Микология и фитопатология, 2021, T. 55, № 2, стр. 148-154
Study of verticillium wilt pathogenesis in different cotton genotypes
M. M. Khotamov 1, *, I. G. Akhmedzhanov 2, **
1 Institute of Genetics and Plant Experimental Biology, Academy of Sciences of the Republic of Uzbekistan
111208 Yukoru-Yuz, Tashkent Region, Uzbekistan
2 Institute of Biophysics and Biochemistry at the National University of Uzbekistan
100047 Tashkent, Uzbekistan
* E-mail: mansurhatamov@mail.ru
** E-mail: iskakhm@mail.ru
Поступила в редакцию 15.10.2020
После доработки 15.11.2020
Принята к публикации 23.12.2020
Аннотация
The pathogenesis of Verticillium wilt in 8 varieties of cotton (Gossypium hirsutum) zoned in Uzbekistan has been established by the method of fluorescence diagnostics. According to the duration of the incubation period, indicating the rate of accumulation of the pathogen, and the presence of ruptures in the fluorescence zone of the vasicentric parenchyma, indicating the termination of its spread through tissues, all varieties were divided into 3 groups. The validity of such a division of varieties in terms of resistance to the pathogen was confirmed by the method of evaluating the indicator that characterizes the degree of staining of the stem vessels cut longitudinally from the soil level. Measurement of the physiological parameters of the hypersensitivity reaction of infected tissues allowed us to divide the studied cotton genotypes in more detail according to the degree of resistance to the causative agent of Verticillium wilt into 4 groups: 1 – unstable, in which there was no hypersensitivity reaction: Ibrat; 2 – susceptible varieties, the quantitative content of phytoalexin – Isohemigossypole in the tissues of which was fixed at a level less than 10 μg/g of raw tissue: Bukhoro-102, S-4727; 3 – medium-resistant varieties: Navbahor-2, Bukhoro-6, Sulton; 4 – resistant varieties: Gulbahor-2, Ishonch.
INTRODUCTION
One of the most important components of the adaptive capabilities of plants and the ability to produce a stable harvest in changing environmental conditions is their resistance to biotic factors, in particular to diseases (Dyakov, 1983; Hanson, 2000; Figen, 2002). Physiological and biochemical study of various aspects of the life of plants makes it possible to use the immune properties of the organism itself, with which help it defends itself against the attack of pathogens in nature.
A distinctive feature of the reaction of plants to the introduction of vascular pathogens is a number of successively developing processes that create barriers to the advancement of the fungus to the xylem vessels. In this case, the browning of the vascular walls occurs, associated with a change in polyphenolic compounds and necrosis of the cells surrounding the vessels, from which polyphenolic compounds diffuse into the vessels, causing lignification of their walls (Avazkhodjaev, Zeltzer, 1980; Avazkhodjaev et al., 1990). As a result, their mechanical strength increases, the penetration and spread of the parasite and the inflow of nutrients to it are limited, which increases the protection of the wall components from the attack of fungus enzymes (Deising et al., 2009).
In this regard, the degree of browning of the longitudinal cut of the stem can be used as a criterion for assessing the wilt resistance of plants. In addition, the property of phenolic compounds and their oxidation products to fluoresce in ultraviolet light is used to diagnose plant diseases with wilt (Subba-Rao, 1954; Cohen, Ibrahim, 1975), and the rate of advancement of fluorescent substances front in the tissues of the infected cotton stem sections and the presence or absence of breaks in the fluorescence zones characterize the degree of its wilt resistance (Lanetskiy, 1970; Avazkhodjaev, Zeltzer, 1980).
The main manifestation of varietal immunity of cotton is the induction of a hypersensitivity reaction in plant tissues infected with the causative agent of Verticillium wilt. This increases the synthesis of fungitoxic substances, post-infectious inhibitors of growth and sporulation of the fungus, phenolic compounds of the gossypole series – phytoalexins (Metlitskiy, Ozerets-kovskaya, 1985; Kodama et al., 1988; Avazkhodjaev et al., 1995; Kuc, 1995; Zeringue, 1995; Hammerschmidt, 1999; Hanson, 2000; Dyakov et al., 2001; Figen, 2002; Mavlanova, 2012; Konan et al., 2014), however, the intensity of phytoalexin formation in different cotton genotypes differs markedly (Agaev et al., 2009; Kurbanbaev et al., 2011; Khotamov, Rejapova, 2019). In this regard, to characterize the resistance of cotton to Verticillium wilt, the generally accepted method for determining the parameters of the hypersensitivity reaction in infected plant tissues is used.
Considering that this method has been successfully tested on samples of the genetic collection of cotton from the Institute of Genetics and Experimental Biological of Plants of the Uzbek Academy of Sciences (Kurbanbaev et al., 2011; Khotamov, Rejapova, 2019), it is of interest to compare the content of phytoalexins, isohemigossypol and phytoalexin-equivalent, toxic to the causative agent of the disease, in tissues artificially infected with the fungus Verticillium dahliae Kleb. A comprehensive study, including various physiological and biochemical methods, can make it possible to establish the degree of wilt resistance of genotypes – representatives of cotton varieties zoned in Uzbekistan with a high probability.
The aim of this work was to study the resistance of cotton varieties to the Verticillium wilt causative agent by measuring the physiological parameters of the hypersensitivity reaction of tissues infected with the pathogen, the degree of staining and ultraviolet fluorescence of the plant stem longitudinal sections.
MATERIALS AND METHODS
In the experiment, 8 promising or zoned varieties of cotton (Gossypium hirsutum) were used: С-4727, Ibrat, Sulton of the Scientific Research Institute of Breeding and Seed Growing of Ministry of Agriculture of Uzbekistan, Bukhoro-6, Bukhoro-102 of the Scientific Research Institute of Cotton Growing of Ministry of Agriculture of Uzbekistan, Ishonch, Novbahor-2, Gulbahor-2 of the Institute of Genetics and Experimental Biology of Plants of the Academy of Sciences of the Republic of Uzbekistan.
The degree of plant injury was studied in artificial infection of cotton in phase with 5–6 true leaves by microcapillary inoculation the root neck zone with spore suspension of Verticillium dahliae race-2.
A monospore culture of V. dahliae from the Collection of Unique Scientific Subjects of Phytopathogens and Other Microorganisms of the Mycology Laboratory of the Institute of Genetics and Experimental Plant Biology of the Academy of Sciences of the Republic of Uzbekistan was grown for 8 days in test tubes on a solid Czapek medium of the following composition: NaNO3 –3 g; KH2PO4 – 1 g; MgSO4 – 0.5 g; KCl – 0.5 g; FeSO4 – 0.01 g, sucrose – 30 g, agar-agar – 20 g per 1 liter of distilled water. The inoculation was carried out by the prick method on the surface of the agar medium. Conidia or microsclerotia of micromycetes, as well as a mixture of these fungal structures, were used separately as inoculum. Then, using the microbiological loop aseptically part conidia were transferred to petri dishes with Czapek medium where they are germinated in a thermostat at a temperature of 27°C in complete darkness. Spore suspension was prepared by shaking 2 ml of sterile distilled water in a test tube with a fungus culture of 10–15 days of age (Mavlanova, 2012). The density of fungal spores in suspension was calculated according to the method described in (Israeli et al., 1968). The suspension of fungal conidia obtained in a test tube, after calculating its density, was diluted at the required concentration.
Plants were infected by inoculum injection (2.5 mln spores/ml) using a triple injection into the stem with a syringe. The inoculum was discharged from the syringe as a drop of suspension at the end of the needle. The needle is inserted into the stem at an angle of 45°. The drop was sucked into the stem, and this gave visible confirmation of inoculation. The appearance of chlorosis on the lower leaves of cotton, yellowing of tissues and necrotization of areas of the leaf blade testified to infection of plants by wilt (Avazkhodjaev, Zeltzer, 1980).
Control over the germination of conidia in the tissues of the infected plant was carried out by the method of fluorescence diagnostics (Lanetsky, 1970). Observations of the development of infection by the formation and advancement of the front of fluorescent substances along the stem of cotton infected with wilt were carried out in a stream of UV rays in darkened conditions. The source of ultra-violet rays were mercury quartz lamp SVDSH-120A with filter UFS in PRK-4 unit, transmitting light waves in the range 3600–4000 Å. Longitudinal sections of cotton stalks were watched and value of the fluorescence band and its progress up to the point of growth of the germ were measured. The infected tissue of the stem fluoresces with bright yellow-green light, and the intact tissue – with violet light. As control served plants, into the root neck of which distilled water was similarly introduced. In all cases, in the control group, formation of a specific fluorescence in the longitudinal sections was not observed.
When assessing the resistance of cotton to the causative agent of Verticillium wilt, an indicator was used that characterizes the degree of staining of the vessels of the stem cut in the longitudinal direction from the soil level (Avazkhodjaev, Zeltzer, 1980). The assessment of the degree of staining was carried out according to the scale for recording the staining of the stem vessels (in points from 0 to 3): 0 – no symptoms; 1 – light brown stripes of reddened vessels; 2 – dark brown stripes of stained vessels are visible; 3 – dark-brown stripes of painted vessels, death of the plant.
When studying the parameters of the hypersensitivity reaction, etiolated hypocotyls of cotton were used. Seedlings were grown in a thermostat at 24°C. The qualitative composition of phytoalexins (FA) was determined by thin layer chromatography on Silufol-UV-254 plates from Kavalier (Czech Republic). For the quantitative determination of isohemigossypol (IHG) and phytoalexin-equivalent (FA-E), cotton seedlings were finely chopped with scissors, filled with chloroform at 1 : 3 ratio, and placed in a refrigerator for 24 hours. Then obtained chloroform extract containing FA was filtered from solid residues and dried with a water jet pump. The dried residue was dissolved in 1 ml of chloroform. The resulting chloroform eluate was used for coating Silufol UV-254 plates. The plates were placed in chromatographic chambers and a single separation was performed in a benzol – methanol (9 : 1) solvent system. Benzol and methanol must be anhydrous because with an admixture of water, the system turns out to be cloudy and unsuitable for analysis. The heat-dried chromatograms were viewed under UV light and developed with fluoroglicin (2% in 96% ethanol). For this, the plate was covered with a developing reagent using a spray gun, then left for several minutes in air to evaporate the solvent, after which the chromatograms were heated for 1 min in a thermostat at 100°C. Phytoalexins IHG and FA-E are stained in a stable dark crimson color. The quantitative determination of FA was carried out by elution of spots from chromatograms with a benzol-methanol mixture (9 : 1) and measurement of the color intensity on a photoelectrocolorimeter with a blue light filter. The eluate from the clean zone of the chromatogram was used as a standard. The quantitative content of IHG was calculated using a pre-drawn calibration graph using pure IHG preparation as a standard. The results obtained were expressed in μg per g of wet tissue of etiolated seedlings.
The experiments were carried out 3–4 times. The results were processed by methods of mathematical statistics according to Dospehov (1985).
RESULTS
The germination of infectious structures and the development of pathological processes in the tissues of infected plants was controlled by the formation and advancement of the fluorescence zone. Fluorescence diagnostics showed that in the infected tissues of all studied cotton genotypes, 48 hours after infection, a pathological process began, as evidenced by the formation of a specific fluorescent zone at the injection sites of the inoculum. Further studies of longitudinal sections of cotton in a stream of ultraviolet rays showed that the zone of fluorescent substances moves up towards the point of plant growth, spreading along the veins of leaves.
The study of the disease incubation period dynamics, the length of which was limited by the dosed inoculum (Avazkhodjaev, Zeltzer, 1980), showed a different degree of activation of plant defense reactions (Fig. 1). Thus, in the cotton varieties Gulbahor-2 and Ishonch, the rate of advancement of the fluorescence zone during the first 5 days of the incubation period was lower than in all other 6 varieties, in which by day 8, fluorescent substances, spreading along the leaf veins, reached the growth point. A week later, i.e. 15–16 days after infection, varieties S-4727, Ibrat and Bukhoro-102 showed symptoms of wilt disease in the form of necrotic spots on plant leaves. Similar symptoms of the disease, but to a somewhat lesser extent, were manifested in varieties Navbahor-2, Sulton and Bukhoro-6 only by day 18–20 after artificial infection of plants. It should be noted that there are no signs of the disease in the cotton varieties Gulbahor-2 and Ishonch.
Fig. 1.
Dynamics of fluorescent substances front advancement along the cotton stem. The ordinate shows the size of the fluorescence zone, in % of the stem length from the site of inoculation to the point of growth. Cotton varieties: 1 – Ibrat; 2 – S-4727; 3 – Bukhoro-102; 4 – Sulton; 5 – Bukhoro-6; 6 – Navbahor-2; 7 – Ishonch; 8 – Gulbahor-2.
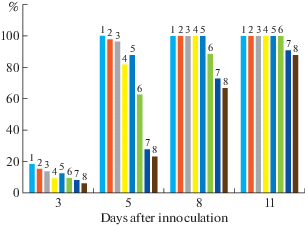
Thus, the differences in the infection of varieties appear at the earliest stages of the incubation period: Gulbahor-2 and Ishonch are characterized by a slower advance of the fluorescence front as compared to other cotton genotypes. At the same time, by the day 11 after infection, varieties Gulbahor-2 and Ishonch are observed numerous ruptures of fluorescence zones, indicating the pathogen spread cessation through plant tissues. To a comparatively lesser extent, ruptures of the fluorescent zones were observed in Navbahor-2 varieties and to a much lesser extent in Bukhoro-2 and Sulton. A continuous, without gaps, fluorescence zone in infected plants of the remaining three varieties (S-4727, Ibrat and Bukhoro-102) spreads along the veins of leaves with the manifestation of external signs of wilt disease. This indicates differences in duration of the incubation period in the studied varieties: its long duration in varieties Gulbahor-2 and Ishonch, to a certain extent, reflects resistance to the pathogen, the accumulation of which is slower (Agaev et al., 2009).
To confirm these conclusions, studies were continued, in which the degree of staining of the stem vessels was used as a criterion for assessing wilt resistance. By the degree of staining, according to the assessment in points, the plants were divided into 4 groups (0, 1, 2, 3) and the percentage of plants in each group was calculated from the total number used in the experiment.
As the obtained data showed, the infection of cotton with the causative agent of Verticillium wilt leads to a significant redistribution of the number of plants of all varieties in groups relative to the control (Table 1). So, for all varieties, the appearance of plants with weak signs of vessels staining (group 1) and with signs characteristic of group 2 was noted. The appearance of plants with traits belonging to the lethal group 3 was not detected only in representatives of 2 varieties (Ishonch, Gulbahor-2). At the same time, the largest number of plants assigned to the lethal group 3 (more than 30%) were characteristic of Ibrat variety and, to a somewhat lesser extent (19–21%) Bukhoro-102 and S-4727. Varieties Sulton, Bukhoro-6 and Navbahor-2 occupied an intermediate position in this indicator, because the number of plants with dark brown stripes of stained vessels did not exceed 10%.
Table 1.
Distribution of cotton plants varieties in 4 groups in accordance with the degree of staining of stem vessels (in % of total plants number in each experimental group)
| Variety | Plant groups (in points from 0 to 3) | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Ibrat (control) | 100 | – | – | – |
| Ibrat (V. d.) | 22.4 | 21.7 | 25.1 | 30.8 |
| S-4727 (control) | 100 | – | – | – |
| S-4727 (V. d.) | 36.9 | 17.7 | 24.2 | 21.2 |
| Bukhoro-102 (control) | 100 | – | – | – |
| Bukhoro-102 (V. d.) | 34.6 | 24.8 | 21.2 | 19.4 |
| Sulton (control) | 100 | – | – | – |
| Sulton (V. d.) | 56.4 | 18.8 | 16.5 | 8.3 |
| Bukhoro-6 (control) | 100 | – | – | – |
| Bukhoro-6 (V. d.) | 4 4.3 | 23.6 | 22.6 | 9.5 |
| Navbahor-2 (control) | 100 | – | – | – |
| Navbakhor-2 (V. d.) | 50.0 | 16.7 | 26.2 | 7.1 |
| Ishonch (control) | 100 | – | – | – |
| Ishonch (V. d.) | 74.1 | 14.8 | 11.1 | – |
| Gulbahor-2 (control) | 100 | – | – | – |
| Gulbahor-2 (V. d.) | 78.0 | 13.1 | 8.9 | – |
The above data correlate with the number of plants with signs of infection by causative agent of Verticillium wilt. Thus, in Ibrat variety, about 35% of diseased plants were detected, in varieties S-4727 and Bukhoro-102, plants with similar signs of infection were noted in 24–26% of cases, in varieties Sulton, Bukhoro-6, Navbahor-2 this indicator did not exceed 14%, and resistant varieties (Ishonch and Gulbahor-2) were characterized, practically, by the absence of plants infected with wilt.
Figure 2 shows thin-layer chromatography on Silufol-UV-254 plates (chromatograms were developed by fluoroglicin) of chloroform extract from etiolated hypocotyls of cotton seedlings, 48 hours after artificial infection of plants with the causative agent of Verticillium wilt.
Fig. 2.
The use of infected cells hypersensitivity biochemical markers in rapid assessment of cotton varieties disease resistance: 1 – Ibrat; 2 – S-4727; 3 – Bukhoro-102; 4 – Sulton; 5 – Bukhoro-6; 6 – Navbahor-2; 7 – Ishonch; 8 – Gulbahor-2; c – control (intact tissue); inf – experiment (infection with Verticillium wilt). IHG content: (+) – less than 10 μg/g of tissue; (++) – more than 10 μg/g of tissue; (+++) – necrogenic concentrations; (–) – no reaction. The arrow indicates the zone corresponding to the IHG.
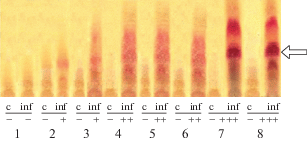
Chromatograms analysis made it possible to reveal that infection of plants with the causative agent of the disease induces a hypersensitivity reaction in the etiolated seedlings hypocotyls tissues of most of the cotton varieties used in the experiments, except for Ibrat variety. To the greatest extent, the hypersensitivity reaction is manifested in infected tissues of cotton varieties Ishonch and Gulbahor-2, the total content of phytoalexins in which reaches concentrations that are practically toxic for the pathogen. The varieties Sulton, Bukhoro-6, and Navbahor-2, under conditions of wilt infection, were characterized by a lower ability to induce phytoalexin formation processes, while IHG concentration exceeded 10 μg/g of raw tissue, but did not reach necrogenic. And a very weak reaction to infection was shown by varieties S-4727 and Bukhoro-102, the content of IHG in the tissues of which was lesser than 10 μg/g (Table 2).
Table 2.
The content of phytoalexins – isohemigossypol (IHG) and phytoalexin-equivalent (FA-E) in etiolated hypocotyls tissues of cotton varieties seedlings 48 hours after infection of plants with causative agent of Verticillium wilt
| Variety | Phytoalexin content, μg/g raw tissue | |
|---|---|---|
| IHG | FA-E | |
| Ibrat | 0.1 ± 0.09 | 0.2 ± 0.15 |
| S-4727 | 9.0 ± 0.95 | 7.8 ± 0.92 |
| Bukhoro-102 | 7.3 ± 0.88 | 6.9 ± 0.72 |
| Sulton | 18.4 ± 1.99 | 15.2 ± 1.61 |
| Bukhoro-6 | 17.7 ± 1.79 | 13.4 ± 1.38 |
| Navbahor-2 | 25.2 ± 2.39 | 19.8 ± 2.07 |
| Ishonch | 34.8 ± 3.53 | 25.7 ± 2.62 |
| Gulbahor-2 | 32.6 ± 3.45 | 24.5 ± 3.01 |
Thus, the assessment of various genotypes by using biochemical markers of the hypersensitivity of infected cells made it possible to divide in more detail the cotton varieties studied in the experiment according to the degree of wilt resistance into 4 groups: 1 – unstable, in which the absence of a hypersensitivity reaction was noted: Ibrat; 2 – susceptible varieties, the quantitative content of IHG in the tissues of which was fixed at a level less than 10 μg/g; Bukhoro-102, S-4727; 3 – medium resistant varieties: Bukhoro-6, Sulton; 4 – resistant varieties: Gulbahor-2, Ishonch. In addition, the Navbahor-2 variety, according to the phytoalexin formation indices, occupies an intermediate position between the groups of medium-resistant and resistant varieties, nevertheless, it was assigned to the group of medium-resistant varieties.
DISCUSSION
Recently, the widespread development of studies of the mechanism of the relationship between the host plant and the pathogen has made it possible to approach the understanding of physiological and biochemical factors from new positions that determine the resistance and susceptibility of cotton to verticillium wilt (Avazkhodjaev et al., 1995; Deising, 2009; Karademira et al., 2012; Le et al., 2020). These factors underlie various methods for identifying pathogen-resistant genotypes (Avazkhodjaev, Zeltzer, 1980; Hanson, 2000; Dyakov et al., 2001; Malinovsky, 2004; Çelik et al., 2019). Among them, one can single out methods for determining the rate of growth of the zone of substances fluorescent in ultraviolet rays in the stems of plants infected with wilt and the degree of brownness of the cotton stem (Subba-Rao, 1954, Lanetsky, 1970; Metlitskiy, Ozeretskovskaya, 1985; Agaev et al., 2009). The use of these methods allowed us to divide a number of varieties zoned in Uzbekistan according to the values of the studied indicators into 3 groups.
Another effective method for determining the wilt resistance of plants is the method of measuring the physiological parameters of the hypersensitivity reaction of the tissues of artificially infected seedlings, which is based on measuring the content of phytoalexins, the particular importance of which in the protective reactions against wilt in cotton and other crops is noted in a number of studies (Cohen, Ibrahim, 1975; Kodama et al., 1988; Avazkhodjaev et al., 1995; Kuc, 1995; Hammerschmidt, 1999; Figen, 2002; Kurbanbaev et al., 2011; Mavlanova, 2012; Khotamov, Rejapova, 2019). Differences in the intensity of phytoalexin formation in the tissues of etiolated seedlings made it possible to assign more correctly 8 cotton genotypes to 4 groups that differ in the degree of resistance to the causative agent of Verticillium wilt.
Comparison of the experimental data on the efficiency of phytoalexin formation in the tissues of infected seedlings and the qualitative and quantitative indicators of the fluorescent zones of stem sections, as well as the degree of their staining in various cotton genotypes, showed the presence of certain dependencies between them. Calculation of the Pearson correlation coefficient (rp) between the content of IHG as the main phytoalexin, which determines the efficiency of the reaction of cotton hypersensitivity to the phytopathogen, and the length of the fluorescence zone made it possible to establish the value rp = –0.92 with an average error of the correlation coefficient mr = 0.058. Thus, a high negative correlation was found between the compared indicators of resistance of different cotton varieties to the causative agent of Verticillium wilt: the shorter the length of the fluorescence zone of the infected tissues, the more effective the system of plant defense reactions, determined by the level of FA content in the tissues of resistant varieties. Conversely, a low level of IHG synthesis contributes to the unimpeded spread of the pathogen through the tissues of susceptible varieties, which is reflected in an increase in their fluorescence level. In addition, a fairly high correlation was found between the indices of the size of the fluorescence zone, as well as the content of phytoalexins on the one hand, and the number of plants with characteristic signs of wilt disease, on the other. The correlation coefficients between them are set at 0.86 ± 0.131 and –0.88 ± 0.091, respectively. This indicates the legitimacy of using these methods in the study of plant wilt resistance and a fairly accurate assessment of the degree of resistance of the studied cotton genotypes to the causative agent of Verticillium wilt – the fungus V. dahliae. A complex of physiological and biochemical studies established differences in the degree of wilt resistance of various varieties of cotton, which, according to this feature, are divided into groups: unstable (Ibrat), susceptible (C-4727, Bukhoro-102), medium-resistant (Sulton, Bukhoro-6, Navbahor-2) and resistant varieties (Ishonch, Gulbahor-2).
Список литературы
Agaev G.M., Avazhodjaev M.H., Nabiev B.A. et al. Realization of immunology control of cotton plant in dependence on cultivation technologies. Uzbekskiy biologi-cheskiy zhurnal. 2009. № 3. P. 49–52 (in Russ.).
Avazkhodjaev M.Kh., Zeltzer S.S., Nuritdinova H. et al. Phytoalexins as a factor in wilt resistance of cotton. In: Handbook of phytoalexin metabolism and action. Marcel Dekker Inc., N.Y. etc., 1995, pp. 129–160.
Avazkhodjaev M.Kh., Nuritdinova H.V., Zeltzer S.S. Dynamics of pathological changes in xylem of the cotton plant under the influence of the fungus Verticillium dahliae. Mikologiya i Fitopatologiya. 1990. V. 24 (3). P. 240–244 (in Russ.).
Avazkhodjaev M.Kh., Zeltzer S.S. Physiological factors of cotton wilt resistance. Tashkent, 1980 (in Russ.).
Çelik S., Bardak A., Erdoğan O. Screening of upland cotton genotypes (Gossypium hirsutum L.) against cotton Verticillium (Verticillium dahliae Kleb) wilt. Bangladesh J. Bot. 2019. V. 48 (4). P. 1185–1192.
Cohen V., Ibrahim R.K. Changes in phenolic compounds of sunflowers infected by Plasmopara halstedii. Can. J. Bot. 1975. V. 53 (22). P. 231–238.
Deising H.B., Horbach R., Ludwig N. et al. Mechanisms of fungal Infection. In: The 3rd International Symposium on plant protection and plant health in Europe “Crop plant resistance to biotic and abiotic factors: Current potential and future demands”. Berlin, Dahlem, 2009, pp. 290–302.
Dospekhov B.A. Field experiment technique (with the basics of statistical processing of research results). Agropromizdat, Moscow, 1985 (in Russ.).
Dyakov Yu. T. Physiological-biochemical mechanisms of resistance of plants to fungal diseases. In: Itogi Nauki i tekhniki. V. 3. Mechanisms of plants resistance to viruses and fungi. Moscow, 1983, pp. 5–90 (in Russ.).
Dyakov Yu.T., Ozeretskovskaya O.L., Javakhia V.G. et al. General and molecular phytopathology. Moscow, 2001 (in Russ.).
Hammerschmidt R. Phytoalexins: What have we learned after 60 years? Ann. Rev. Phytopathol. 1999. V. 37. P. 285–306.
Hanson L.E. Reduction of Verticillium wilt symptoms in cotton following seed treatment with Trichoderma virens. J. Cotton Science. 2000. № 4. P. 224–231.
Izrailskiy V.P., Shklyar S.N., Beltyukova K.I. et al. A guide for studying bacterial plant diseases. General issues. Kolos, Moscow, 1968 (in Russ.).
Karademira E., Karademira Ç., Ekincia R. et al. Effect of Verticillium dahliae Kleb on cotton yield and fiber technological properties. Int. J. Plant Prod. 2012. V. 6 (4). P. 1735–6814.
Khotamov M.M., Rejapova M.M. Resistance of variety Gossypium hirsutum L. species to Verticillium wilt. Int. J. Innovative Research in Multidisciplinary Field. 2019. V. 5 (5). P. 78–80.
Kodama O., Suzuki T., Miyokawa J. et al. Ultraviolet induced accumulation of phytoalexines in rice leaves. Agr. Biol. Chem. 1988. V. 52. P. 2469–2473.
Konan Y.K.F., Kouassi K.M., Kouakou K.L. et al. Effect of methyl jasmonate on phytoalexins biosynthesis and induced disease resistance to Fusarium oxysporum f. sp. vasinfectum in cotton (Gossypium hirsutum L.). Int. J. Agronomy. 2014. https://doi.org/10.1155/2014/806439
Kuc J. Phytoalexins, stress metabolism and disease resistance in plants. Ann. Rev. Phytopathol. 1995. V. 33. P. 275–297.
Kurbanbayev I.Zh., Agaev G.M., Khotamov M.M. et al. Characteristics of some samples of cotton germplasm for resistance to wilt disease. Uzbekskiy Biologicheskiy Zhurnal. 2011. № 2. P. 49–51 (in Russ.).
Lanetsky V.P. Fluorescence of pathological metabolites in the vascular system of cotton plants infected with Verticillium. In: Science for plant protection. Voronezh, 1970, pp. 31–39 (in Russ.).
Le D.P., Gregson A., Tran T.T. et al. Co-occurrence of defoliating and non-defoliating pathotypes of Verticillium dahliae in field-grown cotton plants in New South Wales, Australia. Plants. 2020. V. 9 (6). P. 750–758.
Malinovsky V.I. Plant physiology. Textbook. university manual. Vladivostok, 2004 (in Russ.).
Mavlanova S.A. Physiological and biochemical peculiarities of the induced resistance of cotton to sucking pests-insects and Verticillium wilt exitant. Cand. Biol. Sci. Thesis. Tashkent, 2012 (in Russ.).
Metlitskiy L.V., Ozeretskovskaya O.L. How plants protect themselves from disease. Nauka, Moscow, 1985 (in Russ.).
Mert-Türk F. Phytoalexins: Defense or just a response to stress? J. Cell Mol. Biol. 2002. № 1. P. 1–6.
Subba-Rao N.S. Fluorescence phenomenal in fusariose wilt of cotton. J. Ind. Bot. Soc. 1954. V. 4. P. 33–36.
Zeringue H.J. Cotton (Gossypium hirsutum) strategies of defense expression. In: Handbook of phytoalexin metabolism and action. Marcel Dekker Inc., N.Y. etc., 1995. pp. 161–198.
Авазходжаев М.Х., Зельцер С.Ш. (Avazhojaev, Zeltzer) Физиологические факторы вилтоустойчивости хлопчатника. Ташкент: ФАН, 1980. 122 с.
Авазходжаев М.Х., Нуритдинова Х., Зельцер С.Ш. (Avazhodjaev et al.) Динамика патологических изменений в ксилеме растений хлопчатника под воздействием гриба Verticillium dahliae // Микология и фитопатология. 1990. Т. 24. № 3. С. 240–244.
Агаев Г.М., Авазходжаев М.Х., Набиев Б.А. и др. (Agaev et al.) Реализация иммунологического контроля хлопчатника в зависимости от технологии его возделывания // Узб. биол. ж. 2009. № 3. С. 49–52.
Доспехов Б.А. (Dospekhov) Методика полевого опыта (с основами статистической обработки результатов исследований). М.: Агропромиздат, 1985. 351 с.
Дьяков Ю.Т. (Dyakov) Физиолого-биохимические механизмы устойчивости растений к грибным болезням // Итоги науки и техники. Защита растений. Т. 3. Механизмы устойчивости растений к вирусам и грибам. Москва, 1983. С. 5–90.
Дьяков Ю.Т., Озерецковская О.Л., Джавахия В.Г. и др. (Dyakov et al.) Общая и молекулярная фитопатология. М.: Общество фитопатологов, 2001. 302 с.
Израильский В.П., Шкляр С.Н., Бельтюкова К.И. и др. (Izrailskiy et al.) Руководство для изучения бактериальных болезней растений. Общие вопросы. Москва: Колос, 1968. 343 с.
Курбанбаев И.Ж., Агаев Г.М., Хотамов М.М. и др. (Kurbanbaev et al.) Характеристика некоторых образцов гермоплазмы хлопчатника на устойчивость к вилтовой болезни. Узб. биол. журнал. 2011. № 2. С. 49–51.
Ланецкий В.П. (Lanetskiy et al.) Флуоресценция патологических метаболитов в сосудисто-проводящей системе растений хлопчатника, инфицированного вертициллиумом // Наука – защите растений. Воронеж, 1970. С. 31–39.
Мавланова С.А. (Mavlanova) Физиолого-биохимические особенности индуцированной устойчивости хлопчатника к сосущим насекомым-вредителям и возбудителю вертициллезного вилта. Дисс. … канд. биол. наук. Ташкент, 2012. 132 с.
Малиновский В.И. (Malinovskiy) Физиология растений. Учеб. пособие вузов. Владивосток: ДВГУ, 2004. 94 с.
Метлицкий Л.В., Озерецковская О.Л. (Metlitskiy, Ozertsekovskaya) Как растения защищаются от болезней. М.: Наука, 1985. 114 с.
Дополнительные материалы отсутствуют.
Инструменты
Микология и фитопатология


