica) is known to occur in Africa, Asia and Europe (Labuda, 2001; Bennett
et al., 2011). The virus has been found in Armenia (Failloux et al., 2017), Azerbaijan
(Gromashevsky, Nikimorov, 1973; Lvov, 1994), Iraq (Barakat et al., 2016) and Turkey
(Hubálek, 2008). It has been isolated from different domestic and wild mammals, such as
rodents and insectivores, however it seems that the main reservoirs in Europe are hares and
rabbits. Infection causes a non-fatal flu-like illness in humans. Infections in endemic areas,
such as Central Asia, seem to be very frequent based on serological tests. The virus has
been isolated from different mosquito species of the genera Aedes, Anopheles, Culex and
Culiseta. Transovarial transmission has been documented (Labuda, 2001; Hubálek, 2008;
Atkinson, Hewson, 2018). It seems Aedes vexans is the most important vector. Other known
vectors are Ae. cantans (Meigen) [Ochlerotatus cantans], Ae. caspius s. l., Ae. cinereus
Meigen, Ae. communis (De Geer) [Ochlerotatus communis], Ae. excrucians (Walker)
[Ochlerotatus excrucians], Ae. detritus (Haliday) [Ochlerotatus detritus], Ae. flavescens
(Muller) [Ochlerotatus flavescens], Ae. punctor (Kirby) [Ochlerotatus punctor], Ae. sticticus
378
(Meigen) [Ochlerotatus sticticus], Anopheles hyrcanus (Pallas), Culex modestus, Cx. pallens
Coquillett, Cx. pipiens and Culiseta annulata (Schrank) (Labuda, 2001; Hubálek, 2008;
Li et al., 2010; Hubalek et al., 2014b; Sonnleitner et al., 2014). According to Hannoun
and Rau (1970), the virus has been experimentally transmitted in chickens by the soft
tick Argas reflexus. Based on unpublished data, antibodies for the virus have been found
in humans in Azerbaijan Province of Iran using the serological test (the CDC Arthropod-
there is no verified and published information about the occurrence of the virus in the
country. Among known vectors of the virus, Aedes caspius s. l., Ae. detritus, Ae. flavescens,
Ae. vexans, Anopheles hyrcanus, Culex modestus, Cx. pipiens and Culiseta annulata occur
in Iran (Labuda, 2001; Hubálek, 2008; Li et al., 2010; Azari-Hamidian et al., 2019).
Phenuiviridae
Bhanja virus
Bhanja virus (BHAV) (Phenuiviridae: Phlebovirus) (synonym or subtype: Palma virus)
occurs in Africa, Asia and Europe. Isolation of the virus from mammals is rare, however
serological surveys indicate the highest prevalence of antibodies in domestic mammals, such
as camels, cattle, dogs, goats, horses and sheep, and also antibodies have been detected in
different wild mammals, birds and reptiles (Shah, Work, 1969; Hubálek et al., 1982, 2014a;
Hubálek, 1987; Filipe et al., 1994; Labuda, Nuttall, 2008; Hubálek, Rudolf, 2012; Matsuno
et al., 2013). The virus has been identified in Armenia, Azerbaijan, Pakistan, Somalia and
Turkmenistan (Chunikhin, Karaseva, 1971; Semashko et al., 1973; Matevosyan et al., 1974;
Hubálek et al., 1982, 2014a; Hubálek, 1987; Darwish et al., 1983b; Lvov, 1994; Hubálek,
Rudolf, 2012; Failloux et al., 2017; Atkinson, Hewson, 2018). It has been isolated from
at least 15 species of hard ticks of the genera Amblyomma, Dermacentor, Haemaphysalis,
Hyalomma and Rhipicephalus (Shah, Work, 1969; Hoogstraal, Valdez, 1980; Johnson
et al., 1980; Hoogstraal, 1981; Hubálek et al., 1982, 2014a; Hubálek, 1987; Filipe et al.,
1994; Hubálek, Rudolf, 2012). It seems that the only record of BHAV in Iran is based on
a serological survey of 3000 humans and domestic and wild mammls. A “small proportion”
showed antibodies to BHAV (Saidi, 1975). Also, Arata (1975) mentioned the presence
of BHAV in Iran and listed it as a “representative rodent born [sic] disease”. There are
at least 36 species of hard ticks representing five genera (Dermacentor, Haemaphysalis,
Hyalomma, Ixodes and Rhipicephalus) in Iran (Hosseni-Chegeni et al., 2019), however
there is no more recent information about the virus and its possible vector(s) in the country.
The following 10 species, which are known vectors in other countries, occur in Iran:
Dermacentor marginatus, Haemaphysalis parva (Neumann) (synonym: H. intermedia
Nuttall et Warburton), H. punctata, H. sulcata Canstrini et Fanzago, Hyalomma asiaticum,
H. dromedarii, H. marginatum, H. scupense (synonym: H. detritum), Rhipicephalus
annulatus, Rh. bursa (Hoogstraal, Valdez, 1980; Hubálek et al., 1982, 2014a; Hubálek,
1987; Hubálek, Rudolf, 2012; Hosseni-Chegeni et al., 2019).
379
Rift Valley fever
Rift Valley fever, caused by the Rift Valley fever virus (RVFV) (Phenuiviridae:
Phlebovirus) (synonym: Zinga virus), was reviewed by Azari-Hamidian et al. (2019) and
Kassiri et al. (2020b). Information provided by Fakour et al. (2021) might be added to
those reviews. In addition to Iran, the virus has also been recorded in Djibouti (Andayi et
al., 2014), Iraq (Muhsen, 2012), Saudi Arabia (World Health Organization, 2004; Memish
et al., 2011; Ahmed, 2015; Taha et al., 2015; Kenawy et al., 2018), Somalia (Oldfield
et al., 1993; World Health Organization, 2004; Braak et al., 2018), Sudan (Watts et al.,
1994; McCarthy et al., 1996; Braak et al., 2018; Ahmed et al., 2020), Turkey (Tezcan-Ulger
et al., 2019) and Yemen (World Health Organization, 2004; Kenawy et al., 2018). Among
known principal mosquito vectors, the following species (see Hubálek et al., 2014a and
Azari-Hamidian et al., 2019, 2020) occur in Iran: Aedes caspius s. l., Ae. vexans, Culex
antennatus (Becker), Cx. perexiguus, Cx. pipiens, Cx. theileri, Cx. tritaeniorhynchus and
Mansonia uniformis, however there is no evidence for indigenous transmission of the virus
in the country.
Sandfly fever (papatasi fever, Phlebotomus fever, three-day fever)
Sandfly fever, caused by different sandfly-borne phleboviruses (SFN-SV) (Phenuiviridae:
Phlebovirus), which are transmitted in the Old World by species of the genus Phlebotomus
(Diptera: Psychodidae, Phlebotominae), and probably also the genus Sergentomyia in the
Mediterranean region, Africa, the Indian subcontinent, the Middle East and Central Asia,
and in the New World by species of the genus Lutzomyia. Different vertebrates including
bats, carnivora, insectivora, rodents and sheep, may serve as hosts in nature. Sandfly
fever caused by most of the sandfly-borne phleboviruses is a self-limiting influenza-like
disease without mortality, however acute meningitis or meningo-encephalitis has been
reported for Toscana virus (TOSV) in several European countries (Adler, Theodor, 1957;
Barnett, Suyemoto, 1961; Ashford, 2001; Depaquit et al., 2010; Ready, 2013; Dehghani
et al., 2021). In addition to Iran, viruses that cause sandfly fever have also been found in
Aghanistan, Azerbaijan, Djibouti, Iraq, Pakistan, Saudi Arabia, Somalia, Sudan, Turkey and
Turkmenistan (Tesh et al., 1975, 1976b; Hayes, Burney, 1981; Arsen'eva, 1982; Darwish
et al., 1983b; Tesh, 1989; Gaidamovich et al., 1990a, b; Nikolaev et al., 1991; Lvov,
1994; Watts et al., 1994; Bryan et al., 1996; McCarthy et al., 1996; Wallace et al., 2002;
Riddle et al., 2008; Depaquit et al., 2010; Inci et al., 2013; Andayi et al., 2014; Alkan et
al., 2015; Barakat et al., 2016; Failloux et al., 2017; Atkinson, Hewson, 2018; Ahmed et
al., 2020). The main vector is Phlebotomus papatasi (Scopoli), the distribution of which
coincides closely with the distribution of the disease. Dashli virus (DASHV), Karimabad
virus (KARV), sandfly fever Naples virus (SFNV), sandfly fever Sicilian virus (SFSV) and
Tehran virus (THEV) have been isolated from Ph. papatasi (Tesh et al., 1977; Ashford,
2001; Depaquit et al., 2010; Alkan et al., 2017). Also, SFSV was isolated from Ph. ariasi
Tonnoir in Algeria (Izri et al., 2008). Corfou virus (CFUV), closely related to SFSV, was
380
isolated from Ph. neglectus Tonnoir (as Ph. major Annandale) in Greece (Rodhain et al.,
1985). In Europe, Arbia virus (ARBV), closely related to Salehabad virus (SALV), SFNV
and TOSV were isolated from Ph. perfiliewi Parrot (Ashford, 2001) and Ph. perniciosus
Newstead (Verani et al., 1988; Ashford, 2001). TOSV has been isolated from Sergentomyia
minuta Roundani (Charrel et al., 2006b) and Massilia virus (MASV), closely related to
SFNV, has been isolated from Phlebotomus perniciosus (Charrel et al., 2009). Historically,
the first reports of sandfly fever infection in Iran were by foreign investigators in the
1940s and 1950s (Hertig, Sabin, 1955; Barnett, Suyemoto, 1961; Hyams et al., 1995).
Eight sandfly fever viruses have been found in Iran: DASHV, KARV, SFNV, SALV, SFSV,
THEV, TOSV and sandfly fever Cyprus virus (SFCV). It seems that while Naples and
Sicilian viruses are the most prevalent viruses in most studied areas, Karimabad virus is
most abundant in Isfahan Province, in central Iran, and is also very common in Razavi
Khorasan Province, in northeastern Iran, according to seroepidemiological studies (Saidi,
1974; Tesh et al., 1975, 1976a, b, 1977; Javadian et al., 1977; Saidi et al., 1977; Tesh, 1988,
1989; Mehrabi-Tavana, 1999, 2001; Mehrabi-Tavana et al., 2000; Alkan et al., 2017; Shiraly
et al., 2017). There is doubt about the occurrence of SFCV and TOSV in Iran because
of the rarity of cases and probable cross-reaction between viral serotypes (Shiraly et al.,
2017). Saidi (1974) found seropositive antibodies for KARV in 3% of preschool children
in the Caspian area using hemagglutination inhibition (HI) tests. Tesh et al. (1976a, b)
reported positive neutralization tests for humans in different urban and rural areas of seven
Iranian provinces: East Azerbaijan: SFSV (12%), SFNV (26%), KARV (1%); Guilan: SFSV
(12.9%), SFNV (21.5%); Isfahan: SFSV (14.1-20%), SFNV (6.3-10%), KARV (50-75%);
Kermanshah: SFSV (9.4%), SFNV (28.1%); Khorasan: SFSV (4.1-19%), SFNV (4.2-
33.8%), KARV (1.0-31.1%); Khuzistan: SFSV (9.1-34.2%), SFNV (3.0-42.9%), KARV
(0.8%); Tehran: SFSV (10.8-27.4%), SFNV (19.4-36.6%), KARV (5.9-11.8%). Tesh et al.
(1977) isolated SFSV and KARV from Phlebotomus papatasi and possibly Ph. caucasicus
Marzinowsky in Isfahan Province. Saidi et al. (1977) reported positive neutralization tests
in Isfahan Province for humans: SFNV (17.2%), SFSV (25.4%) and KARV (66.4%); for
sheep: SFSV (5.2%) and for the gerbil Rhombomys opimus: SFSV (34.2%) and KARV
(31.6%). Mehrabi-Tavana (2001) reported positive HI tests for SFSV (60%) and SFNV
(46%) in Ilam Province and SFSV (100%) and SFNV (33.3%) in Kermanshah Province
in limited samples. Seroprevalence of indirect fluorescent antibody (IFA) tests in humans
in Ilam Province gave positive results for SFSV (10.9%), SFNV (5%), SFCV (1.5%) and
TOSV (1%) (Shiraly et al., 2017). Karimabad virus (KARV) and Salehabad virus (SALV)
were found in Phlebotomus species and Tehran virus (THEV) was found in Phlebotomus
papatasi for the first time in Iran in 1959 (International catalog of arboviruses including
aspx). Dashli virus (DASHV) was first isolated and described from Sergentomyia species
and Ph. papatasi collected in Dashliboroun of Golestan Provine, in northern Iran (Alkan
et al.,
2017). Additionally, there are many notes, letters and reviews on sandfly fever
381
in Iran (Mehrabi-Tavana, 2007, 2012, 2015, 2017a, b, c, d, e, f; Khoobdel et al., 2008;
Azari-Hamidian et al., 2023). The most recent checklist of Iranian sandflies (Kasiri et
al., 2000) includes 54 species, 31 species of the genus Phlebotomus and 23 species of
the genus Sergentomyia. While at least 62 species of sandflies occur in Iran (Javadian,
Mesghali, 1975; Artemiev, 1978; Secombe et al., 1993; Kasiri et al., 2000; Badakhshan et
al., 2011; Akhoundi et al., 2012; Zahraei-Ramazani et al., 2013, 2015; Norouzi et al., 2020),
the occurrence of some species and the number of species in Iran are controversial, with
44 to 50 species recorded by different investigators, for example Yaghoobi-Ershadi (2012),
Karimi et al. (2014) and Moradi-Asl et al. (2019).
Poxviridae
Avian (fowl) pox
Avian (fowl or poultry) pox, caused by the avian (fowl) pox virus (FPV) (Poxviridae:
Avipoxvirus), was reviewed by Azari-Hamidian et al. (2019). The papers by Ebrahimi et
al. (2012), Khalesi et al. (2019), Sadat Mousavi et al. (2019), Zarifi et al. (2019), Khalili
Gheidariy et al. (2020), Mehrabadi et al. (2020), Alemian et al. (2021), Mirzazadeh et al.
(2021), Zamani et al. (2021) and Ghodsian et al. (2022) might be added to the Iranian
literature pertaining to the virus. The virus has also been found in Bahrain (Samour et al.,
1996), Iraq (Tantawi et al., 1981), Kuwait (Tarello, 2008), Saudi Arabia (Tarello, 2004)
and the United Arab Emirates (Tarello, 2008), and has been isolated from the poultry red
mite Dermanyssus gallinae in Iran (Eram et al., 2020).
Lumpy skin disease
Lumpy skin disease, caused by the lumpy skin disease virus (LSDV) (Poxviridae:
Capripoxvirus), has been found in Africa, Asia and Europe. The virus infects cattle and
water buffaloes. The infection causes huge economic losses in the livestock industry (Weiss,
1968; Hunter, Wallace, 2001; Tuppurainen, Oura, 2012; Al-Salihi, 2014; Tuppurainen
et al., 2015; Namazi, Khodakaram Tafti, 2021). The disease is known in Azerbaijan,
Bahrain, Djibouti, Iraq, Kuwait, Oman, Saudi Arabia, Somalia, Sudan, Syria, Turkey, the
United Araba Emirates and Yemen (Kumar, 2011; Tuppurainen, Oura, 2012; Al-Salihi,
2014; Tageldin et al., 2014; Tuppurainen et al., 2015; Inci et al., 2016; Sevik, Dogan,
2017). To date, the main route of transmission of LSDV is mechanical, not biological,
through the bites of haematophagous arthropods, therefore it has not been considered an
arbovirus (Hunter, Wallace, 2001; Chihota et al., 2003; Tuppurainen, Oura, 2012; Al-Salihi,
2014; Sprygin et al., 2019). Recently, evidence was found for the biological transmission
of LSDV by Culicoides punctatus in Turkey (Sevik, Dogan, 2017). Mechanical transmission
of LSDV has been reported for different biting, or even non-biting, arthropods, including the
stable fly Stomoxys calcitrans (Weiss, 1968; Baldacchino et al., 2013), the mosquito Aedes
aegypti (Chihota et al., 2001), the hard ticks Amblyomma hebraeum Koch, Rhipicephalus
appendiculatus and Rh. decoloratus (Lubinga et al., 2013a, b, 2014a, b; Tuppurainen
382
et al., 2013a, b), the horn fly Haematobia irritans Linnaeus (Diptera: Muscidae) (Kahana-
Sutin et al., 2017), the house fly Musca domestica Linnaeus (Diptera: Muscidae) (Sprygin
et al., 2018) and Musca (Biomyia) confiscata Speiser (junior homonym: M. fasciata Stein)
(Diptera: Muscidae) (Weiss, 1968; as Biomyia fasciata). Also, transovarial and transtadial
transmission of the virus in ticks has been reported (Lubinga et al., 2013b, 2014a).
In Iran, the disease has been found in Alborz, East Azerbaijan, Fars, Guilan, Ilam, Kerman,
Kermanshah, Khorasan, Khuzistan, Kurdistan, Mazandaran, Qom and West Azerbaijan
Provinces (Norian et al., 2016; Jalili et al., 2017; Sameea Yousefi et al., 2017, 2018;
Karimpour Somedel et al., 2019; Ghalyanchilangeroudi et al., 2021; Hedayati et al., 2021).
There is no information about the possible role that arthropods may play in transmission
in the country.
Sheep and goat pox
Sheep and goat pox is caused, respectively, by the sheep pox virus (SPV) and the
goat pox virus (GPV) (Poxviridae: Capripoxvirus). While, sheep pox is clinically similar
to goat pox, recent molecular findings have shown that these are two separate viruses.
Most strains are host specific and cause severe clinical disease in either sheep or goats,
while some strains have equal virulence in both of these animals. The disease occurs in
Africa, Asia, Europe and the western USA. The virus infects ruminants (especially cattle,
goats and sheep). The main route of transmission is close contact with infected animals
(Rao, Bandyopadhyay, 2000; World Organisation for Animal Health, 2013; Mirzaei et al.,
2015; Tuppurainen et al., 2015; Yune, Abdela, 2017). The stable fly Stomoxys calcitrans
and the sheep head fly Hydrotaea irritans are assumed to play a role via mechanical
transmission (Kitching, Mellor, 1986; Mellor et al., 1987). Infections have occurred in
Afghanistan, Azerbaijan, Djibouti, Iraq, Oman, Pakistan, Somalia, Sudan, Syria, Turkey and
Yemen (Hedger et al., 1980; Kitching, Mellor, 1986; Rao, Bandyopadhyay, 2000; World
Organisation for Animal Health, 2013; Mirzaei et al., 2015; Tuppurainen et al., 2015).
A disease control vaccination program has been ongoing for about 70 years in Iran (Rafyi,
Mirchamsy, 1956; Rafyi, Ramyar, 1959; Ramyar et al., 1974; Sadri, Fallahi, 2010; Ghorani,
Esmaeili, 2022). Despite this, some severe outbreaks still occur in the country with high
morbidity and mortality. Sheep pox outbreaks mostly occur in the northwestern, northeastern
and central provinces of Iran, including Azerbaijan, Hormozgan, Kermanshah, Qom, Fars,
Bushehr, Kerman, Khorasan and Yazd Provinces, and goat pox outbreaks mostly occur in
southern provinces, including Fars, Hormozgan, Kerman and Khorasan Provinces (Mirzaei
et al., 2015; Karimpour Somedel et al., 2019). SPV pathology has been studied in Fars
Province (Khoda Karam Tafti, Namdari, 2000). During a study in six Iranian provinces,
including Azerbaijan, Fars, Hormozgan, Kerman, Khorasan and Khuzistan, 20.75% of goats
were positive for GPV (Sadri, 2012c). A high rate of mortality due to a SPV outbreak was
reported in Qom Province (Mirzaei et al., 2015). There is no information about the possible
vector(s) of the viruses in the country.
383
Reoviridae
African horse sickness
African horse sickness is a non-contagious infection caused by African horse sickness
virus (AHSV) (Reoviridae: Orbivirus). Nine distinct serotypes of the virus are known
(Sailleau et al., 2000; Mellor, 2001a; Mellor, Hamblin, 2004). Equids such as horses, mules,
donkeys and zebras are the most important vertebrate hosts. Dogs may occasionally be
infected, however they do not have an important role in the epidemiology of the disease
and are considered dead-end hosts. The disease is not considered a zoonosis. Infections are
widely distributed in Africa south of the Sahara, including Sudan, and are also enzootic in
Yemen, both countries of the WHO Eastern Mediterranean Region (Mellor, 1994, 2001a;
Mellor, Hamblin, 2004; Tkubet et al., 2016; Carpenter et al., 2017; Dennis et al., 2019). The
mortality rates are 50-95, 50, 5-10% for horses, mules and Europran and Asian donkeys,
respectively; however, mortality is rare in African donkeys and zebras (Tkubet et al., 2016).
The disease is rarely seen as far northward as Algeria, Egypt, Libya, Morocco, Palestine,
Portugal, Spain and Tunisia and eastward to Afghanistan, Cyprus, India, Iran, Iraq, Jordan,
Oman, Pakistan, Saudi Arabia, Syria and Turkey (Rafyi, 1961; Hazrati, Taslimi, 1964;
Hazrati, 1967; Mirchamcy, Hazrati, 1973; Hedger et al., 1980; Anderson et al., 1989;
Mellor et al., 1990a; Mellor, 1994, 2001a). AHSV is biologically and exclusively vectored
by certain species of Culicoides (Mellor et al., 2000), athough different haematophagous
arthropods may be implicated in transmission, such as the mosquitoes Aedes aegypti,
Anopheles stephensi Liston and Culex pipiens, the hard ticks Hyalomma dromedarii and
Rhipicephalus sanguineus (Mellor, 1994) and the horsefly Tabanus pluto Walker (Krinsky,
1976). The only confirmed principal vector is Culicoides imicola, which is present in Africa,
Asia and Europe (Mellor et al., 1990b, 2000; Mellor, 2001a). The species occurs in Bahrain,
Iraq, Oman, Saudi Arabia, Turkey and the United Arab Emirates (Boorman, 1989), however
it has not been reported in Iran (Navai, 1974). Also, C. bolitinos Meiswinkel is considered
as a secondary vector and the North American C. variipennis has been experimentally
found to be an efficient vector (Mellor et al., 1975, 2000; Mellor, 2001a). Two species,
C. obsoletus and C. pulicaris, may be involved in transmission in Europe (Mellor et al.,
1990b; Mellor, Hamblin, 2004). In summer 1959, infections were found in southern Iran
following the outbreak in the Arabian Peninsula, which rapidly spread throughout the
region, including Afghanistan, Cyprus, Iraq, Jordan, Libya, Pakistan, Syria, Turkey and
India, over a period of three years (1959-1961). During the outbreak, the region lost more
than 300,000 equines (Rafyi, 1961; Hazrati, Taslimi, 1964; Mirchamsy, Hazrati, 1973). The
virus was isolated from horses (32.5%), mules (40.0%) and donkeys (20.0%) in Iran during
the outbreak (Hazrati, Taslimi, 1964). After the outbreak, scientists in the Razi Institute
of Iran studied the virus and produced a vaccine, as reviewed by Mirchamsy and Hazrati
(1973). There is no recent record of the disease in Iran. At least 43 species of Culicoides
are known to occur in Iran (Navai, 1974); However, there is no information about
384
the possible vector(s) of AHSV in Iran. Among known vectors, C. pulicaris occurs in
the country (Navai, 1974; Mellor, Hamblin, 2004).
Bluetongue
Bluetongue virus (BLUV) (Reoviridae: Orbivirus) causes the disease of bluetongue,
which has a worldwide distribution. Infections have been found in wild and domestic
ruminants, especially sheep. The virus consists of 26, more likely 27, serotypes. The
BLUV serotypes 1-24 are transmitted almost entirely and biologically by certain species
of Culicoides biting midges, however there is not such certainty about the role of these
midges in the transmission of BLUV-25 and BLUV-26 (Afshar et al., 1989; Wirth, Hubert,
1989; Afshar, 1994; Mellor, 2001d; Hubálek et al., 2014a; Maclachlan et al., 2015). Also,
the sheep ked Melophagus ovinus (Linnaeus) (Diptera: Hippoboscidae) (Gray, Bannister,
1961; Luedke et al., 1965) and a number of mosquitoes, such as Aedes lineatopennis
(Ludlow) [Neomelaniconion lineatopenne], Ae. vigilax (Skuse) [Ochlerotatus vigilax] and
Culex annulirostris, are believed to be possible secondary or mechanical vectors (Weir
et al., 1997; Hubálek et al., 2014a). In addition to biological transmission, BLUV may
be occasionally and directly transmitted via semen and embryo transfer from infected
countries to virus-free regions (Mellor, 2001d). Infections have been found in Aghanistan
(Hassani, Madadgar, 2021), Iraq (Hafez et al., 1978), Kuwait (Maan et al., 2011a, b), Oman
(Hedger et al., 1980; Al-Busaidy, Mellor, 1991a, b), Pakistan (Akhtar et al., 1997), Saudi
Arabia (Hafez, Taylor, 1985; Abu Elzein et al., 1998a; Taha et al., 2015), Sudan (Abu
Elzein, Tageldin, 1985; Abu Elzein, 1986; Mohammad, Taylor, 1987; Mohammad, Mellor,
1990), Syria (Mellor et al., 2008; Hubálek et al., 2014a), Turkey (Gür, 2008; Failloux
et al., 2017) and Yemen (Stanley, 1990). The principal verified vectors are Culicoides
insignis Lutz and species of the C. variipennis complex in the Americas, C. imicola in
Africa and Europe and C. actoni Smith, C. brevitarsis, C. fulvus Sen et Das Gupta and
C. wadai in Australasia and Indonesia (Mohammad, Mellor, 1990; Mellor et al., 2000,
2009). Additionally, the virus has been isolated from C. milnei and C. tororoensis Khamala
et Kettle in Kenya (Walker, Davies, 1971), C. pusillus Lutz in Central America and the
Caribbean (Mo et al., 1994), C. peregrinus Kieffer in Indonesia (Sendow et al., 1996)
and C. dewulfi, C. newsteadi, C. obsoletus, C. pulicaris, C. punctatus and C. scoticus
in Italy (Caracappa et al., 2003; Goffredo et al., 2015; Federici et al., 2019). Afshar and
Kayvanfar (1974) identified precipitating antibodies to BLUV in sera of farm animals
in Iran for the first time. Kargar Moakhar et al. (1988) found the international serotypes
3, 7, 20 and 22 in sheep in different areas of Iran using the agar gel immunodiffusion
(AGID) and microneutralization tests. Serological surveys, using ELISA, found positive
antibodies to BLUV in East Azerbaijan Province (in sheep, 67-76.44%) (Hasanpour
et al., 2008; Imandar et al., 2014), Fars Province (in cattle, 19.77%, in goats, 55.70-85.3%
and in sheep, 70.93-74.4%) (Mohammadi et al., 2012; Oryan et al., 2014; Manavian
et al., 2017; Hashemi et al., 2018), Hamedan Province (in sheep, 46%) (Yavari et al., 2018),
385
Ilam Province (in sheep, 43.88%) (Khezri, Azimi, 2012b), Isfahan Province (in cattle,
2.69%, in goats, 49.19% and in sheep, 53.37%) (Noaman et al., 2008, 2013), Kerman
Province (in camels, 100%, in cattle, 2.13%, in goats, 67.7% and in sheep 6.57%) (Mahdavi
et al., 2006; Mozaffari, Khalili, 2012; Mozaffari et al., 2012, 2014), Khuzistan Province
(in sheep, 55.9%) (Noroozikia et al., 2014), Kohgiluyeh and Boyerahmad Province (in sheep,
77.48%) (Sabaghan et al., 2014), Kurdistan Province (in sheep, 19.3-51.85%) (Khanbabaie
et al., 2011; Khezri, 2012; Khezri, Azimi, 2012a, b; Khezri, Bakhshesh, 2014), Razavi
Khorasan Province (in goats, 87.6% and in sheep, 90.0%) (Najarnezhad, Rajae, 2013), West
Azerbaijan Province (in sheep, 34.7-55.9%) (Jafari-Shoorijeh et al., 2010; Sadri, 2012a;
Hasanpour et al., 2014) and Yazd Province (in camels, 67.8%) (Mozaffari et al., 2013).
In a study conducted in eight Iranian provinces, the total prevalence of BLUV antibodies
found in sheep was 34.9%: the provinces included Ardebil (23.7%), East Azerbaijan
(39.8%), Fars (25.3%), Ilam (42.6%), Khuzistan (15%), Kurdistan (41.7%), Qom (12.1%)
and West Azerbaijan (64.8%) (Khezri, Azimi, 2013). In a study in seven provinces, Ardebil,
East Azerbaijan, Fars, Ilam, Khuzistan, Kurdistan and Qom, the infection was investigated
using RT-PCR for the first time in Iran and 10% of total samples were both seropositive and
RT-PCR positive in sheep (Azimi et al., 2009). During another investigation in nine
provinces, Ardebil, East Azerbaijan, Golestan, Isfahan, Markazi, Qazvin, Qom, West
Azerbaijan and Yazd, 66.43% of all samples were serologically positive in sheep (Fallahi
et al., 2013). In an investigation in three provinces of southeastern Iran, Hormozgan,
Kerman and Sistan and Baluchistan, the total prevalence of BLUV antibodies was 92.67%
in goats and 48.72% in sheep (Ezatkhah et al., 2014). In a study on wild ruminants in
different areas of Iran, 12% of viral serological tests (ELISA) and 8% of PCR results were
positive for BLUV in mouflon (Ovis orientalis) (Hemmatzadeh et al., 2016). Momtaz et al.
(2011) compared the results of ELISA and RT-PCR for BLUV in sheep in Chaharmahal
and Bakhtiari, Isfahan and Khuzistan Provinces. Another study in Chaharmahal and
Bakhtiari Province indicated a significant relationship between seropositivity and topography
(plains or mountains), sex (male or female) and abortion history (Noaman, Arzani, 2017).
Bakhshesh et al. (2020) studied the large-scale seroprevalence and risk factors associated
with the virus in the country. Khezri and Bakhshesh (2014), Oryan et al. (2014) and
Hassani and Madadgar (2021) reviewed infections in Iran. Among known vectors of
the virus, Culicoides pulicaris and C. punctatus occur in the country (Navai, 1974; Goffredo
et al., 2015; Abdigoudarzi, 2016). Although at least 43 species of Culicoides are known to
be present in Iran (Navai, 1974), there is no information about the vector(s) of bluetongue
virus in the country.
Wad Medani virus
Wad Medani virus (WMV) (Reoviridae: Orbivirus) is distributed in Africa and Asia.
The virus has been found in numerous species of hard ticks of the genera Amblyomma,
Dermacentor, Hyalomma and Rhipicephalus. Serological tests have identified infections in
cattle, camels, pigs, buffaloes and rodents (Taylor et al., 1966a; Hoogstraal, Valdez, 1980;
386
Labuda, Nuttall, 2008; Belaganahalli et al., 2015; Atkinson, Hewson, 2018; Dedkov et al.,
2021). The virus has been found in Armenia, Pakistan, Sudan and Turkmenistan (Taylor
et al., 1966a; Lvov et al., 1967; Begum et al., 1970a, d; Skvortsova et al., 1975; Hayes,
Burney, 1981; Darwish et al., 1983b; Lvov, 1994; Alkhovsky et al., 2014c; Atkinson,
Hewson, 2018). It has been isolated from the following ticks: Amblyomma cajennense
(Fabricius) s. l., Dermacentor nuttalli, Hyalomma anatolicum, H. asiaticum, Rhipicephalus
guilhoni Morel et Vassiliades, Rh. microplus (Canstrini), Rh. sanguineus and Rh. turanicus
(see Taylor et al., 1966a; Lvov et al., 1967; Begum et al., 1970d; Hoogstraal, Valdez, 1980;
Hayes, Burney, 1981; Voltsit, 1982; Alkhovsky et al., 2014c; Yadav et al., 2019; Dedkov
et al., 2021). There is a unique record of Wad Medani virus in Iran, which was found in
Hyalomma anatolicum collected from cattle in Razavi Khorasan Province (Sureau, Klein,
1980; Sureau et al., 1980). Among known vectors, Hyalomma asiaticum, Rhipicephalus
sanguineus and Rh. turanicus occur in Iran (Taylor et al., 1966a; Hayes, Burney, 1981;
Hoogstraal, Valdez, 1980; Hosseni-Chegeni et al., 2019).
Retroviridae
Bovine leukemia
Bovine leukemia, caused by the bovine leukemia virus (BLV) (Retroviridae:
Deltaretrovirus), occurs worldwide. The virus consists of ten genotypes. The disease
causes economical losses due to reduction in milk production, reproductive performance
and length of life (Polat et al., 2017). The disease is mainly directly transmitted among
cattle, however there is some evidence for mechanical transmission by horseflies (Tabanus
fuscicostatus Hine, T. nigrovittatus Macquart, T. nipponicus Murdoch et Takahasi and
T. trigeminus Coquillett), the stable fly Stomoxys calcitrans and the hard tick Rhipicephalus
microplus (Foil, 1989; Foil et al., 1988; Foil, Issel, 1991; Baldacchino et al., 2013).
Infections have been found in Saudi Arabia (Hafez et al., 1990) and Turkey (Burgu
et al., 2005). The disease has been found in cattle in the following provinces of Iran based
on ELISA and PCR tests: Alborz (45%) (Kazemimanesh et al., 2012), Ardebil (9.5%)
(Kazemimanesh et al., 2012), Chaharmahal and Bakhtiari (18.4%) (Nekoei et al., 2015a),
East Azerbaijan (50%) (Kazemimanesh et al., 2012), Guilan (100%) (Kazemimanesh
et al., 2012), Isfahan (23.8-81.9%) (Morovati et al., 2012; Nekoei et al., 2015a), Kerman
(15.5%) (Mohammadabadi et al., 2011), Markazi (53.3%) (Kazemimanesh et al., 2012),
North Khorasan (1.5%) (Mousavi et al., 2014), Qom (57%) (Kazemimanesh et al., 2012),
Razavi Khorasan (2.3-29.8%) (Kazemimanesh et al., 2012; Mousavi et al., 2014) and
Tehran (17-88.8%) (Nikbakht Brujeni et al., 2010; Mohammadi et al., 2011; Kazemimanesh
et al., 2012). The virus has also been found in sheep in Chaharmahal and Bakhtiari Province
(2.7%) and Isfahan Province (6.7%) (Nekoei et al., 2015a). Recently, an investigation in
Iran proposed the possible association between the human breast cancer and the BLV
infection in cattle using the nested PCR technique (Khalilian et al., 2019). There is no
information about possible transmission of the virus by arthropods in the country.
387
Equine infectious anaemia (swamp fever)
Equine infectious anaemia, caused by the equine infectious anaemia virus (EIAV)
(Retroviridae: Lentivirus), has a worldwide distribution. The disease is the most important
viral infection in horses. Although EIAV is not an arbovirus, it does not replicate in
the vector and is not vectored biologically, haematophagous insects play an important
role in its transmission and the epidemiology of infection (Issel, 2001; Issel, Foil, 2015).
The virus has been found in Oman (Body et al., 2011), Saudi Arabia (Alnaeem, Hemida,
2019), Sudan (Wegdan et al., 2017) and Turkey (Marenzoni et al., 2013). Horseflies, such as
Chrysops flavidus Wiedemann, Hybomitra frontalis (Walker), H. lasiophthalma (Macquart),
Tabanus fuscicostatus and T. sulcifrons Macquart, and the stable fly Stomoxys calcitrans
(less important), play an important role in mechanical transmission of the virus to horses,
whereas mosquitoes, such as Aedes aegypti and Psorophora columbiae (Dyar et Knab), are
linked to subclinical or inapparent infections (Hawkins et al., 1973; Krinsky, 1976; Foil
et al., 1983; Foil, 1989; Foil, Issel, 1991; Green et al., 1996; Issel, 2001; Baldacchino
et al., 2013). In Iran, the disease has been found in the provinces of Ardebil, East
Azerbaijan, Isfahan, Kurdistan and Tehran (Hazrati et al., 1978; Momtaz, Nejat, 2010;
Rezazadeh et al., 2016). There is no information about the possible role of arthropods in
transmission of the virus in Iran.
Rhabdoiviridae
Bovine ephemeral fever
Bovine ephemeral fever, caused by the bovine ephemeral fever virus (BEFV)
(Rhabdoviridae: Ephemerovirus), was reviewed by Azari-Hamidian et al. (2019). The papers
by Bakhshesh et al. (2018), Pasandideh et al. (2018a, b, c, 2019a, b), Almasi, Bakhshesh
(2019a, b), Mollazadeh et al. (2022) and Rezatofighi et al. (2022) might be added to
the Iranian literature pertaining to the infection. The virus has also been found in
Afghanistan (St George, 1988), Iraq, Kuwait, Pakistan, Saudi Arabia, Somalia, Sudan,
Syria, Turkey, Turkmenistan and Yemen (St George, 1988; Lvov, 1994; Hubálek et al.,
2014a; Walker, Klement, 2015), as well as Iran (Azari-Hamidian et al., 2019).
Isfahan virus
Isfahan virus (ISFV) (Rhabdoviridae: Vesiculovirus) was isolated from pools of
Phlebotomus papatasi for the first time in Isfahan Province of Iran (Tesh et al., 1977;
Calisher et al., 1989). The virus has also been isolated from humans in the provinces of
Isfahan (66.9%), Khuzistan (5.4%) and Tehran (3.3%), and from the rodents Rhombomys
opimus in Isfahan Province (79%) and Tatera indica in Khuzistan Province (8.6%) using
the neutralization test (Tesh et al., 1977). Additionally, the virus has been isolated from
the mosquito Aedes caspius s. l. and the hard tick Hyalomma asiaticum, see Alkhutova
et al. (1981), Alkhutova, Sadykov (1982), Labuda, Nuttall (2008) and Atkinson, Hewson
(2018). Aedes caspius and Hyalomma asiaticum both occur in Iran (Azari-Hamidian et al.,
388
2019; Hosseni-Chegeni et al., 2019). Some isolations of the virus, or its antibodies, have
been made in Central Asia (Tajikistan, Turkmenistan and Uzbekistan) (Gaidamovich et al.,
1980; Lvov, 1994; Atkinson, Hewson, 2018).
Zahedan rhabdovirus
Zahedan rhabdovirus (ZARV) (Rhabdoviridae: Zamolirhabdovirus) was first recovered
and described from the hard tick Hyalomma anatolicum anatolicum collected in Zahedan in
Sistan and Baluchistan Province, southeastern Iran. The virus is lethal for mice and possibly
pathogenic for other mammals. The mammalian host is not known (Dilcher et al., 2015).
Togaviridae
Chikungunya infection
Chikungunya infections caused by Chikungunya virus (CHIKV) (Togaviridae:
Alphavirus) occur widely in sub-Saharan Africa and southern Asia. The virus has been
isolated from different species of monkey, as well as bats and birds. It seems that non-
human primates and humans are the main vertebrate hosts in Africa and Asia, respectively.
The virus has been isolated from different mosquito species of the genera Aedes, Anopheles,
Coquillettidia, Culex and Mansonia, as well as species of soft ticks (Ornithodoros sonrai)
and hard ticks. It is probable that sylvan species of Aedes in Africa, for example Aedes
africanus (Theobald) [Stegomyia africana] and Ae. furcifer (Edwards) [Diceromyia furcifer],
and urban Ae. aegypti and Ae. albopictus in Asia are the main vectors (Hoogstraal, 1985;
McCarthy et al., 1996; Diallo et al., 1999; Woodall, 2001a; Nsoesie et al., 2016; Failloux
et al., 2017; Gould et al., 2017; Wahid et al., 2017; Silva et al., 2018; Simo et al., 2019).
The virus may have been isolated from the tropical bed bug Cimex hemipterus (Fabricius)
(Hemiptera: Cimicidae) (Rao, 1964). Other possible mosquito vectors, which were found
naturally infected or are experimentally assumed to play a role in transmission, include
Aedes calceatus (Edwards) [Stegomyia calceata], Ae. cordellieri (Huang) [Diceromyia
cordellieri], Ae. fulgens (Edwards) [Zavortinkius fulgens], Ae. luteocephalus (Newstead)
[Stegomyia luteocephala], Ae. opok (Corbet et van Someren) [Stegomyia opok], Ae. taylori
(Edwards) [Diceromyia taylori], Ae. vittatus (Bigot) [Fredwardsius vittatus], Coquillettidia
fuscopennata (Theobald), Culex quinquefasciatus, Mansonia africana (Theobald) and
Ma. uniformis (Rao, 1964; Jupp et al., 1981; Jupp, McIntosh, 1990; Diallo et al.,
1999, 2012; Woodall, 2001a; Silva et al., 2018). Chikungunya virus has been found in
Afghanistan, Djibouti, Iraq, Pakistan, Qatar, Saudi Arabia, Somalia, Sudan and Yemen, and
imported cases have been found in Oman and Turkey (Salim, Porterfield, 1973; Darwish
et al., 1983a; Arsen'eva, 1982; Oldfield et al., 1993; Watts et al., 1994; Wallace et al.,
2002; Farnon et al., 2010; Zayed et al., 2012; Andayi et al., 2014; Ciccozzi et al., 2014;
Malik et al., 2014; Rezza et al., 2014; Afzal et al., 2015; Al-Abri et al., 2015; Barakat
et al., 2016; Wahid et al., 2017; Yaqub et al., 2017; Humphrey et al., 2019; Ahmed et al.,
2020; Sawal et al., 2021). Cases of CHIKV imported from Pakistan were recently found
in Sistan and Baluchistan Province in southeastern Iran (Pouriayevali et al., 2019). More
389
recently, Bakhshi et al. (2020
) detected the virus in Anopheles maculipennis Meigen
s. l. in Mazandaran Province and in A. maculipennis s. l., Culex tritaeniorhynchus and
Culisetta longiareolata (Macquart) in North Khorasan Province using primers designed
for CHIKV Asian genotype, however they failed to isolate the virus and whole genome
sequencing was not performed. Also, Tavakoli et al. (2020) detected CHIKV IgM
seropositivity in 16.07% of samples in eight southern provinces of Iran. Among known
vectors of Chikungunya virus, Aedes albopictus, Ae. vittatus and Mansonia uniformis occur
in Iran (Diallo et al., 1999, 2012; Silva et al., 2018; Azari-Hamidian et al., 2019, 2020),
however there is no information about the indigenous transmission of the virus in the
country.
Semliki forest virus
Semliki forest virus (SFV) (Togaviridae: Alphavirus) (synonym or subtype: Me Tri
virus) occurs mostly in Africa south of the Sahara, but the virus has been found in eastern
Russia and Vietnam and antibodies have been detected in Borneo, India, Indonesia,
Malaysia, the Philippines and Thailand. It seems that the main reservoirs are domesticated
mammals, such as cattle, horses and pigs. However, the virus has also been isolated
from monkeys, wild birds, rodents and insectivores. Among arthropods, the virus has
been isolated from species of the mosquito genera Aedes, Culex and Eretmapodites and
the hard tick Rhipicephalus guilhoni (Ha et al., 1995; Pfeffer, 2001; Tan et al., 2008;
Hubálek et al., 2014a). The virus was first isolated from Aedes abnormalis (Theobald)
[Aedimorphus abnormalis], but it seems the main vector in Africa is Ae. africanus. It has
been detected in Ae. vexans and Culex pipiens in eastern Russia and Cx. tritaeniorhynchus
in Vietnam, and has been found in many species in Africa, including Aedes aegypti,
Ae. argenteopunctatus (Theobald) [Catageiomyia argenteopunctata], Ae. fuscinervis
(Edwards) [Neomelaniconion fuscinerve], Ae. jamoti (Hamon et Rickenbach)
[Neomelaniconion jamoti], Ae. opok, Ae. palpalis (Newstead) [Neomelaniconion palpale],
Ae. punctocostalis (Theobald) [Neomelaniconion punctocostale], Ae. togoi (Theobald)
[Tanakaius togoi], Ae. vittatus, Eretmapodites chrysogaster Graham, Er. grahami Edwards,
Culex quinquefasciatus and Mansonia africana (Lee et al., 1974; Gaidamovich et al., 1975;
Ha et al., 1995; Pfeffer, 2001; Hubálek et al., 2014a). According to unpublished data in
Iran, antibodies for the virus were found in humans (3%) using the neutralization test
(the CDC Arthropod-Borne Virus Information Exchange, 1962, available at https://stacks.
cdc.gov). Among known vectors of the virus, Aedes vexans, Ae. vittatus, Culex pipiens,
Cx. quinquefasciatus and Cx. tritaeniorhynchus occur in Iran (Pfeffer, 2001; Hubálek et al.,
2014a; Azari-Hamidian et al., 2019). There is no recently verified and published information
about the occurrence of the virus in the country.
Sindbis fever
Sindbis fever, caused by Sindbis fever virus (SINV) (Togaviridae: Alphavirus) [synonyms
or subtypes: Babanki, Karelian, Kyzylagach (KYZV), Ockelbo, Pogosta and Whataroa],
390
was reviewed by Azari-Hamidian et al. (2019). In addition to Iran, the virus has also been
found in Afghanistan (Arsenʼeva, 1982; Wallace et al., 2002), Armenia (Failloux et al.,
2017), Azerbaijan (Lvov, 1994; Lundström, Pfeffer, 2010; Storm et al., 2013; Alkhovsky
et al., 2014d), Iraq (Riddle et al., 2008; Barakat et al., 2016), Oman (Camp et al., 2019),
Pakistan (Darwish et al., 1983a), Saudi Arabia (Wills et al., 1985; Al-Khalifa et al., 2007;
Lundström, Pfeffer, 2010; Storm et al., 2013), Somalia (Oldfield et al., 1993), Sudan
(Hoogstraal, 1966; Farnon et al., 2010), Turkey (Hubálek et al., 2014a) and Turkmenistan
(Atkinson, Hewson, 2018). Recently, Hanafi-Bojd et al. (2021) detected Sindbis fever virus
in the pools of Culex pipiens and Cx. theileri in West Azerbaijan Province of Iran. Bakhshi
et al. (2022) reviewed the virus in Iran and adjacent countries. Principal vectors of the
virus in other countries that also occur in Iran include Cx. torrentium Martini and Culiseta
morsitans (Theobald) (Hubálek et al., 2014a; Azari-Hamidian et al., 2019).
Unclassified virus
Wanowrie virus
Wanowrie virus (WANV) (unclassified) was first isolated from Hyalomma marginatum
in India (Dandawate et al., 1970; Labuda, Nuttall, 2008). The virus was later found
in Hyalomma impeltatum Schulzae et Schlottke collected from camels in Egypt (Williams
et al., 1973) and identified in the brain of a human in Sri Lanka who died from an
infection (Pavri et al., 1976). Antibodies against WANV have been detected in sera of
domestic abunaks and humans in Pakistan (Darwish et al., 1983b). There is just one record
of Wanorie virus in Iran, isolated from Hyalomma asiaticum collected from goats in Razavi
Khorasan Province (Sureau, Klein, 1980; Sureau et al., 1980). Two other ticks from which
the virus has been isolated, H. impeltatum and H. marginatum, also occur in Iran (Hosseni-
Chegeni et al., 2019).
Other viruses in the countries neighboring Iran
There are other diseases and infections caused by arboviruses or viruses which may
be mechanically transmitted by arthropods in the countries neighboring Iran and in
the WHO Eastern Mediterranean Region that need to be considered. Although these viruses
have not been formally reported in Iran, their eventual occurrence in the country is very
possible, especially in view of tourism, immigration and the presence and/or importation
of possible vectors, hosts, reservoirs and migratory birds. The names of infections, viruses
and country records are as follow:
Flaviviridae
Banzi virus (BANV) (Flaviviridae: Flavivirus), transmitted by mosquitoes, known
in Somalia (Henderson et al., 1968; Cahill, 1971).
Barkedji virus (BJV) (Flaviviridae: Flavivirus), transmitted by mosquitoes, known
in Oman and the United Arab Emirates (Camp et al., 2019).
391
Israel turkey meningoencephalitis virus (ITMV) [synonym or subtype: Bagaza virus
(BAGV)] (Flaviviridae: Flavivirus), transmitted by mosquitoes and possibly biting midges,
known in the United Arab Emirates (Hubálek et al., 2014a; Camp et al., 2019).
Kadam virus (KADV) (Flaviviridae: Flavivirus), transmitted by ticks, known in Saudi
Arabia (Wood et al., 1982; Al-Khalifa et al., 2007; Labuda, Nuttall, 2008).
Karshi virus (KSIV) (Flaviviridae: Flavivirus), transmitted by ticks, known
in Turkmenistan (Hoogstraal, 1985; Labuda, Nuttall, 2008; Atkinson, Hewson, 2018).
Kyasanur Forest disease virus (KFDV) (Flaviviridae: Flavivirus) [synonyms or subtypes:
Alkhurma virus, Aka Alkhumra virus or Alkhurma hemorrhagic fever virus (ALKV or
AHFV)], transmitted by ticks and possibly mosquitoes, known in Djibouti, Saudi Arabia
and Turkey (Hoogstraal, 1985; Zaki, 1997; Charrel et al., 2005, 2006a, 2007; Madani, 2005;
Labuda, Nuttall, 2008; Alzahrani et al., 2010; Carletti et al., 2010; Memish et al., 2010,
2011; Mahdi et al., 2011; Shibl et al., 2012; Ahmed, 2015; Horton et al., 2016; Atkinson,
Hewson, 2018; Hoffman et al., 2018; Shah et al., 2018).
Louping ill virus (LIV) (Flaviviridae: Flavivirus) (synonym: Negishi virus) (Hubálek
et al., 2014a), transmitted by ticks, known in Turkey [as LI-like virus or Turkish sheep
encephalomyelitis virus (TSEV)] (Hartley et al., 1969; Gao et al., 1997; Gould, 2001;
de la Fuente et al., 2008; Hubálek, Rudolf, 2012; Inci et al., 2016; Düzlü et al., 2020).
Royal farm virus (RFV) (Flaviviridae: Flavivirus), transmitted by ticks, known
in Afganistan and Pakistan (Williams et al., 1972; Darwish et al., 1983a; Hoogstraal, 1985;
Labuda, Nuttall, 2008).
Usutu virus (USUV) (Flaviviridae: Flavivirus), transmitted by mosquitoes, known
in Iraq (Barakat et al., 2016).
Yellow fever virus (YFV) (Flaviviridae: Flavivirus), transmitted by mosquitoes, known
in Somalia and Sudan (Henderson et al., 1968; Cahill, 1971; Salim, Porterfield, 1973;
Oldfield et al., 1993; Watts et al., 1994; Monath, 2001; World Health Organization, 2004;
Farnon et al., 2010; Gould et al., 2017; Braak et al., 2018; Ahmed et al., 2020).
Zika virus (ZIKAV) (Flaviviridae: Flavivirus), transmitted by mosquitoes, known
in Pakistan, Saudi Arabia, Somalia, Sudan and Turkey (Henderson et al., 1968; Cahill,
1971; Darwish et al., 1983a; Tomori, 2001; Evans et al., 2017; Kindhauser et al., 2016;
Benelli, Romano, 2017; Dehghani, Amiri, 2017; Epelboin et al., 2017; Gould et al., 2017;
Alayed et al., 2018; Sezen et al., 2018; Tavakoli et al., 2018; Noorbakhsh et al., 2019;
Ahmed et al., 2020; Nikookar et al., 2020; Kassiri et al., 2020a; Saleem et al., 2022).
Nairoviridae
Artashat virus (ARTSV) (Nairoviridae: Orthonairovirus), transmitted by ticks, known
in Armenia and Azerbaijan (Hoogstraal, 1985; Lvov, 1994; Alkhovsky et al., 2014b, 2017).
Caspiy virus (CASV) (Nairoviridae: Orthonairovirus), transmitted by ticks, known
in Azerbaijan and Turkmenistan (Hoogstraal, 1985; Lvov, 1994; Labuda, Nuttall, 2008;
Lvov et al., 2014a; Alkhovsky et al., 2017).
392
Dera Ghazi Khan virus (DGKV) (Nairoviridae: Orthonairovirus), transmitted by ticks,
known in Pakistan (Begum et al., 1970a, c; Hayes, Burney, 1981; Darwish et al., 1983b;
Labuda, Nuttall, 2008; Kuhn et al., 2016).
Geran virus (GERV) (Nairoviridae: Orthonairovirus), transmitted by ticks, known
in Azerbaijan (Lvov et al., 2014c; Alkhovsky et al., 2017).
Hazara virus (HAZV) (Nairoviridae: Orthonairovirus), transmitted by ticks, known
in Pakistan (Begum et al., 1970a, b; Hayes, Burney, 1981; Darwish et al., 1983b; Labuda,
Nuttall, 2008; Hartlaub et al., 2020).
Issyk-Kul virus (ISKV) (Nairoviridae: Orthonairovirus), transmitted by ticks and
mosquitoes, known in Azerbaijan and Turkmenistan (Lvov, 1994; Gavrilovskaya, 2001;
de la Fuente et al., 2008; Labuda, Nuttall, 2008; Atkinson et al., 2015; Atkinson, Hewson,
2018). Gavrilovskaya (2001) noted the possible occurrence of this virus in Iran, Afghanistan
and Pakistan.
Nairobi sheep disease virus (NSDV) (Nairoviridae: Orthonairovirus) [synonym or
Indian subtype: Ganjam virus (GANV)], transmitted by ticks, known in Africa (including
Somalia) and Asia (India and Sri Lanka) (Johnson et al., 1980; Davis, 1997; Peiris, 2001;
de la Fuente et al., 2008; Hubálek et al., 2014a). Hoogstraal and Valdez (1980) considered
the virus as “a prime candidate for investigation in Iran”.
Tamdy virus (TDYV) (Nairoviridae: Orthonairovirus), transmitted by ticks, known
in Armenia, Azerbaijan, Turkey and Turkmenistan (Lvov, 1994; Labuda, Nuttall, 2008;
Lvov et al., 2014b; Failloux et al., 2017; Atkinson, Hewson, 2018).
Zirqa virus (ZIRV) (Nairoviridae: Orthonairovirus), transmitted by ticks, known on
Zirqa Island in the Persian Gulf (the United Arab Emirates) (Hoogstraal et al., 1970;
Varma et al., 1973; Labuda, Nuttall, 2008; Kuhn et al., 2016). The name of the virus was
misspelled as Zirga in the original paper (Varma et al., 1973).
Orthomyxoviridae
Batken virus (BKNV) (Orthomyxoviridae: Thogotovirus), transmitted by mosquitoes
and ticks, known in Azerbaijan (Lvov et al., 1974; Lvov, 1994; Hoogstraal, Valdez, 1980;
Frese et al., 1997; Labuda, Nuttall, 2008; Alkhovsky et al., 2014a). Hoogstraal and Valdez
(1980) considered this virus as “a candidate for investigation in Iranian sheep and goats”.
Dhori virus (DHOV) (Orthomyxoviridae: Thogotovirus), transmitted by ticks, known in
Armenia, Azerbaijan, Pakistan and Saudi Arabia (Williams et al., 1973; Semashko et al.,
1974; Hoogstraal, Valdez, 1980; Darwish et al., 1983b; Jones et al., 1989; Al-Khalifa
et al., 2007; Labuda, Nuttall, 2008; Hubálek, Rudolf, 2012; Failloux et al., 2017).
Peribunyaviridae
Aino virus (AINOV) (Peribunyaviridae: Orthobunyavirus) (synonyms: Kaikalur and
Samford viruses), transmitted by biting midges and mosquitoes, known in Turkey (Mellor,
2001b; Hubálek et al., 2014a; Contigiani et al., 2017).
393
Bakau virus (BAKV) (Peribunyaviridae: Orthobunyavirus), transmitted by mosquitoes
and ticks, known in Pakistan (Hayes, Burney, 1981; Darwish et al., 1983b; Hoogstraal,
1985).
Batai virus (BATV) (Peribunyaviridae: Orthobunyavirus) (synonyms: Calovo, Chitoor,
Olkya, Olyka and UgMP-6830), transmitted by mosquitoes, known in Armenia and Sudan
(Nashed et al., 1993; Failloux et al., 2017).
Ngari virus (NRIV) (Peribunyaviridae: Orthobunyavirus) (synonym: Garissa virus),
transmitted by mosquitoes, known in Somalia and Sudan (Bowen et al., 2001; Braak
et al., 2018).
Phenuiviridae
Arumowot virus (AMTV) (Phenuiviridae: Phlebovirus), transmitted by mosquitoes,
known in Somalia and Sudan (Tesh, 1988; Braak et al., 2018; Ahmed et al., 2020).
Gabek Forest virus (GFV) (Phenuiviridae: Phlebovirus), transmitted by sandflies, known
in Sudan (Tesh et al., 1976b; Tesh, 1988).
Grand Arbaud virus (GAV) (strain Art 363) (Phenuiviridae: Phlebovirus), transmitted
by ticks, known in Afghanistan (Hannoun, Rau, 1970; Williams et al., 1972; Hoogstraal,
1985; Hubálek, Rudolf, 2012; Palacios et al., 2013).
Manawa virus (MWAV) (Phenuiviridae: Phlebovirus), transmitted by ticks, known
in Pakistan (Hayes, Burney, 1981; Darwish et al., 1983b; Hoogstraal, 1985; Labuda, Nuttall,
2008).
Razdan virus (RAZV) (Phenuiviridae: Bandavirus), transmitted by ticks, known
in Armenia (Lvov, 1994; Labuda, Nuttall, 2008; Alkhovsky et al., 2013).
Uukuniemi virus (UUKV) (Phenuiviridae: Phlebovirus), transmitted by mosquitoes
and ticks, known in Azerbaijan (Gromashevsky, Nikimorov, 1973; Labuda, Nuttall, 2008;
Hubálek, Rudolf, 2012).
Reoviridae
Baku virus (BAKUV) (Reoviridae: Orbivirus), transmitted by ticks, known in Azerbaijan
and Turkmenistan (Lvov et al., 1971; Andreev et al., 1973; Gromashevsky, Nikimorov,
1973; Lvov, 1994; Labuda, Nuttall, 2008; Atkinson, Hewson, 2018).
Chenuda virus (CNUV) (Reoviridae: Orbivirus), transmitted by ticks, known
in Turkmenistan (Taylor et al., 1966b; Hoogstraal, 1985; Lvov, 1994; Belaganahalli et al.,
2015; Atkinson, Hewson, 2018).
Epizootic haemorrhagic disease virus (EHDV) (Reoviridae: Orbivirus), transmitted
by biting midges and possibly mosquitoes, known in Bahrain, Oman, Sudan and Turkey
(Al-Busaidy, Mellor, 1991a; Mellor et al., 2000; Mellor, 2001e; Temizel et al., 2009;
Wernery et al., 2013; Hubálek et al., 2014a).
Palyam virus (PALV) (Reoviridae: Orbivirus), transmitted by mosquitoes, known
in Sudan (Mohammad, Mellor, 1990; Mellor et al., 2000).
394
Rhabdoviridae
Barur virus (BARV) (Rhabdoviridae: Vesiculovirus), transmitted by ticks, known
in Somalia (Butenko et al., 1981; Labuda, Nuttall, 2008).
Malakal (MALV) (Rhabdoviridae: Ephemerovirus), transmitted by mosquitoes, known
in Sudan (Calisher et al., 1989; Blasdell et al., 2012b).
Obodhiang virus (OBOV) (Rhabdoviridae: Ephemerovirus), transmitted by mosquitoes,
known in Sudan (Calisher et al., 1989; Blasdell et al., 2012a).
Togaviridae
O'nyong-nyong virus (ONNV) (Togaviridae: Alphavirus), transmitted by mosquitoes,
known in Sudan (Salim, Porterfield, 1973; Woodall, 2001b; Ahmed et al., 2020).
Disscusion
The viruses which are associated with arthropods may be arranged in four ecological
groups: (1) Arthropod-borne viruses (arboviruses), (2) arthropod-transmitted animal viruses,
(3) arthropod viruses and (4) arthropod-transmitted plant viruses. The first two groups
include the viruses of medical and/or veterinary importance (Turell, 2019). Arboviruses
are the viruses which are biologically transmitted from one vertebrate host to another via
the bite of haematophagous arthropods, including biting midges, mosquitoes, sandflies or
ticks. These viruses replicate in both arthropod vectors and vertebrate hosts. Thus, the
viruses which are not transmitted by bite or merely mechanically transmitted by bite are
not among (true) arboviruses. The term “arbovirus” has no taxonomic importance, it is
a vernacular term that signifies a virus transmitted by an arthropod. Nearly all arboviral
infections are zoonotic (World Health Organization, 1967; Hart, 2001; Turell, 2019).
The World Health Organization (1967) provided nine criteria for considering a virus as
an arbovirus (five relating to the transmission cycle and four unrelated to the transmission
cycle). It seems that, based on the aforementioned definition of an arbovirus and the
nine criteria, different viruses may be grouped into four categories: (1) True arboviruses,
(2) probable arboviruses, (3) possible arboviruses and (4) most probably or definitely
not true arboviruses (World Health Organization, 1967; Hart, 2001). The World Health
Organization (1967) also mentioned four criteria for the recognition of a vector of an
arbovirus and classified them as suspected, probable and confirmed vectors based on those
criteria. Arboviruses are generally divided into two groups based on their pathogenicity
to humans: (1) Arthropod-borne viruses pathogenic to humans and (2) arboviruses not
pathogenic to humans (Hubálek, 2008).
Arthropod-transmitted animal viruses do not replicate in the arthropod vectors but do
so in vertebrate hosts and are mechanically transmitted (Turell, 2019). There are a few
important viral infections which arthropods mechanically play an important role in their
transmission and epidemiology, such as equine infectious anaemia, caused by the equine
infectious anaemia virus vectored by horseflies, stable flies and mosquitoes (Foil, Issel,
395
1991; Issel, Foil, 2015), lumpy skin disease, caused by the poxvirus lumpy skin disease
virus transmitted by stable flies, mosquitoes, ticks and Culicoides biting midges (Chihota
et al., 2003; Sprygin et al., 2019) and myxomatosis, caused by the Myxoma virus
(Poxviridae: Leporipoxvirus) transmitted by mosquitoes, fleas and horseflies (Jellison, 1959;
Krinsky, 1976; Fenner, 2001; Brugman et al., 2015).
Arthropod viruses replicate only in arthropods. They cannot cause disease
in vertebrates because they do not replicate in vertebrates, though they may be pathogenic
to the infected arthropod (Turell, 2019). These viruses are isolated only from arthropods,
including mosquito-only (mosquito-specific) flaviviruses (Cella et al., 2019) or those that
are pathogenic only to arthropods (Tinsley, 1979; Beckage et al., 1993). However, there
is no evidence for whether they are pathogenic to humans or domesticated animals and
biologically or mechanically transmitted to vertebrate hosts. Thus, they are not arboviruses
and are not of medical and veterinary significance, however they are important in view
of being a potential agent for biological control (Tinsley, 1979; Beckage et al., 1993)
or because of their impact on the biology of infected arthropods such as flight activity
or reproductivity, and their impact on the circulation of their related vector-borne pathogens
(Goenaga et al., 2015; Cella et al., 2019).
Finally, arthropod-transmitted plant viruses can be transmitted mechanically
or biologically to plants by some arthropods, including certain species of aphids,
leafhoppers, plant bugs and plant mites (Turell, 2019).
With the exception of one unclassified virus, the viruses treated in the present review are
members of 19 genera belonging to 14 families. The taxonomic placements of the viruses
are as follow. (1) Asfaviridae: Asfavirus - ASFV; (2) Flaviviridae: Flavivirus - BANV, BJV,
DENV, ITMV, JEV, KADV, KFDV, KSIV, LIV, RFV, TBEV, USUV, WNV, YFV, ZIKAV;
(3) Hantaviridae: Orthohantavirus - HTNV; (4) Herpesviridae: Varicellovirus - BHV; (5)
Nairoviridae: Orthonairovirus - AHV, ARTSV, CASV, CCHFV, DGKV, GERV, HAZV,
ISKV, NSDV, TDYN, ZIRV; (6) Orthomyxoviridae: Thogotovirus - BKNV, DHOV, QRFV,
THOV; (7) Paramyxoviridae: Morbillivirus - RPV; (8) Peribunyaviridae: Orthobunyavirus -
AINOV, AKAV, BAKV, BATV, NRIV, TAHV, SBV; (9) Phenuiviridae: Bandavirus - RAZV,
Phlebovirus - AMTV, BHAV, GAV, GFV, MWAV, RVFV, SFN-SV, UUKV; (10) Poxviridae:
Avipoxvirus - FPV, Capripoxvirus - LSDV, GPV, SPV; (11) Reoviridae: Orbivirus - AHSV,
BAKUV, BLUV, CNUV, EHDV, WMWV, PALV; (12) Retroviridae: Deltaretrovirus -
BLV, Lentivirus - EIAV; (13) Rhabdoviridae: Ephemerovirus - BEFV, MALV, OBOV,
Vesiculovirus - BARV, ISFV, Zamolirhabdovirus - ZARV; (14) Togaviridae: Alphavirus -
CHIKV, ONNV, SFV, SINV; unclassified virus: WANV.
In addition to various nonspecific signs and symptoms, such as fever, main clinical
syndromes associated with the arboviruses treated herein that are pathogenic to humans and
animals include: (1) Neurological maladies (meningitis, encephalitis, encephalomyelitis):
BHAV, JEV, SINV, TBEV, WNV (Hubálek et al., 2014a), (2) hemorrhagic disease: AHSV,
ASFV, CCHFV, DENV, RVFV (Hubálek et al., 2014a), (3) abortion and congenital disorders
396
such as arthrogryposis and hydranencephaly: AKAV, SBV (Hubálek et al., 2014a; Collins
et al., 2019; Asadolahizoj et al., 2021), (4) vesicular stomatitis: ISFV (Atkinson, Hewson,
2018) and (5) microcephaly: ZIKAV (Kassiri et al., 2020a).
The viral infections treated in the present review may be classified into four categories
based on their main vectors: (1) Biting midge-borne - AHSV, AKAV, BLUV, SBV, (2)
mosquito-borne - AMTV, BANV, BATV, BJV, CHIKV, DENV, JEV, MALV, NRIV, OBOV,
ONNV, PALV, RVFV, SFV, SINV, TAHV, USUV, WNV, YFV, ZIKAV, (3) sandfly-borne -
GFV, ISFV, SFS-NV and (4) tick-borne - AHV, ASFV, ARTSV, BAKUV, BARV, BHAV,
CCHFV, CASV, CNUV, DGKV, DHOV, GAV, GERV, HAZV, KADV, KSIV, LIV,
MWAV, NSDV, QRFV, RAZV, RFV, TDYV, THOV, TBEV, WMV, WANV, ZARV, ZIRV.
It is noteworthy that some other arthropods are involved or assumed to be involved in
the transmission of some viruses, including blackflies (Diptera: Simuliidae) - RVFV
(Bouloy, 2001), fleas - FPV, TBE (Federov et al., 1959; Sotnikova, Soldatov, 1964; Naumov,
Gutova, 1984; Della-Porta, 2001), horseflies - AHSV, BLV, EIAV, RPV, TBE (Krinsky,
1976; Foil, 1989), mites - FPV, HTNV, TBE (Naumov, Gutova, 1984; Della-Porta, 2001;
Houck et al., 2001; Xu, 2001; Sparagano et al., 2014), sheep head fly (Hydrotaea irritans)
- GPV, SPV (Kitching, Mellor, 1986), horn fly (Haematobia irritans) - LSDV (Kahana-
Sutin et al., 2017), face fly (Musca autumnalis) - BHV (Johnson et al., 1991) , house fly
(Musca domestica) - LSDV (Sprygin et al., 2018), sheep ked (Melophagus ovinus) - BLUV
(Gray, Bannister, 1961; Luedke et al., 1965), Musca (Biomyia) confiscata - LSDV (Weiss,
1968), stable fly (Stomoxys calcitrans) - ASFV, BHV, BLV, EIAV, FPV, LSDV, GPV, SPV
(Kitching, Mellor, 1986; Mellor et al., 1987; Della-Porta, 2001; Baldacchino et al., 2013)
and tropical bed bug (Cimex hemipterus) - CHIKV (Rao, 1964).
The role of arthropods in the transmission of different viruses and the epidemiology
of their infections is very disportionate and complicated, summarized as follows: (1) Some
arboviruses are mostly and biologically transmitted by certain arthropods. Other ways
of infection (generally defined as direct route or transmission) such as direct contact with
an infected person (or animal), contact with infected blood, body fluid and tissues, or via
the respiratory route and alimentary tract, do not have an important role in the epidemiology
of infection or their roles are uncertain. AHS is an example of these types of infections
(Mellor, 2001a). (2) While some arboviruses are mostly and biologically transmitted by
certain arthropods, other ways of direct transmission are also important in the epidemiology
of infection in animals or transmission to humans, such as CCHF (Saleem et al., 2020).
(3) There are a few arboviruses which are biologically transmitted by arthropods, but
it seems that the direct route of transmission is more significant in the epidemiology of the
disease, such as ASF (Beltrán-Alcrudo et al., 2017). (4) Exceptionally, some arthropods
mechanically have an important role in the transmission and epidemiology of a few viruses,
such as horseflies, mosquitoes and fleas in relation to myxomatosis (Krinsky, 1976; Bibikova,
1977), stable flies, mosquitoes, ticks and Culicoides biting midges in the case of lumpy
skin disease (Tuppurainen et al., 2015) and horseflies and the stable fly in EIAV infection
397
(Issel, 2001). (5) There are some viruses for which their major transmission route is direct.
Certain arthropods may be mechanically involved in transmission, although their role in
the epidemiology of disease is not important or uncertain such as horseflies in relation to
foot and mouth disease virus (Picornaviridae: Aphtovirus) and rinderpest virus (Krinsky,
1976). It is noteworthy that the late Professor M.P. Chumakov (1909-1993), a famous
Russian virologist, placed more emphasis on other possible ways of transmission (direct
route), even for well-defined and biologically transmitted viral infections (true arboviruses)
(World Health Organization, 1967), which shows the complex and complicated epidemiology
of viral (arboviral) infections. Moreover, the interrupted blood feeding of haematophagous
arthropods and their potential role in the mechanical transmission of infections should not
be neglected, even for the diseases caused by viruses for which biological transmission
is well and undoubtedly defined. That may explain some of the explosive outbreaks of
arboviral infections among vertebrate hosts that occur in just a few weeks (World Health
Organization, 1967). Finally, the role of each of the aforementioned factors (biological
or mechanical transmission via arthropods and/or direct route) associated with every disease
may be different in various foci; thus, the epidemiology of every infection and the role
of possible vectors should be extensively investigated in each focus. In view of the lack
of a specific vaccine or treatment for many viral infections, this basic knowledge will foster
better decisions about how and whether to control diseases by means of vector control
programs, sanitary procedures and/or health education.
Conclusion
The background, definitions and criteria presented herein clearly show that there is
not enough information about many viral (arboviral) infections in the region and available
information is very disportionate when considering countries or different epidemiological
aspects of infections. Even infections that are endemic and widespread in the region, for
which there is relatively more information, such as BEF, CCHF, SFN-S, SIN and WNF,
much more investigation is required, for example, there is little information about the
vectors of BEF in the region. On the other hand, there is little or no information about the
epidemiology of many viral infections and their vectors in the region, including BLU and
many RIDs and EIDs. Additionally, information on viruses in wildlife, which cause many
RIDs and EIDs, is very poor in comparison to information for humans and domesticated
animals. Last but not least, the records of many viral infections are based merely on
serological tests, which have their own limitations such as cross reactions; therefore,
isolation and genetic analysis of those viruses is necessary. The studies of the ecology
of vectors, as well as the epidemiology of related infections are necessary to provide
basic knowledge for vector control programs. This has been a very significant part of
integrated vector control in the One Health approach to decrease the burden of infections in
humans and domestic animals in concert with wildlife conservation. Moreover, expanding
interdisciplinary and international collaborations is necessary for fast detection, monitoring
398
and surveillance of viral infections and the vectors that cause them, in order to conduct
appropriate integrated vector and infection control programs.
Acknowledgements
The authors are grateful to Behzad Norouzi, Research Center of Health and Environment,
Guilan University of Medical Sciences, Rasht, for providing some literatures. Appreciation
is expressed to Professor Ahmad Ali Hanafi-Bojd, Department of Medical Entomology and
Vector Control, School of Public Health, Tehran University of Medical Sciences, Tehran,
for preparing the map of Iran. This research did not receive any specific grant from funding
agencies in the public, commercial or not-for-profit sectors. The authors declare that they
have no conflict of interest.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed
in this study.
References
Abazari M., Adham D., Saghafipour A., Taheri-Kharameh Z., Abbasi-Ghahramanloo A., Asadollahi J., Babaei
Pouya A., Moradi Asl E. 2022. Health beliefs and behaviors of livestock industry workers regarding
Crimean-Congo hemorrhagic fever in Northwest of Iran. BMC Health Services Research 22: 86.
Abbasi A., Moradi A.V. 2005. Six cases report of Crimean Congo hemorrhagic fever (CCHF) in Golestan Province
of Iran. Journal of Gorgan University of Medical Sciences 7: 87-89 (Persian with English abstract).
Abdigoudarzi M. 2016. Some new records of Culicoides species (Diptera: Ceratopogonidae) from Iran. Journal
of Arthropod-Borne Diseases 10: 474-482.
Abdul-Ghani R., Mahdy M.A.K., Alkubati S., Al-Mikhlafy A.A., Alhariri A., Das M., Dave K., Gil-Cuesta J. 2021.
Malaria and dengue in Hodeidah City, Yemen: high proportion of febrile outpatients with dengue or malaria,
Abu Elzein E.M.E., Tageldin M.H. 1985. The first out breaks of sheep bluetongue in Khartoum Province, Sudan.
Abu Elzein E.M.E. 1986. Recovery of bluetongue virus serogroup from sera collected for a serological
survey from apparently healthy cattle, from the Sudan. Journal of Hygiene 96: 529-533.
Abu Elzein E.M.E., Aitchison H., Al-Afaleq A.I., Al-Bashir A.M., Ibrahim A.O., Housawi F.M.T. 1998a. A study
on bluetongue virus infection in Saudi Arabia using sentinel ruminants. Onderstepoort Journal of Veterinary
Research 65: 243-251.
Abu Elzein E.M.E., Al-afaleq A.I., Mellor P.S., Al-Bashir A.M., Hassanien M.M. 1998b. Study of Akabane
infection in Saudi Arabia by the use of sentinel ruminants. Journal of Comparative Pathology 119: 473-478.
Abul-Eis E.S., Mohammad N.A., Wasein S.M. 2012. Crimean-Congo Hemorrhagic Fever in Iraq during 2010.
Iraqi Journal of Veterinary Medicine 36: 99-103.
Adeli E., Pourmahdi Borujeni M., Haji Hajikolaei M.R., Seifi Abad Shapouri M.R. 2017. Bovine Herpesvirus-1
in Khouzestan province in Iran: seroprevalence and risk factors. Iranian Journal of Ruminants Health
Adham D., Moradi-Asl E., Vatandoost H., Saghafipour A. 2019. Ecological niche modeling of West Nile virus
Adham D., Abazari M., Moradi-Asl E., Abbasi-Ghahramanloo A. 2021. Pattern of Crimean-Congo hemorrhagic
fever related high risk behaviors among Iranian butchers and its relation to perceived self-efficacy. BMC
399
Adler S., Theodor O. 1957. Transmission of disease agents by phlebotomine sand flies. Annual Review of
Afshar A., Tadjbakhsh H. 1970. Occurrence of precipitating antibodies to bovine herpes virus (infectious bovine
rhinotracheitis) in sera of farm animals and man in Iran. Journal of Comparative Pathology 80: 307-310.
Afshar A., Kayvanfar H. 1974. Occurrence of precipitating antibodies to bluetongue virus in sera of farm animals
Afshar A., Thomas F.C., Wright P.F., Shapiro J.L., Anderson J. 1989. Comparison of competitive ELISA, indirect
ELISA and standard AGID tests for detecting bluetongue virus antibodies in cattle and sheep. Veterinary
Afshar A. 1994. Bluetongue: laboratory diagnosis. Comparative Immunology, Microbiology, Infectious Diseases
Afzal M.F., Naqvi S.Q., Sultan M.A., Hanif A. 2015. Chikungunya fever among children presenting with
nonspecific febrile illness during an epidemic of dengue fever in Lahore, Pakistan. Merit Research Journal
of Medicine and Medical Sciences 3: 69-73.
Ahmad N., Khan T., Jamal S.M. 2020. A comprehensive study of dengue epidemics and persistence of anti-dengue
Ahmed A.E. 2015. Tropical diseases in Saudi Arabia. SOJ Immunology 3: 1-4.
Ahmed A., Dietrich I., LaBeaud A.D., Lindsay S.W., Musa A., Weaver S.C. 2020. Risks and challenges of
Ahi M.R., Pourmahdi-Borujeni M., Haji-Hajikolaei M.R., Seifi-Abad-Shapouri M.R. 2015. A serological survey
on antibodies against Akabane virus in sheep in southwest of Iran. Iranian Journal of Virology 9: 20-25.
Ahourai P., Gholami M.R., Ezzi A., Kargar R., Khedmati K., Aslani A., Rahmani F., Zarrin-Naal E. 1992. Bovine
congenital arthrogryposis and hydranencephaly outbreaks attributed to Akabane virus infection in Iran.
Archives of Razi Institute 42/43: 51-56.
Aijazi I., Al Shama F.M.A., Shandala Y., Varghese R.M. 2020. Crimean- Congo haemorrhagic fever presenting
with acute compartment syndrome of the extremities (think beyond normal infections). BMJ Case Reports
Akhoundi M., Parvizi P., Baghaei A., Depaquit J. 2012. The subgenus Adlerius Nitzulescu (Diptera, Psychodidae,
Akhtar S., Djallem N., Shad G., Thieme O. 1997. Bluetongue virus seropositivity in sheep flocks
in North West Frontier Province, Pakistan. Preventive Veterinary Medicine 29: 293-298.
Al-Abri S.S., Abdel-Hady D.M., Al Mahrooqi S.S., Al-Kindi H., Al-Jardani A.K., Al-Abaidani I.S. 2015.
Epidemiology of travel-associated infections in Oman 1999-2013: A retrospective analysis. Travel Medicine
Al-Abri S.S., Al Abaidani I., Fazlalipour M., Mostafavid E., Leblebicioglu H., Pshenichnaya N., Ziad A.
Memish Z.A., Hewson R., Petersen E., Mala P., Nhu Nguyen T.M., Malik M.R., Formenty P., Jeffries
R. 2017. Current status of Crimean-Congo haemorrhagic fever in the World Health Organization Eastern
Mediterranean Region: issues, challenges, and future directions. International Journal of Infectious Diseases
Al-Abri S.S., Hewson R., Al-Kindi H., Al-Abaidani I., Al-Jardani A., Al-Maani A., Almahrouqi S., Atkinson
B., Al-Wahaibi A., Al-Rawahi B., Bawikar S., Beeching N.J. 2019. Clinical and molecular epidemiology
of Crimean-Congo hemorrhagic fever in Oman. PLOS Neglected Tropical Diseases 13: 4. e0007100.
Alavi-Naini R., Moghtaderi A., Koohpayeh H.-R., Sharifi-Mood B., Naderi M., Metanat M., Izadi M.
2006. Crimean-Congo hemorrhagic fever in southeast of Iran. Journal of Infection 52: 378-382.
Alayed M.S., Qureshi M.A., Ahmed S., Alqahtani A.S., Al-qahtani A.M., Alshaybari K., Alshahrani M., Asaad
A.M. 2018. Seroprevalence and molecular study of Zika virus among asymptomatic pregnant mothers and
their newborns in the Najran region of southwest Saudi Arabia. Annals of Saudi Medicine 38: 408-412.
Albanese N., Brubo-Smiraglia C., Di Cuonzo G., Lavagninio A., Srihongse S. 1972. Isolation of Thogoto virus
from Rhipicephalus bursa ticks in western Sicily. Acta Virologica 16: 267.
400
Al-Barawary O.L.T. 2018. Serological study for detection of new emerging ectoparasites borne disease
(Schmallenberg Virus) in Duhok Province-Iraq. Assiut Veterinary Medical Journal 64: 39-42.
Al-Baroodi S.Y. 2021. Seroprevelance of Schmallenberg virus infection as emerging disease in cattle in Iraq. Iraqi
Al-Busaidy S.M., Mellor P.S. 1991a. Epidemiology of bluetongue and related orbiviruses in the Sultanate of
Al-Busaidy S.M., Mellor P.S. 1991b. Isolation and identification of arboviruses from the Sultanate of Oman.
Al-Dabal L.M., Rahimi Shahmirzadi M.R., Baderldin S., Abro A., Zaki A., Dessi Z., Al Eassa E., Khan G., Shuri
H., Alwan A.M. 2016 Crimean-Congo hemorrhagic fever in Dubai, United Arab Emirates, 2010: case
Al-Ghamdi G.M. 2014. Incidence of West Nile virus in Al-Ahsa, Saudi Arabia. International Journal of Virology
Alemian A., Khalesi B., Ebrahimi M.M., Masousi S. 2021. Efficacy evaluation of Razi Institute fowl pox vaccine
in laying hen pullet. Veterinary Researches, Biological Products 130: 6-14 (Persian with English abstract).
Alghazali K.A., Teoh B.-T., Loong S.-K., Sam S.-S., Che-Mat-Seri N.-A.-A., Samsudin N.-I., Yaacob C.-N.,
Azizan N.-S., Oo, A., Baharudin N.-A., Tan, K.-K., Abd-Jamil J., Nor’e S.-S., Khor C.-S., Johari J., Mahdy
M.A.K., AbuBakar S. 2019. Dengue outbreak during ongoing civil war, Taiz, Yemen. Emerging Infectious
Alfaresi M., Elkoush A. 2008. West Nile virus in the blood donors in UAE. Indian Journal of Medical
Alkan C., Alwassouf S., Piorkowski G., Bichaud L., Tezcan S., Dincer E., Ergunay K., Ozbel Y., Alten B.,
de Lamballerie X., Charrela R.N. 2015. Isolation, genetic characterization, and seroprevalence of Adana
virus, a novel Phlebovirus belonging to the Salehabad virus complex, in Turkey. Journal of Virology 89:
Alkan C., Moin Vaziri V., Ayhan N., Badakhshan M., Bichaud L., Rahbarian N., Javadian E., Alten B., de
Lamballerie X., Charrel R.N. 2017. Isolation and sequencing of Dashli virus, a novel Sicilian-like virus
in sandflies from Iran; genetic and phylogenetic evidence for the creation of one novel species within
the Phlebovirus genus in the Phenuiviridae family. PLOS Neglected Tropical Diseases 11: 12. e0005978.
Al-Khalifa M.S., Diab F.M., Khalil G.M. 2007. Man-threatening viruses isolated from ticks in Saudi Arabia.
Saudi Medical Journal 28: 1864-1867.
Alkhovsky S.V., Lvov D.K., Shchelkanov M.Y., Shchetinin A.M., Krasnoslobodtsev K.G., Deryabin P.G.,
Samokhvalov E.I., Botikov A.G., Zakaryan V.A. 2013. Molecular-genetic characterization of the Bhanja
virus (BHAV) and the Razdan virus (RAZV) (Bunyaviridae, Phlebovirus) isolated from the Ixodes ticks
Rhipicephalus bursa (Canestrini and Fanzago, 1878) and Dermacentor marginatus (Sulzer, 1776) in
transcaucasus. Voprosy Virusologii 58: 14-19 (Russian with English abstract).
Alkhovsky S.V., Lvov D.K., Shchelkanov M.Y., Shchetinin A.M., Deryabin P.G., Lvov D.N., Lvov S.S.,
Samokhvalov E.I., Gitelman A.K., Botikov A.G., Krasnoslobodtsev K.G. 2014a. Genetic characterization
of the Batken virus (BKNV) (Orthomyxoviridae, Thogotovirus) isolated from the Ixodidae ticks Hyalomma
marginatum Koch, 1844 and the mosquitoes Aedes caspius Pallas, 1771, as well as the Culex hortensis
Ficalbi, 1889 in the Central Asia. Voprosy Virusologii 59(2): 33-37 (Russian with English abstract).
Alkhovsky S.V., Lvov D.K., Shchelkanov M.Y., Shchetinin A.M., Deryabin P.G., Gitelman A.K., Botikov A.G.,
Samokhvalov E.I., Zakarian V.A. 2014b. Taxonomic status of the Artashat virus (ARTSV) (Bunyaviridae,
Nairovirus) isolated from the ticks Ornithodoros alactagalis Issaakjan, 1936 and O. verrucosus Olenev,
Sassuchin et Fenuk, 1934 (Argasidae Koch, 1844) collected in Transcaucasia. Voprosy Virusologii 59(3):
24-28 (Russian with English abstract).
Alkhovsky S.V., Lvov D.K., Shchelkanov M.Y., Shchetinin A.M., Deryabin P.G., Gitelman A.K., Aristova V.A.,
Botikov A.G. 2014c. Genetic characterization of the Wad Medani virus (WMV) (Reoviridae, Orbivirus),
isolated from the ticks Hyalomma asiaticum Schulze et Schlottke, 1930 (Ixodidae: Hyalomminae) in
401
Turkmenistan, Kazakhstan, and Armenia and from the ticks H. anatolicum Koch, 1844 in Tajikistan.
Voprosy Virusologii 59(4): 25-30 (Russian with English abstract).
Alkhovsky S.V., Lvov D.K., Shchelkanov M.Y., Shchetinin A.M., Deryabin P.G., Gitelman A.K., Botikov A.G.,
Samokhvalov E.I. 2014d. Complete genome characterization of the Kyzylagach virus (KYZV) (Togaviridae,
Alphavirus, Sindbis serogroup) isolated from mosquitoes Culex modestus Ficalbi, 1889 (Culicinae) collected
in a colony of herons (Ardeidae Leach, 1820) in Azerbaijan. Voprosy Virusologii 59(5): 27-31 (Russian
with English abstract).
Alkhovsky S.V., Lvov D.K., Shchetinin A.M., Deriabin P.G., Shchelkanov M.Y., Aristova V.A., Morozova T.N.,
Gitelman A.K., Palacios G.F., Kuhn J.H. 2017. Complete genome coding sequences of Artashat, Burana,
Caspiy, Chim, Geran, Tamdy, and Uzun-Agach viruses (Bunyavirales: Nairoviridae: Orthonairovirus).
Alkhutova L.M., Sadykov V.G., Ponirovsky E.N., Listovskaya E.K. 1981. Isolation of strains identical to Isfahan
virus from Hyalomma asiaticum ticks in Turkmenistan. Sborn. Nauch. Tr. Inst. Virus Im. Ivanov. Akad.
Med. Nauk SSSR. pp. 29-32 (Russian, English translation, NAMRU3-T1566).
Alkhutova L.M., Sadykov V.G. 1982. New dta on ecology of Isfahan virus. Sborn. Nauch. Tr. Inst. Virus Im.
Ivanov. Akad. Med. Nauk SSSR. pp. 144-147 (Russian, English translation, NAMRU3-T1665).
Almasi S., Bakhshesh M. 2019a. Antigenic variation of bovine ephemeral fever viruses isolated in Iran, 2012-
Almasi S., Bakhshesh M. 2019b. Laboratory production of hyperimmune serum against bovine ephemeral fever
virus isolated in Iran. Journal of Animal Research 32: 79-84 (Persian with English abstract).
Alnaeem A.A., Hemida M.G. (2019) Surveillance of the equine infectious anemia virus in eastern and central Saudi
Al-Nakib W., Lloyd G., El-Mekki A., Platt G., Beeson A., Southee T. 1984. Preliminary report on
arbovirus-antibody prevalence among patients in Kuwait: evidence of Congo/Crimean virus
infection. Transactions of the Royal Society of Tropical Medicine and Hygiene 78: 474-476.
Alqahtani A.S. 2020. Investigation of some zoonotic viruses at south western Saudi Arabia. American Journal of
Animimal and Veterinary Sciences 15: 108-112.
Alsaad K.M., Alautaish H.H.N., Alamery M.A.Y. 2017. Congenital arthrogryposis-hydranencephaly syndrome
caused by Akabane virus in newborn calves of Basrah Governorate, Iraq. Veterinary World 10: 1143-1148.
Al-Salihi K.A. 2014. Lumpy skin disease: Riview of literature. Mirror of Research in Veterinary Sciences and
Animals 3: 6-23.
Al-Salihi K.A., Al-Dabhawi A.H. 2019. Congenital abnormalities and arthrogryposis in newly born lambs in Al
Muthanna province Iraq. Suspicion of Akabane virus infection. Brazilian Journal of Veterinary Research
Al-Samadi M.M., Ali K.S. 2020. Evaluation of some hematological and serological changes in dengue patients
of Lahj-Yemen. Electronic Journal of University of Aden for Basic and Applied Sciences 1: 25-29.
Al-Tikriti S.K., Al-Ani F., Jurji F.J., Tantawi H., Al-Moslih M., Al-Janabi N., Mahmud M.I.A., Ai-Bana A., Habib
H., Al-Munthri H., Al-Janabi Sh., Al-Jawahry K., Yonan M., Hassan F., Simpson D.I.H. 1981. Congo/
Crimean haemorrhagic fever in Iraq. Bulletin of the World Health Organization 59: 85-90.
Al-Zadjali M., Al-hashim H., Al-Ghailani M., Balkhair A. 2013. A Case of Crimean-Congo hemorrhagic fever in
Alzahrani A.G., Al Shaiban H.M., Al Mazroa M.A., Al-Hayani O., MacNeil A., Rollin P.E., Memish Z.A. 2010.
Alkhurma hemorrhagic fever in humans, Najran, Saudi Arabia. Emerging Infectious Diseases 16: 1882-
Amin M., Zaim M., Edalat H., Basseri H.R., Yaghoobi-Ershadi M.R., Rezaei F., Azizi K., Salehi-Vaziri M., Ghane
M., Yousefi S., Dabaghmanesh S., Kheirandish S., Najafi M.E., Mohammadi J. 2020. Seroprevalence study
on West Nile virus (WNV) infection, a hidden viral disease in Fars Province, southern Iran. Journal of
402
Amini M., Hanafi-Bojd A.A., Asghari S., Chavshin A.R. 2019. The potential of West Nile virus transmission
regarding the environmental factors using geographic information system (GIS), West Azerbaijan Province,
Aminol-Achrafi T., Noraniyan A. 1966a. Allergy and hemorrhagic syndrome, the report of seven cases of
observation of purpura and allergic hemorrhagy. Journal of Faculty of Medicine and Farmacology,
University of Tabriz 5: 293-316 (Persian).
Aminol-Achrafi T., Noraniyan A. 1966b. The report of first observation of hemorrhagic fever in one region
located in vills of Azarbaijan-e-Sharqi. Journal of Faculty of Medicine and Farmacology, University of
Tabriz 6: 182-188 (Persian).
Anastos G. 1957. The Ticks, or Ixidides, of the USSR. Public Health Service Publication No. 548, USA.
Andayi F., Charrel R.N., Kieffer A., Richet H., Pastorino B., Leparc-Goffart I., Ahmed A.A., Carrat F., Flahault
A., de Lamballerie X. 2014. A sero-epidemiological study of arboviral fevers in Djibouti, horn of Africa.
Anderson E.C., Mellor P.S., Hamblin C. 1989. African horse sickness in Saudi Arabia. Veterinary Record 125:
Andreev V.P., Gromashevsky V.L., Veselovskaya O.V., Vasilev V.I., Shcherbina A.A. 1973. Isolation of Baku
virus in western Turkmenia. Sborn. Izuch. Trud. Ekol. Virus 1: 107-110 (Russian, English translation,
NAMRU3-T714).
Arab-Ameree M., Mirshafee M. 2006 Crimean-Congo hemorrhagic fever. Journal of Semnan University of Medical
Sciences (Koomesh) 7: 107-110 (Persian with English abstract).
Aradaib I.E., Erickson B.R., Karsany M.S., Khristova M.L., Elageb R.M., Mohamed M.E.H., Nichol S.T. 2011.
Multiple Crimean-Congo hemorrhagic fever virus strains are associated with disease outbreaks in Sudan,
Arata A.A. 1975. The Importance of Small Mammals in Public Health. In: Golley F.B., Petrusewicz K., Ryszkowski
L. (eds). Small Mammals: Their Productivity and Population Dynamics. International Biological Programme
5, Cambridge University Press. 349-359.
Ardalan M.R., Tubbs R.S., Chinikar S., Shoja M.M. 2006. Crimean-Congo haemorrhagic fever presenting as
thrombotic microangiopathy and acute renal failure. Nephrology Dialysis Transplantation 21: 2304-2307.
Ardoin A., Karimi Y. 1982. Spot of thrombocytopenia purpura in Iran in East Azerbaijan (1974 to 1975). Médecine
Tropicale 42: 319-326 (French).
Aristova V.A., Neronov V.M., Veselovskaya O.V., Lushchekina A.A., Kurbanov M. 1973. Investigation of Crimean
hemorrhagic fever natural foci in southeastern Turkmenia. Sborn. Izuch. Trud. Ekol. Virus 1: 115-118
(Russian, English translation, NAMRU3-T719).
Arsen'eva L.P. 1982 Natural focal zoonoses in Afghanistan (review of literature). Medical Parasitology and
Parasitic Diseases 51: 54-59 (Russian, English translation, NAMRU3-T1706).
Artemiev M.M. 1978. Sandflies (Diptera, Psychodidae, Phlebotominae) of Afghanistan. Malaria and Leishmania
Institute, Ministry of Public Health, Afghanistan, Kabul.
Asadolahizoj S., Jafari A., Jafari-Nozad A.M., Rasekh M., Sarani A., Bakhshi H. 2021. A systematic review on
the spread of Schmallenberg virus (SBV) in Iran and neighboring countries. New Findings in Veterinary
Asefi V. 1974. Etude Clinique de 60 patients atteints d'un syndrome infectieux et hemorragique en Azerbaijan
(Iran). Iranian Journal of Public Health 3: 140-146.
Asefi V. 1977. Arboviroses. Ferdowsi University Press, Publication No. 56, Mashhad.
Ashford, R.W. 2001. Phlebotomus fevers. In: Service M.W. (ed). Encyclopedia of Arthropod-Transmitted Infections
of Man and Domesticated Animals. CABI Publishing, Wallingford. 397-401.
Atkinson B., Marston D.A., Ellis R.J., Fooks A.R., Hewson R. 2015. Complete genomic sequence of Issyk-Kul
Atkinson B., Hewson R. 2018. Emerging arboviruses of clinical importance in Central Asia. Journal of General
Azari-Hamidian S., Norouzi B., Noorallahi A., Hanafi-Bojd A.A. 2018. Seasonal activity of adult mosquitoes
(Diptera: Culicidae) in a focus of dirofilariasis and West Nile infection in northern Iran. Journal of
403
Azari-Hamidian S., Norouzi B., Harbach R.E. 2019. A detailed review of the mosquitoes (Diptera:
Culicidae) of Iran and their medical and veterinary importance. Acta Tropica 194: 106-122.
Azari-Hamidian S., Abai M.R., Norouzi B. 2020. Mansonia uniformis (Diptera: Culicidae), a genus and species
new to southwestern Asia, with a review of its medical and veterinary importance. Zootaxa 4772: 385-395.
Azari-Hamidian S., Norouzi B., Maleki H. 2023. The checklist and distribution of sand flies (Diptera: Psychodidae)
of Guilan Province and their medical importance with a taxonomic note on the name Sergentomyia
Azkur A.K., Albayrak H., Risvanli A., Pestil Z., Ozan E., Yılmaz O., Tonbak S., Cavunt A., Kadi H.,
Macun H.C., Acar D., Özenç E., Alparsalan S., Bulut H. 2013. Antibodies to Schmallenberg
virus in domestic livestock in Turkey. Tropical Animal Health and Production 45: 1825-1828.
Azimi S.M., Kayvanfar H., Mahravani H. 2009. Bluetongue virus detection in sheep by RT-PCR. Journal of
Veterinary Research 64: 141-146 (Persian with English abstract).
Azmi K., Tirosh-Levy S., Manasrah M., Mizrahi R., Nasereddin A., Al-Jawabreh A., Ereqat S., Abdeen Z., Lustig
Y., Gelman B., Schvartz G., Steinman A. 2017. West Nile virus: seroprevalence in animals in Palestine and
Badakhshan M., Sadraei J., Moin-Vaziri V. 2011. Morphometric and morphological variation between two different
populations of Phlebotomus major s.l. from endemic and non-endemic foci of visceral leishmaniasis in
Bagheri M., Terenius O., Oshaghi M.A., Motazakker M., Asgari S., Dabiri F., Vatandoost H., Mohammadi
Bavanim M., Chavshin A.R. 2015. West Nile virus in mosquitoes of Iranian wetlands. Vector-Borne and
Bahari A., Gharekhani J., Zandieh M., Sadeghi-Nasab A., Akbarein H., Karimi-Makhsous A., Yavari M. 2013.
Serological study of bovine herpes virus type 1 in dairy herds of Hamedan province, Iran. Veterinary
Research Forum 4: 111-114.
Bakhshesh M., Ranjbar M.M., Almasi S. 2018. Immunoinformatic analysis of glycoprotein from
bovine ephemeral fever virus. Biomedical and Biotechnology Research Journal 2: 208-212.
Bakhshesh M., Otarod V., Fallah Mehrabadi M.H. 2020. Large-scale seroprevalence and risk factors
associated with Bluetongue virus in Iran. Preventive Veterinary Medicine 179:
104994.
Bakhshi H., Mousson L., Moutailler S., Vazeille M., Piorkowski G., Zakeri S., Raz A., de Lamballerie
X., Dinparast-Djadid N., Failloux A.-B. 2020. Detection of arboviruses in mosquitoes: evidence
of circulation of chikungunya virus in Iran. PLOS Neglected Tropical Diseases 14: 6. e0008135.
Bakhshi H., Beck C., Lecollinet S., Monier M., Mousson L., Zakeri S., Raz A., Arzamani K., Nourani L.,
Dinparast-Djadid N., Failloux A.-B. 2021. Serological evidence of West Nile virus infection among
birds and horses in some geographical locations of Iran. Veterinary Medicine and Science 7: 204-209.
Bakhshi H., Shirazitabar S.F., Khajehmohammadi M., Moallem S.M., Arzamani K., Jafari A. 2022. Sindbis virus
in Iran and adjacent countries - a systematic review. Journal of Isfahan Medical School 40: 602-610.
Baldacchino F., Muenworn V., Desquesnes M., Desoli F., Theeraphap Charoenviriyaphap T., Duvallet, G.
2013. Transmission of pathogens by Stomoxys flies (Diptera, Muscidae): a review. Parasite 20: 26.
Baniasadi V., Salehi-Vaziri M., Jalali T., Azad-Manjiri S., Mohammadi T., Khakifirouz S., Fazlalipour
M. 2016. An imported case of Dengue fever in Iran, 2015. Iranian Journal of Virology 10: 31-34.
Barakat A.M., Smura T., Kuivanen S., Huhtamo E., Kurkela S., Putkuri N., Hasony H.J., Al-Hello H., Vapalahti
O. 2016. The presence and seroprevalence of arthropod-borne viruses in Nasiriyah Governorate,
404
southern Iraq: a cross-sectional study. American Journal of Tropical Medicine and Hygiene 94: 794-799.
Barnett H.C., Suyemoto W. 1961. Field studies on sandfly fever and Kala-Azar in Pakistan, in Iran, and in
Baltistan (Little Tibet) Kashmir. Transactions of the New York Academy of Sciences, Ser. II 23: 609-617.
Barrett A.D.T. 2001. Japanese encephalitis. In: M.W. Service M.W. (ed). Encyclopedia of Arthropod-Transmitted
Infections of Man and Domesticated Animals. CABI Publishing, Wallingford. 239-246.
Baskerville A., Satti A.G.O., Murphy F.A., Simpson D.I.H. 1981. Congo-Crimean haemorrhagic fever in Dubai:
Beckage N.E., Thompson S.N., Federici, B.A. 1993. Parasites and Pathogens of Insects. Volume 1: Parasites,
Volume 2: Pathogens. Academic Press, San Diego.
Begum F., Wissemen C.L., Traub R. 1970a. Tick-borne viruses of west Pakistan. I. Isolation
and general characteristics. American Journal of Epidemiology
92:
180-191.
Begum F., Wissemen C.L., Casals J. 1970b. Tick-borne viruses of west Pakistan. II. Hazara virus, a new
agent isolated from Ixodes redikorzevi ticks from the Kaghan Valley, W. Pakistan. American Journal
Begum F., Wissemen C.L., Casals J. 1970c. Tick-borne viruses of west Pakistan. III. Dera Ghazi Khan virus, a
new agent isolated from Hyalomma dromedarii ticks in the D. G. Khan District of West Pakistan. American
Begum F., Wissemen C.L., Casals J. 1970d. Tick-borne viruses of west Pakistan. IV. Viruses similar to, or identical
with, Crimean hemorrhagic fever (Congo-Semunya), Wad Medani and Pak Argas 461 isolated from ticks
of the Changa Manga forest, Lahore District, and of Hunza, Gilgit agency, W. Pakistan. American Journal
Belaganahalli M.N., Maan S., Maan N.S., Brownlie J., Tesh R., Attoui H., Mertens P.P.C. 2015. Genetic
Beltrán-Alcrudo D., Arias M., Gallardo C., Kramer S., Penrith M.L. 2017. African Swine Fever: Detection and
Diagnosis - A Manual for Veterinarians. FAO Animal Production and Health Manual No. 19. Food and
Agriculture Organization of the United Nations (FAO), Rome.
Benelli G., Romano D. 2017. Mosquito vectors of Zika virus. Entomologia Generalis 36: 309-318.
Bennett R.S., Gresko A.K., Murphy B.R., Whitehead S.S. 2011. Tahyna virus genetics, infectivity, and
Bente D.A., Forrester N.L., Watts D.M., Mcauley A.J., Whitehouse C.A., Bray, M. 2013. Crimean-Congo
hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral
Bi Z., Formently P.B.H., Roth C.E. 2008. Hantavirus Infection: a review and global update. Journal of Infection
Bibikova V.A. 1977. Contemporary views on the interrelationships between fleas and the
pathogens of human and animal diseases. Annual Review of Entomology 22: 23-32.
Blair P.W., Kuhn J.H., Pecor D.B., Apanaskevich D.A., Kortepeter M.G., Cardile A.P., Ramos, A.P.,
Keshtkar-Jahromi M. 2019. An emerging biothreat: Crimean-Congo hemorrhagic fever virus in
southern and western Asia. American Journal of Tropical Medicine and Hygiene 100: 16-23.
Blasdell K.R., Voysey R., Bulach D., Joubert D.A., Tesh R.B., Boyle D.B., Walker P.J. 2012a. Kotonkan and
Obodhiang viruses: African ephemeroviruses with large and complex genomes. Virology 425: 143-153.
Blasdell K.R., Voysey R., Bulach D.M., Trinidad L., Tesh R.B., Boyle D.B., Walker P.J. 2012b. Malakal virus from
Africa and Kimberley virus from Australia are geographic variants of a widely distributed ephemerovirus.
405
Body M., Rawahi A.A., Hussain M.H., Lamki K.A., Habsy S.A., Almaawali M., Alrawahi Q.A. 2011. Sero-survey
of equine infectious anemia in the sultanate of Oman during 2007-2009. Pakistan Veterinary Journal 31:
235-238
Body M.H.H., Alrawahi A.H., Hussain H.M., Ahmed M.S., Alhabsi S.S., Al-Maklady S., Al-
Maewaly M., Rajamony S. 2016. Cross-sectional survey of Crimean-Congo hemorrhagic fever
virus in the Sultanate of Oman. Journal of Veterinary Medicine and Animal Health 8: 44-49.
Boinas F.S., Hutchings G.H., Dixon L.K., Wilkinson P.J. 2004. Characterization of pathogenic and non-pathogenic
African swine fever virus isolates from Ornithodoros erraticus inhabiting pig premises in Portugal. Journal
Boinas F.S., Wilson A.J., Hutchings G.H., Martins C., Dixon L.J. 2011. The persistence of African swine fever
virus in field-infected Ornithodoros erraticus during the ASF endemic period in Portugal. PLOS One 6:
Bokaie S., Mstafavi E., Haghdoost A.A., Keyvanfar H., Gooya M.M., Meshkat M., Davari A., Chinikar S. 2008.
Crimean Congo hemorrhagic fever in northeast of Iran. Journal of Animal and Veterinary Advances 7:
354-361.
Boorman J. 1989. Culicoides (Diptera: Ceratopogonidae) of the Arabian Peninsula with notes on their medical
and veterinary importance. Fauna of Saudi Arabia 10: 160-224.
Borkent A., Dominiak P. 2020. Catalog of the biting midges of the world (Diptera: Ceratopogonidae). Zootaxa
Bouloy M. 2001. Rift Valley fever virus. In: Service M.W. (ed). Encyclopedia of Arthropod-Transmitted Infections
of Man and Domesticated Animals. CABI Publishing, Wallingford. 426-434.
Bowen M.D., Trappier S.G., Sanchez A.J., Meyer R.F., Goldsmith C.S., Zaki S.R., Dunster L.M., Peters C.J.,
Ksiazek T.G., Nichol S.T. 2001. A reassortant bunyavirus isolated from acute hemorrhagic fever cases in
Braack L., Gouveia de Almeida A.P., Cornel A.J., Swanepoel R., de Jager, C. 2018. Mosquito-borne
arboviruses of African origin: review of key viruses and vectors. Parasites and Vectors 11: 29.
Brugman V.A., Hernández-Triana L.M., Prosser S.W.J., Weland C., Westcott D.G., Fooks A.R., Johnson N.
2015. Molecular species identification, host preference and detection of myxoma virus in the Anopheles
maculipennis complex (Diptera: Culicidae) in southern England, UK. Parasites and Vectors 8: 421.
Bryan J.P., Iqbal M., Ksiazek T.G., Ahmed A., Duncan J.F., Awan B., Krieg R., Riaz M., Leduc J.W., Nabi S.,
Shuaib Qureshi M., Malik I.A., Legters L.J. 1996. Prevalence of sand fly fever, West Nile, Crimean-Congo
hemorrhagic fever, and leptospirosis antibodies in Pakistani military personnel. Military Medicine 161,
Bryant J.E., Crabtree M.B., Nam V.S., Yen N.T., Duc H.M., Miller B.R. 2005. Isolation of arboviruses from
mosquitoes collected in northern Vietnam. American Journal of Tropical Medicine and Hygiene 73: 470-
Burgu I., Alkan F., Karaoglu T., Bilge-Dagalp S., Can-Sahna K., Gungor B., Demir B. 2005. Control and
eradication programme of enzootic bovine leucosis (EBL) from selected dairy herds in Turkey. Deutsche
Tierarztliche Wochenschrift 112: 271-274.
Butenko A.M., Gromashevsky V.L., L’vov D.K., Popov V.F. 1981. First isolations of Barur virus
(Rhabdoviridae) from ticks (Acari: Ixodidae) in Africa. Journal of Medical Entomology 18: 232-234.
Cahill K.M. 1971. Studies in Somalia. Transactions of the Royal Society of Tropical Medicine and Hygiene 65:
Camp J.V., Karuvantevida N., Chouhna H., Safi E., Shah J.N., Nowotny N. 2019. Mosquito biodiversity
and mosquito-borne viruses in the United Arab Emirates. Parasites and Vectors 12:
153.
Camp J.V., Kannan D.O., Osman B.M., Shah M.S., Howarth B., Khafaga T., Weidinger P., Karuvantevida
N., Kolodziejek J., Mazrooei H., Wolf N., Loney T., Nowotny N. 2020. Crimean-Congo hemorrhagic
406
fever virus endemicity in United Arab Emirates, 2019. Emerging Infectious Diseases 26: 1019-1021.
Calisher C.H., Karabatsos N., Zeller H., Digoutte J.-P., Tesh R.B., Shope R.E., Travassos da Rosa A.P.A., St.
George T.D. 1989. Antigenic relationships among rhabdoviruses from vertebrates and hematophagous
Caracappa S., Torina A., Guercio A., Vitale F., Calabro A., Purpari G., Ferrantelli V., Vitale M., Mellor P.S. 2003.
Identification of a novel bluetongue virus vector species of Culicoides in Sicily. Veterinary Record 153:
Carletti F., Castilletti C., Di Caro A., Capobianchi M.R., Nisii C., Suter F., Rizzi M., Tebaldi A., Goglio A., Tosi
C.P., Ippolito G. 2010. Alkhurma hemorrhagic fever in travelers returning from Egypt, 2010. Emerging
Carpenter S., Mellor P.S., Fall A.G., Garros C., Venter G.J. 2017. African horse sickness virus:
history, transmission, and current status. Annual Review of Entomology 62: 343-358.
Casals J., Tignor G.H. 1980. The Nairovirus genus: serological relationships. Intervirology 14: 144-147.
Cecaro M., Isolani L., Cuteri, V. 2013. European monitoring plans for the management of outbreak
of Crimean Congo haemorrhagic fever (CCHF). Occupational Medicine, Health Affairs 1: 123.
Cella E., Benvenuto D., Donati D., Garilli F., Angeletti S., Pascarella S., Ciccozzi M. 2019. Phylogeny of Culex
theileri virus flavivirus in Spain, Myanmar, Portugal and Turkey. Asian Pacific Journal of Tropical Medicine
Champour M., Mohammadi G.R., Chinikar S., Razmi G.R., Shah-Hosseini N., Khakifirouz S., Mostafavi E., Jalali
T. 2014. Seroepidemiology of Crimean-Congo hemorrhagic fever virus in one-humped camels (Camelus
dromedarius) population in northeast of Iran. Journal of Vector Borne Diseases 51: 62-65.
Charrel R.N., Zaki A.M., Fakeeh M., Yousef A.I., De Chesse R., Attoui H., de Lamballerie X. 2005. Low diversity
of Alkhurma hemorrhagic fever virus, Saudi Arabia, 1994-1999. Emerging Infectious Diseases 11: 683-688.
Charrel R.N., Zaki A.M., Fagbo S., de Lamballerie X. 2006a. Alkhurma hemorrhagic fever virus is an emerging
Charrel R.N., Izri A., Temmam S., de Lamballerie X., Parola P. 2006b. Toscana virus RNA in Sergentomyia minuta
Charrel R.N., Fagbo S., Moureau G., Alqahtani M.H., Temmam S., de Lamballerie X. 2007. Alkhurma
hemorrhagic fever virus in Ornithodoros savignyi ticks. Emerging Infectious Diseases 13: 153-155.
Charrel R.N., Moureau G., Tammam S., Izri A., Marty P., Parola P., da Rosa A.T., Tesh R.B., de Lamballerie
X. 2009. Massilia virus, a novel Phlebovirus (Bunyaviridae) isolated from sandflies in the Mediterranean.
Chatterjee A., Bakshi S., Sarkar S.N., Mitra J., Chowdhury S. 2016. Bovine herpes virus-1 and its infection in
India-A review. Indian Journal of Animal Health 55: 21-40.
Chihota C.M., Rennie L.F., Kitching R.P., Mellor P.S. 2001. Mechanical transmission of lumpy skin
disease virus by Aedes aegypti (Diptera: Culicidae). Epidemiology, Infection 126: 317-321.
Chihota C.M., Rennie L.F., Kitching R.P., Mellor P.S. 2003. Attempted mechanical transmission of
lumpy skin disease virus by biting insects. Medical and Veterinary Entomology 17: 294-300.
Chinikar S., Fayaz A., Mirahmadi R., Mazaheri V., Mathiot C., Saron M.F. 2002. The specific serological
investigation of suspected humans and domestic animals to have Crimean-Congo hemorrhagic fever in
various parts of Iran using ELISA techniques. Hakim Research Journal 4: 294-300 (Persian with English
abstract).
Chinikar S. 2003. Seroepidemiology of Crimean-Congo haemorrhagic fever in human and domestic animals in Iran
by analyzing the quantities of specific IGM and IGG against the virus of the disease by ELISA method.
Journal of Veterinary Organization 3: 69-73 (Persian with English abstract).
407
Chinikar S., Persson S.M., Johansson M., Bladh L., Goya M., Houshmand B., Mirazimi A., Plyusnin A., Lundkvist
A., Nilsson M. 2004. Genetic analysis of Crimean-Congo hemorrhagic fever virus in Iran. Journal of
Chinikar S., Mazaheri V., Mirahmadi R., Nabeth P., Saron M.F, Salehi P., Hosseini N., Bouloy M., Mirazimi A.,
Lundkvist A., Nilsson M., Mehrabi-tavana A. 2005. A serological survey in suspected human patients of
Crimean-Congo hemorrhagic fever in Iran by determination of IGM-specific ELISA method during 2000
- 2004. Archives of Iranian Medicine 8: 52-55.
Chinikar S., Goya M.M., Shirzadi M.R., Ghiasi S.M., Mirahmadi R., Haeri A., Moradi M., Afzali N.,
Rahpeyma M., Zeinali M., Meshkat M. 2008. Surveillance and laboratory detection system of
Crimean-Congo haemorrhagic fever in Iran. Transboundary and Emerging Diseases 55: 200-204.
Chinikar S., Ghiasi S.M., Ghalyanchi-Langeroudi A., Goya M.M., Shirzadi M.R., Zeinali M., Haeri A. 2009. An
overview of Crimean-Congo hemorrhagic fever in Iran. Iranian Journal of Microbiology 1: 7-12.
Chinikar S., Ghiasi S.M., Moradi M., Goya M.M., Shirzadi M.R., Zeinali M., Meshkat M., Bouloy M. 2010a.
Geographical distribution and surveillance of Crimean-Congo hemorrhagic fever in Iran. Vector-Borne and
Chinikar S., Ghiasi S., Hewson R., Moradi M., Haeri A. 2010b. Crimean-Congo hemorrhagic fever in Iran and
Chinikar S., Ghiasi S.M., Moradi M., Goya M.M., Shirzadi M.R., Zeinali M., Mostafavi E., Pourahmad M., Haeri
A. 2010c. Phylogenetic analysis in a recent controlled outbreak of Crimean-Congo haemorrhagic fever in the
Chinikar S., Ghiasi S.M., Naddaf S., Piazak N., Moradi M., Razavi M.R., Afzali N., Haeri A., Mostafavizadeh
K., Ataei B., Khalififard-Brojeni M., Husseini S.M., Bouloy M. 2012a. Serological evaluation of Crimean-
Congo hemorrhagic fever in humans with high-risk professions living in enzootic regions of Isfahan
Province of Iran and genetic analysis of circulating strains. Vector Borne and Zoonotic Diseases 12:
Chinikar S., Moghadam A.H., Parizadeh S.J., Moradi M., Bayat N., Zeinali M., Mostafavi E. 2012b.
Seroepidemiology of Crimean-Congo hemorrhagic fever in slaughterhouse workers in north eastern Iran.
Iranian Journal of Public Health 41: 72-77.
Chinikar S., Shayesteh M., Khakifirouz S., Jalali T., Rasi Varaie F.S., Rafigh M., Mostafavi E., Shah-Hosseini
N. 2013a. Nosocomial infection of Crimean-Congo haemorrhagic fever in eastern Iran: case report. Travel
Chinikar S., Shah-Hosseini N., Bouzari S., Jalali T., Shokrgozar M.A., Mostafavi E. 2013b. New circulating
genomic variant of Crimean-Congo hemorrhagic fever virus in Iran. Archives of Virology 158: 1085-1088.
Chinikar S., Javadi A.A., Hajiannia A., Ataei B., Jalali T., Khakifirouz S., Nowotny N., Schmidt-Chanasit J.,
Shahhosseini N. 2014. First evidence of Hantavirus in central Iran as an emerging viral disease. Advances
Chinikar S., Shah-Hosseini N., Bouzari S., Shokrgozar M.A., Mostafavi E., Jalali T., Khakifirouz S., Groschup
M.H., Niedrig M. 2016a. Assessment of recombination in the s-segment genome of Crimean-Congo
hemorrhagic fever virus in Iran. Journal of Arthropod-Borne Diseases 10: 12-23.
Chinikar S., Bouzari S., Shokrgozar M.A., Mostafavi E., Jalali T., Khakifirouz S., Nowotny N., Fooks A.R.,
Shah-Hosseini N. 2016b. Genetic diversity of Crimean Congo hemorrhagic fever virus strains from Iran.
Journal of Arthropod-Borne Diseases 10: 127-140.
Chitimia-Dobler L., Lemhöfer G., Król N., Bestehorn M., Dobler G., Pfeffer, M. 2019. Repeated isolation of
tick-borne encephalitis virus from adult Dermacentor reticulatus ticks in an endemic area in Germany.
Chumakov M.P., Ismailova S.T., Rubin S.G., Smirnova S.E., Zgurskaya G.N., Khankishiev A.Sh., Berezin V.V.,
Solovey E.A. 1970. Detection of Crimean hemorrhagic fever foci in Azerbaijan SSR from results from
serological investigations of domestic animals. Trudy Inst. Polio. Virus. Entsef. Akad. Med. Nauk SSSR
18: 120-122 (Russian, English translation, NAMRU3-T941).
408
Chumakov M.P., Smirnova S.E. 1972. Detection of antibodies to CHF virus in wild and domestic animal blood
sera from Iran and Africa. Tezisy 17. Nauchn. Sess. Inst. Posvyashch. Aktual. Probl. Virus Profilakt. Virus.
Zabolev. Moscow, Ocrober 1972, pp. 367-376 (Russian, English translation, NAMRU3-T1072).
Chunikhin S.P., Karaseva P.S. 1971. Study of Bhanja virus foci in Central Asia. Vop. Med. Virus 11: 128-131
(Russian, English translation, NAMRU3-T1323).
Ciccozzi M., Lo Presti A., Cella E., Giovanetti M., Lai A., El-Sawaf G., Faggioni G., Vescio F., Al Ameri R.,
De Santis R., Helaly G., Pomponi A., Metwally D., Fantini M., Qadif H., Zehender G., Lista F., Rezza
G. 2014. Phylogeny of dengue and chikungunya viruses in Al Hudayda governorate, Yemen. Infection,
Collins A.B., Doherty M.L., Barrett D.G., Mee J.F. 2019. Schmallenberg virus: a systematic international
literature review (2011-2019) from an Irish perspective. Irish Veterinary Journal 72: 9 .
Contigiani M.S., Diaz L.A., Tauro L.B. 2017. Bunyaviruses. In: Marcondes C.B. (ed). Arthropod Borne Diseases.
Springer, Switzerland. 137-154.
Converse J.D., Hoogstraal H., Moussa M.I., Stek M.Jr., Kaiser M.N. 1974. Bahig virus (Tete Group) in naturally-
and transovarially-infected Hyalomma marginatum ticks from Egypt and Italy. Archiv fur die Gesamte
Converse J.D., Moussa, M.I. 1982. Quaranfil virus from Hyalomma dromedarii (Acari: Ixodoidea)
collected in Kuwait, Iraq and Yemen. Journal of Medical Entomology 19: 209-210.
Conway M.J., Colpitts T.M., Fikrig, E. 2014. Role of the vector in arbovirus transmission. Annual Review of
Dagalp S.B., Dik B., Dogan F., Aligholipour Farzani T., Ataseven V.S., Acar G., Sahinkesen I., Ozkul
A. 2021. Akabane virus infection in Eastern Mediterranean Region in Turkey: Culicoides
(Diptera: Ceratopogonidae) as a possible vector. Tropical Animal Health and Production 53: 231.
Dandawate C.N., Shah K.V., D'lima L.V. 1970. Wanowrie virus: a new arbovirus isolated from Hyalomma
marginatum. Indian Journal of Medical Research 58: 985-989.
Dargham S.R., Al-Sadeq D.W., Yassine H.M., Ahmed M., Kunhipurayil H., Humphrey J.M., Abu-Raddad L.J.,
Nasrallah G.K. 2021. Seroprevalence of West Nile virus among healthy blood donors from different
national populations residing in Qatar. International Journal of Infectious Diseases 103: 502-506.
Darwish M.A., Imam I.Z., Omar F.M. 1976. Complement-fixing antibodies against Abu Hammad and Abu Mina
viruses in mammalian sera from Egypt. Journal of the Egyptian Public Health Association 51: 51-54.
Darwish M.A., Hoogstraal H., Roberts T.J., Ahmed I.P., Omar F. 1983a. A sero-epidemiological survey for certain
arboviruses (Togaviridae) in Pakistan. Transactions of the Royal Society of Tropical Medicine and Hygiene
Darwish M.A., Hoogstraal H., Roberts T.J., Ghazi R., Amer T. 1983b. A sero-epidemiological survey for
Bunyaviridae and certain other arboviruses in Pakistan. Transactions of the Royal Society of Tropical
Davis F.G. 1997. Nairobi sheep disease. Parassitologia 39: 95-98.
DeCarlo C., Omar A.H., Haroun M.I., Bigler L., Bin Rais M.N., Abu J., Omar A.R., Mohammad H.O. 2017.
Potential reservoir and associated factors for West Nile virus in three distinct climatological zones. Vector-
Dedkov V.G., Dolgova A.S., Safonova M.V., Samoilov A.E., Belova O.A., Kholodilov I.S., Matsvay
A.D., Speranskaya A.S., Khafizov K., Karganova G.G. 2021. Isolation and characterization of Wad
Medani virus obtained in the tuva Republic of Russia. Ticks and Tick-Borne Diseases 12: 101612.
Dehghani R., Amiri M. 2017. Is Iran threated by Zika virus? International Journal of Epidemiologic Research
4: 91-93.
Dehghani R., Kassiri H., Kasiri N., Dehghani M. 2020. A review on epidemiology and ecology of West Nile fever:
409
Dehghani R., Kassiri H., Khodkar I., Karami S. 2021. A comprehensive overview on sandfly fever. Journal of
Dehghan Rahimabadi P., Raoofi A., Gorjidooz M., Mardjanmehr S.H., Masoudifard M. 2020. Detection of
Akabane virus with clinical and necropsy findings in suckling calves in Tehran suburb. Journal of Veterinary
Microbiology 16: 33-42 (Persian with English abstract).
Della-Porta A.J. 2001. Fowl pox. In: Service M.W. (ed). Encyclopedia of Arthropod-Transmitted Infections of
Man and Domesticated Animals. CABI Publishing, Wallingford. 187-190.
de la Fuente J., Estrada-Pena A., Venzal J.M., Kocan K.M., Sonenshine D.E. 2008. Overview: Ticks as
vectors of pathogens that cause disease in humans and animals. Frontiers in Bioscience 13: 6938-6946.
Dennis S.J., Meyers A.E., Hitzeroth I.I., Rybicki E.P. 2019. African horse sickness: a review of current
Depaquit J., Grandadam M., Fouque F., Andry P.E., Peyrefitte C. 2010. Arthropod-borne viruses transmitted by
phlebotomine sandflies in Europe: a review. Eurosurveillance 15(10): pii=19507.
Depoortere E., Kavle J., Keus K., Zeller H., Murri S., Legros D. 2004. Outbreak of West Nile virus causing severe
neurological involvement in children, Nuba Mountains, Sudan, 2002. Tropical Medicine, International
De Regge N., Deblauwe I., De Deken R., Vantieghem P., Madder M., Geysen D., Smeets F., Losson B., van den
Berg T., Cay A.B. 2012. Detection of Schmallenberg virus in different Culicoides spp. by real-time RT-
Dhanda V., Thenmozhi V., Kumar N.P., Hiriyan J., Arunachalam N., Balasubramanian A., Ilango A., Gajanana
A. 1997. Virus isolation from wild-caught mosquitoes during a Japanese encephalitis outbreak in Kerala
in 1996. Indian Journal of Medical Research 106: 4-6.
Diallo M., Thonnon J., Traore-Lamizana M., Fontenille D. 1999. Vectors of Chikungunya virus in Senegal:
current data and transmission cycles. American Journal of Tropical Medicine and Hygiene 60: 281-286.
Diallo D., Sall A.A., Buenemann M., Chen R., Faye O., Diagne C.T., Faye O., Ba Y., Dia I., Watts D.,
Weaver S.C., Hanley K.A., Diallo M. 2012. Landscape ecology of sylvatic Chikungunya virus
and mosquito vectors in southeastern Senegal. PLOS Neglected Tropical Diseases 6: 6. e1649.
Dilcher M., Faye O., Faye O., Weber F., Koch A., Chinikar S., Manfred Weidmann M., Alpha Sall A.
2015. Zahedan rhabdovirus, a novel virus detected in ticks from Iran. Virology Journal: 12: 183.
Dixon L.K., Sun H., Roberts H. 2019. African swine fever. Antiviral Research 165: 34-41.
Dominiak P., Alwin A. 2013. Five new species and new records of biting midges of the genus
Dasyhelea Kieffer from the Near East (Diptera: Ceratopogonidae). Zootaxa 3683: 133-144.
Doosti S., Yaghoobi-Ershadi M.R., Schaffner F., Moosa-Kazemi S.H., Akbarzadeh K., Gooya M.M., Vatandoost
H., Shirzadi M.R., Mosta-Favi E. 2016. Mosquito surveillance and the first record of the invasive mosquito
species Aedes (Stegomyia) albopictus (Skuse) (Diptera: Culicidae) in Southern Iran. Iranian Journal of
Public Health 45: 1064-1073.
Düzlü Ö., İnci A., Yıldırım A., Doğanay M., Özbel Y., Aksoy S. 2020. Vector-borne zoonotic
diseases in Turkey: rising threats on public health. Türkiye Parazitoloji Dergisi 44: 168-175.
Ebadiazar F., Esmaeili R.A., Zohoori A. 2011. Epidemiological survey of Crimean Congo hemorrhagic fever in
Khorasan Razavi (2009). Medical Science Journal of Islamic Azad University Tehran Medical Branch 21:
61-66 (Persian with English abstract).
Ebrahimi M.M., Shahsavandi S., Masoudi S., Ghodsian N., Hashemi A., Hablalvarid M.H., Hatami A.R. 2012.
Development of a multiplex polymerase chain reaction for differential diagnosis of canary pox virus.
Iranian Journal of Virology 6: 19-23.
410
Elata A.T., Karsany M.S., Elageb R.M., Hussain M.A., Eltom K.H., Elbashir M.I., Aradaib I.E. 2011. A nosocomial
transmission of crimean-congo hemorrhagic fever to an attending physician in north kordufan, Sudan.
El-Azazy O.M., Scrimgeour E.M. 1997. Crimean-Congo haemorrhagic fever virus infection in the western province
of Saudi Arabia. Transactions of the Royal Society of Tropical Medicine and Hygiene 91: 275-278.
Elbers A.R.W., Meiswinkel R., van Weezep E., Sloet van Oldruitenborgh-Oosterbaan M.M., Kooi E.A. 2013.
Schmallenberg virus in Culicoides spp. biting midges, the Netherlands, 2011. Emerging Infectious Diseases
Elyan D.S., Moustafa L., Noormal B., Jacobs J.S., Aziz M.A., Hassan K.S., Wasfy M.O., Monestersky J.H.,
Oyofo, B.A. 2014. Serological evidence of flaviviruses infection among acute febrile illness patients in
Emadi-Kouchak H., Yalda A.R., Haji Abd Albaghi M., Soudbakhsh A.A.R. 2003. Crimean-Congo hemorrhagic
fever. Tehran University Medical Journal 61: 343-358.
Epelboin Y., Talaga S., Epelboin L., Dusfour I. 2017. Zika virus: an updated review of competent
or naturally infected mosquitoes. PLOS Neglected Tropical Diseases 11: 11, e0005933.
Eram N., Peighambari S., Madani S., Razmyar J., Barin, A. 2020. Sequence and phylogenetic study
of two fowlpox virus isolates obtained from layer chickens and red mite (Dermanyssus
gallinae) in 2016. Journal of Veterinary Research 75: 218-225 (Persian with English abstract).
Erfani A.M., Bakhshesh M., Fallah M.H., Hashem M. 2019. Seroprevalence and risk factors associated with
bovine viral diarrhea virus and bovine herpes virus-1 in Zanjan Province, Iran. Tropical Animal Health
Ergunay K., Whitehouse C.A., Ozkul A. 2011. Current status of human arboviral diseases in Turkey. Vector-Borne
Evans M., Dallas T.A., Han B.A., Murdock C.C., Drake, J.M. 2017. Data-driven identification of potential Zika
Eybpoosh S., Fazlalipour M., Baniasadi V., Pouriayevali M.H., Sadeghi F., Ahmadi Vasmehjani A., Karbalaie
Niya M.H., Hewson R., Salehi-Vaziri M. 2019. Epidemiology of West Nile virus in the Eastern
Mediterranean Region: a systematic review. PLOS Neglected Tropical Diseases 13: 1. e0007081.
Ezatkhah M., Shamsaddini Bafti M., Alimolaei M., Amini M. 2014. High seroprevalence of bluetongue
virus in small ruminants in southeast Iran. Online Journal of Veterinary Research 18: 260-267.
Ezzi A., Hatami A., Bakhshesh M., Shoukri M.R., Gharaghozloyan M. 2013. Serological study of bovine
herpesvirus type 1 and parainfluenza type 3 in cow farms of Qazvin Province based on different ages and
seasons. Archives of Razi Institute 68: 53-57.
Faghihi F., Chinikar S., Telmadarraiy Z., Bakhshi H., Khakifirooz S., Jalali T., Nutifafa G.G. 2015. Crimean-congo
hemorrhagic fever: a seroepidemiological and molecular survey in north of Iran. Journal of Entomology
and Zoology Studies 3: 156-159.
Faghihi F., Telmadarraiy Z., Chinikar S., Nowotny N., Fooks A.R., Shahhosseini N. 2018. Spatial and
phylodynamic survey on Crimean-Congo hemorrhagic fever virus strains in northeast of Iran. Jundishapur
Journal of Microbiology 11: 3. e59412.
Failloux A.-B., Bouattour A., Faraj C., Gunay F., Haddad N., Harrat Z., Jancheska E., Kanani K., Kenawy M.A.,
Kota M., Pajovic I., Paronyan L., Petric D., Sarih M., Sawalha S., Shaibi T., Sherifi K., Sulesco T., Velo
E., Gaayeb L., Victoir K., Robert V. 2017. Surveillance of arthropod-borne viruses and their vectors in
the Mediterranean and Black Sea Regions within the MediLabSecure network. Current Tropical Medicine
Fakoorziba M.R., Golmohammadi P., Moradzadeh R., Momenbellah-Fard M.D., Azizi K., Davari B., Alipour H.,
Ahmadnia S., Chinikar S. 2012. Reverse transcription PCR-based detection of Crimean-Congo hemorrhagic
fever virus isolated from ticks of domestic ruminants in Kurdistan Province of Iran. Vector-Borne and
411
Fakoorziba M.R., Naddaf-Sani A.A., Momenbellah-Fard M.D., Azizi K., Ahmadnia S., Chinikar S. 2015.
First phylogenetic analysis of a Crimean-Congo hemorrhagic fever virus genome in naturally
infected Rhipicephalus appendiculatus ticks (Acari: Ixodidae). Archives of Virology 160: 1197-1209.
Fakour S., Naserabadi S., Ahmadi E. 2021. A serological and hematological study on Rift Valley fever and
associated risk factors in aborted sheep at Kurdistan Province in west of Iran. Comparative Immunology,
Fallahi R., Yaghini M., Kargar Moakhar R., Khedmati K. 2013. Virological and werological wurvey on bluetongue
disease in sheep in some parts of Iran. Veterinary Journal (Pajouhesh, Sazandegi) 99: 36-43 (Persian with
English abstract).
Farnon E.C., Gould L.H., Griffith K.S., Osman M.S., Kholy A.E., Brair M.-E., Panella A.J., Kosoy O., Laven
J.J., Godsey M.S., Perea W., Hayes E.B. 2010. Household-based sero-epidemiologic survey after a yellow
fever epidemic, Sudan, 2005. American Journal of Tropical Medicine and Hygiene 82: 1146-1152.
Farhadpour F., Telmadarraiy Z., Chinikar S., Akbarzadeh K., Fakoorziba M., Moemenbel-lahFard M. 2015.
Molecular detection of Crimean-Congo hemorrhagic fever (CCHF) virus in tick species collected from
livestock in Marvdasht, Fars Province during 2012-2013. Armaghane-danesh, Yasuj University of Medical
Sciences Journal 19: 1049-1057 (Persian with English abstract).
Farzinnia B., Saghafipour A., Telmadarraiy Z. 2013. Study of the epidemiological status of Crimean-Congo
hemorrhagic fever disease in Qom Province, 2011, Iran. Qom University Medical Sciences Journal 7:
42-48 (Persian with English abstract).
Fazlalipour M., Baniasadi V., Pouriayevali M.H., Jalali T., Mohammadi T., Azad-Manjiri S., Azizzadeh S., Hosseini
M., Fereydouni Z., Tavakoli M., Ghalejoogh M., Khakifirouz S., Salehi-Vaziri M. 2019. Crimean-Congo
hemorrhagic fever virus Asia 2 genotype in Qeshm Island, southern Iran: a case report. Journal of Vector
Borne Diseases 56: 276-279.
Federici V., Goffredo M., Mancini G., Quaglia M., Santilli A., Di Nicola F., De Ascentis M., Cabras P.,
Volpicelli C., De Liberato C., Satta G., Federico G., Leone A., Pisciella M., Portanti O., Pizzurro F.,
Teodori L., Savini G. 2019. Vector competence of Italian populations of Culicoides for some bluetongue
virus strains responsible for recent northern African and European outbreaks. Viruses 11: 941.
Federov J.V., Igolkin N.I., Tiushniakova M.K. 1959. Some data on virus-carrying fleas in areas of tick-borne
encephalitis and lymphocytic choriomeningitis. Medical Parasitology and Parasitic Diseases 28: 149-152
(Russian).
Fenner F. 2001. Myxomatosis. In: Service M.W. (ed). Encyclopedia of Arthropod-Transmitted Infections of Man
and Domesticated Animals. CABI Publishing, Wallingford. 356-363.
Filipe A.R., Calisher C.H. 1984. Isolation of Thogoto virus from ticks in Portugal. Acta Virologica 28: 152-155.
Filipe A.R., Alves M.J, Karabatsos N., Alves de Matos A.P., Núncio M.S., Bacellar F. 1994. Palma virus, a new
Firooziyan S., Sadeghi R., Sabouri M., Tol A., Rikhtehgar E., Fathi B., Sedaghat M.M. 2022. Predictors
of dengue preventive practices based on precaution adoption process model among health
care professionals in northwest of Iran. Journal of Arthropod-Borne Diseases 16: 340-349.
Firouzmanesh P., Fakour S., Ahmady E. 2017. Serologic survey of Crimean-Congo hemorrhagic fever in high
risk people in slaughter house, livestock owners and livestock in Kurdistan Province. Scientific Journal of
Kurdistan University of Medical Sciences 22: 1-9 (Persian with English abstract).
Foil L.D., Meek C.L., Adams W.V., Issel C.J. 1983. Mechanical transmission of equine infectious anemia virus
by deer flies (Chrysops flavidus) and stable flies (Stomoxys calcitrans). American Journal of Veterinary
Research 44: 155-156.
Foil L.D., Seger C.L., French D.D., Issel C.J., McManus J.M., Ohrberg C.L., Ramsey R.T. 1988. Mechanical
transmission of bovine leukemia virus by horse flies (Diptera: Tabanidae). Journal of Medical Entomology
Foil L.D. 1989. Tabanids as vectors of disease agents. Parasitology Today 5: 88-96.
412
Foil L.D., Issel C.J. 1991. Transmission of retroviruses by arthropods. Annual Review of Entomology 36: 355-381.
Frese M., Weeber M., Weber F., Speth V., Haller O. 1997. Mx 1 sensitivity: Batken virus is an
orthomyxovirus closely related to Dhori virus. Journal of General Virology 78: 2453-2458.
Gaidamovich S.Ia., Melnikova E.E., Agafonov V.I., Lokhova M.D., Rodnia V.Ia. 1975. Identification of a group
A arbovirus isolated in the far east. Voprosy Virusologii 3: 317-320 (Russian).
Gaidamovich S.Ia., Altukhova L.M., Obukhova V.R., Ponirovskii E.N., Sadykov V.G. 1980. Isolation of the Isfahan
virus in Turkmenia. Voprosy Virusologii 5: 618-620 (Russian with English abstract).
Gaidamovich S.Ia., Khutoretskaya N.V., Asyamov Y.V., Tsyupa V.I., Melnikova E.E. 1990a. Sandfly fever in
Central Asia and Afghanistan. Archives of Virology [Suppl 1]: 287-293.
Gaidamovich S.Ia., Khutoretskaia N.V., Aziamov I.V., Tsiupa V.I., Mel'nikova E.E. 1990b. Virological study of
cases of sandfly fever in Afghanistan. Voprosy Virusologii 35: 45-47 (Russian).
Galati E.A.B., Galvis-Ovallos F., Lawyer P., Léger N., Depaquit J. 2017. An illustrated guide for characters
and terminology used in descriptions of Phlebotominae (Diptera, Psychodidae). Parasite 24: 1-35.
Gallardo C., Okoth E., Pelayo V., Anchuelo R., Martin E., Simon A., Llorente A., Nieto R., Soler A., Martin R.,
Arias M., Bishop R.P. 2011. African swine fever viruses with two different genotypes, both of which occur
in domestic pigs, are associated with ticks and adult warthogs, respectively, at a single geographical site.
Gao G.F., Zanotto P.M.De.A., Holmes E.C., Reid H.W., Gould E.A. 1997. Mokecular variation, evolution and
geographical distribution of Louping ill virus. Acta Virologica 41: 259-268.
Gavrilovskaya I.N. 2001. Issyk-Kul virus disease. In: Service M.W. (ed). Encyclopedia of Arthropod-Transmitted
Infections of Man and Domesticated Animals. CABI Publishing, Wallingford. 231-234.
Gevorgyan H., Grigoryan G.G., Atoyan H.A., Rukhkyan M., Hakobyan A., Zakaryan H., Aghayan S.A. 2019.
Evidence of Crimean-Congo haemorrhagic fever virus occurrence in Ixodidae ticks of Armenia. Journal
Ghalyanchilangeroudi A., Ziafati Kafi Z., Rajeoni A., Ataii J., Sadri N., Hajizamani N., Aghaeean
L., Majidi S., Sadeghi H., Ghorani M. 2021. Molecular detection and phylogenetic analysis
of lumpy skin disease virus in Iran. Iranian Journal of Veterinary Medicine 15: 168-174.
Ghasemian S.O., Fazlalipour M., Hosseini G., Pouryaievali M.H., Azad-Manjiri S., Khakifirouz S., Ahmadi
Vasmehjani A., Salehi-Vaziri M. 2021. Serosurvey of Crimean-Congo hemorrhagic fever virus in livestock,
Kohgiluyeh and Boyer-Ahmad, Iran, 2017. Journal of Vector Borne Diseases 58: 70-73.
Ghodsian N., Khalesi B., Motamed N., Ebrahimi M.M., Abdoshah M., Mojahedi Z., Shiri, N. 2022. Evaluation
of the possibility of reversion of virulence and safety tests of Razi Institute‘s live attenuated fowl
pox vaccine. Veterinary Researches, Biological Products 136: 12-17 (Persian with English abstract).
Ghorani M., Esmaeili H. 2022. Comparison of susceptibility of different goat breeds to live attenuated goatpox
Gibbens N. 2012. Schmallenberg virus: a novel viral disease in northern Europe. Veterinary Record 170: 58.
Gibbs E.P., Johnson R.H., Osborne A.D. 1972. Field observations on the epidemiology of bovine herpes
mammillitis. Veterinary Record 91: 395-401.
Gibbs, E.P., Johnson R.H., Osborne A.D. 1973a. Experimental studies of the epidemiology of bovine herpes
mammillitis. Research in Veterinary Science 14: 139-144.
Gibbs E.P., Johnson R.H., Gatehouse A.G. 1973b. A laboratory technique for studying the mechanical transmission
of bovine herpes mammillitis virus by the stable fly (Stomoxys calcitrans L.). Research in Veterinary
Science 14: 145-147.
Gibbs E.P.J. 2001. African swine fever. In: Service M.W. (ed). Encyclopedia of Arthropod-Transmitted Infections
of Man and Domesticated Animals. CABI Publishing, Wallingford. 7-13.
413
Goenaga S., Kenney J.L., Duggal N.K., Delorey M., Ebel G.D., Zhang B., Levis S.C., Enria D.A., Brault A.C.
2015. Potential for co-infection of a mosquito-specific flavivirus, Nhumirim virus, to block West Nile virus
Goffredo M., Catalani M., Federici V., Portanti O., Marini V., Mancini G., Quaglia M., Santilli A., Teodori L.,
Savini G. 2015. Vector species of Culicoides midges implicated in the 2012-214 bluetongue epidemics in
Gould E.A. 2001. Louping ill, sheep. In: Service M.W. (ed). Encyclopedia of Arthropod-Transmitted Infections
of Man and Domesticated Animals. CABI Publishing, Wallingford. 290-295.
Gould E., Pettersson J., Higgs S., Charrel R, de Lamballerie X. 2017. Emerging arboviruses: Why today? One
Gozalan A., Kalaycioglu H., Uyar Y., Sevindi D.F., Turkyilmaz B., Cakir V., Cindemir C., Unal B., Yagci-Caglayik
D., Korukluoglu G., Ertek M., Heyman P., Lundkvist A. 2013. Human Puumala and Dobrava Hantavirus
infections in the Black Sea Region of Turkey: a cross-sectional study. Vector-Borne and Zoonotic Diseases
Gray D.P., Bannister G.L. 1961. Studies on blue tongue. I. Infectivity of the virus in the sheep ked, Melophagus
ovinus (L.). Canadian Journal of Comparative Medicine and Veterinary Science 25: 230-232.
Green B.E., Foil L.D., Hagius S.D., Issel C.J. 1996. Stability of equine infectious anemia virus in Aedes aegypti
(Diptera: Culicidae), Stomoxys calcitrans (Diptera: Muscidae), and Tabanus fuscicostatus (Diptera:
Tabanidae) stored at -70 degrees C. Journal of the American Mosquito Control Association 12: 334-336.
Gromashevsky V.L., Nikimorov L.P. 1973. Arboviruses in Azerbaijan. Sborn. Trud. Ekol. Virus 1: 119-122.
Gugliemone A.A., Robbins R.G., Apanaskevich D.A., Petney T.N., Estrada-Pena A., Horak I.G., Shao R., Barker
S.C. 2010. The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida) of the world: a list of valid species
Gugliemone A.A., Robbins R.G., Apanaskevich D.A., Petney T.N., Estrada-Pena A., Horak I.G. 2014. The Hard
Ticks of the World (Acari: Ixodida: Txodidae). Springer, Dordrecht.
Gür S. 2008. A serologic investigation of blue tongue virus (BTV) in cattle, sheep and Gazella subgutturosa
subgutturosa in southeastern Turkey. Tropical Animal Health and Production 40: 217-221.
Ha D.Q., Calisher C.H., Tien P.H., Karabatos N., Gubler D.J. 1995. Isolation of a newly recognized alphavirus
from mosquitoes in Vietnam and evidence for human infection and disease. American Journal of Tropical
Habibzadeh S., Mohammadshahi J., Bakhshzadeh A., Moradi-Asl E. 2021. The First Outbreak of Crimean-
Congo hemorrhagic fever disease in northwest of Iran. Acta Parasitologica 66: 1086-1088.
Hadinia A., Ilami O., Mousavizadeh A., Akbartabar Tori M., Khosravani S.A. 2012. Seroepidemiology of Crimean-
Congo hemorrhagic fever in high risk professions. Journal of Mazandaran University of Medical Sciences
22(92): 45-50 (Persian with English abstract).
Hafez S.M., Pollis E.G., Mustafa S.A. 1978. Serological evidence of the occurrence of bluetongue in Iraq. Tropical
Hafez S.M., Taylor W.P. 1985. Serotypes of bluetongue virus present in Saudi Arabia. Progress in Clinical and
Biological Research 178: 531-537.
Hafez S.M., Sharif M., Al-Sukayran A., Dela-Cruz D. 1990. Preliminary studies on enzootic bovine leucosis in
Saudi dairy farms. Deutsche Tierarztliche Wochenschrift 97: 61-63.
Haig D.A., Woodall J.P., Danskin D. 1965. Thogoto virus: a hitherto undescribed agent isolated from ticks in
Hanafi-Bojd A.A., Motazakker M., Vatandoost H., Dabiri F., Chavshin A.R. 2021. Sindbis virus infection of
mosquito species in the wetlands of northwestern Iran and modeling the probable ecological niches of
Hannoun C., Rau U. 1970. Experimental transmission of certain arboviruses by Argas reflexus reflexus (Fabricius,
1794). Folia Parasitologia 17: 365-366.
Harbach R.E. 1988. The mosquitoes of the subgenus in southwestern Asia and Egypt (Diptera: Culicidae).
Contributions of the American Entomological Institute 24: vi + 1-236.
414
Hart C.A. 2001. Arboviruses. In: Service M.W. (ed). Encyclopedia of Arthropod-Transmitted Infections of Man
and Domesticated Animals. CABI Publishing, Wallingford. 48-50.
Hartley W.J., Martin W.B., Hakioglu F., Chifney S.T.E. 1969. A viral encephalitis of sheep in Turkey. Pendik
Institute Journal 2: 89-95.
Hartlaub J., von Arnim F., Fast C., Somova M., Mirazimi A., Groschup M.H., Keller M. 2020. Sheep and cattle
are not susceptible to experimental inoculation with Hazara Orthonairovirus, a tick-borne arbovirus closely
Harwood R.F., James M.T. 1979. Entomology in Human and Animal Health. Seventh edition. Macmillan Publishin
Co., Inc., New York.
Hasanpour A., Mosakhani F., Mirzaii H., Mostofi S. 2008. Seroprevalence of bluetongue virus infection in sheep
in East-Azarbagan Province in Iran. Research Journal of Biological Sciences 3: 1265-1270.
Hasanpour A., Najafi M.S., Khakpour M. 2014. Seroprevalence of bluetongue virus infection in sheep in Tekab
area in Iran. Indian Journal of Fundamental and Applied Life Science 4: 634-640.
Hashemi M., Manavian M., Nikoo D., Bakhshesh M., Tavan F. 2018. Seroprevalence rate of bluetongue virus
in sheep and goat populations of Fars Province. Iranian Veterinary Journal 14: 112-119 (Persian with
English abstract).
Hashemi M., Bakhshesh M., Manavian M. 2022. Bovine viral diarrhea virus and bovine herpes virus-1 in dairy
cattle herds in Fars Province, southern Iran: seroprevalence and evaluation of risk factors. Archives of
Hashemian M., Ebrahimi M. 2010. A case report of Crimean Congo hemorrhagic fever with brucellosis. Journal
of Sabzevar University of Medical Sciences 17: 63-66 (Persian with English abstract).
Hassani M., Madadgar O. 2021. Serological evidence of bluetongue in Iran: a meta-analysis study. Veterinary
Hassanein K.M., El-Azazy O.M.E., Yousef H.M. 1997. Detection of Crimean-Congo haemorrhagic fever virus
antibodies in humans and imported livestock in Saudi Arabia. Transactions of the Royal Society of Tropical
Hawkins J.A., Adams W.V., Cook L., Wilson B.H., Roth, E.E. 1973. Role of horse fly (Tabanus fuscicostatus
Hine) and stable fly (Stomoxys calcitrans L.) in transmission of equine infectious anemia to ponies in
Louisiana. American Journal of Veterinary Research 34: 1583-1586.
Hayes C.C., Burney M.I. 1981. Arboviruses of public health importance in Pakistan. Journal of Pakistan Medical
Association 31: 16-26.
Hayes C.G., Baqar S., Ahmed T., Chowdhry M.A., Reisen W.K. 1982. West Nile virus in Pakistan. 1. Sero-
epidemiological studies in Punjab Province. Transactions of the Royal Society of Tropical Medicine and
Hazrati A., Taslimi H. 1964. Study on horse sickness virus strains isolated in Iran. Archives of Razi Institute
16: 90-99.
Hazrati A. 1967. Identification and typing of horse-sickness virus strains isolated in the recent epizootic of the
disease in Morocco, Tunisia, and Algeria. Archives of Razi Institute 19: 131-143.
Hazrati A., Kargar Moakhar R., Mahinpoor M., Dayhim, F. 1978. Serological survey on the presence of distribution
of equine infectious anaemia in Iran. Archives of Razi Institute 30: 17-23.
Hazrati A., Bazargan T.T., Shahrabadi M.S., Amjadi A.R. 1981. Isolation and characterization of a bovine
herpesvirus 3 from cases of calf pneumonia in Iran. Archives of Razi Institute 32: 21-35.
Hedger R.S., Barnett I.T.R., Gray, D.F. 1980. Some virus diseases of domestic animals in the Sultanate of Oman.
Hedayati Z., Varshovi H.R., Mohammadi A., Tabatabaei M. 2021. Molecular characterization
of lumpy skin disease virus in Iran (2014-2018). Archives of Virology 166: 2279-2283.
Heinz F.X., Holzmann H. 2001. Tick-borne encephalitis. In: Service M.W. (ed). Encyclopedia of Arthropod-
Transmitted Infections of Man and Domesticated Animals. CABI Publishing, Wallingford. 507-512.
Hemida M.G., Perera R.A.P.M., Chu D.K.W., Ko R.L.W., Alnaeem A.A., Peiris M. 2019. West Nile
virus infection in horses in Saudi Arabia (in 2013-2015). Zoonoses Public Health 66: 248-253.
415
Hemmatzadeh F., Boardman W., Alinejad A., Hematzade A., Kharazian Moghadam M. 2016. Molecular and
serological survey of selected viruses in free-ranging wild ruminants in Iran. PLOS One 11: 12. e0168756.
Henderson B.E., Metselaar D., Cahill K., Timms G.L., Tukei P.M., Williams M.C. 1968. Yellow fever immunity
surveys in northern Uganda and Kenya and eastern Somalia, 1966-67. Bulletin of the World Health
Organization 38: 229-237.
Hertig M., Sabin A.B. 1955. Chapter IX Sandfly Fever (Pappataci, Phlebotomus, Three-Day Fever). In: Hoff E.C.
(ed). Preventive Medicine in World War II. V. VII. Communicable Diseases. Arthropodborne Diseases Other
Than Malaria. Medical Department, United States Army, The Surgeon General, Washinton, DC. 109-174.
Hess W.R., Endris R.G., Haslett T.M., Monahan M.J., McCoy J.P. 1987. Potential arthropod vectors of African
swine fever virus in North America and the Caribbean basin. Veterinary Parasitology 26: 145-155.
Heydari, A., Movahed Danesh M. 2013. Crimean Congo hemorrhagic fever in the Razavi Khorasan Province of
Iran. Medical Journal of Mashhad University of Medical Sciences 56: 85-92 (Persian with English abstract).
Heydari M., Metanat M., Rouzbeh-Far M.A., Tabatabaei S.M., Rakhshani M., Sepehri-Rad N., Keshtkar-Jahromi
M. 2018. Dengue fever as an emerging infection in southeast Iran. American Journal of Tropical Medicine
Hoffmann B., Scheuch M., Höper D., Jungblut R., Holsteg M., Schirrmeier H., Eschbaumer M., Goller K.V.,
Wernike K., Fischer M., Breithaupt A., Mettenleiter T.C., Beer, M. 2012. Novel Orthobunyavirus in cattle,
Hoffman T., Lindeborg M., Barboutis C., Erciyas-Yavuz K., Evander M., Fransson T., Figuerola J., Jaenson
T.G.T., Kiat Y., Lindgren P.-E., Lundkvist Å., Mohamed N., Moutailler S., Nyström F., Olsen B., Salaneck
E. 2018. Alkhurma Hemorrhagic fever virus RNA in Hyalomma rufipes ticks infesting migratory birds,
Hoogstraal H. 1966. Ticks in relation to human diseases caused by viruses. Annual Review of Entomology 11:
Hoogstraal H., Oliver R.M., Guirgis S.S. 1970. Larva, nymph, and life cycle of Ornithodoros (Alectorobius)
muesebecki (Ixodoidea: Argasidae), a virus-infected parasite of birds and petroleum industry
employees in the Arabian Gulf [sic]. Annals of the Entomological Society of America 63: 1762-1768.
Hoogstraal H. 1979. The epidemiology of tickborne Crimean-Congo hemorrhagic fever in Asia, Europe, and
Hoogstraal H., Valdez R. 1980. Ticks (Ixodoidea) from wild sheep and goats in Iran and medical and veterinary
implications. Fieldiana Zoology 6: 1-16.
Hoogstraal H. 1981 Changing patterns of tickborne diseases in modern society. Annual Review of Entomology
Hoogstraal H. 1985. Argasid and nuttalliellid ticks as parasites and vectors. Advances in Parasitology 24: 135-238.
Horton K.C., Fahmy N.T., Watany N., Zayed A., Mohamed A., Ahmed A.A., Rolin P.E., Dueger E.L. 2016.
Crimean Congo hemorrhagic fever virus and Alkhurma (Alkhumra) virus in ticks in Djibouti. Vector Borne
Hosseni-Chegeni A., Tavakoli M., Telmadarraiy Z. 2019. The updated list of ticks (Acari: Ixodidae and Argasidae)
occurring in Iran with a key to the identification of species. Systematic and Applied Acarology 24: 2133-
Hosseni-Chegeni A., Tavakoli M. 2020. Argas hermanni Audouin (Acari: Argasidae), a new member of Iranian
Houck M.A., Qin H., Roberts H.R. 2001. Hantavirus transmission: potential role of ectoparasites. Vector Borne
Hubálek Z., Cerny V., Rodl P. 1982. Possible role of birds and ticks in the dissemination of Bhanja virus. Folia
Parasitologia 29: 85-9Hubálek Z. 1987. Geographic distribution of Bhanja virus. Folia Parasitologia 34:
77-86.
Hubálek Z., Juricová Z., Halouzka J., Pellantová J., Hudec K. 1989. Arboviruses associated with birds in southern
Moravia, Czechoslovakia. Acta scientiarum naturalium Academiae Scientiarum Bohemicae, Brno 7: 1-50.
416
Hubálek Z. 2008. Mosquito-borne viruses in Europe. Parasitology Research 103 (Suppl 1): 29-43.
Hubálek Z., Rudolf I. 2012. Tick-borne viruses in Europe. Parasitology Research 111: 9-36.
Hubálek Z., Rudolf I., Nowotny N. 2014a. Arbovirus pathogenic for domestic and wild animals. Advances in
Hubalek Z., Sebesta O., Pesko J., Betasova L., Blazejova H., Venclikova K., Rudolf I. 2014b. Isolation of
Tahyna virus (California Encephalitis Group) from Anopheles hyrcanus (Diptera, Culicidae), a mosquito
species new to, and expanding in, central Europe. Journal of Medical Entomology 51: 1264-1267.
Humphrey J.M., Al-Absi E.S., Hamdan M.M., Okasha S.S., Al-Trmanini D.M., El-Dous H.G.,
Dargham S.R., Schieffelin J., Abu-Raddad L.J., Nasrallah G.K. 2019. Dengue and chikungunya
seroprevalence among Qatari nationals and immigrants residing in Qatar. PLOS One 14: 1. e0211574.
Hunter P., Wallace D. 2001. Lumpy skin disease in southern Africa: a review of the disease and aspects of control.
Hyams K.C., Hanson K., Stephen Wingall F., Escamilla J., Oldfield E.C.III. 1995. The impact of infectious
diseases on the health of U.S. troops deployed to the Persian Gulf during operations Desert Shield and
Ibrahim A.M., Adam I.A., Osman B.T., Aradaib I.E. 2015. Epidemiological survey of Crimean Congo
hemorrhagic fever virus in cattle in East Darfur State, Sudan. Ticks and Tick-Borne Diseases 6: 439-444.
Ibrahim I.T., Badri A.M., Arbab M.H., Elrasoul R.M.H., Mohamed S.G. 2017. Seroprevalence of Hanta virus IgM
antibody in febrile patients in West Kurdofan State, Sudan. EC Microbiology 11(4): 138-142.
Igarashi A., Tanaka M., Morita K., Takasu T., Ahmed A., Ahmed A., Akram D.S., Anwar Waqar M. 1994.
Detection of West Nile and Japanese encephalitis viral genome sequences in cerebrospinal fluid
from acute encephalitis cases in Karachi, Pakistan. Microbiology and Immunology 38: 827-830.
Inci A., Yazar S., Tuncbilek A.S., Canhilal R., Doganay M., Aydin L., Aktas M., Vatansever Z., Ozdarendeli A.,
Ozbel Y., Yildirim A., Duzlu O. 2013. Vectors and vector-borne diseases in Turkey. Ankara Üniversitesi
Veteriner Fakültesi Dergisi 60: 281-296.
Inci A., Yildirim A., Duzlu O., Doganay M., Aksoy S. 2016. Tick-borne diseases in Turkey: a review
based on One Health perspective. PLOS Neglected Tropical Diseases 10: 12. e0005021.
Inci A., Doğanay M., Özdarendeli A., Düzlü Ö., Yıldırım A. 2018. Overview of zoonotic diseases
in Turkey: the One Health concept and future threats. Türkiye Parazitoloji Dergisi 42: 39-80.
Im J.H., Baek J.-H., Durey A., Kwon H.Y., Chung M.-H., Lee J.-S. 2020. Geographic distribution
of tick-borne encephalitis virus complex. Journal of Vector Borne Diseases 57: 14-22.
Imandar M., Pourbakhsh S., Hassanpour A., Moosakhani F. 2014. Evaluate of efficacy clinical signs, physiological
and environmental factors on the seroprevalence rate of bluetongue virus in sheep flocks. Journal of
Veterinary Medicine and Research 5: 31-41.
Issel C.J. 2001. Equine infectious anaemia. In: Service M.W. (ed). Encyclopedia of Arthropod-Transmitted
Infections of Man and Domesticated Animals. CABI Publishing, Wallingford. 176-178.
Issel C.J., Foil L.D. 2015. Equine infectious anaemia and mechanical transmission: man and the wee beasties.
Revue Scientifique et Technique OIE 34: 513-523.
Izadi S., Holakouie-Naieni K., Madjdzadeh S.R., Chinikar S., Rakhshani F., Nadim A., Hooshmand B. 2003. The
prevalence of Crimean-Congo hemorrhagic fever in Sistan and Baluchestan Province, Iran: a serologic
study. Payesh 2: 87-95 (Persian with English abstract).
Izadi S., Holakouie-Naieni K., Madjdzadeh S.R., Nadim A. 2004. Crimean-Congo hemorrhagic fever in Sistan
and Balouchestan Province of Iran, a case-control study on epidemiological characteristics. International
417
Izadi S., Holakouie-Naieni K., Madjdzadeh S.R., Chinikar S., Nadim A., Rakhshani F., Hooshmand B. 2006.
Seroprevalence of Crimean-Congo hemorrhagic fever in Sistan-va-Baluchistan Province of Iran. Japanese
Journal of Infectious Diseases 59: 326-328.
Izadi M., Salehi H., Chinikar S., Mostafavizadeh K., Darvishi M., Jonaidi N., Ranjbar R., Khorvash F., Bidar R.,
Ataei B. 2007. A geographical distribution survey on CCHF positive antibody ovines of Isfahan Province
in 1383-1384. Journal of Military Medicine 9: 97-102 (Persian with English abstract).
Izri A., Tammam S., Moureau G., Hamrioui B., de Lamballerie X., Charrel R.N. 2008. Sandfly fever Sicilian
Jafari A., Rasekh M., Saadati D., Faghihi F., Fazlalipour M., Khakifirouz S., Jalili T., Ahmadi, Z. 2020. Molecular
detection of Crimean Congo hemorrhagic fever in tick vectors in rural areas of eastern Iran. New Findings
in Veterinary Microbiology 3: 1-9 (Persian with English abstract).
Jafari-Shoorijeh S., Ramin A.G., Maclachlan N.J., Osburn B.I., Tamadon A., Behzadi M.A., Mahdavi M.,
Araskhani A., Samani D., Rezajou N., Amin-Pour A. 2010. High seroprevalence of bluetongue virus
infection in sheep flocks in West Azerbaijan, Iran. Comparative Immunology, Microbiology and Infectious
Jalili S.M., Rasooli A., Seifi Abad-Shapouri M.R., Daneshi M. 2017. Clinical, hematologic, and biochemical
findings in cattle infected with lumpy skin disease during an outbreak in southwest Iran. Archives of Razi
Institute 72: 255-263.
Javadian E., Mesghali A. 1975. Check-list of phlebotomine sand flies (Diptera: Psychodidae) of Iran. Bulletin de
la Société de Pathologie Exotique 68: 207-209.
Javadian E., Tesh R., Saidi S., Nadim A. 1977. Studies on the epidemiology of sandfly fever in Iran III. Host-
feeding patterns of Phlebotomus papatasi in an endemic area of the disease. American Journal of Tropical
en.04.010159.002133
Jemeršić L., Dežđek D., Brnić D., Prpić J., Janicki Z., Keros T., Roic B., Slavica A., Terzic S., Konjevic D.,
Beck R. 2014. Detection and genetic characterization of tick-borne encephalitis virus (TBEV) derived
from ticks removed from red foxes (Vulpes vulpes) and isolated from spleen samples of red deer (Cervus
Jennings M., Mellor P.S. 1989. Culicoides: biological vectors of akabane virus. Veterinary Microbiology 21:
Johnson B.K., Chanas A.C., Squires E.J., Shockley P., Simpson D.I.H., Parsons J., Smith D.H., Casals J. 1980.
Arbovirus isolations from ixodid ticks infesting livestock, Kano Plain, Kenya. Transactions of the Royal
Johnson G.D., Campbell J.B., Minocha H.C., Broce A.B. 1991. Ability of Musca autumnalis (Diptera:
Muscidae) to Acquire and Transmit Bovine Herpesvirus-1. Journal of Medical Entomology 28: 841-846.
Jones L.D., Davies C.R., Steel G.M., Nuttall P.A. 1989. Vector capacity of Rhipicephalus appendiculatus and
Amblyomma variegatum for Thogoto and Dhori viruses. Medical and Veterinary Entomology 3: 195-202.
Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L., Daszak P. 2008. Global trends in
Jorjani S.E. 2001. Zakhireye Kharazmshahi. Islamic Acad. Med. Sci. Iran, Tehran.
Jupp P.G., McIntosh B.M., Dos Santos I. 1981. Laboratory vector studies on six mosquito and one tick species
with chikungunya virus. Transactions of the Royal Society of Tropical Medicine and Hygiene 75: 15-19.
Jupp P.G., McIntosh B.M. 1990. Aedes furcifer and other mosquitoes as vectors of chikungunya virus at Mica,
northeastern Transvaal, South Africa. Journal of the American Mosquito Control Association 6: 415-420.
Kahana-Sutin E., Klement E., Lensky I., Gottlieb Y. 2017. High relative abundance of the stable fly Stomoxys
calcitrans is associated with lumpy skin disease outbreaks in Israeli dairy farms. Medical and Veterinary
Kamali K., Ostovan H., Atamehr A. 2001. A Catalog of Mites and Ticks (Acari) of Iran. Islamic Azad University
Scientific Publication Center, Tehran.
418
Karami Boldaji S., Pourmahdi Borujeni M., Haji Hajikolaei M.R., Seifi Abad Shapouri M.R. 2016. Seroprevalence
and risk factors of Akabane virus infection in cattle from Khouzestan Province of Iran. Iranian Journal
of Virology 10: 14-20.
Karapetyan R.M., Vorobev A.G., Semashko I.V., Matevosyan K.Sh., Vopyan D.S. 1974. A case of Crimean
hemorrhagic fever in Armenian SSR. In: Chumakov M.P. (ed). Medical virology. Trudy Inst. Polio. Virus.
Entsef. Akad. Med. Nauk SSSR, 22: 260-265 (Russian, English translation, NAMRU3-T1115).
Kargar Moakhar R., Taylor W.P., Ghaboussi B., Hessami M. 1988. Serological survey of sheep in Iran for type
specific antibody to bluetongue virus. Archives of Razi Institute 38-39: 92-99.
Kargar Moakhar R., Bokaie S., Akhavizadegan M.A., Charkhkar S., Meshkot M. 2001. Seroepidemological
survey for antibodies against infectious bovine rhinotracheitis and bovine herpes 4 viruses among cattle
in different provinces of Iran. Archives of Razi Institute 52: 93-102.
Kargar Moakhar R., Ghorashi S.A., Sadeghi M.R., Morshedi D., Masoudi S., Pourbakhsh S.A. 2003. Detection of
different Iranian isolates of bovine herpes virus type-1 (BHV-1) using polymerase chain reaction. Archives
of Razi Institute 55: 11-18.
Karimi A., Hanafi-Bojd A.A., Yaghoobi-Ershadi M.R., Akhavan A.A., Ghezelbash Z. 2014. Spatial and temporal
distributions of phlebotomine sand flies (Diptera: Psychodidae), vectors of leishmaniasis, in Iran. Acta
Karimpour Somedel A., Varshoiee H.R., Aghaiypour K., Hedayati Z., Aghaebrahimian M. 2019. Phylogenetic
analysis of Iran capripoxvirus isolates during 2013-2016. Veterinary Researches and Biological Products
122: 2-16 (Persian with English abstract).
Kasi K.K., Sas M.A., Sauter-Louis C., von Arnim F., Gethmann J.M., Schulz A., Wernike K., Groschup
M.H., Conraths F.J. 2020. Epidemiological investigations of Crimean-Congo haemorrhagic fever virus
infection in sheep and goats in Balochistan, Pakistan. Ticks and Tick-Borne Diseases 11: 101324.
Kasiri H., Javadian E., Seyedi-Rashti M.A. 2000. Check-list of Phlebotominae sandflies (Diptera: Psychodidae)
of Iran. Bulletin de la Société de Pathologie Exotique 93: 129-130 (French with English abstract).
Kassiri H., Dehghani R. 2020. Hantavirus infections as zoonotic emerging viral diseases: current status with an
emphasis on data from Iran. Entomology and Applied Science Letters 7(4): 9-17.
Kassiri H., Dehghani R., Kasiri M., Dehghani M., Kasiri R. 2020a. A survey on Zika virus infection as a global
emergency, a mosquito-borne flavivirus. Entomology and Applied Science Letters 7: 51-57.
Kassiri H., Dehghani R., Kasiri M., Dehghani M. 2020b. Neglected tropical disease of Rift Valley fever and its
impact on human, and animal health with emphasis on Iran: a review article. Entomology and Applied
Science Letters 7: 68-75.
Kassiri H., Dehghani R., Kasiri M., Dehghani M., Kasiri R. 2020c. A review on the reappearance of Crimean-
Congo hemorrhagic fever, a tick-borne Nairovirus. Entomology and Applied Science Letters 7: 81-90.
Kaveh A.A., Merat E., Samani S., Danandeh R., Soltannezhad S. 2017. Infectious Causes
of Bovine Abortion in Qazvin Province, Iran. Archives of Razi Institute 72: 225-230.
Kayedi M.H., Chinikar S., Mostafavi E., Khakifirouz S., Jalali T., Hosseini-Chegeni A., Naghizadeh A., Niedrig
M., Fooks A.R., Shahhosseini N. 2015. Crimean-Congo hemorrhagic fever virus clade IV (Asia 1) in
Kazemimanesh M., Madadgar O., Mahzoonieh M.R., Zahraei-Salehi T., Steinbach F. 2012. A serological study
on bovine leukemia virus infection in ten provinces of Iran between 2010 and 2012. Iranian Journal of
Virology 6: 1-7.
Kenawy M.A., Abdel-Hamid Y.M., Beier J.C. 2018. Rift Valley Fever in Egypt and other African countries:
Historical review, recent outbreaks and possibility of disease occurrence in Egypt. Acta Tropica 181: 40-49.
Keshtkar-Jahromi M., Sajadi M.M., Ansari H., Mardani M., Holakouie-Naini K. 2013. Crimean-Congo hemorrhagic
Keshtkar Jahromi M. 2014. Crimean-Congo hemorrhagic fever treatment and preventive strategies. International
419
Khalafalla A.I., Li Y., Uehara A., Hussein N.A., Zhang J., Tao Y., Bergeron E., Ibrahim I.H., Al Hosani M.A.,
Yusof M.F., Alhammadi Z.M., Alyammahi S.M., Gasim E.F., Ishag H.Z.A., Hosani F.A.I., Gerber
S.I., Almuhairi S.S., Tong S. 2021. Identification of a novel lineage of Crimean-Congo haemorrhagic
fever virus in dromedary camels, United Arab Emerates. Journal of General Virology 102: 2.
Khalesi B., Ebrahimi M.M., Ghodsian N., Kaffashi A., Shahkarami M.K., Ebrahimzadeh M.S. 2019. Evaluation of
efficacy of Razi fowl pox vaccine in comparison with the commercial fowl pox vaccine in SPF chickens
by challenge Test. Iranian Journal of Virology 13: 1-8.
Khalili Gheidariy M., Khalesi B., Ghaderi M., Taghizadeh M., Shahkarami M.K., Motamed N., Karimi Razakani
H. 2020. Evaluation and optimization of chick embryo fibroblasts for production of a fowl pox vaccine
based on cell culture. Iranian Journal of Virology 14: 6-15.
Khalilian M., Hosseini S.M., Madadgar O. 2019. Bovine leukemia virus detected in the breast tissue and blood
Khan A.S., Maupin G.O., Rollin P.E., Noor A.M., Shurie H.H.M., Shalabi A.G.A., Wasef S., Haddad Y.M.A.,
Sadek R., Ijaz K., Peters C.J., Ksiazek T.G. 1997. An outbreak of Crimean-Congo hemorrhagic fever in
the United Arab Emirates, 1994-1995. American Journal of Tropical Medicine and Hygiene 57: 519-525.
Khan N.A., Azhar E.I., El-Fiki S., Madani H.H., Abuljadial M.A., Ashshi A.M., Turkistani A.M., Hamouh E.A.
2008. Clinical profile and outcome of hospitalized patients during first outbreak of dengue in Makkah,
Khan E., Farooq J.Q., Barr K.L., Prakoso D., Nasir A., Kanji A., Shakoor S., Riaz Malik F., Hasari R., Lednicky
J.A., Long M.T. 2016. Flaviviruses as a cause of undifferentiated fever in Sindh Province, Pakistan:
Khanbabaie, H., Fakur, S., Khezri, M., Mohammadian, B., Rokhzad, B. (2011) Serological survey of bluetongue
disease in sheep of Sanandaj City by ELISA. Journal of Veterinary Medicine 5, 11-18.
Khezri M. 2012. Seroprevalence of bluetongue virus antibodies in sheep in Kurdistan Province in west of Iran.
International Journal for Agro Veterinary and Medical Sciences 6: 183-188.
Khezri M., Azimi S.M. 2012a. Investigation of bluetongue virus in Kurdish sheep in Kurdistan province of
Khezri M., Azimi S.M. 2012b. Seroprevalence and S7 gene characterization of bluetongue virus in the west of
Khezri M., Azimi S.M. 2013. Epidemiological investigation of bluetongue virus antibodies in sheep in Iran.
Khezri M., Bakhshesh M. 2014. Investigation of bluetongue in sheep in western Iran with an overview of infection
Khoda Karam Tafti A.A., Namdari I. 2000. Clinicopathological study of natural outbreaks of sheep pox in Fars
Province of Iran. Iranian Journal of Veterinary Research 1: 139-144.
Khoobdel M., Mehrabi Tavana A., Vatandoost H., Abaei M.R. 2008. Arthropod borne diseases in imposed war
during 1980-88. Iran. Journal of Arthropod-Borne Diseases 2: 28-36.
Kim S.Y., Jeong Y.E., Yun S.-M., Lee I.Y., Han M.G., Ju Y.R. 2009. Molecular evidence for tick-
borne encephalitis virus in ticks in South Korea. Medical and Veterinary Entomology 23: 15-20.
Kindhauser M.K., Allen T., Frank V., Shankar Santhana R., Dye C. 2016. Zika: the origin and
spread of a mosquito-borne virus. Bulletin of the World Health Organization 94: 675-686.
Kirkland P.D. 2015. Akabane virus infection. Revue Scientifique et Technique OIE 34: 403-410.
Kislenko G.S., Korotkov Iu.S., Shmakov L.V. 1987. The meadow tick Dermacentor reticulatus in natural foci of
tick-borne encephalitis in Udmurtia. Parazitologyia 21: 730-735 (Russian with English abstract).
Kitching R.P., Mellor P.S. 1986. Insect transmission of capripoxvirus. Research in Veterinary Science 40: 255-258.
Klein J.-M., Sureau P., Casals J., Piazak N., Kourouri C., Calvo M.-A. 1979. Isolment du virus Quaranfil en Iran
a partir de tiques Argas vulgaris. Cab. O.R.S.T.O.M., Ser. Ent. Med. Et Parasitol 17: 201-206 (French
with English abstract).
420
Kouhpayeh H. 2019. An overview of complications and mortality of Crimean-Congo hemorrhagic fever.
Kojouri G.A., Davoodi Z., Momtaz H. 2015. Serological and molecular detection of Akabane virus in Iran. Iranian
Journal of Applied Animal Science 5: 737-740.
Krinsky W.L. 1976. Animal disease agents transmitted by horse flies and deer flies (Diptera: Tabanidae). Journal
Krivanec K., Kopecky J., Tomkova E., Grubhoffer L. 1988. Isolation of TBE virus from the tick Ixodes hexagonus.
Folia Parasitologica 35: 273-276.
Kuhn J.H., Wiley M.R., Rodriguez S.E., Bào Y., Prieto K., Travassos da Rosa A.P.A., Guzman H., Savji N., Ladner
J.T., Tesh R.B., Wada J., Jahrling P.B., A. Bente D.A., Palacios G. 2016. Genomic characterization of the
Kumar S.M. 2011. An outbreak of lumpy skin disease in a Holtein dairy herd in Oman: A clinical report. Asian
Journal of Animal and Veterinary Advances 6: 851-859.
Kurogi H., Akiba K., Inaba Y., Matumoto M. 1987. Isolation of Akabane virus from the biting midge Culicoides
oxystoma in Japan. Veterinary Microbiology 15: 243-248.
Labuda M. 2001. Tahyna virus. In: Service M.W. (ed). Encyclopedia of Arthropod-Transmitted Infections of Man
and Domesticated Animals. CABI Publishing, Wallingford. 482-483.
Labuda M., Nuttall P.A. 2008. Viruses transmitted by ticks. In: Bowman A.S., Nuttall P.A. (eds). Ticks Biology,
Disease and Control. Cambridge University Press, Cambridge. 253-280.
Lane R.P., Crosskey R.W. 1993. Medical Insects and Arachnids. Chapman and Hall, London.
Larska M., Lechowski L., Grochowska M., Zmudzinski J.F. 2013. Detection of the Schmallenberg virus in
nulliparous Culicoides obsoletus/scoticus complex and C. punctatus - the possibility of transovarial
virus transmission in the midge population and of a new vector. Veterinary Microbiology 166: 467-473.
Lee V.H., Monath T.P., Tomori O., Fagbami A., Wilson D.C. 1974. Arbovirus studies in Nupeko forest, a possible
natural focus of yellow fever virus in Nigeria II. Entomological investigations and viruses isolated.
Transactions of the Royal Society of Tropical Medicine and Hygiene 68: 39-43.
Li F.Q. 1986. Experimental study on natural infection, biting and transovarial transmission of epidemic
haemorrhagic fever virus in gamasid mites. Chinese Journal of Epidemiology 7: 200-202 (Chinese).
Li W.J., Wang J.L., Li M.H., Fu S.H., Wang H.Y., Wang Z.Y., Jiang S.Y., Wang X.W., Guo P., Zhao S.-C., Shi
Y., Lu N.-N., Nasci R.S., Tang Q., Liang G.-D. 2010. Mosquitoes and mosquito-borne arboviruses in the
Qinghai-Tibet plateau-focused on the Qinghai area, China. American Journal of Tropical Medicine and
Lichard M., Kozuch O. 1967. Persistence of tick-borne encephalitis virus in nymphs and adults of Ixodes
arboricola and its transmission to white mice. Acta Virologica 11: 480.
Linley R.J., Hoch A.L., Pinheiro F.P. 1983. Biting midges (Diptera: Ceratopogonidae) and human health. Journal
Lockwood J.A. 2012. Insects as weapons of war, terror, and torture. Annual Review of Entomology 57: 205-227.
Lotfollahzade S., Nikbakht Brujen G., Mokhber Dezfouli M.R., Mahdavi Pak S., Raoofi A., Tajik P., Mostafavii
E. 2009. Study on the detection of specific antibody (IgG) to Crimean-Congo hemorrhagic fever (CCHF)
virus in blood serum of dairy cows in Khorasan. Journal of Veterinary Research 63: 311-316.
Lotfollahzadeh S., Nikbakht Boroujeni G.R., Mokhber Dezfouli M.R., Bokaei S. 2011. A Serosurvey of Crimean-
Congo haemorrhagic fever virus in dairy cattle in Iran. Zoonoses Public Health 58: 54-59.
Lubinga J.C., Tuppurainen E.S.M., Stoltsz W.H., Ebersohn K., Coetzer J.A.W., Venter E.H. 2013a. Detection of
lumpy skin disease virus in saliva of ticks fed on lumpy skin diseae virus-infected cattle. Experimental
Lubinga J.C., Tuppurainen E.S.M., Mahlare R., Coetzer J.A.W., Stoltsz W.H., Venter E.H. 2013b. Evidence of
transstadial and mechanical transmission of lumpy skin disease virus by Amblyomma hebraeum ticks.
Lubinga J.C., Tuppurainen E.S.M., Coetzer J.A.W., Stoltsz W.H., Venter E.H. 2014a. Evidence of lumpy
skin disease virus over-wintering by transstadial persistence in Amblyomma hebraeum and transovarial
421
persistence in Rhipicephalus decoloratus ticks. Experimental and Applied Acarology 62: 77-90.
Lubinga J.C., Clift S.J., Tuppurainen E.S.M., Stoltsz W.H., Babiuk S., Coetzer J.A.W., Venter E.H. 2014b.
Demonstration of lumpy skin disease virus infection in Amblyomma hebraeum and Rhipicephalus
appendiculatus ticks using immunohistochemistry. Ticks and Tick-Borne Diseases 5: 113-120.
Luedke A.J., Jochim M.M., Bowne J.G. 1965. Preliminary bluetongue transmission with the sheep ked Melophagus
ovinus (L.). Canadian Journal of Comparative Medicine And Veterinary Science 29: 229-231.
Lundström J.O., Pfeffer M. 2010. Phylogeographic structure and evolutionary history of Sindbis virus. Vector-
Lvov D.K., Kurbanov M.M., Neronov V.M., Gromashevsky V.L., Skvortsova T.M., Gofman Yu.P., Klimenko
S.M., Berdyev A., Kiseleva N.V., Vatolin V.P., Aristova V.A. 1967. Isolation of Wad Medani arbovirus
from Hyalomma asiaticum Sch. and Schl. 1929 ticks in Turkmen SSR. Medical Parasitology and Parasitic
Diseases 45: 452-455 (Russian, English translation, NAMRU3-T1236).
Lvov D.K., Gromashevsky V.L., Sidorova G.A., Tsirkin Yu.M., Chervonsky V.I., Gostinshchikova G.V., Aristova
V.A. 1971. Isolation of a new arbovirus Baku of Kemerovo group from argasid ticks Ornithodoros coniceps
in Azerbaijan. Voprosy Virusologii 16: 434-437 (Russian, English translation, NAMRU3-T1401).
Lvov D.K., Karas F.R., Tsyrkin Yu.M., Vargina S.G., Timofeev E.M., Osipova N.Z., Veselovskaya O.V., Grebenyuk
Yu.I., Gromashevski V.L., Fomina K.B. 1974. Batken virus, a new arbovirus isolated from ticks and
mosquitoes in Kirghiz S.S.R. Archiv fur die Gesamte Virusforschung 44: 70-73.
Lvov D.K. 1994. Arboviral Zoonoses of Northern Eurasia (Eastern Europe and the Commonwealth of Independent
States). In: Beran G.W. (ed). Handbook of Zoonoses. Section B: Viral. Second Edition. CRC Press, Boca
Raton. 237-260.
Lvov D.K., Alkhovsky S.V., Shchelkanov M.Iu., Shchetinin A.M., Deriabin P.G., Samokhvalov E.I., Gitelman
A.K., Botikov A.G. 2014a. Genetic characterization of the Caspiy virus (CASV) (Bunyaviridae, Nairovirus)
isolated from the Laridae (Vigors, 1825) and Sternidae (Bonaparte, 1838) birds and the Argasidae (Koch,
1844) ticks Ornithodoros capensis Neumann, 1901, in western and eastern coasts of the Caspian Sea.
Voprosy Virusologii 59(1): 24-29 (Russian with English abstract).
Lvov D.K., Alkhovsky S.V., Shchelkanov M.Iu., Shchetinin A.M., Aristova V.A., Gitelman A.K., Deriabin P.G.,
Botikov A.G. 2014b. Taxonomy of previously unclassified Tamdy virus (TAMV) (Bunyaviridae, Nairovirus)
isolated from the Hyalomma asiaticum asiaticum Schülce et Schlottke, 1929 (Ixodidae, Hyalomminae) in
the Middle East and Transcaucasia. Voprosy Virusologii 59(2): 15-22 (Russian with English abstract).
Lvov D.K., Alkhovsky S.V., Shchelkanov M.Iu., Deriabin P.G., Shchetinin A.M., Samokhvalov E.I., Aristova V.A.,
Gitelman A.K., Botikov A.G. 2014c. Genetic characterization of the Geran virus (GERV, Bunyaviridae,
Nairovirus, Qalyub group) isolated from the ticks Ornithodoros verrucosus Olenev, Zasukhin and Fenyuk,
1934 (Argasidae) collected in the burrow of Meriones erythrourus Grey, 1842 in Azerbaijan. Voprosy
Virusologii 59(5): 13-18 (Russian with English abstract).
Maan S., Maan N.S., Nomikou K., Batten C., Antony F., Belaganahalli M.N., Samy A.M., Abdel Reda A.,
Al-Rashid S.A., El Batel M., Oura C.A.L., Mertens P.P.C. 2011a. Novel bluetongue virus serotype from
Maan S., Maan N.S., Nomikou K., Veronesi E., Bachanek-Bankowska K., Belaganahalli M.N., Attoui H., Mertens
P.P.C. 2011b. Complete genome characterisation of a novel 26th bluetongue virus serotype from Kuwait.
Maclachlan N.J., Mayo C.E., Daniels P.W., Savini G., Zientara S., Gibbs E.P.J. 2015. Bluetongue. Revue
Scientifique et Technique OIE 34: 329-340.
Madani T.A. 2005. Alkhumra virus infection, a new viral hemorrhagic fever in Saudi Arabia. Journal of Infection
Maes P., Alkhovsky S.V., Bào Y., Beer M., Birkhead M., Briese T. et al. (2018) Taxonomy of the family
Arenaviridae and the order Bunyavirales: update 2018. Archives of Virology 163: 2295-2310.
Maghsood H., Nabian S., Shayan P., Jalali T., Saboor Darbandi M., Ranjbar M.M. 2020. Molecular epidemiology
and phylogeny of Crimean-Congo haemorrhagic fever (CCHF) virus of ixodid ticks in Khorasan Razavi
422
Mahdavi S, Khedmati K., Pishraft-Sabet L. 2006. Serologic evidence of bluetongue infection in one humped
camels (Camelus dromedarius) in Kerman Province, Iran. Iranian Journal of Veterinary Research 7: 85-87.
Mahdi M., Erickson B.R., Comer J.A., Nichol S.T., Rollin P.E., AlMazroa M.A., Memish Z.A. 2011. Kyasanur
Forest disease virus Alkhurma subtype in ticks, Najran Province, Saudi Arabia. Emerging Infectious
Mahzounieh M., Dincer E., Faraji A., Akin H., Akkutay A.Z., Ozkul A. 2012. Relationship between Crimean-
Congo hemorrhagic fever virus strains circulating in Iran and Turkey: possibilities for transborder
Malik M.R., Mnzava A., Mohareb E., Zayed A., Al Kohlani A., Thabet A.A.K.,, El Bushra H. 2014.
Chikungunya outbreak in Al-Hudaydah, Yemen, 2011: epidemiological characterization and key lessons
learned for early detection and control. Journal of Epidemiology and Global Health 4: 203-211.
Manavian M., Hashemi M., Nikoo Farhang D., Hosseini S.M.H., Bakhshesh M., Marhamatizade M.H. 2017.
Seroprevalence of bluetongue virus infection and associated risk factors in domestic ruminants in the south
of Iran. The Thai Journal of Veterinary Medicine 47: 225-231.
Mardani M. 2019. Two-decade experience of Crimean-Congo hemorrhagic fever (CCHF) management in Iran.
Marenzoni M.L., Cuteri V., de Parri F., Denzetta M.L., Yilmaz Z., Yarmis C.P., Kennerman E., Or M.E., Marchi
S., Casciari C., de Mia G.M., Valente C., Costarelli S. 2013. A pilot study on the epidemiological status
of equine infectious anaemia, equine viral arteritis, glanders, and dourine in Turkey. Turkish Journal of
Veterinary, Animal Sciences 37: 76-80.
Matevosyan K.Sh., Semashko I.V., Chumakov M.P. 1974. Isolation of Bhanja virus from Dermacentor marginatus
ticks in Armenian SSR. Zh. Eksp. Klin. Med 14: 9-13 (Russian, English translation, NAMRU3-T1385).
Matsuno K., Weisend C., Travassos da Rosa A.P.A., Anzick S.L., Dahlstrom E., Porcella S.F., Dorward D.W.,
Yu X.-J., Tesh R.B., Ebihara H. 2013. Characterization of the Bhanja serogroup viruses (Bunyaviridae):
a novel species of the genus Phlebovirus and its relationship with other emerging tick-borne phleboviruses.
Journal of Virology 87: 3719-3728.
McCarthy M.C., Haberberger R.L., Salib A.W., Soliman B.A., El-Tigani A., Khalid I.O., Watts D.M.
1996. Evaluation of arthropod-borne viruses and other infectious disease pathogens as the causes
of febrile illnesses in the Khartoum Province of Sudan. Journal of Medical Virology 48: 141-146.
Mehrabadi F., Ghalyanchilangeroudi A., Charkhkar S., Hosseini H., Zabihipetroudi T., Shayganmehr
A., Esmaeelzadeh Dizaji R., Aghaeean L. 2020. Fowlpox outbreak in a laying farm: up to
date data on phylogenetic analysis in Iran, 2018. Archives of Razi Institute 75: 501-508.
Mehrabi-Tavana A. 1999. The seroepidemiological studies of sand fly fever during the imposed war, 1980-88.
Hakim Research Journal 2: 14-17 (Persian).
Mehrabi Tavana A., Javadian E., Nategh R., Shojaei A. 2000. First report of sandfly fever in west of Iran. Journal
of Tropical Medicine and Infectious Diseases 5: 59-62.
Mehrabi-Tavana A. 2001. The seroepidemiological studies of sand fly fever in Iran during imposed war. Iranian
Journal of Public Health 30: 145-146.
Mehrabi-Tavana A. 2007. Minireview on sand fly fever. Journal Entomolology 4: 401-403.
Mehrabi-Tavana A. 2012. Sand fly fever: the disease which must be introduced to doctors, health care workers
and public now. Health Med 6: 3657-3659.
Mehrabi-Tavana A. 2015. Sandfly fever in the world. Annals of Tropical Medicine and Public Health 8: 83-87.
Mehrabi-Tavana A. 2017a. Sand fly fever with different names. Journal of Arthropod-Borne Diseases 11: 171.
Mehrabi-Tavana A. 2017b. Sand fly fever: an important vector-borne disease for travelers? Annals of Tropical
Medicine and Public Health 10: 13.
Mehrabi-Tavana A. 2017c. May sandfly fever be seen with leishmaniasis as coinfection or not? Annals of Tropical
Medicine and Public Health 10: 263-264.
Mehrabi-Tavana A. 2017d. Could sandfly fever be isolated from HIV/AIDS patients as coinfection or not? Annals
of Tropical Medicine and Public Health 10: 1070-1071.
423
Mehrabi-Tavana A. 2017e. Can sandfly fever be mistaken with influenza? Annals of Tropical Medicine and Public
Health 10: 1085-1086.
Mehrabi-Tavana A. 2017f. Still sand fly fever is unknown: the disease which must be introduced to medical,
nurse, and health-care workers at their courses in particular in tropical and semitropical regions. Annals
of Tropical Medicine and Public Health 10: 1093-1094.
Mehravaran A., Moradi M., Telmadarraiy Z., Mostafavi E., Moradi A.R., Khakifirouz S., Shah-Hosseini N., Sadat
Rasi Varaie F., Jalali T., Hekmat S., Ghiasi S.M., Chinikar S. 2013. Molecular detection of Crimean-Congo
haemorrhagic fever (CCHF) virus in ticks from southeastern Iran. Ticks and Tick-Borne Diseases 4: 35-38.
Mellor P.S., Boorman J., Jennings M. 1975. The multiplication of African horse-sickness virus in two species of
Culicoides (Diptera, Ceratopogonidae). Archives of Virology 47: 351-356.
Mellor P.S., Kitching R.P., Wilkinson, P.J. 1987. Mechanical transmission of capripox virus and African swine
fever virus by Stomoxys calcitrans. Research in Veterinary Science 43: 109-112.
Mellor P.S., Hamblin C., Graham S.D. 1990a. African horse sickness in Saudi Arabia. Veterinary Record 127:
41-42.
Mellor P.S., Boned J., Hamblin C., Graham S.D. 1990b. Isolations of African horse sickness virus from vector
insects made during the 1988 epizootic in Spain. Epidemiology, Infection 105: 447-454.
Mellor P.S. 1994 Epizootiology and vectors of African horse sickness virus. Comparative Immunology,
Microbiology, Infectious Diseases 17: 287-296.
Mellor P.S., Boorman J., Baylis M. 2000. Culicoides biting midges: their role as arbovirus vectors. Annual Review
of Entomology 45: 307-340.
Mellor P.S. 2001a. African horse sickness. In: Service M.W. (ed). Encyclopedia of Arthropod-Transmitted
Infections of Man and Domesticated Animals. CABI Publishing, Wallingford. 3-7.
Mellor P.S. 2001b. Aino virus. In: Service M.W. (ed). Encyclopedia of Arthropod-Transmitted Infections of Man
and Domesticated Animals. CABI Publishing, Wallingford. 23-25.
Mellor P.S. 2001c. Akabane virus. In: Service M.W. (ed). Encyclopedia of Arthropod-Transmitted Infections of
Man and Domesticated Animals. CABI Publishing, Wallingford. 25-27.
Mellor P.S. 2001d. Bluetongue virus. In: Service M.W. (ed). Encyclopedia of Arthropod-Transmitted Infections
of Man and Domesticated Animals. CABI Publishing, Wallingford. 78-83.
Mellor P.S. 2001e. Epizotic haemorrhagic disease. In: Service M.W. (ed). Encyclopedia of Arthropod-Transmitted
Infections of Man and Domesticated Animals. CABI Publishing, Wallingford. 174-176.
Mellor P.S., Hamblin C. 2004. African horse sickness. Veterinary Research 35: 445-466.
Mellor P.S., Carpenter S., Harrup L., Baylis M., Mertens P.P.C. 2008. Blutongue in Europe and the
Mediterranean Basin: history of occurrence prior to 2006. Preventive Veterinary Medicine 87: 4-20.
Mellor P.S., Baylis M., Mertens P.P.C. 2009. Bluetongue. Academic Press, London.
Memish Z.A., Charrel R.N., Zaki A.M., Fagbo S.F. 2010. Alkhurma haemorrhagic fever—a viral haemorrhagic
disease unique to the Arabian Peninsula. International Journal of Antimicrobial Agents 36: S53-S57.
Memish Z.A., Albarrak A., Almazroa M.A., Al-Omar I., Alhakeem R., Assiri A., Fagbo S., MacNeil A., Rollin
P.E., Abdullah N., Stephens G. 2011. Seroprevalence of Alkhurma and other hemorrhagic fever viruses,
Mikryukova T.P., Moskvitina N.S., Kononova Y.V., Korobitsyn I.G., Kartashov M.Y., Tyuten′kov O.Y., Protopopova
E.V., Romanenko V.N., Chausov E.V., Gashkov S.I., Konovalova S.N., Moskvitin S.S., Tupota N.L.,
Sementsova A.O., Ternovoi V.A., Loktev V.B. 2014. Surveillance of tick-borne encephalitis virus in wild
birds and ticks in Tomsk city and its suburbs (Western Siberia). Ticks and Tick-Borne Diseases 5: 145-151.
Mirchamsy H., Hazrati A. 1973. A review of aetiology and pathology of African horsesickness. Archives of Razi
Institute 25: 23-46.
Mirzaei K., Barani S.M., Bokaie S. 2015. A review of sheep pox and goat pox: perspective of their control and
eradication in Iran. Journal of Advanced Veterinary and Animal Research 2: 373-381.
Mirzazadeh A., Matos M., Emadi-Jamali S., Dieter Liebhart D., Hess M. 2021. Atypical manifestation of cutaneous
fowlpox in broiler chickens associated with high condemnation at a processing plant. Avian Diseases 65:
424
Mirzoeva N.M., Sultanova Z.D., Kanbai I.G., Obukhova V.R., Gaidamovich S.Ya., Sokolova E.I., Kulieva N.M.
1974. Biological properties of West Nile virus strains isolated in Azerbaijan. In: Gaidamovich S.Ya. (ed).
Arboviruses. Sborn. Trud. Inst. Virus. Imeni S.I. Ivanovsky, Akad. Med. Nauk SSSR, 1: 119-122 (Russian,
English translation, NAMRU3-T1159).
Mo C.L., Thompson L.H., Homan E.J., Oviedo M.T., Greiner E.C., González J., Sáenz M.R. 1994. Bluetongue
virus isolations from vectors and ruminants in Central America and the Caribbean. American Journal of
Veterinary Research 55: 211-215.
Mohajer F., Sheikh Y., Staji H., Keyvanloo M., Hashemzadeh H. 2019. Evaluation of the seroprevalence of
Akabane and bluetongue viruses using competitive-ELISA in dairy cattle from industrial herds, Semnan
Mohamed M.E.H., Mellor P.S., Taylor W.P. 1996. Akabane virus: serological survey of antibodies in livestock in
the Sudan. Revue d'Elevage et de Médecine Vétérinaire des Pays Tropicaux 49: 285-288.
Mohammad M.E., Taylor W.P. 1987. Infection with bluetongue and related orbiviruses in the
Sudan detected by the study of sentinel calf herds. Epidemiology, Infection 99: 533-545.
Mohammad M.E., Mellor P. 1990. Further studies on bluetongue and bluetongue-related orbiviruses in the Sudan.
Mohammadabadi M.R., Soflaei M., Mostafavi H., Honarmand M. 2011. Using PCR for early diagnosis of
bovine leukemia virus infection in some native cattle. Genetics and Molecular Research 10: 2658-2663.
Mohammadi V., Atyabi N., Nikbakht Brujeni Gh., Lotfollahzadeh S., Mostafavi E. 2011. Seroprevalence of bovine
leukemia virus in some dairy farms in Iran. Global Veterinaria 7: 305-309.
Mohammadi A., Tanzifi P., Nemati Y. 2012. Seroepidemiology of bluetongue disease and risk factors in small
ruminants of Shiraz suburb, Fars Province, Iran. Tropical Biomedicine 29: 632-637.
Mohammadian M., Chinikar S., Telmadarraiy Z., Vatandoost H., Oshaghi M.A., Hanafi-Bojd A.A., Sedaghat
M.M., Noroozi M., Faghihi F., Jalali T., Khakifirouz S., Shahhosseini N., Farhadpour F. 2016. Molecular
assay on Crimean Congo hemorrhagic fever virus in ticks (Ixodidae) collected from Kermanshah Province,
western Iran. Journal of Arthropod-Borne Diseases 10: 383-393.
Mollazadeh S., Bakhshesh M., Keyvanfar H., Nikbakht Brujeni G. 2022. Identification of cytotoxic T
lymphocyte (CTL) Epitope and design of an immunogenic multi-epitope of bovine ephemeral fever
virus (BEFV) glycoprotein G for vaccine development. Research in Veterinary Science 144: 18-26.
Momtaz H., Nejat S. 2010. Detection of proviral sequences of equine infectious anemia virus in peripheral blood
cells of horses in Iran. Bulgarian Journal of Veterinary Medicine 13: 18-22.
Momtaz H., Nejat S., Souod N., Momeni M., Safari S. 2011. Comparisons of competitive enzyme-linked
immunosorbent assay and one step RT-PCR tests for the detection of bluetongue virus in south west of
Iran. African Journal of Biotechnology 10 (36): 6857-6862.
Monath T.P. 2001. Yellow fever. In: Service M.W. (ed). Encyclopedia of Arthropod-Transmitted Infections of
Man and Domesticated Animals. CABI Publishing, Wallingford. 571-577.
Moradi-Asl E., Rassi Y., Hanafi-Bojd A.A., Saghafipour A. 2019. Spatial distribution and infection rate of
leishmaniasis vectors (Diptera: Psychodidae) in Ardabil Province, northwest of Iran. Asian Pacific Journal
Morovati H., Shirvani E., Noaman V., Lotfi M., Kamalzadeh M., Hatami A., Bahreyari M., Shahramyar Z.,
Morovati M.H., Azimi M., Sakhaei D. 2012. Seroprevalence of bovine leukemia virus (BLV) infection
in dairy cattle in Isfahan Province, Iran. Tropical Animal Health and Production 44: 1127-1129.
Morovvati A., Ghalyanchi-Langeroudi A., Soleimani M., Mousavi-Nasab S.D., Majidzadeh A.K. 2012. Emergence
of a new genotype of Crimean-Congo hemorrhagic fever virus in Iran. Iranian Journal of Virology 6: 24-29.
Mostafavi E., Chinikar S., Esmaeili S., Bagheri Amiri F., Tabrizi A.M.A., Khakifirouz S. 2012. Seroepidemiological
survey of Crimean-Congo hemorrhagic fever among sheep in Mazandaran Province, northern Iran. Vector-
425
Mostafavi E., Haghdoost A.A., Khakifirouz S., Chinikar S. 2013a. Spatial analysis of Crimean Congo
hemorrhagic fever in Iran. American Journal of Tropical Medicine and Hygiene 89: 1135-1141.
Mostafavi E., Chinikar S., Moradi M., Bayat N., Meshkat M., Khalili Fard M., Ghiasi S.M. 2013b. A case
report of Crimean Congo hemorrhagic fever in ostriches in Iran. Open Virology Journal 7: 81-83.
Mostafavi E., Pourhossein B., Chinikar S. 2014. Clinical symptoms and laboratory findings supporting early
diagnosis of Crimean-Congo hemorrhagic fever in Iran. Journal of Medical Virology 86: 1188-1192.
Mostafavi E., Pourhossein B., Esmaeili S., Bagheri Amiri F., Khakifirouz S., Shah-Hosseini N., Tabatabaei
S.M. 2017. Seroepidemiology and risk factors of Crimean-Congo hemorrhagic fever among butchers and
slaughterhouse workers in southeastern Iran. International Journal of Infectious Diseases 64: 85-89.
Moucha J. 1976. Horse-flies (Diptera: Tabanidae) of the World Synoptic Catalogue. Acta Entomologica Musei
Nationalis Pragae, Supplementum 7. National Museum (Natural History), Pragae.
Mourya D.T., Yadav P.D., Nyayanit D.A., Majumdar T.D., Jain S., Sarkale P., Shete A. 2019. Characterization
of a strain of quaranfil virus isolated from soft ticks in India. Is quaranfil virus an unrecognized cause of
Mousavi S. Haghparast A., Mohammadi G., Tabatabaeizadeh S.E. 2014. Prevalence of bovine leukemia virus
(BLV) infection in the northeast of Iran. Veterinary Research Forum 5: 135-139.
Mozaffari A.A., Khalili M., Yahyazadeh F. 2012. A serological investigation of bluetongue virus in cattle of
south-east Iran. Veterinaria Italiana 48: 41-44.
Mozaffari A.A., Khalili M. 2012. The first survey for antibody against bluetongue virus in sheep flocks
in southeast of Iran. Asian Pacific Journal of Tropical Biomedicine 2(Suppl 3): S1808-S1810.
Mozaffari A.A., Sakhaee E., Khalili M., Ardakani A.P. 2013. High seroprevalence of bluetongue virus (BTV)
antibodies in camel in Yazd Province of Iran. Journal of Camel Practice and Research 20: 171-173.
Mozaffari A.A., Khalili M., Sabahi S. 2014. High seroprevalence of bluetongue virus antibodies in
goats in southeast Iran. Asian Pacific Journal of Tropical Biomedicine 4(Suppl 1): S275-278.
Muhsen R.K. 2012. Seroepidemiology of Rift Valley fever in Basrah. Kufa Journal for Veterinary Medical Sciences
3: 91-95.
Mullen G., Durden L. 2019. Medical and Veterinary Entomology. 3rd Edition. Academic Press, Burlington.
Mustafa A.S., Elbishbishi E.A., Grover S., Pacsa A.S., Al-Enezi A.A., Chaturvedi U.C. 2001. A study of dengue
imported to Kuwait during 1997-1999. Acta Virologica 45: 125-128.
Naderi H.R., Sheybani F., Bojdi A., Khosravi N., Mostafavi I. 2013. Fatal nosocomial spread of Crimean-Congo
hemorrhagic fever with very short incubation period. American Journal of Tropical Medicine and Hygiene
Najarnezhad V., Rajae M. 2013. Seroepidemiology of bluetongue disease in small ruminants of north-east of Iran.
Namazi F., Khodakaram Tafti A. 2021. Lumpy skin disease, an emerging transboundary viral disease: a review.
Nashed N.W., Olson J.G., El-Tigani A. 1993. Isolation of Batai virus (Bunyaviridae: Bunyavirus) from the blood
of suspected malaria patients in Sudan. Americam Journal of Tropical Medicine and Hygiene 48: 676-681.
Nasirian H. 2019. Crimean-Congo hemorrhagic fever (CCHF) seroprevalence: a systematic review and meta-
Nasirian H. 2020. New aspects about Crimean-Congo hemorrhagic fever (CCHF) cases and associated fatality
trends: a global systematic review and meta-analysis. Comparative Immunology, Microbiology, Infectious
Navai S. 1974. Studies of the Culicoides of Iran. Annales de Parasitologie Humaine et Comparée 49: 645-648.
Naumov R.L., Gutova V.P. 1984. Experimental study of the participation of gamasid mites and fleas in circulating
the tick-borne encephalitis virus (a review). Parazitologyia 18: 106-115 (Russian).
426
Nekoei S., Taktaz Hafshejani T., Doosti A., Khamesipour F. 2015a. Molecular detection of bovine leukemia virus
in peripheral blood of Iranian cattle, camel and sheep. Polish Journal of Veterinary Sciences 18: 703-707.
Nekoei S., Fattahian A., Momeni H., Raki A. 2015b. Advances in Schmallenberg virus research: a review.
Biosciences Biotechnology Research Asia 12: 931-938.
Niazi S.K., Alam M., Yazdani M.S., Ghani E., Rathore M.A. 2017. Nucleic acid amplification test for detection
of West Nile virus infection in Pakistani blood donors. Journal of Ayub Medical College Abbottabad 29:
547-550.
Nikbakht G., Tabatabaei S., Lotfollahzadeh S., Nayeri Fasaei B., Alireza Bahonar A., Khormali M. 2015.
Seroprevalence of bovine viral diarrhoea virus, bovine herpesvirus 1 and bovine leukaemia virus in
Iranian cattle and associations among studied agents. Journal of Applied Animal Research 43: 22-25.
Nikbakht Brujeni G., Poorbazargani T., Nadin-Davis S., Tolooie M., Barjesteh N. 2010. Bovine immunodeficiency
virus and bovine leukemia virus and their mixed infection in Iranian Holstein cattle. Journal of Infection
in Developing Countries 4: 576-579.
Nikolaev V.P., Perepelkin V.S., Raevski K.K., Prusakova Z.M. 1991. A natural focus of sandfly fever in the
Republic of Afghanistan. Zhurnal Mikrobiologii, Epidemiologii i Immunobiologii 3: 39-41 (Russian).
Nikoonejad A.R., Bijani B. 2016. Report of the first case of Crimean Congo hemorrhagic fever in Qazvin Province
(2016). Journal of Qazvin University of Medical Sciences 20: 67-73 (Persian with English abstract).
Nikookar S.H., Fazeli-Dinan M., Azari-Hamidian S., Mousavi Nasab S.N., Aarabi M., Ziapour S.P., Enayati A.,
Hemingway J. 2018. Fauna, ecological characteristics, and checklist of the mosquitoes in Mazandaran
Nikookar S.H., Fazeli-Dinana M., Enayati A., Zaim M. 2020. Zika; a continuous global threat to public health.
Nili S., Khanjani N., Jahani Y., Bakhtiari B. 2020. The effect of climate variables on the incidence of
Crimean Congo hemorrhagic fever (CCHF) in Zahedan, Iran. BMC Public Health 20: 1893.
Njeumi F., Taylor W., Diallo A., Miyagishima K., Pastoret P.-P., Vallat B., Traore M. 2012. The long journey:
a brief review of the eradication of rinderpest. Revue Scientifique et Technique OIE 31: 729-746.
Noaman V., Kargar-Moakhar R., Shahmoradi A.H., Heidari M.R., Tabatabaei J., Nabinejad A.R. 2008. Use of
competitive ELISA for serological detection of bluetongue virus antibody in sheep and goats of Isfahan
province, Iran. Animal Science Journal 21: 39-48.
Noaman V., Shirvani E., Hosseini S.M., Shahmoradi A.H., Heidari M.R., Raiszadeh H., Kamalzadeh M., Bahreyari
M. 2013. Serological surveillance of bluetongue virus in cattle in central Iran. Veterinaria Italiana 49:
141-144.
Noaman V., Arzani H. 2017. Environmental and host factors affecting seroprevalence of bluetongue virus infections
of sheep. Comparative Clinical Pathology 26: 397-403.
Noaman V., Nabinejad A.R. 2020. Seroprevalence and risk factors assessment of the three main infectious agents
associated with abortion in dairy cattle in Isfahan Province, Iran. Tropical Animal Health and Production
Noorbakhsh F., Abdolmohammadi K., Fatahi Y., Dalili H., Rasoolinejad M., Rezaei F., Salehi-Vaziri M., Shafiei-
Jandaghi N.Z., Gooshki E.S., Zaim M., Nicknam M.H. 2019. Zika virus infection, basic and clinical
aspects: a review article. Iranian Journal of Public Health 48: 20-31.
Norian R., Afzal Ahangran N., Varshovi H.R., Azadmehr A. 2016. Evaluation of humoral and cell-mediated
immunity of two capripoxvirus vaccine strains against lumpy skin disease virus. Iranian Journal of Virology
10: 1-11.
Noroozikia S., Pourmahdi-Borujeni M., Haji Hajikolaei M.R., Seifi M.R. 2014. Seroepidemiological survey of
bluetongue disease in sheep in Khuzestan Province. Iranian Veterinary Journal 10: 103-111 (Persian with
English abstract).
Norouzi B., Hanafi-Bojd A.A., Moin-Vaziri V., Noorallahi A., Azari-Hamidian S. 2020. An inventory of the
sand flies (Diptera: Psychodidae) of Rudbar County, a new focus of leishmaniasis in northern Iran,
with a taxonomic note on the subgenus Larroussius. Journal of Arthropod-Borne Diseases 14: 302-316.
427
Nosek J., Ciampor F., Kozuch O., Rajcani J. 1972. Localization of tick-borne encephalitis virus in alveolar cells of
salivary glands of Dermacentor marginatus and Haemaphysalis inermis ticks. Acta Virologica 16: 493-497.
Nosek J., Kožuch O. 1985. Replication of tick-borne encephalitis virus in ticks Dermacentor marginatus.
Angewandte Parasitologie 26: 97-101.
Nsoesie E.O., Kraemer M.U., Golding N., Pigott D.M., Brady O.J., Moyes C.L., Johansson M.A.,
Gething P.W., Velayudhan R., Khan K., Hay S.I., Brownstein J.S. 2016. Global distribution
and environmental suitability for chikungunya virus, 1952 to 2015. Eurosurveillance 21: 20.
Nuttall P.A. 2001. Crimean-Congo haemorrhagic fever. In: Service M.W. (ed). Encyclopedia of Arthropod-
Transmitted Infections of Man and Domesticated Animals. CABI Publishing, Wallingford. 126-132.
Oldfield III E.C., Rodier G.R., Gray G.C. 1993. The Endemic Infectious Diseases of Somalia. Clinical infectious
diseases 16(Suppl. 3): S132-S157.
Olsen A.S., Hansen M.F., Rasmussen T.B., Belsham G.J., Bødker R., Bøtner A. 2018a. Survival and localization
of African swine fever virus in stable flies (Stomoxys calcitrans) after feeding on viremic blood using
Olsen A.S., Lohse L., Hansen M.F., Boklund A., Halasa T., Belsham G.J., Rasmussen T.B., Bøtner A., Bødker R.
2018b. Infection of pigs with African swine fever virus via ingestion of stable flies (Stomoxys calcitrans).
Oncul O., Atalay Y., Onem Y., Turhan V., Acar A., Uyar Y., Caglayik D.Y., Ozkan S., Gorenek L.
2011. Hantavirus infection in Istanbul, Turkey. Emerging Infectious Diseases 17: 303-304.
Oryan A., Amrabadi O., Mohagheghzadeh M. 2014. Seroprevalence of bluetongue in sheep and goats in southern
Iran with and overview of four decades of its epidemiological status in Iran. Comparative Clinical Pathology
Osman H.A.M., Eltom K.H., Musa N.O., Bilal N.M., Elbashir M.I., Aradaib I.E. 2013. Development and evaluation
of loop-mediated isothermal amplification assay for detection of Crimean Congo hemorrhagic fever virus
Owaysee Oskooei H., Eini P., Nasiroghli Khiyabani F. 2008. A pregnant woman with Crimean-Congo hemorrhagic
fever. Avicenna Journal of Clinical Medicine (Former Scientific Journal of Hamedan University of Medical
Sciences) 14: 64-67 (Persian with English abstract).
Oya A., Okuno T., Ogata T., Kobayashi I., Matsuyama T. 1961. Akabane, a new arbor virus isolated in Japan.
Japanese Journal of Medical Science and Biology 14: 101-108.
Pacsa A.S., Elbishbishi E.A., Chaturvedi Chu K.Y., Mustafa A.S. 2002. Hantavirus-specific antibodies
in rodents and human living in Kuwait. FEMS Immunology, Medical Microbiology 33: 139-142.
Pacsa A.S., Chaturvedi U.C., Mustafa A.S. 2003. Seroprevalence of three emerging arboviral infections in Kuwaiti
Pages N., Talavera S., Verdun M., Pujol N., Valle M., Bensaid A., Pujol J. 2018. Schmallenberg virus detection
in Culicoides biting midges in Spain: first laboratory evidence for highly efficient infection of Culicoides
of the Obsoletus complex and Culicoides imicola. Transboundary and Emerging Diseases 65: 1-6.
Palacios G., Savij N., da Rosa A.T., Guzman H., Yu X., Desai A., Rosen G.E., Hutchinson S., Lipkin W.I., Tesh
R. 2013. Characterization of the Uukuniemi virus group (Phlebovirus: Bunyaviridae): evidence for seven
Parhizgari N., Gouya M.M., Mostafavi E. 2017. Emerging and re-emerging infectious diseases in Iran. Iran
Journal of Microbiology 9: 122-142.
Parhizgari N., Piazak N., Mostafavi E. 2021. Vector-borne diseases in Iran: epidemiology and key challenges.
Pavri K.M., Anandarajah M., Hermon Y.E., Nayar M., Wikramsinghe M.R., Dandawate C.N. 1976. Isolation of
Wanowrie virus from brain of a fatal human case from Sri Lanka. Indian Journal of Medical Research
64: 557-561.
428
Pasandideh R., Seyfi Abad Shapouri M.R., Beigi Nassiri M.T. 2018a. Immunogenicity of a plasmid DNA vaccine
encoding G1 epitope of bovine ephemeral fever virus G glycoprotein in mice. Onderstepoort Journal of
Veterinary Research 85: 1.
Pasandideh R., Beigi Nassiri M.T., Seyfi Abad Shapouri M.R. 2018b. Expression of the G1 epitope of bovine
ephemeral fever virus G glycoprotein gene by pET24-G1 recombinant construct in Escherichia coli. Iranian
Veterinary Journal 15: 15-24 (Persian with English abstract).
Pasandideh R., Beigi Nassiri M.T., Seyfi Abad Shapouri M.R., Fayazi J., Roshanfekr H., Lotfi M. 2018c. Designing
of the expressing eukaryotic plasmid of the G1 epitope of bovine ephemeral fever virus G glycoprotein
in human embryonic kidney cells. Iranian Veterinary Journal 15: 19-27 (Persian with English abstract).
Pasandideh R., Seyfi Abad Shapouri M.R., Beigi Nassiri M.T. 2019a. Production of monoclonal antibody against
prokaryotically expressed G1 protein of bovine ephemeral fever virus. Iranian Journal of Applied Animal
Science 9: 51-57.
Pasandideh R., Beigi Nassiri M.T., Seyfi Abad Shapouri M.R. 2019b. Expression of the G1 epitope of bovine
ephemeral fever virus G glycoprotein gene by pET24-G1 recombinant construct in Escherichia coli. Iranian
Peiris J.S.M., Amerasinghe P.H., Amerasinghe F.P., Calisher C.H., Parakrama Perera L., Arunagiri
C.K., Munasingha N.B., Parakrama Karunaratne S.H.P. 1994. Viruses isolated from mosquitoes
collected in Sri Lanka. American Journal of Tropical Medicine and Hygiene 51: 154-161.
Peiris J.S.M. 2001. Nairobi sheep disease. In: Service M.W. (ed). Encyclopedia of Arthropod-Transmitted
Infections of Man and Domesticated Animals. CABI Publishing, Wallingford. 364-368.
Pfeffer M. 2001. Semliki forest virus. In: Service M.W. (ed). Encyclopedia of Arthropod-Transmitted Infections
of Man and Domesticated Animals. CABI Publishing, Wallingford. 462-464.
Pilvari M., Goldasteh Sh., Modarres Najafabadi S.S. 2016. Faunestic study of biting midge (Diptera:
Ceratopogonidae) from Markazi Province, Iran. IAU Entomological Research Journal 8: 15-23.
Plowright W., Perry C.T., Peirce M.A. 1970. Transovarial infection with African swine fever virus in the
argasid tick, Ornithodoros moubata porcinus, Walton. Research in Veterinary Science 11: 582-584.
Polat M., Takeshima S.-N., Aida, Y. 2017. Epidemiology and genetic diversity of bovine leukemia virus. Virology
Pouriayevali M.H., Rezaei F., Jalali T., Baniasadi V., Fazlalipour M., Mostafavi E., Khakifirouz S., Mohammadi
T., Fereydooni Z., Tavakoli M., Azad-Manjiri S., Hosseini M., Ghalejoogh M., Gouya M.M., Failloux
A.-B., Salehi-Vaziri M. 2019. Imported cases of Chikungunya virus in Iran. BMC Infectious Diseases 19:
Presti R.M., Zhao G., Beatty W.L., Mihindukulasuriya K.A., Travassos da Rosa A.P.A., Popov V.L., Tesh R.B.,
Virgin H.W., Wang, D. 2009. Quaranfil, Johnston Atoll, and Lake Chad viruses are novel members of
Pukhovskaya N.M., Morozova O.V., Vysochina N.P., Belozerova N.B., Bakhmetyeva S.V., Zdanovskaya N.I.,
Seligman S.J., Ivanov L.I. 2018. Tick-borne encephalitis virus in arthropod vectors in the Far East of
Qassem M.A.M., Jaawal A.A.T. 2014. Dengue fever or West Nile virus outbreak? Yemen 2013. International
Rafyi A., Mirchamsy H. 1956. Seven years control of sheep pox in Iran with an adsorbed tissue vaccine on
aluminium gel. British Veterinary Journal 112: 541.
Rafyi A., Ramyar H. 1959. Goat pox in lran serial passage in goats and the developing egg, and relationship with
sheep pox. Archives of Razi Institute 11: 57-64.
Rafyi A. 1961. Horse-sickness. Archives of Razi Institute 13: 60-106.
Rahbari S., Nabian S., Shayan P. 2007. Primary report on distribution of tick fauna in Iran. Parasitology Research
Rahimi P., Sohrabi A., Ashrafihelan J., Edalat R., Alamdari M., Masoudi M., Mostofi S., Azadmanesh K. 2010.
Emergence of African swine fever virus, northwestern Iran. Emerging Infectious Diseases 16: 1946-1948.
doi: 10.3201/eid1612.100378
429
Ramezankhani R., Kaveh F. 2014. Data and Statistics of Communicable Disease during 1385-1390. Andishmand
Press, Tehran (Persian).
Ramyar H., Hessami M., Ghaboussi B. 1974. Goat pox: immunogenicity of vaccine virus modified in cell culture.
Recueil de Medecine Veterinaire 150: 131-133 (French).
Rao T.R. 1964. Vectors of dengue and chikungunya viruses: a brief review. Indian Journal of Medical Research
52: 719-726.
Rao T.V.S., Bandyopadhyay S.K. 2000. A comprehensive review of goat pox and sheep pox and their diagnosis.
Raoofi A., Hemmatzadeh F., Ghanaei A.M. 2012a. Serological survey in camels (Camelus dromedarius) to detect
antibodies against bovine herpesvirus type-1 and Mycobacterium avium paratuberculosis in Iran. Journal
of Camel Practice and Research 19: 65-68.
Raoofi R., Pourahmad M., Nazer M.R., Pournia Y., Chinikar S. 2012b. Case series of Crimean-Congo disease:
an outbreak in south of Fars, Iran. Journal of Babol University of Medical Sciences 14: 96-100 (Persian
with English abstract).
Rasekh M., Sarani A., Hashemi S.H. 2018. Detection of Schmallenberg virus antibody in equine population of
Rasekh M., Sarani A., Jafari A. 2022. First detection of Schmallenberg virus antibody in cattle population of
Ravaomanana J., Michaud V., Jori F., Andriatsimahavandy A., Roger F., Albina E., Vial L. 2010. First detection of
African swine fever virus in Ornithodoros porcinus in Madagascar and new insights into tick distribution
Rezatofighi S.E., Mirzadeh K., Mahmoodi F. 2022. Molecular characterization and phylogenetic analysis of bovine
ephemeral fever viruses in Khuzestan Province of Iran in 2018 and 2020. BMC Veterinary Research 18:
Ready P.D. 2013. Biology of phlebotomine sand flies as vectors of disease agents. Annual Review of Entomology
Reinert J.F. 2009. List of abbreviations for currently valid generic-level taxa in family Culicidae (Diptera).
European Mosquito Bulletin 27: 68-76.
Reisen W.K., Hayes C.G., Azra K., Niaz S., Mahmood F., Parveen T., Boreham P.F.L. 1982. West Nile virus in
Pakistan. II. Entomological studies at Changa Manga National Forest, Punjab Province. Transactions of the
Reuben R., Tewari S.C., Hiriyan J., Akiyama J. 1994. Illustrated keys to species of Culex (Culex) associated with
Japanese encephalitis in Southeast Asia (Diptera: Culicidae). Mosquito Systematics 26: 75-96.
Rezaei F., Rezazadeh A., Moghaddami M., Mir Ahmadizadeh A.R., Rezazadeh F. 2012. Reported 5 cases of
Crimean-Congo hemorrhagic fever in Fars Province in 2011. Iranian South Medical Journal 3: 241-247
(Persian with English abstract).
Rezazadeh F., Chinikar S., Bageri Amiri F. 2012. A seroprevalance survey of anti CCHFV IgG by ELISA in
sheep from some area in northwest of Iran. Global Veterinaria 9: 655-658.
Rezazadeh F., Chinikar S., Bageri Amiri F. 2013. Seroprevalance survey of anti-CCHFV IgG by ELISA
in sheep from some area in northwest of Iran. World Applied Sciences Journal 28: 1757-1760.
Rezazadeh F., Hosseinzadeh N., Khodabande E., Ezazi A. 2016. Seroprevalence of equine infectious anemia virus
in Iran. Online Journal of Veterinary Research 20: 596-601.
Rezza G., El-Sawaf G., Faggioni G., Vescio F., Al Ameri R., De Santis R., Helaly G., Pomponi A.,
Metwally D., Fantini M., Qadi H., Ciccozzi M, Lista F. 2014. Co-circulation of dengue and
chikungunya viruses, Al Hudaydah, Yemen, 2012. Emerging Infectious Diseases 20: 1351-1354.
Riddle M.S., Althoff J.M., Earhart K., Monteville M.R., Yingst S.L., Mohareb E.W., Putnam S.D.,
Sanders J.W. 2008. Serological evidence of arboviral infection and self-reported febrile
illness among U.S. troops deployed to Al Asad, Iraq. Epidemiology, Infection 136: 665-669.
Rodhain F., Madulo-Leblond G., Hannoun C., Tesh R.B. 1985. Le virus Corfou: un nouveau Phlebovirus isole
de phlebotomes en Grece. Annales de l'Institut Pasteur / Virologie 136E: 161-166.
430
Rodriguez L.L., Maupin G.O., Ksiazek T.G., Rollin P.E., Khan A.S., Schwarz T.F., Lofts R.S., Smith J.F., Noor
A.M., Peters C.J., Nichol S.T. 1997. Molecular investigation of a multisource outbreak of Crimean-Congo
hemorrhagic fever in the United Arab Emirates. American Journal of Tropical Medicine and Hygiene 57:
Roeder P., Mariner J., Kock R. 2013. Rinderpest: the veterinary perspective on eradication. Philosophical
Sabaghan M., Pourmahdi-Borujeni M., Seifi Abad Shapouri M., Rasooli A. Norouzi M., Samimi S., Mansouri S.
2014. Seroprevalence of bluetongue in sheep in Kohgiluyeh and Boyer-Ahmad Province, Iran. Veterinary
Research Forum 5: 325-328.
Sadat Mousavi F., Ghalyanchilangeroudi A., Abdollahi H., Hosseini H., Rajeoni A., Aghayian L., Ghorani M.,
Gholamian B., Modiri A., Sadri N., Ziafati Kafi Z. 2019. Isolation and phylogenetic characterization of
avipoxvirus causing outbreaks in Iran, 2019. Iranian Journal of Virology 13: 29-34.
Sadeghi M., Asgharzadeh S.A., Bayani M., Alijanpour E., Javaniyan M., Jabbari A. 2013. Crimean Congo
hemorrhagic fever appearance in the north of Iran. Caspian Journal of Internal Medicine 4: 617-620.
Sadri R., Fallahi R. 2010. A new approach to develop a vaccine against capropox infection in sheep and goats
using a new strain of sheep pox virus in Iran. International Journal of Veterinary Research 4: 221-224.
Sadri R. 2012a. Seasonal effects on the prevalence of bluetongue in small ruminants in West Azarbaijan, Iran.
Iranian Journal of Veterinary Medicine 6: 19-22.
Sadri R. 2012b. A new way of occurrence and serodiagnosis for infectious bovine rhinotrchitis in Iranian cattle
herds. Iranian Journal of Veterinary Medicine 6: 99-103.
Sadri R. 2012c. Prevalence and economic significance of goat pox virus disease in semi-arid provinces of Iran.
Iranian Journal of Veterinary Medicine 6: 187-190.
Safarpoor Dehkordi F., Haghighi N., Momtaz H., Salari Rafsanjani M., Momeni M. 2013. Conventional vs
real-time PCR for detection of bovine herpes virus type 1 in aborted bovine, buffalo and camel fetuses.
Bulgarian Journal of Veterinary Medicine 16: 102-111.
Saghafipour A., Noroozi M., Zia sheikholeslami N., Haidarpour A. 2012a. A rare case of Crimean-Congo
Hemorrhagic fever. Journal of Army University of Medical Sciences 10: 180-184 (Persian with English
abstract).
Saghafipour A., Norouzi M., Zia Sheikholeslami N., Mostafavi R. 2012b. Epidemiologic status of the patients
with Crimean Congo hemorrhagic fever and its associated risk factors. Iranian Journal of Military Medicine
14: 1-5 (Persian with English abstract).
Saidi S. 1974. Viral antibodies in preschool children from the Caspian area, Iran. Iranian Journal of Public
Health 3: 83-91.
Saidi S. 1975. Survey for antibodies to arboviruses in various animals in Iran. 3rd International Congress of
Virology, Madrid, Sep 1975, 3, 274.
Saidi S., Casals J., Faghih M. 1975. Crimean hemorrhagic fever-Congo (CHF-C) virus antibodies in man, and in
domestic and small mammals in Iran. American Journal of Tropical Medicine and Hygiene 24: 353-357.
Saidi S., Tesh R., Javadian E., Sahabi Z., Nadim A. 1977. Studies on the epidemiology of sandfly fever
in Iran II. The prevalence of human and animal infection with five Phlebotomus fever virus
serotypes in Isfahan Province. American Journal of Tropical Medicine and Hygiene 26: 288-293.
Sailleau C., Hamblin C., Paweska J.T., Zientara S. 2000. Identification and differentiation of the nine African horse
sickness virus serotypes by RT-PCR amplification of the serotype-specific genome segment 2. Journal of
Sakhaee E., Khalili M., Kazemi nia S. 2009. Serological study of bovine viral respiratory diseases in dairy herds
in Kerman province, Iran. Iranian Journal of Veterinary Research, Shiraz University 10: 49-53.
Saleem M., Tanvir M., Akhtar M.F., Saleem A. 2020. Crimean-Congo hemorrhagic fever: etiology, diagnosis,
management and potential alternative therapy. Asian Pacific Journal of Tropical Medicine 13: 143-151.
Saleem T., Akhtar H., Jamal S.B., Maryam F., Faheem M. 2022. Zika Virus from the perspective
of observational studies: a review. Journal of Arthropod-Borne Diseases 16: 262-277.
431
Salehi-Vaziri M., Fazlalipour M., Baniasadi V. 2016. Solving the mystery of dengue in Iran; are we close to an
answer? Iranian Journal of Virology 10: 39-40.
Salehi-Vaziri M., Kaleji A.S., Fazlalipour M., Jalali T., Mohammadi T., Khakifirouz S., Baniasadi V., Pouriayevali
M.H., Mahmoudi A., Tordo N., Mostafavi E. 2019. Hantavirus infection in Iranian patients suspected to
Salehi-Vaziri M., Pouriayevali M.H., Azad-Manjiri S., Ahmadi Vasmehjani A., Baniasadi V., Fazlalipour M. 2020.
The seroprevalence of tick-borne encephalitis in rural population of Mazandaran Province, Northern Iran
Salehi-Vaziri M., Sarvari J., Mansurnejadan M., Shiri A., Joharinia N., Khoshbakht R., Jaberi O., Pouriayevali
M.H., Azad-Manjiri S., Jalali T., Fazlalipour M., Hosseini S.Y. 2021. Evidence of Hantavirus
circulation among municipal street sweepers, southwest of Iran. Virus Disease 32: 251-254.
Salim A.R, Porterfield J.S. 1973. A serological survey on arbovirus antibodies in the Sudan. Transactions of the
Salim Abadi Y., Chinikar S., Telmadarraiy Z., Vatandoost H., Moradi M., Oshaghi M.A., Ghiasi S.M. 2011.
Crimean-Congo hemorrhagic fever: a molecular survey on hard ticks (Ixodidae) in Yazd province, Iran.
Sameea Yousefi P., Mardani K., Dalir-Naghadeh B., Jalilzadeh-Amin G. 2017. Epidemiological study of lumpy
skin disease outbreaks in north-western Iran. Transboundary and Emerging Diseases 64: 1782-1789. https://
doi.org/10.1111/tbed.12565
Sameea Yousefi P., Dalir-Naghadeh B., Mardani K., Jalilzadeh-Amin G. 2018. Phylogenetic analysis of the lumpy
skin disease viruses in northwest of Iran. Tropical Animal Health and Production 50: 1851-1858. https://
doi.org/10.1007/s11250-018-1634-3
Samour J.H., Kaaden O.-R., Wernery U., Baily T.A. 1996. An epornitic of avian pox in houbara bustards:
(Chlamydotis undulata macqueenii). Journal of Veterinary Medicine, Series B 43: 287-292.
Sawal H.A., Niazi S.K., Ghani E., Noor M. 2021. Phylogenetic and sequencing analysis of Chikungunya
virus strains from local Pakistani population. Journal of Pakistan Medical Association 71: 2335-2339.
Schwarz T.E., Nsanze H., Ameen A.M. 1997. Clinical features of Crimean-Congo haemorrhagic fever in the
Scrimgeour E.M., Zaki A., Mehta F.R., Abraham A.K., Al-Busaidy S., El-Khatim H., Al-Rawas S.F.S., Kamal
A.M., Mohammed A.J. 1996. Crimean-Congo haemorrhagic fever in Oman. Transactions of the Royal
Scrimgeour E.M., Mehta F.R., Suleiman A.J.M. 1999. Infectious and tropical diseases in Oman: a review. American
Secombe A.K., Ready P.D., Huddleston L.M. 1993. A Catalogue of Old World Phlebotomine Sandflies (Diptera:
Psychodidae, Phlebotominae). Occasional Papers on Systematic Entomology No. 8, The Natural History
Museum, London.
Sedaghat M.M., Sarani M., Chinikar S., Telmadarraiy Z., Salahi Moghaddam A., Azam K., Nowotny N., Fooks
A.R., Shahhosseini N. 2017. Vector prevalence and detection of Crimean-Congo haemorrhagic fever virus
in Golestan Province, Iran. Journal of Vector Borne Diseases 54: 353-357.
Segard A., Gardes L., Jacquier E., Grillet C., Mathieu B., Rakotoarivony I., Setier-Rio M.-L., Chavernac D.,
Cetre-Sossah C., Balenghien T., Garros C. 2018. Schmallenberg virus in Culicoides Latreille (Diptera:
Ceratopogonidae) populations in France during 2011-2012 outbreak. Transboundary and Emerging Diseases
Semashko I.V., Matevosyan K.Sh., Pivanova G.P., Chumakov M.P. 1973. Isolation of Bhanja virus from
Dermacentor marginatus ticks collected from sheep in the area of Sevan Lake, Armenia. Trudy Inst.
Polio. Virus. Entsef. Akad. Med. Nauk SSSR, 21: 160-164 (Russan, English translation, NAMRU3-T1216).
Semashko I.V., Chumakov M.P., Safarov R.K., Tkachenko E.A., Bashkirtsev V.N., Chunikhin S.P. 1974. Isolation
and identification of Crimean hemorrhagic fever and Dhori viruses from Hyalomma plumbeum plumbeum
ticks collected in Azerbaijan SSR. In: Chumakov M.P. (ed). Medical virology. Trudy Inst. Polio. Virus.
Entsef. Akad. Med Nauk SSSR, 22: 57-60 (Russian, English translation, NAMRU3-T1031).
432
Sendow I., Sukarshi, Soleha E., Pearce M., Bahri S., Daniels P.W. 1996. Bluetobgue virus research in Indonesia.
In: St George T.D., Peng K. (eds). Bluetongue Disease in Southeast Asia and the Pacific. ACIAR, Canberra.
28-32.
Sevik M., Dogan M. 2017. Epidemiological and molecular studies on lumpy skin disease outbreak in Turkey
Seyfi Abad Shapouri M., Ghiami Rad M., Haji Hajikolaei M.R., Mahmoodi P., Karami A., Daghari M. 2016.
Isolation of Bovine Herpesvirus-1 (BoHV-1) From Latently Infected/Carrier Cattle in Ahvaz. Iranian Journal
of Ruminants Health Research 1: 11-20.
Sezen A.I., Yildirim M., Kultur M.N., Pehlivanoglu F., Menemenlioglu D. 2018. Cases of Zika virus
infection in Turkey: newly married couple returning from Cuba. Mikrobiyoloji Bülteni 52: 308-315.
Shafei E., Dayer M.S., Telmadarraiy Z. 2016. Molecular epidemiology of Crimean-Congo hemorrhagic fever virus
in ticks in northwest of Iran. Journal of Entomology and Zoology Studies 4: 150-154.
Shah K.V., Work T.H. 1969. Bhanja virus: a new arbovirus from ticks Haemaphysalis intermedia Warburton and
Nuttall, 1909 in Orissa, India. Indian Journal of Medical Research 57: 793-798.
Shah S.Z., Jabbar B., Ahmed N., Rehman A., Nasir H., Nadeem S., Jabbar I., Rahman Z., Azam S.
2018. Epidemiology, pathogenesis, and control of a tick-borne disease-Kyasanur Forest disease:
current status and future directions. Frontiers in Cellular and Infection Microbiology 8:
149.
Shahbazi N., Khaki Firouz S., Karimi M., Mostafavi E. 2019. Seroepidemiological survey of Crimean-Congo
haemorrhagic fever among high-risk groups in the west of Iran. Journal of Vector Borne Diseases 56:
Shahhosseini N., Chinikar S. 2016. Genetic evidence for circulation of Kunjin-related West Nile virus strain in
Iran. Journal of Vector Borne Diseases 53: 384-386.
Shahhosseini N., Chinikar S., Moosa-Kazemi S.H., Sedaghat M.M., Kayedi M.H., Lühken R., Schmidt-Chanasit
J. 2017. West Nile Virus lineage-2 in Culex specimens from Iran. Tropical Medicine, International Health
Shahhosseini N., Azari-Garmjan G.A., Rezaiyan M.K., Haeri A., Nowotny N., Fooks A.R., Chinikar S., Youssefi
M. 2018. Factors affecting transmission of Crimean-Congo hemorrhagic fever among slaughterhouse
employees: a serosurvey in Mashhad. Iran. Jundishapur Journal of Microbiology 11. e57980.
Shahhosseini N., Moosa-Kazemi S.H., Sedaghat M.M., Wong G., Chinikar S., Hajivand Z., Mokhayeri H.,
Nowotny N., Kayedi M.H. 2020. Autochthonous transmission of West Nile virus by a new vector
in Iran, vector-host interaction modeling and virulence gene determinants. Viruses 12(12): 1449.
Shamsizadeh Y., Roodbari F., Arab Soleymani N. 2015. Prevalence of West Nile virus infection in the cities of
Neka and Shiraz, Iran. Medical Laboratory Journal 9: 141-145 (Persian with English abstract).
Sharififard M., Alavi S.M., Salmanzadeh S., Safdari F., Kamali A. 2016. Epidemiological survey of Crimean-
Congo hemorrhagic fever (CCHF), a fatal infectious disease in Khuzestan Province, southwest Iran, during
1999-2015. Jundishapur Journal of Microbiology 9: 5. e30883.
Sharifinia N., Rafinejad J., Hanafi-Bojd A.A., Chinikar S., Piazak N., Baniardalani M., Biglarian A., Sharifinia
F. 2015. Hard ticks (Ixodidae) and Crimean-Congo hemorrhagic fever virus in south west of Iran. Acta
Medica Iranica 53: 177-181.
Sharifi Mod B., Metanat M. 2006. Clinical menifestations, laboratory results and clinical outcome in six pregnant
women with Crimean-Congo hemorrhagic fever. The Iranian Journal of Obstetrics, Gynecology and
Infertility 9: 81-85 (Persian with English abstract).
Sharifi-Mood B., Metanat M., Rakhshani F., Shakeri A. 2011. Co-infection of malaria and Crimean-Congo
hemorrhagic fever. Iranian Journal of Parasitology 6: 113-115.
Sharifi-Mood B., Metanat M., Alavi-Naini R. 2014. Prevalence of Crimean-Congo hemorrhagic fever among
high risk human groups. International Journal of High Risk Behaviors, Addiction 3: 1. e11520.
Sharifzadeh A., Namazi M.-J., Mokhtari-Farsani A., Doosti A. 2015. Bovine herpesvirus type 5 in semen samples
433
Shi L ., Fu S., Wang L., Li X., Gu D., Liu C., Zhao C., He J., Liang G. 2016. Surveillance of mosquito-borne
infectious diseases in febrile travelers entering China via Shenzhen ports, China, 2013. Travel Medicine
Shibl A., Senok A., Memish Z. 2012. Infectious diseases in the Arabian Peninsula and Egypt. Clinical microbiology
Shiraly R., Khosravi A., Farahangiz S. 2017. Seroprevalence of sadfly fever virus infection in military
personnel on the western border of Iran. Journal of Infection and Public Health 10: 59-63.
Shirvani E., Lotfi M., Kamalzadeh M., Noaman V., Bahriari M., Morovati H., Hatami A. 2012. Seroepidemiological
study of bovine respiratory viruses (BRSV, BoHV-1, PI-3V, BVDV, and BAV-3) in dairy cattle in
central region of Iran (Esfahan province). Tropical Animal Health and Production 44: 191-195.
Silva J.V.J.Jr., Ludwig-Begall L.F., de Oliveira-Filhoa E.F., Oliveira R.A.S., Durães-Carvalhoa R., Lopese
T.R.R., Silva D.E.A., Gil L.H.V.G. 2018. A scoping review of Chikungunya virus infection: epidemiology,
clinical characteristics, viral co-circulation complications, and control. Acta Tropica 188: 213-224.
Simo F.B.N., Bigna J.J., Well E.A., Kenmoe S., Sado F.B.Y., Weaver S.C., Moundipa P.F., Demanou M. 2019.
Chikungunya virus infection prevalence in Africa: a contemporaneous systematic review and meta-analysis.
Simpson D.I.H., Bowen E.T.W., Platt G.S., Way H., Smith C.E.G., Peto S. 1970. Japanese encephalitis in Sarawak:
virus isolation and serology in a land Dyak Village. Transactions of the Royal Society of Tropical Medicine
Simpson D.I.H., Bowen E.T.W., Way H. J., Platt G.S., Hill M.N. 1974. Arbovirus infections in Sarawak, October
1968 - February 1970: Japanese encephalitis virus isolations from mosquitoes. Annals of Tropical Medicine,
Skvortsova T.M., Kurbanov M.M., Gromashevsky V.L., Lvov D.K., Aristova V.A., Neronov V.M., Berdiev A.
1975. Identification of Wad Medani virus in Turkmen SSR. Mater. 9. Simp. Ekol. Virus. Dushanbe, October
1975. 45-46 (Russian, English translation NAMRU3-T1130).
Sofizadeh A., Shoraka H.R., Mesgarian F., Ozbaki G.M., Gharaninia A., Sahneh E., Dankoob R., Malaka A.,
Fallah S., Nemani S. 2018. Fauna and larval habitats characteristics of mosquitoes (Diptera: Culicidae)
in Golestan Province, northeast of Iran, 2014-2015. Journal of Arthropod-Borne Diseases 12: 240-251.
Sonnleitner S.T., Lundstrom J., Baumgartner R., Simeoni J., Schennach H., Zelger R., Prader A., Schmutzhard
E., Nowotny N., Walder G. 2014. Investigations on California serogroup Orthobunya viruses in the
Tyrols: first description of Tahyna virus in the Alps. Vector-Borne and Zoonotic Diseases 14: 272-277.
Sotnikova A.N., Soldatov G.M. 1964. Isolation of tick-borne encephalitis virus from fleas Ceratophyllus tamias
Wagn. Medical Parasitology and Parasitic Diseases 33: 622-624 (Russian).
Sparagano O.A.E., George D.R., Harrington D.W.J., Giangaspero A. 2014. Significance and control
of the poultry red mite, Dermanyssus gallinae. Annual Review of Entomology 59: 447-466.
Spengler J.R., Bergeron E., Rollin P.E. 2016. Seroepidemiological studies of Crimean-Congo hemorrhagic
fever virus in domestic and wild animals. PLOS Neglected Tropical Diseases 10: 1, e0004210.
Sprygin A., Pestova Ya., Prutnikov P., Kononov A.V. 2018. Detection of vaccine-like lumpy skin disease virus in
cattle and Musca domestica L. flies in an outbreak of lumpy skin disease in Russia in 2017. Transboundary
Sprygin A., Pestova Ya., Wallace D.B., Tuppurainen E., Kononov A.V. 2019. Transmission of lumpy skin disease
Staji H., Keyvanlou M., Geraili Z., Shahsavari H., Jafari E. 2021. The First Study of West Nile Virus in Feral
Pigeons (Columba livia domestica) Using Conventional Reverse Transcriptase PCR in Semnan and
Khorasane-Razavi Provinces, Northeast of Iran. Journal of Arthropod-Borne Diseases 15: 126-132.
434
Stanley M.J. 1990. Prevalence of bluetongue precipitating antibodies in domesticated animals in Yemen Arab
Stekolnikov A.A., Saboori A., Shamsi M., Hakimitabar M. 2019. Chigger mites (Acariformes: Trombiculidae) of
St George T.D., Stanfast H.A., Cybinski D.H. 1978. Isolations of Akabane virus from sentinel cattle and Culicoides
St George T.D. 1988. Bovine ephemeral fever: A review. Tropical Animal Health and Production 20: 194-202.
Storm N., Weyer J., Markotter W., Leman P.A., Kemp A., Nel L.H., Paweska J.T. 2013. Phyogeny of Sindbis
virus isolates from South Africa. Southern African Journal of Epidemiology and Infection 28: 207-214.
Sugamata M. 1988. Dependence on the birth season of the antibody level against West Nile virus in the Pakistani
population. Acta Virologica 32: 138-147.
Sugamata M., Ahmed A., Miura T., Takasu T., Kono R., Ogata T., Kimura-Kuroda J., Yasui K. 1988.
Seroepidemiological study of infection with West Nile virus in Karachi, Pakistan, in 1983 and 1985.
Suleiman M.N.E.H., Muscat-Baron J.M., Harries J.R., Satti A.G.O., Platt G.S., Bowen E.T.W., Simpson D.I.H.
1980. Congo/Crimean haemorrhagic fever in Dubai an outbreak at the Rashid Hospital. Lancet 316: 939-
Suliman H.M., Adam I.A., Saeed S.I., Abdelaziz S.A., Haroun E.M., Aradaib I.E. 2017. Crimean Congo
hemorrhagic fever among the one-humped camel (Camelus dromedaries) in Central Sudan. Virology Journal
Sureau P., Klein J.-M. 1980. Arbovirus en Iran. Médecine Tropicale 40: 549-554 (French with English abstract).
Sureau P., Klein J.-M., Casals J., Digoutte J.-P., Salaun J.-J., Piazak N., Calvo M.A. 1980. Isolment des virus
Thogoto, Wad Medani, Wanowrie et de la fever hemorragique de Crimee-Congo en Iran a partir de
tiques d'animaux domestiques. Annales de l'Institut Pasteur / Virologie 131: 185-200 (French with English
abstract).
Tageldin M.H., Wallace D.B., Hermanna Gerdes G., Putterill J.F., Greyling R.R., Phosiwa M.N., Al Busaidy
R.M., Al Ismaaily S.I. 2014. Lumpy skin disease of cattle: an emerging problem in the Sultanate of Oman.
Taha H.A., Shoman S.A., Alhadlag N.M. 2015. Molecular and serological survey of some haemoprotozoan,
rickettsial and viral diseases of small ruminants from Al-Madinah Al Munawarah, KSA. Tropical
Biomedicine 32: 511-523.
Tahmasebi F., Ghiasi S.M., Mostafavi E., Moradi M., Piazak N., Mozafari A., Haeri A., Fooks A.R., Chinikar S.
2010. Molecular epidemiology of Crimean-Congo hemorrhagic fever virus genome isolated from ticks of
Hamadan province of Iran. Journal of Vector Borne Diseases 47: 211-216.
Takeda T., Ito T., Chiba M., Takahashi K., Niioka T., Takashima I. 1998. Isolation of tick-borne encephalitis
virus from Ixodes ovatus (Acari: Ixodidae) in Japan. Journal of Medical Entomology 35: 227-231.
Tan L.V., Ha D.Q., Hien V.M., van der Hoek L., Farrar J., de Jong M.D. 2008. Me Tri virus: a Semliki Forest virus
Tantawi H.H., Al Sheikhly S., Hassan F.K. 1981. Avian pox in buzzard (Accipiter nisus) [sic] in Iraq. Journal of
Tarello W. 2004. Avian pox in psittacine birds from Saudi Arabia. Revue de Médecine Vétérinaire 155: 483-485.
Tarello W. 2008. Prevalence and clinical signs of avipoxvirus infection in falcons from the Middle East. Veterinary
Tavakoli A., Esghaei M. Karbalaie Niya M.H., Marjani A. Tabibzadeh A., Karimzadeh M., Monavari S.H. 2018.
A comprehensive review of Zika virus infection. Journal of Qazvin University of Medical Sciences 22:
87-105 (Persian with English abstract).
Tavakoli F., Rezaei F., Shafiei-Jandaghi N.Z., Shadab A., Mokhtari-Azad T. 2020. Seroepidemiology of dengue
and chikungunya fever in patients with rash and fever in Iran, 2017. Epidemiology and Infection 148: e42,
435
Taylor R.M., Hoogstraal H., Hurlbut H.S. 1966a. Isolation of a virus (Wad Medani) from
Rhipicephalus sanguineus in Sudan. American Journal of Tropical Medicine and Hygiene 14: 75.
Taylor R.M., Hurlbut H.S., Work T.H., Kingston J.R., Hoogstraal H. 1966b. Arboviruses isolated from Argas
ticks in Egypt: Quaranfil, Chenuda, and Nyamanini. American Journal of Tropical Medicine and Hygiene
Taylor R.E.L., Seal B.S., St Jeor S. 1982. Isolation of infectious bovine rhinotracheitis virus from the soft-shelled
Taylor W.P., Mellor P.S. 1994. The distribution of Akabane virus in the Middle East. Epidemiology and Infection
Telmadarraiy Z., Moradi A.R., Vatandoost H., Mostafavi E., Oshaghi M.A., Zahirnia A.H., Haeri A.,
Chinikar S. 2008. Crimean-Congo hemorrhagic fever: a seroepidemiological and molecular survey
in Bahar, Hamadan Province of Iran. Asian Journal of Animal and Veterinary Advances 3: 321-327.
Telmadarraiy Z., Ghiasi S.M., Moradi M., Vatandoost H., Eshraghian M.R., Faghihi F. 2010. A survey of Crimean-
Congo haemorrhagic fever in livestock and ticks in Ardabil Province, Iran during 2004-2005. Scandinavian
Telmadarraiy Z., Saghafipour A., Farzinnia B., Chinikar S. 2012. Molecular detection of Crimean-Congo
hemorrhagic fever virus in ticks in Qom Province, Iran, 2011-2012. Iranian Journal of Virology 6: 13-18.
Telmadarraiy Z., Chinikar S., Vatandoost H., Faghihi F., Hosseini-Chegeni A. 2015. Vectors of Crimean Congo
hemorrhagic fever virus in Iran. Journal of Arthropod-Borne Diseases 9: 137-147.
Temizel E.M., Yesilbağ K., Batten C., Senturk S., Maan S.A., Clement Mertens P.P., Batmaz H. 2009.
Epizootic hemorrhagic disease in cattle, western Turkey. Emerging Infectious Diseases 15: 317-319.
Tesh R.B., Peralta P.H., Shope R.E., Chaniotis B.N., Johnson K.M. 1975. Antigenic relationships among
Phlebotomus fever group arboviruses and their implications for the epidemiology of sandfly fever. American
Tesh R., Saidi S., Javadian E., Nadim A., Seyedi-Rashti M.A. 1976a. The distribution and prevalence of human
infection with Phlebotomus fever group viruses in Iran. Iranian Journal of Public Health 5: 1-7.
Tesh R.B., Saidi S., Gajdamovic S.Ja., Rodhain F., Vesenjak-Hirjan J. 1976b. Serological studies on the
epidemiology of sandfly fever in the Old World. Bulletin of the World Health Organization 54: 663-674.
Tesh R., Saidi S., Javadian E., Nadim A. 1977. Studies on the epidemiology of sandfly fever in Iran I. Virus
isolates obtained from Phlebotomus. American Journal of Tropical Medicine and Hygiene 26: 282-287.
Tesh R.B. 1988. The genus Phlebovirus and its vectors. Annual Review of Entomology 33: 169-181.
Tesh R.B. 1989. Chapter 37 Phlebotomus fevers. In: Monath T.P. (ed). The Arboviruses: Epidemiology and
Ecology Volume IV. Taylor, Francis, London. 15-27.
Tezcan-Ulger S., Kurnaz N., Ulger M., Aslan G., Emekdas G. 2019. Serological evidence of Rift Valley fever
virus among humans in Mersin province of Turkey. Journal of Vector Borne Diseases 56: 373-379.
Tinsley T.W. 1979. The potential of insect pathogenic viruses as pesticidal agents. Annual Review of Entomology
Tkubet G., Firesbhat A., Demessie Y., Bezie G., Yohannes A.G. 2016. A review on African horse sickness. World
Journal of Agricultural Sciences 12: 357-363.
Tomori O. 2001. Zika virus. In: Service M.W. (ed). Encyclopedia of Arthropod-Transmitted Infections of Man
and Domesticated Animals. CABI Publishing, Wallingford. 578-579.
Tonbak Ş., Azkur A.K., Pestil Z., Biyikli E., Abayli H., Baydar E., van der Poel W.H.M., Bulut H. 2016.
Circulation of Schmallenberg virus in Turkey, 2013. Turkish Journal of Veterinary, Animal Sciences 40:
Tuppurainen E.S.M., Oura C.A.L. 2012. Review: Lumpy skin disease: An emerging threat to
Europe, the Middle East and Asia. Transboundary and Emerging Diseases 59: 40-48.
436
Tuppurainen E.S.M., Lubinga J.C., Stoltsz W.H., Troskie M. 2013a. Mechanical transmission of lumpy skin
disease virus by Rhipicephalus appendiculatus male ticks. Epidemiology, Infection 141: 425-430.
Tuppurainen E.S.M., Lubinga J.C., Stoltsz W.H., Troskie M., Carpenter S.T., Coetzer J.A.W., Venter E.H., Oura
C.A.L. 2013b. Evidence of vertical transmission of lumpy skin disease virus in Rhipicephalus decoloratus
Tuppurainen E.S.M., Venter E.H., Shisler J.L., Gari G., Mekonnen G.A., Juleff N., Lyons N.A., De Clercq
K., Upton C., Bowden T.R., Babiuk S., Babiuk L.A. 2015. Review: Capropoxvirus diseases:
current status and opportunities for control. Transboundary and Emerging Diseases 64: 729-745.
Turell M.J. 2019. Arthropod-Related Viruses of Medical and Veterinary Importance. In: Mullen G.R., Durden L.A.
(eds). Medical and Veterinary Entomology. Third edition. Academic Press, Elsevier, London. 695-703.
Valarcher J.F., Hagglud S., Juremalm M., Blomqvist G., Renstrom L., Zohari S., Leijion M., Chirico J. 2015.
Tick-borne encephalitis. Revue Scientifique et Technique OIE 34: 453-466.
Varma M.G.R., Bowen E.T.W., Simpson D.I.H., Casals J. 1973. Zirga virus, a new arbovirus isolated from bird-
Verani P., Ciufolini M.G., Caciolli S., Renzi A., Nicoletti L., Sabatinelli G., Bartolozzi D., Volpi G., Amaducci
L., Coluzzi M., Paci P., Balducci M. 1988. Ecology of viruses isolated from sand flies in Italy and
characterization of a new Phlebovirus (Arbia virus). American Journal of Tropical Medicine and Hygiene
Veronesi E., Henstock M., Gubbins S., Batten C., Manley R., Barber J., Hoffmann B., Beer M., Attoui H., Mertens
P.P.C., Carpenter S. 2013. Implicating Culicoides biting midges as vectors of Schmallenberg virus using
Vlasova N.N., Kazakova A.S., Varentsova A.A., Akopian T.A., Kostryukova E.S. 2012. Comparative sequence
analysis of genes encoding outer proteins of African swine fever virus isolates from different regions
of Russian Federation and Armenia. International Journal of Virology and M olecular Biology 1: 1-11.
Voltsit O.V. 1982. Review of Arboviruses Isolation from Ixodides Ticks in Afghanistan, Pakistan and India. In:
Lvov D.K. (ed). Ecology of Viruses. Acad. Med. Sci. USSR, Moscow. 111-119 (Russian).
Wahid B., Ali A., Rafique S., Idrees M. 2017. Global expansion of chikungunya: mapping the 64-year history.
Walker A.R., Davies F.G. 1971. A preliminary survey of the epidemiology of bluetongue in Kenya. Journal of
Walker P.J., Klement E. 2015. Epidemiology and control of bovine ephemeral fever. Veterinary Research 46: 124.
Wallace M.R., Hale B.R., Utz G.C., Olson P.E., Earhart K.C., Thornton A.A., Hyams,K.C. 2002.
Endemic infectious diseases of Afghanistan. Clinical Infectious Diseases 34 (Suppl. 5): S171-207.
Wang H.Y., Takasaki T., Fu S.H., Sun X.H., Zhang H.L., Wang Z.X., Hao Z.Y., Zhang J.K., Tang Q.,
Kotaki A., Tajima S., Liang X.F., Yang W.Z., Kurane I., Liang G.D. 2007. Molecular epidemiological
analysis of Japanese encephalitis virus in China. Journal of General Virology 88: 885-894.
Watts D.M., El-Tigani A., Botros B.A.M., Salib A.W., Olson J.G., McCarthy M., Ksiazek T.G. 1994. Arthropod-
borne viral infections associated with a fever outbreak in the Northern Province of Sudan. Journal of
Tropical Medicine and Hygiene 97: 228-230.
Wegdan H.A., Sahar M.E., Ballal A., Intisar K.S., Shaza M.M., Algezoli A., Ihsan H.A., Baraa A.M., Taha
K.M., Nada E.M., Manan A.A., Ali Y.H., Nouri Y.M. 2017. Sero prevalence of equine infectious
anemia (EIA) virus in selected regions in Sudan. Microbiology Research Journal International 18: 1-6.
Weir R.P., Hyatt A.D., Calisher C.H., Whelan P.I. 1997. New records of arboviruses isolated from
mosquitoes in the northern territory, 1982-1992. Arbovirus Research in Australia 7: 311-321.
437
Weiss K.E. 1968. Lumpy skin disease virus. Virology Monographs 3: 111-131.
Wentink G.H., van Oirschot J.T., Verhoeff J. 1993. Risk of infection with bovine herpes virus 1 (BHV1): A review.
Wernery U., Kettle T., Moussa M., Babiker H., Whiting J. 2007. West Nile fever in the United Arab Emirates.
Wild Life Middle East 2: 3.
Wernery U., Thomas R., Raghavan R., Syriac G. 2013. Serological evidence of epizootic haemorrhagic disease
and Schmallenberg virus in dromedaries. Journal of Camel Practice and Research 20: 135-137.
Williams R.E., Abdel Wahab K.S.E., Hoogstraal H. 1970. Viruses in ticks. V. Viruses isolated from Afghanistan
ticks during 1968. Folia Parasitologia 17: 359-363.
Williams R.E., Casals J., Moussa M.I., Hoogstraal H. 1972. Royal farm virus: a new tickborne group B agent
related to the RSSE Complex. American Journal of Tropical Medicine and Hygiene 21: 582-586.
Williams R.E., Hoogstraal H., Casals J., Kaiser M.N., Moussa M.I. 1973. Isolation of Wanowrie, Thogoto,
and Dhori viruses from Hyalomma ticks infesting camels in Egypt. Journal of Medical Entomology 10:
Williams R.J., Al-Busaidy S., Mehta F.R., Maupin G.O., Wagoner K.D., Al-Awaidy S., Suleiman A.J.M.,
Khan A.S., Peters C.J., Ksiazek T.G. 2000. Crimean-Congo haemorrhagic fever: a seroepidemiological
and tick survey in the Sultanate of Oman. Tropical Medicine, International Health 5: 99-106.
Wills W.M., Jakob W.L., Francy D.B., Oertley R.E., Anani E., Calisher C.H., Monath T.P. 1985. Sindbis virus
isolations from Saudi Arabian mosquitoes. Transactions of the Royal Society of Tropical Medicine and
Wirth W.W., Hubert A.A. 1989. The Culicoides of Southeast Asia (Diptera: Ceratopogonidae). Memoirs of the
American Entomological Institute 44: 1-508.
Wójcik-Fatla A., Cisak E., Zając V., Zwoliński J., Dutkiewicz J. 2011. Prevalence of tick-borne encephalitis virus
in Ixodes ricinus and Dermacentor reticulatus ticks collected from the Lublin region (eastern Poland). Ticks
Wood O.L., Moussa M.I., Hoogstraal H., Büttiker W. 1982. Kadam virus (Togaviridae, Flavivirus) infecting
camel-parasatizing Hyalomma dromedarii ticks (Acari: Ixodidae) in Saudi Arabia. Journal of Medical
Woodall J. 2001a. Chikungunya virus. In: Service M.W. (ed). Encyclopedia of Arthropod-Transmitted Infections
of Man and Domesticated Animals. CABI Publishing, Wallingford. 115-119.
Woodall J. 2001b. O'nyong-nyong virus. In: Service M.W. (ed). Encyclopedia of Arthropod-Transmitted Infections
of Man and Domesticated Animals. CABI Publishing, Wallingford. 388-390.
Woodall J. 2001c. Thogoto virus. In: Service M.W. (ed). Encyclopedia of Arthropod-Transmitted Infections of
Man and Domesticated Animals. CABI Publishing, Wallingford. 504-506.
World Health Organization 1967. Arboviruses and Human Disease. Technical Report Series, No. 369. WHO,
Geneva.
World Health Organization 2004. Integrated Vector Management. WHO Regional Officer for Eastern Mediterranean,
Cairo.
World Health Organization 2008. The Global Burden of Disease: 2004 update. WHO, Geneva.
World Health Organization 2010. Working to Overcome the Global Impact of Neglected Tropical Diseases.
WHO, Geneva.
World Health Organization 2017. World Malaria Report 2017. WHO, Geneva.
World Organisation for Animal Health 2013. Sheep and goat pox. OIE, Paris.
Xu R. 2001. Haemorrhagic fever with renal syndrome. In: Service M.W. (ed). Encyclopedia of Arthropod-
Transmitted Infections of Man and Domesticated Animals. CABI Publishing, Wallingford. 206-208.
Yadav P.D., Whitmer S.L.M., Sarkale P., Ng T.F.F., Goldsmith C.S., Nyayanit D.A., Esona M.D., Shrivastava-
Ranjan P., Lakra R., Pardeshi P., Majumdar T.D., Francis A., Klena J.D., Nichol S.T., Ströher U., Mourya
D. 2019. Characterization of novel reoviruses [Wad Medani virus (Orbivirus) and Kundal (Coltivirus)]
collected from Hyalomma antolicum ticks in India during CCHF surveillance. Journal of Virology 93: 13.
438
Yaghoobi-Ershadi M.R. 2012. Phlebotomine sand flies (Diptera: Psychodidae) in Iran and their role on Leishmania
transmission. Journal of Arthropod-Borne Diseases 6: 1-17.
Yaqub T., Shabbir M.Z., Mukhtar N., Tahir Z., Abbas T., Amir E., Defang G. 2017. Detection of selected
arboviral infections in patients with history of persistent fever in Pakistan. Acta Tropica 176: 34-38.
Yavari M., Gharekhani J., Mohammadzadeh A. 2018. Bluetongue virus seropositivity and some risk factors
affecting bluetongue virus infection in sheep flocks. Comparative Clinical Pathology 27: 1017-1022.
Yildirim Y., Yilmaz V., Yazici K., Öziç C., Ozkul A., Çağırgan A.A. 2021. Phylogenetic analysis of West Nile
virus: first report of lineage 1 in donkey in Turkey. Tropical Animal Health and Production 53: 453.
Yilmaz H., Hoffmann B., Turan N., Cizmecigil U.Y., Richt J.A., Van der Poel W.H.M. 2014. Detection and partial
sequencing of Schmallenberg virus in cattle and sheep in Turkey. Vector-Borne and Zoonotic Diseases 14:
Yousof Y.S., Ahmed S.E., Noor M.H., Nour M.M., Saleh M.S., Garbi M.I., Eltayeb E.S. 2018. Seroprevalence
of West Nile virus among blood donors at central blood bank, Khartoum State, Sudan. Annals of Medical
and Biomedical Sciences 4: 8-10.
Yoshii K., Okamoto N., Nakao R., Hofstetter R.K., Yabu T., Masumoto H., Someya A., Kariwa H., Maeda A.
2015. Isolation of the Thogoto virus from a Haemaphysalis longicornis in Kyoto City, Japan. Journal of
Yun S.-M., Song B.G., Choi W.Y., Park W.I., Kim S.Y., Roh J.Y., Ryou J., Ju Y.R., Park C., Shin E.-
H. 2012. Prevalence of tick-borne encephalitis virus in ixodid ticks collected from the Republic
of Korea during 2011-2012. Osong Public Health and Research Perspectives 3: 213-221.
Yune N., Abdela N. 2017. Epidemiology and economic importance of sheep and gaot pox: a
review on past and current aspects. Journal of Veterinary Science and Technology 8:
2.
Zahraei-Ramazani A.R., Kumar D., Yaghoobi-Ershadi M.R., Naghian A., Jafari R., Shirzadi M.R., Abdoli
H., Soleimani H., Shareghi N., Ghanei M., Arandian M.H., Hanafi-Bojd A.A. 2013. Sand flies of the
subgenus Adlerius (Diptera: Psychodidae) in an endemic focus of visceral leishmaniasis and introduction
of Phlebotomus (Adlerius) comatus as a new record for Iran. Journal of Arthropod-Borne Diseases 7: 1-7.
Zahraei-Ramazani A., Kumar D., Mirhendi H., Sundar S., Mishra R., Moin-Vaziri V., Soleimani H., Shirzadi
M.R., Jafari R., Hanafi-Bojd A.A., Hamedi Shahraky S., Yaghoobi-Ershadi M.R. 2015. Morphological and
genotypic variations among the species of the subgenus Adlerius (Diptera: Psychodidae, Phlebotomus) in
Iran. Journal of Arthropod-Borne Diseases 9: 84-97.
Zaim M. 1987. The distribution and larval habitat characteristics of Iranian Culicinae. Journal of the American
Mosquito Control Association 3: 568-573.
Zaki A.M. 1997. Isolation of a flavivirus related to the tick-borne encephalitis complex from human cases
in Saudi Arabia. Transactions of the Royal Society of Tropical Medicine and Hygiene 91: 179-181.
Zaki A., Perera D., Jahan S.S., Cardosa M.J. 2008. Phylogeny of dengue viruses circulating in
Jeddah, Saudi Arabia: 1994 to 2006. Tropical Medicine, International Health 13: 584-592.
Zamani T., Montazeri M., Hosseini H., Ghalyanchi Langroudi A., Ziafati Kafi Z., Sadri N., Hojabr Rajeoni A.
2021. Atypical fowl pox in silkie, Iran, 2021. Iranian Journal of Virology 15: 53-58.
Zarifi F., Nakhaei P., Nourani H., Mirshokraei P., Razmyar, J. 2019. Characterization of Iranian canarypox and
Zayed A., Awash A.A., Esmail M.A., Al-Mohamadi H.A., Al-Salwai M., Al-Jasari A., Medhat, I., Morales-Betoulle
M.E., Mnzava A. 2012. Detection of Chikungunya virus in Aedes aegypti during 2011 outbreak in Al
Zeynalova S., Vatani M., Asarova A., Lange C.E. 2019. Schmallenberg virus in Azerbaijan 2012-2018. Archives
439
Ziyaei M., Azarkar Gh., Shayesteh M. 2011. Tow cases of Crimean-Congo hemorrhagic fever and a suspected
case in South Khorasan Province, 2011. Modern Care 8: 173-178.
Ziyaeyan M., Behzadi M.A., Leyva-Grado V.H., Azizi K., Pouladfar G., Dorzaban H., Ziyaeyan A., Salek S., Jaber
Hashemi A., Jamalidoust M. 2018. Widespread circulation of West Nile virus, but not Zika virus in southern
Zohaib A., Saqib M., Beck C., Hussain M.H., Lowenski S., Lecollinet S., Sial A., Asi M.N., Mansoor
M.K., Saqalein M., Sajid M.S., Ashfaq K., Muhammad G., Cao S. 2015. High prevalence of West
Nile virus in equines from the two provinces of Pakistan. Epidemiology, Infection 143: 1931-1935.
Zowghi E., Bayat M., Kousha A., Nissiani M. 2008. Emerging and re-emerging zoonoses. World Journal of
Zoology 3: 71-76.
Вирусы,
прямо или косвенно передаваемые членистоногими,
в Иране и соседних странах
Ш. Азари-Хамидиан, Р. Е. Харбаш
Ключевые слова: арбовирусы, биологическая передача патогена, механическая
передача патогена, мобовирусы, очаги, переносчики, зоонозы
резюме
Членистоногие являются очень важной группой для медицины и ветеринарной
медицины из-за огромного количества переносимых ими патогенов. В данной работе
были проанализированы базы данных, включающие Web of Science, PubMed, Scopus,
Google Scholar, CABI, Scientific Information Database, IranMedex и Magiran, на период
конца декабря
2002 г. в отношении арбовирусных инфекций, выявленных в Иране.
В Иране были обнаружены тридцать три инфекции, прямо или косвенно переносимые
членистоногими. Для каждого заболевания приводятся данные об агентах (вирусах),
распространении (в 31 иранской провинции), хозяевах (людях и животных) и известных
переносчиках в Иране. В дополнение приведен список арбовирусов для соседних стран,
включающих Афганистан, Армению, Азербайджан, Бахрейн, Ирак, Кувейт, Оман, Пакистан,
Катар, Саудовская Аравия, Турция, Туркменистан и Объединенные Арабские Эмираты, а также
Джибути, Сомали, Судан, Сирию и Йемен, которые, хотя и не граничат с Ираном, но, подобно
Ирану, входят в Восточно-средиземноморский регион, выделенный Всемирной Организацией
Здравоохранения (ВОЗ). Список включает 40 вирусов, формально не зарегистрированных
в Иране. Эти вирусы относятся к 19 родам 14 семейств, из которых 3 вируса переносятся
москитами, четыре - мокрецами, 20 - комарами, и 29 - иксодовыми клещами.
440