Микология и фитопатология, 2020, T. 54, № 3, стр. 221-227
Triazole and strobilurin fungicides sensitivity of Pyrenophora tritici-repentis isolates originated from Eastern Algeria
D. Mehamdia 1, *, T. Merad 2, **, L. Tichati 1, ***
1 Badji Mokhtar University, Faculty of Science, Department of Biochemestry, Laboratory of Microbiology
23000 Annaba, Algeria
2 Badji Mokhtar University, Faculty of Science, Laboratory of Genetic Amelioration of Plants
23000 Annaba, Algeria
* E-mail: doniazed23@gmail.com
** E-mail: meradtarek@yahoo.fr
*** E-mail: lazharbio@hotmail.com
Поступила в редакцию 31.05.2019
После доработки 2.11.2019
Принята к публикации 20.12.2019
Аннотация
Tan spot of wheat, caused by the fungus Pyrenophora tritici-repentis, is a destructive disease worldwide that can cause serious losses in quality and quantity of wheat grain production. The fungus induces two distinct symptoms: tan necrosis and extensive chlorosis, on susceptible wheat cultivars.This disease is widespread in the cereals production areas Algeria, causing considerable losses of yield and obliging farmers to use different class of fungicides. In this study, 100 isolates of P. tritici-repentis collected from different fields in eastern of Algeria during four successive agricultural seasons (2012–2015) were tested in vitro for their sensitivity to the fungicides that commonly in use in the country: azoxystrobin (Amistar), propiconazol (Tilt250EC) and azoxystrobin combined with prothioconazole (Amistar Xtra). In vitro assays showed a decrease in the sensitivity of the isolates of P. tritici-repentis to these three fungicides during the four years of testing. For Amistar, IC 50 values range from 7.5 ppm to 15 ppm, between 5 ppm and 12.5 ppm for Tilt 250EC and between 5 ppm and 10 ppm for Amistar Xtra, the combination of triazoles (Cyproconazole) with strobilurins (azoxystrobin) appears to have good antifungal activity on P. tritici-repentis, whereas the strobilurins or triazoles used alone show low antifungal activity.
INTRODUCTION
The fungus Pyrenophora tritici-repentis (Died.) Drechs. [anamorph: Drechslera tritici-repentis (Died.) Shoem.] is a homothallic ascomycete that causes tan spot (syn. yellow spot) of wheat (Drechsler, 1993; Agrios, 2005). This pathogen has been reported worldwide, including Europe, southwest Asia, central Asia, north and south America and Africa (Freisen et al., 2006; Perello, 2014). Severe tan spot epidemics tend to cause 10–20% yield losses, and may cause losses of over 50% under favorable conditions for disease development (Bhatal, 2003; Singh et al., 2008). The disease has been reported in Algeria (Benbelkacem, Bendif, 2011; Benslimane, 2011), but the extent of economic losses is unknown. Intensive wheat production, reduced tillage practices, susceptible varieties, and shorter crop rotations have contributed to tan spot epidemics in many regions.
P. tritici-repentis reproduces both sexually and asexually, sexual reproduction occurs on wheat stubble between crops whereas asexual reproduction is predominant during the growing season on the wheat crop (Ali, Francl, 2003; Cao et al., 2009).
Eight races of P. tritici-repentis have been identified on the basis of necrosis (browning) and/or chlorosis (yellowing) symptoms induced on a set of specific wheat cultivars (Lamari et al., 2003; Perello, 2011). These races produce different host-selective toxins (HSTs) on susceptible wheat varieties that work as pathogenicity factors.
Effective management of tan spot is acheived by integrating available strategies, that is, a combination of chemical, cultural and genetic controls (Strelkov, Lamari, 2003; Jorgensen, Olsen, 2007).
To date, the farming community is very dependent on effective fungicides being available in order to reduce the losses from the development of disease epidemics in cereals and to stabilize yields. Intensive use of fungicides for control of tan spot disease in wheat has over the years been found to give rise to development of fungicide resistance. Since 2003, P. tritici-repentis isolates has been reported as insensitive to fungicides commonly used to manage tan spot on wheat such as benzimidazols (SDHI), triazoles (DMIs) and strobilurins (QoI) (Bockus, 1998; Bread et al., 2009). The degree of risk associated with resistance to a fungicide is dependent on its mode of action, the way it is used and the evolutionary potential of the target fungus (Fraaije et al., 2003; Cools et al., 2013).
In Algeria, tan spot is the most important disease of durum wheat (Gisi et al., 2005; Benbelkacem, Bendif, 2011; Benslimane, 2011), the climatic conditions of the country play a major role in the persistence of the disease, especially in the eastern regions which are characterized by high rainfall and temperature suitable for fungus growth during the winter period (Benslimane et al., 2006; Benbelkacem, Bendif, 2011). The yield losses and the low quality of seeds (red smudge) incurred each year by this disease force farmers to use different fungicides to protect their crops. Little is known regarding the sensitivity of the P. tritici-repentis population to fungicides in Algeria, the following study is the first one witch treat this problem, the aim of this work was to evaluate the sensitivity of 100 strains of P. tritici-repentis collected from infected durum wheat fields in the eastern cereal growing areas of Algeria during four growing seasons (2012–2015) to three class of fungicides: Tilt 250E (strobilurin), Amistar (triazole) and Amistar Xtra (combination of strobilurin and triazole) by following the increase in their IC50 and IC90 values during the four years.
MATERIALS AND METHODS
Fungal isolates. One hundred strains of P. tritici-repentis were obtained from infected wheat leaves collected from seven different eastern wheat producing areas of Algeria during four successive years (2012–2015) (Table 1).
Table 1.
The list of Pyrenophora tritici-repentis isolates studied
| Isolate number | Region | Variety of durum wheat | Date of collection | Isolate number | Region | Variety of durum wheat | Date of collection |
|---|---|---|---|---|---|---|---|
| 1 | Annaba | Siméto | 2012 | 51 | Om-Elbouagui | Simeto | 2015 |
| 2 | Annaba | GTA DUR | 2012 | 52 | Om-Elbouagui | GTA DUR | 2015 |
| 3 | Annaba | Simeto | 2013 | 53 | Om-Elbouagui | Simeto | 2015 |
| 4 | El-Tarf | HD1220 | 2012 | 54 | Khenchela | Waha | 2015 |
| 5 | El-Tarf | Simeto | 2012 | 55 | Khenchela | Waha | 2015 |
| 6 | Skikda | Vitron | 2012 | 56 | Khenchela | Semito | 2015 |
| 7 | Skikda | Simeto | 2013 | 57 | Annaba | Semito | 2014 |
| 8 | Skikda | HD1220 | 2013 | 58 | S. ahras | GTA DUR | 2014 |
| 9 | Annaba | GTA DUR | 2013 | 59 | S. ahras | GTA DUR | 2014 |
| 10 | S. ahras | Simeto | 2012 | 60 | Guelma | Semito | 2013 |
| 11 | S. ahras | GTA DUR | 2013 | 61 | Guelma | Vitron | 2012 |
| 12 | S. ahras | HD1220 | 2013 | 62 | Guelma | Semito | 2013 |
| 13 | Guelma | Simeto | 2012 | 63 | Om-Elbouagui | Simeto | 2012 |
| 14 | El-Tarf | Vitron | 2012 | 64 | Khenchela | Simeto | 2012 |
| 15 | Skikda | Siméto | 2013 | 65 | Skikda | GTA DUR | 2013 |
| 16 | Annaba | Siméto | 2013 | 66 | Skikda | GTA DUR | 2013 |
| 17 | El-Tarf | Chagra | 2013 | 67 | Om-Elbouagui | Vitron | 2014 |
| 18 | Guelma | Simeto | 2012 | 68 | Annaba | HD1220 | 2014 |
| 19 | Guelma | HD1220 | 2013 | 69 | S. ahras | Vitron | 2012 |
| 20 | S. ahras | Citra | 2013 | 70 | Khenchela | Waha | 2012 |
| 21 | Annaba | Siméto | 2014 | 71 | Annaba | Vitron | 2013 |
| 22 | Annaba | HD1220 | 2014 | 72 | S.ahras | Waha | 2013 |
| 23 | S. ahras | Citra | 2013 | 73 | El-Tarf | GTA DUR | 2012 |
| 24 | Skikda | Siméto | 2014 | 74 | El-Tarf | Simeto | 2013 |
| 25 | Skikda | GTA DUR | 2014 | 75 | Guelma | HD1220 | 2012 |
| 26 | El-Tarf | Chagra | 2013 | 76 | Guelma | GTA DUR | 2012 |
| 27 | Annaba | Simeto | 2015 | 77 | Annaba | Simeto | 2014 |
| 28 | Annaba | GTA DUR | 2015 | 78 | Annaba | HD1220 | 2015 |
| 29 | Guelma | Simeto | 2014 | 79 | Om-Elbouagui | Siméto | 2014 |
| 30 | Guelma | HD1220 | 2014 | 80 | S. ahras | Simeto | 2014 |
| 31 | S. ahras | Siméto | 2014 | 81 | S. ahras | Vitron | 2014 |
| 32 | Skikda | Simeto | 2014 | 82 | Khenchela | HD1220 | 2015 |
| 33 | Skikda | GTA DUR | 2014 | 83 | El-Tarf | Vitron | 2013 |
| 34 | El-Tarf | GTA DUR | 2013 | 84 | Guelma | Simeto | 2013 |
| 35 | El-Tarf | Simeto | 2014 | 85 | Guelma | Waha | 2014 |
| 36 | El-Tarf | Vitron | 2014 | 86 | Khenchela | Simeto | 2014 |
| 37 | Annaba | Simeto | 2015 | 87 | El-Tarf | Vitron | 2012 |
| 38 | Guelma | Vitron | 2014 | 88 | El-Tarf | Waha | 2012 |
| 39 | Guelma | Siméto | 2014 | 89 | Annaba | Simeto | 2013 |
| 40 | Guelma | GTA DUR | 2015 | 90 | El-Tarf | Siméto | 2014 |
| 41 | S. ahras | HD1220 | 2014 | 91 | Skikda | Vitron | 2014 |
| 42 | S. ahras | Simeto | 2015 | 92 | Skikda | Waha | 2015 |
| 43 | S. ahras | GTA DUR | 2015 | 93 | Khenchela | Waha | 2013 |
| 44 | Skikda | Siméto | 2015 | 94 | S. ahras | Siméto | 2013 |
| 45 | El-Tarf | Siméto | 2015 | 95 | S. ahras | Vitron | 2013 |
| 46 | Guelma | Vitron | 2015 | 96 | Om-Elbouagui | Siméto | 2014 |
| 47 | S. ahras | Simeto | 2015 | 97 | Guelma | HD1220 | 2015 |
| 48 | Guelma | GTA DUR | 2015 | 98 | El-Tarf | Waha | 2015 |
| 49 | Skikda | Waha | 2015 | 99 | S. ahras | GTA DUR | 2013 |
| 50 | El-Tarf | Siméto | 2015 | 100 | Skikda | Simeto | 2012 |
Leaves of symptomatic plants were cut into 3 cm fragments and surfaces were sterilized with 5% hypochlorite sodium solution for 2 min then washed with sterile distilled water. Leaf segments were placed in plastic Petri dishes with wet sterile paper, and the dishes were incubated in the dark for 3 days at 25°C in order to stimulate the production of conidia. A single spore was picked of under binoculaire microscope with a sterile needle then transferred to malt extract agar growing medium (10 g malt extract and 20 g agar per litre of medium autoclaved for 20 minutes at 120°C) and incubated at 25°C for 7 days in the dark.
Fungicides tested. Three classes of fungicides commonly used in Algeria for the chemical control of the tan spot were used in this study: 1) Tilt 250E (triazoles) with propiconazole (250 g/l) as active ingredient, 2) Amistar (strobilurine) with azoxystrobine (250 g/l) as active ingredient, and 3) Amistar Xtra witch is a mixture of triazoles and strobilurins [Azoxystrobin (200 g/l) + cyproconazole (80 g/l)]. A final doses of fungicides tested was 2.5 ppm, 5 ppm, 7.5 ppm, 10 ppm, 12.5 ppm, 15 ppm, 17.5 ppm, and 20 ppm, respectively.
Antifungal activity on mycelial growth. The technique used was the incorporation of the various doses of the fungicides in the malt extract agar medium (MEA) heating at 45°C, after solidification, a mycelial disc of 5 mm harvested from 7 days old culture on MEA was placed in the center of Petri dish contaning the medium MEA-fungicide and MEA medium alone for the control. The dishs were incubated in the dark for 7 days at 25°C. All treatments were made in two replics.
Fungicides sensitivity testing. The diameter of the colony of P. tritici-repentis containing a fungicide and that of the control was measured after 7 days of incubation at 25°C. The effect of different doses of fungicides on mycelial growth were estimated by comparing the measured values with those of the control. The percent inhibition of mycelial growth (Ic) was calculated according to the following formula: IC (%) = (D0 – ‒ Dc)/D0 × 100, where D0 – the diameter growth of the control, Dc – the diameter growth of the fungus in the presence of a dose (C) of the fungicide.
Statistical analysis. The statistical analysis was carried out using the procedures of the general linear models (GLM) of the SPSS software (10.0). The experiments were analyzed using standard analysis of variance (Anova) with interactions. For all tests, the level of the significance was assessed at the 5%, the averages of IC 50 (dose of fungicide that inhibits 50% of mycelial growth) and IC 90 (dose of fungicide that inhibits 90% of mycelial growth) were compared by means of the test of Student-Newman-Keuls.
RESULTS AND DISCUSSION
In total, 100 isolates of P. tritici-repentis were regrouped into four groups according the year of their collection: the group 2012 (33 isolates), the group 2013 (27 isolates), the group 2014 (18 isolates), and the group 2015 (22 isolates).
For Tilt 250EC fungicide (Propiconazole), 100% of the 2012 group isolates showed an IC50 of 5 ppm while the IC90 was estimated at 12.5 ppm. In 2013, 70% of the isolates tested retained an IC50 of 5 ppm, while 20% of the isolates showed an IC50 of 7.5 ppm, IC90 was estimated at 12.5 ppm for isolates of this group. In 2014, the IC50 for 80% of isolates was 7.5 ppm and 10 ppm for 20% of other isolates, and IC90 was also increased to 15 ppm for all isolates. 60% of the 2015 isolates had an IC50 of 10 ppm and 40% with an IC50 of 12.5 ppm, the IC90 was estimated to be 15 ppm.
The Tilt 250EC fungicide has been used in Algeria since the 1980s for the control of fungal diseases of cereals, it is a unisite fungicide based on propiconazole, a triazole product also called 14-α-demethylase inhibitors (IDM) (Ezzahiri, 2001; Dutzmann, Suty-Heiz, 2004). Inhibition of the synthesis of ergosterol, a major component of the fungal plasma membrane (Fraaije et al., 2003; Caraisse, 2010). Results of antifungal activity for Tilt 250EC fungicide on P. tritici-repentis isolates show a significantly (p < 0.05) increase in IC50 and IC90 values during the four farming seasons (2012 to 2015) (Fig. 1), which means that the isolates of P. tritici-repentis has become increasingly insensitive to this type of fungicide. These results have been proved by the officials of the Regional Plant Protection Institution (INPV) of the EL-Tarf wilaya who confirmed the persistence of the tan spot after treatment with the Tilt 250EC.
Fig. 1.
Dynamic changes in the concentration of the Tilt 250EC for IC50 and IC90 values for Pyrenophora tritici-repentis isolates between 2012 and 2015.
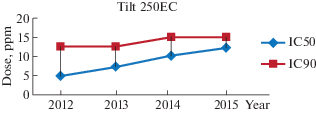
Triazole resistance was detected a few years after the start of use (Clarck, Paveley, 2005; Russell, 2005), it appears that resistance to triazole generally induces resistance to other active ingredients in the same group. According to Jorgensen and Olsen, the percentage of the tan spot in fields after treatment with a dose of 125 g/l Tilt was estimated at 67.44% (Walker, Leroux, 2004), this resistance was also reported by the Arvalis Institute which during tests carried out in 2012, triazoles such as cyproconazole at 100 g/l or propiconazole at 125 g/l had a low efficacy on P. tritici-repentis. Harvy and Craigie (2010) showed that the IC50 for prothioconazole was 4.4 ppm and 6 ppm for propiconazole in 2011 (Sierotzki and Fery, 2007; Jorgensen and Olsen, 2007), isolates of P. tritici-repentis tested in France and Germany showed an IC50 of 38 ppm to prothiocon azole (Pawn et al., 2012). Resistance to IDM also affected other phytopathogenic agents of durum wheat such as Septoria: IC50 was 12 ppm for cyproconazole and 10 ppm for prothioconazole in 2011 (Cousin, Mornval, 2012). Similar results have been reported for Erysiphe graminis (Clarck, Paveley, 2005; Marvroeidi, Shaw, 2005) and also for Puccinia recondita (Leroux et al., 2007; Ronis et al., 2014).
Based on molecular studies of resistant isolates of P. tritici-repentis, the researchers found that resistance to IDM is induced by a mutation in the CYP51 gene that codes for the enzyme ebicicol 14 α-demethylase (Russell, 2005; Chin et al., 2001), in this mutation named Y136F, the amino acid tyrosine was replaced by the phenylalanine at position 136 of the gene (Chin et al., 2001; Cousin, Mornval, 2012). They also discovered in these resistant isolates the presence of efflux pumps at the level of the plasma membrane, these pumps have a role of detoxification, they export the fungicide outside the fungal cell (Chin et al., 2001).
For Amistar based on azoxystrobin, results of antifungal activity on P. tritici-repentis isolates showed a highly significant difference (p < 0.01) of the IC 50 and IC 90 values, 100% of the 2012 group isolates had an IC50 of 7.5 ppm and an IC50 of 15 ppm in 2013, 60% of the isolates showed an IC50 of 7.5 ppm and 40% had an IC50 of 10 ppm, all isolates in this group showed an IC50 of 15 ppm. In 2014, 70% of the isolates showed an IC50 of 10 ppm while the 30% had an IC50 of 12.5 ppm, IC90 was also increased to 17.5 ppm for this group of isolates. 80% of the 2015 isolates had an IC50 of 12.5 ppm and 20% had an IC50 of 15 ppm, the IC90 also increased to 20 ppm.
Amistar has been used in Algeria since the 1990s, and the results of the antifungal activity of this fungicide on the isolates of P. tritici-repentis from the four companions showed a remarkable increase in the IC50 and IC90 values (Fig. 2). These results indicate that isolates of P. tritici-repentis isolated in eastern Algeria during the period (2012–2015) have developed resistance to strobilurins. Amistar Fungicide belongs to the strobilurin class or cellular respiration inhibitors (Qol) (Dutzmann, Suty-Heinz, 2004), it is a unisite fungicide containing azoxystrobin, its role is the attachment to the cytochrome b component of the complex Mitochondrial III of the fungal cell (Bartlett et al., 2002), this attachment blocks the transfer of electrons and consequently prevents the production of ATP in the cell (Cools et al., 2013; Harvey et al., 2015).
Fig. 2.
Dynamic changes in the concentration of the Amistar for IC50 and IC90 values for Pyrenophora tritici-repentis isolates between 2012 and 2015.
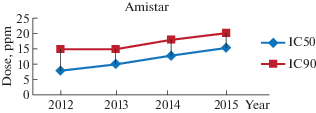
The resistance of P. tritici-repentis to azoxystrobin has been reported by Colsen and his collaborators, based on their field trials, the percentage of tan spot was 88.48% after treatment with azoxystrobin (125 g/l) and it was 71.33% for a dose of 250 g/l (Russell, 2005). Isolates of P. tritici-repentis tested in France with strobilurins showed an IC50 of 25 ppm (Russell, 2005), other isolates tested in Denmark, Canada and Australia showed an IC50 greater than 10 ppm (Clarck, Paveley, 2005). The problem of strobilurin resistance is currently reported for all phytopathogenic fungi on cereals including Septoria tritici, Blumeria graminis, and Puccinia recondita on durum wheat, Pyricularia grisea on rice and Helminthosporium on barley (Kim et al., 2003; Leadbeater, 2005).
According to some researches, the resistance to strobilurins is induced by two mutations at the level of the gene which codes cytochrome b (Fernandez-Ortuno et al., 2008; Patel et al., 2012), in position F129L where phenylalanine becomes leucine, this mutation manifests itself by low resistance, or in position G143A where the glycine becomes alanine and the mutation manifests itself by high resistance (Bokus, 1998). It was demonstrated that isolates of P. tritici-repentis containing both mutations at the same time showed IC50 values between 80 and 100 ppm (Bokus, 1998; Harvey et al., 2015), those mutants escape from the action of strobilurins by using another cellular respiration pathway called alternative respiration (Patel et al., 2012).
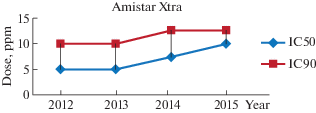
For Amistar Xtra based on azoxystrobin and cyproconazole, the 2012 and 2013 isolates showed an IC50 of 5 ppm and an IC90 of 10 ppm by 2014, 80% of the isolates of P. tritici-repentis had an IC50 Of 5 ppm and 20% showed an IC50 equal to 7.5 ppm, the IC90 was increased to 12.5 ppm for these isolates. All isolates in group 2015 showed an IC50 of 7.5 ppm and an IC50 of 12.5 ppm.
Amistar Xtra is a multisite fungicide (Syngenta) used recently in Algeria (since 2004), it is a mixture of triazoles (cyproconazole) and strobilurins (azoxystrobin), this combination of two different active ingredients makes it possible to diversify the modes of action for this fungicide and the attack of several targets in the fungal cell. Firstly, Amistar Xtra acts on cell respiration and ATP production by azoxystrobin, secondly on the structure of the plasma membrane by cyproconazole, the fungus is strongly weakened (Jorgensen, Olsen, 2007).
The results of the antifungal activity of Amistar Xtra on the isolates of P. tritici-repentis of the four groups showed a slow increase in IC50 and IC90 values during the four seasons (Fig. 3). It appears that the isolates of P. tritici- repentis have a good sensitivity to this fungicide compared to Tilt 250EC and Amistar.
Fig. 3.
Dynamic changes in the concentration of the Amistar Xtra for IC50 and IC90 values for Pyrenophora tritici-repentis isolates between 2012 and 2015.
The Anova test showed highly significant differences (p < 0.01) for the CI 50 and IC 90 values of the three fungicides between the isolates of P. tritici-repentis. During the four agricultural seasons, IC50 values for Tilt 250EC increased from 5 ppm in 2012 to 12.5 ppm in 2015 and from 12.5 ppm to 15 ppm for IC90, while the increase was significantly for Amistar where IC50 values had increased from 7.5 ppm in 2012 to 15 ppm in 2015 and from 15 ppm to 20 ppm for IC90.
The efficacy of the combination of triazoles with strobilurins against P. tritici-repentis has been demonstrated by several studies (Caraisse, 2010). In Australia, the severity of the tan spot is reduced when treated with triazoles combined with strobilurins and with a yield gain of 2.6 tons/hr (Russell 2008; Beard et al., 2009; Cools et al., 2013), similar results were obtained in New Zealand in 2015 (Grasso et al., 2006; Harvey et al., 2015).
CONCLUSION
Durum wheat is the main crop grown in Algeria; this culture is constantly threatened by fungal diseases which predominantly is tan spot. This study aims is to evaluated in vitro the sensitivity of 100 isolates of P. tritici-repentis, collected during four agricultural seasons (2012–2015) from different cereal areas in eastern of Algeria to three groups of fungicides: Amistar (azoxystrobin) that belongs to the strobilurin class, Tilt 250EC (propiconazole) from the triazoles class and Amistar Xtra (azoxystrobin plus cyproconazole) which is a combination of the two class.
The results of the antifungal activity in vitro show a decreasing in the sensitivity of isolates from one year to another. This decrease resulted in the increase of the IC50 values of the three fungicides used; the IC50 values for Amistar vary between 7.5 ppm and 15 ppm, between 5 ppm and 12.5 ppm for Tilt 250EC and between 5 ppm and 10 ppm for Amistar Xtra. It seems that P. tritici-repentis isolates have developed a resistance against strobilurins and triazoles. Mixture of triazoles and strobilurins in a single product (Amistar Xtra) has made it possible to diversify the modes of action and thus the targets of attack in the fungal cell (ATP production and plasma membrane structure) as result, the fungus is strongly weakened. Several factors may contributed to the emergence of resistance among isolates of P. tritici-repentis the main ones being the use of sensitive durum wheat varieties, poor farming practices especially monoculture of wheat, abandonment of plowing and burial of residues of previous crops, repeated application of the same fungicide or mode of action in the same area and season, finally, the use of reduced doses of fungicides allows the pathogen to adapt and become resistant to those fungicides.
Список литературы
Agrios G.N., Plant Pathology, 5me ed., Burlington, Academic Press, 2005.
Ali S., Francl L.J. Population race structure of Pyrenophora tritici-repentis prevalent on wheat and non cereal grasses in the Great Plains. Plant Disease. 2003. V. 87. P. 418–422.
Andonova R., Todorova M . In vitro characteristics of different Pyrenophora tritici-repentis isolates. Bulgarian J. Agric. Sci. 2007. V. 13. P. 673–678.
Barlett D.W., Clough J.M., etc. Review the strobilurin fungicides. Pest. Manag. Sci. 2002. V. 58. P. 649–662.
Beard C., Loughman R., Smith A, Speijers J. Baseline sensitivity to three triazoles fungicides in Pyrenophora tritici- repentis. Australasian Plant Pathol. 2009. V. 38. P. 168–172.
Benbelkacem A., Bendif N. Results of survey of cereal diseases and insects in the eastern region of Algeria. Bilan PNAB 2009/2010. Cereal Culture. 2010. V. 45. P. 12–19.
Benslimane H. Distrubition of races of Pyrenophora tritici-repentis in Algeria. Phytopathologia Mediterranea. 2011. V. 50. P. 203−211.
Benslimane H., Bouznad Z., Aouali S., Khalfi A., Benbelkacem K., Sayoud R. Prevalence in Algeria of the bronze spot of wheat caused by Pyrenophora tritici-repentis. In: 6me Journées Scientifiques et Techniques Phytosanitaires, 20–21 juin 2006, El-Harrach, Alger, Algeria.
Bhathal J.S., Loughman R., Speijers J. Yield reduction in wheat in relation to leaf disease from yellow (tan) spot and Septoria nodorum blotch. Eur. J. Plant Pathol. 2003. V. 109. P. 435–443.
Bokus W.W. Control strategies for stubble-borne pathogen of wheat. Can. J. Plant Pathol. 1998. V. 20. P. 371–375.
Cao T., Yong M.K., Kav N.V., Strelkov S.E. A proteomic evaluation of Pyrenophora tritici-repentis, causal agent of tan spot of wheat, reveals major differences between virulent and avirulent isolates. Proteomics. 2009. V. 9. P. 1177–1196.
Caraisse O. Fungicides. Tech. Rijeka, Croatia. 2010. V. 23. P. 69–201.
Chin K.M., Chavaillaz D., Staub T. Characterizing resistance risk of Erysiphe graminis to strobilurine. Crop Protection. 2001. V. 20. P. 87–96.
Clarck W. S., Paveley N. Wheat disease management guide – update: Homegrown cereals authority. London, 2005.
Cools H., Mullins G.L., Fraaije B.A., Parker J.E., Kelly D.E., Lucas J.A., Kelly S.L. Impact of recently emerged strerol 14-α-demethylase (CYP51) variants of Mycospharealla graminicola on azoles fungicides sensitivity. Appl. Env. Microbiol. 2013. V. 77. P. 3830–3837.
Cousin A., Mornval M.H. Septoria and triazoles: management of modes of action for sustainable wheat protection. Phytoma-la défense des végétaux. 2012. V. 652. P. 7–10.
Drechsler C. Some graminicolous species of Helminthosporium. J. Agricult. Res. 1923. V. 24. P. 641–740.
Dutzmann S., Suty-Heinze A. Prothioconazole: A broad spectrum demethylation inhibitor for arable crops. Flanzenschutz-Nacnirichten Bayer. 2004. V. 57. P. 249–264.
Ezzahiri B. Wheat diseases: Identification, development factors and control methods. Bulletin de Transfert de Tchnologie en Agriculture. 2001. V. 77. P. 4.
Fernández-Ortuño D., Torés J.A., De Vicente A., Pérez-García A. Mechanisms of resistance to QoI fungicides in phytopathogenic fungi. International Microbiol. 2008. V. 11. P. 1–9.
Fraaije B.A., Lucas J.A., Clarck W.S., Burnett F.J. QoI resistance developement in population of cereal pathogen in UK. In: Proccedings of the BCPC Congress. Crop science and technology. V. 2. Glasgow, 2003, pp. 689–694.
Friesen T.L., Klein K.K., Rasmussen J.B. Population genetic analysis of a global collection of Pyrenophora tritici-repentis, causal agent of tan spot of wheat. Phytopathology. 2005. V. 95. P. 1144–1150.
Gisi U., Pvic L., Stanger C., Hugelshofer U., Sierotzki H. Dynamics of Mycosphaerella graminicola population in response to selection by différent fungicides. In: Dehne H.W et al. (eds). Moderne fungicides and antifungal compounds. V. 4, 2005, pp. 82–101.
Grasso N., Sierotzki H., Garibaldi A., Gisi U. Characterization of the cytochrome b gene fragment of Puccinia species responsible for the binding site of QoI fungicides. Pesticides Biochem. Physiol. 2006. V. 84. P. 72–82.
Harvey I.C., Craigie R.A., Mc Cloy B.L. The control of tan spot of wheat: a possible emerging disease in New Zealand. N.Z. Plant Protect. 2015. V. 68. P. 428–433.
Jorgensen L.N., Olsen L.V. Control of tan spot (Drechslera tritici-repentis) using cultivar resistance, tillage methods and fungicides. Crop Protection. 2007. V. 26. P. 1606–1616.
Kim Y.S., Dixon E.W., Vincelli P., Farman M. Field resistance to strobilurine fungicides in Pyricularia grisea caused by mutation in the mitochondrial cytochrome b gene. Phytopathology. 2003. V. 93. P. 891–900.
Lamari L., Strelkov S.E., Yahyaoui A., Orabi J., Smith R.B. The identification of two new races of Pyrenophora tritici-repentis from the host center of diversity confirms a one-to-one relationship in tan spot of wheat. Phytopathology. 2003. V. 93. P. 391–396.
Leadbeater A.J. Ensuring the long term effectiveness of fungicides an industry perspective. Crop Sci. Technol. 2005. V. 22. P. 253–266.
Lepoivre P. Phytopathology. De Boeck les presses agronomiques de Gembloux. 2003.
Leroux P., Albertini C., Gautier A., Gredt M., Walker A.S. Mutation in the CYP51 gene correlated with changes in sensibility to IDM in field isolates of Mycosphaerella graminicola. Pesticides Management Science. 2007. V. 63. P. 688–698.
Marvroeidi V.I., Shaw M.W. Sensitivity distribution and cross-resistance patterns of Mycosphaerella graminicola to fluquinconazole, prochloraz and azoxystrobine over a period of 9 years. Crop Protection. 2005. V. 24. P. 259–266.
Patel J.S., Gudmestad N.C., Meinhardt S.W., Adhikari T.B. Pyraclostrobine sensitivity of baseline and fungicides expose isolates of Pyrenophora tritici repentis. Crop Protection. 2012. V. 34. P. 37–41.
Pawn K., Singh S., Duveiller E. Resistance breeding for tan sot (Pyrenophora tritici-repentis) of wheat. In: Disease resistance in wheat, edition Sharma. Mexico, 2012, pp. 136–140.
Perello A. Genetic analysis and comparative virulence of isolates of Pyrenophora tritici-repentis from wheat in Argentina. African J. Microbiology Research. 2012. V. 6. P. 5558–5567.
Ronis A., Jorgensen L.N., Semaskiene R., Gaurilcikiene I., Ramanauskiene J. Sensitivity of Mycosphaerella graminicola isolates to demethylation-inhibiting (DMI) fungicides. Zemderbyste agriculture. 2014. V. 101. P. 177–184.
Russell P.E. A centry of fungicides evolution. J. Agricult. Sci. 2005. V. 143. P. 11–25.
Sierotzki H., Frey R. Pyrenophora tritici-repentis and implication for QoI resistance. Pest. Management Science. 2007. V. 63. P. 225–233.
Singh S., Bockus W.W., Sharma I., Bowden R.L. A new source of resistance in wheat to Pyrenophora tritici-repentis race 1. Plant Diseases. 2008. V. 92. P. 91–95.
Strelkov S.E., Lamari L. Host-parasite interaction in tan spot Pyrenophora tritici-repentis of wheat. Can. J. Plant Pathol. 2003. V. 25. P. 339–349.
Tekauz A., Mueller E., Stulzer M., Schultz D. Leaf spot diseases of winter wheat in Manitoba in 2003. Can. Plant Diseases. 2004. V. 83. P. 73–74.
Walker A.S., Leroux P. Wheat powdery mildew: What resistant to fungicides in French? Phytoma-la défense des végétaux. 2004. V. 571. P. 16–18.
Дополнительные материалы отсутствуют.
Инструменты
Микология и фитопатология


