Микология и фитопатология, 2019, T. 53, № 4, стр. 223-228
Tensidols A and B from Aspergillus tubingensis strain and their biological activity
H. A. Mohamed 1, 2, *, W. Ebrahim 3, 4, **, A. M. Peterson 1, ***, F. C. Özkaya 3, ****, P. Proksch 3, *****
1 Saratov State University
410012 Saratov, Russia
2 Al-Azhar University
71524 Assiut, Egypt
3 Institute of Pharmaceutical Biology and Biotechnology, Heinrich-Heine University
40225 Dusseldorf, Germany
4 Department of Pharmacognosy, Faculty of Pharmacy, Mansoura University
35516 Mansoura, Egypt
* E-mail: hassanmohamed85@azhar.edu.eg
** E-mail: weaamnabil@hotmail.de
*** E-mail: alexandra.peterson@yandex.ru
**** E-mail: fcanozkaya@gmail.com
***** E-mail: proksch@uni-duesseldorf.de
Поступила в редакцию 7.07.2018
После доработки 17.11.2018
Принята к публикации 24.12.2018
Аннотация
Two furopyrrols, namely tensidols A and B, were purified from EtOAc extract of the rice culture medium of the endophyte, Aspergillus tubingensis (strain AN103) that resides the fresh tree branches of apple plant (Malus domestica), collected from Volga region, Saratov, Russian Federation. Morphologically, the strain is a black fungus belonging to the Aspergillus section Nigri. Moreover, this endophytic fungal isolate (AN103) was characterized by performing cotton blue staining. The identity of A. tubingensis (strain AN103) was unambiguously confirmed based on microbiological study of its DNA sequence and this sequence was submitted and approved by GenBank and it was given the GenBank accession number (KR184138). To find out a possible explanation for the endophytism of A. tubingensis (strain AN103) in apple tree branches, tensidols A and B, the major components in the HPLC chromatogram of EtOAc extract of the rice cultures of this fungal endophyte were purified to study their biological effect. Chromatographic investigation and purification of this extract revealed the unequivocal determination of the chemical structures of both compounds. Interestingly, both compounds were found to inhibit the growth of Brevibacterium halotolerans and Bacillus methylotrophicus, the two common pathogens that attack apple tree. Moreover, the lack of cytotoxic activity of both the crude extract and its purified major compounds tensidols A and B against lymphoma cell lines (L5178Y), highlights their specificity in action.
INTRODUCTION
Fungal natural products are secondary metabolites that are not needed or involved in metabolic growth of the microorganism. It is well known that microorganisms including fungi have the potential to synthesize a plethora of biochemical molecules. Some of these metabolites showed a clear mechanism of action either in vitro or in vivo, while, the obvious mechanism of action for others is still anonymous (Coleman et al., 2011).
One explanation for the microbial biosynthesis of secondary metabolites is that these metabolites could help the producing-microorganisms to survive when there is a competition with other organisms in the environment (Fox, Howlett, 2008). Black aspergilli (Aspergillus section Nigri; Gams et al., 1985) are microorganisms that belong to kingdom Fungi and proved to exert a pivotal role in many aspects in modern society. For example, many species of this section are involved in the fermentation industry to obtain hydrolytic enzymes, such as lipases and, amylases and many organic acids, such as citric and tartaric acids. On the other hand, some species of this section are known to deteriorate foods (Varga et al., 2000). Aspergillus niger is widespread and found in the surrounding environment such as in soil and on decaying plants and it was found that this species has the ability to produce some mycotoxins such as ochratoxins (Saber et al., 2016). Moreover, this species is known to grow on foods (Sorensen et al., 2009). Generally, endophytic fungi are known to thrive in fresh plant cells under normal conditions but without interfering with growth of plants. It is well known that these special fungi are unique and are untapped source for many pharmaceutically-valuable secondary metabolites (Mohamed et al., 2017).
The World Health Organization (WHO, 2017) declared that one of the most serious problems is the spread of antimicrobial resistance which influence many biological and economic threats in many countries. This was clear from recent plague outbreak on Madagascar which highlighted that even infections which are defeated by humanity can reappear again causing death to humans and this is mainly caused because of resistance of pathogenic microorganisms to drugs (Chanteau et al., 1998). An example form England is that leprosy infection of red squirrels created a reservoir of dangerous pathogenic bacteria to humans (Charlotte et al., 2016).
The fungus A. niger is a well-known organism that is used in many industrial aspects. This particular fungal species is known to be a vital industrial source for many natural products along with some useful enzymes. Examples are, the antimicrobial secondary metabolites tensyuic acid, nigerazine B, ochratoxin which are produced by this fungus (Nielsen et al., 2009). As a result of culturing the microorganisms in nutrient media, the microorganisms secrete some secondary metabolites and these metabolites could have biological activities such as antimicrobial effect (Natarajan et al., 2010).
Taxonomically, many scientists investigated Aspergillus section Nigri (Abarca et al., 2004). As a result of PCR based techniques and nuclear and mitochondrial DNA (mtDNA) polymorphisms, identification of at least two species within the A. niger species complex which are A. tubingensis and A. niger (Kusters-van Someren et al., 1991; Varga et al., 1994). Moreover, the D1–D2 region of the 28S rRNA gene and phylogenetic analyses of sequences of the ITS and the 5.8S rRNA gene revealed the identification of five extra species belonging to the section Nigri; A. carbonarius, A. japonicus, A. heteromorphus, A. ellipticus, and A. aculeatus (Varga et al., 2000; Parenicova et al., 2001). Later on, many species of the genus Aspergillus have been reported, such as A. vadensis (De Vries et al., 2005), A. piperis, A. lacticoffeatus, A. costaricaensis, and A. sclerotioniger (Samson et al., 2004) and A. ibericus (Serra et al., 2006).
As said before, fungi are fascinating microorganisms which have the ability to produce many therapeutic secondary metabolites such as insecticides, antibiotics, cytotoxic agents, growth-inhibitory compounds, etc. These secondary metabolites which are produced from fungi differ in production, function and specificity from one fungus to the other (Kishore et al., 2007).
In the present study, we describe in addition to the taxonomy of the fungal strains A. tubingensis isolated from apple plant, the phytochemical and biological investigation along with the fermentation are also presented.
MATERIALS AND METHODS
Isolation of fungal culture. A. niger strain AN103 was isolated from the fresh Apple plant stems, Saratov city, Russia in December 2013. Small tissues of the stems were cut aseptically inserted into malt agar plates (15 g/L malt extract, 15 g/L agar, and 0.2 g/L chloramphenicol in distilled water, pH 7.4–7.8) containing chloramphenicol to suppress bacterial growth and these plates were incubated for two weeks at room temperature. The growing fungal hyphae were further periodically cultivated on fresh malt agar medium until we get pure fungal strains. All voucher specimens are deposited at Department of Microbiology and Plant Physiology, Faculty of Biology, Saratov State University, Russia.
Identification of fungal strain. The isolated fungal strain was identified as A. tubingensis performing the standard protocol of molecular biological which involves DNA amplification and sequencing using ITS1 (TCCGTAGGTGAACCTGCGG) and ITS4 (TCCTCCGCTTATTGATATGC) as primers. The total genomic DNA of A. tubingensis was subjected to PCR reactions utilizing forward and reverse primers. After incubation for 1 min at 95°C, thirty-five PCR cycles (94°C, 1 min; 56°C, 1 min and 72°C, 1 min) were performed, followed by one cycle of 10 min at 72°C. The PCR product was separated by electrophoresis and sequenced (Kjer et al., 2010). A homology search was performed with GenBank database. This fungal strain was stored with ID code (AN103) at Institute of Pharmaceutical Biology and Biotechnology at the Heinrich Heine University Düsseldorf, Germany.
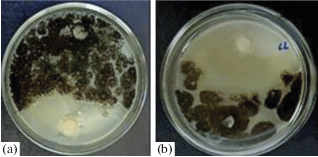
In vitro screening of isolates for antagonism. The EtOAc extract along with the isolated major compounds of A. tubingensis (AN103) were screened in vitro against Brevibacterium halotolerans and Bacillus methylotrophicus, the two common pathogens that attack apple tree utilizing a dual culture technique using vancomycin as positive control (Fig. 1) (Rana et al., 2016).
Fig. 1.
Inhibition of Brevibacterium halotolerans (а) and Bacillus methylotrophicus (b) growth with Aspergillus tubingensis (AN103).
Cultivation, extraction and isolation of tensidols. The fungal strain fermented statically on solid rice culture media (100 g Rice in 110 ml distilled water, autoclaved for 20 min at 121°C) in 1L Erlenmeyer flask for 21 days at 28°C. Extraction of fungal cultures was performed using EtOAc. The combined extracts were then evaporated in vacuum and the residue was partitioned between n-hexane and 90% aqueous methanol. The 90% MeOH fraction was evaporated to give 1.6 g of solid residue which was chromatographed over Sephadex LH-20 using MeOH as eluting solvent. Further purification was achieved by semi preparative HPLC (Merck Hitachi L7100).
General experimental procedures. NMR spectra (chemical shifts in ppm) were recorded on a Bruker AVANCE HD III 300 MHz spectrometer (Switzerland). NMR samples were dissolved in CDCl3 (Sigma Aldrich, Germany). HPLC analyses were performed using Dionex® ultimate 300 LC system coupled with photodiode array detector (UVD340S) with detection wavelengths of 235, 254, 280 and 340 nm using reversed phase ODS column (Knauer 4 mm × 125 mm) with the following gradient (MeOH, 0.01% HCOOH in H2O) 0–5 min. (10% MeOH); 5–35 min. (10% MeOH-100% MeOH) and 35–45 (100% MeOH) and 1 ml/min flow rate was applied for the analysis. Final purification steps were performed using semi-preparative HPLC (Knauer, Germany) on Kromasil ODS preparative column (10 mm × 250 mm) at flow rates 4 ml/min and UV detection at 254 and 340 nm, LC-ESIMS analysis were performed using a HP-1100 Agilant Finnigan LCQ Deca XP Thermoquest mass spectrometer. Normal phase column chromatography was performed using silica gel 60 (0.04–0.063, Merck, Germany). TLC analysis was performed using normal phase silica gel precoated plates F254 (Merck, Germany).
Testing the biological activity. The isolated compounds and fungal crude extract were assessed for their cytotoxicity against murine lymphoma cell line L5178Y at 10µg/mL concentration by standard microculture tetrazolium (MTT). This test was carried out in institute of Physiological Chemistry, University of Mainz. The antibacterial activity was evaluated against Staphyloccocus aureus ATCC 25922. This antibacterial assay was done by the broth microdilution method. It was carried out by Prof. Dr. Kassack, M. in Institute for Pharmaceutical and Medicinal Chemistry, Heinrich-Heine University, Düsseldorf, Germany (Chen et al., 2015a,b). In addition, the EtOAc extract along with the isolated major compounds of Aspergillus tubingensis (AN103) were screened in vitro against Brevibacterium halotolerans and Bacillus methylotrophicus, the two common pathogens that attack apple tree utilizing a dual culture technique using vancomycin as positive control (Rana et al., 2016).
RESULTS AND DISCUSSION
Isolation and identification of fungal culture. After culturing the fungal isolate on malt extract agar for 7 days at 28°C, fungus was identified based on morphological features and sequencing of the ITS region. The results of the DNA sequence were submitted to GenBank with access number KR184138. The DNA sequence showed 100% similarity with Aspergillus tubingensis.
Antagonism effect of fungal isolate. To examine the antagonistic properties of the A. tubingensis obtained from the Perti dishes against pathogenic bacteria. Results showed that the antagonist network size were enriched between these strains when A. tubingensis gave the highest antagonism effect and inhibition zone (34, 31) mm against Brevibacterium halotolerans and Bacillus methylotrophicus, respectively (Fig. 1) with. The MICs of tensidol A against Brevibacterium halotolerans and Bacillus methylotrophicus were 32 and 64 µg/mL, respectively. While, tensidol B possesses MICs against Brevibacterium halotolerans and Bacillus methylotrophicus 16 and 32 µg/mL. Vancomycin was used as a positive control (MIC was 5 µg/mL against both bacteria).
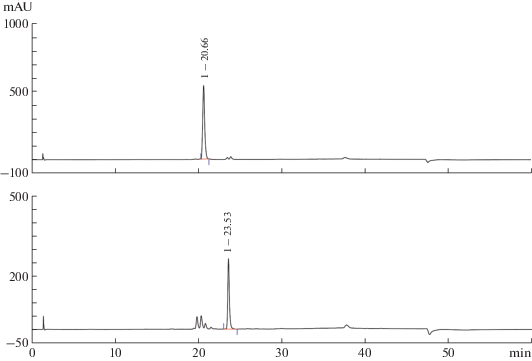
Elucidation of structure of extracted compounds. The fungal extract was chromatographed over silica gel and Sephadex LH-20, followed by purification by semi-preparative HPLC (Fig. 2) to yield the two known natural product tensidols A and B (Fukuda et al., 2006).
Fig. 2.
A chromatographic profiles of purification of tensidols A (above) and B (below) by semi-preparative HPLC.
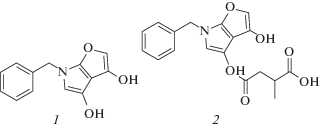
Compounds 1 and 2 were isolated as yellow powder and molecular formula of 1 was determined as C13H11NO3 on the basis of the pseudomolecular ion peak observed at m/z 230 [M + H]+ in the ESIMS spectrum. Compound 2 had a molecular formula of C18H17NO6 as indicated by ESIMS and showed a positive ion signal at m/z 344 [M + H]+. The chemical structure of 1 and 2 were identified as tensidols A and B by comparison of their spectroscopic data including optical rotations (Fukuda et al., 2006) (Fig. 3) (Table 1).
Table 1.
NMR Data of tensidols A and B in CDCI3
| Position | Tensidol A | Tensidol B |
|---|---|---|
| 1H chemical shifts (ppm)a | ||
| 2 | 8.75 (1H, s) | 8.74 (1H, s) |
| 5 | 6.25 (1H, s) | 6.30 (1H, s) |
| 7 | 3.90 (2H, s) | 3.90 (2H, s) |
| 9 | 7.29 (2H, m) | 7.36 (2H, m) |
| 10 | 7.25 (2H, m) | 7.24 (2H, m) |
| 11 | 7.29 (1H, m) | 7.36 (1H, m) |
| 2' | 2.92 (1H, dd, J = 8.5, 5.0) | |
| 3.28 (1H, dd, J = 8.5, 5.0) | ||
| 3' | 3.07 (1H, m) | |
| 5' | 1.30 (3H, d, J_7.5) | |
| 3-OH | 5.90 (1H, br s)b | 11.79 (1H, s) |
| 4-OH | 9.05 (1H, br s)b | |
The production of secondary metabolites is usually consistent in a species, and they are commonly useful for species identification (Samson et al., 2007). Asperazine, funalenone, malformins, naphtho-γ-pyrones, pyranonigrin A, and tensidol A and B are extrolites expected to be produced by Aspergillus.
Biological properties. The crude extract of Aspergillus tubingensis was inactive against mouse lymphoma cell line L5178Y. Moreover, tensidols A and B did not show any cytotoxicity. However, crude extract exhibited weak antimicrobial against Staphyllococcus aureus ATCC 25922 (MIC is 64 µg/mL).
CONCLUSION
In the current study, we isolated an endophytic fungal strain of Aspergillus tubingensis (AN 103) from Malus domestica. The strain produced tensidols A and B as major components under growth in sterilized solid rice medium. Both compounds were found to inhibit the growth of Brevibacterium halotolerans and Bacillus methylotrophicus, the two common pathogens that attack Malus domestica. Moreover, the lack of cytotoxic activity of both the crude extract and its purified major compounds tensidols A and B against lymphoma cell lines (L5178Y), highlights their specificity in antibacterial action.
Список литературы
Abarca M.L., Accensi F., Cano J., Cabanes F.J. Taxonomy and significance of black aspergilli. Antonie. Van. Leeuwenhoek. 2004. V. 86. P. 33–49.
Chanteau S., Ratsifasoamanana L., Rasoamanana B., Rahalison L., Randriambelosoa J., Roux J., Rabeson D. Plague, a reemerging disease in Madagascar. Emerging Infectious Disease. 1998. V. 4 (1). P. 101–104.
Charlotte A., Jorge D., Andrej B., Karen S., Red squirrels in the British Isles are infected with Leprosy bacilli. Science. 2016. V. 354 (6313). P. 744–747.
Chen H., Aktas N., Konuklugil B., Mándi A., Daletos G., Lin W., Dai H., Kurtán T., Proksch P. A new fusarielin analogue from Penicillium sp. isolated from the Mediterranean sponge Ircinia oros. Tetrahedron Letters. 2015a. V. 56. P. 5317–5320.
Chen H., Daletos G., Abdel-Aziz M.S., Thomy D., Dai H., Brötz-Oesterhelt H., Lin W., Proksch P. Inducing secondary metabolite production by the soil-dwelling fungus Aspergillus terreus through bacterial co-culture. Phytochemistry Letters. 2015b. V. 12. P. 35–41.
Coleman J., Ghosh S., Okoli I., Mylonakis E. Antifungal Activity of Microbial Secondary Metabolites. PLoS One. 2011. V. 6 (9). e25321. P. 1–9.
De Vries R.P., Frisvad J.C., van de Vondervoort P.I., Burgers K., Kuijpers F.A., Samson R.A., Visser J. Aspergillus vadensis, a new species of the group of black Aspergilli. Antonie. Van Leeuwenhoek. 2005. V. 87. P. 195–203.
Fox E.M., Howlett B.J. Secondary metabolism: Regulation and role in fungal biology. Current Opin. Microbiol. 2008. V. 11 (6). P. 481–487.
Fukuda T., Hasegawa Y., Hagimori K., Yamaguchi Y., Masuma R., Tomoda H., Omura S. Tensidols, new potentiators of antifungal miconazole activity, produced by Aspergillus niger FKI-2342. Antibiotics. 2006. V. 59. P. 480–485.
Gams W., Christensen M., Onions A.S., Pitt J.I., Samson R.A. Infrageneric taxa of Aspergillus. In Advances in Penicillium and Aspergillus Systematics, Edited by Samson, R.A., Pitt, J.I. New York, Plenum. 1985. V. 102. P. 55–62.
Kishore H.K., Misra S., Chandra D.R., Prakash K.V., Murty S.U. Antimicrobial efficiency of secondary metabolites from Glomerella cingulata. Brazilian Journal of Microbiology. 2007. V. 38. P. 150–152.
Kjer J., Debbab A., Aly A.H., Proksch P. Methods for isolation of marine-derived endophytic fungi and their bioactive secondary products. Natural Products. 2010. V. 5. P. 479−490.
Kusters-van Someren M.A., Samson R.A., Visser J. The use of RFLP analysis in classification of the black Aspergilli, reinterpretation of Aspergillus niger aggregate. Current Genetics. 1991. V. 19. P. 21–26.
Mohamed H.A., Ebrahim W., Peterson A.M., Proksch P. Production of Phytotoxic Polyketide Spiciferone A by Phoma fungicola, Mikologiya i fitopatologiya. 2017. V. 51 (1). P. 41–46.
Natarajan K., Rabiya S.S., Sathish R. Screening for antibacterial compound in broth extracts of Aspergillus niger MTCC 2208. Drug Invention Today. 2010. V. 2 (8). P. 385–386.
Nielsen K.F., Mogensen J.M., Johansen M., Larsen T.O., Frisvad J.C. Review of secondary metabolites and mycotoxins from the Aspergillus niger group. Analytical and Bioanalytical Chemistry. 2009. V. 395 (5). P. 1225–1242.
Parenicova L., Skouboe P., Frisvad J., Samson R.A., Rossen L., Hoor-Suykerbuyk M., Visser J. Combined molecular and biochemical approach identifies Aspergillus japonicus and Aspergillus aculeatus as two species. Applied and Environmental Microbiology. 2001. V. 67. P. 521–527.
Rana H., Jean R., Olivier B., Marie D., Marie F., Corio-Costet F., Thomas G., Alain D., Marc F. Screening and modes of action of antagonistic bacteria to control the fungal pathogen Phaeomoniella chlamydospora involved in grapevine trunk diseases. Microbiological Research. 2016. V. 192. P.172–184.
Saber S.M., Youssef M.S., Arafa R.F., Hassane A.M. Mycotoxins production by Aspergillus ostianus Wehmer and using phytochemicals as control agent. Journal of Scientific and Engineering Research. 2016. V. 3(2). P. 198–213.
Samson R.A., Houbraken J.A., Kuijpers M.P., Frank A.F., Frisvad J.C. New ochratoxin or sclerotium producing species in Aspergillus section Nigri. 2004. V. 50. P. 45–61.
Samson R.A., Noonim P., Meijer M., Houbraken J., Frisvad J., Varga J. Diagnostic tools to identify black aspergilla. 2007. V. 59. P. 129–145.
Serra R., Cabanes F.J., Perrone G., Castella G., Venancio A., Mule G., Kozakiewicz Z. Aspergillus ibericus, a new species of section Nigri isolated from grapes. Mycologia. 2006. V. 98. P. 295–306.
Sorensen L.M., Lametsch R., Andersen M.R., Nielsen P.V., Frisvad J.C. Proteome analysis of Aspergillus niger, Lactate added in starch containing medium can increase production of the mycotoxin fumonisin B2 by modifying acetyl-CoA metabolism. BMC Microbiology. 2009. V. 9. P. 255.
Varga J., Kevei F., Debets F., Kozakiewicz Z., Croft J.H. Mitochondrial DNA restriction fragment length polymorphisms in field isolates of the Aspergillus niger aggregate. Canadian Journal of Microbiology. 1994. V. 40. P. 612–621.
Varga J., Kevei F., Hamari Z., Toth B., Teren J., Croft J.H., Kozakiewicz Z. Genotypic and phenotypic variability among black Aspergilli, In Integration of Modern Taxonomic Methods for Penicillium and Aspergillus Classification. Edited by Samson, R.A. Pitt, J.I. Amsterdam. Harwood Academic Publishers. 2000. P. 397–411.
WHO publishes list of bacteria for which new antibiotics are urgently needed, 27 February 2017, News Release, Geneva.
Дополнительные материалы отсутствуют.
Инструменты
Микология и фитопатология