Коллоидный журнал, 2023, T. 85, № 5, стр. 655-667
Концентрация наночастиц как важный параметр для характеристики дисперсий и ее применение в биомедицине
Т. Н. Паширова 1, *, З. М. Шайхутдинова 1, Э. Б. Соуто 2, 3, П. Массон 4, В. Ф. Миронов 1
1 Институт органической и физической химии им. А.Е. Арбузова,
ФИЦ Казанский научный центр РАН
420088 Казань, ул. Акад. Арбузова, 8, Россия
2 UCIBIO – Applied Molecular Biosciences Unit, MEDTECH, Laboratory of Pharmaceutical Technology,
Department of Drug Sciences, Faculty of Pharmacy, University of Porto
4050-313 Porto, Portugal
3 Associate Laboratory i4HB – Institute for Health and Bioeconomy,
Faculty of Pharmacy, University of Porto
4050-313 Porto, Portugal
4 Казанский (Приволжский) федеральный университет
420008 Казань, ул. Кремлевская, 18, Россия
* E-mail: tatyana_pashirova@mail.ru
Поступила в редакцию 26.06.2023
После доработки 03.08.2023
Принята к публикации 03.08.2023
- EDN: DLPFDE
- DOI: 10.31857/S0023291223600487
Аннотация
В настоящее время стоит острая необходимость в стандартизации методов определения концентрации наночастиц и создания эталонных материалов для калибровки измеряемой величины. Точное определение концентрации наночастиц необходимо для оценки максимальной дозы вводимых нанопрепаратов в области диагностики и терапии in vivo, определения порядка реакции при использовании ферментативных нанореакторов. Кроме того, данный параметр обуславливает биологические эффекты, такие как образование белковой короны, улучшение поглощения и интернализации с клетками и т.д. В обзоре представлены наиболее часто встречающиеся способы определения концентрации наночастиц, основанные на их прямой визуализации с использованием методов микроскопии, на поглощении или рассеянии света, прямого подсчета наночастиц и гравиметрии, обсуждены их достоинства, недостатки и способы усовершенствования. Показано, что для более надежного и достоверного определения концентрации наночастиц следует использовать комбинацию нескольких методов.
ВВЕДЕНИЕ
Наноразмерные системы доставки лекарственных веществ (НСДЛВ) представляют большой интерес в области биомедицины и фармацевтики. Как правило, при создании новых НСДЛВ в публикациях описывается их рецептура с точки зрения способа получения, физико-химических свойств и биологической активности. Ключевыми параметрами физико-химических свойств нанопрепаратов являются размер наночастиц (НЧ), полидисперсность, площадь поверхности, загрузка лекарственного вещества, стабильность, кинетика высвобождения, в некоторых случаях указывается дзета-потенциал, например, в случае доставки генного материала в клетку, когда важен заряд НЧ.
Концентрация НЧ (количество НЧ в объеме, частиц/мл) − важный параметр для всесторонней характеристики нанопрепарата. Так, например, не только размер, но и количественная (численная) концентрация НЧ влияет на абсорбцию, клиренс и место расположения НСДЛВ в организме человека. Известно, что данный параметр сказывается на эффективном поглощении нанопрепаратов клетками, например, фагоцитами, т.е. содержание лекарства внутри НЧ будет определять их биологическую активность. Концентрация дает информацию о количественной оценке аффинных сайтов на каждой функционализированной НЧ, что важно при создании вирусов, для генной терапии, визуализирующих зондов и вакцин. Данный параметр позволяет точно оценить концентрацию лекарственного вещества в каждой частице или его распределение между фазами, что определяeт, будет ли система являться растворенной или дисперсной, и, следовательно, будет свидетельствовать о кинетике и механизме высвобождения. Известно, что эффективность визуализации зависит от мощности лазерного излучения и концентрации наноагентов, поэтому точное определение концентрации НЧ необходимо для оценки максимальной дозы вводимых нанопрепаратов в системный кровоток, что является основополагающим для диагностики in vivo и терапии заболеваний [1, 2].
Кроме того, при конструировании эффективного нанореактора концентрация загруженного компонента, например, фермента, будет определять порядок реакции, протекающей внутри замкнутых систем [3–5], что чрезвычайно важно при создании искусственных органелл, клеток и тканей [6], работе ферментных наноустройств [5]. Концентрация нанопрепарата требуется для тщательной характеристики продукта при получении разрешения регулирующих органов [1]. Так, фармакопейные требования, касающиеся безопасности лекарственных форм, ограничивают номинальное содержание числа частиц в объеме для инъекционных фармацевтических суспензий на основе твердых НЧ [7]. Регулирующие органы FDA по составам наноносителей в дополнение к таким характеристикам, как средний размер НЧ и полидисперсность, требуют сообщать их численную концентрацию.
Таким образом, точное определение и контроль количественной концентрации НСДЛВ является ключевой характеристикой в наномедицине для достижения максимальной эффективности лекарства и минимизации его токсичности при введении in vivo [8]. Известно, что существуют теоретические способы расчета концентрации НЧ, например, предложенные авторами в работе [9, 10]. Однако математические модели достаточно просты, существуют ограничения для расчетов и расхождения теоретического значения по числу НЧ с эмпирическими результатами [11], и самое главное, – не учитывается сложность биологической среды. Поэтому в настоящее время наблюдается острая потребность в надежных эмпирических методах определения численной концентрации НЧ в биологических средах и органах. В настоящем обзоре кратко представлены существующие методы, типы НЧ и их применение.
МЕТОДЫ ИЗМЕРЕНИЯ КОНЦЕНТРАЦИИ НАНОЧАСТИЦ
Несмотря на то, что в настоящее время нет сертифицированных стандартов для определения концентрации НЧ или эталонных материалов для калибровки измеряемой концентрации [12–14], в литературе встречаются методы, основанные на поглощении или рассеянии света, а также способы прямого подсчета НЧ. Некоторые методы специфичны для определенного типа НЧ, в то время как другие могут быть адаптированы для широкого спектра нанообъектов. Например, УФ-видимая спектрофотометрия (UV Vis) [15] и масс-спектрометрия с индуктивно-связанной плазмой [16, 17] достаточно точны для определения концентрации металлических и полупроводниковых НЧ. Для углеродных наноточек лучшим методом определения их концентрации является УФ-видимая, флуоресцентная и атомно-эмиссионная спектрометрия [18]. Метод турбидиметрии дает хорошие результаты для монодисперсных НЧ с известным показателем преломления и коэффициентом рассеяния, таких как частицы латекса или кремнезема, однако чувствительность метода снижается с уменьшением размера НЧ [19, 20].
Методы визуализации
Способ подсчета НЧ методами прямой визуализации (микроскопии) обеспечивает опре-деление количественнoй концентрации частиц без использования каких-либо стандартов. Однако в этом случае трудно получить высокую статистическую достоверность и требуется достаточно сложная пробоподготовка, так как методы микроскопии не предназначены для измерений в жидких средах. Атомно-силовая (AFM) и трансмиссионная электронная микроскопия (TEM) имеют огромный потенциал, обеспечивающий точное измерение числа НЧ. Долгое время эти методы считались наиболее подходящими для высокого разрешения вплоть до нанометрового диапазона. Однако они склонны к артефактам, и их применение ограничено для “мягких” органических образцов с низкой электронной плотностью. Для увеличения контраста таких образцов требуeтся использование окрашивающиx молекул. Более того, идентификация НЧ усложняется, когда образцы подвергаются деформации и агрегации в процессе сушки. Отметим, что процедура сушки в процессе пробоподготовки постоянно совершенствуется, так, например, в работе [21] использовали сочетание функционализации субстрата и ультрацентрифугирования для обеспечения высокого и равномерного извлечения НЧ на субстрате AFM и количественного определения количества НЧ и их распределения по размерам.
Среди методов подсчета, не основанных на микроскопии, известен метод настраиваемого резистивного измерения импульсов (tunable resistive pulse sensing, TRPS), который позволяет связывать количество НЧ с обнаруженными изменениями тока или сопротивления, когда частицы проходят через электрическое поле.
Оптические методы
УФ-видимая спектроскопия, турбидиметрия и динамическое рассеяние света (DLS) – оптические методы, измеряющие интенсивность света при поглощении или рассеянии НЧ. Способы измерения массовой или счетной концентрации НЧ с помощью данных методов, как правило, предусматривают построение градуировочного графика (зависимости оптической плотности образца на выбранной длине волны от концентрации НЧ) с помощью серии образцов с известными значениями концентрации частиц (образцов сравнения). Затем измеряют оптическую плотность анализируемого образца на той же длине волны и с помощью градуировочного графика определяют соответствующее ему значение концентрации. Для реализации любого из этих способов необходима серия образцов сравнения с заранее известными значениями концентрации.
Известен также оптический способ, не требующий образцов сравнения и использующий для определения счетной концентрации спектры экстинкции. Способ предполагает расчет концентрации НЧ по значению их экстинкции при длине волны, находящейся на достаточном расстоянии от пика плазмонного резонанса. Для такого расчета необходимо знать размер частиц, который определяется по положению пика плазмонного резонанса по шкале длин волн. Этот способ описан в работах [22, 23] для золотых и [24] для серебряных НЧ. Однако данный метод может быть использован только длямонодисперсных частиц (один максимум в распределении частиц по размерам), имеющих пик плазмонного резонанса в доступной для наблюдения области. Для полидисперсных систем, в которых имеются частицы двух и более характерных размеров, сильно отличающихся друг от друга, рассматриваемый способ непригоден, за исключением частиц с размером <20 нм [23]. Кроме того, положение пика плазмонного резонанса зависит как от размеров НЧ, так и их поверхности, а также от свойств среды.
Достаточно простой метод турбидиметрии для определения концентрации силикатных НЧ с использованием экспериментально определенных показателя преломления и спектра мутности представлен в работе [25]. Авторы рекомендуют данный метод и для других коллоидных систем, которые агрегативно устойчивы в среде. Одно из требований к используемым приборам – это ограничение апертуры приемника света для устранения эффектов малоуглового рассеяния [25].
DLS как метод определения относительной численной концентрации модельных систем монодисперсных сферических НЧ латекса размером 60 нм, а также коллоидных НЧ серебра и золота диаметром от 4.5 до 50 нм в растворах был применен в работе [26]. Авторы этой работы отмечают, что используемый прибор ZetasizerNano (Malvern) не позиционируется для указанной цели, однако с помощью него можно производить сравнительный анализ образцов по численной концентрации НЧ. Данный способ основан на измерении среднего гидродинамического диаметра НЧ для двух образцов и измерения на том же приборе дополнительной оптической характеристики – скорости счета фотонов рассеянного света для каждого из образцов. Расчет относительной концентрации НЧ в одном из образцов по отношению к другому основан на предположении, что интенсивность рассеяния прямо пропорциональна шестой степени диаметра частиц, т.е. в предположении, что интенсивность рассеяния I подчиняется закону Релея: I ~ $d_{{\text{H}}}^{{\text{6}}}n$.
Наиболее близким к вышеописанному способу является метод, описанный в работе [27], включающий измерение среднего гидродинамического диаметра частиц методом DLS, далее – расчет по измеренному значению эффективности экстинкции частиц, измерение оптической плотности на одной из длин волн видимого диапазона и расчет по полученным данным счетной концентрации НЧ с использованием формул для сечения экстинкции общей теории рассеяния Ми, справедливых для частиц с диаметром до 10 000 нм. Формула для расчета представлена ниже:
За последние несколько лет были созданы установки, основанные на новой оптической технологии, получившей название лазерная спектроскопия пропускания (LTS). Данный метод позволяет определить концентрацию НЧ и их геометрический размер путем однократного измерения зависящего от длины волны коэффициента пропускания лазерного луча через суспензию НЧ золота и полистирола [29–31], а также частиц в нормальных и раковых клеточных лизатах [32]. LTS оказалaсь достаточно перспективной по чувствительности, в особенности для полистирольных НЧ. Метод LTS может быть адаптирован для характеристики НЧ различной формы и геометрии (например цилиндрoв, дискoв, эллипсoв и т.д.) с использованием соответствующих алгоритмов, что также дает возможность реализации данного метода для НЧ различной природы. Применение LTS для природных везикул, таких как экзосомы и везикулы, представлено в работе [33]. Синтетические липосомные дисперсии различного состава были исследованы в работе [31]. Необходимо отметить, что с помощью данных, полученных LTS, авторам удалось количественно оценить способность захвата гидрофильного противотуберкулезного препарата (изониазидa) липосомами и накопление лекарства в фосфолипидном бислое благодаря взаимодействиям липид – лекарство.
Анализ траектории НЧ (NTA) является надежным методом определения концентрации, который основан на рассеянии света и наблюдении за броуновским движением каждой НЧ в отдельности с целью определения среднеквадратичного смещения отдельных НЧ. Анализ осуществляется без разделения популяций НЧ по размерам [34–36]. Данный метод подходит для различных типов НЧ таких как клеточные везикулы, вирусные частицы, микровезикулы и экзосомы, НЧ золота, фуллерены, липосомы и белковые агрегаты [37–41]. Меньше известно о его использовании для анизотропных частиц. Метод NTA был применен для образцов белка, в качестве стандарта использовались НЧ полистирола. Установлено, что для белков более подходящим является режим мониторинга в реальном времени [42]. Ограничением данного метода является пониженная чувствительность при достижении нижнего предела обнаружения размера частиц. Для получения статистически достоверных результатов рекомендуется работать в области концентраций от 107 до 109 частиц/мл [43]. Наличие агломерации частиц и неоднородности усложняют измерение концентрации данным методом [34, 44, 45]. Результаты, полученные для везикул методом NTA, зависят от настроек измерения [46], программного и аппаратного обеспечения ZetaView или NanoSight NS300 [47]. Авторы полагают, что дальнейшее развитие метода NTA должно быть сосредоточено на стандартизации измерений в биологических средах для того, чтобы свести к минимуму влияние оператора на измерение концентрации НЧ. Для исследования стержнеобразных структур (диаметр 10 нм, длина 35–250 нм) и белковых, фибриллярных структур на примере белков миозина (L/d = 80, гидродинамический размер dh ~ 30–35 нм) Hoover и Murphy рекомендуют использовать NTA в комбинации с другими методами, такими как статическое рассеяние света (SLS), DLS и TEM. Метод NTA в дополнение к информации о распределении частиц по размерам и концентрации НЧ может быть использован для детектирования изменения морфологии от глобулярной к фибриллярной в режиме реального времени [48].
Гравиметрические методы
По сравнению с аналитическими методами преимуществом гравиметрических измерений является то, что они позволяют получить абсолютную концентрацию НЧ, используя совокупное количество НЧ в суспензии, деленное на вес одной НЧ (рис. 1) по формулам [19]:
Рис. 1.
Абсолютная концентрация НЧ, представленная как совокупное количество НЧ в суспензии, деленное на вес одной НЧ.
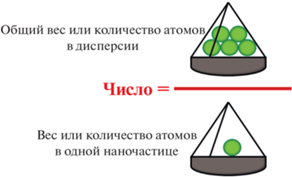
Гравиметрический метод определения концентрации НЧ достаточно просто реализуем, если известна плотность исследуемого наноматериала, и может применяться ко всем типам НЧ. Известно, что концентрацию коммерческиx эталонныx образцов НЧ полистирола, использующиxся в качестве стандартов, производители определяют количественно именно гравиметрическими методами [49]. Тем не менее использование данного метода ограничено неточностью измерения таких параметров, как плотность и количество атомов на одну частицу. Последнее часто приводит к неточности и только грубой оценки концентрации НЧ, например, в случае новых полимерных нано-материалов и т.д. Данный метод постоянно улучшается, так, например, в работе [50] плотность НЧ – наносфер сополимеров поли(стирола/акриламида) определяли центрифугированием, что способствовало повышению точности гравиметрического метода. После определения концентрации НЧ, модифицированных стрептавидином и антителами, успешно удалось определить активные аффинные сайты на каждой биофункциональной НЧ.
Комбинированные подходы
Для обеспечения точного и достоверного определения концентрации НЧ рекомендуется сравнительная оценка с применением нескольких методов, а именно использующих различные свойствa НЧ, например, поглощение света, массу, светорассеяние и/или, например, совместное применение микроскопии высокого разрешения и оптико-спектральных методов. В частности, данный подход применялся для внеклеточных везикул, пегилированных липосом с использованием таких методов как NTA, TRPS, когда НЧ проходят через нанопоры с регулируемым размером) [51], nFCM (нанопроточная цитофлуориметрия), CLS (дифференциальное центробежное осаждение) [52], AF4-MALS (фракционирование потока в режиме онлайн с многоугловым детектором светорассеяния) и многоугловое динамическое светорассеяние (MADLS). Наблюдалась достаточно хорошая сходимость полученных результатов для всех 6 методов, например, для полистирольных НЧ она составила около 90%, за исключением метода NTA, который завышал концентрацию НЧ. Для пегилированных липосом метод nFCM значительно снижал концентрацию НЧ в сравнении с другими методами. Для внеклеточных везикул наблюдалось сложное поведение, только для трех методов (NTA, TRPS и nFCM) значение концентрации НЧ находилось в пределах одного порядка [12].
Разрабатываются инновационные комбинации методов, такие как настраиваемое резистивное измерение импульсов [53], нанопроточная цитометрия и MADLS [54, 55], малоугловое рентгеновское рассеяние (SAXS) [56], центробежные методы осаждения и AF4-MALS [56] и т.д. Описан способ, сочетающий иммобилизацию НЧ на анизотропно-коллапсированный агарозный гель с последующим использованием оптической микроскопии и подсчета НЧ. Эталоном служил метод TEM. В качестве объектов исследовали НЧ кремнезема с флуоресцентным красителем, квантовые точки CdSe/ZnS и субмикронные частицы кремнезема [57]. Встречается комбинация электроспрея (ES) для создания парофазного дисперсного материала с использованием сканирующего измерителя подвижности частиц (SMPS). Подвижность анализировалась с помощью дифференциальной подвижности (DMA), газофазного электрофоретического молекулярного анализа (GEMMA) или масс-спектрометрии (MS) [11, 58]. Наноэлектроспрей газофазный анализ молекулярной подвижности (nES GEMMA) используется как альтернативный метод [59].
Метод масс-спектрометрии с индуктивно-связанной плазмой (ICP-MS) чувствителен к ультранизким концентрациям, однако его точность ограничена для частиц субмикронного размера [60], в связи с этим данный метод рекомендуется сочетать с динамическим потоком массы [61]. Сочетание газофазного электрофореза и спектроскопии открывает возможности для тщательного углубленного исследования материала “мягких” НЧ, например, липосом, с точки зрения размера и формы [62]. В табл. 1 представлены методы определения концентрации НЧ, наиболее широко освещенные в литературе.
Таблица 1.
Физико-химические методы определения концентрации НЧ
| Тип НЧ | Метод | Пределы измерения, точность измерения |
Недостатки и ограничения | Сссылка |
|---|---|---|---|---|
| НЧ золота | UV Vis | До 100 нм, 5% | Сложная форма и размеры >100 нм | [15, 22] |
| НЧ серебра | UV Vis | От 8 до 100 нм | Наличие коэффициента экстинкции | [24] |
| НЧ золота | ICP-MS | От 5 до 100 нм, 10% |
НЧ сложной формы | [63] |
| НЧ золота и серебра | DLS | От 4.5 нм до 50 нм | Наличие эталонного образца | [26] |
| НЧ золота | LTS | От 5 до 40 нм | Влияние геометрии НЧ на спектр экстинкции | [29] |
| НЧ оксида кремния | Турбидиметрия | От 80 до 250 нм | Наличие показателя преломления и устранение эффектов малоуглового рассеяния | [23] |
| Липосомы | LTS | От 30 до 300 нм | Наличие выборки для получения статистически значимых результатов | [31] |
| НЧ полистирола | LTS | От 50 3000 нм от 0.5 до 4% |
– | [29] |
| НЧ золота, НЧ кремнезема, вирусные НЧ |
NTA | От 80 до 250 нм | Рекомендуется работать в области концентраций от 107 до 109 НЧ/мл | [64] |
| НЧ полистирола НЧ альбумина |
NTA | От 20 до 1000 нм | Наличие полидисперсности или агрегации | [42] |
| НЧ полистирола, липосомы Внешние везикулы |
NTA | Около 100 нм | Проверка линейности диапазона концентраций | [65] |
| НЧ монокл. антител | NTA | Субмикронные НЧ | Контроль стабильности | [37, 38] |
КОНЦЕНТРАЦИЯ НАНОЧАСТИЦ ДЛЯ ПРИЛОЖЕНИЙ НАНОМЕДИЦИНЫ
Наночастицы и их концентрация в биологических средах
Исследование поведения НЧ в биологических средах, органах и их гомогенатах усложняется ввиду наличия в них макромолекул, изменения свойств НЧ, образования “белковой короны”, процессов агрегации и агломерации. Метод абсорбционной спектрофотометрии и спектроскопии резонансного рассеяния были применены для определения счетной концентрации НЧ золота [63]. Для НЧ из благородных металлов в качестве характеристики выбирается длина волны, соответствующая пику поверхностного плазмонного резонанса, что дает возможность отличить рассеяние НЧ от рассеяния другими нанообъектами в биологических пробах. ICP-MS [66] был использован для определения концентрации введенных внутривенно НЧ золота с различным размером 10, 50, 100 и 250 нм, дзета-потенциалом и дозой в крови и органах.
Метод NTA был использован для визуализации и отслеживания поведения НЧ в жидких биологических средах [36]. Оценка цитотоксичности показала, что НЧ серебра вызывали значительное снижение целостности мембран и клеточной метаболической активности в зависимости от их концентрации. Определение концентрации липосом в сыворотке крови проводилось для анализа их стабильности в кровотоке [67].
Данные о концентрации НЧ информативны для полиплексов и полидисперсных систем, склонных к агрегации [68]. Так, в работе [69] проведено сравнение НЧ, полученных с использованием новых технологий, таких как микрофлюидика, как в отсутствие, так и в присутствии ДНК, и определение количества плазмид-содержащих НЧ. С биологической точки зрения физико-химический параметр нанопрепаратов – количество НЧ имеет бóльшее значение, чем площадь поверхности или масса, поскольку доза эффективно соответствует количеству рецепторов, которые связывают НЧ. Недавно описана доза нанопрепаратов, введенная инъекционно мыши и представленная с точки зрения количества НЧ, и оценено ее пороговое значение (рис. 2).
Рис. 2.
Пороговое значение дозы нанопрепаратов, введенной инъекционно мыши и представленной с точки зрения количества НЧ [2].
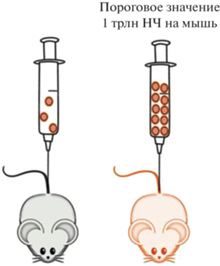
Установлено, что введение дозы НЧ (золотые и силикатные НЧ и липосомы), превышающей пороговое значение 1 триллион НЧ/на мышь, значительно улучшало терапевтическую эффективность независимо от дозы активного лекарства. Авторами как экспериментально, так и с учетом данных 40 публикаций [2], был проведен пересчет доз НЧ по их количеству и было установлено, что наиболее важным параметром является именно количество НЧ, которое играет определяющую роль при доставке в опухоли по сравнению с другими параметрами, такими как размер, дизайн таргетинга (нацеливания), тип НЧ или модель рака [2]. Далее в работе [70] с учетом дозы 1.5 × 1015 НЧ для человека была пересчитана доза НЧ в мг/кг для различных биоматериалов.
Клеточное поглощение и интернализация
Во всех методах лечения, существует пороговая или терапевтическая доза лекарства, которая должна быть усвоена отдельной клеткой, чтобы эта клетка считалась “вылеченной” [2]. Крайне важно знать, сколько НЧ попадает в (целевые) клетки, чтобы понять, сколько лекарства фактически доставлено. Доза лекарства, доставляемая в клетки, будет зависеть от интернализации, кинетики поглощения, распределения, времени накопления лекарства в органеллах. В то же время необходимо отметить, что поглощение НЧ клетками происходит неравномерно [71]. Эффективное проникновение и клеточная интернализация зависят от различных физико-химических свойств НЧ, включая поверхностный заряд, размер, состав и содержание липидов, а также наличие лигандов, нацеленных на поверхность и т.д. [72]. Тем не менее кинетика поглощения НЧ клетками человека и их последующее внутриклеточное распределение являются ключевыми параметрами [73]. По-прежнему отсутствуют стандартизированные протоколы для измерения клеточных взаимодействий и цитотоксичности сконструированных НЧ. Чтобы охарактеризовать внутриклеточный перенос НЧ с лекарствами, необходимы методы, позволяющие отслеживать их внутри клеток с течением времени и определять их внутриклеточное местоположение, а также время, необходимое для достижения определенного местоположения [74]. Часто внутри одних и тех же клеток некоторые НЧ достигают лизосом за короткое время, в то время как другим требуется гораздо больше времени [75, 76]. Дополнительным и часто игнорируемым параметром, оказывающим большое влияние на степень интернализации наноносителей фагоцитирующими клетками, является концентрация НЧ, т.е. их вводимое количество, а не концентрация действующего лекарственного вещества [11]. Однако в работе [77] было показано, что для лучшего клеточного поглощения необходимо увеличивать концентрацию инкапсулированного лекарства в каждой везикуле, а не количество везикул.
На двух типах НЧ – липосомах и квантовых точках – было проведено исследование о взаимосвязи цитотоксичности и концентрации НЧ [78]. С помощью комбинации теоретических и эмпирических методов (TEM, сканирующий анализатор подвижности частиц), описанных в работе [11], удалось определить концентрацию НЧ, связанных с поверхностью, и концентрацию интернализированных НЧ. Воздействие на клетки различного количества НЧ может подразумевать разную активность поглощения, последствия эндо/фагоцитоза или другие явления [79]. В работах [18, 80] авторы подчеркивают, что только тщательная и полная физико-химическая характеристика НЧ, включая их концентрацию, позволяет объяснить разницу в биологических эффектах (образование белковой короны, взаимодействие, поглощение клетками) для различных типов НЧ, например, для наноматериалов с различной хиральностью.
В литературе нет общепринятой кинетической схемы, лишь обзорно описана кинетика поглощения НЧ и их внутриклеточное распределение [73]. Автор этой работы подчеркивает, что для описания кинетических аспектов необходимым условием является определение количества НЧ. В общую кинетику поглощения он вносит вклады трех процессов: адсорбция/десорбция и клеточная интернализация. В другой работе [81] процесс поглощения полистирольных НЧ был описан кинетической моделью, которая схематично представлена на рис. 3. Для простых молекул поглощение описывается кинетикой первого порядка с равновесными константами скорости (рис. 3а). В случае НЧ (рис. 3б) импорт в клетку определяется независимыми временными потоками (J01 − течение через мембрану, J12 − течение в сторону эндосом и J23 − течение между эндосомами и лизосомами), при этом процесс выхода для полистирольных НЧ отсутствует, т.е. они попадают в лизосомы и остаются там [82].
Рис. 3.
Модели клеточного поглощения (a) молекулы – флуоресцентного красителя; и (б) НЧ, помеченных флуоресцентным красителем. Клетка состоит из двух контейнеров, представляющих собой общее внутриклеточное пространство (цитоплазму) и эндоплазматический ретикулум в случае красителя и эндосомальный и лизосомальный компартменты в случае НЧ. Адаптировано из [82].
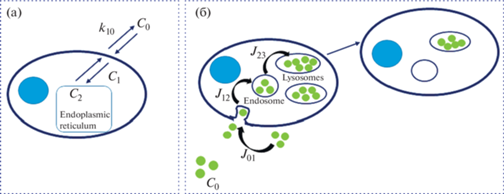
При накоплении НЧ в лизосомах следует обратить внимание на цитотоксичность самих НЧ и лизосомально-индуцированную нанотоксичность [70] для развития наноприложений [83]. В то же время, например, для липосомальных частиц возможен их экспорт из клеток и/или их деградация, а также высвобождение и выход загруженных молекул в окружающую среду [84], т.е. необходимо учитывать и другие вклады в кинетику, такие как деградация, эндосомальное ускользание, клеточное деление, а также транспорт во внеклеточной среде. Например, исходя из времени удвоения клеточной популяции, равного 22 ч (для клеточной линии ATCC), наблюдаемое затухание флуоресценции можно отнести к клеточному делению, т.е. деление клеток приводит к экспоненциальному спаду внутриклеточной концентрации НЧ. В результате непрерывного поступления НЧ устойчивое состояние наступает через некоторое время, так как деление клеток будет конкурировать с процессом поглощения НЧ [85].
Диагностика и биовизуализация
Количественные измерения с помощью техники флуоресценции в биологических средах достаточно сложны ввиду того, что флуорофоры чувствительны к изменению их окружения. Тем не менее абсолютное количество НЧ в органе может быть получено при помощи калибровочных зависимостей, то есть систематических измерений флуоресценции НЧ, растворенных в гомогенатах, полученных из органов при различных известных концентрациях НЧ. Надежность полученных данных увеличивается с использованием комбинации методов микроскопии и проточной цитометрии [86]. Существуют подходы с определением интенсивности флуоресценции одной частицы и измерений флуоресценции клеток, инкубированных с частицами, далее с использованием данных проточной цитометрии и изображений конфокальной сканирующей лазерной микроскопии можно рассчитать среднее количество интернализованных частиц на клетку [87].
С целью диагностики и фототермической терапии были охарактеризованы модифицированные липопротеинами твердые липидные НЧ с загруженным металлом Cu(II), в том числе определена концентрация НЧ с использованием комбинации методов спектроскопии и микроскопии с сочетанием теории Mie и Drude [88]. В другой работе авторы отметили, что фототермическая эффективность может зависеть от численной концентрации НЧ (золотых наностержней) [89].
Наноустройства для детоксикации
Кинетика ферментативной реакции, протекающей в гомогенной среде с идеально перемешанными реагентами, подчиняется уравнению Михаэлиса–Ментен. Однако эти условия сильно отличаются в случае работы ферментативных нанореакторов (NR): 1) высокая концентрация фермента в среде НЧ, которая не соответствует условиям кинетики Михаэлиса–Ментен; 2) скопление большого количества молекул фермента (E), так как они инкапсулированы внутри НЧ. Тем самым наблюдается аномальная диффузия веществ, которая изменяет кинетические законы действия масс. Таким образом, в нанореакторах локальная концентрация фермента [E] выше, чем концентрация субстрата [S], особенно в закрытых наноразмерных системах, такие “замкнутые условия” близки к тем, которые встречаются в клетках. Основной проблемой кинетики ферментативных нанореакторов является точное измерение и управление [E]. Высокая концентрация активных центров фермента в нанореакторах (особенно когда [E] ≥ [S]) влияет на равновесие. В нанореакторах вместо этого следует учитывать равновесие между субстратом, связанным с ферментом, и продуктом (P) (KNR = [EP]/[ES]) [90].
В условиях инъекционного нанореактора для детоксикации токсикант (Т), присутствующий в кровотоке, диффундирует через мембрану нанореактора, где далее в его герметичном отсеке проходит ферментативная реакция детоксикации. Для эффективной работы нанореактора [E] внутри наноносителя должна значительно превышать концентрацию токсиканта [T], тогда реакция внутри нанореактора будет протекать в условиях [E] $ \gg $ [T], или [E] ≈ [T]. В работе [3] был оценен размер нанореакторов тремя независимыми методами: TEM, DLS и NTA. Все три метода свидетельствовали о монодисперсности образца, что позволило методом NTA определить численную концентрацию нанореакторов и далее, с учетом сферической формы НЧ [4] и эффективности капсулирования, рассчитать объем нанореакторa, концентрацию фермента внутри нанореактора и порядок протекающей реакции.
ЗАКЛЮЧЕНИЕ
Рассмотренные выше методы определения концентрации НЧ и примеры ее использования для биомедицины свидетельствуют о важности данной характеристики для дальнейшего применения НЧ в доклинических исследованиях и успешного применения в клинике. Только полная характеристика НЧ, включая их концентрацию, может обеспечить дополнительную оценку получаемых биологических эффектов наносистем. Наиболее перспективным и надежным подходом для определения концентрации НЧ является сочетание нескольких методов с учетом прямой визуализации, спектроскопии и гравиметрии. Одним из наиболее удобных и несложных в использовании методов можно рекомендовать анализ траектории движения НЧ.
Список литературы
Clement S., Gardner B., Razali W.A.W. et al. Quantification of nanoparticle concentration in colloidal suspensions by a non-destructive optical method // Nanotechnology. 2017. V. 28. № 47. P. 475702. https://doi.org//10.1088/1361-6528/aa8d89
Ouyang B., Poon W., Zhang Y.-N. et al. The dose threshold for nanoparticle tumour delivery // Nat. Mater. 2020. V. 19. № 12. P. 1362–1371. https://doi.org//s41563-020-0755-z
Pashirova T., Shaihutdinova Z., Mansurova M. et al. Enzyme nanoreactor for in vivo detoxification of organophosphates // ACS Appl. Mater. Interfaces. 2022. V. 14. № 17. P. 19241–19252. https://doi.org/10.1021/acsami.2c03210
Shajhutdinova Z., Pashirova T., Masson P. Kinetic processes in enzymatic nanoreactors for in vivo detoxification // Biomedicines. 2022. V. 10. № 4. P. 784. https://doi.org/10.3390/biomedicines10040784
Pashirova T.N., Shaihutdinova Z.M., Mironov V.F., Masson P. Biomedical nanosystems for in vivo detoxification: From passive delivery systems to functional nanodevices and nanorobots // Acta Naturae. 2023. V. 15. № 1. P. 4–12. https://doi.org//2 10.32607/actanaturae.15681
Qian X., Nymann Westensee I., Brodszkij E., Städler B. Cell mimicry as a bottom-up strategy for hierarchical engineering of nature-inspired entities // Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology. 2020. V. 13. № 3. P. e1683. https://doi.org/10.1002/wnan.1683
Driscoll D.F., Nicoli D.F. Analytical methods for determining the size (distribution) in parenteral dispersions // Non-Biological Complex Drugs. The Science and the Regulatory Landscape. 2015. V. 20. P. 193–259. https://doi.org/10.1007/978-3-319-16241-6_7
Soema P.C., Willems G.-J., Jiskoot W., Amorij J.-P., Kersten G.F. Predicting the influence of liposomal lipid composition on liposome size, zeta potential and liposome-induced dendritic cell maturation using a design of experiments approach // Eur. J. Pharm. Biopharm. 2015. V. 94. P. 427–435. https://doi.org/10.1016/j.ejpb.2015.06.026
Mozafari M.R., Mazaheri E., Dormiani K. Simple equations pertaining to the particle number and surface area of metallic, polymeric, lipidic and vesicular nanocarriers // Sci. Pharm. 2021. V. 89. № 2. P. 15. https://doi.org/10.3390/scipharm89020015
Pidgeon C., Hunt C.A. Calculating number and surface area of liposomes in any suspension // J. Pharm. Sci. 1981. V. 70. № 2. P. 173–176. https://doi.org/10.1002/jps.2600700215
Epstein H., Afergan E., Moise T. et al. Number-concentration of nanoparticles in liposomal and polymeric multiparticulate preparations: Empirical and calculation methods // Biomaterials. 2006. V. 27. № 4. P. 651–659. https://doi.org/10.1016/j.biomaterials.2005.06.006
Vogel R., Savage J., Muzard J. et al. Measuring particle concentration of multimodal synthetic reference materials and extracellular vesicles with orthogonal techniques: Who is up to the challenge? // J. Extracell. Vesicles. 2021. V. 10. № 3. P. e12052. https://doi.org/10.1002/jev2.12052
Mourdikoudis S., Pallares R.M., Thanh N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties // Nanoscale. 2018. V. 10. № 27. P. 12871–12934. https://doi.org/10.1039/C8NR02278J
Minelli C., Bartczak D., Peters R. et al. Sticky measurement problem: Number concentration of agglomerated nanoparticles // Langmuir. 2019. V. 35. № 14. P. 4927–4935. https://doi.org/10.1021/acs.langmuir.8b04209
Shard A.G., Wright L., Minelli C. Robust and accurate measurements of gold nanoparticle concentrations using UV-visible spectrophotometry // Biointerphases. 2018. V. 13. № 6. P. 061002. https://doi.org/10.1116/1.5054780
Chithrani B.D., Ghazani A.A., Chan W.C.W. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells // Nano Lett. 2006. V. 6. № 4. P. 662–668. https://doi.org/10.1021/nl052396o
Cho E.C., Xie J., Wurm P.A., Xia Y. Understanding the role of surface charges in cellular adsorption versus internalization by selectively removing gold nanoparticles on the cell surface with a I2/KI etchant // Nano Lett. 2009. V. 9. № 3. P. 1080–1084. https://doi.org/10.1021/nl803487r
Yan H., Cacioppo M., Megahed S. et al. Influence of the chirality of carbon nanodots on their interaction with proteins and cells // Nat. Commun. 2021. V. 12. № 1. P. 7208. https://doi.org/10.1038/s41467-021-27406-1
Shang J., Gao X. Nanoparticle counting: Towards accurate determination of the molar concentration // Chem. Soc. Rev. 2014. V. 43. № 21. P. 7267–7278. https://doi.org/10.1039/C4CS00128A
Khlebtsov B.N., Khanadeev V.A., Khlebtsov N.G. Determination of the size, concentration, and refractive index of silica nanoparticles from turbidity spectra // Langmuir. 2008. V. 24. № 16. P. 8964–8970. https://doi.org/10.1021/la8010053
Baalousha M., Prasad A., Lead J.R. Quantitative measurement of the nanoparticle size and number concentration from liquid suspensions by atomic force micro-scopy // Environ. Sci. Process. Impacts. 2014. V. 16. № 6. P. 1338–1347. https://doi.org/10.1039/C3EM00712J
Haiss W., Thanh N.T.K., Aveyard J., Fernig D.G. Determination of size and concentration of gold nanoparticles from UV−Vis spectra // Anal. Chem. 2007. V. 79. № 11. P. 4215–4221. https://doi.org/10.1021/ac0702084
Khlebtsov N.G. Determination of size and concentration of gold nanoparticles from extinction spectra // Anal. Chem. 2008. V. 80. № 17. P. 6620–6625. https://doi.org/10.1021/ac800834n
Paramelle D., Sadovoy A., Gorelik S. et al. A rapid method to estimate the concentration of citrate capped silver nanoparticles from UV-visible light spectra // Analyst. 2014. V. 139. № 19. P. 4855. https://doi.org/10.1039/C4AN00978A
Хлебцов Б.Н., Ханадеев В.А., Хлебцов Н.Г. Определение размера, концентрации и показателя преломления наночастиц оксида кремния методом спектротурбидиметрии // Оптика и спектроскопия. 2008. Т. 105. № 5. С. 801–808.
Высоцкий В.В., Урюпина О.Я., Гусельникова А.В., Ролдугин В.И. О возможности определения концентрации наночастиц методом динамического светорассеяния // Коллоид. журн. 2009. Т. 71. № 6. С. 728–733.
Левин А.Д., Садагов А.Ю. Способ оптического измерения счетной концентрации дисперсных частиц в жидких средах и устройство для его осуществления // Патент № 2610942 C Российская Федерация, МПК G01N 21/00. № 2015151702, заявл. 02.12.2015, опубл. 17.02.2017.
Борен К., Хафмен Д. Поглощение и рассеяние света малыми частицами. М.: Мир, 1986.
Li F., Schafer R., Hwang C.-T., Tanner C.E., Ruggiero S.T. High-precision sizing of nanoparticles by laser transmission spectroscopy // Appl. Opt. 2010. V. 49. № 34. P. 6602. https://doi.org/10.1364/AO.49.006602
Li F., Mahon A.R., Barnes M.A. et al. Quantitative and rapid DNA detection by laser transmission spectroscopy // PLoS One. 2011. V. 6. № 12. P. e29224. https://doi.org/10.1371/journal.pone.0029224
Sennato S., Sarra A., La Capria C.P. et al. Quantification of particle number concentration in liposomal suspensions by Laser Transmission Spectroscopy (LTS) // Colloid. Surf. B. 2023. V. 222. P. 113137. https://doi.org/10.1016/j.colsurfb.2023.113137
Sun N., Johnson J., Stack M.S. et al. Nanoparticle analysis of cancer cells by light transmission spectroscopy // Anal. Biochem. 2015. V. 484. P. 58–65. https://doi.org/10.1016/j.ab.2015.05.004
Sarra A., Stanchieri G.D.P., De Marcellis A. et al. Laser Transmission Spectroscopy based on tunable-gain dual-channel dual-phase LIA for biological nanoparticles characterization // IEEE Trans. Biomed. Circuits Syst. 2021. V. 15. № 1. P. 177–187. https://doi.org/10.1109/TBCAS.2021.3060569
Filipe V., Hawe A., Jiskoot W. Critical evaluation of nanoparticle tracking analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates // Pharm. Res. 2010. V. 27. № 5. P. 796–810. https://doi.org/10.1007/s11095-010-0073-2
Griffiths D., Carnell-Morris P., Wright M. Nanoparticle tracking analysis for multiparameter characterization and counting of nanoparticle suspensions // Methods Mol. Biol. 2020. P. 289–303. https://doi.org/10.1007/978-1-0716-0319-2_22
Gallego-Urrea J.A., Tuoriniemi J., Hassellöv M. Applications of particle-tracking analysis to the determination of size distributions and concentrations of nanoparticles in environmental, biological and food samples // TrAC Trends Anal. Chem. 2011. V. 30. № 3. P. 473–483. https://doi.org/10.1016/j.trac.2011.01.005
Tian X., Nejadnik M.R., Baunsgaard D. et al. Comprehensive evaluation of nanoparticle tracking analysis (NanoSight) for characterization of proteinaceous submicron particles // J. Pharm. Sci. 2016. V. 105. № 11. P. 3366–3375. https://doi.org/10.1016/j.xphs.2016.08.009
Sediq A.S., van Duijvenvoorde R.B., Jiskoot W., Nejadnik M.R. No Touching! Abrasion of adsorbed protein is the root cause of subvisible particle formation during stirring // J. Pharm. Sci. 2016. V. 105. № 2. P. 519–529. https://doi.org/10.1016/j.xphs.2015.10.003
Bickel F., Herold E.M., Signes A. et al. Reversible NaCl-induced aggregation of a monoclonal antibody at low pH: Characterization of aggregates and factors affecting aggregation // Eur. J. Pharm. Biopharm. 2016. V. 107. P. 310–320. https://doi.org/10.1016/j.ejpb.2016.07.020
Chen C., Zhu S., Huang T., Wang S., Yan X. Analytical techniques for single-liposome characterization // Anal. Methods. 2013. V. 5. № 9. P. 2150.https://doi.org/10.1039/c3ay40219c
Barcelos J.M., Hayasaki T.G., de Santana R.C. et al. Photothermal properties of IR-780-based nanoparticles depend on nanocarrier design: A comparative study on synthetic liposomes and cell membrane and hybrid biomimetic vesicles // Pharmaceutics. 2023. V. 15. № 2. P. 444. https://doi.org/10.3390/pharmaceutics15020444
Gross J., Sayle S., Karow A.R., Bakowsky U., Garidel P. Nanoparticle tracking analysis of particle size and concentration detection in suspensions of polymer and protein samples: Influence of experimental and data evaluation parameters // Eur. J. Pharm. Biopharm. 2016. V. 104. P. 30–41. https://doi.org/10.1016/j.ejpb.2016.04.013
Anderson W., Kozak D., Coleman V.A., Jämting Å.K., Trau M. A comparative study of submicron particle sizing platforms: Accuracy, precision and resolution analysis of polydisperse particle size distributions // J. Colloid Interface Sci. 2013. V. 405. P. 322–330. https://doi.org/10.1016/j.jcis.2013.02.030
Malloy A., Carr B. NanoParticle tracking analysis – The HaloTM System // Part. Part. Syst. Charact. 2006. V. 23. № 2. P. 197–204. https://doi.org/10.1002/ppsc.200601031
Takechi-Haraya Y., Usui A., Izutsu K., Abe Y. Atomic force microscopic imaging of mRNA-lipid nanoparticles in aqueous medium // J. Pharm. Sci. 2023. V. 112. № 3. P. 648–652. https://doi.org/10.1016/j.xphs.2022.11.026
Usfoor Z., Kaufmann K., Rakib A.S.H., Hergenröder R., Shpacovitch V. Features of sizing and enumeration of silica and polystyrene nanoparticles by nanoparticle tracking analysis (NTA) // Sensors. 2020. V. 20. № 22. P. 6611. https://doi.org/10.3390/s20226611
Bachurski D., Schuldner M., Nguyen P.-H. et al. Extracellular vesicle measurements with nanoparticle tracking analysis – An accuracy and repeatability comparison between NanoSight NS300 and ZetaView // J. Extracell. Vesicles. 2019. V. 8. № 1. P. 1596016. https://doi.org/10.1080/20013078.2019.1596016
Hoover B.M., Murphy R.M. Evaluation of nanoparticle tracking analysis for the detection of rod-shaped particles and protein aggregates // J. Pharm. Sci. 2020. V. 109. № 1. P. 452–463. https://doi.org/10.1016/j.xphs.2019.10.006
Reipa V., Purdum G., Choi J. Measurement of nanoparticle concentration using quartz crystal microgravimetry // J. Phys. Chem. B. 2010. V. 114. № 49. P. 16112–16117. https://doi.org/10.1021/jp103861m
Wen C.-Y., Tang M., Hu J. et al. Determination of the absolute number concentration of nanoparticles and the active affinity sites on their surfaces // Anal. Chem. 2016. V. 88. № 20. P. 10134–10142. https://doi.org/10.1021/acs.analchem.6b02613
Maas S.L.N., De Vrij J., Broekman M.L.D. Quantification and size-profiling of extracellular vesicles using tunable resistive pulse sensing // J. Vis. Exp. 2014. № 92. P. e51623. https://doi.org/10.3791/51623
Shard A.G., Sparnacci K., Sikora A. et al. Measuring the relative concentration of particle populations using differential centrifugal sedimentation // Anal. Methods. 2018. V. 10. № 22. P. 2647–2657. https://doi.org/10.1039/C8AY00491A
Vaclavek T., Prikryl J., Foret F. Resistive pulse sensing as particle counting and sizing method in microfluidic systems: Designs and applications review // J. Sep. Sci. 2019. V. 42. № 1. P. 445–457.https://doi.org/10.1002/jssc.201800978
Austin J., Minelli C., Hamilton D., Wywijas M., Jones H.J. Nanoparticle number concentration measurements by multi-angle dynamic light scattering // J. Nanoparticle Res. 2020. V. 22. № 5. P. 108. https://doi.org/10.1007/s11051-020-04840-8
Marques S.S., Ramos I.I., Silva C. et al. Lab-on-Valve automated and miniaturized assessment of nanoparticle concentration based on light-scattering // Anal. Chem. 2023. V. 95. № 10. P. 4619–4626. https://doi.org/10.1021/acs.analchem.2c04631
Pauw B.R., Kästner C., Thünemann A.F. Nanoparticle size distribution quantification: Results of a small-angle X-ray scattering inter-laboratory comparison // J. Appl. Crystallogr. 2017. V. 50. № 5. P. 1280–1288. https://doi.org/10.1107/S160057671701010X
Hlaváček A., Křivánková J., Brožková H. et al. Absolute counting method with multiplexing capability for estimating the number concentration of nanoparticles using anisotropically collapsed gels // Anal. Chem. 2022. V. 94. № 41. P. 14340–14348. https://doi.org/10.1021/acs.analchem.2c02989
Li M., Guha S., Zangmeister R., Tarlov M.J., Zachariah M.R. Method for determining the absolute number concentration of nanoparticles from electrospray sources // Langmuir. 2011. V. 27. № 24. P. 14732–14739. https://doi.org/10.1021/la202177s
Urey C., Weiss V.U., Gondikas A. et al. Combining gas-phase electrophoretic mobility molecular analysis (GEMMA), light scattering, field flow fractionation and cryo electron microscopy in a multidimensional approach to characterize liposomal carrier vesicles // Int. J. Pharm. 2016. V. 513. № 1–2. P. 309–318. https://doi.org/10.1016/j.ijpharm.2016.09.049
Tuoriniemi J., Moreira B., Safina G. Determining number concentrations and diameters of polystyrene particles by measuring the effective refractive index of colloids using surface plasmon resonance // Langmuir. 2016. V. 32. № 41. P. 10632–10640. https://doi.org/10.1021/acs.langmuir.6b02684
Cuello-Nuñez S., Abad-Álvaro I., Bartczak D. et al. The accurate determination of number concentration of inorganic nanoparticles using spICP-MS with the dynamic mass flow approach // J. Anal. At. Spectrom. 2020. V. 35. № 9. P. 1832–1839. https://doi.org/10.1039/c9ja00415g
Weiss V.U., Wieland K., Schwaighofer A., Lendl B., Allmaier G. Native nano-electrospray differential mobility analyzer (nES GEMMA) enables size selection of liposomal nanocarriers combined with subsequent direct spectroscopic analysis // Anal. Chem. 2019. V. 91. № 6. P. 3860–3868. https://doi.org/10.1021/acs.analchem.8b04252
Левин А.Д., Нагаев А.И., Рукин Е.М. и др. Проблемы методического обеспечения биомедицинских нанотехнологий // Измерительная техника. 2010. № 8. С. 29–34.
Du S., Kendall K., Morris S., Sweet C. Measuring number-concentrations of nanoparticles and viruses in liquids on-line // J. Chem. Technol. Biotechnol. 2010. V. 85. № 9. P. 1223–1228. https://doi.org/10.1002/jctb.2421
Yahata S., Hirose M., Ueno T., Nagumo H., Sakai-Kato K. Effect of sample concentration on nanoparticle tracking analysis of small extracellular vesicles and liposomes mimicking the physicochemical properties of exosomes // Chem. Pharm. Bull. 2021. V. 69. № 11. P. 1045–1053. https://doi.org/10.1248/cpb.c21-00452
De Jong W.H., Hagens W.I., Krystek P. et al. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration // Biomaterials. 2008. V. 29. № 12. P. 1912–1919. https://doi.org/10.1016/j.biomaterials.2007.12.037
Reshetov V., Zorin V., Siupa A. et al. Interaction of liposomal formulations of meta-tetra(hydroxyphenyl)chlorin (Temoporfin) with serum proteins: Protein binding and liposome destruction // Photochem. Photobiol. 2012. V. 88. № 5. P. 1256–1264. https://doi.org/10.1111/j.1751-1097.2012.01176.x
Wilson D.R., Green J.J. Nanoparticle tracking analysis for determination of hydrodynamic diameter, concentration, and zeta-potential of polyplex nanoparticles // Methods Mol. Biol. 2017. P. 31–46. https://doi.org/10.1007/978-1-4939-6840-4_3
Wilson D.R., Mosenia A., Suprenant M.P. et al. Continuous microfluidic assembly of biodegradable poly(be-ta-amino ester)/DNA nanoparticles for enhanced gene delivery // J. Biomed. Mater. Res. Part A. 2017. V. 105. № 6. P. 1813–1825. https://doi.org/10.1002/jbm.a.36033
Uzhytchak M., Smolková B., Lunova M. et al. Lysosomal nanotoxicity: Impact of nanomedicines on lysosomal function // Adv. Drug Deliv. Rev. 2023. V. 197. P. 114828. https://doi.org/10.1016/j.addr.2023.114828
Kato H. Tracking nanoparticles inside cells // Nat. Nanotechnol. 2011. V. 6. № 3. P. 139–140. https://doi.org/10.1038/nnano.2011.25
Rennick J.J., Johnston A.P.R., Parton R.G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics // Nat. Nanotechnol. 2021. V. 16. № 3. P. 266–276. https://doi.org/10.1038/s41565-021-00858-8
Åberg C. Kinetics of nanoparticle uptake into and distribution in human cells // Nanoscale Adv. 2021. V. 3. № 8. P. 2196–2212. https://doi.org/10.1039/D0NA00716A
Salvati A., Poelstra K. Drug targeting and nanomedicine: Lessons learned from liver targeting and opportunities for drug innovation // Pharmaceutics. 2022. V. 14. № 1. P. 217. https://doi.org/10.3390/pharmaceutics14010217
Vtyurina N., Åberg C., Salvati A. Imaging of nanoparticle uptake and kinetics of intracellular trafficking in individual cells // Nanoscale. 2021. V. 13. № 23. P. 10436–10446. https://doi.org/10.1039/D1NR00901J
Åberg C., Piattelli V., Montizaan D., Salvati A. Sources of variability in nanoparticle uptake by cells // Nanoscale. 2021. V. 13. № 41. P. 17530–17546. https://doi.org/10.1039/D1NR04690J
Aizik G., Waiskopf N., Agbaria M. et al. Delivery of liposomal quantum dots via monocytes for imaging of inflamed tissue // ACS Nano. 2017. V. 11. № 3. P. 3038–3051. https://doi.org/10.1021/acsnano.7b00016
Labouta H.I., Sarsons C., Kennard J. et al. Understanding and improving assays for cytotoxicity of nanoparticles: What really matters? // RSC Adv. 2018. V. 8. № 41. P. 23027–23039. https://doi.org/10.1039/C8RA03849J
Fan Y., Marioli M., Zhang K. Analytical characterization of liposomes and other lipid nanoparticles for drug delivery // J. Pharm. Biomed. Anal. 2021. V. 192. P. 113642. https://doi.org/10.1016/j.jpba.2020.113642
Ma B., Bianco A. Regulation of biological processes by intrinsically chiral engineered materials // Nat. Rev. Mater. 2023. V. 8. № 6. P. 403–413. https://doi.org/10.1038/s41578-023-00561-1
Salvati A., Åberg C., dos Santos T. et al. Experimental and theoretical comparison of intracellular import of polymeric nanoparticles and small molecules: Toward models of uptake kinetics // Nanomedicine Nanotechnology, Biol. Med. 2011. V. 7. № 6. P. 818–826. https://doi.org/10.1016/j.nano.2011.03.005
Shi H., He X., Yuan Y., Wang K., Liu D. Nanoparticle-based biocompatible and long-life marker for lysosome labeling and tracking // Anal. Chem. 2010. V. 82. № 6. P. 2213–2220. https://doi.org/10.1021/ac902417s
Chen Y.-C., Chen K.-F., Lin K.-Y.A. et al. Evaluation of the pulmonary toxicity of PSNPs using a Transwell-based normal human bronchial epithelial cell culture system // Sci. Total Environ. 2023. V. 895. P. 165213. https://doi.org/S0048969723038366
Yang K., Tran K., Salvati A. Tuning liposome stability in biological environments and intracellular drug release kinetics // Biomolecules. 2022. V. 13. № 1. P. 59. https://doi.org/10.3390/biom13010059
Faria M., Noi K.F., Dai Q. et al. Revisiting cell–particle association in vitro: A quantitative method to compare particle performance // J. Control. Release. 2019. V. 307. P. 355–367. https://doi.org/10.1016/j.jconrel.2019.06.027
Simonsen J.B., Kromann E.B. Pitfalls and opportunities in quantitative fluorescence-based nanomedicine studies – A commentary // J. Control. Release. 2021. V. 335. P. 660–667. https://doi.org/10.1016/j.jconrel.2021.05.041
Gottstein C., Wu G., Wong B.J., Zasadzinski J.A. Precise quantification of nanoparticle internalization // ACS Nano. 2013. V. 7. № 6. P. 4933–4945. https://doi.org/10.1021/nn400243d
Vischio F., Fanizza E., De Bellis V. et al. Near-infrared absorbing solid lipid nanoparticles encapsulating plasmonic copper sulfide nanocrystals // J. Phys. Chem. C. 2019. V. 123. № 37. P. 23205–23213.https://doi.org/10.1021/acs.jpcc.9b05897
Салаватов Н.А., Большакова А.В., Морозов В.Н. и др. Золотые наностержни с функционализированной органокремнеземной оболочкой: синтез и перспективы применения в тераностике опухолей // Коллоидный журнал. 2022. Т. 84. № 1. С. 97–104. https://doi.org/10.31857/S0023291222010104
Chauhan K., Zárate-Romero A., Sengar P., Medrano C., Vazquez-Duhalt R. Catalytic kinetics considerations and molecular tools for the design of multienzymatic cascade nanoreactors // ChemCatChem. 2021. V. 13. № 17. P. 3732–3748. https://doi.org/10.1002/cctc.202100604
Дополнительные материалы отсутствуют.
Инструменты
Коллоидный журнал


