Экология, 2023, № 4, стр. 245-260
Стохастические и детерминистические процессы в формировании таксономического, функционального и филогенетического разнообразия сообществ: обзор современных представлений
a Институт проблем экологии и эволюции им. А.Н. Северцова РАН
119071 Москва, Ленинский просп., 33, Россия
* E-mail: v.d.leonov@gmail.com
Поступила в редакцию 05.12.2022
После доработки 15.03.2023
Принята к публикации 21.03.2023
- EDN: RQCVLG
- DOI: 10.31857/S0367059723040054
Аннотация
Оценка биологического разнообразия и процессов, которые его определяют, – важная составляющая экологических исследований и природоохранной деятельности. В обзоре перечислены основные обобщающие теории и изложена современная концепция механизмов формирования экологических сообществ. Дано представление о детерминистических (абиотическом и биотическом фильтрах среды) и стохастических (экологическом дрейфе, расселении, видообразовании) процессах, которые участвуют в формировании таксономического, функционального и филогенетического аспектов разнообразия. Приведены примеры воздействия тех или иных процессов (влияние отдельных факторов среды, биотических взаимодействий, ограничения расселения) на α- и β-компоненты каждого из аспектов биоразнообразия, что позволяет оценить вклад тех или иных процессов в формирование биоразнообразия изучаемых локальных сообществ.
Определение механизмов формирования экологических сообществ и закономерностей изменения биологического разнообразия – одна из главных задач экологии как биологической науки, изучающей жизнь на надорганизменном уровне организации [1–3]. Знание этих механизмов открывает перед человечеством перспективы в области защиты и сохранения существующих сообществ, а также создания и поддержания искусственных экосистем, основанных на природных принципах [4]. Понимание этих механизмов помогает предсказать, как сообщества могут трансформироваться в результате изменений климата [5], в том числе и на заповедных территориях [6], прогнозировать инвазии чужеродных видов, их масштабы и последствия для экосистем [7].
В первых фундаментальных исследованиях экологи уже стремились объяснить наблюдаемое разнообразие и установить процессы, которые его формируют [8–13]. К настоящему времени разработано много частных концепций регуляции состава сообществ, а также предложен ряд подходов, которые их объединяют [14, 15].
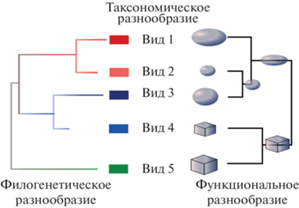
Само по себе биологическое разнообразие – это сложный феномен, включающий несколько измерений (рис. 1). Традиционно биоразнообразие определяют в контексте таксономического разнообразия (~видового богатства) и обилия видов (соотношения их численности). Однако виды, входящие в состав сообществ, во-первых, различаются и выполняют разные экологические функции, поэтому потеря одних видов влечет за собой иные последствия для экосистем, нежели потеря других, во-вторых, виды имеют разную эволюционную историю, а значит, вносят разный вклад в существующее разнообразие сообществ [16, 17]. Для наиболее полной оценки биологического разнообразия, помимо его таксономического аспекта, необходимо учитывать функциональное и филогенетическое разнообразие [18]. Ввиду того, что механизмы формирования сообществ могут по-разному влиять на разные аспекты биоразнообразия, функциональное и филогенетическое разнообразие могут не коррелировать с таксономическим [19].
Цель данного обзора – описать наиболее общие концепции, объясняющие формирование разнообразия сообществ, и привести примеры оценки роли отдельных процессов, исходя из наблюдаемых паттернов таксономического, функционального и филогенетического разнообразия.
МЕХАНИЗМЫ ФОРМИРОВАНИЯ СООБЩЕСТВ
Основные теории
Экология – сравнительно молодая наука, но за время ее существования предложено множество частных механизмов, действие которых определяет состав и численность видов в локальных сообществах, и количество таких механизмов составляет как минимум несколько сотен [20]. Несмотря на такое количество, можно выделить несколько наиболее важных обобщающих теоретических моделей, которые призваны объяснить формирование и современное состояние сообществ, изучаемых эклогами (табл. 1).
Таблица 1.
Описание наиболее общих концепций, объясняющих наблюдаемое разнообразие сообществ
| Теория | Задействованные процессы высокого уровня (по М. Велленду [15]) | Описание |
|---|---|---|
| Экологические ниши | Отбор видов в сообщество: абиотический и биотический фильтры | Локальные сообщества сформированы видами, которые приспособлены к среде обитания, длительному сосуществованию друг с другом и имеют экологические отличия – занимают разные экологические ниши [21]. Разделение ниш между видами – основополагающий механизм поддержания биоразнообразия [12] |
| Нейтральная теория | Экологический дрейф, расселение, видообразование | Локальные сообщества сформированы случайными событиями расселения видов и стохастическими демографическими колебаниями – экологическим дрейфом. Все виды сообщества имеют одинаковые свойства демографической динамики. Разнообразие локальных сообществ – результат баланса между расселением, видообразованием и дрейфом [14, 22] |
| Метасообщество | Расселение, абиотический и биотический фильтры | Локальные сообщества являются частью региональной системы высшего порядка – метасообщества и связаны между собой через расселение множества потенциально взаимодействующих видов. Разнообразие локальных сообществ формируется ограничением расселения, разделением экологических ниш видов, постоянной иммиграцией в сообщество с неблагоприятными условиями из окружающих сообществ, где существует стабильная популяция, и нейтральным сосуществованием видов [23, 24] |
| Теория экологических сообществ М. Велленда | Отбор – абиотический и биотический фильтры, расселение особей, экологический дрейф, видообразование | Взаимодействие детерминистических и стохастических процессов определяет состав сообщества. Любой частный экологический процесс регуляции состава сообщества можно свести к одному из четырех процессов высшего порядка – фильтру среды, расселению, экологическому дрейфу, видообразованию [15] |
Виды, входящие в состав локальных сообществ, могут иметь разные экологические ниши, представляющие собой набор биотических и абиотических характеристик местообитания, в которых виды могут выживать и сосуществовать длительное время [9, 11, 12]. Экологическое сообщество определяется особенностями видов, которые позволяют им добывать ресурсы, избегать хищников, выдерживать конкуренцию и выживать в определенных условиях окружающей среды [21]. В соответствии с правилом конкурентного исключения виды с одинаковыми экологическими нишами не могут сосуществовать в одном местообитании [10].
С другой стороны, многие наблюдаемые сообщества не соответствуют теории ниш, согласно которой виды сосуществуют потому, что значительно различаются и поэтому избегают конкуренции и исключения. Ярким примером является “загадка разнообразия почвенной фауны”: например, микробо- и детритофаги среди орибатид (весьма насыщенная видами функциональная группа, представители которой потребляют схожие ресурсы) способны длительное время поддерживать стабильные популяции в ограниченном пространстве [25, 26]. Нейтральная модель биоразнообразия предполагает, что виды сосуществуют благодаря тому, что схожи по своим экологическим характеристикам и не имеют конкурентного преимущества друг перед другом, в результате чего сообщество контролируется случайными процессами: демографической стохастичностью динамики популяций – экологическим дрейфом, расселением и видообразованием, которые противостоят процессам вымирания [14, 27]. При этом экологически идентичные виды способны сосуществовать длительное время, если в расчете на одну особь у них сохранятся близкая вероятность размножиться, вымереть, заселить свободное пространство и эволюционировать [28].
Теория “метасообществ” объединяет перечисленные выше частные механизмы, учитывая взаимодействие между локальными сообществами, иерархическую организацию экологических систем и сложную разномасштабную структуру процессов, которые влияют на формирование разнообразия. Связанные между собой процессами расселения локальные сообщества формируют метасообщество и региональный пул видов, окружающая среда фильтрует расселяющиеся виды в локальных сообществах, при этом виды могут взаимодействовать друг с другом в процессе разделения ниш, сосуществовать согласно нейтральной теории, но также могут поддерживаться в локальных сообществах иммиграцией, если ее темпы достаточно велики [23]. Теория метасообществ представляет собой взгляд на локальное сообщество как на часть системы более высокого порядка и использует для объяснения наблюдаемого локального разнообразия сообществ процессы, которые работают в разных масштабах [24].
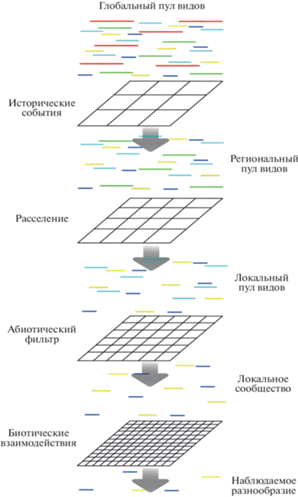
Наиболее общее описание процессов, под влиянием которых формируются локальные сообщества, предложил Марк Велленд в монографии “The theory of ecological communities” [15]. В рамках этого обобщения локальные сообщества формируются под воздействием четырех основных процессов высокого порядка – фильтра окружающей среды, расселения особей, экологического дрейфа и видообразования (рис. 2). Каждый из этих процессов на уровне экологических сообществ аналогичен одной из четырех сил, действующих в эволюции: отбору, миграции, дрейфу и мутациям. Эти высокоуровневые процессы, как и частные низкоуровневые, можно разделить на стохастические и детерминистические в зависимости от определенности состояния системы (локального сообщества) в результате их влияния.
Рис. 2.
Наблюдаемые паттерны биоразнообразия – результат взаимодействия стохастических и детерминистических процессов (по [3], с изменениями и дополнениями).

Детерминистические механизмы не предполагают случайности в формировании локальных сообществ. Состав и соотношение видов в них определены свойствами среды обитания и качествами видов, которые должны быть приспособлены к условиям среды и друг к другу. Стохастические механизмы предполагают формирование локальных сообществ под воздействием случайных процессов – случайных событий расселения и экологического дрейфа, в результате чего невозможно предсказать точный состав локальных сообществ, но можно оценить множество будущих состояний и вероятность каждого из них. При этом экологическая нейтральность, часто привлекаемая для объяснения паттернов биоразнообразия изучаемых сообществ, подразумевает, что организмы одной функциональной или экологической группы на индивидуальном уровне идентичны по вероятности рождения, смерти, миграции и видообразования [14]. Это весьма широкое определение не исключает сложных экологических взаимодействий между особями видов в сообществе. В основном экологическая нейтральность подразумевает, что популяции видов должны подчиняться одним и тем же экологическим правилам демографической динамики. При этом детерминированные процессы в сообществах могут быть экологически нейтральными и наоборот [29].
Детерминистические механизмы. Отбор
Детерминистические процессы определяют состав сообществ путем отбора видов, способных выжить в конкретном местообитании. М. Велленд [15] выделяет “отбор” как основной детерминистический механизм высшего порядка, принимающий участие в формировании локального сообщества. Отбор регулирует присутствие тех или иных видов в сообществе на основе различий в приспособленности особей разных видов. Отбор можно разделить на два основных процесса: абиотическую фильтрацию и биотические взаимодействия. Несмотря на то, что эти процессы могут приводить к одинаковым последствиям для сообществ – исключению видов, не соответствующих абиотическому и биотическому окружению, они действуют на разных масштабах, и эти процессы необходимо различать [30]. Виды, присутствующие в сообществе, приспособлены к условиям окружающей среды (абиотический фильтр, фильтр окружающей среды, фильтр местообитания) и существованию с другими видами сообщества (т.е. способны выдержать конкуренцию, пресс паразитов, хищников – биотические взаимодействия, биотический фильтр). Состав сообщества в рамках этой концепции имеет неслучайный характер и зависит от условий среды [31].
В отношении динамики популяций видов, присутствующих в сообществе, отбор может быть частотно-зависимым. При положительном частотно-зависимом отборе приспособленность вида возрастает по мере увеличения его численности. Например, некоторые виды растений способны изменять эдафические условия, делая их менее подходящими для других видов. При отрицательном частотно-зависимом отборе приспособленность вида падает с увеличением относительного обилия вида (например, хищник с большей вероятностью будет охотиться на наиболее многочисленную добычу, ограничивая ее численность и высвобождая часть ресурсов для других видов той же экологической группы). Обсуждение этих двух типов отбора в контексте экологии сообществ важно, поскольку они приводят к разным результатам – отрицательный частотно-зависимый отбор поддерживает разнообразие видов (фенотипов, генотипов) в сообществе (например, сложные биотические взаимодействия), в то время как положительный частотно-зависимый отбор снижает разнообразие [15].
Фильтр окружающей среды (абиотический фильтр) является первым механизмом, определяющим появление видов регионального пула в локальных сообществах [32]. Согласно этой концепции, видовой состав сообществ определяется комплексом абиотических факторов среды, толерантностью видов по отношению к этим факторам – набором признаков, которые позволяют выживать в определенных условиях, способностью к размножению и поддержанию стабильной популяции в условиях локального сообщества. Фильтр окружающей среды не только прямо влияет на выживание видов в локальном сообществе, но и модифицирует взаимодействия между видами [33]. Например, суровые условия окружающей среды, с одной стороны, отбирают в сообщество экологически близкие виды, способные выжить в таких условиях, с другой, препятствуют доминирующим видам захватить подавляющее количество ресурсов, что могло бы привести к исчезновению других видов [34, 35]. Пространственная и временна́я неоднородность абиотических факторов среды (сезонность, вариация эдафических условий), как правило, повышает разнообразие сообществ в случае, если виды не испытывают ограничений в расселении [36].
Взаимодействия между видами (биотический фильтр). Если особи вида способны существовать в конкретных абиотических условиях, они должны выжить в условиях конкуренции с другими видами, выдержать пресс паразитов и хищников. Взаимодействие видов (биотическая фильтрация) регулирует конечное количество видов, обитающих в локальном сообществе, за счет конкурентного исключения и других биотических взаимодействий [37]. Состав современных сообществ и появление в них новых видов подвержены “эффекту приоритета”, при котором уже имеющиеся в сообществе виды оказывают положительное или отрицательное влияние на появление новых видов путем модификации нишевого пространства или его существенного сокращения [38].
Характер биотических взаимодействий зависит от суровости абиотических факторов, неоднородности (гетерогенности) среды и нарушений, вызванных разными причинами [39]. Важность экологического содействия как одного из основных биотических взаимодействий возрастает с увеличением суровости среды и снижением продуктивности сообществ, тогда как интенсивность конкуренции падает. Например, с нарастанием суровости условий среды в альпийских сообществах биомасса, рост и размножение альпийских видов растений выше в окружении других растений [40]. Это происходит по причине изменения лимитирующих факторов: в экстремальных условиях лимитирующими являются климатические факторы (температура, ветер) и развитость почвенного покрова, в то время как в умеренных условиях основным лимитирующим фактором становится доступность ресурсов, что порождает конкуренцию. Таким образом, на формирование конечного состава сообщества сильное влияние оказывают и положительные биотические взаимодействия, например средообразующая деятельность некоторых видов по отношению к другим [41].
Когда новые виды не встречаются с естественными сдерживающими факторами (оказываются более конкурентными, чем существующие виды, имеют специфические защитные приспособления, не сталкиваются с прессом хищников и паразитов), они становятся инвазивными и значительно влияют на разнообразие локальных сообществ. Их появление может приводить к исчезновению нативных видов сообщества и замещению их другими видами [33, 42].
Фильтр окружающей среды отражает фундаментальную экологическую нишу вида, а биотические взаимодействия – реализуемую экологическую нишу. В экспериментальных исследованиях часто тяжело установить влияние конкретного процесса, поскольку и абиотический фильтр, и биотические взаимодействия могут приводить к отсутствию определенных видов в локальном сообществе [30].
Стохастические механизмы
Стохастические механизмы формирования сообществ связаны прежде всего со случайными событиями, влияние которых в итоге приводит к некоторому состоянию сообщества в рассматриваемый момент времени. Виды случайно появляются в сообществе и исчезают, а наблюдаемое соотношение их обилия может быть обусловлено случайными колебаниями – экологическим дрейфом [14].
Многие процессы на разных уровнях организации биологических систем носят случайный характер, являясь одним из источников наблюдаемого разнообразия живой природы: ошибки при копировании ДНК, процессы формирования иммунитета и путей передачи нервных импульсов, выработка новых моделей поведения [43]. Случайные (вероятные, но непредсказуемые) события – это не просто шум, а одно из фундаментальных свойств природы. Таким образом, “случайность” во многом лежит в основе формирования живой и неживой природы. Среди стохастических процессов, участвующих в формировании экологических сообществ, выделяют случайное расселение особей на новые территории, экологический дрейф и видообразование [15].
Расселение видов и колонизация новых территорий – важные фундаментальные экологические процессы. Расселение видов подразумевает перемещение особей между локальными сообществами [3]. Этот процесс является основополагающим для многих частных экологических концепций, особенно первичной сукцессии и теории островной биогеографии [13]. В паре с видообразованием расселение – это процесс, в результате которого в сообществе появляются новые виды [15]. При этом если детерминистические процессы стремятся сократить число видов в сообществе, то расселение видов способствует появлению новых видов и увеличивает биологическое разнообразие локальных сообществ [3, 44]. В результате расселения локальные сообщества перестают быть независимыми и объединяются во взаимосвязанную систему [15].
Распространение видов в целом невозможно считать абсолютно случайным. Однозначно стохастическое расселение видов зависит только от размера популяции: виды с большей численностью имеют большую вероятность расселиться, но поскольку виды регионального пула отличаются по своим способностям к расселению, вероятность расселения одних видов может быть существенно выше, чем других. Расселение может быть активным или пассивным, и, вероятно, истинно стохастическим можно считать именно пассивное расселение [45].
Виды, которые не в состоянии поддерживать стабильную (самовоспроизводящуюся) популяцию внутри какого-либо сообщества, тем не менее могут поддерживать свою численность при постоянной иммиграции из окружающих сообществ [46]. Высокая интенсивность расселения и отсутствие ограничений приводят к увеличению локального разнообразия сообществ (α-разнообразия), в то время как различия между локальными сообществами снижаются (уменьшается β-разнообразие) [47].
Экологический дрейф – результат случайных изменений в сообществе, связанных с рождением и смертью особей и колебаниями численности видов, вызванных случайными причинами [3]. Экологический дрейф можно наблюдать на уровне случайных событий рождения и смертности особей, случайного определения (и соотношения) полов в популяциях и различий в жизнеспособности особей в популяции [48]. Влияние дрейфа на сообщество тем выше, чем меньше размер популяций видов, входящих в сообщество [48, 49]. Экологический дрейф может приводить к исчезновению небольших изолированных популяций, что упрощает структуру сообщества [50]. Этот процесс имеет тенденцию снижать разнообразие внутри сообществ и таким образом увеличивать различия (β-разнообразие) между схожими в остальном сообществами, что особенно выражено в регионах с большим пулом видов [31, 51].
Видообразование. Определение видообразования как стохастического процесса условно. К числу стохастических процессов относятся генетический дрейф, поток генов и появление мутаций, в то время как отбор носит детерминистический характер и зависит от функциональных свойств видов. Видообразование играет важную роль в формировании регионального пула видов, являющегося источником разнообразия локальных сообществ [52]. В меньшем масштабе, например на изолированных океанических островах, этот процесс становится важным источником локального разнообразия [15]. Регионы с более высокими темпами видообразования (или низкими темпами вымирания) имеют более крупные пулы видов, что увеличивает влияние экологического дрейфа: в регионах с более крупным пулом видов экологический дрейф будет вносить больший вклад в разницу между локальными сообществами [31]. Видообразование, которое происходит независимо в разных локальных сообществах, увеличивает разнообразие этих сообществ и повышает разницу между локальными сообществами одного региона [15].
Биологическое разнообразие – результат взаимодействия детерминистических и стохастических процессов
В формировании сообществ участвуют все описанные выше процессы, но установить относительный вклад каждого из них часто не просто [33]. Определение роли того или иного процесса в наблюдаемой структуре сообщества зависит от пространственного и временно́го охвата исследований, конкретного сообщества и истории его развития [53]. Описанные выше процессы можно представить как несколько фильтров разного масштаба (рис. 3), которые определяют состав наблюдаемых локальных сообществ [54].
На разнообразие локальных сообществ оказывают влияние геологическая история региона и крупные события планетарного масштаба. Появление Панамского перешейка около 3 млн лет назад и Великий межамериканский обмен привели к значительным изменениям в фауне млекопитающих Южной Америки. Ныне около половины южно-американских видов имеют североамериканское происхождение, при этом многие виды южно-американских сумчатых не выдержали конкуренции с плацентарными млекопитающими [56]. В то же время фауна Австралии, не имевшая в своей истории подобных по масштабу событий, сохранила значительную самобытность фауны до прихода людей [57]. Геологическая и эволюционная истории региона, масштабные катастрофические события, видообразование и вымирание видов формируют региональный пул видов крупных регионов – большое количество видов, популяции которых присутствуют в локальных местообитаниях, объединенных процессами расселения [58].
Расселение видов и появление их в локальных сообществах региона – следующий процесс, который определяет локальное разнообразие. Обмен особями между локальными сообществами и появление в сообществе новых видов из регионального пула модифицируют влияние фильтра среды и экологического дрейфа в формировании сообществ [3, 31]. Расселение способствует насыщению локальных сообществ видами и появлению особей видов уже существующих популяций, уменьшая относительное влияние экологического дрейфа, увеличивая роль абиотического фильтра и особенно биотических взаимодействий [59]. Появление новых видов в локальных сообществах может как стабилизировать их динамику, так и вывести их из состояния равновесия [60, 61].
Биотические взаимодействия и абиотический фильтр, по-видимому, действуют в разных пространственных масштабах. Биотические взаимодействия, например конкуренция, наблюдаются на уровне взаимодействия между особями, в то время как абиотический фильтр определяет состав видов, входящих в сообщество, в более крупном масштабе [62]. Но некоторые биотические взаимодействия, например симбиоз, могут влиять на состав сообщества и в более крупных масштабах [63]. Виды могут исчезать из сообщества не только под воздействием фильтра среды, но и в результате случайных событий и экологического дрейфа [15].
В результате видовой состав современных локальных сообществ зависит от расселения видов и существующих барьеров расселения, а также от того, обладают ли виды определенными свойствами, позволяющими им выжить в условиях окружающей среды и сосуществовать с другими видами в сообществе. В случае слабого действия фильтра среды (как абиотических факторов, так и биотических взаимодействий) в формировании сообществ увеличивается роль стохастических процессов и наоборот [31]. Относительная важность стохастических процессов растет с увеличением продуктивности сообществ. Также предполагается, что более благоприятные для жизни условия, большая доступность ресурсов и, таким образом, ослабление влияния фильтра среды и биотических взаимодействий лежат в основе положительной связи между увеличением продуктивности сообществ с уменьшением географической широты и увеличением биоразнообразия [64].
Как отмечено выше, при изучении и оценке влияния данных процессов на формирование сообществ велика роль пространственного и временнóго масштабов исследований, а также конкретных целей и задач [65]. Роль тех или иных процессов изменяется с развитием сообщества. Например, при восстановительной сукцессии после пожаров состав мхов и сосудистых растений в сообществе в первые годы определяется случайными процессами, роль которых постепенно падает, а детерминистических возрастает [66]. Однако если исследователь сосредоточен на изучении конкретного локального сообщества и процессов, которые протекают внутри него в данный момент времени, для объяснения наблюдаемых паттернов биоразнообразия может хватить нескольких низкоуровневых процессов, при этом для интерпретации результатов будет не важно, что некий низкоуровневый процесс порождает процесс высокого порядка [67].
АСПЕКТЫ РАЗНООБРАЗИЯ
Виды, представленные в сообществе, не являются функционально равнозначными и эволюционно эквивалентными, поэтому сообщества, включающие сходное количество видов (сходное таксономическое разнообразие), тем не менее могут иметь разное функциональное (ввиду экологических особенностей видов и их неравнозначности в выполнении экологических функций) и филогенетическое (из-за разной эволюционной истории видов) разнообразие [68, 69]. Так как виды имеют разные экологические, функциональные и физиологические особенности, описанные выше процессы могут по-разному влиять на различные аспекты биоразнообразия [70].
Функциональное разнообразие определяется как разнообразие функциональных признаков организмов, присутствующих в сообществе [71]. Функциональные признаки (трейты, traits) – это особенности организмов, в отношении которых предполагается или известно, что они влияют на приспособленность организмов: их рост, размножение и выживание [72]. Функциональные особенности определяют многомерное пространство признаков, в котором существуют виды (например, масса тела, диета, форма и размер клюва, листовая поверхность) [73]. Функциональные признаки видов определяют отношения между организмами и их биотической и абиотической средой, определяют экологическую нишу вида, т.е. его способность переносить определенные условия. Некоторые функциональные признаки определяют не только способность организмов выживать в конкретных условиях (response traits), но и возможность оказывать влияние на окружающую среду (effect traits). Такие признаки могут влиять на другие виды, представленные в сообществе, например через трофические взаимодействия (хищник – жертва, паразитизм), мутуалистические отношения либо такие экологические процессы, как круговорот питательных веществ, опыление, потребление опада и т.п. [71].
Оценка функционального разнообразия дает представление об устойчивости экосистем [74]. Функциональная избыточность, означающая, что популяции разных видов выполняют аналогичную или одинаковую функцию в сообществе, характерна для наиболее устойчивых к потере таксономического биоразнообразия сообществ [75]. В то же время популяции функционально избыточных сообществ более восприимчивы к экологическому дрейфу.
Виды вносят уникальный вклад в филогенетическое разнообразие сообществ, которое отражает их уникальную эволюционную историю [76]. Оценка филогенетического разнообразия основана на измерении длины ветвей филогенетического древа сообщества и учитывает эволюционную историю входящих в него видов [77]. Филогенетическое разнообразие часто предлагают использовать как косвенную меру для оценки функционального разнообразия, поскольку в отношении многих признаков предполагают эволюционное наследование, а в отношении более близких в эволюционном плане видов – экологическое сходство [78–80]. Помимо этого, взаимодействия между видами и их функциональные особенности зависят от комплекса признаков, часть из которых может быть не известна исследователям. Филогенетическое разнообразие в этом случае может служить мерой косвенной оценки функционального разнообразия [81]. Но филогенетическое разнообразие ценно само по себе и отражает в целом морфологические, экологические, генетические и физиологические различия, которые накопились между видами в ходе эволюции [82].
Функциональное разнообразие может не коррелировать с филогенетическим, если функциональные признаки находятся под сильным стабилизирующим отбором или из-за интенсивных конкурентных взаимоотношений между эволюционно близкими (и функционально похожими) видами [83, 84]. Оценка этого аспекта биоразнообразия важна для его сохранения во всей полноте, поскольку филогенетическое разнообразие отражает уникальную эволюционную историю видов. Например, потеря сравнительно небольшого числа видов может критически сказаться на филогенетическом разнообразии, если потерянные виды были единственными представителями своей клады в сообществе [85]. Оценка филогенетического разнообразия важна в качестве одного из критериев природоохранной деятельности для оценки экосистем [86].
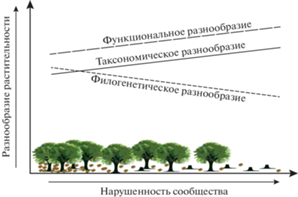
Функциональный и филогенетический аспекты разнообразия могут не коррелировать с таксономическим, поскольку ограничение расселения и фильтр окружающей среды по-разному влияют на эти компоненты разнообразия, а виды и сообщества имеют разную эволюционную историю [73]. Например, таксономическое разнообразие птиц лесов Центральной Европы положительно и наиболее сильно коррелирует с увеличением площади лесных массивов, возрастом древостоя и его составом, в то время как филогенетическое разнообразие уменьшается, а функциональное имеет положительную, но не столь выраженную корреляцию с этими факторами [87]. Все три аспекта биоразнообразия могут по-разному меняться при сукцессионных изменениях (рис. 4). На ранней стадии сукцессии видовое богатство увеличивается, но не наблюдается увеличения функционального и филогенетического разнообразия – виды имеют высокий уровень функционального сходства. На поздней стадии сукцессии не происходит дальнейшего увеличения видового богатства, но наблюдается рост функционального разнообразия за счет замены функционально избыточных видов, что свидетельствует о возрастающей роли биотических взаимодействий [88].
Рис. 4.
Изменения аспектов разнообразия растительности напочвенного яруса в градиенте вырубки: увеличение количества неспециализированных видов приводит к снижению филогенетического разнообразия (по [89]).
ОПРЕДЕЛЕНИЕ МЕХАНИЗМОВ ФОРМИРОВАНИЯ ЛОКАЛЬНЫХ СООБЩЕСТВ
Оценка функционального и филогенетического разнообразия по отношению к таксономическому позволяет лучше понять, в какой степени формирование сообщества обусловлено стохастическими или детерминистическими механизмами [88, 90]. Процессы, участвующие в формировании биологического разнообразия локальных сообществ, действуют на разных иерархически организованных пространственных масштабах [65]. Согласно описанным выше теоретическим моделям, биологическое разнообразие с точки зрения пространственной организации делится на региональный пул видов (региональное разнообразие – γ‑разнообразие), разнообразие локальных сообществ (локальное разнообразие – α-разнообразие) и изменение биологического разнообразия между локальными сообществами (β-разнообразие) [91]. Совместные оценки таксономического, функционального и филогенетического α- и β-разнообразия дополняют друг друга при определении процессов, которые влияют на формирование локальных сообществ в градиенте факторов среды, и позволяют определить те процессы, которые в наибольшей степени объясняют наблюдаемые паттерны разнообразия [19, 92].
Биотические взаимодействия, абиотический фильтр и экологический дрейф действуют на локальном уровне и определяют α-разнообразие локальных сообществ, тогда как изменение факторов среды в региональном масштабе, исторические и эволюционные процессы могут в значительной степени влиять на β-разнообразие [69, 93]. Например, таксономическое, функциональное и филогенетическое α-разнообразие локальных сообществ может оставаться неизменным, несмотря на существенное изменение видового состава, функциональных признаков или филогенетического древа от сообщества к сообществу, что демонстрирует влияние того или иного процесса на формирование локальных сообществ в региональном масштабе – изменение в локальных сообществах в градиенте факторов среды или в результате ограниченного расселения [17, 19].
Детерминистические механизмы: фильтр среды и биотические взаимодействия
Поскольку функциональные признаки позволяют видам адаптироваться к условиям окружающей среды, то фильтр окружающей среды преимущественно влияет на функциональную структуру сообществ, в то время как таксономический и филогенетический аспекты разнообразия могут не отражать влияния факторов [17]. Если роль абиотического фильтра в формировании сообщества велика (например, суровость условий среды), то в сообщество будут отобраны виды, обладающие сходными чертами, которые позволяют им выживать в определенных условиях, и мы будем наблюдать функционально кластеризованное (“clustered”) сообщество, функциональное α-разнообразие которого снижено (рис. 5) по отношению к таксономическому разнообразию [73]. Если велика роль биотического фильтра (интенсивны биотические взаимодействия, например конкуренция), то ожидается, что сообщество будет сформировано наиболее функционально разнообразными видами, сильные различия признаков которых будут способствовать сосуществованию благодаря лучшему разделению ресурсов, и мы будем наблюдать функционально сверхразнообразное (“overdispersed”) сообщество. В случае выраженной гетерогенности локального сообщества (вследствие нарушений или присутствия участков с разной продуктивностью) могут одновременно наблюдаться высокие уровни таксономического и функционального α-разнообразия [71, 90].
Рис. 5.
Влияние процессов формирования локальных сообществ на α- и β-компоненты различных
аспектов разнообразия:  и
и  – увеличение и уменьшение наблюдаемого аспекта разнообразия соответственно;
– увеличение и уменьшение наблюдаемого аспекта разнообразия соответственно;  –как увеличение, так уменьшение уровня аспекта разнообразия в зависимости от условий
(рассматриваемой группы организмов, неоднородности локального сообщества, выраженности
градиента факторов в регионе и различий между локальными сообществами);
–как увеличение, так уменьшение уровня аспекта разнообразия в зависимости от условий
(рассматриваемой группы организмов, неоднородности локального сообщества, выраженности
градиента факторов в регионе и различий между локальными сообществами);  и
и  – аспекты разнообразия, в отношении которых воздействие рассматриваемого процесса
выражено наиболее сильно по сравнению с другими;
– аспекты разнообразия, в отношении которых воздействие рассматриваемого процесса
выражено наиболее сильно по сравнению с другими;  – существенное влияние случайных причин в определении аспекта разнообразия, ввиду
чего тенденции в изменении тяжело прогнозировать.
– существенное влияние случайных причин в определении аспекта разнообразия, ввиду
чего тенденции в изменении тяжело прогнозировать.
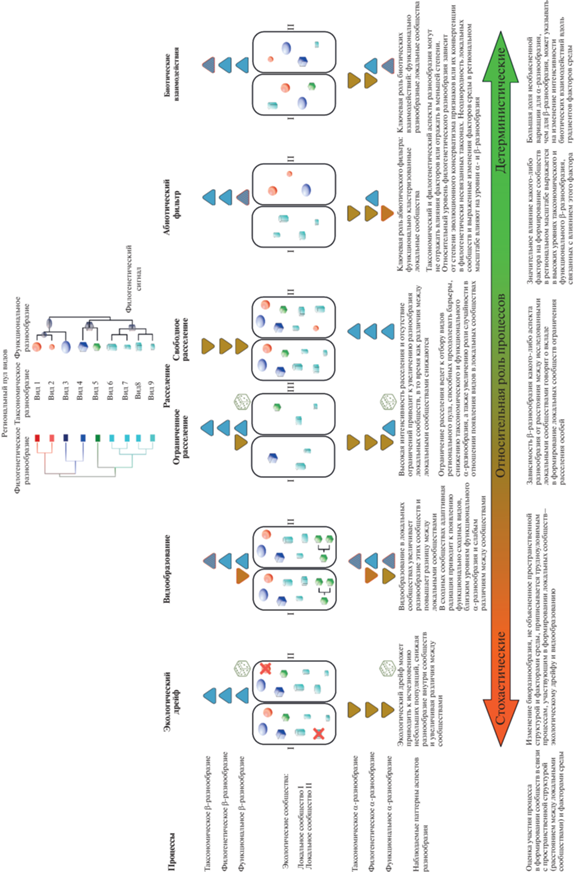
Если функциональные признаки эволюционно сохраняются, т.е. существует филогенетический сигнал по отношению к функциональным признакам, будет наблюдаться корреляция между функциональным и филогенетическим разнообразием [69, 79, 94]. Степень эволюционного консерватизма ниши и уровень филогенетического сигнала могут различаться в отношении факторов среды, из-за чего закономерности изменения рассматриваемых аспектов разнообразия могут отличаться в градиентах отдельных факторов [19]. При конвергенции функциональных признаков в филогенетически не связанных таксонах или низком эволюционном консерватизме функциональных признаков между филогенетическим и функциональным аспектами разнообразия ожидается обратная зависимость [71].
Абиотический и биотический фильтры влияют на формирование локальных сообществ, “пропуская” виды с определенными функциональными признаками, что должно отразиться на уровне функционального, филогенетического и таксономического β-разнообразия. Если какой-либо фактор значительно влияет на формирование сообществ в региональном масштабе, то ожидается высокое таксономическое и функциональное β-разнообразие, связанное с влиянием этого фактора [19]. Если влияние фильтра окружающей среды не столь выражено, то серьезного изменения функционального разнообразия не ожидается, но могут отмечаться высокие темпы изменения таксономического разнообразия из-за влияния стохастических процессов или интенсивных биотических взаимодействий [90]. Бо́льшая доля необъясненной факторами среды вариации для α-разнообразия, чем для β-разнообразия, может указывать на изменение интенсивности биотических взаимодействий вдоль градиентов факторов среды [19].
Благоприятные локальные условия и высокий уровень первичной продуктивности могут ослабить конкуренцию за ресурсы и снизить темпы вымирания видов, что в конечном итоге приводит к функциональной избыточности и снижению функционального α-разнообразия локальных сообществ по сравнению с таксономическим и филогенетическим аспектами разнообразия [90]. В этом случае наблюдается увеличение функционального β-разнообразия в градиенте факторов, которые обусловливают степень благоприятности условий среды, по сравнению с таксономическим β-разнообразием [19].
Стохастические механизмы. Расселение
Случайное, но ограниченное в силу разных причин расселение видов может влиять на разнообразие локальных сообществ и определять пространственно-структурированные паттерны изменения биоразнообразия [93]. Если перемещение видов не ограничено какими-либо барьерами или расстоянием, то виды можно найти во всех локальных сообществах, в которых они могут существовать. В этом случае фильтр окружающей среды и биотические взаимодействия определяют распространение видов [27]. Свободное расселение видов регионального пула приводит к увеличению таксономического и функционального α-разнообразия локальных сообществ, в то же время уменьшая различия (β-разнообразие) между локальными сообществами [90].
Существующие препятствия к расселению (например, сложный рельеф) даже без изменения факторов среды могут влиять на изменение функционального и филогенетического разнообразия локальных сообществ, уменьшая таксономическое α-разнообразие и приводя к формированию локальных сообществ, виды в которых обладают случайным набором функциональных признаков или функционально сходны по способности к расселению [90]. Ограничение расселения может влиять на определенные функциональные группы, более явно проявляясь в функциональном и филогенетическом β-разнообразии [70]. В этом случае ограничение расселения будет действовать как экологический фильтр, отбирая в сообщества виды, функционально схожие по своим способностям к расселению [71]. Зависимость β-разнообразия от пространственной структуры (расстояния между исследованными локальными сообществами) говорит о значительном вкладе в формирование локальных сообществ ограничения расселения особей [19].
Влияние расстояния между исследуемыми биотопами (определяемого пространством разнообразия) на изменение аспектов биологического разнообразия, помимо ограничений расселения, может свидетельствовать о существовании не учтенных в исследовании пространственно-структурированных факторов. Влияние учтенных в исследовании пространственно-структурированных факторов окружающей среды (например, изменения температуры с широтой) может выражаться в одновременном влиянии ограничения расселения и пространственно-структурированных факторов на разнообразие сообществ [95].
Стохастические механизмы. Видообразование и экологический дрейф
Необъясненное пространственной структурой и факторами среды изменение биоразнообразия приписывается трудноуловимым процессам, которые тем не менее участвуют в формировании локальных сообществ – экологическому дрейфу, видообразованию, а также не учтенным в модели, но влияющим на аспекты разнообразия факторам среды.
Видообразование
Видообразование – один из источников пополнения регионального пула видов наряду с масштабными событиями расселения. Несмотря на то, что видообразование происходит достаточно медленно и региональный пул видов можно считать сравнительно постоянным в современных исследованиях, в некоторых случаях его влияние невозможно игнорировать. Например, заполнение свободных экологических ниш на изолированных островах в большой мере достигается за счет видообразования, в то время как вблизи материка обычно благодаря расселению и иммиграции [96].
Видообразование, происходящее независимо в разных локальных сообществах, повышает α-разнообразие локальных сообществ и определяет случайные различия между этими сообществами – β-разнообразие [15]. Широкая адаптивная радиация может выражаться в низком уровне филогенетического разнообразия по отношению к другим аспектам разнообразия и высоком уровне эндемизма [90]. В экологически сходных локальных сообществах адаптивная радиация может приводить к появлению набора функционально сходных видов, близким уровням функционального α-разнообразия и слабым различиям между сообществами [97]. Темпы видообразования и влияние на аспекты разнообразия зависят от условий, в которых оно протекает: изолированности локального сообщества, его гетерогенности, силы действия абиотического фильтра и биотических взаимодействий, а также конкретной рассматриваемой группы организмов, которой свойственны собственные темпы видообразования и возможности давать широкую адаптивную радиацию [96, 98, 99].
Экологический дрейф
В изолированных локальных сообществах, не связанных расселением, обилие видов случайно и независимо “дрейфует” в каждом из локальных сообществ, что ведет к изменению их видового состава и различиям между сообществами (т.е. к увеличению β-разнообразия). В небольших сообществах значение экологического дрейфа возрастает: его воздействие влечет за собой случайное исчезновение видов, что существенно уменьшает локальное разнообразие. Случайное влияние экологического дрейфа позволяет видам доминировать даже там, где им не вполне благоприятствуют условия среды, что ослабляет наблюдаемую связь между составом сообществ и факторами среды. В общем виде экологический дрейф, как правило, может стать причиной уменьшения локального разнообразия и исчезновения видов, и ввиду случайности таких изменений увеличивает различия между сообществами – β-разнообразие [15].
В случае экологического дрейфа мы имеем дело со случайными изменениями состава сообществ, которые трудно предсказать. Эти случайные изменения могут определять не связанные с другими процессами (расселением, видообразованием, фильтром среды) различия между исследуемыми сообществами.
ОПРЕДЕЛЕНИЕ ПРОЦЕССОВ ФОРМИРОВАНИЯ ЭКОЛОГИЧЕСКИХ СООБЩЕСТВ: ПЕРСПЕКТИВЫ РАЗВИТИЯ
В обзоре изложены представления о формировании экологических сообществ в контексте одной из механистических обобщающих теорий, в наиболее явном виде формализованной Марком Веллендом [15]. Этот класс теорий основан на нашем понимании экологических и биологических процессов (фильтры среды, ограниченное и свободное расселение, видообразование, экологический дрейф), которые обусловливают формирование паттернов биологического разнообразия.
Другой класс теорий базируется на представлении, что наблюдаемые паттерны определяются наличием статистических ограничений в формировании сообществ. Данные теории не включают явных предположений о вкладе биологических или экологических процессов в формирование локальных сообществ [100]. Одним из таких подходов является Maximum Entropy Theory of Ecology (МЕТЕ) [101]. Он учитывает информацию о четырех параметрах сообществ для прогнозов их состояния: общая площадь сообщества, общее количество особей, общее количество видов и общая скорость метаболизма всех особей. Эти переменные используются для формирования биологических ограничений системы, и прогнозируемые состояния сообществ должны иметь плотность особей, среднее количество особей на вид и среднюю скорость метаболизма, соответствующие исходным ограничениям [101, 102]. METE рассчитывает наиболее вероятное состояние сообществ согласно этим ограничениям.
В литературе отмечается хорошая эмпирическая поддержка прогнозов METE для распределения обилия видов (species-abundance distribution – SAD), закономерностей, связанных с пространственным распределением особей видов, взаимосвязи между видами и территорией (species-area relationship – SAR) и вклада видов в метаболизм сообщества [102–104]. Однако в экосистемах с изменяющимися во времени переменными состояния предсказания METE часто не соответствуют наблюдаемым данным. Развернутая Maximum Entropy Theory of Ecology – DynaMETE – предусматривает учет механизмов и экологических процессов (например, природных и антропогенных нарушений или быстрого видообразования на островах), которые ответственны за изменение сообществ во времени, с чем классическая теория METE справлялась не очень хорошо, так как в классическом виде предполагает относительно статичное состояние сообществ. Как и классическая METE, DynaMETE включает два функциональных признака видов – скорость метаболизма (или размер тела) и численность видов. Но в этот подход можно включить другие функциональные признаки видов и связанные с ними факторы среды. Можно учесть и филогенетическое разнообразие на основании индексов расстояния между видами на филогенетическом древе [105].
Интеграция биологических процессов и экологических механизмов формирования экологических сообществ и статистических подходов к прогнозированию состояния экосистем, вероятно, позволит создать новую синтетическую теорию экологических сообществ, снабженную мощным математическим инструментарием, который даст возможность предсказывать паттерны различных аспектов разнообразия и оценивать роль тех или иных экологических процессов в формировании сообществ.
ЗАКЛЮЧЕНИЕ
Необходимо помнить, что описанные выше механизмы формирования локальных сообществ – удобные механистические модели, которые облегчают наше понимание устройства, формирования и изучение таких сложных систем, как экологические сообщества. Представленная схема взаимодействия процессов в виде иерархически-организованных фильтров, безусловно, полезна, но многие описанные выше процессы действуют, скорее, одновременно. Например, фильтры являются абстракциями экологических процессов и игнорируют тот факт, что эти процессы постоянно взаимодействуют сложным образом [33]. Сообщества могут быть одновременно ограничены фильтром среды и интенсивными биотическими взаимодействиями, ввиду чего функциональные признаки, связанные с фильтрацией среды, будут функционально избыточными, а черты, связанные с биотическими взаимодействиями, разнообразными [106]. Однако описанные теории позволяют выдвигать и тестировать гипотезы, которые уточняют наши представления о формировании сообществ и роли факторов среды, которые определяют существующие паттерны биоразнообразия.
Важное свойство теории экологических сообществ Марка Велленда [15] – ее инклюзивность, благодаря чему предложенные ранее отдельные концепции и механизмы формирования экологических сообществ рассматриваются как порождающие четыре процесса формирования сообществ высшего порядка и могут быть сведены к ним. Необходимо учитывать пространственный и временно́й масштаб исследований. Влияние многих факторов на состав локального сообщества можно установить только при проведении крупномасштабных региональных исследований [19, 107] или при изучении сообществ в разных масштабах [108]. Влияние многих процессов может ускользать от экологов, поскольку мы сосредотачиваем свою исследовательскую работу и интерпретацию полученных результатов на уровне объекта исследования – локального сообщества [67]. С другой стороны, в зависимости от задач исследования при изучении локальных сообществ вне контекста их взаимосвязи с другими сообществами для объяснения наблюдаемого разнообразия может быть достаточно только низкоуровневых процессов, проявление которых можно установить в наблюдаемом локальном сообществе.
Исследование выполнено за счет гранта Российского научного фонда № 22-74-00101 “Роль факторов среды в формировании сообществ почвенных орибатид (Acari: Oribatida) Европы”, https://rscf.ru/project/22-74-00101/.
Список литературы
Гиляров М.С., Винберг Г.Г., Чернов Ю.И. Экология – задачи и перспективы // Природа. 1977. № 5. С. 3.
Courchamp F., Dunne J.A., Le Maho Y. et al. Fundamental ecology is fundamental // Trends Ecol. Evol. 2015. V. 30. № 1. P. 9–16. https://doi.org/10.1016/j.tree.2014.11.005
Vellend M. Conceptual synthesis in community ecology // Q. Rev. Biol. 2010. V. 85. № 2. P. 183–206. https://doi.org/10.1086/652373
Hinterman E., Moccia A., Baber S. et al. MarsGarden: Designing an ecosystem for a sustainable multiplanetary future // Acta Astronaut. 2022. V. 195. P. 445–455. https://doi.org/10.1016/j.actaastro.2022.03.011
Wiens J.A., Stralberg D., Jongsomjit D. et al. Niches, models, and climate change: Assessing the assumptions and uncertainties // Proc. Natl. Acad. Sci. 2009. V. 106. P. 19729. https://doi.org/10.1073/pnas.0901639106
Semenchuk P., Moser D., Essl F. et al. Future representation of species’ climatic niches in protected areas: A case study with Austrian endemics // Front. Ecol. Evol. 2021. V. 9:685753. https://doi.org/10.3389/fevo.2021.685753
Arnan X., Angulo E., Boulay R. et al. Introduced ant species occupy empty climatic niches in Europe // Sci. Rep. 2021. V. 11. № 1. P. 3280. https://doi.org/10.1038/s41598-021-82982-y
Clements F.E. Plant succession: An analysis of the development of vegetation. Washington: Carnegie Institution of Washington, 1916. 512 p.
Elton C.S. Animal Ecology. New York: The Macmillan Company, 1927. 207 p.
Gause G.F. The struggle for existence. Baltimore: The Williams & Wilkins Company, 1934. 163 p.
Grinnell J. The niche-relationships of the California thrasher // The Auk. American Ornithological Society. 1917. V. 34. № 4. P. 427–433. https://doi.org/10.2307/4072271
Hutchinson G.E. Concluding remarks. Population studies: Animal ecology and demography // Cold Spring Harbor Symposia on Quantative Biology. 1957. V. 22. P. 415–427.
MacArthur R.H., Wilson E.O. The theory of island biogeography. Princeton, New Jersey: Princeton University Press, 1967. 203 p.
Hubbell S. The unified neutral theory of biodiversity and biogeography. Princeton, Oxford: Princeton University Press, 2001. V. 32. 448 p.
Vellend M. The theory of ecological communities (MPB-57). Princeton, Oxford: Princeton University Press, 2016. 248 p.
Faith D.P. Phylogenetic diversity, functional trait diversity and extinction: avoiding tipping points and worst-case losses. // Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015. V. 370. № 1662. P. 20140011. https://doi.org/10.1098/rstb.2014.0011
Mouchet M.A., Villéger S., Mason N.W.H. et al. Functional diversity measures: An overview of their redundancy and their ability to discriminate community assembly rules // Funct. Ecol. 2010. V. 24. № 4. P. 867–876. https://doi.org/10.1111/j.1365-2435.2010.01695.x
Jarzyna M.A., Jetz W. Detecting the multiple facets of biodiversity // Trends Ecol. Evol. 2016. V. 31. № 7. P. 527–538. https://doi.org/10.1016/j.tree.2016.04.002
Arnan X., Cerda X., Retana J. Partitioning the impact of environment and spatial structure on alpha and beta components of taxonomic, functional, and phylogenetic diversity in European ants // PEERJ. 2015. V. 3. https://doi.org/10.7717/peerj.1241
Palmer M.W. Variation in species richness: Towards a unification of hypotheses // Folia Geobot. Phytotaxon. 1994. V. 29. № 4. P. 511–530. https://doi.org/10.1007/BF02883148
Chase J.M., Leibold M.A. Ecological niches: Linking classical and contemporary approaches. Chicago, London: University of Chicago Press, 2009. 221 p. https://doi.org/10.7208/9780226101811
Bell G. Neutral Macroecology // Science. 2001. V. 293. № 5539. P. 2413–2418. https://doi.org/10.1126/science.293.5539.2413
Leibold M.A., Holyoak M., Mouquet N. et al. The metacommunity concept: A framework for multi-scale community ecology // Ecol. Lett. 2004. V. 7. № 7. P. 601–613. https://doi.org/10.1111/j.1461-0248.2004.00608.x
Logue J.B., Mouquet N., Peter H. et al. Empirical approaches to metacommunities: A review and comparison with theory // Trends Ecol. Evol. 2011. V. 26. № 9. P. 482–491. https://doi.org/10.1016/j.tree.2011.04.009
Anderson J.M. The enigma of soil animal species diversity // Progress in Soil Zoology: Proceedings of the 5th International Colloquium on Soil Zoology Held in Prague September 17–22, 1973. Dordrecht: Springer Netherlands, 1975. P. 51–58. https://doi.org/10.1007/978-94-010-1933-0_5
Walter D.E., Proctor H.C. Mites: Ecology, Evolution and Behaviour. Wallingford - New York - Sydney: CABI Publishing, 2013. 494 p. https://doi.org/10.1007/978-94-007-7164-2_1
Hubbell S.P. Neutral theory in community ecology and the hypothesis of functional equivalence // Funct. Ecol. 2005. V. 19. № 1. P. 166–172. https://doi.org/10.1111/j.0269-8463.2005.00965.x
Гиляров А.М. В поисках универсальных закономерностей организации сообществ: прогресс на пути нейтрализма // Журнал общей биологии. 2010. Т.71. № 5. С. 386–401. EDN MUKAWP
Zombie ideas in ecology: “neutral” = “stochastic” [Electronic resource] // Oikos Blog. 2012. URL: https://oikosjournal.wordpress.com/2012/01/23/-zombie-ideas-in-ecology-neutral-stochastic/ (accessed: 10.11.2022).
Kraft N.J.B., Adler P.B., Godoy O. et al. Community assembly, coexistence and the environmental filtering metaphor // Funct. Ecol. 2015. V. 29. № 5. P. 592–599. https://doi.org/10.1111/1365-2435.12345
Chase J.M., Myers J.A. Disentangling the importance of ecological niches from stochastic processes across scales // Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011. V. 366. № 1576. P. 2351–2363. https://doi.org/10.1098/rstb.2011.0063
Boet O., Arnan X., Retana J. The role of environmental vs. biotic filtering in the structure of European ant communities: A matter of trait type and spatial scale // PLOS ONE. 2020. V. 15. № 2. P. e0228625. https://doi.org/10.1371/journal.pone.0228625
Cadotte M.W., Tucker C.M. Should environmental filtering be abandoned? // Trends Ecol. Evol. 2017. V. 32. № 6. P. 429–437. https://doi.org/10.1016/j.tree.2017.03.004
Grime J.P., Pierce S. The evolutionary strategies that shape ecosystems. John Wiley & Sons, Ltd., 2012. 263 p.
Weiher E., Keddy P.A. Assembly rules, null models, and trait dispersion: New questions from old patterns // Oikos. 1995. V. 74. № 1. P. 159–164. https://doi.org/10.2307/3545686
Questad E.J., Foster B.L. Coexistence through spatio-temporal heterogeneity and species sorting in grassland plant communities // Ecol. Lett. 2008. V. 11. № 7. P. 717–726. https://doi.org/10.1111/j.1461-0248.2008.01186.x
Bauer B., Berti E., Ryser R. et al. Biotic filtering by species’ interactions constrains food-web variability across spatial and abiotic gradients // Ecol. Lett. 2022. V. 25. № 5. P. 1225–1236. https://doi.org/10.1111/ele.13995
Fukami T. Historical contingency in community assembly: Integrating niches, species pools, and priority effects // Annu. Rev. Ecol. Evol. Syst. 2015. V. 46. № 1. P. 1–23. https://doi.org/10.1146/annurev-ecolsys-110411-160340
Piculell B.J., Hoeksema J.D., Thompson J.N. Interactions of biotic and abiotic environmental factors in an ectomycorrhizal symbiosis, and the potential for selection mosaics // BMC Biol. 2008. V. 6. № 1. P. 11. https://doi.org/10.1186/1741-7007-6-23
Callaway R.M., Brooker R.W., Choler P. et al. Positive interactions among alpine plants increase with stress // Nature. 2002. V. 417. № 6891. P. 844–848. https://doi.org/10.1038/nature00812
Callaway R.M. Positive interactions and interdependence in plant communities. Dordrecht: Springer, 2007. 404 p.
Arnan X., Andersen A.N., Gibb H. et al. Dominance–diversity relationships in ant communities differ with invasion // Glob. Change Biol. 2018. V. 24. № 10. P. 4614–4625. https://doi.org/10.1111/gcb.14331
Noble D. The role of stochasticity in biological communication processes // Prog. Biophys. Mol. Biol. 2021. V. 162. P. 122–128. https://doi.org/10.1016/j.pbiomolbio.2020.09.008
Vandvik V., Goldberg D.E. Distinguishing the roles of dispersal in diversity maintenance and in diversity limitation // Folia Geobot. 2005. V. 40. № 1. P. 45–52. https://doi.org/10.1007/BF02803043
Lowe W.H., McPeek M.A. Is dispersal neutral? // Trends Ecol. Evol. 2014. V. 29. № 8. P. 444–450. https://doi.org/10.1016/j.tree.2014.05.009
Heinrichs J.A., Lawler J.J., Schumaker N.H. Intrinsic and extrinsic drivers of source˗sink dynamics // Ecol. Evol. 2016. V. 6. № 4. P. 892–904. https://doi.org/10.1002/ece3.2029
Mouquet N., Loreau M. Community patterns in source-sink metacommunities // Amer. Nat. 2003. V. 162. № 5. P. 544–557. https://doi.org/10.1086/378857
Melbourne B.A., Hastings A. Extinction risk depends strongly on factors contributing to stochasticity // Nature. 2008. V. 454. № 7200. P. 100–103. https://doi.org/10.1038/nature06922
Legendre S., Clobert J., Møller A.P. et al. Demographic stochasticity and social mating system in the process of extinction of small populations: The case of passerines introduced to New Zealand // Amer. Nat. 1999. V. 153. № 5. P. 449–463. https://doi.org/10.1086/303195
Lehmann L., Perrin N. On metapopulation resistance to drift and extinction // Ecology. 2006. V. 87. № 7. P. 1844–1855. https://doi.org/10.1890/0012-9658(2006)87[1844:-OMRTDA]2.0.CO;2
Gilbert B., Levine J.M. Ecological drift and the distribution of species diversity // Proc. R. Soc. B Biol. Sci. 2017. V. 284. № 1855. P. 20170507. https://doi.org/10.1098/rspb.2017.0507
Vellend M., Srivastava D.S., Anderson K.M. et al. Assessing the relative importance of neutral stochasticity in ecological communities // Oikos. 2014. V. 123. № 12. P. 1420–1430. https://doi.org/10.1111/oik.01493
Gan H., Zak D.R., Hunter M.D. Scale dependency of dispersal limitation, environmental filtering and biotic interactions determine the diversity and composition of oribatid mite communities // Pedobiologia. 2019. V. 74. P. 43–53. https://doi.org/10.1016/j.pedobi.2019.03.002
Lambers H., Oliveira R.S. Plant physiological ecology. Cham: Springer International Publishing, 2019. 736 p. https://doi.org/10.1007/978-3-030-29639-1
Götzenberger L., de Bello F., Bråthen K.A. et al. Ecological assembly rules in plant communities – approaches, patterns and prospects // Biol. Rev. 2012. V. 87. № 1. P. 111–127. https://doi.org/10.1111/j.1469-185X.2011.00187.x
Cione A.L., Gasparini G.M., Soibelzon E. et al. The great american biotic interchange: A south american perspective. Dordrecht: Springer, 2015. 97 p.
Ebach M.C. Handbook of Australasian biogeography. Boca Raton: CRC Press, 2017. 404 p.
Cornell H.V., Harrison S.P. What are species pools and when are they important? // Annu. Rev. Ecol. Evol. Syst. 2014. V. 45. № 1. P. 45–67. https://doi.org/10.1146/annurev-ecolsys-120213-091759
Ron R., Fragman-Sapir O., Kadmon R. Dispersal increases ecological selection by increasing effective community size // Proc. Natl. Acad. Sci. 2018. V. 115. № 44. P. 11280–11285. https://doi.org/10.1073/pnas.1812511115
Gallien L., Zimmermann N.E., Levine J.M. et al. The effects of intransitive competition on coexistence // Ecol. Lett. 2017. V. 20. № 7. P. 791–800. https://doi.org/10.1111/ele.12775
Henriksson A., Wardle D.A., Trygg J. et al. Strong invaders are strong defenders – implications for the resistance of invaded communities // Ecol. Lett. 2016. V. 19. № 4. P. 487–494. https://doi.org/10.1111/ele.12586
Swenson N.G., Enquist B.J., Pither J. et al. The problem and promise of scale dependency in community phylogenetics. // Ecology. 2006. V. 87. № 10. P. 2418–2424. https://doi.org/10.1890/0012-9658(2006)87[2418:-tpapos]2.0.co;2
Wisz M.S., Pottier J., Kissling W.D. et al. The role of biotic interactions in shaping distributions and realised assemblages of species: Implications for species distribution modelling // Biol. Rev. 2013. V. 88. № 1. P. 15–30. https://doi.org/10.1111/j.1469-185X.2012.00235.x
Chase J.M. Stochastic community assembly causes higher biodiversity in more productive environments // Science. 2010. V. 328. № 5984. P. 1388–1391. https://doi.org/10.1126/science.1187820
Mahon M.B., Penn H.J., Campbell K.U. et al. Differential patterns of taxonomic and functional diversity for two groups of canopy arthropods across spatial scales // bioRxiv. 2022. P. 2022.08.03.502641. https://doi.org/10.1101/2022.08.03.502641
Måren I.E., Kapfer J., Aarrestad P.A. et al. Changing contributions of stochastic and deterministic processes in community assembly over a successional gradient // Ecology. 2018. V. 99. P. 148–157. https://doi.org/10.1002/ecy.2052
Fox J. Book review: The theory of ecological communities by Mark Vellend [Electronic resource] // Dynamic Ecology. 2016. URL: https://dynamicecology.wordpress.com/2016/12/19/book-review-the-theory-of-ecological-communities-by-mark-vellend/ (accessed: 09.11.2022).
Hooper D.U., Chapin III F.S., Ewel J.J. et al. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge // Ecol. Monogr. 2005. V. 75. № 1. P. 3–35. https://doi.org/10.1890/04-0922
Webb C.O., Ackerly D.D., McPeek M.A. et al. Phylogenies and community ecology // Annu. Rev. Ecol. Syst. 2002. V. 33. № 1. P. 475–505. https://doi.org/10.1146/annurev.ecolsys.33.010802.-150448
Meynard C.N., Devictor V., Mouillot D. et al. Beyond taxonomic diversity patterns: how do α, β and γ components of bird functional and phylogenetic diversity respond to environmental gradients across France? // Glob. Ecol. Biogeogr. 2011. V. 20. № 6. P. 893–903. https://doi.org/10.1111/j.1466-8238.2010.00647.x
de Bello F., Carmona C.P., Dias A.T.C. et al. Handbook of trait-based ecology: From theory to R tools. Cambridge: Cambridge University Press, 2021. 308 p. https://doi.org/10.1017/9781108628426
Violle C., Navas M.L., Vile D. et al. Let the concept of trait be functional! // Oikos. 2007. V. 116. № 5. P. 882–892. https://doi.org/10.1111/j.0030-1299.2007.15559.x
Mazel F., Thuiller W. Functional and phylogenetic diversity˗area relationships // The species˗area relationship: Theory and application / Eds. Triantis K.A., Whittaker R.J., Matthews T.J. Cambridge: Cambridge University Press, 2021. P. 107–132. https://doi.org/10.1017/9781108569422.009
Naeem S. Species redundancy and ecosystem reliability // Conserv. Biol. 1998. V. 12. № 1. P. 39–45. https://doi.org/10.1111/j.1523-1739.1998.96379.x
Biggs C.R., Yeager L.A., Bolser D.G. et al. Does functional redundancy affect ecological stability and resilience? A review and meta-analysis // Ecosphere. 2020. V. 11. № 7. P. e03184. https://doi.org/10.1002/ecs2.3184
Owen N.R., Gumbs R., Gray C.L. et al. Global conservation of phylogenetic diversity captures more than just functional diversity // Nat. Commun. 2019. V. 10. № 1. P. 859. https://doi.org/10.1038/s41467-019-08600-8
Faith D.P. Conservation evaluation and phylogenetic diversity // Biol. Conserv. 1992. V. 61. № 1. P. 1–10. https://doi.org/10.1016/0006-3207(92)91201-3
Головатюк Л.В., Шитиков В.К., Зинченко Т.Д. Анализ связи филогенетического разнообразия донных сообществ с минерализацией равнинных рек бассейна Нижней Волги // Экология. 2022. № 2. С. 129–139. https://doi.org/10.31857/S0367059722010048
Blomberg S.P., Garland Jr T. Tempo and mode in evolution: phylogenetic inertia, adaptation and comparative methods // J. Evol. Biol. 2002. V. 15. № 6. P. 899–910. https://doi.org/10.1046/j.1420-9101.2002.00472.x
Potapov A.M., Scheu S., Tiunov A.V. Trophic consistency of supraspecific taxa in below-ground invertebrate communities: Comparison across lineages and taxonomic ranks // Funct. Ecol. 2019. V. 33. № 6. P. 1172–1183. https://doi.org/10.1111/1365-2435.13309
Cadotte M.W., Cavender-Bares J., Tilman D. et al. Using phylogenetic, functional and trait diversity to understand patterns of plant community productivity // PLOS ONE. 2009. V. 4. № 5. P. e5695. https://doi.org/10.1371/journal.pone.0005695
Gerhold P., Cahill J.F., Jr. Winter M. et al. Phylogenetic patterns are not proxies of community assembly mechanisms (they are far better) // Funct. Ecol. 2015. V. 29. № 5. P. 600–614. https://doi.org/10.1111/1365-2435.12425
Devictor V., Mouillot D., Meynard C. et al. Spatial mismatch and congruence between taxonomic, phylogenetic and functional diversity: The need for integrative conservation strategies in a changing world // Ecol. Lett. 2010. V. 13. № 8. P. 1030–1040. https://doi.org/10.1111/j.1461-0248.2010.01493.x
Prinzing A., Reiffers R., Braakhekke W.G. et al. Less lineages – more trait variation: phylogenetically clustered plant communities are functionally more diverse // Ecol. Lett. 2008. V. 11. № 8. P. 809–819. https://doi.org/10.1111/j.1461-0248.2008.01189.x
Faith D.P., Richards Z.T. Climate change impacts on the tree of life: Changes in phylogenetic diversity illustrated for Acropora corals // Biology. 2012. V. 1. № 3. P. 906–932. https://doi.org/10.3390/biology1030906
Cadotte M.W., Dinnage R., Tilman D. Phylogenetic diversity promotes ecosystem stability // Ecology. 2012. V. 93. № sp8. P. S223–S233. https://doi.org/10.1890/11-0426.1
Bełcik M., Lenda M., Amano T. et al. Different response of the taxonomic, phylogenetic and functional diversity of birds to forest fragmentation // Sci. Rep. 2020. V. 10. № 1. P. 20320. https://doi.org/10.1038/s41598-020-76917-2
Purschke O., Schmid B.C., Sykes M.T. et al. Contrasting changes in taxonomic, phylogenetic and functional diversity during a long-term succession: insights into assembly processes // J. Ecol. 2013. V. 101. № 4. P. 857–866. https://doi.org/10.1111/1365-2745.12098
Hupperts S.F., Webster C.R., Froese R.E. et al. Increasing ground-layer plant taxonomic diversity masks declining phylogenetic diversity along a silvicultural disturbance gradient // Can. J. For. Res. 2020. V. 50. P. 1259–1267.https://doi.org/10.1139/cjfr-2020-0055
Pavoine S., Bonsall M.B. Measuring biodiversity to explain community assembly: a unified approach // Biol. Rev. 2011. V. 86. № 4. P. 792–812. https://doi.org/10.1111/j.1469-185X.2010.00171.x
Ricotta C., Szeidl L. Diversity partitioning of Rao’s quadratic entropy // Theor. Popul. Biol. 2009. V. 76. № 4. P. 299–302. https://doi.org/10.1016/j.tpb.2009.10.001
Jones H.P., Barber N.A., Gibson D.J. Is phylogenetic and functional trait diversity a driver or a consequence of grassland community assembly? // J. Ecol. 2019. V. 107. № 5. P. 2027–2032. https://doi.org/10.1111/1365-2745.13260
Dambros C., Zuquim G., Moulatlet G.M. et al. The role of environmental filtering, geographic distance and dispersal barriers in shaping the turnover of plant and animal species in Amazonia // Biodivers. Conserv. 2020. V. 29. № 13. P. 3609–3634. https://doi.org/10.1007/s10531-020-02040-3
Pagel M. Inferring the historical patterns of biological evolution // Nature. 1999. V. 401. № 6756. P. 877–884. https://doi.org/10.1038/44766
Jones M.M., Tuomisto H., Borcard D. et al. Explaining variation in tropical plant community composition: influence of environmental and spatial data quality // Oecologia. 2008. V. 155. № 3. P. 593–604. https://doi.org/10.1007/s00442-007-0923-8
Emerson B.C., Gillespie R.G. Phylogenetic analysis of community assembly and structure over space and time // Trends Ecol. Evol. 2008. V. 23. № 11. P. 619–630. https://doi.org/10.1016/j.tree.2008.07.005
Losos J.B., Jackman T.R., Larson A. et al. Contingency and determinism in replicated adaptive radiations of island lizards // Science. 1998. V. 279. № 5359. P. 2115–2118. https://doi.org/10.1126/science.279.5359.2115
Barajas-Barbosa M.P., Craven D., Weigelt P. et al. Assembly of functional diversity in an oceanic island flora // bioRxiv. 2022. P. 2022.03.04.482684. https://doi.org/10.1101/2022.03.04.482684
Weigelt P., Daniel Kissling W., Kisel Y. et al. Global patterns and drivers of phylogenetic structure in island floras // Sci. Rep. 2015. V. 5. № 1. P. 12213. https://doi.org/10.1038/srep12213
McGlinn D.J., Xiao X., Kitzes J. et al. Exploring the spatially explicit predictions of the Maximum Entropy Theory of Ecology // Glob. Ecol. Biogeogr. 2015. V. 24. № 6. P. 675–684. https://doi.org/10.1111/geb.12295
Harte J. Maximum Entropy and Ecology: A Theory of Abundance, Distribution, and Energetics. Oxford University Press, 2011. 264 p. https://doi.org/10.1093/acprof:oso/9780199593415.-001.0001
Harte J., Newman E.A., Rominger A.J. Metabolic partitioning across individuals in ecological communities // Glob. Ecol. Biogeogr. 2017. V. 26. № 9. P. 993–997. https://doi.org/10.1111/geb.12621
Harte J., Zillio T., Conlisk E. et al. Maximum entropy and the state-variable approach to macroecology // Ecology. 2008. V. 89. № 10. P. 2700–2711. https://doi.org/10.1890/07-1369.1
Harte J., Smith A.B., Storch D. Biodiversity scales from plots to biomes with a universal species – area curve // Ecol. Lett. 2009. V. 12. № 8. P. 789–797. https://doi.org/10.1111/j.1461-0248.2009.01328.x
Harte J., Umemura K., Brush M. DynaMETE: a hybrid MaxEnt-plus-mechanism theory of dynamic macroecology // Ecol. Lett. 2021. V. 24. № 5. P. 935–949. https://doi.org/10.1111/ele.13714
Weiher E., Freund D., Bunton T. et al. Advances, challenges and a developing synthesis of ecological community assembly theory // Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011. V. 366. № 1576. P. 2403–2413. https://doi.org/10.1098/rstb.2011.0056
Le Bagousse-Pinguet Y., Gross N., Maestre F.T. et al. Testing the environmental filtering concept in global drylands // J. Ecol. 2017. V. 105. № 4. P. 1058–1069. https://doi.org/10.1111/1365-2745.12735
Thakur M.P., Phillips H.R.P., Brose U. et al. Towards an integrative understanding of soil biodiversity // Biol. Rev. 2020. V. 95. № 2. P. 350–364. https://doi.org/10.1111/brv.12567
Дополнительные материалы отсутствуют.