Микология и фитопатология, 2020, T. 54, № 6, стр. 446-451
CULTURE CHARACTERISTICS AND ENZYMATIC ACTIVITY OF SARCODONTIA CROCEA (BASIDIOMYCOTA) STRAINS COLLECTED FROM THE CENTRAL RUSSIAN UPLAND
N. V. Shakhova 1, *, S. V. Volobuev 1, **
1 Komarov Botanical Institute of the Russian Academy of Sciences
197376 St. Petersburg, Russia
* E-mail: nshakhova@binran.ru
** E-mail: sergvolobuev@binran.ru
Поступила в редакцию 20.04.2020
После доработки 3.05.2020
Принята к публикации 11.05.2020
Аннотация
The biological properties of the xylotrophic fungus Sarcodontia crocea (Polyporales, Basidiomycota), which develops basidiocarps on the trunks of fruit trees (mainly Malus domestica) and exhibits phytopathogenic activity, have been investigated under pure culture conditions. Morphological and molecular verification was performed for Sarcodontia crocea strains isolated in 2019 by seeding the basidiospores and directly from specimens of fresh basidiocarps growing on Malus domestica in Belgorod and Oryol Oblasts (the Central Russian Upland). New data on micromorphological (presence of chlamydospores and anastomoses on dikaryotic mycelium) and molecular (complete ITS1–5.8S–ITS2 nrDNA sequences) characteristics of this species have been obtained. The maximum growth rate of mycelium on the agarized MEA medium was determined (up to 5.5 mm/day) and the oxidative and cellulolytic activity of Sarcodontia crocea was assessed. It was revealed that all strains had the ability to ABTS oxidation as a result of activity of oxidative enzymes. Strains of S. crocea LE-BIN 4342, 4343, 4346, 4350, and 4365 showed the high activity of oxidoreductases.
INTRODUCTION
Basidial macromycetes represent one of the key evolutionary-established groups of living organisms that actively decompose wood. Among xylotrophic basidiomycetes, so-called xyloparasites can be distinguished that grow on the trunks, branches or roots of living trees, both wild and cultivated Sarcodontia crocea (Schwein.) Kotl. (Polyporales, Basidiomycota) is a xylotrophic basidiomycete with pronounced phytopathogenic activity that develops predominantly on fruit trees, mainly Malus (Eriksson et al., 1981) and rarely on Pyrus, Sorbus, and Prunus, causing white rot. The most obvious features of infestation of fruit trees with phytopathogenic xylotrophs, as S. crocea, in the absence of developed fungal fruit bodies are the individual branches drying up and crown asymmetry, while the tree retains its limited ability to fruit. The dead basidiomata of S. crocea have also been found on thick, dry branches and dry tree trunks, for which this species is the main cause of drying and death (Volobuev et al., 2019). For this reason, S. crocea is considered to be a saprotroph. S. crocea is relatively rare in Central and Northern Europe (Szczepkowski et al., 2017), and the species is assessed as Vulnerable (VU) A2c+3c+4c according to the IUCN criteria because the habitat for S. crocea has declined and continues to be so due to the intensification of orchard and garden management (Iršėnaitė, 2019). At the same time, in the conditions of the Central Russian Upland (Eastern Europe), there are significant areas of neglected gardens or orchards that do not receive proper horticultural and phytosanitary care and thus are areas of mass distribution of this phytopathogen (Fig. 1). To date, the biology and ecology of S. crocea have not been sufficiently studied, although it is important for developing measures to control and prevent its spread in orchard agrocoenoses.
The aim of this study is to verify new strains of Sarcodontia crocea, to obtain their cultural characteristics and to evaluate oxidative and cellulolytic enzymes that determine the xylotrophic activity of the species and its ecology. This study is also intended to supplement the current knowledge on the biology of the xylotrophic basidial fungus S. crocea by obtaining new DNA sequences and ex situ collection.
MATERIALS AND METHODS
Isolation and verification of fungal cultures. We stu-died nine strains of Sarcodontia crocea obtained in 2019 from basidiocarps growing on different parts of Malus domestica as a host tree on the territories of Belgorod and Oryol Oblasts (Table 1). Ex situ isolation was carried out by traditional methods of solid-phase culturing: by seeding of basidiospores or by placing small fragments of basidiocarps on an agarized medium (4% ale-wort “Severnaya Pivovarnya”, pH 5.8 and 2% w/v agar “Difco”), with addition of kanamycin water solution (final concentration in the medium – 0.5 mg/ml) in order to prevent bacterial contamination. Dikaryotic strains were deposited in the Komarov Botanical Institute Basidiomycetes Culture Collection (LE-BIN, St. Petersburg, Russia) and are stored using the subculture method, the disk method (in distilled water at 4°C), and the cryopreservation method at –80°C (Psurtseva et al., 2012; Shakhova, Volobuev, 2020). The morphological description and photo-documentation were carried out for 14 days old colonies using the AxioScope A1 microscope (Carl Zeiss) with ×40 and ×60 magnifications. Strains of S. crocea were characterized by cultural and morphological parameters using the method and terminology of J.A. Stalpers (1978).
Table 1.
Characteristics of fungal strains studied
| Strain numbers in LE-BIN | Origin of strains | The growth in the standard MEA medium | GenBank accessions | |||
|---|---|---|---|---|---|---|
| Locality | Substrate | 10 days diam., (mm) | Petri dish (days) | AGR (mm/days) | ||
| 4342 | Russia, Belgorod Oblast, Korochansky District, vicinity of the Popovka village | alive tree of M. domestica | 50.7 ± 1.8 | 21 | 4.2 | MW042103 |
| 4343 | Russia, Belgorod Oblast, Korochansky District, vicinity of the Popovka village | dry branches of alive tree of M. domestica | 52.5 ± 2.2 | 17 | 5.5 | MW042104 |
| 4346 | Russia, Belgorod Oblast, Korochansky District, vicinity of the Popovka village | trunk of alive tree of M. domestica | 36.7 ± 3.1 | 27 | 3.3 | – |
| 4350 | Russia, Oryol Oblast, Glazunovsky District, vicinity of the Lovchikovo village | trunk of a dying tree of M. domestica | 39.5 ± 2.5 | 19 | 4.9 | MW042105 |
| 4355 | Russia, Oryol Oblast, Glazunovsky District, vicinity of the Lovchikovo village | branch of alive tree of M. domestica | 58.7 ± 2.3 | 19 | 4.8 | MW042106 |
| 4365 | Russia, Oryol Oblast, Mtsensky District, vicinity of the Volya village | trunk of alive tree of M. domestica | 32.3 ± 2.1 | 25 | 3.5 | MW042107 |
| 4367 | Russia, Oryol Oblast, Mtsensky District, vicinity of the Volya village | trunk of alive tree of M. domestica | 29.3 ± 2.2 | 23 | 4.0 | MW042108 |
| 4378 | Russia, Oryol Oblast, Orlovsky District, vicinity of the Zhilina village | trunk of a dying tree of M. domestica | 17.2 ± 1.5 | 30 | 3.0 | MW042109 |
| 4382 | Russia, Oryol Oblast, Orlovsky District, vicinity of the Zhilina village | dead tree of M. domestica | 56.3 ± 4.2 | 19 | 4.9 | – |
Molecular study of pure cultures. Genomic DNA was extracted using the FitoSORB DNA extraction kit (Syntol, Russia) according to the manufacturer’s instructions from 14 days old cultures, which were grown on standard liquid medium Malt Extract (ME, Conda) in the dark at 25°C. Amplification of the ITS1–5.8S–ITS2 region of the nrDNA, and sequencing were reformed as described in Zmitrovich et al. (2019). Newly generated sequences were deposited in GenBank.
Growth measurement. To characterize the linear growth rate, pure cultures of S. crocea were grown in Petri dishes 90 mm diam. on standard Malt Extract Agar (MEA, Conda) in the dark at 25°C. Cultivation of strains was carried out with mycelial blocks (7 mm diam.) cut from the edge zone of the actively growing colony, by placing them on the nutrient medium in the center of Petri dishes with the mycelial layer down wards. The growth of strains was characterized by the diameter of the colony (mm), measuring them every two days starting from the third day until the Petri dish was completely overgrown. The average growth rate of the vegetative mycelium of S. crocea strains (AGR, mm/day) was calculated according to the formula (Badalyan et al., 2015): AGR = (D1 – D0)/(t1 – t0), where D1 is the diameter of the colony at the end of the growth, mm; D0 – colony diameter at the beginning phase of linear growth, mm; t1 – t0 – the duration of the linear phase of the colony growth, days.
Detection of enzymatic activity. The activity of oxidative and cellulolytic enzymatic complexes was studied using the rapid screening method. The strains were grown on the MEA medium in a thermostat at 25°C for 2 weeks. Cultivation of strains was carried out with mycelial blocks 7 mm diam. cut from the edge zone of the actively growing colony, by placing them in the center of the Petri dish with the mycelial layer upwards. The qualitative activity of oxidative enzymes in the studied strains was determined by the method of application with modifications. The activity of oxidoreductases was registered 48 hours after placing the mycelial blocks on Petri dishes with plain agar (1% w/v; Difco) containing ABTS (in 0.1% concentration) by the presence of emerald-greenish staining around inoculum (d, mm). The criteria to determine the intensity of the substrate oxidation reaction were described in Shakhova and Volobuev (2020). The qualitative activity of cellulolytic enzymes in cultures was determined using the medium containing carboxymethyl cellulose (1% w/v, CMC; Chemapol) and agar (1% w/v; Difco) by the application method and estimated by the presence of a clear zone around inoculum (d, mm). The clear zone was detected 48 hours after inoculation using solution I in KI (0.5% w/v I in 2% KI) (Shakhova, Volobuev, 2020). The same parameters were used to estimate the intensity of the reaction as in determining the activity of oxidoreductases.
RESULTS AND DISCUSSION
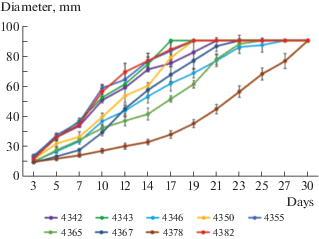
The growth characteristics of the studied strains on the standard MEA medium are presented in Table 1 and Fig. 2. When comparing the data on the linear growth rate of the vegetative mycelium of S. crocea strains with the results of a study on the growth rate of other cultivated xylotrophic fungi obtained previously (Shakhova, Volobuev, 2020) and the literature data (Petre, Tănase, 2013), it should be noted that S. crocea belongs to slow-growing species. The most rapidly growing strains were LE-BIN 4343, LE-BIN 4350, LE-BIN 4355, and LE-BIN 4382. By the 10th day of cultivation, the diameter of the colonies of these strains exceeded 50 mm, and the Petri dishes (d ≥ ≥ 75 mm) were completely overgrown in 17–19 days. The average mycelium growth rate (AGR) of these strains was 4.8–5.5 mm/day. The strain LE-BIN 4378 had the lowest growth rate, with a colony diameter of 17.2 mm after 10 days, AGR was 3.0 mm/day, and the colonies of the strain covered the whole Petri dish after 30 days (Table 1, Fig. 2).
Morphological characteristics of cultures of studied strains are listed in Table 2, photos of the colonies are shown in Fig. 3. The main features of S. crocea in the cultivation on the MEA medium are floccose (occasionally pinnate as in LE-BIN 4343 and 4346) colonies with a strong characteristic sweet-fruity odour (Table 2, Fig. 3). The fruity odour, which indicates the presence of volatile compounds in strains (Kokubun et al., 2007), can play an important physiological role in the development of S. crocea basidiocarps and the spread of its substrate mycelium (in particular, in the distant inhibition of S. crocea fungal opponents during wood colonization). The vast majority of strains had unclear colony zones (except for LE-BIN 4343 and 4346). More than half of the strains had a bleached reverse. The colour of the colonies ranged from white to creamy (LE-BIN 4343, 4346, 4367, 4378) and lemon creamy (LE-BIN 4355) (Fig. 3). It should be noted that pigmentation manifested itself well with the age of the colonies. The outline of the colonies also varied significantly from fringe to wavy (LE-BIN 4382) and flat (LE-BIN 4365).
Table 2.
Culture characteristics of the Sarcodontia crocea strains
| LE-BIN strains | Macromorphology | Micromorphological features |
|---|---|---|
| 4342 | The aerial mycelium is white. Reverse bleached. Colony growing edge pressed. Outlines of colonies fringed. Mycelial mat has an approximately zonal development. The colony has a floccose texture with small hyphal tufts, standing out from the agar. Odor is very intense, strong, sweetish-fruity | Hyphal system monomitic. The mycelium has two types of hyphae: leading hyphae d 3.5–4.8 μm and thin-walled and branched exploiting hyphae d 2.3–3.0 μm. Branched generative hyphae, differentiated with septate and rare clamp connections, d 4.3–5.8 μm |
| 4343 | The aerial mycelium is white (becomes dark cream color with age). Reverse bleached. Colony growing edge pressed. Outlines of colonies fringed, broken. Mycelial mat immersed (sometimes downy in more differentiated hyphal). The colony has a plumose texture, mycelial tufts with long groups of hyphae radiating from the central axis, fan-like arrangement. Odor is very intense, strong, sweetish-fruity | Hyphal system monomitic. The mycelium consists of straight, smooth, rarely branching hyphae d 2.5–5.0 μm, often arranged with partitions, with thickened walls. Generative hyphae, differentiated with rare clamp connections, d 5.6–8.1 μm |
| 4346 | The aerial mycelium is white (becomes cream color with age). Reverse unchanged. Colony growing edge pressed. Outlines of colonies fringed, broken. Mycelial mat has an approximately zonal development. The colony has a floccose texture with small hyphal tufts, standing out from the agar. Odor is very intense, strong, sweetish-fruity | Hyphal system monomitic. The mycelium has thin-walled and branched hyphae (d 1.7–3.0 μm) forming anastomosis. Branched generative hyphae, differentiated with septate and frequent clamp connections, d 4.3–5.8 μm |
| 4350 | The aerial mycelium is white (becomes cream color with age). Reverse unchanged. Colony growing edge pressed. Outlines of colonies fringed. Mycelial mat has an approximately zonal development. The colony has a floccose texture with small hyphal tufts, standing out from the agar. Odor is very intense, strong, sweetish-fruity | Hyphal system monomitic. The mycelium consists of straight, smooth, rarely branching hyphae d 3.3–4.7 μm, often arranged with partitions, with thickened walls. Generative hyphae, differentiated with rare clamp connections, 6.0–8.2 μm in diameter. Chlamydospores broadly ellipsoid: d 5.0–6.6 µm, length –15.0–24.0 µm |
| 4355 | The aerial mycelium is white (becomes lemon-cream color with age). Reverse bleached. Colony growing edge pressed. Outlines of colonies fringed. Mycelial mat has an approximately zonal development. The colony has a floccose texture with small hyphal tufts, standing out from the agar. Odor is very intense, strong, sweetish-fruity | Hyphal system monomitic. The mycelium has thin-walled and branched hyphae (d 1.7–2.7 μm) forming frequent curling. Branched generative hyphae, differentiated with septate and rare clamp connections, d 5.2–6.3 μm |
| 4365 | The aerial mycelium is white. Reverse bleached. Colony growing edge pressed. Outlines of colonies smooth. Mycelial mat has an approximately zonal development. The colony has a floccose texture with small hyphal tufts, standing out from the agar. Odor is very intense, strong, sweetish-fruity | Hyphal system monomitic. The mycelium consists of straight, smooth, rarely branching hyphae d 3.4–4.9 μm, often arranged with partitions, with thickened walls. Generative hyphae, differentiated with rare clamp connections, 5.3–8.5 μm in diameter. Chlamydospores globose (d 9.0–12.6 µm) or broadly ellipsoid (d 5.0–6.6 µm, length –15.0–24.0 µm) |
| 4367 | The aerial mycelium is white (becomes cream color with age). Reverse unchanged. Colony growing edge pressed. Outlines of colonies fringed. Mycelial mat has an approximately zonal development. The colony has a floccose texture with small hyphal tufts, standing out from the agar. Odor is very intense, strong, sweetish-fruity | Hyphal system monomitic. The mycelium has thin-walled and branched hyphae (d 3.2–4.3 μm) forming anastomosis. Sometimes hyphae forming curling. Sometimes there are empty swollen hyphae with thickened walls. Branched generative hyphae, differentiated with septate and frequent clamp connections, 4.9–6.3 μm in diameter |
| 4378 | The aerial mycelium is white (becomes cream color with age). Reverse unchanged. Colony growing edge pressed. Outlines of colonies fringed. Mycelial mat has an approximately zonal development. The colony has floccose-cottony texture, rather long, single mycelial hyphae spreading in all directions. Odor is very intense, strong, sweetish-fruity | Hyphal system monomitic. The mycelium has thin-walled and branched hyphae (d 1.8–2.8 μm) forming frequent curling. Branched generative hyphae, differentiated with septate and rare clamp connections, d 4.5–5.6 μm |
| 4382 | The aerial mycelium is white. Reverse bleached. Colony growing edge pressed. Outlines of colonies wavy. Mycelial mat has an approximately zonal development. The colony has a floccose texture with small hyphal tufts, standing out from the agar. Odor is very intense, strong, sweetish-fruity | Hyphal system monomitic. The mycelium has thin-walled and branched hyphae (d 2.3–3.7 μm) forming frequent curling. Branched generative hyphae, differentiated with septate and rare clamp connections, 4.5–5.6 μm in diameter |
Fig. 3.
Morphology of Sarcodontia crocea cultures which were incubated for 14 days on Malt Extract Agar (MEA). Scale bars = 1 cm. A – LE-BIN 4342, B – LE-BIN 4343, C – LE-BIN 4346, D – LE-BIN 4350, E – LE-BIN 4355, F – LE-BIN 4365, G – LE-BIN 4367, H – LE-BIN 4378, I – LE-BIN 4382.
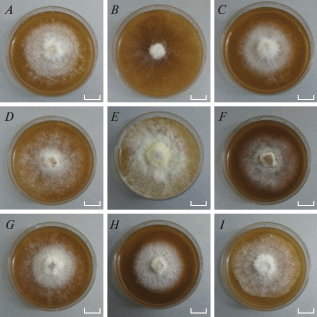
Among the characteristic features of the micromorphology of the vegetative mycelium of S. crocea is the presence of asexual reproduction structures on the dikaryotic mycelium, such as chlamydospores, along with the presence of clamps and anastomoses (LE-BIN 4346 and 4367). Terminal chlamydospores were found in strains LE-BIN 4350 and 4365. The beginning of growth of apical chlamydospores occurred after their separation from the parent hyphae by a transverse septum. No thickening of the protoplasm was observed. The presence of chlamydospores on the dikaryotic mycelium of S. crocea strains which we have described is in accordance with the micromorphological study of this basidiomycete by J.A. Stalpers (1978). Thus, the macromorphological features of different strains of S. crocea on MEA fluctuated significantly, while their micromorphology remained more stable. The results obtained can be used to verify strains and control the purity of vegetative mycelium in a culture.
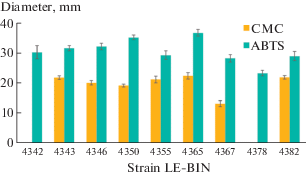
S. crocea is among the very few organisms that colonize fruit trees, mainly Malus spp., less often Pyrus spp. and members of the genus Prunus (Eriksson et al., 1981, Volobuev et al., 2015), and thanks to the complex enzymatic system can degrade lignin, one of the most abundant and resistant biopolymers. However, the enzymatic activity of this species has not been sufficiently studied. Therefore, we performed an assessment of lignocellulose-converting enzyme activity among S. crocea cultures. The results of a rapid test for the presence of cellulolytic enzymes showed that most of the strains studied have a medium activity of these enzymes. The cellulolytic enzyme activity was not revealed for the strains LE-BIN 4343 and LE-BIN 4378. It should be noted that in S. crocea strains the activity level of cellulolytic enzymes was significantly lower than that of oxidative enzymes (by 25–45%). This is probably due to the fact that during selective delignification with white rot fungi at an early stage of wood decomposition, more lignin breaks down than hemicellulose or cellulose (Źółciak, 2019). As can be seen in Fig. 4, all strains had the ability to oxidize ABTS as a result of the oxidative enzymes. Strains LE-BIN 4342, 4343, 4346, 4350, and 4365 showed high oxidoreductase activity in qualitative spot-test (Fig. 4).
Fig. 4.
Results of the express assays of oxidative (ABTS) and cellulolytic (CMC) enzymes in pure cultures of Sarcodontia crocea.
As a result of the research, a collection of pure cultures of S. crocea with the expressed potential of ligno- and cellulolytic enzymes was formed and conditions were selected for reliable maintenance of strains in the LE-BIN collection with preservation of their growth and biosynthetic activity were selected. This study also allowed us to ensure a sufficient set of strains to further solve the problems of S. crocea ecology, for instance, connected with interspecies relations of different xylotrophic pathogens.
This study was carried out within the framework of the institutional research project of the Komarov Botanical Institute RAS (AAAA-A19-119020890079-6). The work of S.V. Volobuev was partially supported by the Grant of the President of the Russian Federation (MK-3216.2019.11). Molecular researches of pure cultures were performed using the equipment of the Core Facility Centre “Cell and Molecular Technologies in Plant Science” at the Komarov Botanical Institute, RAS (St. Petersburg, Russia).
Список литературы
Badalyan S.M., Shnyreva A.V., Iotti M. et al. Genetic resources and mycelial characteristics of several medicinal polypore mushrooms (Polyporales, Basidiomycetes). Int. J. Medicinal Mushrooms. 2015. V. 17 (4). P. 371–384. https://doi.org/10.1615/IntJMedMushrooms.v17.i4.60
Eriksson J., Hjortstam K., Ryvarden L. Vol. 6. Phlebia – Sarcodontia. The Corticiaceae of North Europe. Oslo: Fungiflora, 1981. P. 1051–1276.
Iršėnaitė R. 2019. Sarcodontia crocea. The IUCN Red List of Threatened Species 2019: e.T147533826A148058863.
Kokubun T., Rozwadowski Z., Duddeck H. Benzaldehyde derivatives from Sarcodontia crocea. J. Natur. Prod. 2007. V. 70 (9). P. 1539–1541. https://doi.org/10.1021/np070305s
Petre C.V., Tănase C. Culture characteristics of 20 lignicolous basidiomycetes species that synthesize volatile organic compounds. Analele Ştiinţifice ale Universităţii “Al. I. Cuza” Biologie vegetală. 2013. V. 59 (2). P. 37–51.
Psurtseva N.V., Barinova K.V., Yakovleva N.S. Basidiomycetes culture collection LE-BIN: conservation methods and problems in maintaining the gene pool. In: Dyakov Yu.T., Sergeev Yu.V. (eds) Current mycology in Russia. Vol. 3. National Academy of mycology, Moscow, 2012, p. 135 (in Russ.).
Shakhova N.V., Volobuev S.V. Revealing new active and biotechnologically perspective producers of oxidative and cellulolytic enzymes among pure cultures of xylotrophic Agaricomycetes from the Southern Non-Chernozem zone of the European part of Russia. Current Res. Envir. Appl. Mycol. 2020. V. 10 (1). P. 113–119. https://doi.org/10.5943/cream/10/1/12
Stalpers J.A. Identification of wood-inhabiting Aphyllophorales in pure culture. Stud. Mycol. 1978. V. 16. P. 1–248.
Szczepkowski A., Gierczyk B., Borowski J., Neubauer G. New localities of Sarcodontia crocea (Polyporales, Basidiomycota) in Poland. Acta Mycologica. 2017. V. 52 (1). Art. 1090. https://doi.org/10.5586/am.1090
Volobuev S.V., Bolshakov S.Yu., Shakhova N.V. Monitoring of xylotrophic basidiomycetes – phytopathogens of fruit trees in the Belgorod region. In: Materialy XXI Mezhdunarodnoy konferentsii “Biologicheskoe raznoobrazie Kavkaza i yuga Rossii”. Magas, 2019. P. 42–45 (in Russ.).
Volobuev S.V., Logachev A.A., Mushnikov N.V. et al. New records of aphyllophoroid fungi (Agaricomycetes, Basidiomycota) from the Les na Vorskle area of the Belogorye Nature Reserve (Belgorod Region, Russia). Folia Cryptogamica Estonica. 2015. V. 52. P. 89–93. https://doi.org/10.12697/fce.2015.52.11
Zmitrovich I.V., Volobuev S.V., Dudka V.A. et al. Ganoderma applanatum (Polyporales, Basidiomycota) at the Saint Petersburg area. Mikologiya i fitopatologiya. 2019. V. 53 (6). P. 354–362. https://doi.org/10.1134/S0026364819060084
Źółciak A. Ligninolytic activity of Phlebiopsis gigantea strains in cultivation on Norway spruce wood. Baltic Forestry. 2019. V. 25 (1). P. 15–24. https://doi.org/10.46490/vol25iss1pp015
Волобуев С.В., Большаков С.Ю., Шахова Н.В. (Volobuev et al.) Мониторинг ксилотрофных базидиомицетов – фитопатогенов семечковых плодовых культур в Белгородской области // Материалы XXI Международной научной конференции “Биологическое разнообразие Кавказа и юга России”. Магас, 2019. С. 42–45.
Псурцева Н.В., Баринова К.В., Яковлева Н.С. (Psurtseva et al.) Коллекция культур базидиомицетов LE-BIN: методы сохранения и проблемы поддержания генофонда // Современная микология в России. Том 3 / Ред. Ю.Т. Дьяков, Ю.В. Сергеев. М.: Нац. акад. микол., 2012. С. 135.
Дополнительные материалы отсутствуют.
Инструменты
Микология и фитопатология